Abstract
Signaling through the high affinity receptor for immunoglobulin E (FcεRI) results in the coordinate activation of tyrosine kinases before calcium mobilization. Receptors capable of interfering with the signaling of antigen receptors, such as FcεRI, recruit tyrosine and inositol phosphatases that results in diminished calcium mobilization. Here, we show that antibodies recognizing CD81 inhibit FcεRI-mediated mast cell degranulation but, surprisingly, without affecting aggregation-dependent tyrosine phosphorylation, calcium mobilization, or leukotriene synthesis. Furthermore, CD81 antibodies also inhibit mast cell degranulation in vivo as measured by reduced passive cutaneous anaphylaxis responses. These results reveal an unsuspected calcium-independent pathway of antigen receptor regulation, which is accessible to engagement by membrane proteins and on which novel therapeutic approaches to allergic diseases could be based.
Crosslinking of high affinity receptor for immunoglobulin E (FcεRI)1–IgE complexes on mast cells and basophils by multivalent antigens initiates a signaling cascade characterized by tyrosine kinase activation, calcium release and influx and, later, by degranulation and release of inflammatory mediators (1–5). Like the B and T cell antigen receptors, FcεRI lacks endogenous signaling capacity and uses tyrosine phosphorylation to recruit signaling effector molecules. Receptor aggregation leads to phosphorylation and/or activation of several protein tyrosine kinases (PTKs), Lyn, Syk, Btk, Itk, Fer, and FAK (1–4, 6–8), as well as protein kinase C isoenzymes (9), MAP kinase (10), and other signaling molecules such as Cbl and Shc (11, 12). The precise role of many of these proteins in degranulation remains undefined. However, it is clear that FcεRI-mediated calcium mobilization, degranulation, and leukotriene and cytokine synthesis depend on early tyrosine kinase activation events, especially the activation of the PTK Syk. FcεRI signaling is initiated by tyrosine phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAM; defined by the sequence [D/E]x2Yx2Lx6–7Yx2[L/I]; references 13, 14), found in FcεRIβ and FcRγ chains upon receptor aggregation (1, 3, 4). The primary function of FcεRIβ is to amplify FcRγ signals, as it has no autonomous signaling capacity (4). Phosphorylated ITAMs facilitate binding of src homology (SH) domain–containing proteins to FcεRI (15, 16). The dimeric FcRγ phosphorylated ITAMs bind Syk via its tandem SH2 domains, leading to Syk phosphorylation and activation (3, 4, 15, 16). The importance of Syk recruitment to calcium mobilization, degranulation, and leukotriene synthesis has been demonstrated in mast cells lacking Syk expression or by introduction of dominant negative Syk proteins. FcεRI-mediated calcium mobilization and degranulation are absent in Syk-negative mast cells despite the FcεRI-mediated tyrosine phosphorylation of receptor subunits (17). In addition, expression of kinase-inactive Syk blocks FcεRI-induced calcium release from endoplasmic reticulum (ER) stores (3) and introduction of kinase-negative Syk SH2 domains inhibits both degranulation and leukotriene release in FcεRI-stimulated cells (18).
In addition to activation events, receptor-activated PTKs initiate the regulation of antigen receptor signaling by phosphorylating tyrosine-based motifs on membrane receptors known as inhibitory receptors (19, 20). These proteins bind SH2-containing tyrosine phosphatases (SHP-1 and SHP-2), and the polyphosphatidylinositol (3,4,5) 5′ phosphatase (SHIP), upon coengagement with antigen or growth factor receptors. Although the molecular targets are still being defined, phosphatase recruitment to inhibitory receptors has one of two general effects on signaling. Engagement of inhibitory receptors that preferentially bind SHIP, such as the low affinity receptor for IgG (FcγRIIb1; references 21, 22), results in selective inhibition of calcium influx with little or no effect on receptor-mediated calcium release or tyrosine phosphorylation. On the other hand, killer cell inhibitory receptors (KIR) bind SHP-1 upon receptor costimulation, resulting in reduced tyrosine phosphorylation, calcium release from the ER, and calcium influx (23, 24). In both mechanisms, calcium mobilization is inhibited along with downstream signaling events.
In this report, we isolated mAbs that inhibited FcεRI-induced mast cell degranulation. Through protein isolation, peptide sequencing, cloning, and gene expression, we have identified CD81 as a novel inhibitory receptor for FcεRI. Anti-CD81 mAbs also inhibited passive cutaneous anaphylaxis (PCA) reactions, a model of IgE-dependent, mast cell activation in vivo.
Materials and Methods
Cell Culture, Reagents, and Antibodies.
