Abstract
B cell receptor (BCR)-mediated antigen processing is a mechanism that allows class II–restricted presentation of specific antigen by B cells at relatively low antigen concentrations. Although BCR-mediated antigen processing and class II peptide loading may occur within one or more endocytic compartments, the functions of these compartments and their relationships to endosomes and lysosomes remain uncertain. In murine B cells, at least one population of class II– containing endocytic vesicles (i.e., CIIV) has been identified and demonstrated to be distinct both physically and functionally from endosomes and lysosomes. We now demonstrate the delivery of BCR-internalized antigen to CIIV within the time frame during which BCR-mediated antigen processing and formation of peptide–class II complexes occurs. Only a fraction of the BCR-internalized antigen was delivered to CIIV, with the majority of internalized antigen being delivered to lysosomes that are largely class II negative. The extensive colocalization of BCR-internalized antigen and newly synthesized class II molecules in CIIV suggests that CIIV may represent a specialized subcellular compartment for BCR-mediated antigen processing. Additionally, we have identified a putative CIIV-marker protein, immunologically related to the Igα subunit of the BCR, which further illustrates the unique nature of these endocytic vesicles.
The recognition of MHC class II–restricted antigens by antigen-specific T cells requires the proteolytic processing of protein antigens to immunogenic peptides by class II–positive antigen-presenting cells (1, 2). The first step in antigen processing by B cells involves B cell receptor (BCR)1–mediated internalization of antigen (3–5). BCR-internalized antigen is then proteolytically processed and the resultant peptides preferentially loaded onto newly synthesized class II molecules (6–8) from which the class II– associated invariant chain has been removed by the concerted action of acid proteases and the protein HLA-DM/ H-2M (9). The resultant peptide–class II complexes are then transported to the surface of the B cell.
The intracellular compartments where antigen processing occurs have only recently been characterized and there is considerable variation in the intracellular localization of class II molecules among different cell types. Many cells, such as human lymphoblasts and macrophages, sequester much of their class II in lysosomes or lysosome-like structures referred to as the MHC class II–enriched compartment (MIIC; reference 10). Although delivery of BCR-internalized antigen to MIIC has been demonstrated (11), the fate of the antigen delivered to these structures (i.e., complete degradation versus processing and binding to class II molecules) remains unknown.
In other professional antigen-presenting cells such as many murine B cell lines, there is little accumulation of class II in lysosomes under normal conditions (12–14). Instead, class II is found in endosomes and endosome-related structures, at least one population of which (class II vesicles [CIIV]) can be purified and physically separated from conventional endocytic and secretory organelles by cell fractionation techniques (14).
Although many or all endocytic, class II–containing vesicle populations may host the loading of peptides onto class II molecules, there may be important qualitative differences regarding the subcellular compartments where antigenic peptides are generated and efficiently loaded onto class II molecules. Specifically, although BCR-mediated antigen presentation appears to involve binding of peptide to newly synthesized class II molecules (6–8), presentation of fluid phase proteins by B cells appears to be able to occur via both newly synthesized and recycling class II molecules (7, 8, 15, 16), possibly reflecting differences in the intracellular sites of peptide generation and class II loading.
Additionally, not all receptors are equivalent at mediating antigen processing and presentation. In murine B cells, antigen internalized via the transferrin receptor (while presented more efficiently than soluble antigen) is presented 10–100 times less efficiently than the same antigen internalized via the BCR (17). This result may reflect the fact that the transferrin receptor has far more restricted access to intracellular class II compartments in B cells than does the BCR (11). Even more dramatic is the demonstration that a single amino acid substitution in the transmembrane region of the human IgM BCR (huBCR) can completely abolish the ability of this receptor to mediate efficient antigen processing and presentation without affecting BCR-mediated antigen endocytosis and bulk antigen degradation (18, 19). Thus, antigen uptake and degradation is necessary, but not sufficient, for antigen processing and presentation.
Thus, it has become important to determine the intracellular compartments to which physiologically important receptors (e.g., the BCR) deliver antigens. In this paper, we demonstrate that, within the time frame during which the intracellular events of BCR-mediated antigen processing are known to occur, BCR molecules and BCR-internalized antigen have access not only to predominantly class II– negative endosome and lysosomes, but also to a novel population of endocytic vesicles that are highly enriched in newly synthesized class II molecules (i.e., CIIV). Moreover, CIIV contain a putative marker protein, immunologically related to the Igα subunit of the BCR, further illustrating the distinct nature of these endocytic vesicles.
Materials and Methods
Cell Culture and Fractionation.
A20μWT (i.e., A20 cells expressing a transfected, phosphorylcholine (PC)–specific human mIgM BCR (huBCR); reference 19) were cultured in αMEM, 5% FBS, 50 μM 2-mercaptoethanol, and 500 μg/ml G418. A20μWT cells were homogenized, fractionated by free flow electrophoresis (FFE), and the distribution of plasma membrane, lysosomes, and CIIV was determined as previously reported (14).
Distribution of huBCR-internalized Antigen in A20μWT FFE Fractions.
A20μWT cells (2 × 108 total cells) were incubated at 4 × 107 cells/ml for 30 min at 37°C in media containing 2 μg/ml PC-modified Fab fragments of rabbit γ globulin labeled with 125I (PC–RGG–125I [2μCi 125I/μg PC–RGG]), homogenized, fractionated by FFE, and the distribution of the plasma membrane and lysosomes was determined. The distribution of PC–RGG–125I was determined by counting each FFE fraction in a γ counter. Background counts (<100 cpm) were subtracted from the counts for each sample and the results normalized to a maximum value of 1.00.
Immuno-electron Microscopy Localization of huBCR-internalized Antigen and BCR Molecules to Isolated CIIV.
A20μWT cells were incubated in 400-nM PC-modified ovalbumin (PC–OVA) for 20 min at 37°C, homogenized, and fractionated by FFE. Isolated CIIV, as well as endosomes–lysosomes, were processed for immuno-electron microscopy (immunoEM) as previously described (14). Cryosections were stained with rabbit IgG specific for either murine class II (14), murine IgG (315-005-046; Jackson Immunologicals, West Grove, PA), human IgM (309-005-095; Jackson Immunologicals; 309-005-095), or ovalbumin (RaOVALBUMI; East Acres Biologicals, Southbridge, MA). Antibody to class II molecules, anti-ovalbumin, and both anti-Ig antibodies were visualized with either 1, 5, or 10 nm protein A–gold (14).
