Abstract
Bicyclams are a novel class of antiviral compounds that are highly potent and selective inhibitors of the replication of HIV-1 and HIV-2. Surprisingly, however, when the prototype compound AMD3100 was tested against M-tropic virus strains such as BaL, ADA, JR-CSF, and SF-162 in human peripheral blood mononuclear cells, the compound was completely inactive. Because of the specific and potent inhibitory effect of AMD3100 on T-tropic viruses, but not M-tropic viruses, it was verified that AMD3100 interacts with the CXC-chemokine receptor CXCR4, the main coreceptor used by T-tropic viruses. AMD3100 dose dependently inhibited the binding of a specific CXCR4 monoclonal antibody to SUP-T1 cells as measured by flow cytometry. It did not inhibit the binding of the biotinylated CC-chemokine macrophage inflammatory protein (MIP) 1α or MIP-1β, ligands for the chemokine receptor CCR5 (the main coreceptor for M-tropic viruses). In addition, AMD3100 completely blocked (a) the Ca2+ flux at 100 ng/ml in lymphocytic SUP-T1 and monocytic THP-1 cells, and (b) the chemotactic responses of THP-1 cells induced by stromal cell–derived factor 1α, the natural ligand for CXCR4. Finally, AMD3100 had no effect on the Ca2+ flux induced by the CC-chemokines MIP-1α, regulated on activation normal T cell expressed and secreted (RANTES; also a ligand for CCR5), or monocyte chemoattractant protein 3 (a ligand for CCR1 and CCR2b), nor was it able to induce Ca2+ fluxes by itself. The bicyclams are, to our knowledge, the first low molecular weight anti-HIV agents shown to act as potent and selective CXCR4 antagonists.
The bicyclam derivatives were described several years ago as potent and selective inhibitors of HIV type 1 and type 2 replication (1, 2). AMD3100, previously called JM3100 (2) or SID791 (3), exhibits anti-HIV potency at concentrations of 1–10 ng/ml, with a selectivity index ⩾100,000 (2). Based on time-of-addition experiments, the compound has been assumed to interact with the HIV fusion-uncoating process, but does not inhibit virus binding to the CD4 receptor (1, 2). AMD3100 blocks syncytium formation at a concentration that is 10–100-fold higher than the concentration required to inhibit virus infection (1). The env glycoprotein (gp)120 has been considered the major target molecule for this class of compounds because, for viruses that were made resistant to the bicyclams, a number of mutations accumulated in the gp120, especially in the V3-V4 region (3, 4).
Numerous publications over the last year have demonstrated the importance of chemokine receptors for HIV entry. Chemokines are chemotactic cytokines, which are classified as CC or CXC, depending on the positioning of conserved cysteine residues. Fusin/LESTR, now designated CXC-chemokine receptor 4 (CXCR4), mediates entry of T-tropic viruses (5, 6) which can be inhibited by its natural ligand, the CXC-chemokine stromal cell–derived factor 1α (SDF-1α) (7, 8). The CC-chemokine receptor, CCR5, mediates entry of M-tropic viruses (9–13) and the CC-chemokines regulated on activation normal T cell expressed and secreted (RANTES), macrophage inflammatory protein (MIP) 1α and MIP-1β have been shown to inhibit the replication of M-tropic viruses (14). Moreover, M-tropic env proteins can interact directly with CCR5 (15, 16).
In previous studies AMD3100 was shown to inhibit the replication of T-tropic HIV strains or clinical isolates in T cell lines (such as MT-4, MOLT-4, or CEM cells; references 1–4). While verifying whether AMD3100 was active against M-tropic viruses in PBMCs, we found that AMD3100 does not inhibit M-tropic viruses such as BaL, ADA, JR-CSF, and SF-162. Here we show that AMD3100 selectively inhibits the binding of a CXCR4-specific mAb, but not the binding of biotinylated human MIP-1α or MIP-1β. The bicyclam was also found to inhibit the Ca2+ flux and the chemotactic response induced by SDF-1α but not such effects induced by RANTES, MIP-1α, or monocyte chemoattractant protein 3 (MCP-3).
Materials and Methods
Viruses, Cells, Cell Lines, and Cell Culture.