The rat basophilic leukemia cell line (RBL-2H3) was cultured in Eagle's minimum essential medium supplemented with 16% heat-inactivated FCS, 2 mM l-glutamine, and penicillin (100 U/ml)/streptomycin (50 μg ml−1) (Biofluids, Rockville, MD). NS-1 myeloma cells were cultured in RPMI-1640 supplemented with 20% FCS, glutamine, and antibiotics. C1.MC/C57.1 cells were cultured as described (25). DNP–human serum albumin (DNP–HSA) (30–40 mol DNP/mol albumin) was purchased from Sigma Chemical Co. (St. Louis, MO). DNP-specific IgE supernatants were used to saturate FcεRI as described (26). For PCA experiments, MOPC 31c (IgG1) and anti-DNP mouse IgE (clone SPE-7) were purchased from Sigma Chemical Co. and anti–rat β2 integrin (anti-LFA-1β, CD18; clone WT.3) was purchased from PharMingen (San Diego, CA). MOPC 31c and anti-DNP IgE were dialyzed to remove sodium azide before in vivo injections. Anti–rat CD81 (5D1, IgG1) was purified from ascites on protein G–Sepharose (Pharmacia, Uppsala, Sweden).
Production of Anti-RBL-2H3 Antibodies and Flow Cytometry.
Spleens from BALB/c immunized with whole RBL-2H3 cells were fused with NS-1 myeloma cells and plated onto normal BALB/c spleen feeder cells. To enhance the development of the hybridomas, Saccharomyces typhimurium mitogen (Ribi ImmunoChem Research, Inc., Hamilton, MT) was included in the culture medium from days 0–10. Hybridoma supernatants were tested after day 14 by flow cytometry for binding to RBL-2H3 using FITC-conjugated goat anti–mouse F(ab′)2-specific antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and analyzed by flow cytometry on a FACScan® flow cytometer (Becton Dickinson, San Jose, CA). From three separate fusions, a total of 2,160 wells were plated and 622 supernatants from wells with hybridoma growth were screened by FACS® for reactivity with RBL-2H3 cells. In all, 283 of 622 (45%) elicited detectable reactivity by FACS® with membrane antigens of RBL-2H3 (data not shown). The screening of RBL-2H3–reactive mAbs by serotonin release assay lead to the identification of 1A12 (IgG2b) and 5D1 (IgG1), which were characterized further. Rat CD81 transfectants of C1.MC/C57.1 cells were stained with purified 1A12 and 5D1 (1 μg/106 cells), counterstained with goat anti–mouse F(ab′)2-specific antibody, and analyzed by flow cytometry on a FACScan® flow cytometer.
Serotonin Release and Leukotriene C4 Assays.
RBL-2H3 cells were loaded with [3H]5-hydroxytryptamine ([3H]serotonin; 0.3 μCi/ 105 cells) and saturated with DNP-specific IgE in 96-well microtiter tissue culture plates (105 cells well −1, 37°C, 5% CO2) as described (27, 28). Monolayers were washed three times with buffer (glucose–saline, Pipes buffer (pH 7.2) containing (in mM) 25 Pipes, 110 NaCl, 5 KCl, 5.6 glucose, 0.4 MgCl2, 1 CaCl2, and 0.1% BSA; and 25 μl of a dilution of purified antibody was added to the monolayers and plates were incubated for 30 min (or as indicated) at room temperature. Triggering of FcεRI was performed by the addition of DNP–HSA (final concentration 10–250 ng ml−1) and plates were incubated at 37°C (30 min except as indicated in Fig. 1 D) with control samples present on each plate. Degranulation was stopped by placing the plates on ice and by the addition of 150 μl of cold culture medium/well. 100-μl aliquots were taken from replicate wells for scintillation counting. Total cellular incorporation was determined from 1% SDS, 1% NP-40 lysates.
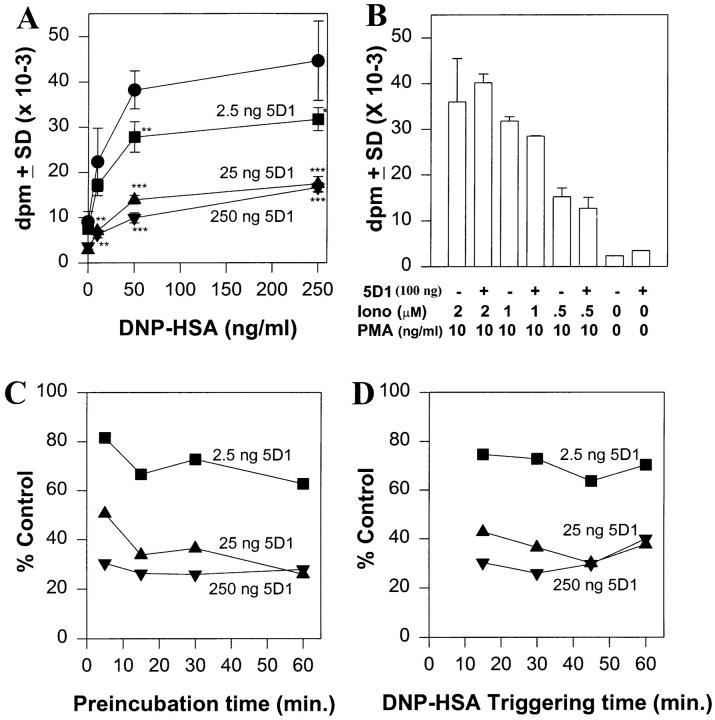
Figure 1.
