Abstract
In the thymus, T cells are selected according to their T cell receptor (TCR) specificity. After positive selection, mature cells are exported from primary lymphoid organs to seed the secondary lymphoid tissue. An important question is whether survival of mature T cells is an intrinsic property or requires continuous survival signals, i.e., engagement of the TCR by major histocompatibility complex (MHC) molecules in the periphery, perhaps in a similar way as occurring during thymic positive selection. To address this issue we used recombination-activating gene (Rag)-deficient H-2b mice expressing a transgenic TCR restricted by I-Ed class II MHC molecules. After engraftment with Rag−/− H-2d fetal thymi, CD4+8− peripheral T cells emerged. These cells were isolated and transferred into immunodeficient hosts of H-2b or H-2d haplotype, some of the latter being common cytokine receptor γ chain deficient to exclude rejection of H-2b donor cells by host natural killer cells. Our results show that in the absence, but not in the presence, of selecting MHC molecules, peripheral mature T cells are short lived and disappear within 7 wk, indicating that continuous contact of the TCR with selecting MHC molecules is required for survival of T cells.
Positive selection of T cells in the thymus requires the interaction of the TCR on immature thymocytes with self-MHC molecules expressed on thymic epithelial cells (1–3). This process is dependent on the continual interaction of TCRs to MHC molecules since the transfer of thymocytes exhibiting only the first signs of positive selection (i.e., TCR upregulation and CD69 expression) into hosts lacking appropriate MHC molecule expression results in the loss of the transferred cells (4, 5). The presence of certain intermediate stages of T cell development in different gene-deficient mice also indicated that T cells were selected by a multistep process (6–8). Nevertheless, T cells do acquire functional maturity within the thymus since they can be stimulated to divide (9–11), as was found for recent thymic emigrants (12).
After the export to secondary lymphoid organs, some naive T cells do not divide but are long lived with an intermitotic lifespan of the order of 8 wk in euthymic mice, and much longer in athymic mice (13, 14). These experiments were performed in mice expressing the same MHC molecules in peripheral lymphoid tissue that induced positive selection in the thymus. Recently, some evidence was presented that MHC molecules in the peripheral lymphoid organs may have a role in sustaining long-term survival of mature T cells (15). We have addressed the same question in a different experimental system and, even though we agree that MHC molecules do support the survival of mature T cells, we have obtained results that differ from those obtained in the previous report (15). In our experiments, we have grafted recombination activating gene (Rag)1-deficient mice of H-2b haplotype that express a transgenic I-Ed-restricted TCR with thymi from fetal H-2d Rag−/− mice. This allowed the positive selection of CD4+8− T cells expressing the transgenic TCR. These cells were transferred into immunodeficient hosts of either H-2b or H-2d haplotype, some of the latter being deficient in expression of the common cytokine receptor γ chain (IL-2Rγ−/−) and therefore devoid of NK cells (16, 17). The results show that H-2d-restricting MHC molecules are required for survival of the transferred T cells in secondary lymphoid tissue.
Materials and Methods
Mice.
BALB/c and BALB/c nu/nu mice were from IFFA-Credo (Orléans, France). BLACK nu/nu mice were from Bomholtgart (Ry, Denmark) and screened for H-2b homozygosity. Rag-2– (18) and Rag-1– (19) deficient mice were H-2b homozygous. Hemagglutinin-specific TCR transgenic mice (ABII TCR) on Rag-2−/− background have been described (8, 20). H-2d Rag-2−/− mice were obtained from Drs. Antonius Rolink and Shunichi Takeda (Basel Institute for Immunology, Basel, Switzerland; reference 15). These mice were crossed with IL-2Rγ−/− mice to obtain H-2d Rag-2−/− IL-2Rγ−/− mice. All breeding was done in the animal colonies at the Basel Institute for Immunology (Basel, Switzerland) and at the Netherlands Cancer Institute (Amsterdam, The Netherlands).
B Cell Depletion, Cell Sorting, and FACS Analysis.
Single cell suspensions of thymus, lymph nodes, and/or spleen (RBCs lysed or removed by Ficoll density gradient centrifugation) were prepared in PBS with 2% FCS. Where applicable, surface immunoglobulin-positive (sIg+) cells were depleted using Dynabeads (Milan, Switzerland).
6.5 (anti-ABII-TCR; reference 8) and MKD6 (anti-I-Aβ d; reference 21) mAbs were labeled with FLUOS (Boehringer Mannheim, Mannheim, Germany). Heat stable antigen (HSA)-specific mAbs M1/69 (22) were biotinylated. FITC-labeled 104.2.1 mAbs (anti-Ly-5b; reference 23) were a gift of Dr. Hans-Reimer Rodewald (Basel Institute for Immunology, Basel, Switzerland). Anti-CD4-PE, anti-CD8-Red613 (GIBCO BRL, Gaithersburg, MD), and streptavidin-allophycocyanin (Molecular Probes Inc., Eugene, OR) conjugates were obtained commercially.