The HIV-1 T-tropic viruses IIIB strain and RF strain, the HIV-2 T-tropic ROD strain, and the HIV-1 M-tropic strains BaL, SF-162, ADA, and JR-FL were all obtained through the Medical Research Council AIDS reagent project (Herts, UK). The HIV-1 T-tropic molecular clone NL4-3 was obtained from the National Institute of Allergy and Infectious Disease AIDS reagent program (Bethesda, MD). The CD4+ lymphocytic SUP-T1 and the CD4+ monocytic THP-1 cell lines were obtained from the American Type Culture Collection (Rockville, MD). PBMC from healthy donors were isolated by density gradient centrifugation and stimulated with PHA at 1 μg/ml (Sigma Chemical Co., Bornem, Belgium) for 3 d at 37°C. The activated cells (PHA-stimulated blasts) were washed three times with PBS, and viral infections were done as described by Cocchi et al. (14). HIV-infected or mock-infected PHA-stimulated blasts were cultured in the presence of 25 U/ml of IL-2 and varying concentrations of AMD3100, SDF-1α, and RANTES. Supernatant was collected at days 6 and 10, and HIV-1 core antigen in the culture supernatant was analyzed by the p24 ELISA kit from DuPont-Merck Pharmaceutical Co. (Wilmington, DE) and for HIV-2 detection the INNOTEST from Innogenetics (Temse, Belgium) was used.
Chemokines and mAbs.
Recombinant human SDF-1α was purchased from PeproTech (London, UK) and human RANTES and human MIP-1α were purchased from R & D Systems, Inc. (Abingdon, UK). MCP-3 was chemically synthesized according to the published protein sequence (17). The biotinylated human MIP-1α and MIP-1β fluorokineTM kits were purchased from R & D Systems Inc. The mAb, termed 12G5, reacts specifically with the human CXCR4 and was initially provided by Dr. James A. Hoxie (University of Pennsylvania, Philadelphia, PA) and later purchased from R & D Systems Inc.
Analysis of CXCR4 Expression.
SUP-T1 cells were incubated with AMD3100 or SDF-1α (at different concentrations) or PBS for different time periods (1 or 15 min) and at different temperatures (on ice or at room temperature) and the cells were washed once with PBS. The 12G5 mAb (10 μg/ml) was then added for 30 min at room temperature. The cells were washed twice in PBS and then incubated with FITC-conjugated goat anti–mouse Ab (Caltag Labs, San Francisco, CA) for 30 min at room temperature and washed twice in PBS. The binding of the biotinylated human MIP-1α was performed according to the protocol of the manufacturer. Cells were analyzed by a FACScan® flow cytometer. The percentage of positive cells and the mean fluorescence intensity (MFI) values are indicated in each histogram. The region for positivity was defined using a control isotype mAb (Becton Dickinson, San Jose, CA). The percentage of inhibition of mAb binding in the presence of different concentrations of chemokine or compound was calculated using the MFI values, as previously described (18).
Measurement of Intracellular Calcium Concentrations and Chemotactic Assay.
The determination of intracellular calcium concentrations [Ca2+]i was carried out as previously described (19). In brief, THP-1 cells or SUP-T1 cells were loaded with Fura-2 (Molecular Probes, Leiden, The Netherlands). Fura-2 fluorescence was measured in a luminescence spectrophotometer, fitted with a water-thermostatable, stirred four-position cuvette holder (Perkin-Elmer, Norwalk, CT). Cells were first stimulated with dilution buffer (control) or AMD3100 or 12G5 mAb at different concentrations. As a second stimulus, chemokines were used at an optimal concentration to induce a maximal [Ca2+]i increase. The second stimulus was added 100 s after the first stimulus. The percentage of inhibition of the [Ca2+]i increase in response to the second stimulus was calculated. Chemotaxis of THP-1 cells was measured in the microchamber assay (5 μm pore membrane) essentially as previously described (17, 20). For inhibition of chemokine activity by AMD3100, the compound was added to the cells just before transfer to the upper compartment of the chemotaxis chamber containing chemokine in the lower compartment (20). Chemotactic activities are expressed as indexes ± SEM (17).
Results
Antiretroviral Activity Profile of AMD3100.
AMD3100 was active in PHA-stimulated blasts against T-tropic virus strains such as IIIB, RF, and NL4-3, and also against the HIV-2 ROD strain. The 50% inhibitory concentration (IC50) was between 2 and 7 ng/ml (Table 1). Surprisingly, AMD3100 was completely inactive against four different M-tropic virus strains (IC50 >25 μg/ml; Table 1). These M-tropic virus strains mainly use the chemokine receptor CCR5, but some can also use other chemokine receptors such as CCR2b and CCR3 (but not CXCR4) to enter the target cells (9–13). As controls, SDF-1α and RANTES were included and, as can be seen in Table 1, there was no activity of RANTES (up to 1 μg/ml) against the T-tropic virus strains, whereas the IC50 of SDF-1α varied between 20 and 100 ng/ml against the T-tropic virus strains. The opposite activity profile of these two chemokines was observed with M-tropic viruses. Here, RANTES had IC50 values between 4 and 25 ng/ml, whereas SDF-1α was not active up to 1 μg/ml. AMD3100 was not active against several simian immunodeficiency virus (SIV) strains such as MAC, MND, and AGM in MT-4 or MOLT-4 cells (2), which use CCR5 rather than CXCR4 as the main coreceptor to enter human T cells (21).
