Abstract
Fas is an apoptosis-inducing surface receptor involved in controlling tissue homeostasis and function at multiple sites. Here we show that β cells from the pancreata of newly diagnosed insulin-dependent diabetes mellitus (IDDM) patients express Fas and show extensive apoptosis among those cells located in proximity to Fas ligand–expressing T lymphocytes infiltrating the IDDM islets. Normal human pancreatic β cells that do not constitutively express Fas, become strongly Fas positive after interleuken (IL)-1β exposure, and are then susceptible to Fas-mediated apoptosis. NG-monomethyl-l-arginine, an inhibitor of nitric oxide (NO) synthase, prevents IL-1β–induced Fas expression, whereas the NO donors sodium nitroprusside and nitric oxide releasing compound (NOC)-18, induce functional Fas expression in normal pancreatic β cells. These findings suggest that NO-mediated upregulation of Fas contributes to pancreatic β cell damage in IDDM.
The interaction of Fas (CD95/Apo-1) with its ligand promotes the deletion of potentially harmful, damaged, or unnecessary cells during the immune response. This interaction also regulates tissue remodelling and homeostasis. Impaired Fas-induced apoptosis results in abnormal cell proliferation and accumulation, whereas inappropriate expression or excessive Fas activity causes tissue damage (1–3).
Insulin-dependent diabetes mellitus (IDDM)1 is a T cell– mediated autoimmune disease resulting from a selective destruction of pancreatic β cells (4). Two complementary lytic pathways mediate T cell cytotoxicity: the exocytosis of perforin-containing granules on cognate target cells, and the engagement of Fas on cognate or neighboring target cells by membrane-bound or released Fas ligand (FasL; 5, 6).
Normal pancreatic β cells do not express Fas (7). However, we have recently shown that the exposure of human β cells to IL-1β induces Fas expression on these cells, suggesting the existence of an additional pathway that promotes β cell destruction (8). The possible role of FasL-based cytotoxicity in the pathogenesis of autoimmune diabetes is suggested by recent findings showing protection from both spontaneous and T cell–transferred diabetes in Fas negative NODlpr/lpr mice (9). IL-1β has been reported to be abundantly present during the insulitis process and selectively toxic for pancreatic β cells, as its secretion by activated macrophages induces transcription and translation of inducible nitric oxide synthase (iNOS) and nitric oxide (NO) production (10, 11). During the insulitis process, iNOS is highly expressed in β cells and islet-infiltrating macrophages (12). Although IL-1β–mediated NO production may result in β cell damage, and islet-infiltrating macrophages are a major source of NO, it seems unlikely that this direct cytotoxic effect of NO is responsible for massive and selective β cell destruction (13). We therefore studied the requirement for functional Fas expression in pancreatic β cells, and investigated the possible involvement of Fas and FasL in the pathogenesis of human IDDM.
Materials and Methods
Subjects.
The IDDM pancreata were from two boys (ages 12 and 11), who died in 1992 (patient No. 1) and 1993 (patient No. 2), respectively, after a diagnosis of type I diabetes mellitus complicated by diabetic ketoacidosis (14). Despite appropriate treatment based on fluid rehydration combined with insulin infusion therapy, both children developed severe brain edema with cerebellar tonsillar herniation. Upon admission to Children's Hospital of Pittsburgh (Pittsburgh, PA), they were found to be brain dead. After obtaining an informed consent from the parents, the tail, body, and part of the head of the pancreas were obtained at autopsy. In both cases, molecular HLA typing revealed heterozygosity at both DQA1 (i.e., patient No. 1: *0501, *0301; and patient No. 2: *0101, *0501) and DQB1 (i.e., patient No. 1: *0301, *0302; and patient No. 2: *0201, *0501) loci. Thus, both patients had the possibility of forming “diabetogenic” (αArg-52 and β-non-Asp57) DQ heterodimers (15). Islet cell antibodies were >20 Juvenile Diabetes Foundation (JDF) units in the serum of patient No. 1, and <20 in the serum of patient No. 2 (14). 10 normal human pancreas (NHP) specimens were provided by the Consorzio Centro Sud Trapianti (Palermo, Italy) and the Thomas E. Starzl Transplantation Center (Pittsburgh, PA) under their respective Institutional Review Board approvals. They were obtained from three female and seven male nondiabetic cadaveric donors (mean age was 25 yr old).
Flow Cytometry Analysis of Islet Cells.