Cells were stained with mAbs at optimal dilution as determined before. Three and four-color flow cytometry was performed on FACStar+® or FACS-Vantage® (Becton Dickinson, Mountain View, CA) instruments.
Data (up to 5 × 105 events) were stored in list mode and analyzed with Lysys II or CellQuest software (Becton Dickinson). Dead cells were excluded using forward- and side-scatter parameters. Data are presented as dot plots with numbers in quadrants or regions indicating the proportion of cells within that area. Data from lymph nodes or spleen gave similar results whenever analyzed.
The recovery of transferred cells was calculated from the absolute number of cells recovered from spleen plus two times the number of cells recovered from lymph nodes each multiplied by the percentage of cells within the region of interest as determined by FACS® analysis. In case of staining before and after depletion of sIg+ cells, the average of both determinations, taking into account sIg+ cell depletion, was used for calculation. Note that from the experience of others, the recovery of lymphocytes immediately after transfer is estimated to be ∼20% after intravenous transfer (14, 24).
Lymphocyte Proliferation Assay.
Cell sorter purified responder cells were cultured with 5 × 105 X-irradiated (2,200 rad) stimulator cells in 200 μl Iscove's modified Dulbecco's medium supplemented with FCS (10%), β-mercaptoethanol (5 × 10−5 M), penicillin (100 IU/ml), and streptomycin (100 mg/ml). To some cultures, peptide 107-119 of influenza hemagglutinin (SVSSFERFEIFPK) was added at a final concentration of 5 μM. Cultures were kept in a water-saturated atmosphere of 6% CO2 in air at 37°C. After 48–60 h, 1 μCi [3H]thymidine (Amersham Corp., Arlington Heights, IL) was added and cells were cultured for a further 12–24 h when they were harvested. Incorporated radioactivity was measured by standard liquid scintillation counting.
Fetal Thymus Transplantation and Adoptive Transfers.
Fetal H-2d Rag-2−/− thymi were isolated at days 14–16 of gestation (plug day = day 0). For some experiments (Fig. 1), thymus lobes were isolated from BALB/c mice and cultured 5 d in medium containing 1.35 mM 2′-deoxyguanosine (dGuo; Sigma Chemical Co., St. Louis, MO). 1–2 lobes were transplanted under the kidney capsule of H-2b or H-2b/d ABII TCR Rag-2−/− mice. 6–16 wk later, cells were isolated from lymph nodes and spleen, stained with CD4, CD8, and I-Aβ d-specific mAbs, and cell sorter purified. CD4+8− I-Aβ d− and CD4−8low/+I-Aβ d− cells were injected intravenously into various 4–6-wk-old recipient mice.
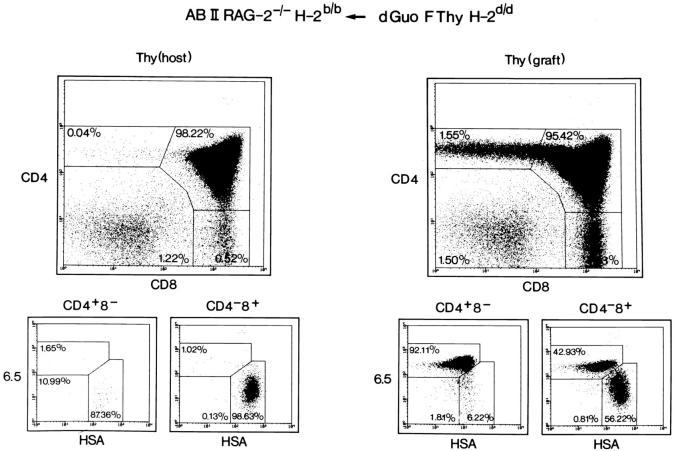
Figure 1.
Thymic development of CD4+8− T cells in H-2d fetal thymus–grafted H-2b ABII TCR Rag−/− mice. H-2b ABII TCR Rag−/− mice were transplanted with BALB/c fetal thymi that had been treated with dGuo. Several weeks after transplantation, thymocytes from grafted and host thymi were recovered and analyzed by four-color flow cytometry including the ABII TCR–specific mAb 6.5. Similar data were obtained when grafting H-2d Rag−/− thymi that, however, grew much more extensively (average of 3.5 × 107 thymocytes).
Results
H-2d-restricted CD4+8− T Cells in Thymus Grafted ABII TCR Rag−/− H-2b Mice.
