Abstract
Heat shock protein (HSP) preparations derived from cancer cells and virus-infected cells have been shown previously to elicit cancer-specific or virus-specific immunity. The immunogenicity of HSP preparations has been attributed to peptides associated with the HSPs. The studies reported here demonstrate that immunogenic HSP–peptide complexes can also be reconstituted in vitro. The studies show that (a) complexes of hsp70 or gp96 HSP molecules with a variety of synthetic peptides can be generated in vitro; (b) the binding of HSPs with peptides is specific in that a number of other proteins tested do not bind synthetic peptides under the conditions in which gp96 molecules do; (c) HSP–peptide complexes reconstituted in vitro are immunologically active, as tested by their ability to elicit antitumor immunity and specific CD8+ cytolytic T lymphocyte response; and (d) synthetic peptides reconstituted in vitro with gp96 are capable of being taken up and re-presented by macrophage in the same manner as gp96– peptides complexes generated in vivo. These observations demonstrate that HSPs are CD8+ T cell response–eliciting adjuvants.
Immunization with heat shock protein (HSP)1 preparations isolated from cancer cells or virus-infected cells has been reported to elicit protective antitumor or antiviral cellular immune response (1–8). This paradigm has also been substantiated in other antigenic systems, such that gp96 HSP preparations isolated from a cell expressing a transfected cytosolic protein can immunize and elicit specific CTLs against that antigen (9). Similarly, gp96 preparations isolated from cells expressing a given set of minor H antigens can be used to immunize and elicit CTL response against the minor antigens expressed by the cells that were the source of the immunizing gp96 preparation (9). HSPs are not polymorphic molecules and do not differ in their primary structure among normal tissues and cancers, or among normal and virus-infected cells. In this light, the remarkably general immunizing ability of HSP preparations has been explained on the basis of the suggestion that the HSP molecules are associated with peptides generated in the cells from which the HSPs are isolated (10). Peptides associated with gp96 and hsp70 have since been demonstrated (6, 11) and it has been shown that dissociation of the HSP-bound peptides leads to abrogation of immunogenicity of the HSP preparation (6). Confirmation of these results has also been obtained in a viral system, as a recent study has demonstrated that gp96 preparations isolated from vesicular stomatitis virus (VSV)-infected cells contain VSV-derived peptides (12). It has been suggested that cytosolic and endoplasmic reticular HSPs chaperone antigenic peptides during antigen processing and presentation by MHC class I molecules (13). The mechanism by which such noncovalent HSP–peptide complexes elicit protective cellular immune responses has recently been elucidated (14, 15).
The HSP–peptide interaction is at the center of this newly emerging immunological paradigm. In this report, we demonstrate that HSP–peptide complexes can also be generated in vitro and that the biological activity of these complexes is comparable to that of HSP–peptide complexes generated in vivo. Further, the HSP–peptide complexes reconstituted in vitro elicit immunity by a mechanism apparently identical to that implicated in the immunogenicity of the complexes generated in vivo.
Materials and Methods
Mice and Cell Lines.
Female C57BL/6 (6–8 wk old) were purchased from The Jackson Laboratory (Bar Harbor, ME). EL4 cells are a thymoma of C57BL/6 origin. N1 is a clone of EL4 transfected with the nucleocapsid gene of VSV (16). VSV-specific CTLs were derived from mice that were immunized with irradiated (7,500 rads) N1 cells. 7 d after immunization, splenocytes (8 × 106) from immune mice were cultured with irradiated N1 cells (5 × 104) in 24-well plates. CTLs were restimulated every 7 d. Pristane- induced peritoneal exudate cells were used for macrophage enriched population.
Purification of HSPs.
gp96 was purified from C57BL/6 liver cells, as described (2). In brief, 15 livers were homogenized in 40 ml of hypotonic buffer (30 mM NaHCO3, 0.1 mM phenylmethylsulfonyl fluoride, pH 7.1) by a tissue tearor, and a 100,000 g supernatant was obtained. The supernatant was fractionated by 50– 70% ammonium sulfate precipitation, applied to a concanavalin A–agarose column, and glycoproteins were eluted by 10% α-methylmannoside. The eluate was applied to a DEAE–agarose column, equilibrated with 0.3 M NaCl, and was eluted with 0.7 M NaCl. Hsp70 was purified as described by Peng et al. (17).
HSP–Peptide Binding.
gp96 and 125I-labeled peptides (synthesized by Bio-Synthesis, Inc., Lewisville, TX), were mixed in the quantities indicated, and incubated for 10 min at the indicated temperatures in a binding buffer (20 mM Hepes, pH 7.2, 20 mM NaCl, and 2 mM MgCl2). The samples were then incubated for 30 min at room temperature. Alternatively, gp96 and peptides were coincubated in sodium phosphate buffer at 25 or 50°C, as indicated, for 10 min at various salt concentrations, followed by incubation at room temperature for 30 min. In the case of hsp70, high temperatures and high salt concentrations were unnecessary; hsp70 and peptides were coincubated at 37°C in sodium phosphate buffer containing 1 mM ADP and 1 mM MgCl2. Free peptide was removed completely using a microcon 50 (Amicon, Inc., Beverly, MA). The removal of free peptides was monitored by electrophoretic analysis of the labeling mixture, followed by quantitative autoradiography; if peptides were not removed, they were visible on the dye front. Samples were also analyzed by silver staining or immunoblotting with anti-gp96 antibody (anti-GRP94, SPA-850, clone 9G10; NeoMarkers, Fremont, CA) or anti-hsp70 antibody (clone BRM22 from NeoMarkers). Peptide quantification was determined by densitometry using the Quantity 1 (version 2.2) program with the PDI Discovery series system (Sun Microsystems).
Tumor Rejection Assay.
Mice were injected subcutaneously with 10 μg or 25 μg reconstituted HSP–peptide complexes, or gp96 alone, peptide alone (75 nM), or buffer twice at weekly intervals. Mice were challenged intraperitoneally with 5,000 live N1 tumor cells 7 d after the second immunization.