Pancreatic islets were isolated by the intraductal pancreatic distension method as previously described (8). Single cell suspensions were obtained by dissociating islets with 3 mM EGTA, 20 mg/ml BSA, 2.8 mM glucose, and 0.83 μg/ml trypsin (8). Human IFN-γ (specific activity of 106 U/mg; Sigma Chemical Co., St. Louis, MO), TNF-α (specific activity of >2 × 107 U/mg; Boehringer Mannheim, Indianapolis, IN), IL-1β (specific activity of ∼5 × 108 U/ mg; Genzyme, Cambridge, MA), sodium nitroprusside (SNP), NG-monomethinyl-l-arginine (L-NMMA), and NG-monomethyl- d-arginine (D-NMMA; Sigma Chemical Co.), and NO releasing compound (Z)-1-[2-(2-aminoethyl)-(N)-(2-ammonioethyl)- ammo]diazen-1-ium-1,2-diolate] (NOC-18), l-arginine, and d-arginine (Alexis Corp., Laufelfingen, Switzerland) were added to the cultures at time 0. Cells were maintained in culture for 72 h and harvested for evaluation of Fas expression at different time points. Islet cells were first permeabilized in a fixative solution containing 0.25% paraformaldehyde and 0.2% Tween 20 for the labeling with anti-insulin mAb (IgG2a; Novo Nordisk, Bagsvaerd, Denmark), revealed by an FITC-conjugated goat anti– mouse Ig antibody. Cells were then incubated for 10 min with 6% normal mouse serum, treated with phycoerythrin-conjugated anti-CD95 (DX2, IgG1; PharMingen, San Diego, CA), washed twice in cold PBS/azide, and resuspended at 106 cells/ml for cytofluorometric analysis. An isotype-matched antibody was used as a negative control. Relative fluorescent intensities of individual cells were analyzed using a FACScan® flow cytometer (Becton Dickinson, Mountain View, CA).
Apoptosis Detection of Islet Cells.
After a 24-h culture in the presence of 50 U/ml IL-1β, 0.5 mM SNP, or IL-1β plus L-NMMA, dispersed islet cells were incubated with an isotype-matched control mAb or with 200 ng/ml of anti-Fas mAb (IgM, clone CH-11; Upstate Biotechnology Incorporated, New York, NY), or 10 μg/ml of anti-Fas–blocking mAb (IgG1, ZB4; Kamiya Biomedical Company, Tukwila, WA) for a total of 48 h. The percentage of cells undergoing apoptosis was measured by hypotonic fluorochrome solution staining (50 μg/ml propidium iodide in 0.1% sodium citrate plus 0.1% Triton X-100; Sigma Chemical Co.) detected with a FACScan® flow cytometer. Cell recovery was determined by evaluating the number of surviving insulin or glucagon positive cells recognized by specific mAbs (Novo Nordisk) out of 500 cells counted, revealed by a FITC-conjugated goat anti–mouse Ig antibody and observed by fluorescence microscopy.
Immunostaining Procedure.
Pancreas fragments (0.5 cm) were snap frozen in isopentane, chilled at −150°C and kept at −80°C until used. Serial cryostat pancreatic sections (5 μm) were allowed to equilibrate to room temperature and exposed to absolute acetone for 10 min before starting the alkaline phosphatase anti–alkaline phosphatase (APAAP) complex procedure, or to a hydrogen peroxide methanol solution for double staining (Dako Double Stain Kit; Dako Corp., Santa Barbara, CA). Detection of antiglucagon antibody (Dako Corp.) binding was accomplished using a horseradish peroxidase–rabbit anti–horseradish peroxidase complex (Dako Corp.) and revealed by 3,3′-diamino benzidine tetrahydrochloride. Bound anti-CD3 (Dako Corp.), anti-CD14 (Dako Corp.), anti–human perforin (provided by E.R. Podack, University of Miami, Miami, FL), anti-CD95 (DX2, IgG1; PharMingen), and anti-FasL (NOK-1, IgG1; PharMingen) mAbs were detected with APAAP mouse (Dako Corp.) and revealed by a Fast Red colorimetric substrate. Hematoxylin was used as a counterstain. Control tissue sections were subjected to identical treatment and analysis using irrelevant isotype-matched mAbs.
FasL Messenger RNA Analysis.
Total cytoplasmic RNA was prepared from the NHP-purified islets, IDDM-purified islets, and resting and PMA/ionomycin-activated PBLs from healthy individuals. The RNA was extracted using TRIZOL reagent (GIBCO BRL, Gaithersburg, MD) according to the manufacturer's instructions. For each preparation, 3 μg of total RNA was reverse transcribed into a single-stranded cDNA (GeneAmp RNA PCR Kit; Perkin-Elmer, Roche Molecular Systems, Inc., Branchburg, NJ).
In Situ Apoptosis Studies.
Pancreas cryostat sections (5 μm) were mounted onto microscope slides and fixed with 4% paraformaldehyde. Permeabilization of the tissue was determined by incubation with 0.1% Triton X-100, 0.1% sodium citrate (16). The labeling of 3′-OH fragmented DNA ends (TdT-mediated dUTP-X nick end labeling; TUNEL) was carried out by an in situ apoptosis detection kit (In Situ Cell Death Detection, AP; Boehringer Mannheim) following the instructions of the manufacturer. Detection of labeled ends was done with an antifluorescein antibody (Fab′2 fragment) conjugated with alkaline phosphatase. 5-bromo-4-chloro-3-indolylphosphate (Dako Corp.) was used as substrate. Sections were then counterstained with hematoxylin.