Table 1.
The Anti-HIV Activity Profile of AMD3100 Correlated with Coreceptor Use
| IC50 (ng/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Strain | Coreceptor used | ADM3100 | SDF-1α | RANTES | ||||
| T-tropic | ||||||||
| HIV-1 IIIB | CXCR4 | 2 | 20 | >1,000 | ||||
| HIV-1 RF | CXCR4 | 5 | 50 | >1,000 | ||||
| HIV-1 NL4-3 | CXCR4 | 3 | 100 | >1,000 | ||||
| HIV-2 ROD | CXCR4 | 7 | 55 | >1,000 | ||||
| M-tropic | ||||||||
| HIV-1 BaL | CCR5 | >25,000 | >1,000 | 25 | ||||
| HIV-1 SF-162 | CCR5 | >25,000 | >1,000 | 5 | ||||
| HIV-1 ADA | CCR5 (CCR2b, CCR3) | >25,000 | >1,000 | 10 | ||||
| HIV-1 JR-FL | CCR5 (CCR2b, CCR3) | >25,000 | >1,000 | 4 | ||||
Effect of AMD3100, SDF-1α, and RANTES on the replication of T-tropic and M-tropic HIV strains in PHA-stimulated blasts. Virus yield was monitored in the cell-free supernatant 6–10 d after infection by viral Ag ELISA. Results represent mean values for three separate experiments from three different PBMC donors.
AMD3100 Dose Dependently Inhibits the Binding of a CXCR4-specific mAb.
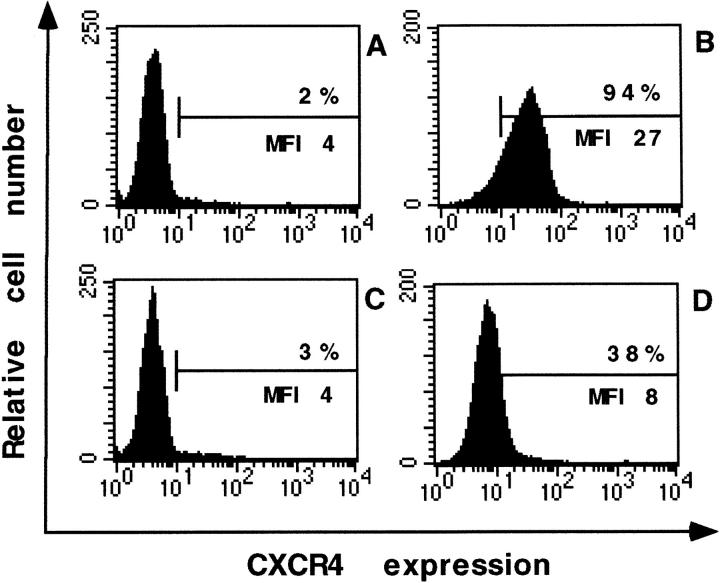
Because of the specific and potent inhibitory effect of AMD3100 on T-tropic viruses and not on M-tropic viruses (or SIV), it was verified that AMD3100 interacts with CXCR4. The mAb 12G5 reacts specifically with the human CXCR4 protein and recognizes this receptor on many T cell lines such as the SUP-T1 cells (22). AMD3100 dose dependently interacted with the CXCR4 receptor, as shown in Fig. 1. Indeed, AMD3100 at 1 μg/ml completely inhibited the binding of the mAb 12G5 to CXCR4 on SUP-T1 cells, as measured by flow cytometry. At 100, 10, 1, and 0.1 ng/ml, AMD3100 still blocked the mAb binding for 79, 70, 24, and 9% respectively. SDF-1α competed, as expected, with the binding of the CXCR4 mAb to its receptor. SDF-1α inhibited the binding of the mAb for 83% at 2 μg/ml and for 54% at 200 ng/ml. Even when washed away before addition of the mAb, AMD3100 inhibited the binding of the CXCR4 mAb as efficiently as when the compound was present during the whole incubation period with the mAb. Adding AMD3100 only 1 min before the CXCR4 mAb still blocked the binding of the mAb as efficiently as adding the compound 15 min before the mAb (data not shown). Irrespective of whether the staining was performed on ice or at room temperature, identical results were obtained with AMD3100 for inhibition of the binding of the CXCR4 mAb.