CTL Assay.
Spleen cells (8 × 106/well) from immunized mice (day 96) were cultured in mixed lymphocyte tumor culture with 7,500 rads irradiated antigen-positive cells or cognate peptide-pulsed cell (5 × 104/well) in 24-well plates. After 5 d, mixed lymphocyte tumor cultures were tested for cytotoxicity in a chromium release assay.
TNF-α Bioassay.
Macrophages (1.5 × 104) and VSV-specific CTLs (5 × 104) were cultured with serially diluted reconstituted VSV–gp96 complexes for 24 h at 37°C. Supernatants were collected and assayed for TNF-α production in a cytotoxicity assay as described (15).
Results
Exchange of Peptides Naturally Bound to HSPs with Exogenous Peptides.
The ability of gp96 molecules to bind peptides in vitro was analyzed using an electrophoretic assay. The rationale for the use of this assay was as follows: it had been demonstrated earlier that gp96 preparations obtained from preparative sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) could still be used to elicit limited but significant tumor-specific immunity (2). This observation seen in the context of subsequent studies, which suggested that gp96 preparations are immunogenic because of association of gp96 with antigenic peptides (11, 12), indicated that gp96–peptide interaction would be expected to be stable under conditions of SDS-PAGE.
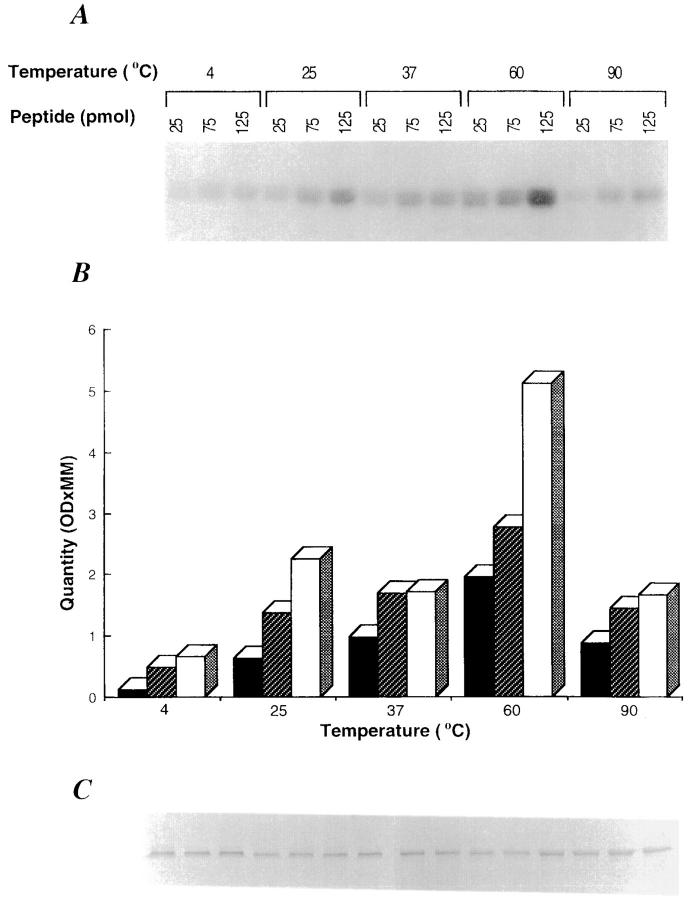
The peptide A (KRQIYTDLEMNRLGK) derived from G protein of the VSV was used for the initial studies. This peptide has been shown previously to bind hsp70 molecules in vitro (18). Apparently homogeneous, unlabeled gp96 preparations were incubated at 37°C with iodinated peptide A as described in Materials and Methods. The sample was analyzed by SDS-PAGE with or without additional heating of the sample in SDS-PAGE sample buffer, followed by autoradiography. The expectation from such an experiment is that SDS-resistant binding of unlabeled gp96 to labeled peptide will result in a labeled 96-kD band. However, no binding of gp96 to peptide A is detected under these conditions. The possibility was considered that incubation of gp96 with exogenous peptides at higher temperatures might permit dissociation of naturally bound peptides followed by reannealing of a proportion of exogenously added radiolabeled peptides at lower temperatures. Iodinated preparations of peptide A were incubated with unlabeled gp96 at 4, 25, 37, 60, or 90°C for 10 min and allowed to cool to room temperature for an additional 30 min. The samples were analyzed by SDS-PAGE without further heating and the gels were stained for proteins and autoradiographed. It was observed (Fig. 1) that exogenously added labeled peptide A could associate with gp96 in a temperature-dependent manner with optimal binding at 60°C. Little binding is detected at 4, 25, 37, or 90°C. Although the intensity of label in the gp96 band varies at different temperatures and at different peptide concentrations (Fig. 1, A and B), the quantity of gp96 as detected by silver staining is constant in all lanes (Fig. 1 C). It was also observed that the gp96–peptide binding can be dissociated, if the complexes are heated in a boiling water bath (data not shown).
Figure 1.
gp96 binds to peptides in vitro. gp96 (10 pmol) was incubated with increasing concentrations of radioiodinated peptide A (25, 75, and 125 pmol) for 10 min at different temperatures in 20 μl reaction buffer, followed by 30 min at room temperature. The reaction was terminated by mixing with sample buffer (0.1% SDS, 20% glycerol, and 5% bromophenol blue) and analyzed by SDS-PAGE. (A) Autoradiogram after 48-h exposure. (B) Densitometric quantification of results in A. An aliquot of each reaction was analyzed in parallel by SDS-PAGE and silver staining (C).