Results and Discussion
IL-1β Induces Selective Fas Expression in β Cells.
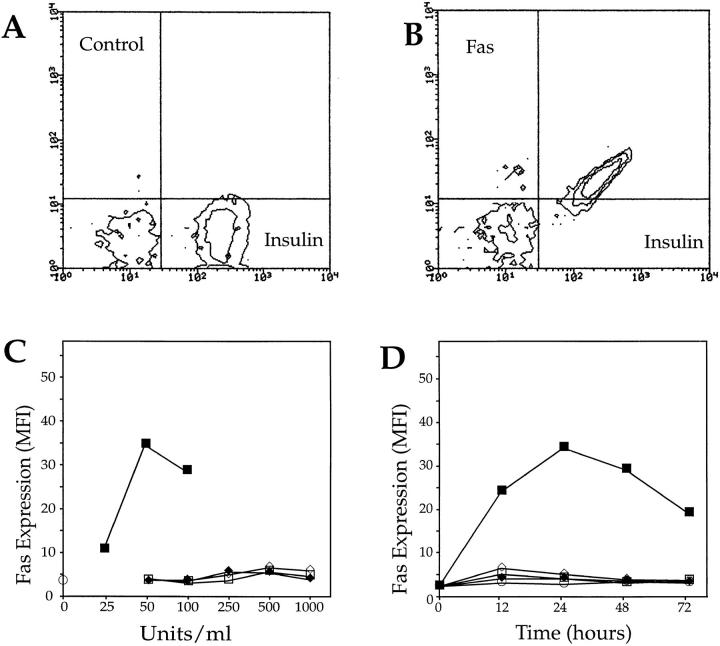
To determine whether IL-1β selectively induces Fas expression in β cells but not in other pancreatic endocrine cells, normal dispersed islet cells were treated 24 h with 50 U/ml IL-1β, double stained with anti-insulin and anti-Fas mAbs, and analyzed by flow cytometry. Fig. 1, A and B shows that IL-1β–induced Fas expression in islet cells was restricted to insulin-producing cells. The induction of Fas expression in β cells peaked after ∼24 h of IL-1β stimulation, and was not observed in any cell type after exposure to TNF-α or IFN-γ, alone or in combination (Fig. 1, C and D). These data suggest that some molecular component generated by IL-1β stimulation may be responsible for Fas induction in β cells.
Figure 1.
Fas expression induced by IL-1β on enzymatically dispersed NHP islet cells. Control (A) or Fas (B) expression on insulin-negative and -positive islet cells exposed for 24 h to 50 U/ml IL-1β. (C) Fas expression on β cells exposed for 24 h to varying (i.e., ranging from 25 to 1,000 U/ml) doses of recombinant IL-1β (closed squares), TNF-α (open diamonds), IFN-γ (closed diamonds), TNF-α plus IFN-γ (open squares), or tissue culture medium alone (open circle). (D) Time-course experiment (one of eight performed on eight different human pancreata) demonstrating Fas expression in β cells incubated with IL-1β (50 U/ ml), TNF-α, IFN-γ, and IFN-γ plus TNF-α (500 U/ml).
NO Induces Fas Expression in β Cells and Mediates IL-1β– induced Fas Upregulation.