Steady-state Distribution of the PC-specific huBCR in A20μWT Cells.
A20μWT cells were homogenized and fractionated by FFE. 200 μl of each FFE fraction, along with 50 μl of homogenization buffer containing 5% Triton X-100 and 0.5 mg/ml BSA, was added to a PC–BSA-coated 96-well plate and the samples allowed to bind for 6 h at 4°C. The plates were washed and probed with rabbit anti–human IgM (1:1,000; 309-005-095; Jackson Immunologicals) followed by horseradish peroxidase (HRP)–labeled goat anti–rabbit IgG (1:1,000; 31462; Pierce Chemical Co., Rockford, IL). Bound goat anti–rabbit Ig–HRP was detected by addition of 200 μl of 0.5 mg/ml O-phenylenediamine and 0.015% H2O2 in borate buffer. After sufficient time, 50 μl of 1N HCl was added and the absorbance (OD 490 nm) measured. The absorbance above background is reported (background = ∼0.200 OD 490 nm).
Surface Labeling and Endocytosis.
A20μWT cells were collected by centrifugation and washed two times with PBS. Cells were labeled for 15 min at 108 viable cells/ml in PBS pH 7.5 containing 1 mg/ml sulfosuccinimidyl-6-(biotinamido) hexanoate (NHS– LC–biotin; Pierce Chemical Company; 21335). The labeling was quenched by addition of 5–10 vol of 10 mM lysine in PBS. The labeled cells were pelleted and then washed twice in PBS 0.1% BSA before incubation at 37°C in complete media containing 1 μM PC–OVA (Cells were >98% viable after labeling, washing, and incubation).
Detection of Biotin-labeled huBCR Molecules in A20μWT FFE Fractions.
FFE fractions (200 μl) from biotin-labeled/PC–OVA pulsed A20μWT cells were added to NeutrAvidin (50 μg/ml; 31000; Pierce)-coated plates along with 50 μl of homogenization buffer containing 5% Triton X-100 and 0.5 mg/ml BSA and allowed to bind for 6 h at 4°C. The plate was washed and probed with rabbit anti–human IgM (1:1,000, 309-005-095; Jackson Immunologicals) followed by HRP-labeled goat anti–rabbit Ig (1:1,000; 31462; Pierce). Bound goat anti–rabbit Ig–HRP was detected as described above. The absorbance above background is reported (background = ∼0.100 OD 490 nm).
Steady-state Distribution of Igα in A20μWT Cells.
Individual A20μWT FFE fractions were concentrated by centrifugation and analyzed by SDS-PAGE and Western blotting (14) with a rabbit antiserum raised against intact, full length, bacterially expressed murine Igα (rabbit anti-Igα, 1:5,000). Binding of rabbit anti-Igα was detected with HRP-labeled goat anti–rabbit Ig (1:5,000; 31462; Pierce) and enhanced chemiluminescence (ECL; 34080; Pierce).
Immunological Relationship Between Igα and p50Igα.
A20μWT low density membranes (LDM) were separated by preparative SDS-PAGE and electroblotted onto nitrocellulose. The blot was probed with rabbit anti-Igα (1:1,000) and then extensively washed. The regions of the blot containing either Iga and p50Igα were individually excised and bound antibodies eluted with 500 μl of 0.1 M glycine, pH 2.5. The eluted antibody was neutralized with 100 μl, 1.0 M Tris, pH 8.0, and 4.5 ml of blotting buffer. The affinity-purified antibody was used to probe Western blots of total A20μWT LDM and binding revealed with HRP-labeled goat anti–rabbit Ig (1:5,000; 31462; Pierce) and ECL (34080; Pierce).
Results and Discussion
BCR-mediated Delivery of Antigen to Endosome and CIIV.
The murine A20μWT cell line (19) was used as a model cell for these studies. A20μWT cells express a PC-specific huBCR as well as an endogenous murine IgG2a BCR (muBCR) and the ability of these cells to process and present antigens via both BCRs has been well characterized (4, 19). Importantly, A20μWT cells localize only a small fraction (<10%) of their total class II to intracellular membranes (13, 14), with little class II present in high density, hydrolase-rich lysosomes (14). Owing to the lack of class II molecules in the lysosomes of these cells, it is easy to distinguish, by FFE, a distinctive population of low density, novel endocytic vesicles (i.e., CIIV) that are enriched in newly synthesized class II molecules (14). Moreover, comparative analysis of A20 (14, 23) and A20μWT cells (Figs. 1, 2 A, and 4; data not shown) demonstrates that CIIV isolated from both cells possesses the same morphological, biophysical, biochemical, and immunological characteristics (e.g., class II–positive, lgp110-negative, β-Hexosaminidase– negative multivesicular membrane structures with a characteristic electrophoretic mobility). Importantly, in A20μWT cells, BCR-mediated antigen processing and peptide loading of class II molecules can occur exclusively in low density endocytic structures without the involvement of high density lysosomal structures (13, 14).
Figure 1.