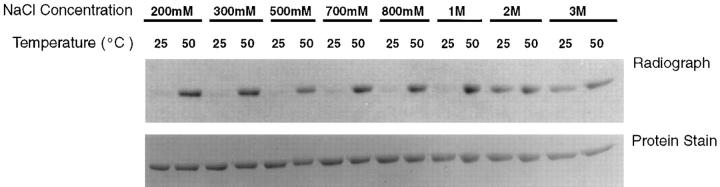
The exchange of exogenous and native-bound peptides could also be achieved by incubation of gp96 with exogenous peptides at high salt concentrations. Gp96 preparations were incubated at 25 or 50°C with radio-iodinated peptide VSV19 (extended on both termini of Kb-binding VSV nucleocapsid protein (NP)-derived octamer VSV8) for 10 min in sodium phosphate buffer containing 200 mM, 300 mM, 500 mM, 700 mM, 800 mM, 1 M, 2 M, or 3 M NaCl, followed by 30 min at room temperature. The samples were desalted and analyzed by SDS-PAGE, followed by staining as well as autoradiography. It was observed (Fig. 2) that significant quantities of labeled peptides formed an SDS-resistant association with gp96 after incubation at 2 M or higher NaCl concentration, but not at lower concentrations. In presence of high salt, the extent of association of gp96 with peptides was comparable at the low and high temperatures, whereas at low salt concentrations, gp96–peptide interaction was detected only at the higher temperature. The quantity of gp96 in each lane, as judged by Coomassie blue staining and scanning, was identical.
Figure 2.
Exchange of exogenous and native-bound peptides at high salt concentrations. gp96 (40 pM) and iodinated synthetic peptide (2 nM, NH2–Ser–Leu–Ser–Asp–Leu–Arg–Gly–Tyr–Val–Tyr–Gln– Gly– Leu– Lys– Ser–Gly–Asn–Val–Ser–COOH) were mixed in phosphate buffer in 20 μl reaction volume and incubated at 25 or 50°C for 10 min. After centrifugation, the mixtures were incubated at 25°C for another 30 min. Samples were analyzed by SDS-PAGE and staining, followed by autoradiography of the stained gel (24-h exposure).
Reconstitution of peptides with hsp70 molecules was observed to require neither a heating and cooling cycle, nor exposure to high salt concentrations. Incubation of apparently homogeneous preparations of hsp70 with radiolabeled peptide A in sodium phosphate buffer containing 1 mM ADP and 1 mM MgCl2 at 37°C was found to be sufficient to generate SDS-stable hsp70–peptide complexes as judged by autoradiography (see Fig. 5 A).
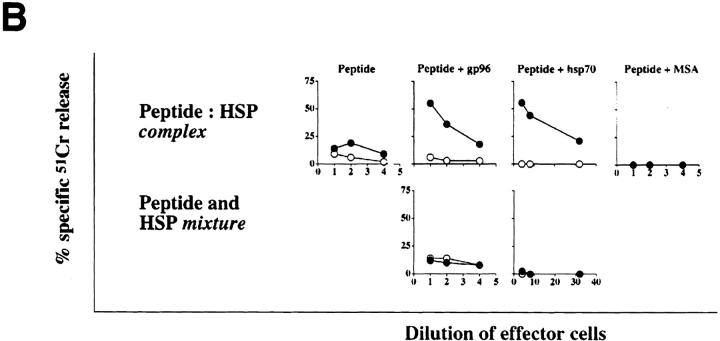
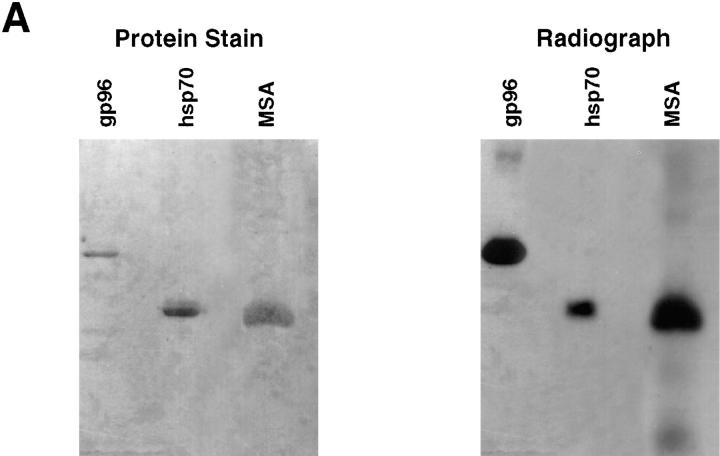
Figure 5.
Chaperoning of peptides by HSPs is required for generation of an effective CD8+ T cell response. gp96, hsp70, or mouse serum albumin (MSA) were complexed with radiolabeled VSV9 and analyzed by (A) SDS-PAGE followed by Coomassie blue staining and autoradiography. In addition, mice were immunized (B) with peptides complexed or simply mixed with each of the proteins. Splenocytes of these mice were tested for induction of CD8+ T lymphocytes, as described in legend to Fig. 4. N1 (closed circle) and EL4 (open circle) were used as targets.