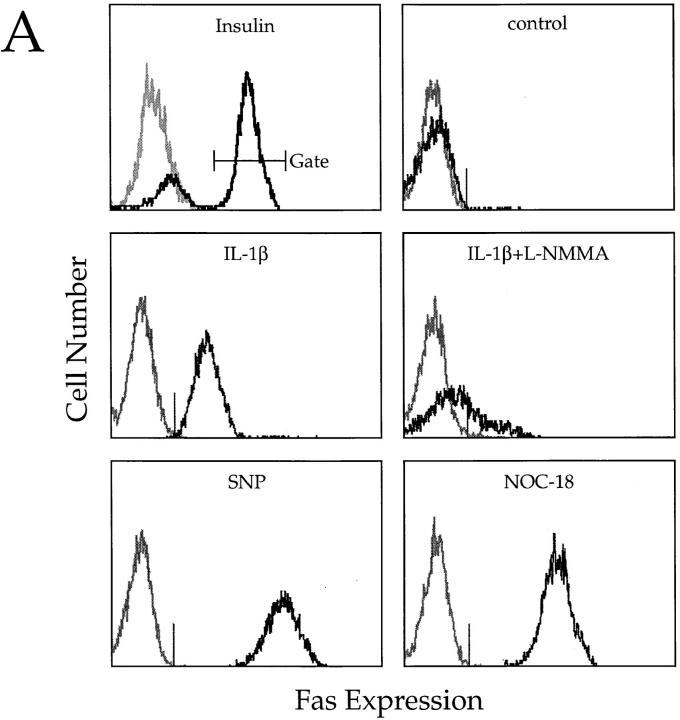
NO is a reactive nitrogen intermediate with multiple biological effects (17). Different from TNF-α or IFN-γ, IL-1β is a potent inducer of iNOS in islet cells (11). To investigate the possible contribution of NO in IL-1β–induced Fas expression, we treated normal dispersed islet cells with IL-1β in the presence or absence of the nitric oxide synthase inhibitor L-NMMA. Targeting NOS activation by L-NMMA but not D-NMMA (18), treatment inhibited IL-1β–induced Fas expression (Fig. 2, A and B). Also, inhibition by L-NMMA was completely reversed by excess of l-arginine, but not by d-arginine (19), suggesting that NO generation is directly responsible for IL-1β–induced Fas upregulation. To confirm this hypothesis, we exposed islet cells to the NO donors SNP and NOC-18 for 24 h and examined them for Fas expression. Both SNP and NOC-18 were extremely effective in inducing Fas expression in β cells (Fig. 2 A). As expected, L-NMMA did not interfere with NO-induced (NOC-18) Fas expression (Fig. 2 B). Taken together, these results suggest a primary role for NO in sensitizing β cells to Fas-induced apoptosis.
Figure 2.
Cytofluorometric analysis of enzymatically dispersed islet cells from NHPs. (A) Insulin-positive cells were gated for subsequent analyses. Untreated cells are Fas negative (control). Treatment with 50 U/ ml IL-1β or 0.5 mmol/liter SNP or 0.5 mg/ml NOC-18, induces Fas expression on gated insulin-positive cells. Exposure to 3 mmol/liter L-NMMA suppresses IL-1β–induced Fas on β cells. Reactivity with irrelevant isotype-matched mAbs is shown in gray. (B) Mean values of two experiments confirming results shown in A and demonstrating that exposure to 3 mmol/liter D-NMMA was not able to antagonize IL-1β–mediated Fas expression on β cells. The addition of l-arginine (15 mM) overrode L-NMMA inhibitory effect on IL-1β–induced Fas, whereas d-arginine (15 mM) did not. Also, L-NMMA did not reverse NOC-18–promoted Fas upregulation.
NO Primes Pancreatic β Cells for Fas-mediated Destruction.
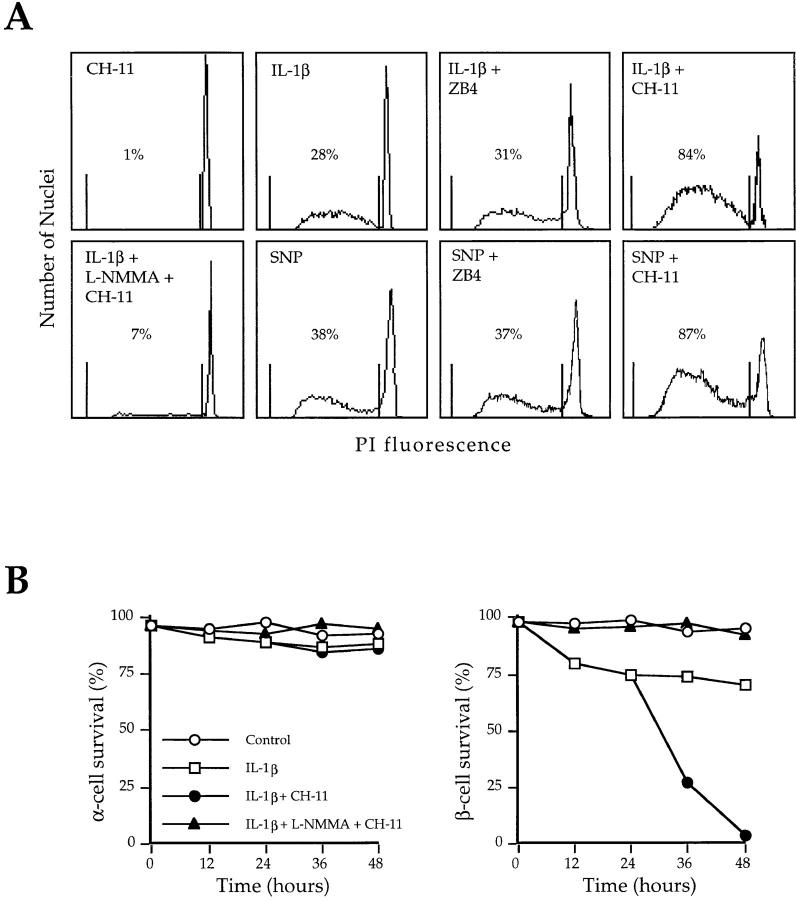
To determine whether Fas is functional and able to induce a death signal in β cells, we incubated IL-1β– or SNP– treated and untreated islet cells with an agonist (CH-11) or a blocking anti-Fas (ZB4) mAb and examined them for evidence of apoptosis. Treatment with CH-11 mAb in the absence of IL-1β or SNP did not trigger apoptosis. By contrast, incubation with CH-11 dramatically increased apoptosis in SNP– or IL-1β–treated-cells (Fig. 3 A). Immunofluorescence staining and fluorescence microscopy analysis revealed that after 24 h, essentially all cells surviving IL-1β and anti-Fas treatment were glucagon-positive α cells, whereas the recovery of insulin-producing cells was extremely low, indicating that only β cells underwent apoptosis (Fig. 3 B). Moreover, L-NMMA was able to completely suppress IL-1β–induced apoptotic cell death, whereas the anti-Fas antagonist ZB4 did not interfere with IL-1β– or SNP–induced apoptosis (Fig. 3 A). Thus, NO mediates both Fas-independent and Fas-dependent β cell apoptosis induced by IL-1β.