In our studies, we used ABII TCR transgenic mice that express a transgenic TCR specific for peptide 111-119 of influenza hemagglutinin presented by I-Ed class II MHC molecules. These mice were crossed onto the Rag−/− background to exclude the interference of TCRs with unknown specificity due to lack of allelic exclusion of the TCR-α locus (25, 26). To obtain T cells that are selected in the thymus but not able to encounter the selecting MHC molecules in peripheral lymphoid tissue, we transplanted H-2b ABII TCR Rag−/− mice with fetal thymi of H-2d haplotype that had been depleted of hematopoietic cells or were from Rag−/− background. (It is possible that some donor thymus-derived I-Ed–expressing cells emigrate from the graft. This will be addressed below.) Under such conditions, the thymus graft is seeded by T cell precursors of the host such that mature CD4+8− T cells with the transgenic TCR develop in the grafted H-2d, but not the host H-2b thymus (Fig. 1). Subsequently, CD4+8− T cells from the grafted thymus are exported to the periphery (Fig. 2). Such CD4+8− peripheral T cells are not present in H-2b ABII TCR Rag−/− mice, since cells with the I-Ed-restricted ABII TCR cannot develop in a H-2b thymus (8). There are also CD4−8low T cells in these mice that will be discussed below.
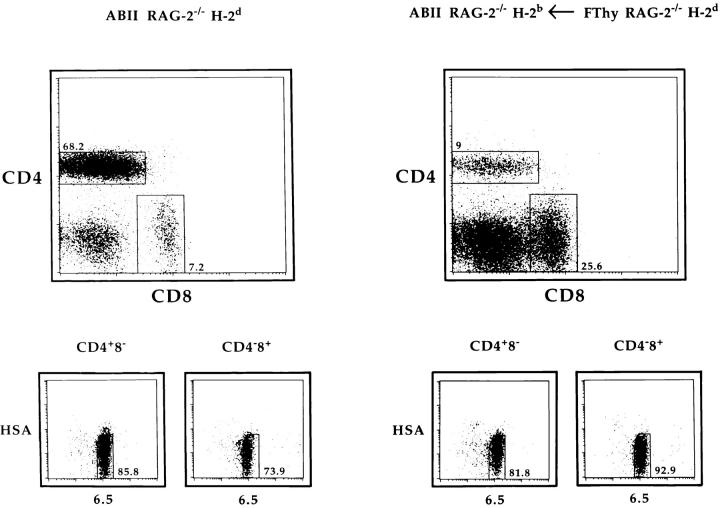
Figure 2.
CD4+8− lymphocytes are present in H-2b ABII TCR Rag−/− mice grafted with an H-2d fetal thymus. Lymphocytes were recovered from H-2d ABII TCR Rag−/− and H-2b ABII TCR Rag−/− mice that had previously been transplanted with fetal thymus lobes from H-2d Rag−/− mice. Cells were analyzed by four-color flow cytometry as shown.
To confirm that the CD4+8− T cells in grafted mice were functionally mature, cells from such mice as well as from H-2d ABII TCR Rag−/− mice were isolated and stimulated with antigen presented by H-2d APCs. As shown in Table 1, both populations of cells gave proliferative responses that were in the same order of magnitude, indicating that they had acquired functional competence.
Table 1.
Proliferation of CD4+8− ABII TCR Lymphocytes Positively Selected in Endogenous or Transplanted H-2d Rag−/− Thymi
| Stimulators (2,200 rad) | ||||
|---|---|---|---|---|
| CD4+8− responders of | BALB/c nu/nu | BALB/c nu/nu + peptide | ||
| ABII RAG-2−/− H-2d | 600 | 15,000 | ||
| ABII RAG-2−/−H-2b ← FThy RAG-2−/− H-2d | 260 | 23,000 | ||
| None | 160 | 170 | ||
CD4+8− lymphocytes were cell sorter purified from H-2d ABII TCR Rag−/− and H-2b ABII TCR Rag−/− mice that had previously been transplanted with an H-2d Rag−/− fetal thymus. 5 × 104 responder cells were cultured with irradiated BALB/c nu/nu stimulators. To some cultures, peptide had been added as source of antigen.
There was, however, a clear difference in the absolute number of CD4+8− T cells in the thymus-grafted H-2b ABII TCR Rag−/− mice and the H-2d ABII TCR Rag−/− mice in that the former contained far fewer cells than the latter (2.9 × 106; n = 3); and ranging between 107 to 108, respectively). This difference could be due to the fact that fewer CD4+8− T cells are produced/exported from the grafted thymus and/or the fact that the lack of I-Ed class II MHC molecules in the peripheral lymphoid organs of grafted H-2b ABII TCR Rag−/− mice resulted in a shorter life span of these cells. Further, the few CD4+8− T cells might be continuously renewed and/or depend on few I-Ed–expressing cells originating from the fetal thymus graft. To address the issue of whether peripheral expression of MHC molecules the CD4+8− T cells were selected on in the thymus was required for their peripheral survival, we performed transfer experiments into immunodeficient hosts expressing different MHC molecules.
CD4+8− Peripheral T Cells from H-2d Thymus-Grafted H-2b ABII TCR Rag−/− Mice Disappear in H-2b but Not in H-2d Adoptive Hosts.