Figure 1.
Inhibition of the binding of the anti-CXCR4 mAb to SUP-T1 cells in the presence of AMD3100 at 1 μg/ml (C) and SDF-1α (2 μg/ ml; D). In A an isotype control mAb and in B the specific anti-CXCR4 mAb were used. The percentage of positive cells and the MFI values are indicated in each histogram.
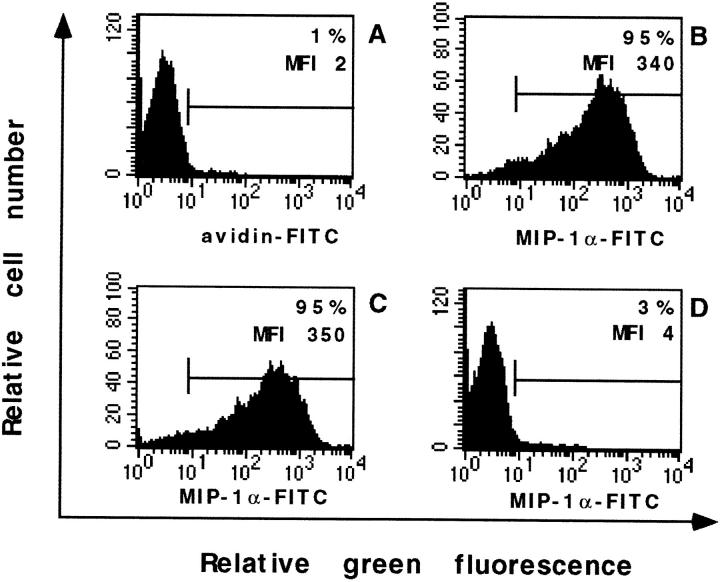
In contrast, even at 25 μg/ml AMD3100 did not inhibit the binding of biotinylated human MIP-1α to THP-1 cells, whereas, as control, the anti-human MIP-1α blocking Ab included in the fluorokineTM kit almost completely blocked the binding of the biotinylated MIP-1α (Fig. 2). Identical results were obtained with the biotinylated human MIP-1β fluorokineTM kit (data not shown).
Figure 2.
Lack of inhibition of the binding of biotinylated MIP-1α to THP-1 cells in the presence of AMD3100 (25 μg/ml; C). In A only the avidin-FITC was added, in B the biotinylated MIP-1α and avidin-FITC were added, in C AMD 3100 (25 μg/ml) was added, and in D the blocking Ab was added. The percentage of positive cells and MFI values are indicated in each histogram.
AMD3100 Specifically Blocks SDF-1α–induced Ca2+ Fluxes and Chemotaxis.
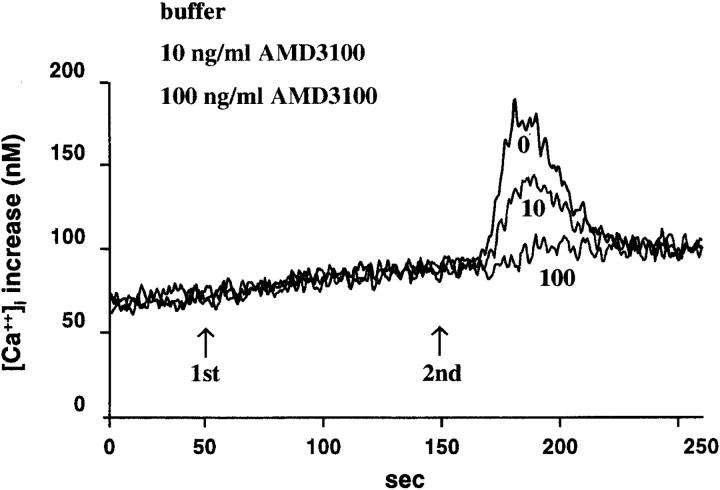
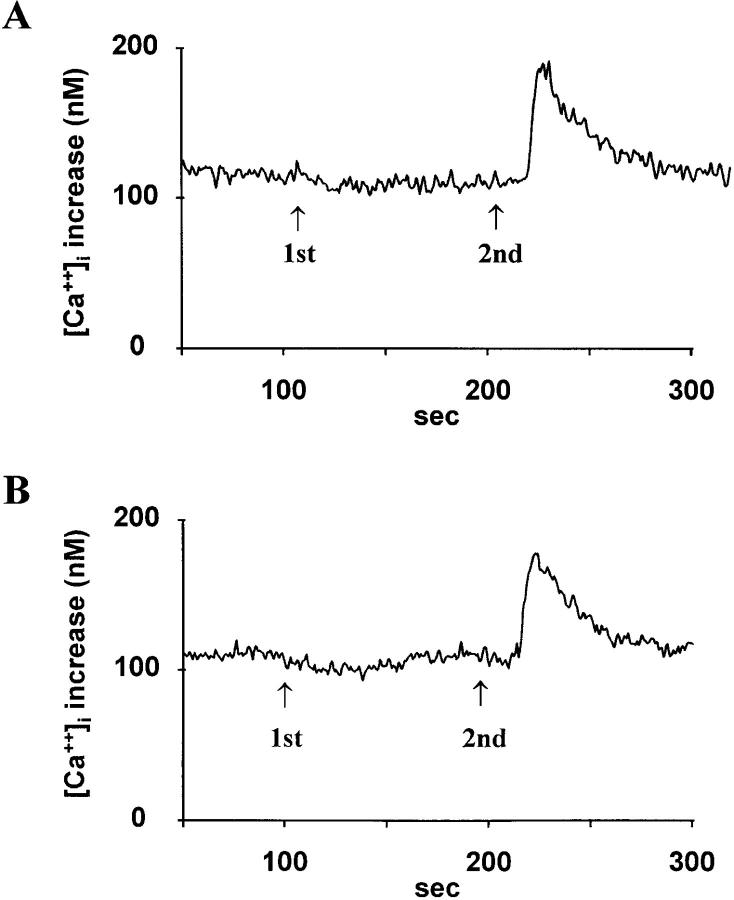
We next examined the inhibitory effect of AMD3100 on the SDF-1α–induced increase in [Ca2+]i (Ca2+ flux). Because the lymphocytic SUP-T1 cells did not respond in the Ca2+ flux assays to the CC-chemokines RANTES and MIP-1α, we used the monocytic THP-1 cell line, which is responsive to these chemokines. This allowed us to test the chemokine receptor specificity of AMD3100. In addition, the THP-1 cells were positive for CXCR4 expression, as measured by flow cytometry with the CXCR4 mAb (data not shown). THP-1 cells also dose dependently responded in the Ca2+ flux assay to SDF-1α, and half-maximal increases in [Ca2+]i were obtained with 10 ng/ml (data not shown). As a control for receptor usage, 10 μg/ml of the CXCR4 mAb was found to completely inhibit the SDF-1α–induced Ca2+ flux and at 1 