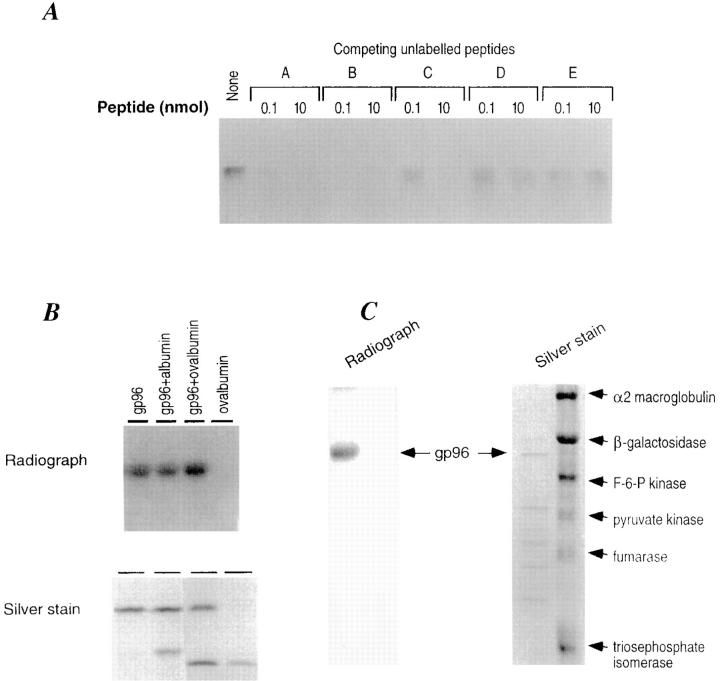
Binding of gp96 or hsp70 to peptides is not restricted to peptide A and can also be demonstrated for an array of other peptides, such as peptide B, LSSLFRPKRRPIYKS (derived from VSV G protein; reference 18); peptide C, SLSDLRGYVYQGLKSGNVS (derived from VSV nucleoprotein; reference 18); peptide D, IASNENMETMESSTLE (derived from nucleoprotein of influenza virus strain A/PR/8/34); and peptide E, SFIRGTKVSPRGKLST (derived from nucleoprotein of influenza virus A/NY/60/ 68) (data not shown). To evaluate the specificity of binding of peptides to gp96, unlabeled peptides A, B, C, D, and E were tested for their ability to compete with labeled peptide A in the gp96–peptide A binding assay. gp96, 25 pmol radiolabeled peptide A, and 0.1 or 10 nmol unlabeled peptides A, B, C, D, or E were coincubated at 50°C, followed by a 30-min incubation at room temperature. It was observed that all peptides could compete with peptide A in binding to gp96, although with different efficiencies (Fig. 3 A). As expected, higher quantities (10 nmol) of competing unlabeled peptides were more effective in displacing labeled peptide A than the lower quantities in the case of all peptides except peptide E, in which case the competition was already saturating at the lower quantity.
Figure 3.
Specificity of peptide binding by gp96. (A) Unlabeled peptides A, B, C, D, and E (0.1 and 10 nM) were used to compete with labeled peptide A (25 pmol) for the binding to gp96 (10 pmol). The sequences of these peptides are described in the text. The binding assay described in the legend to Fig. 1 was used, except that binding was carried out at 50°C. (B) Albumin and ovalbumin do not compete with gp96 for binding to peptide A. gp96 (10 pmol) was incubated with 25-pmol radiolabeled peptide A and analyzed as in the legend to Fig. 1. Albumin and ovalbumin (10 pmol each) were included in the binding assay. The autoradiogram and the silver stained gel are shown. (C) A partially degraded preparation of gp96 and a mixture of six purified proteins (i.e., α–2 macroglobulin, β-galactosidase, fructose-6-phosphokinase, pyruvate kinase, fumarase, and triosephosphate isomerase) were tested for binding to iodinated peptide A. Only the intact gp96 molecule is able to form a stable complex with radioactive peptide A. None of the other six proteins tested are able to bind peptide A.
The specificity of binding of HSPs with the exogenous peptide was demonstrated in the following additional ways, as shown here for gp96, but also observed for hsp70. (a) Inclusion of BSA or OVA in gp96–peptide A binding reaction had no influence on gp96–peptide A binding (Fig. 3 B); (b) a number of proteins, i.e., α–2 macroglobulin, β-galactosidase, fructose-6-phosphate kinase, OVA, pyruvate kinase, fumarase, and triosephosphate isomerase were tested for their ability to bind peptide A and were observed to not bind it (Fig. 3 C); and (c) it was demonstrated that only the intact gp96 and not any of its various degradation products could bind peptide A (Fig. 3 C).
The quantity of peptide bound to the HSPs in vitro was determined. The specific radioactivity of the peptides (cpm/ mol of peptide) was measured; using this number, the number of moles of peptides bound to a given quantity of gp96 were determined by measuring the cpm in the HSP band after autoradiography, by cutting out the band and counting it in a γ counter. This calculation revealed that under the conditions tested, and assuming a stoichiometry of one peptide per HSP molecule, ∼1% of HSP molecules were loaded with the exogenous peptide. The assumption of a 1:1 stoichiometry between HSP and peptides was made on the basis of the recent demonstration of a single peptide-binding pocket in a bacterial hsp70 molecule (19).
Immunogenicity of HSP–peptide Complexes Reconstituted In Vitro.
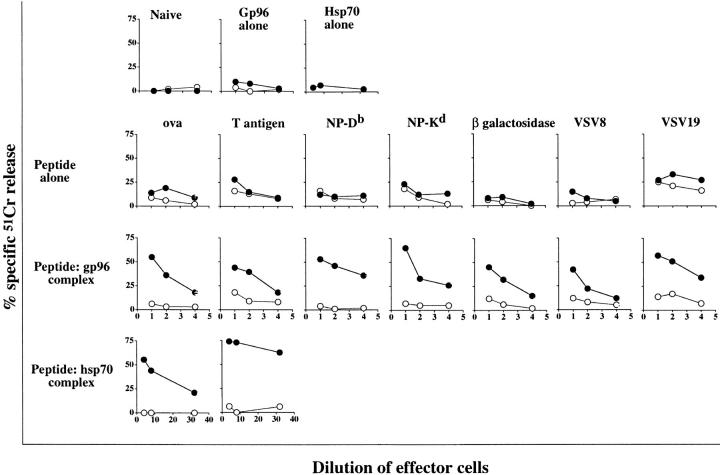
gp96–peptide complexes and hsp70–peptide complexes generated in vitro were tested in a variety of models for their ability to elicit CTLs and tumor immunity. For generation of CTLs, seven model peptides, which bind to different MHC class I alleles, were tested. These derive from OVA (Kb), SV40 T antigen (Db), NP antigen of influenza virus (Db and Kb), NP antigen of VSV (Kb), and β-galactosidase (Ld). The peptides were complexed with gp96, hsp70, or both and mice of the appropriate haplotype (b or d) were immunized twice at weekly intervals, with the peptides alone (10 μg peptide in PBS), the uncomplexed HSPs alone (20–50 μg HSP in PBS), or the HSP– peptide complexes (20–50 μg HSP complexed with ∼2 ng peptide, determined as described in the previous section). Spleen cells from the immunized mice were put in culture and were stimulated with the cognate peptide and tested for cytotoxic activity on target cells pulsed or unpulsed with relevant peptides. It was observed (Fig. 4) that T cells obtained from mice immunized with peptides alone or with gp96 or hsp70 alone showed no cytotoxic activity, whereas T cells obtained from mice immunized with HSP– peptide complexes showed significant and consistent peptide-specific CTL activity. The precise MHC class I–binding peptides were used in these studies. To test whether larger peptides than the exact epitopes could be complexed with HSPs and used to immunize successfully, a 19-mer precursor of Kb-binding VSV8 (VSV19) was complexed with hsp70 and gp96 and the complexes used to immunize. The HSP–VSV19 complexes were found to be as effective at eliciting antigen-specific CTL response as the HSP– VSV8 complexes were (Fig. 4).