We have shown previously that CD4−8low cells with the transgenic ABII TCR are an abundant subset in peripheral lymphoid organs of H-2b ABII TCR Rag−/− mice and therefore do not need I-Ed MHC molecules for selection and survival (8). This population most likely results from the unusual early expression of the α/β-TCR in TCR transgenic mice leading to the formation of γ/δ lineage T cells that express the transgenic α/β-TCR on the cell surface as discussed by Bruno et al. (27). Here, this subset serves as a convenient internal control to determine engraftment.
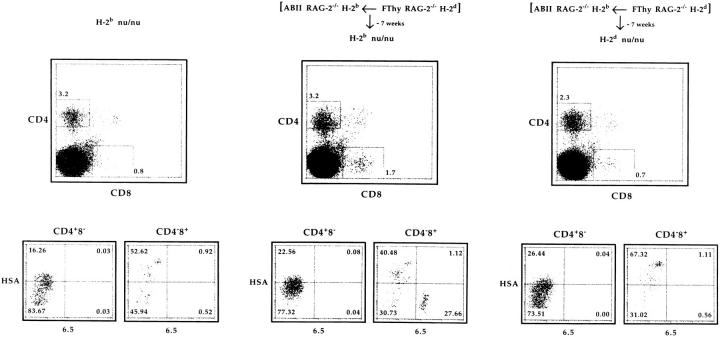
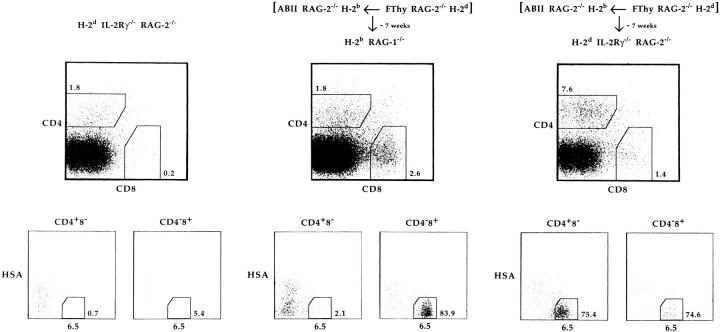
Cell sorter–purified CD4+8− and CD4−8low cells from thymus-grafted H-2b ABII TCR Rag−/− mice were transferred into either H-2b or H-2d immunodeficient recipient mice. As shown in Fig. 3, no 6.5+CD4+8− cells could be detected in either H-2b nu/nu or H-2d nu/nu recipient mice 7 wk after transfer, whereas 6.5+CD4−8low cells were found in the H-2b but not H-2d recipients. (Similar results were obtained after transfer into H-2b Rag−/− or H-2d Rag−/− recipient mice, data not shown.) In terms of engraftment, this result would be compatible with the notion that the transferred cells expressing H-2b MHC molecules only, could engraft in H-2b hosts, but not in H-2d hosts because of rejection by host NK cells (28–30). To circumvent this problem, a similar transfer of cells from thymus-grafted H-2b ABII TCR Rag−/− mice was performed into H-2b Rag−/− and H-2d Rag−/− IL-2Rγ−/− mice, devoid of NK cells (16, 17). As shown in Fig. 4, 6.5+CD4+8− cells survived only in H-2d recipients, whereas 6.5+CD4−8low cells survived both in H-2b and H-2d recipients when analyzed 7 wk after transfer. Taken together, these results show that in H-2b recipients, NK cells do not reject the transferred cells as 6.5+CD4−8low cells survive, but 6.5+CD4+8− cells vanish due to the lack of H-2d MHC molecules. In contrast, 6.5+CD4+8− cells survive long term in the presence of H-2d MHC molecules once not rejected by NK cells. 6.5+CD4−8low cells survived then as well.
Figure 3.
CD4+8− lymphocytes from H-2d thymus-grafted H-2b ABII TCR Rag−/− mice disappear in H-2b adoptive hosts. 7.5 × 105 CD4+8− and 5 × 105 CD4−8low lymphocytes from H-2d Rag−/− fetal thymus–transplanted H-2b ABII TCR Rag−/− mice were adoptively transferred into H-2b and H-2d nu/nu mice. 7 wk later, cells from lymph nodes and spleen (RBCs lysed) were isolated, depleted of sIg+ cells, and analyzed by four-color flow cytometry. Data from lymph nodes and spleen gave similar results. Calculated numbers of 6.5+HSA− cells were 5,600, 6,500, and 1,800 for CD4+8− and 1,800, 463,600, and 400 for CD4−8low cells in unmanipulated H-2b (one mouse), injected H-2b (average of two mice), and injected H-2d (average of two mice) nu/nu mice, respectively. (Note that numbers also contain the calculated background values of mice not injected.) Another experiment and two experiments using H-2b Rag−/− and H-2d Rag−/− recipients gave similar results.
Figure 4.