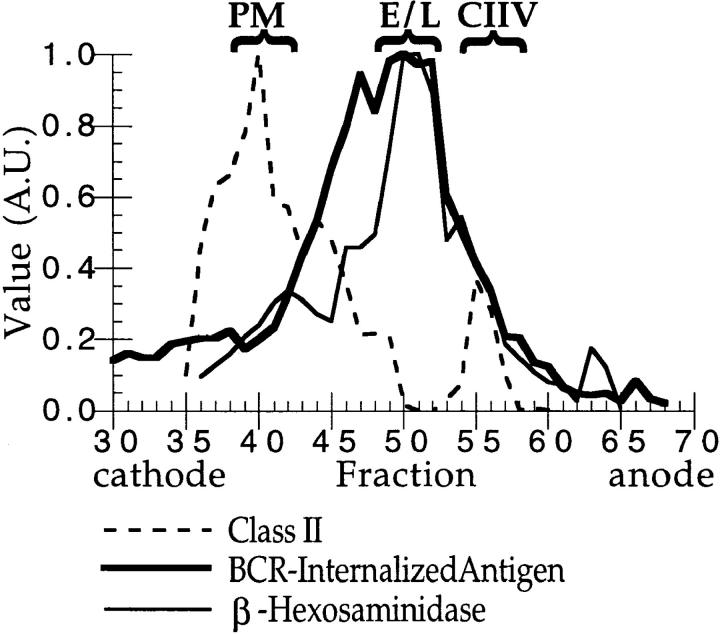
The intracellular distribution of BCR-internalized antigen in A20μWT cells. A20μWT cells were pulsed with 2 μg/ml antigen (i.e., PC–RGG– 125I) for 30 min at 37°C before homogenization and fractionation by FFE. The distribution of plasma membrane (PM, major peak of class II), endosomes and lysosomes (E/L, β-Hexosaminidase; thin solid line), and CIIV (minor, anodally shifted peak of class II) are indicated above the graph. (The distribution of class II (broken line) was determined by quantitative Western blot of every FFE fraction [reference 14].) Additionally, density gradient analysis of A20μWT cells has demonstrated that, as in A20 cells (14), β-Hexosaminidase–positive lysosomes are devoid of class II molecules (data not shown), demonstrating that the β-Hexosaminidase activity present in the CIIV-containing FFE fractions likely represents contamination by β-Hexosaminidase–positive, class II–negative lysosomes that were occasionally observed during ImmunoEM analysis of these FFE fractions (Table 1). The distribution of huBCR-internalized antigen (i.e., PC–RGG–125I; thick solid line) was determined by counting each fraction in a γ counter. (A value of 1.00 = 16,000 cpm above background in the experiment shown.) The major peak of huBCR-internalized antigen comigrated with markers for endosomes and lysosomes with a small amount present in CIIV-containing FFE fractions. Illustrated are results representative of three independent experiments.
Figure 2.
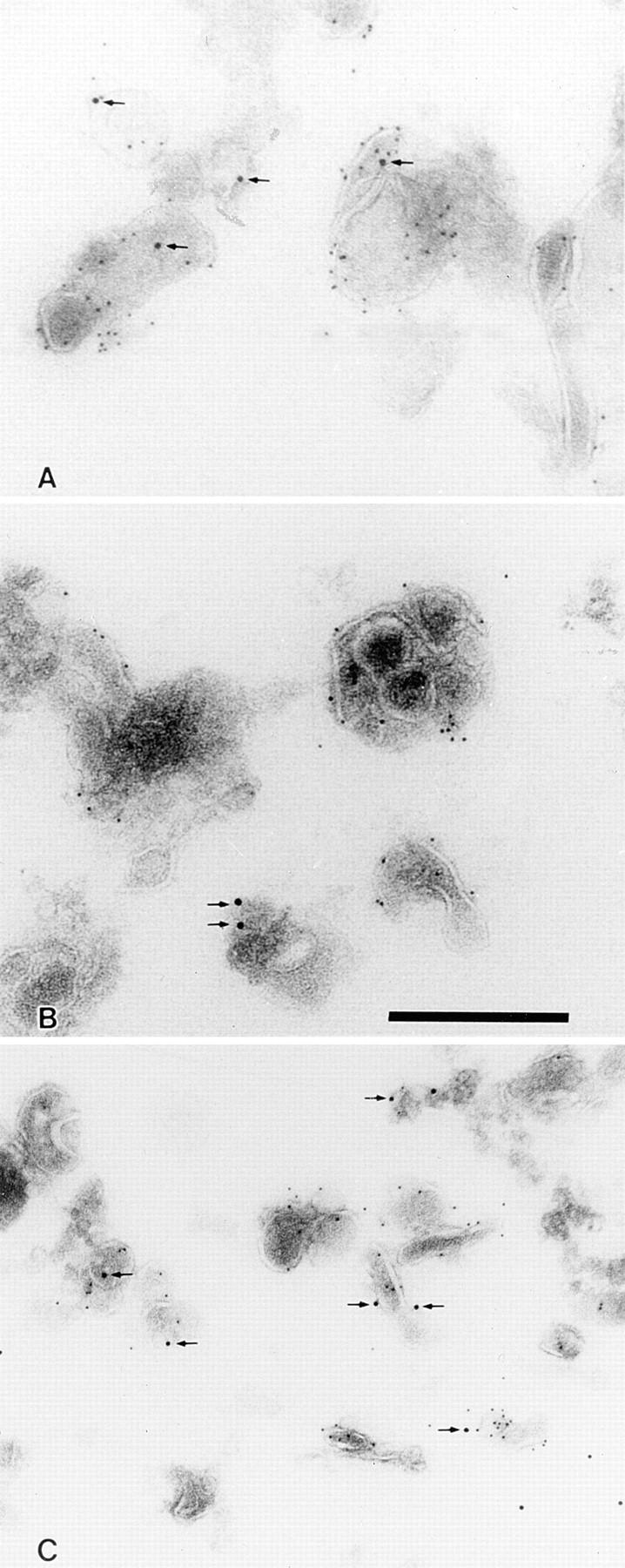
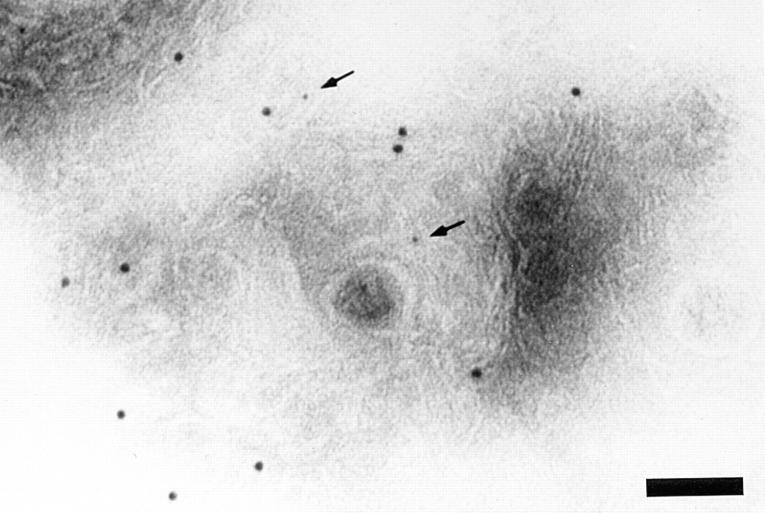
ImmunoEM localization of BCR-internalized antigen in isolated CIIV as well as endosomes and lysosomes. Bar: 300 nm. (A) ImmunoEM localization of BCR-internalized antigen in isolated CIIV. A20μWT cells were pulsed with antigen (i.e., 20 μg/ml PC–OVA) for 30 min at 37°C and then fractionated by FFE. Isolated CIIV were then analyzed by multiple label immunoEM with rabbit anti-class II and 5 nm protein A–gold followed by rabbit anti-OVA and 10 nm protein A–gold. Isolated CIIV were found to be doubly positive for both class II and BCR-internalized antigen (PC–OVA; arrows), indicating that a portion of the BCR-internalized antigen is delivered to CIIV. The specificity of the staining for class II molecules and BCR-internalized antigen is demonstrated by the lack of label over areas of the section that do not contain vesicular structures. (B and C) ImmunoEM localization of BCR-internalized antigen in isolated endosomes and lysosomes. Endosome/lysosome– containing FFE fractions from PC–OVA pulsed A20μWT cells were double labeled for class II and BCR-internalized antigen as in A. BCR-internalized antigen (PC–OVA; arrows) could be detected in both class II– negative (B) and class II–positive (C) vesicles.