μg/ml of the mAb there was still 36% inhibition (data not shown). AMD3100 at 100 ng/ml completely blocked [Ca2+]i increases induced by 30 ng/ml SDF-1α in both SUP-T1 and THP-1 cells (Table 2). Lower doses of AMD3100 (10 and 1 ng/ml) still conferred partial inhibition (35–69%) of Ca2+ increase induced by SDF-1α (Fig. 3; Table 2). In addition, the THP-1 cells responded to RANTES, MIP-1α, and MCP-3, but no inhibition whatsoever was seen when AMD3100 was added at 100 ng/ml before addition of the chemokines (Fig. 4; data not shown). Finally, to confirm the inhibitory effect of AMD3100 on functional CXCR4 binding and signaling, chemotaxis assays were performed. Similar to monocytes (23), THP-1 cells dose dependently responded to SDF-1α. On THP-1 cells, 100 ng/ml resulted in a half-maximal chemotactic index (11 ± 4; n = 3). The chemotactic effect of SDF-1α (100 ng/ml) was completely blocked in the presence of AMD3100 at 10 μg/ml (chemotactic index: 1.3 ± 0.6; n = 3), whereas 1 μg/ml of AMD3100 resulted in a partial reduction of the chemotactic index (4.3 ± 0.7; n = 3). AMD3100 alone induced no chemotactic response on THP-1 cells when tested at a concentration range from 0.01 to 10 μg/ml.
Table 2.
Inhibition of SDF-1α–induced Ca2+ Flux by AMD3100 in SUP-T1 and THP-1 Cells
| Stimulus | SUP-T1 cells [Ca2+]i increase | THP-1 cells [Ca2+]i increase | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMD3100(first) | SDF-1α(second) | exp. 1 | exp. 2 | exp. 3 | mean percentage of inhibition | exp. 1 | exp. 2 | exp. 3 | mean percentage of inhibition | |||||||||
| ng/ml | ng/ml | nM | % | nM | % | |||||||||||||
| 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||
| 100 | 30 | <10* | <10 | <10 | ⩾90 | <8 | <8 | <8 | >90 | |||||||||
| 10 | 30 | 83 | 33 | 49 | 45 | 31 | 37 | ND | 69 | |||||||||
| 1 | 30 | ND | 39 | 78 | 35 | ND | 56 | ND | 59 | |||||||||
| 0.1 | 30 | ND | ND | 90 | <10 | ND | ND | ND | ||||||||||
| 0 | 30 | 110 | 89 | 91 | 0 | 87 | 136 | 65 | 0 | |||||||||
Results of individual Ca2+ flux assays are given.