CD4+8− lymphocytes from H-2d thymus-grafted H-2b ABII TCR Rag−/− mice survive in H-2d NK cell–deficient adoptive hosts. 105 CD4+8− and 105 CD4−8low lymphocytes from H-2d Rag−/− fetal thymus–transplanted H-2b ABII TCR Rag−/− mice were adoptively transferred into H-2b Rag−/− and H-2d Rag−/− IL-2Rγ−/− mice. 7 wk after transfer, cells from lymph nodes and spleen were analyzed as in Fig. 3. Calculated numbers of 6.5+HSA− cells were 900, 13,500, 5,900, and 3 × 106 for CD4+8− and 600, 6,100, 7.6 × 105, and 1.5 × 106 for CD4−8low cells in unmanipulated H-2d Rag−/− IL-2Rγ−/−, unmanipulated H-2b Rag−/− (not shown), injected H-2b Rag−/−, and injected H-2d Rag−/− IL-2Rγ−/− mice, respectively. Expansion in Rag-deficient H-2d mice was consistent with previous data (41). We also performed this type of experiment with two thymectomized H-2d Rag−/− IL-2Rγ−/− mice and obtained similar results.
In other experiments, we have followed the fate of CD4+8− and CD4−8low cells from thymus-grafted H-2b/d ABII TCR Rag−/− mice upon transfer into either H-2b or H-2d immunodeficient recipient mice. (The H-2b/d ABII TCR Rag−/− mice themselves cannot select the ABII TCR in the thymus due to preferential pairing of Eα with Eβb chains; references 8, 31, 32.) 3 d after transfer, both populations were present in H-2b nu/nu and H-2d nu/nu recipients. 7 wk later, both subsets were present in H-2d nu/nu recipients but could no longer be found in H-2b nu/nu recipients (data not shown, made available to reviewers). This might indicate that the shared class I MHC molecule expression between H-2b/d hybrid donor and homozygous host protected the donor cells from NK cell– mediated lysis in H-2d homozygous hosts, but that the reduced class I H-2b MHC molecule expression of H-2b/d hybrid cells was not sufficient to circumvent NK cell–mediated lysis in H-2b homozygous hosts. Such differences might become evident only upon transfer of relatively small numbers of cells as performed here. An inverse correlation between the level of class I expression and NK cell lysis has indeed been observed (33), and NK cell–mediated lysis of hybrid cells did not always occur to the same extent in each of the parental strains (34). Further, H-2b cells expressing transgene-encoded Dd MHC class I molecules were rejected by NK cells in otherwise syngenic H-2b hosts (35).
Discussion
Our data show that appropriate MHC molecules are required to support the survival of mature α/β T cells in peripheral lymphoid tissue; a conclusion in line with previously published experiments (15). In the latter study, however, polyclonal CD4+8− T cells in thymus-grafted Rag class II MHC double deficient mice appeared to survive much longer since significant numbers of cells could still be found 16 wk after export from the thymus ceased. This may be due to the fact that in these experiments, CD4+8− T cells with class I MHC–restricted TCRs (36–38) could interact with class I MHC molecules expressed in the peripheral lymphoid tissue or due to the fact that some class II MHC–positive cells had migrated from the transplanted thymus into the periphery. In both of these cases, T cells could have been stimulated by antigen. The high proportion of proliferating CD4+8− T cells at various points in time after thymus transfer could indicate that this was indeed the case, and some CD4+8− T cells might have rather disappeared because of exhaustion (39, 40). These possibilities were excluded in our experiments; the CD4+8− T cells were of known antigen specificity and MHC restriction. Any potential class II MHC molecule–expressing cells originating from the thymus graft were removed by cell sorting before transfer into adoptive recipients.
In line with our previous observations, we noticed expansion of CD4+8− ABII TCR-expressing cells in H-2d mice (reference 41 and this report). Currently, it is not clear whether T cell survival in the absence (13, 14) or the presence (41) of proliferation (or both possibilities) reflects the normal physiologic situation. Interestingly, normal mice that had been thymectomized have some cells of naive phenotype that label with BrdU (42).
Irrespective of the MHC environment, CD4−8low cells that are not dependent on positive selection in the thymus, did expand after transfer. Presently, the biology of these cells is not well understood and it has been speculated that these cells represent γ/δ lineage T cells expressing the transgenic α/β-TCR (27).
While it becomes established that T cells require the interaction of their TCRs with selecting MHC molecules for survival in the peripheral lymphoid tissue, the mechanism behind this requirement is unknown. In the thymus, immature CD4+8− cells express low levels of the cell death– repressing bcl-2 protein. They have a half life of 3 d (43) unless their TCR binds with sufficient affinity to self-MHC molecules resulting in maturation that is accompanied by bcl-2 upregulation (44–47). One might then speculate that the level of bcl-2 expression and, hence, survival is (indirectly) regulated by TCR ligation with selecting MHC molecules in the absence of antigen. In that respect, peripheral survival could be similar to thymic positive selection. The data reported on T cells from bcl-2–deficient mice are compatible with such a hypothesis (48–51). We have investigated bcl-2 expression by intracellular staining of CD4+8− cells from thymus-grafted H-2b ABII TCR Rag−/− and H-2d ABII TCR Rag−/− mice. However, the differences we observed were far less dramatic than during thymic positive selection (mean fluorescence reduced to 74 compared to 98 in controls, whereas in the thymus, a threefold difference was detectable: 44 versus 120 in an independent experiment). This could be due to the fact that cells with low bcl-2 expression are rapidly dying and eliminated and escape detection.