Figure 4.
Hsp–peptide complexes reconstituted in vitro prime mice for CD8+ T cell response. Mice were immunized with HSPs alone (20–50 μg), peptides alone (10 μg), or HSP–peptide complexes (20–50 μg), as indicated. 1 wk after the last immunization, spleens were removed and stimulated with the cognate peptide or with cells transfected with the gene encoding the relevant antigen. The lymphocyte cultures were tested for their ability to lyse cells transfected with the antigen of interest (closed circle) and the nontransfected parental line (open circle). For the top panel, HSPs alone were tested for their immunizing ability in each antigenic system and were found to be consistently negative. The CTL responses were tested in many but not all systems and where tested, were found to be MHC class I and CD8 restricted.
A number of parameters of the adjuvant activity of gp96 and hsp70 were tested. The possibility that complexing of HSPs with peptides is unnecessary and HSP–peptide mixtures (instead of complexes) may be equally immunogenic was tested. It was observed that, similar to the results of immunization with HSPs or peptides alone, mixtures of HSPs and peptides were consistently nonimmunogenic. Similarly, when mice were immunized with HSPs on one flank and peptides on the other, no CTL response was detected (data not shown).
The possibility that immunization with peptides mixed or complexed with any large molecule, particularly other carrier molecules, might also elicit a potent and specific CTL response was investigated. The VSV9 peptide (Tyr– VSV8) was mixed with a traditional carrier protein, mouse serum albumin, under conditions that facilitate binding with gp96 and hsp70. The complexed material was analyzed by SDS-PAGE, and mouse serum albumin, like gp96 and hsp70, was found to form an SDS-resistant complex with the peptide (Fig. 5 A). Mice were immunized with mouse serum albumin–VSV9 complex, and were tested for CTL response as described previously. No CTL response was detected (Fig. 5 B). On the other hand, Gp96–VSV9 and hsp70–VSV9 complexes elicited significant specific CTL responses (Fig. 5 B).
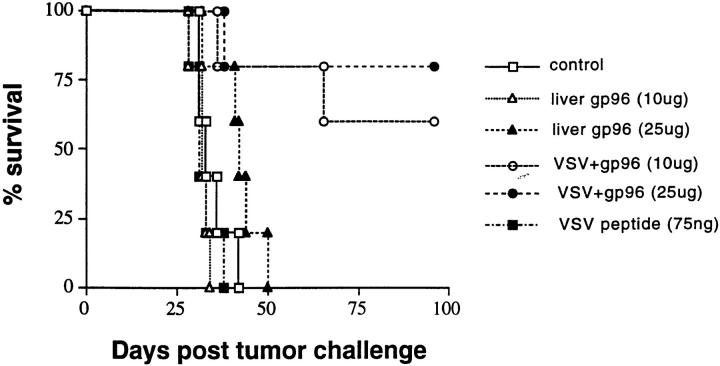
These observations demonstrate that the HSPs gp96 and hsp70 possess an adjuvant activity effective for eliciting a CD8+ T cell response. The efficacy of HSP–peptide vaccination in eliciting tumor rejection was tested in an artificial model, because the identity of peptides that can elicit rejection of natural tumors is yet unknown. Tumor rejection studies were carried out using the N1 tumor, which has been derived by transfection of the EL4 lymphoma of the C57BL/6 mouse with the gene encoding the NP of the VSV. Therefore, VSV–NP is a model tumor rejection antigen for this tumor (16). (In the past, we have actively refrained from using tumor models in which foreign genes have been transfected into tumors, as they reveal little about tumor immunity. However, this model is used in the present study to measure an antigen-specific T cell response in vivo.) Thus, gp96 molecules obtained from normal livers of C57BL/6 mice were complexed with the VSV8 known to bind Kb. C57BL/6 mice were immunized with such reconstituted complexes, or with VSV8 alone, or with liver gp96 alone, and were challenged with N1 cells. Survival of mice was monitored (Fig. 6). It was observed that there was no difference in the survival kinetics of unimmunized mice and mice immunized with liver gp96 or the VSV8 alone: all mice died within 30–50 d of tumor challenge. In contrast, 8 of 10 mice immunized with gp96–VSV8 complexes reconstituted in vitro, survived beyond 100 d after tumor challenge. Spleens of the immunized mice were also tested for antigen-specific CTL response to the VSV8 epitope. It was observed that mice immunized with the gp96–VSV8 complex generated effective antigen-specific, CD8+ CTL response, whereas mice immunized with gp96 alone, or the VSV8 alone, did not (data not shown). These results indicate that the peptides complexed with gp96 in vitro elicit tumor immunity in a manner consistent with the gp96– peptide complexes generated in vivo. Similar antitumor activity has been shown for hsp70–peptide complexes (data not shown).