Figure 3.
Inhibition of SDF-1α–induced Ca2+ fluxes in SUPT-1 cells by AMD3100 pretreatment (first stimulus; 1st) at 100 ng/ml and 10 ng/ml. SDF-1α was given as second stimulus (2nd) at 30 ng/ml.
Figure 4.
AMD3100 fails to inhibit Ca2+ fluxes induced by RANTES in THP-1 cells. Buffer was added in A and AMD3100 at 100 ng/ml in B as first stimulus (1st), then RANTES was added at 100 ng/ml as second stimulus (2nd).
Discussion
CXCR4 is the coreceptor that promotes entry of T-tropic HIV strains (7, 8), whereas CCR5 allows entry of M-tropic HIV strains (9–13). SDF-1α, the natural ligand for CXCR4, has been shown to inhibit T-tropic viruses and primary HIV isolates through CXCR4 blockage (6, 7). AMD3100 is a bicyclam active against a broad range of T-tropic HIV-1 and HIV-2 strains (IC50: 1–10 ng/ml), but not against M-tropic HIV-1 strains (Table 1) such as BaL, ADA, SF-162, and JR-FL (IC50 >25 μg/ml). AMD3100 is able to inhibit the replication of HIV-2 strains such as ROD, but these virus strains also use CXCR4 to enter the cells (24). In addition, AMD3100 is not active against several SIV strains (2), and recently it was demonstrated that not only the M-tropic SIV strains but also the T-tropic SIV strains use CCR5, and not CXCR4, as the main coreceptor to enter human T cells (21).
The specific antiviral activity profile of AMD3100 suggests that it might directly interact with the CXCR4 receptor. This study brings evidence to support this hypothesis, at both the level of binding and of signaling. AMD3100 not only inhibits the binding of CXCR4 mAb to its receptor (Fig. 1), it also inhibits the intracellular SDF-1α signaling in a dose-dependent fashion (Table 2). The CXCR4 mAb, 12G5, is reported to inhibit HIV-1 and HIV-2 infection at 1–20 μg/ml, although the ability of this mAb to block infection of T-tropic isolates of HIV-1 is highly dependent on the viral isolate and the target cell; occasionally, it is even inactive against T-tropic viruses (23). Very potent and far less variable antiviral activity is seen with AMD3100 (IC50: 1–10 ng/ml, 50% cytotoxic concentration [CC50] >100 μg/ml; reference 2), indicating a very strong interaction of AMD3100 with the CXCR4 receptor.
Although the interaction of the bicyclams with CXCR4 has been unequivocally demonstrated in this study, interference either with other (still unknown) CXCR4-like or other chemokine receptors used by SDF-1α cannot be excluded at this moment. At present there is no evidence that the CXCR4 mAb and SDF-1α can recognize receptors other than CXCR4. AMD3100 does not appear to interfere with CCR5 as there is no inhibitory effect of AMD3100 on the replication of SIV and M-tropic HIV-1 strains in PBMC. Moreover, AMD3100 does not inhibit the binding of either of the biotinylated CC-chemokines, MIP-1α (Fig. 2) and MIP-1β, nor does it block the Ca2+ flux induced by RANTES (Fig. 4) or MIP-1α, although it markedly inhibited the Ca2+ flux induced by SDF-1α (Fig. 3). The bicyclam also did not inhibit the Ca2+ flux induced by MCP-3, a natural ligand for CCR1 and CCR2b (25). By itself AMD3100 did not induce [Ca2+]i increases even at a concentration of 100 μg/ml (data not shown). The IC50 of AMD3100 required to inhibit binding of the CXCR4 mAb and to desensitize the SDF-1α–induced Ca2+ flux is between 1 and 10 ng/ml. This dose nicely correlates with the IC50 of the compound for the replication of T-tropic viruses in T cell lines and PBMCs, whereas a relatively higher concentration (10 μg/ml) was necessary to completely block SDF-1α–induced chemotaxis. This illustrates that AMD3100 can funtion as a potent antiviral compound in vitro (1, 2) and in vivo (100 ng/ml in plasma of SCID-hu mice is sufficient to reduce the viral load significantly; reference 26), rather than acting as an antagonist of leukocyte chemoattraction.