Figure 3.
Induction of apoptosis in human pancreatic islet cells by anti-Fas mAb (CH-11). (A) Islet cells incubated for 24 h in tissue culture medium and then exposed for an additional 24 h to 200 ng/ml CH-11 mAb, or in medium supplemented with IL-1β (50 U/ml) or 0.5 mmol/liter SNP followed by control IgM, or in medium containing IL-1β or SNP followed by 10 μg/ml ZB4 or CH-11 mAb, or in medium containing IL-1β plus 3 mmol/liter L-NMMA followed by CH-11 mAb, were processed for DNA content analysis by staining. Percentages of apoptotic (i.e., hypodiploid) nuclei are indicated between shift markers, plotted on log histograms as red fluorescence intensity (x-axis) versus relative nuclei number (y-axis). (B) Evaluation of cell survival of human α (left) and β cells (right) cultured in culture medium for 24 h and then for 24 h in medium in the absence of IL-1β, in medium supplemented with IL-1β (50 U/ml; open squares), and control mAb (open circles), or in medium with IL-1β and CH-11 mAb (closed circles), or in medium with IL-1β plus L-NMMA and CH-11 mAb (closed triangles). Data shown represent the values at the indicated time points of a representative experiment out of a total of seven independent experiments performed.
Fas Expression in Pancreatic β Cells During Insulitis.
To investigate whether Fas is expressed in pancreatic β cells during the insulitis process, we next analyzed expression and distribution of Fas in endocrine and infiltrating cells in pancreatic specimens from newly diagnosed IDDM patients. Immunohistochemical analysis of frozen pancreatic sections from IDDM patients revealed that the few remaining insulin-secreting β cells were strongly Fas positive, whereas the β cells from NHP were negative (Fig. 4). The cellular infiltrate in the diabetic pancreata was composed of a minor population of CD14-positive cells with the morphology of macrophages, and a number of CD3-positive lymphocytes (Fig. 4). The majority of islet-infiltrating T cells were CD4 positive, whereas CD8-positive cells were occasionally observed (14). All infiltrating T lymphocytes were perforin negative (Fig. 4), suggesting that, at the time of our analysis, perforin-mediated T lymphocyte cytotoxicity was not playing a major role in the destruction of the pancreatic β cells.
Figure 4.
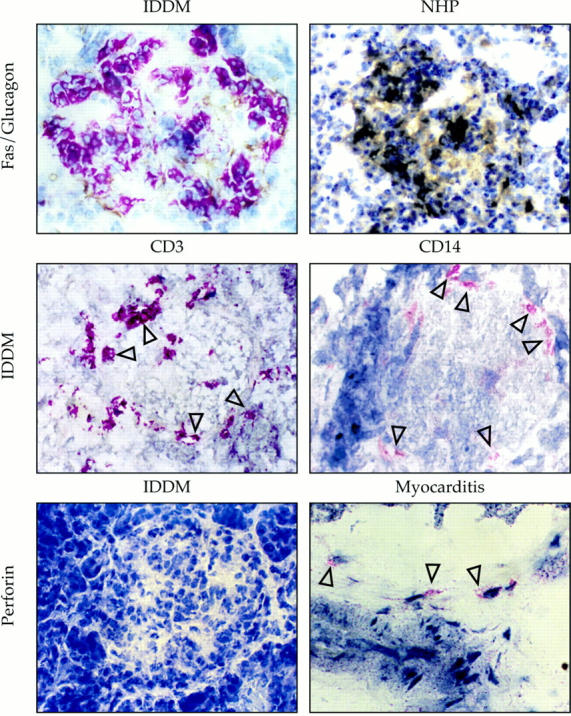
Fas expression on human pancreatic islet cells from NHP and newly diagnosed IDDM patients. Double immunohistochemical analysis of pancreas cryostat serial sections (top) exposed to anti-Fas mAb revealed by immunoenzymatic APAAP complex (red), and antiglucagon antibody revealed by peroxidase-anti-peroxidase complex (brown), shows that Fas is specifically expressed on the non–glucagon-stained, insulin-producing β cells. CD3 and CD14 expression (middle, red) on leukocyte subpopulations (arrows) present in pancreas cryostat sections from IDDM patients. Reactivity of pancreatic islets from an IDDM patient (bottom left) and of the cellular infiltrate within the heart of a patient with myocarditis as a positive control (arrows, bottom right) with an antiperforin antibody.