Figure 4.
ImmunoEM localization of huBCR molecules in isolated CIIV. CIIV were isolated from A20μWT cells by FFE and analyzed by multiple label immunoEM with rabbit anti-huBCR (anti-human IgM) and 1 nm protein A–gold followed by rabbit anti–class II and 10 nm protein A–gold. Isolated CIIV stained for class II and huBCR (arrows). Bar: 100 nm.
If CIIV are involved in BCR-mediated antigen processing, then BCR-internalized antigens should be delivered to CIIV within 30–60 min after BCR-mediated internalization, the time required for BCR-mediated antigen processing in A20μWT cells (18). To determine whether this was the case, A20μWT cells were incubated with PC–RGG–125I for 30 min under conditions where antigen internalization and processing occur exclusively via cell-surface huBCR molecules (18, 19). The antigen-pulsed A20μWT cells were then homogenized, fractionated by FFE, and the distribution of huBCR-internalized PC–RGG–125I, as well as that of markers for plasma membrane (PM), endosomes, lysosomes, and CIIV (14), was determined. As shown in Fig. 1, the vast majority of the huBCR-internalized antigen was found in FFE fractions that contained endosomes and lysosomes (fractions 45–53). However, a small but significant amount of labeled antigen was present in the anodally shifted CIIV-containing FFE fractions (fractions 54–58). Thus, BCR-bound antigen could be found in CIIV-containing FFE fractions within the time frame during which BCR-mediated antigen processing is occurring.
This biochemical analysis, along with our previous observations that CIIV-containing FFE fractions consist almost entirely of class II–positive vesicles, strongly suggested that BCR-internalized antigen can gain access to CIIV. To demonstrate this point directly, and rule out the possibility that the BCR-internalized antigen present in the CIIV-containing FFE fractions was contained exclusively in class II–negative, β-Hexosaminidase–positive lysosomes, CIIV-containing FFE fractions from antigen (i.e., PC–OVA)– pulsed A20μWT cells were examined by multiple label immunoEM (14). As shown in Fig. 2 A, huBCR-internalized antigen (arrow) was present in CIIV isolated from antigen-pulsed B cells. Additionally, immunoEM analysis of endosome/lysosome–enriched FFE fractions demonstrated that BCR-internalized antigen was present both in class II–negative endosomes and lysosomes as well as class II–positive endosomes (Fig. 2, B and C, respectively). Quantitation of these immunoEM samples (Table 1) revealed that the vast majority (71–85%) of CIIV were endocytic (i.e., accessible by BCR-internalized antigen) and that the bulk (58–90%) of the endocytic vesicles within the CIIV-containing FFE fractions were class II positive. On the contrary, only 34% of the endocytic vesicles in the endosome/lysosome–containing FFE fractions were class II–positive endosomes, with the majority of the antigen-containing vesicles being class II–negative endosomes and lysosomes.
Table 1.
Quantitative ImmunoEM Analysis of FFE Fractions Isolated from Antigen-pulsed A20 μWT Cells
| FFE Fraction | Fraction of total vesicles that contain class II molecules | Fraction of class II–positive vesicles* that also contain BCR-internalized antigen | Fraction of BCR-internalized antigen-containing vesicles that are class II positive* | |||
|---|---|---|---|---|---|---|
| % | % | % | ||||
| CIIV | 75 | 71‡ | 58‡ | |||
| E/L | 72 | 21§ | 34 |
CIIV- and endosome/lysosome (E/L)–containing FFE fractions from PC–OVA pulsed A20μWT cells were analyzed by multiple label immunoEM for the presence of class II molecules and BCR-internalized antigen (i.e., PC–OVA). The percent of vesicles labeled for one or both markers determined by an unbiased sampling technique (26). For CIIV FFE fractions, a total of 531 vesicle profiles (from four independent experiments) were analyzed. Values are reported as percentages.
Class II–positive endocytic vesicles represent either CIIV (CIIV FFE fractions) or class II–positive endosomes (endosome/lysosome FFE fractions).
In a parallel set of experiments, the distribution of a ligand (i.e., HRP-labeled goat anti-murine IgG) internalized via the endogenous muBCR of A20μWT cells was determined in CIIV FFE fractions. Quantitative analysis of these samples (292 total vesicle profiles) revealed that 90% of the vesicles that contained muBCR-internalized ligand were class II–positive structures (i.e., CIIV) and 85% of CIIV contain muBCR-internalize ligand.
The nonantigen-containing, class II–positive vesicles in the E/L-containing FFE fractions most likely represent contaminating, class II–containing Golgi-derived vesicles.
CIIV Contain BCR Molecules Internalized from the Cell Surface.
The presence of BCR-internalized antigen in CIIV raised the question of whether antigen was delivered to this compartment while still bound to the BCR or after dissociation of BCR–antigen complexes. To determine whether antigen is delivered to CIIV while still bound to the BCR, we first determined whether BCR molecules could be found in CIIV at steady-state. To this end, A20μWT cells were homogenized, fractionated by FFE, and the distribution of PC-binding huBCR molecules was determined by an antigen-specific anti–human IgM ELISA. As shown in Fig. 3 A, most of the PC-binding huBCR was present in PM-containing FFE fractions (fractions 35–44) with lower but significant levels also detected in endosome and lysosome, as well as CIIV-containing FFE fractions (fractions 45–54 and 55–64, respectively). A similar steady-state distribution was observed for the endogenous muBCR as determined by Western blotting (data not shown).