Some individuals who were repeatedly exposed to HIV infection and remained uninfected were found to be homozygous for a 32-bp deletion in the CCR5 (27–29). Perhaps mutations in CXCR4 and other coreceptors may also be identified in individuals who are less susceptible to HIV infection and/or in individuals who have been infected but do not proceed to AIDS, the so-called long-term nonprogressors (30), who have a predominance of M-tropic viruses. CCR5-binding viruses are important during early stages of infection, whereas the CXCR4-binding viruses emerge later in the progression to AIDS (31). AMD3100, because of its strong interaction with CXCR4, may become an important antiviral drug in vivo, because of its potential to block infection with T-tropic viruses, which in most cases precedes the decline in CD4+ T cells and the development of AIDS.
Recently, derivatives of the CC-chemokine RANTES have been described as CCR5 antagonists with activity against M-tropic HIV-1 strains (32, 33). However, the bicyclams are the first low molecular weight chemicals among the anti-HIV agents described as CXCR4 antagonists.
Acknowledgments
We thank Sandra Claes, Erik Fonteyn, and Jean-Pierre Lenaerts for their excellent technical assistance. We thank Ghislain Opdenakker for critical comments on the manuscript and are grateful to James A. Hoxie for kindly providing the anti-CXCR4 mAb 12G5.
This work was supported by grants from the Fonds voor Wetenschappelijk Onderzoek (FWO) Vlaanderen, the Belgian Geconcerteerde Onderzoekacties, the Belgian Fonds voor Geneeskundig Wetenschappelijk Onderzoek (FGWO), and the Janssen Research Foundation. J.A. Esté and S. Struyf hold fellowships from the BID-CONICIT (Venezuela) and FWO, respectively.
References
- 1.De Clercq E, Yamamoto N, Pauwels R, Baba M, Schols D, Nakashima H, Balzarini J, Murrer BA, Schwartz D, Thornton D, et al. Potent and selective inhibition of human immunodeficiency virus (HIV)-1 and HIV-2 replication by a class of bicyclams interacting with a viral uncoating event. Proc Natl Acad Sci USA. 1992;89:5286–5290. doi: 10.1073/pnas.89.12.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Clercq E, Yamamoto N, Pauwels R, Balzarini J, Witvrouw M, De Vreese K, Debyser Z, Rosenwirth B, Peichl P, Datema R, et al. Highly potent and selective inhibition of human immunodeficiency virus by the bicyclam derivative JM3100. Antimicrob Agents Chemother. 1994;38:668–674. doi: 10.1128/aac.38.4.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Vreese K, Reymen D, Griffin P, Steinkasserer A, Werner G, Bridger GJ, Esté J, James W, Henson G, Desmyter J, et al. The bicyclams, a new class of potent human immunodeficiency virus inhibitors, block viral entry after binding. Antivir Res. 1996;29:209–219. doi: 10.1016/0166-3542(95)00837-3. [DOI] [PubMed] [Google Scholar]
- 4.De Vreese K, Kofler-Mongold V, Leutgeb C, Weber V, Vermeire K, Schacht S, Anné J, De Clercq E, Datema R, Werner G. The molecular target of bicyclams, potent inhibitors of human immunodeficiency virus replication. J Virol. 1996;70:689–696. doi: 10.1128/jvi.70.2.689-696.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein–coupled receptor. Science (Wash DC) 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 6.Berson JF, Long D, Doranz BJ, Rucker J, Jirik FR, Doms RW. A seven-transmembrane domain receptor involved in fusion and entry of T cell–tropic human immunodeficiency virus type–1 strains. J Virol. 1996;70:6288–6295. doi: 10.1128/jvi.70.9.6288-6295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature (Lond) 1996;382:829–832. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 8.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, Schwartz O, Heard JM, Clark-Lewis I, Legler DF, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature (Lond) 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 9.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science (Wash DC) 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 10.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu L, Mackay CR, LaRosa G, Newman W, et al. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 11.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Marzio PD, Marmon S, Sutton RE, Hill CM, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature (Lond) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 12.Doranz BJ, Rucker J, Yi Y, Smyth RJ, Samson M, Peiper SC, Parmentier M, Collman RG, Doms RW. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 13.Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. HIV-1 entry into CD4+cells is mediated by the chemokine receptor CC-CKR-5. Nature (Lond) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 14.