Detection of Apoptosis in Pancreatic β Cells Located in Proximity to Reactive FasL+ T Lymphocytes Infiltrating the Diabetic Pancreata.
Fas requires the interaction with its ligand to transduce the death signal. We therefore searched for FasL expression in normal and IDDM pancreata. Different from thyroid follicular cells (20), both the endocrine and the exocrine cells from NHP were FasL negative, as were the islet cells from IDDM patients (Fig. 5, A and B). However, activated T cells infiltrating the islets were strongly FasL positive (Fig. 5 A), as shown by the colocalization of FasL and CD3 positivity in serial sections (Fig. 4 and 5 A). Moreover, FasL transcripts were not detected in cDNA prepared from purified islets of NHP, but were found in cDNA preparations from diabetic pancreata (Fig. 5 B). Finally, TUNEL analysis of IDDM pancreas specimens showed extensive apoptosis among Fas-positive β cells that appears to proceed from the islet periphery (as shown in Fig. 5 C) to encompass the entire islet (not shown), most frequently in proximity to the CD3-positive cells producing FasL. β cells from NHP were consistently Fas negative and showed no evidence of apoptosis. As expected, apoptosis in infiltrating T lymphocytes was also observed. This may be the direct consequence of the autoregulated immune process in which T cells, expressing both Fas and FasL, are not only able to kill Fas positive target cells, but also themselves or one another (21, 22).
Figure 5.
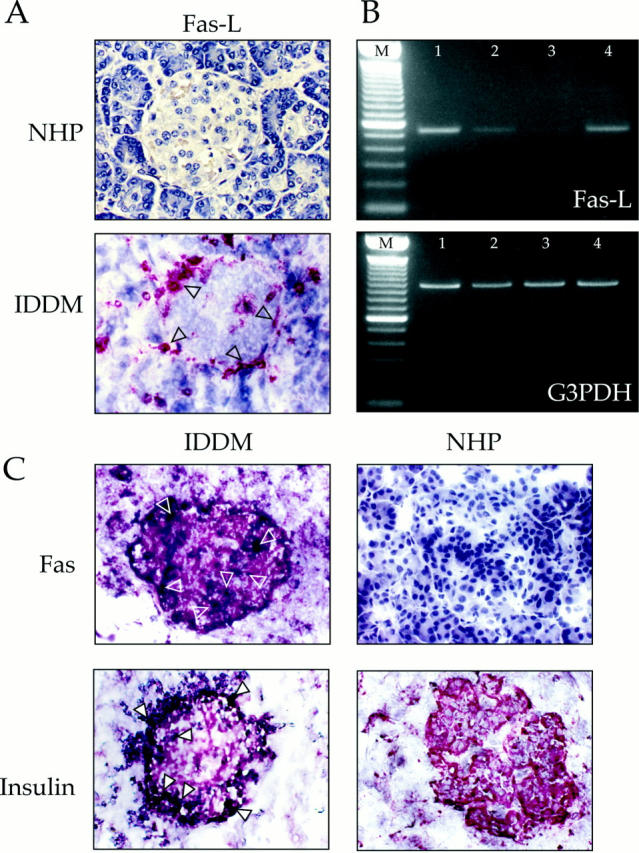
Detection of Fas ligand and apoptosis in vivo. (A) FasL expression on human pancreatic sections from NHP and from IDDM patients. (B) Lane M, marker (100-bp DNA ladder; GIBCO BRL, Gaithersburg, MD). Two primers specific for the FasL coding sequence (5′-CCA CCG CCA CCA CTA CCA-3′, nucleotides 212–229, and 5′-TCT TCC CCT CCA TCA TCA CC-3′, complementary to nucleotides 749–730; These sequence data are available from EMBL/GenBank/DDBJ under accession number U11821) were selected to specifically amplify FasL cDNA prepared from: lane 1, PMA/ionomycin on activated T lymphocytes from PBLs; lane 2, nonactivated PBLs; lane 3, islets purified from NHP; and lane 4, islets purified from IDDM patients' pancreata. A 983-bp segment of the glyceraldehyde 3-phosphate dehydrogenase (G3PDH) gene (Clontech, Palo Alto, CA) was amplified from duplicate aliquots of the same cDNA preparations. (C) In situ TUNEL reaction revealed by 5-bromo-4-chloro-3-indolyl phosphate (black) staining on IDDM and NHP pancreas sections prelabeled with anti-Fas or anti-insulin mAbs, using 3-amino-9-ethylcarbozole as a substrate (red). Arrows indicate apoptotic nuclei among Fas- or insulin-positive cells.