Further, it will be of interest to determine whether TCR contact with selecting MHC molecules on any type of cell is sufficient for T cell survival or whether the selecting MHC molecules have to be encountered on a specific cell type. The latter would be analogous to the requirement of TCR–MHC molecule interaction on thymic epithelial cells for thymic positive selection (3). Interestingly, RelB-deficient mice that lack dendritic cells have an increased proportion of activated T cells, whereas absolute numbers of T cells are reduced (52, 53). The former might be due to limited self-censorship in the thymus followed by peripheral activation by self-antigens the T cells were not tolerized for in the thymus (52–55). The latter, however, could indicate that (naive) peripheral T cell survival requires TCR interaction with selecting MHC molecules on dendritic cells. The data of DeKoning et al. on transfer of naive TCR transgenic T cells into RelB−/− mice support this theory (53).
Acknowledgments
We thank M. Dessing, S. Meyer, and E. Noteboom for expert help with flow cytometry and cell sorting; the animal care takers (especially E. Wagner and W. Metzger, and L. Tolkamp in Basel, Switzerland and Amsterdam, The Netherlands, respectively) for making possible these experiments; H.-P. Stahlberger for art work; P. Krimpenfort for providing the IL-2Rγ−/− mice; and John D. Allen, Thomas Brocker, and Hergen Spits for reading the manuscript. The TCR clonotype specific mAb 6.5 was produced by B. Riwar and H. Kishi. The Basel Institute for Immunology was founded and is supported by F. Hoffmann-La Roche Ltd. (Basel, Switzerland). J. Kirberg receives a fellowship from the Boehringer Ingelheim Foundation (Stuttgart, Germany).
Footnotes
Abbreviations used in this paper: dGuo, 2′-deoxyguanosine; Rag, recombination activating gene; sIg, surface Ig; HSA, heat stable antigen.
References
- 1.Kisielow P, Teh HS, Blüthmann H, von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature (Lond) 1988;335:730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- 2.Scott B, Blüthmann H, Teh HS, von Boehmer H. The generation of mature T cells requires interaction of the alpha beta T-cell receptor with major histocompatibility antigens. Nature (Lond) 1989;338:591–593. doi: 10.1038/338591a0. [DOI] [PubMed] [Google Scholar]
- 3.Anderson G, Owen JJ, Moore NC, Jenkinson EJ. Thymic epithelial cells provide unique signals for positive selection of CD4+CD8+thymocytes in vitro. J Exp Med. 1994;179:2027–2031. doi: 10.1084/jem.179.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kisielow P, Miazek A. Positive selection of T cells: rescue from programmed cell death and differentiation require continual engagement of the T cell receptor. J Exp Med. 1995;181:1975–1984. doi: 10.1084/jem.181.6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pircher H, Ohashi PS, Boyd RL, Hengartner H, Brduscha K. Evidence for a selective and multi-step model of T cell differentiation: CD4+CD8lowthymocytes selected by a transgenic T cell receptor on major histocompatibility complex class I molecules. Eur J Immunol. 1994;24:1982–1987. doi: 10.1002/eji.1830240907. [DOI] [PubMed] [Google Scholar]
- 6.van Meerwijk JP, Germain RN. Development of mature CD8+ thymocytes: selection rather than instruction? . Science (Wash DC) 1993;261:911–915. doi: 10.1126/science.8102208. [DOI] [PubMed] [Google Scholar]
- 7.Chan SH, Cosgrove D, Waltzinger C, Benoist C, Mathis D. Another view of the selective model of thymocyte selection. Cell. 1993;73:225–236. doi: 10.1016/0092-8674(93)90225-f. [DOI] [PubMed] [Google Scholar]
- 8.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+single positive cells with a class II major histocompatibility complex–restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson A, Day LM, Scollay R, Shortman K. Subpopulations of mature murine thymocytes: properties of CD4−CD8+ and CD4+CD8−thymocytes lacking the heat-stable antigen. Cell Immunol. 1988;117:312–326. doi: 10.1016/0008-8749(88)90121-9. [DOI] [PubMed] [Google Scholar]
- 10.Chen WF, Ewing T, Scollay R, Shortman K. Growth of single T cells and single thymocytes in a high cloning efficiency filler-cell free microculture system. Thymus. 1988;12:51–68. [PubMed] [Google Scholar]
- 11.Dyall R, Nikolic-Zugic J. The majority of postselection CD4+single-positive thymocytes requires the thymus to produce long-lived, functional T cells. J Exp Med. 1995;181:235–245. doi: 10.1084/jem.181.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scollay R, Chen WF, Shortman K. The functional capabilities of cells leaving the thymus. J Immunol. 1984;132:25–30. [PubMed] [Google Scholar]