Figure 3.
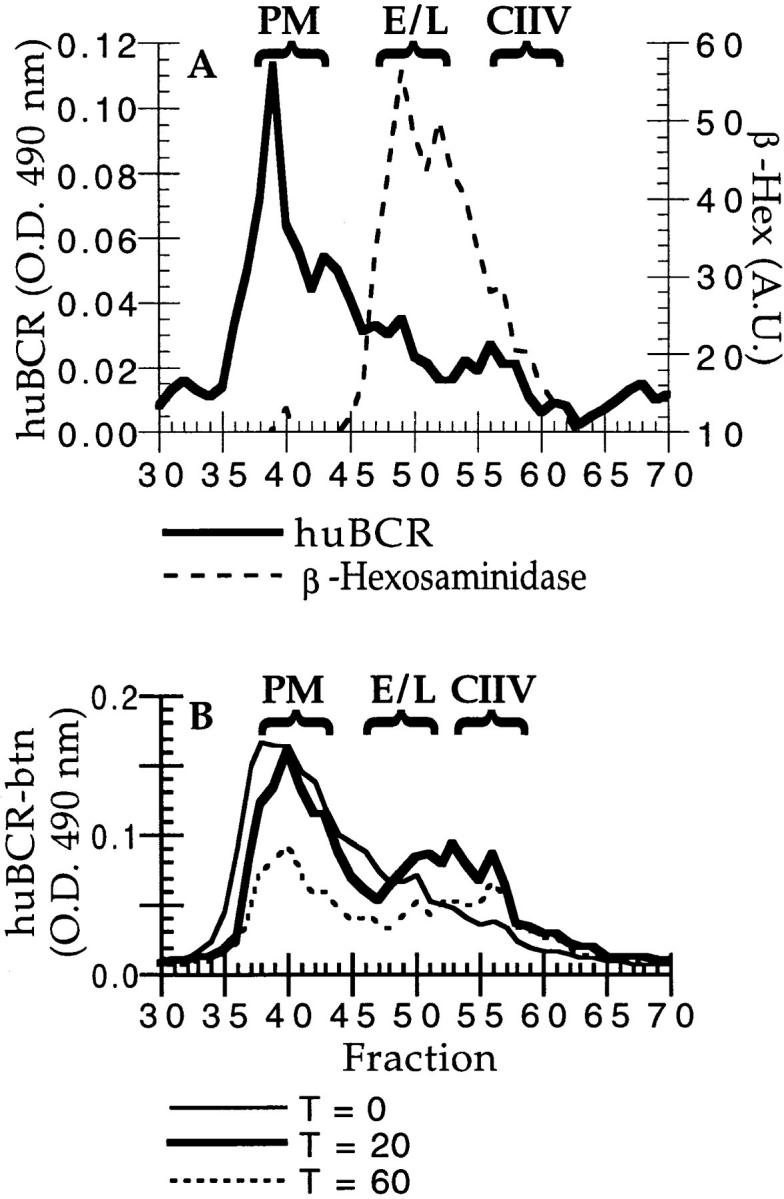
Subcellular distribution of BCR molecules in A20μWT cells. (A) Steady-state distribution of huBCR molecules in A20μWT cells. A20μWT cells were homogenized, fractionated by FFE, and the steady-state distribution of the PC-specific human BCR was determined by a solid phase ELISA using PC–BSA-coated plates. The background absorbance (i.e., all reagents added except a human BCR-containing sample; 0.150 OD 490 nm) was subtracted from the value for each sample. The major peak of huBCR (thick solid line) was coincident with plasma membrane (PM) markers (fraction 40). Some huBCR was also found in FFE fractions containing endosome–lysosome (β-Hexosaminidase; broken line) as well as the anodally shifted CIIV (see Fig. 1). Illustrated are results representative of three independent experiments. (B) Distribution of internalized huBCR molecules in A20μWT cells. A20μWT cells were surface labeled with NHS–LC–biotin and then incubated for 0 (thin solid line), 20 (thick solid line), or 60 (broken line) min at 37°C in the presence of 100 nM antigen (i.e., PC–OVA) before homogenization and fractionation by FFE. The distribution of biotin-labeled huBCR was determined by a solid-phase, human IgM-specific avidin-capture ELISA. After 20 min of internalization at 37°C, biotin-labeled huBCR, internalized from the cell surface, could be detected both in FFE fractions containing CIIV (fractions 56–65) as well as endosomes and lysosomes (fractions 46–55). Illustrated are results representative of four independent experiments.
To confirm that the huBCR molecules detected in the CIIV-containing FFE fractions were actually localized to CIIV, we performed multiple label immunoEM analysis of FFE-isolated CIIV for the presence of huBCR molecules. As shown in Fig. 4, CIIV do contain huBCR molecules (arrows) at steady-state. Similar analysis of endosome/lysosome–containing FFE fractions demonstrated that huBCR molecules could be found in both class II–positive as well as class II–negative vesicles in these fractions (data not shown).
Because we have previously demonstrated that newly synthesized BCR molecules do not traffic through CIIV before arrival at the cell surface (14), these results strongly suggest that the BCR molecules found in CIIV are derived from the PM by endocytosis and suggest that antigen is delivered to this compartment while bound to these internalized BCR molecules. To demonstrate directly that cell surface BCR molecules, internalized in the presence of polyvalent antigen, are delivered to CIIV, A20μWT were surface labeled with biotin, incubated for various times at 37°C in the presence of polyvalent antigen (i.e., PC–OVA), homogenized, and then fractionated by FFE. The level of biotin-labeled (i.e., internalized) huBCR molecules in each FFE fraction was then determined by a human IgM-specific, avidin-capture ELISA. As shown in Fig. 3 B, after 20 min of incubation, internalized huBCR molecules could be detected in CIIV-containing FFE fractions (fractions 53–60) as well as those enriched in endosomes and lysosomes (fractions 45–52). Surprisingly, even though we had found that, under similar conditions, a vast majority of the BCR-internalized antigen was ultimately delivered to endosomes and lysosomes (see Fig. 1), a significant fraction of the internalized huBCR molecules were found in CIIV-containing FFE fractions. Therefore, we suggest that a portion, and possibly all, of the huBCR molecules detected in CIIV by immunoEM (Fig. 4) were derived from the PM after endocytosis, further supporting the contention that antigen is delivered to CIIV while bound to the BCR. Interestingly, as suggested by the presence of huBCR molecules in CIIV isolated from nonantigen-pulsed A20μWT cells, preliminary analysis of the constitutive endocytosis and trafficking of the huBCR of A20μWT cells suggests that BCR endocytosis and delivery to CIIV can occur in the absence of antigen cross-linking (Drake, J.R., unpublished results).