Figure 6.
gp96–VSV8 complexes reconstituted in vitro elicit peptide-specific, protective immunity. Mice were immunized twice at weekly intervals with gp96–VSV8 complexes (10 μg or 25 μg liver gp96 complexed with 1–2 ng VSV8 peptide), liver gp96 alone (10 or 25 μg), VSV8 peptide alone (75 ng), or RPMI medium control. gp96–VSV8 complexes were washed extensively using a minicon 50 to remove unbound peptides. All mice were challenged intraperitoneally with 5,000 live N1 cells 1 wk after the second immunization; survival was monitored.
Re-presentation of Peptides Reconstituted with gp96 In Vitro, by MHC Class I Molecules of Macrophages.
The mechanism whereby immunization with gp96–peptide complexes generated in vivo leads to a protective CTL response has been elucidated (15). It has been shown that gp96–peptide complexes are taken up by macrophages and the chaperoned peptides are re-presented by the MHC class I molecules of the macrophage through a novel pathway. The immunological activity of the gp96–peptide complexes generated in vitro was tested in this assay. gp96 preparations were reconstituted with the VSV8 peptide at different temperatures and the resulting complexes were used to pulse pristane-induced macrophages of C57BL/6 mice in vitro. The pulsed macrophages were tested for their ability to stimulate anti-VSV CTLs, as measured by the secretion of TNF-α by the CTLs (Fig. 7). It was observed that the macrophage pulsed with complexes reconstituted at 60°C were effective in this re-presentation assay, whereas those reconstituted at 37, 80, or 98°C were not. As we have shown previously (15), the quantity of VSV8 complexed with gp96 in these experiments is ∼2 log scales lower than that necessary for direct charging of the empty surface MHC class I molecules by the VSV8 peptides. Data in Fig. 7 show that the gp96–peptide complexes reconstituted in vitro appear to be re-presented by the antigen-presenting cells in the same manner as shown previously (15) for the natural HSP–peptide complexes.
Figure 7.
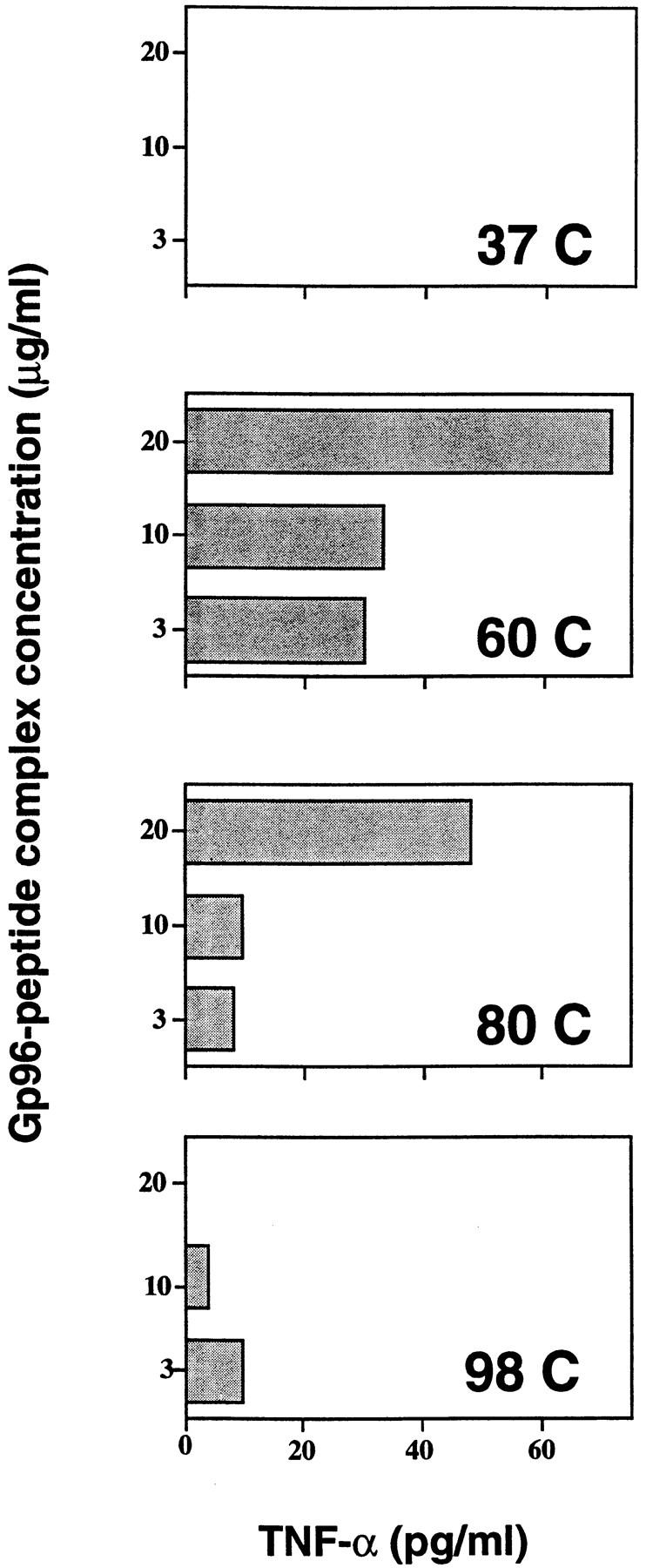
VSV8 peptide chaperoned by gp96 is re-presented by macrophage. Pristane-induced peritoneal exudate cells (104) and VSV peptide-specific CTL (5 × 104) were cocultured with gp96– VSV8 complexes (3–20 μg/ml) reconstituted at 37, 60, 85, or 98°C in a 96-well U-bottomed plate at 37°C. After 24 h, supernatants were collected and assayed for TNF-α production in a cytotoxicity assay as described (15).