- 13.von Boehmer H, Hafen K. The life span of naive alpha/beta T cells in secondary lymphoid organs. J Exp Med. 1993;177:891–896. doi: 10.1084/jem.177.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science (Wash DC) 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 15.Takeda S, Rodewald HR, Arakawa H, Bluethmann H, Shimizu T. MHC class II molecules are not required for survival of newly generated CD4+T cells, but affect their long-term life span. Immunity. 1996;5:217–228. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]
- 16.DiSanto JP, Muller W, Guy-Grand D, Fischer A, Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor γ chain. Proc Natl Acad Sci USA. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, Drago J, Noguchi M, Grinberg A, Bloom ET, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor γ chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 18.Shinkai Y, Koyasu S, Nakayama K, Murphy KM, Loh DY, Reinherz EL, Alt FW. Restoration of T cell development in RAG-2–deficient mice by functional TCR transgenes. Science (Wash DC) 1993;259:822–825. doi: 10.1126/science.8430336. [DOI] [PubMed] [Google Scholar]
- 19.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1–deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 20.Kirberg J, Brocker T. CD45 up-regulation during lymphocyte maturation. Int Immunol. 1996;8:1743–1749. doi: 10.1093/intimm/8.11.1743. [DOI] [PubMed] [Google Scholar]
- 21.Kappler JW, Skidmore B, White J, Marrack P. Antigen-inducible, H-2–restricted, interleukin-2–producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981;153:1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Springer T, Galfre G, Secher DS, Milstein C. Monoclonal xenogeneic antibodies to murine cell surface antigens: identification of novel leukocyte differentiation antigens. Eur J Immunol. 1978;8:539–551. doi: 10.1002/eji.1830080802. [DOI] [PubMed] [Google Scholar]
- 23.Shen, F.-W. 1981. Monoclonal antibodies to mouse lymphocyte differentiation antigens. In Monoclonal Antibodies and T Cell Hybridomas. G.J. Hämmerling, U. Hämmerling, and J.F. Kearney, editors. Elsevier, Amsterdam. 25–31.
- 24.Rocha BB. Population kinetics of precursors of IL 2-producing peripheral T lymphocytes: evidence for short life expectancy, continuous renewal, and post-thymic expansion. J Immunol. 1987;139:365–372. [PubMed] [Google Scholar]
- 25.Borgulya P, Kishi H, Uematsu Y, von Boehmer H. Exclusion and inclusion of alpha and beta T cell receptor alleles. Cell. 1992;69:529–537. doi: 10.1016/0092-8674(92)90453-j. [DOI] [PubMed] [Google Scholar]
- 26.Padovan E, Casorati G, Dellabona P, Meyer S, Brockhaus M, Lanzavecchia A. Expression of two T cell receptor alpha chains: dual receptor T cells. Science (Wash DC) 1993;262:422–424. doi: 10.1126/science.8211163. [DOI] [PubMed] [Google Scholar]
- 27.Bruno L, Fehling HJ, von Boehmer H. The α/β T cell receptor can replace the γ/δ receptor in the development of γ/δ lineage cells. Immunity. 1996;5:343–352. doi: 10.1016/s1074-7613(00)80260-5. [DOI] [PubMed] [Google Scholar]
- 28.Snell GD. Recognition structures determined by the H-2 complex. Transplant Proc. 1976;8:147–156. [PubMed] [Google Scholar]
- 29.Bix M, Liao NS, Zijlstra M, Loring J, Jaenisch R, Raulet D. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature (Lond) 1991;349:329–331. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- 30.Gumperz JE, Parham P. The enigma of the natural killer cell. Nature (Lond) 1995;378:245–248. doi: 10.1038/378245a0. [DOI] [PubMed] [Google Scholar]
- 31.Conrad PJ, Lerner EA, Murphy DB, Jones PP, Janeway C., Jr Differential expression of Ia glycoprotein complexes in F1 hybrid mice detected with alloreactive cloned T cell lines. J Immunol. 1982;129:2616–2620. [PubMed] [Google Scholar]
- 32.Conrad PJ, Janeway C., Jr The expression of I–Ed molecules in F1 hybrid mice detected with antigen-specific, I-Ed–restricted cloned T-cell lines. Immunogenetics. 1984;20:311–319. doi: 10.1007/BF00364212. [DOI] [PubMed] [Google Scholar]
- 33.Storkus WJ, Howell DN, Salter RD, Dawson JR, Cresswell P. NK susceptibility varies inversely with target cell class I HLA antigen expression. J Immunol. 1987;138:1657–1659. [PubMed] [Google Scholar]
- 34.Heslop BF, McNeilage LJ. The F1 hybrid effect in allogeneic lymphocyte cytotoxicity. Points of similarity between hybrid resistance and ALC. Transplantation (Baltimore) 1989;48:634–639. [PubMed] [Google Scholar]