Subcellular Distribution of BCR Subunits and Identification of a Putative CIIV Marker Protein Immunologically Related to Igα.
Although both BCR molecules and BCR-internalized antigen clearly gained access to CIIV, as well as endosomes and class II–negative lysosomes, the extent to which these molecules are, or are not, selectively targeted to CIIV remains unclear. To begin to address whether there is any selective targeting of BCR molecules or antigen–BCR complexes to CIIV, we first attempted to determine whether there is any difference in the subunit composition of the BCR molecules present in PM, endosome and lysosome, or CIIV-containing FFE fractions.
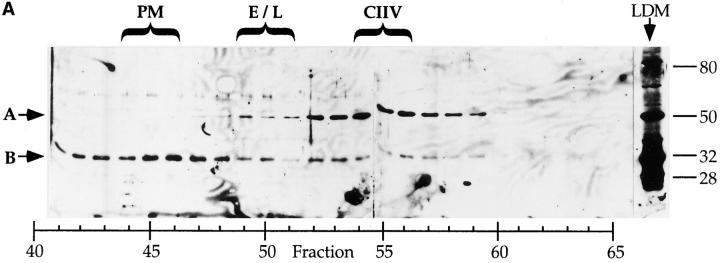
To this end, we examined the steady state distribution of the Igα subunit of the BCR. As shown in Fig. 5 A, the majority of the 32-kD Igα protein (arrow B) is present in PM-containing FFE fractions with lesser amounts detected in endosome–lysosome and CIIV-containing fractions. The same distribution was also found for the Igβ subunit of the BCR as well as the heavy and light chain subunits of both the huBCR and muBCR (data not shown), suggesting that the subunit composition of the BCRs in these compartments is similar. Surprisingly, the anti-Igα antiserum also recognized a second, 50-kD protein (Fig. 5 A, arrow A) that appears to be selectively enriched in CIIV-containing FFE fractions.
Figure 5.
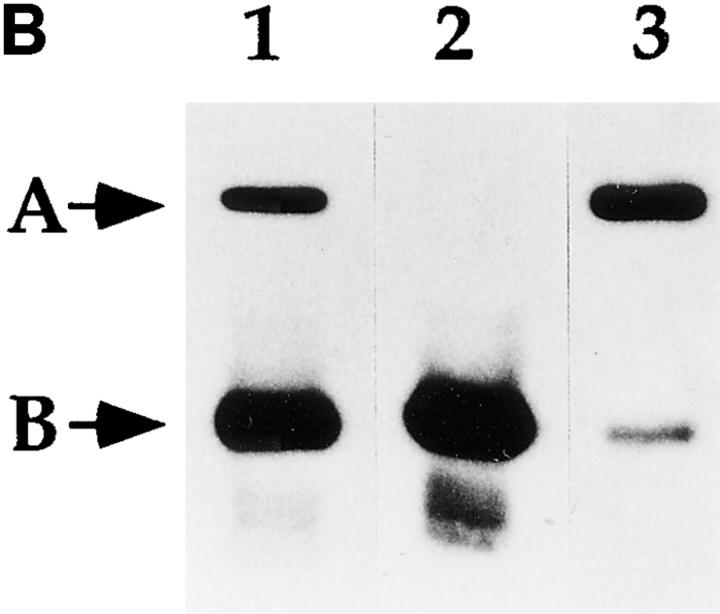
Identification of a putative CIIV-marker protein that is immunologically related to Igα subunit of the BCR. (A) Identification of a 50-kD protein enriched in CIIV-containing FFE fractions. A20μWT cells were fractionated by FFE and the distribution of markers for plasma membrane (PM, unshifted class II), endosomes/lysosomes (E/L, β-Hexosaminidase), and CIIV (anodally shifted class II) were determined. The position and size in kilodaltons, of the molecular mass standards is indicated at the right. The distribution of the Igα subunit of the BCR was determined by Western blot analysis using a rabbit antiserum against Igα. The 32-kD Igα protein (indicated by B) was detected at the highest levels in PM-containing FFE fractions containing and to a lesser extent in fractions containing endosomes–lysosomes and CIIV. Additionally, a 50-kD protein (A) was selectively enriched in the CIIV-containing FFE fractions (fractions 52–59). The 50-kD protein is also detectable in the unfractionated LDM. Illustrated are results representative of five independent experiments. (B) The 50-kD putative CIIV-marker protein is immunologically related to the Igα subunit of the BCR. Preparative Western blots of A20 LDMs were probed with the rabbit anti-Igα antiserum and washed. The antibodies against Igα and the 50-kD protein were affinity purified by excising the regions of the blot containing the anti-Igα and anti-50-kD proteins (and bound antibodies) and then eluting the bound antibodies. The affinity-purified antibodies, as well as uncut antiserum, were then used to probe a Western blot of unfractionated LDM. The uncut serum (lane 1) recognized both the 32-kD Igα (arrow B) as well as the 50-kD protein (arrow A) in the LDM. Although the affinity-purified anti-Igα (lane 2) did recognize Igα, it failed to recognize the 50-kD protein present in the LDM. (In some experiments, the affinity-purified anti-Igα antibodies did demonstrate reactivity towards the 50-kD protein.) Importantly, antibodies affinity purified on the 50-kD protein (lane 3) recognized both the 32-kD Igα protein (arrow B) as well as the 50-kD protein (arrow A), indicating an immunological relationship between these two proteins. (Immunoreactivity of the affinity-purified anti-50-kD antibodies against Igα was observed in every experiment.) Illustrated are results representative of three independent experiments.