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+T cells. Science (Wash DC) 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 15.Trkola A, Dragic T, Arthos J, Binley JM, Olson WC, Allaway GP, Cheng-Mayer C, Robinson J, Maddon PJ, Moore JP. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature (Lond) 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 16.Wu L, Gerard NP, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso AA, Desjardin E, Newman W, et al. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature (Lond) 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 17.Van Damme J, Proost P, Lenaerts J-P, Opdenakker G. Structural and functional identification of two human, tumor-derived monocyte chemotactic proteins (MCP-2 and MCP-3) belonging to the chemokine family. J Exp Med. 1992;176:59–65. doi: 10.1084/jem.176.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schols D, Pauwels R, Baba M, Desmyter J, De Clercq E. Specific interaction of aurintricarboxylic acid with the human immunodeficiency virus/CD4 cell receptor. Proc Natl Acad Sci USA. 1989;86:3322–3326. doi: 10.1073/pnas.86.9.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wuyts A, Van Osselaer N, Haelens A, Samson I, Herdewijn P, Ben-Baruch A, Oppenheim JJ, Proost P, Van Damme J. Characterization of synthetic human granulocyte chemotactic protein 2: usage of chemokine receptors CXCR1 and CXCR2 and in vivoimflammatory properties. Biochemistry. 1997;36:2716–2723. doi: 10.1021/bi961999z. [DOI] [PubMed] [Google Scholar]
- 20.Masure S, Paemen L, Proost P, Van Damme J, Opdenakker G. Expression of a human mutant monocyte chemotactic protein 3 in Pichia pastorisand characterization as an MCP-3 receptor antagonist. J Interferon Cytokine Res. 1995;15:955–963. doi: 10.1089/jir.1995.15.955. [DOI] [PubMed] [Google Scholar]
- 21.Edinger AL, Amedee A, Miller K, Doranz BJ, Endres M, Sharron M, Samson M, Lu Z-H, Clements JE, Murphy-Corb M, et al. Differential utilization of CCR5 by macrophage and T cell tropic simian immunodeficiency virus strains. Proc Natl Acad Sci USA. 1997;94:4005–4010. doi: 10.1073/pnas.94.8.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Endres MJ, Clapham PR, Marsh M, Ahuja M, Turner JD, McKnight A, Thomas JF, Stoebenau-Haggarty B, Choe S, Vance PJ, et al. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 23.McKnight A, Wilkinson D, Simmons G, Talbot S, Picard L, Ahuja M, Marsh M, Hoxie JA, Clapham PR. Inhibition of human immunodeficiency virus fusion by a monoclonal antibody to a coreceptor (CXCR4) is both cell type and virus strain dependent. J Virol. 1997;71:1692–1696. doi: 10.1128/jvi.71.2.1692-1696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant stromal cell–derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Combadiere C, Ahuja SK, Van Damme J, Tiffany HL, Gao J-L, Murphy PM. Monocyte chemoattractant protein–3 is a functional ligand for CC chemokine receptors 1 and 2b. J Biol Chem. 1995;270:29671–29675. doi: 10.1074/jbc.270.50.29671. [DOI] [PubMed] [Google Scholar]
- 26.Datema R, Rabin L, Hincenbergs M, Moreno MB, Warren S, Linquist V, Rosenwirth B, Seifert J, McCune JM. Antiviral efficacy in vivoof the anti-human immunodeficiency virus bicyclam SDZ SID 791 (JM3100), an inhibitor of infectious cell entry. Antimicrob Agents Chemother. 1996;40:750–754. doi: 10.1128/aac.40.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dean M, Carrington M, Winkler C, Huttley GA, Smith MW, Allikmets R, Goedert JJ, Buchbinder SP, Vittinghoff E, Gomperts E, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science (Wash DC) 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 28.Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR. Homozygous defect in HIV-1 co-receptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 29.Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber C-M, Saragosti S, Lapouméroulie C, Cognaux J, Forceille C, et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature (Lond) 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 30.Schuitemaker H, Koot M, Kootstra NA, Dercksen MW, de Goede RE, van Steenwijk RP, Lange JM, Schattenkerk JK, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell–tropic virus population. J Virol. 1994;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connor RI, Sheridan KE, Ceradini D, Choe S, Landau NR. Change in coreceptor use correlates with disease progression in HIV-1 infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arenzana-Seisdedos F, Virelizier J-L, Rousset D, Clark-Lewis I, Loetscher P, Moser B, Baggiolini M. HIV blocked by chemokine antagonist. Nature (Lond) 1996;383:400. doi: 10.1038/383400a0. [DOI] [PubMed] [Google Scholar]
- 33.Simmons G, Clapham PR, Picard L, Offord RE, Rosenkilde MM, Schwartz TW, Buser R, Wells TNC, Proudfoot AEI. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science (Wash DC) 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]