- 35.Ohlen C, Kling G, Hoglund P, Hansson M, Scangos G, Bieberich C, Jay G, Karre K. Prevention of allogeneic bone marrow graft rejection by H-2 transgene in donor mice. Science (Wash DC) 1989;246:666–668. doi: 10.1126/science.2814488. [DOI] [PubMed] [Google Scholar]
- 36.Macphail S, Stutman O. L3T4+cytotoxic T lymphocytes specific for class I H-2 antigens are activated in primary mixed lymphocyte reactions. J Immunol. 1987;139:4007–4015. [PubMed] [Google Scholar]
- 37.McKisic MD, Sant AJ, Fitch FW. Some cloned murine CD4+ T cells recognize H-2Ld class I MHC determinants directly. Other cloned CD4+ T cells recognize H-2Ld class I MHC determinants in the context of class II MHC molecules. J Immunol. 1991;147:2868–2874. [PubMed] [Google Scholar]
- 38.De Bueger M, Bakker A, Goulmy E. Existence of mature human CD4+ T cells with genuine class I restriction. Eur J Immunol. 1992;22:875–878. doi: 10.1002/eji.1830220338. [DOI] [PubMed] [Google Scholar]
- 39.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells (erratum published 364:262) Nature (Lond) 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 40.Rocha B, Grandien A, Freitas AA. Anergy and exhaustion are independent mechanisms of peripheral T cell tolerance. J Exp Med. 1995;181:993–1003. doi: 10.1084/jem.181.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruno L, von Boehmer H, Kirberg J. Cell division in the compartment of naive and memory T lymphocytes. Eur J Immunol. 1996;26:3179–3184. doi: 10.1002/eji.1830261251. [DOI] [PubMed] [Google Scholar]
- 42.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huesmann M, Scott B, Kisielow P, von Boehmer H. Kinetics and efficacy of positive selection in the thymus of normal and T cell receptor transgenic mice. Cell. 1991;66:533–540. doi: 10.1016/0092-8674(81)90016-7. [DOI] [PubMed] [Google Scholar]
- 44.Veis DJ, Sentman CL, Bach EA, Korsmeyer SJ. Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. J Immunol. 1993;151:2546–2554. [PubMed] [Google Scholar]
- 45.Moore NC, Anderson G, Williams GT, Owen JJ, Jenkinson EJ. Developmental regulation of bcl-2 expression in the thymus. Immunology. 1994;81:115–119. [PMC free article] [PubMed] [Google Scholar]
- 46.Linette GP, Grusby MJ, Hedrick SM, Hansen TH, Glimcher LH, Korsmeyer SJ. Bcl-2 is upregulated at the CD4+ CD8+stage during positive selection and promotes thymocyte differentiation at several control points. Immunity. 1994;1:197–205. doi: 10.1016/1074-7613(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 47.Gratiot-Deans J, Merino R, Nunez G, Turka LA. Bcl-2 expression during T-cell development: early loss and late return occur at specific stages of commitment to differentiation and survival. Proc Natl Acad Sci USA. 1994;91:10685–10689. doi: 10.1073/pnas.91.22.10685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakayama K, Nakayama K, Negishi I, Kuida K, Shinkai Y, Louie MC, Fields LE, Lucas PJ, Stewart V, Alt FW, et al. Disappearance of the lymphoid system in Bcl-2 homozygous mutant chimeric mice. Science (Wash DC) 1993;261:1584–1588. doi: 10.1126/science.8372353. [DOI] [PubMed] [Google Scholar]
- 49.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2–deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 50.Nakayama K, Nakayama K, Negishi I, Kuida K, Sawa H, Loh DY. Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc Natl Acad Sci USA. 1994;91:3700–3704. doi: 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakayama K, Nakayama K, Dustin LB, Loh DY. T-B cell interaction inhibits spontaneous apoptosis of mature lymphocytes in bcl-2-deficient mice. J Exp Med. 1995;182:1101–1109. doi: 10.1084/jem.182.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weih F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck RP, Lira SA, Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-κB/ Rel family. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 53.DeKoning J, DiMolfetto L, Reilly C, Wei Q, Havran WL, Lo D. Thymic cortical epithelium is sufficient for the development of mature T cells in relB-deficient mice. J Immunol. 1997;158:2558–2566. [PubMed] [Google Scholar]
- 54.Weih F, Durham SK, Barton DS, Sha WC, Baltimore D, Bravo R. Both multiorgan inflammation and myeloid hyperplasia in RelB-deficient mice are T cell dependent. J Immunol. 1996;157:3974–3979. [PubMed] [Google Scholar]
- 55.Laufer TM, DeKoning J, Markowitz JS, Lo D, Glimcher LH. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex. Nature (Lond) 1996;383:81–85. doi: 10.1038/383081a0. [DOI] [PubMed] [Google Scholar]