To determine whether this 50-kD putative CIIV-marker protein was immunologically related to Igα (as opposed to being recognized by antibodies of a second specificity present in the rabbit anti-Igα antiserum), we affinity-purified antibodies to both Igα and the 50-kD protein on Western blots of A20μWT LDM, and tested the specificity of these purified antibodies. As shown in Fig. 5 B, the unfractionated rabbit anti-Igα antiserum recognized both Igα as well as the 50-kD protein present in unfractionated A20μWT LDM. Although affinity-purified anti-Igα antibody failed to recognize the 50-kD protein (occasionally, reactivity of the affinity-purified anti-Igα toward the 50-kD protein was observed although the results were variable, possibly owing to removal of low affinity/highly cross-reactive antibodies by the affinity purification protocol), affinity-purified antibody against the 50-kD protein recognized both Igα as well as the 50-kD protein. Because this antiserum was originally generated against recombinant whole murine Igα, these results demonstrate that at least some anti-Igα antibodies specifically recognize the 50-kD putative CIIV-marker protein, demonstrating that these proteins are immunologically related (i.e., that Igα and the 50-kD protein minimally share one cross-reactive epitope). Additionally, an antiserum raised against the cytoplasmic tail of Igα (20) also demonstrated cross-reactivity to the 50-kD protein, suggesting an immunological relationship between the cytoplasmic tail of Igα and some region of the 50-kD protein (data not shown).
Because the 50-kD putative CIIV-marker protein does not exhibit a decrease in apparent molecular weight upon treatment with either endoglycosidase H or F (Drake, J.R., unpublished results), we have foregone the more traditional gp50 designation in favor of p50Igα (the Igα superscript indicates the immunological relationship of the protein to the Igα subunit of the BCR). Although the structure and function of p50Igα remains unknown, it is unlikely to represent a highly modified form of Igα, since it was also detected in Igα-negative J774 macrophage-like cell line (Drake, J.R., unpublished results). Moreover, p50Igα is unlikely to be an artifact of proteolytic activity because it can be detected in detergent extracts of whole cells prepared in the presence of a cocktail of protease inhibitors (Drake, J.R., unpublished results). Additionally, preliminary analysis of J774 cells and the murine B cell hybridoma 2C3E1 (21) suggests a restricted distribution of p50Igα to CIIV in these cells (Drake, J.R., and P. Webster, unpublished results).
Considering the presence of p50Igα in the BCR-negative J774 macrophage-like cell line, p50Igα, unlike Igα, may not be a component of the BCR protein complex. Correspondingly, preliminary analysis has failed to reveal any physical association between p50Igα and the BCR of A20μWT cells (Drake, J.R., unpublished results). Therefore, a more thorough understanding of the possible function of p50Igα will have to await its eventual purification and sequencing or cDNA cloning. Interestingly, previous Southern blot analysis of the murine MB-1 gene (which codes for Igα) indicated the presence of an additional, Igα-related gene (22), possibly that coding for p50Igα. Most importantly, the identification of p50Igα as a putative marker for CIIV graphically demonstrates the unique biochemical nature of these novel class II-containing vesicles and provides us with a tool to study their origin, fate, and relationship to other intracellular compartments.
Previously, we demonstrated that in murine B cells, class II molecules are restricted to relatively early endocytic compartments (i.e., endosomes and CIIV), with little or no class II found in high density lysosomes (14). Because peptide–class II complexes have been demonstrated to form only in low density (i.e., nonlysosomal) compartments in these cells (13, 14), the presence of BCR-internalized antigen in both class II–positive endosomes as well as CIIV suggests that antigen processing and class II peptide loading may occur at either or both of these sites. Given the predominant role of newly synthesized class II molecules in BCR-mediated antigen processing and presentation (6–8) and the fact that CIIV are an intermediate in the pathway of transport of newly synthesized class II molecules to the cell surface (14, 23), our results strongly suggest a role for CIIV in BCR-mediated antigen processing and class II peptide loading.
Why might B cells possess a novel endocytic compartment for the processing of BCR-internalized antigens? In contrast with endosomes, lysosomes, and MIIC, which readily accumulate nonselectively internalized fluid phase endocytic tracers (10, 24), CIIV do not readily accumulate proteins internalized by fluid phase endocytosis (14). Within endosomes, lysosomes, and MIIC, the vast excess of nonantigenic peptides (i.e., peptides derived from the proteolytic degradation of fluid phase plasma proteins) may effectively compete with antigenic peptides (i.e., peptides derived from BCR-internalized antigen) for binding to class II molecules, preventing the efficient formation of antigenic peptide–class II complexes within these compartments. The absence of these nonantigenic peptides from CIIV may allow for more efficient formation of antigenic peptide–class II complexes in these vesicles. Additionally, because peptide loading onto class II is a relatively slow process (25), the relatively slow transport of newly synthesized class II molecules through CIIV, with class II molecules residing in CIIV for up to 2 h (14), may provide the necessary time for the loading of these class II molecules with antigen-derived peptides.
Acknowledgments
The authors would like to thank L. Schaefer for technical assistance, L. Ryan for photographic darkroom assistance, and L. Chicoine for assistance with sectioning and labeling samples for immunoEM analysis. Additionally, the authors would like to thank R. Mitchell (Harvard University, Cambridge, MA) and M. Nussenzweig (Rockefeller University, New York) who generously provided A20μWT cells and careful guidance as to their use.
Footnotes
The research described in this paper was supported by grants from the Public Health Service.
Abbreviations used in this paper: BCR, B cell receptor; CIIV, class II vesicles; ECL, enhanced chemiluminescence; FFE, free flow electrophoresis; huBCR, phosphorylcholine-specific human mIgM BCR; immunoEM; immuno-electron microscopy; LDM, low density membranes; MIIC, MHC class II–enriched compartment; muBCR, murine IgG2a BCR; NHS–LC–biotin, sulfosuccinimidyl-6-(biotinamido) hexanoate; PC, phosphorylcholine; PC–RGG–125I, PC-modified Fab fragments of rabbit γ globulin labeled with 125I; PC–OVA, PC-modified ovalbumin; PM, plasma membrane.