Concluding Remarks.
Fas-mediated cytotoxicity can be induced by both CD4- and CD8-positive T cells, being the major cytotoxic effector mechanism of CD4 cells (23, 24), which constitute the dominant subset of T cells infiltrating the IDDM pancreata we have examined (14). Multiple islet antigens have been claimed as major targets of the autoimmune aggression in IDDM, as both humoral and cell-mediated autoimmune responses are polyclonal and not β cell specific (25). However, the selective expression of Fas in β cells primed by NO may be responsible for their specific killing as T cells expressing FasL may promote an MHC unrestricted destruction of Fas-positive bystander β cells, while sparing neighbor Fas-negative α and δ cells. In accordance with these results, it has been recently demonstrated that transgenic NOD mice expressing FasL in β cells show a higher rate of spontaneous diabetes and are more sensitive to injection of diabetogenic T cells (9). By contrast, NODlpr/lpr mice, expressing a mutated Fas receptor, are resistant to both spontaneous and T cell transferred diabetes, suggesting that Fas-induced β cell destruction mediates both human and experimental autoimmune diabetes.
During the insulitis process, NO production is probably not confined to IL-1β–primed β cells, as high levels of NO could be released by several periinsulitis cell types, including dendritic cells, macrophages, and endothelial cells (10, 26). The produced NO may directly induce apoptosis in β cells, but NO-induced Fas expression may also mediate a new mechanism of β cell destruction. The simultaneous presence in IDDM pancreas of a number of apoptotic Fas-positive β cells and infiltrating FasL-positive lymphocytes suggests a major role for the aberrant Fas expression on pancreatic β cells in the pathogenesis of human autoimmune diabetes.
Footnotes
Dr. Stassi is personally indebted to GianDomenico Bompiani for his encouragement and support. The authors are also grateful to Dr. Susan Faas for her suggestions and constructive criticism, Read Fritsch who prepared the figures, and Patrick Hnidka who edited the manuscript.
The work was supported in part by National Institutes of Health grant DK46864 (M. Trucco) and CNR, Comitato Nazionale per le Biotecnologie e la Biologia Molecolare: Patologia Molecolare della malattia diabetica, 1996 (A. Galluzzo). R. De Maria is the recipient of an Associazione Italiana Ricerca sul Cancro fellowship.
Abbreviations used in this paper: APAAP, alkaline phosphatase antialkaline phosphatase; D-NMMA, NG-monomethyl-d-arginine; FasL, Fas ligand; IDDM, insulin-dependent diabetes mellitus; iNOS, inducible nitric oxide synthase; L-NMMA, NG-monomethyl-l-arginine; NHP, normal human pancreas; NO, nitric oxide; NOC, NO releasing compound; SNP, sodium nitroprusside; TUNEL, TdT-mediated dUTP-X nick end labeling.
References
- 1.Nagata S, Golstein P. The Fas death factor. Science (Wash DC) 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 2.Krammer PH. The CD95(APO-1/Fas) receptor/ ligand system: death signals and disease. Cell Death Differ. 1996;3:159–160. [PubMed] [Google Scholar]
- 3.Adachi M, Suematsu S, Kondo T, Ogasawara J, Tanaka T, Yoshida N, Nagata S. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nat Med. 1995;11:294–299. doi: 10.1038/ng1195-294. [DOI] [PubMed] [Google Scholar]
- 4.Bach JF. Insulin-dependent diabetes mellitus as an autoimmune disease. Endocr Rev. 1994;15:516–542. doi: 10.1210/edrv-15-4-516. [DOI] [PubMed] [Google Scholar]
- 5.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature (Lond) 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 6.Kägi D, Vignaux F, Ledermann B, Bürki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell–mediated cytotoxicity. Science (Wash DC) 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 7.Leithauser F, Dhein J, Mechtersheimer G, Koretz K, Bruderlein S, Henne C, Schmidt A, Debatin KM, Krammer PH, Moller P. Constitutive and induced expression of Apo-1, a member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest. 1993;69:415–429. [PubMed] [Google Scholar]
- 8.Stassi G, Todaro M, Richiusa P, Giordano M, Mattina A, Sbriglia S, Lo A, Monte, Buscemi G, Galluzzo A, Giordano C. Expression of apoptosis-inducing CD95 (Fas/Apo-1) on human β-cells sorted by flow-cytometry and cultured in vitro. Transplant Proc. 1995;27:3271–3275. [PubMed] [Google Scholar]