Discussion
The studies described here indicate that HSP–peptide complexes can be reconstituted in vitro and that, by all parameters tested, such complexes show immunological activity similar to the HSP–peptide complexes generated in vivo. The results also show significant differences between gp96 and hsp70 with respect to the conditions in vitro, under which they bind peptides. These differences presumably reflect the fact that although hsp70–ATP interaction plays a crucial role in hsp70–peptide interaction in vivo, the identity of the corresponding ligand for gp96 is presently unknown. In contrast with the situation with hsp70, gp96–ATP interaction does not strip gp96 of its associated peptides (data not shown), even though gp96, like hsp70, is an ATP-binding protein and is an ATPase (11). Exposure to high temperature and high salt apparently causes the gp96 molecule to assume an open conformation, which permits dissociation from and association with exogenous peptides. The identity of the ligands that catalyze this process in vivo would be of interest in this regard.
The observations reported here have several implications. First, they support the hypothesis that immunogenicity of tumor-derived gp96 preparations results from a physical association of gp96 with antigenic peptides. The HSP–peptide complex elicits immunity under conditions in which the HSP molecules alone, or the peptides alone, do not. Second, these observations show that one does not have to rely on HSP–peptide complexes generated in vivo to elicit immunity; instead, such complexes can be generated reproducibly in vitro, provided the identity of the immunogenic peptides is known. A variety of peptides of different lengths, compositions, and hydrophobicity can bind the HSPs, suggesting that the nature of an epitope is not a limiting factor in its suitability as a vaccine in the form of a HSP–peptide complex. The ability of the gp96 to bind peptides in vitro has also been independently demonstrated recently (20). The quantity of peptide that is required to be conjugated to the HSPs is extremely small and 1–2 ng of peptides complexed to the HSPs elicit potent cellular immune response. At first sight, this quantity may appear to be unrealistically small; however, when it is considered that the peptides chaperoned by the HSPs are targeted specifically to the professional antigen-presenting cells (15, 21), 1–2 ng or ∼6 × 1011 molecules of specific peptide targeted to the relevant antigen-presenting cells are actually a large number, as argued in more detail elsewhere (22). This observation has significant implications for vaccination against infectious diseases in which the protective epitopes are known, and for any cancers, such as those of viral etiology, that may share antigenic epitopes.
Essentially, these results show that HSPs are adjuvants. This adjuvanticity has a number of unique characteristics: in contrast with other nonlive adjuvants, the adjuvanticity of HSPs generates MHC class I–restricted T cell responses. No serological antipeptide response has ever been detected among the tens of immunized mice tested (data not shown). The quantitative requirements of antigens administered with HSPs are log scales lower than corresponding requirements for other adjuvants. Finally, HSPs are the first adjuvants of mammalian origin. We have suggested previously that the immunogenicity of HSP–peptide complexes may reflect the role in vivo of such complexes in priming of cellular immune responses (23). In this view, the observed adjuvanticity of HSPs is simply a reflection of the natural role of HSPs in vivo.
The structural basis of the ability of gp96 molecules to bind a variety of peptides is presently unclear and requires further study. Obviously, there are certain rules for the HSP–peptide interaction as seen in the observation that peptides differ in their ability to compete with a given peptide for binding to gp96 (Fig. 3). However, the studies carried out here are not of a broad enough scope to permit elucidation of these rules. Broadly speaking, HSP–peptide interaction is reminiscent of MHC–peptide interaction, which was equally mysterious as to its structural basis until the rules of interaction were identified (24). The MHC and the HSPs share a number of crucial properties, such as the ability to bind peptides, a ubiquitous tissue distribution, high degree of phylogenetic conservation, inducibility of the respective genes by IFN-γ (25) and, finally, the ability to prime CTL responses against the peptides chaperoned by them. These considerations led us in the past (26) to suggest a phylogenetic relationship between the MHC and the HSPs, and a number of recent observations (19, 27–28) have not been inconsistent with that suggestion. The association of peptides with HSPs of the cytosol (hsp70 and hsp90) and the endoplasmic reticulum (gp96) had also led us to suggest that HSPs constitute a relay line of molecules that chaperones the peptides and ultimately delivers them to the MHC class I molecules (23). Therefore, the HSPs were suggested to be accessories to antigen presentation by MHC class I molecules. Our recent results, which show that peptides precursors to the MHC class I–binding epitopes are found in specific association with hsp70, hsp90, and gp96 (Ishii et al., manuscript submitted for publication), are in accord with our suggestion. The recent demonstration by Lammert et al. (29) that the HSP gp96 acts as a major peptide acceptor for peptides transported into the lumen of the endoplasmic reticulum through transport-associated protein molecules, also supports the relay-line hypothesis.
Acknowledgments
The authors are grateful to Antoine Menoret and Ping Peng for critical reading of the manuscript.
This work was supported by National Institutes of Health grants CA44786 and CA64394, and a sponsored research agreement with Antigenics, Inc (New York, NY). R. Suto was a postdoctoral fellow of the Cancer Research Institute, New York.
Footnotes
N.E. Blachere and Z. Li contributed equally to this work and are listed in alphabetical order.
Abbreviations used in this paper: HSP, heat shock protein; NP, nucleocapsoid protein; VSV, vesicular stomatitis virus.