References
- 1.Wolf PR, Ploegh HL. How MHC class II molecules acquire peptide cargo: biosynthesis and trafficking through the endocytic pathway. Annu Rev Cell Dev Biol. 1995;11:267–306. doi: 10.1146/annurev.cb.11.110195.001411. [DOI] [PubMed] [Google Scholar]
- 2.Germain RN, Margulies DH. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- 3.Grey HM, Colon SM, Chesnut RW. Requirements for the processing of antigen by antigen-presenting B cells. II. Biochemical comparison of the fate of antigen in B cell tumors and macrophages. J Immunol. 1982;129:2389–2395. [PubMed] [Google Scholar]
- 4.Chesnut RW, Colon SM, Grey HM. Requirements for the processing of antigens by antigen-presenting B cells. I. Functional comparison of B cell tumors and macrophages. J Immunol. 1982;129:2382–2388. [PubMed] [Google Scholar]
- 5.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature (Lond) 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 6.Davidson HW, Reid PA, Lanzavecchia A, Watts C. Processed antigen binds to newly synthesized MHC class II molecules in antigen-specific B lymphocytes. Cell. 1991;67:105–116. doi: 10.1016/0092-8674(91)90575-j. [DOI] [PubMed] [Google Scholar]
- 7.Kakiuchi T, Watanabe M, Hozumi N, Nariuchi H. Differential sensitivity of specific and nonspecific antigen-presentation by B cells to a protein synthesis inhibitor. J Immunol. 1990;145:1653–1658. [PubMed] [Google Scholar]
- 8.Kakiuchi T, Takatsuki A, Watanabe M, Nariuchi H. Inhibition by brefeldin A of the specific B cell antigen presentation to MHC class II–restricted T cells. J Immunol. 1991;147:3289–3295. [PubMed] [Google Scholar]
- 9.Roche PA. HLA-DM: an in vivo facilitator of MHC class II peptide loading. Immunity. 1995;3:259–262. doi: 10.1016/1074-7613(95)90111-6. [DOI] [PubMed] [Google Scholar]
- 10.Peters PJ, Neefjes JJ, Oorschot V, Ploegh HL, Geuze HJ. Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature (Lond) 1991;349:669–676. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- 11.West MA, Lucocq JM, Watts C. Antigen processing and class II MHC peptide-loading compartments in human B-lymphoblastoid cells. Nature (Lond) 1994;369:147–151. doi: 10.1038/369147a0. [DOI] [PubMed] [Google Scholar]
- 12.Brachet V, Raposo G, Amigorena S, Mellman I. Ii chain controls the transport of major histocompatability complex class II molecules to and from lysosomes. J Cell Biol. 1997;137:51–65. doi: 10.1083/jcb.137.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnes KA, Mitchell RN. Detection of functional class II–associated antigen: role of a low density endosomal compartment in antigen processing. J Exp Med. 1995;181:1715–1727. doi: 10.1084/jem.181.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amigorena S, Drake JR, Webster P, Mellman I. Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature (Lond) 1994;369:113–120. doi: 10.1038/369113a0. [DOI] [PubMed] [Google Scholar]
- 15.Soreng KM, Moore JC, Sherman MA, Jensen PE. Requirement for protein synthesis in antigen processing by B cells. Cell Immunol. 1994;157:277–290. doi: 10.1006/cimm.1994.1222. [DOI] [PubMed] [Google Scholar]
- 16.St. Pierre Y, Watts TH. MHC class II–restricted presentation of native protein antigen by B cells is inhibitable by cycloheximide and brefeldin A. J Immunol. 1990;145:812–818. [PubMed] [Google Scholar]
- 17.Niebling WL, Pierce SK. Antigen entry into early endosomes is insufficient for MHC class II processing. J Immunol. 1993;150:2687–2697. [PubMed] [Google Scholar]
- 18.Mitchell RN, Barnes KA, Grupp SA, Sanchez M, Misulovin Z, Nussenzweig MC, Abbas AK. Intracellular targeting of antigens internalized by membrane immunoglobulin in B lymphocytes. J Exp Med. 1995;181:1705–1714. doi: 10.1084/jem.181.5.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw AC, Mitchell RN, Weaver YK, Campos TJ, Abbas AK, Leder P. Mutations of immunoglobulin transmembrane and cytoplasmic domains: effects on intracellular signaling and antigen presentation. Cell. 1990;63:381–392. doi: 10.1016/0092-8674(90)90171-a. [DOI] [PubMed] [Google Scholar]
- 20.Clark MR, Campbell KS, Kazlauskas A, Johnson SA, Hertz M, Potter TA, Pleiman C, Cambier JC. The B cell antigen receptor complex: association of Ig-alpha and Ig-beta with distinct cytoplasmic effectors. Science (Wash DC) 1992;258:123–126. doi: 10.1126/science.1439759. [DOI] [PubMed] [Google Scholar]
- 21.Drake JR, Repasky EA, Bankert RB. Endocytosis of antigen, anti-idiotype, and anti-immunoglobulin antibodies and receptor re-expression by murine B cells. J Immunol. 1989;143:1768–1776. [PubMed] [Google Scholar]
- 22.Kashiwamura S, Koyama T, Matsuo T, Steinmetz M, Kimoto M, Sakaguchi N. Structure of the murine mb-1 gene encoding a putative sIgM-associated molecule. J Immunol. 1990;145:337–343. [PubMed] [Google Scholar]
- 23.Amigorena S, Webster P, Drake J, Newcomb J, Cresswell P, Mellman I. Invariant chain cleavage and peptide loading in major histocompatibility complex class II vesicles. J Exp Med. 1995;181:1729–1741. doi: 10.1084/jem.181.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters PJ, Raposo G, Neefjes JJ, Oorschot V, Leijendekker RL, Geuze HJ, Ploegh HL. Major histocompatibility complex class II compartments in human B lymphoblastoid cells are distinct from early endosomes. J Exp Med. 1995;182:325–334. doi: 10.1084/jem.182.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen PE. Enhanced binding of peptide antigen to purified class II major histocompatibility glycoproteins at acidic pH . J Exp Med. 1991;174:1111–1120. doi: 10.1084/jem.174.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw MK, Tilney LG, Musoke AJ. The entry of Theileria parva sporozoites into bovine lymphocytes: evidence for MHC class I involvement. J Cell Biol. 1991;113:87–101. doi: 10.1083/jcb.113.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]