- 9.Chervonsky AV, Wang Y, Wong FS, Visintin I, Flavell RA, Janeway CA, Matis LA. The role of Fas in autoimmune diabetes. Cell. 1997;89:17–24. doi: 10.1016/s0092-8674(00)80178-6. [DOI] [PubMed] [Google Scholar]
- 10.Kolb H, Kolb-Bachofen V. Nitric oxide: a pathogenetic factor in autoimmunity. Immunol Today. 1992;13:157–160. doi: 10.1016/0167-5699(92)90118-Q. [DOI] [PubMed] [Google Scholar]
- 11.Reddy S, Kaill S, Poole CA, Ross J. Inducible nitric oxide synthase in pancreatic islets of the non-obese diabetic mouse: a light and confocal microscopical study of its ontogeny, co-localization and up-regulation following cytokine administration. Histochem J. 1997;29:53–64. doi: 10.1023/a:1026416918339. [DOI] [PubMed] [Google Scholar]
- 12.Rabinovitch A, Suarez-Pinzon WL, Sorensen O, Bleackley RC. Inducible nitric oxide synthase (iNOS) in pancreatic islets of nonobese diabetic mice: identification of iNOS expressing cells and relationships to cytokines expressed in the islets. Endocrinology. 1996;137:1907–1912. doi: 10.1210/endo.137.5.8612552. [DOI] [PubMed] [Google Scholar]
- 13.Kolb H, Kolb-Bachofen V, Roep B. Autoimmune versus inflammatory type I diabetes: a controversy? . Immunol Today. 1995;16:170–172. doi: 10.1016/0167-5699(95)80115-4. [DOI] [PubMed] [Google Scholar]
- 14.Conrad B, Weidmann E, Trucco G, Rudert WA, Behboo R, Ricordi C, Rodriquez-Rilo H, Finegold D, Trucco M. Evidence of superantigen involvement in insulin-dependent diabetes mellitus aetiology. Nature (Lond) 1994;371:351–354. doi: 10.1038/371351a0. [DOI] [PubMed] [Google Scholar]
- 15.Trucco M. To be, or not to be Asp 57, that is the question. Diabetes Care. 1992;15:705–715. doi: 10.2337/diacare.15.5.705. [DOI] [PubMed] [Google Scholar]
- 16.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacMicking J, Xie Q, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 18.Palmer RMJ, Rees DD, Ashton DS, Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. BBRC (Biochemical and Biophysical Research Communications) 1988;153:1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- 19.Sakuma I, Stuehr DJ, Gross SS, Nathan C, Levi R. Identification of arginine as a precursor of endothelium-derived relaxing factor. Proc Natl Acad Sci USA. 1988;85:8664–8667. doi: 10.1073/pnas.85.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giordano C, Stassi G, De Maria R, Todaro M, Richiusa P, Papoff G, Ruberti G, Bagnasco M, Testi R, Galluzzo A. Potential involvement of Fas and its ligand in the pathogenesis of Hashimoto's thyroiditis. Science (Wash DC) 1997;275:960–963. doi: 10.1126/science.275.5302.960. [DOI] [PubMed] [Google Scholar]
- 21.Abbas AK. Die and let live: eliminating dangerous lymphocytes. Cell. 1996;85:291–297. doi: 10.1016/s0092-8674(00)81042-9. [DOI] [PubMed] [Google Scholar]
- 22.De Maria R, Boirivant M, Cifone MG, Roncaioli P, Hahne M, Tschopp J, Pallone F, Santoni A, Testi R. Functional expression of Fas and Fas ligand on human gut lamina propria lymphocytes. A potential role for the acidic sphingomyelinase pathway in normal immunoregulation. J Clin Invest. 1996;97:316–322. doi: 10.1172/JCI118418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Parijs L, Abbas AK. Role of Fas-mediated cell death in the regulation of immune responses. Curr Opin Immunol. 1996;8:355–361. doi: 10.1016/s0952-7915(96)80125-7. [DOI] [PubMed] [Google Scholar]
- 24.Kurrer MO, Pakala SV, Hanson HL, Katz JD. β cell apoptosis in T cell–mediated autoimmune diabetes. Proc Natl Acad Sci USA. 1997;94:213–218. doi: 10.1073/pnas.94.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roep BO. T-cell responses to autoantigens in IDDM: the search for the holy grail. Diabetes. 1996;45:1147–1156. doi: 10.2337/diab.45.9.1147. [DOI] [PubMed] [Google Scholar]
- 26.Corbett JA, Wang JL, Sweetland MA, Lancaster JR, McDaniel ML. Interleukin-1 β induces the formation of nitric oxide by β-cells purified from rodent islets of Langerhans. Evidence for the beta-cell as a source and site of action of nitric oxide. J Clin Invest. 1992;90:2384–2391. doi: 10.1172/JCI116129. [DOI] [PMC free article] [PubMed] [Google Scholar]