References
- 1.Srivastava PK, Das MR. The serologically unique cell surface antigen of Zajdela ascitic hepatoma is also its tumor associated transplantation antigen. Int J Cancer. 1984;33:417–422. doi: 10.1002/ijc.2910330321. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava PK, DeLeo AB, Old LJ. Tumor rejection antigens of chemically induced tumors of inbred mice. Proc Natl Acad Sci USA. 1986;83:3407–3411. doi: 10.1073/pnas.83.10.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ullrich SJ, Robinson EA, Law LW, Willingham M, Appella E. A mouse tumor-specific transplantation antigen is a heat shock–related protein. Proc Natl Acad Sci USA. 1986;83:3121–3125. doi: 10.1073/pnas.83.10.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palladino MA, Srivastava PK, Oettgen HF, DeLeo AB. Expression of a shared tumor-specific antigen by two chemically induced BALB/c sarcomas. Cancer Res. 1987;47:5074–5079. [PubMed] [Google Scholar]
- 5.Udono H, Srivastava PK. Comparison of tumor-specific immunogenicities of stress-induced proteins gp96, hsp90, and hsp70. J Immunol. 1994;152:5398–5403. [PubMed] [Google Scholar]
- 6.Udono H, Srivastava PK. Heat shock protein 70–associated peptides elicit specific cancer immunity. J Exp Med. 1993;178:1391–1396. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldweg AM, Srivastava PK. Molecular heterogeneity of tumor rejection antigen/heat shock protein gp96. Int J Cancer. 1995;63:310–314. doi: 10.1002/ijc.2910630227. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava PK. Peptide-binding heat shock proteins in the endoplasmic reticulum: role in immune response to cancer and in antigen presentation. Adv Cancer Res. 1993;62:153–177. doi: 10.1016/s0065-230x(08)60318-8. [DOI] [PubMed] [Google Scholar]
- 9.Arnold D, Faath S, Rammensee H, Schild H. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunization with the heat shock protein gp96. J Exp Med. 1995;182:885–889. doi: 10.1084/jem.182.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava PK, Maki R. Stress-induced proteins in immune response to cancer. Curr Top Microbiol Immunol. 1991;167:109–123. doi: 10.1007/978-3-642-75875-1_7. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Srivastava PK. Tumor rejection antigen gp96/grp94 is an ATPase: implication for protein folding and antigen presentation. EMBO (Eur Mol Biol Organ) J. 1993;12:3143–3151. doi: 10.1002/j.1460-2075.1993.tb05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nieland TJF, Tan MCA, Monnee-van Muijen M, Koning F, Kruisbeek AM, Van Bleek GM. Isolation of an immunodominant viral peptide that is endogenously bound to the stress protein gp96/grp94. Proc Natl Acad Sci USA. 1996;93:6135–6139. doi: 10.1073/pnas.93.12.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastava PK, Udono H, Blachere N, Li Z. Heat shock proteins transfer peptide during antigen processing and CTL priming. Immunogenetics. 1994;39:93–98. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- 14.Udono H, Levey D, Srivastava PK. Cellular requirements for tumor-specific immunity elicited by heat shock proteins: tumor rejection antigen gp96 primes CD8+T cells in vivo. Proc Natl Acad Sci USA. 1994;91:3077–3081. doi: 10.1073/pnas.91.8.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein–chaperoned peptides. Science (Wash DC) 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 16.Puddington L, Bevan MJ, Rose JK, Lefrancois L. N protein is the predominant antigen recognized by vesicular stomatitis virus-specific cytotoxic T cells. J Virol. 1986;60:708–717. doi: 10.1128/jvi.60.2.708-717.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng P, Menoret A, Srivastava PK. Purification of hsp70 molecules with associated antigenic peptides. J Immunol Methods. 1997;204:13–21. doi: 10.1016/s0022-1759(97)00017-3. [DOI] [PubMed] [Google Scholar]
- 18.Flynn GC, Chappell TG, Rothman JE. Peptide binding and release by proteins implicated as catalysts of protein assembly. Science (Wash DC) 1989;245:385–390. doi: 10.1126/science.2756425. [DOI] [PubMed] [Google Scholar]
- 19.Zhu X, Zhao X, Burkholder WF, Gragerov A, Ogata CM, Gottesman ME, Hendrickson WA. Structural analysis of substrate binding by the molecular chaperone DnaK. Science (Wash DC) 1996;272:1606–1614. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wearsch PA, Nicchitta CV. Interaction of endoplasmic reticulum chaperone grp94 with peptide substrates is adenine nucleotide–independent. J Biol Chem. 1997;272:5152–5156. doi: 10.1074/jbc.272.8.5152. [DOI] [PubMed] [Google Scholar]
- 21.Heike, M., H. Bernhardt, T. Wolfel, and K.-H. Meyer zum Buschenfeld. 1996. International Conference on Cancer Vaccines. New York. (Abstr. M13.)
- 22.Srivastava PK, Udono H. Heat shock proteins in immune response to cancer: the fourth paradigm. Experientia. 1994;50:1054–1060. doi: 10.1007/BF01923461. [DOI] [PubMed] [Google Scholar]
- 23.Srivastava PK, Udono H, Blachere NE, Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39:93–98. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- 24.Rammensee HGR, Friede T, Stevanovic S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 25.Anderson SL, Sheng T, Lou J, Xing L, Blachere NE, Srivastava PK, Rubin BY. The endoplasmic reticular heat shock protein gp96 is transcriptionally up-regulated in interferon-treated cells. J Exp Med. 1994;180:1565–1569. doi: 10.1084/jem.180.4.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srivastava PK, Heike M. Tumor-specific immunogenicity of stress-induced proteins: convergence of two evolutionary pathways of antigen presentation? . Semin Immunol. 1991;3:57–64. [PubMed] [Google Scholar]
- 27.Flajnik MF, Canel C, Kramer J, Kasahara M. Which came first: MHC class I or class II? . Immunogenetics. 1991;33:295–300. doi: 10.1007/BF00216688. [DOI] [PubMed] [Google Scholar]
- 28.Morshauser RC, Wang H, Flynn GC, Zuiderweg ERP. The peptide-binding domain of the chaperone protein hsc70 has an unusual secondary structure topology. Biochemistry. 1995;34:6261–6266. doi: 10.1021/bi00019a001. [DOI] [PubMed] [Google Scholar]
- 29.Lammert E, Arnold D, Nijenhuis M, Momburg F, Hammerling GJ, Brunner J, Stevanovic S, Rammensee H-G, Schild H. The endoplasmic reticulum–resident stress protein gp96 binds peptides translocated by TAP. Eur J Immunol. 1997;27:923–927. doi: 10.1002/eji.1830270418. [DOI] [PubMed] [Google Scholar]