Effect of preincubation of purified mAb 5D1 on FcεRI-mediated degranulation in RBL-2H3 cells. (A–D) Degranulation of IgE-saturated, RBL-2H3 cells after incubation with buffer (closed circles) or purified 5D1 mAb (mouse IgG1) at 2.5 ng (closed squares), 25 ng (closed triangles), or 50 ng (closed inverted triangles) (A, C, D) or with 100 ng (B) of 5D1 mAb per 105 cells before triggering with the indicated concentrations of DNP–HSA (A), 50 ng/ml−1 DNP–HSA (C and D), or with PMA and ionomycin (B). Cells were preincubated with 5D1 or buffer for 30 min (A, B, D) or for the indicated times (C) at room temperature before triggering for 30 min (A–C; 37°C, 5% CO2) or as indicated (D). The data shown are representative of >10 experiments with the 5D1 mAb. Data are expressed as mean dpm ± standard deviation or as percentages of control (no antibody) mean dpm. Statistical significance versus untreated controls was determined using an unpaired Student's t test: *, P <0.05; **, P <0.01; ***, P <0.001 for A. All data points in C and D were found to be significantly different from controls (P <0.02) with the exception of the 5-min preincubation time point with 2.5 ng mAb 5D1 (C, P = 0.067).
Leukotriene C4 was measured from 106 anti-DNP IgF-saturated RBL-2H3 treated with 1 μg 5D1 or buffer before triggering with 30 ng ml−1 DNP–HSA. Supernatants were stored at −80°C until measurement of LTC4 by specific enzyme immunoassay (Cayman Chemical, Ann Arbor, MI).
Immunoaffinity Chromatography, Electrophoresis, and Western Blotting.
RBL-2H3 cells were cultured in routine culture medium in spinner flasks to a cell density of ∼106/ml, harvested by centrifugation, and washed twice with cold PBS. Washed cells (1010 RBL-2H3 cells were obtained from spinner flasks [12 l]) were extracted in 0.5 M K2HPO4 (pH 7.5) with proteinase inhibitors (10 μg/ml pepstatin, 5 μg/ml leupeptin, and 10 μg/ml aprotinin) at 5.0 × 107 ml−1 (60 min, 4°C) with frequent mixing. n-octylglucoside (10 mM) was added during the extraction to ensure protein solubility. Postnuclear lysates were prepared by centrifugation at 15,000 g (20 min, 4°C), desalted, and passed several times over protein G–Sepharose coupled to 1A12 (2 mg ml−1 bed volume), washed with PBS (10 mM n-octylglucoside), and eluted with 0.2 M glycine (pH 2.3). Tris-neutralized, concentrated extracts were reduced with β-mercaptoethanol, resolved on 12.5% preparative SDS-PAGE, and transferred to ImmobilonS Q (Millipore, Bedford, MA). The membrane was stained with amido black and the Mr 25-kD band was excised, eluted, alkylated, and digested overnight with Lys-C. Peptides were separated by reverse phase-HPLC and the peptide peak eluting at 36 min was sequenced.
For antiphosphotyrosine Western blots, 0.5% Triton X-100 (BBS, proteinase inhibitors) extracts were immunoprecipitated overnight with 2 μg of antiphosphotyrosine mAb 4G10 bound to protein A–Sepharose beads (4°C with rotation). Beads were washed with lysis buffer, eluted, resolved on 12.5% SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted with 1 μg ml−1 4G10 mAb followed by incubation with HRP-conjugated anti–mouse IgG secondary antibodies and development with chemiluminescence substrates (Renaissance, Dupont/New England Nuclear, Boston, MA).
Construction and Screening of RBL-2H3 cDNA Library in UNI-ZAP™.
Poly (A)+ messenger RNA was isolated from RBL-2H3, reverse-transcribed into cDNA, size-fractionated on Sephacryl S-500 spin columns, and ligated into UNI-ZAP–XR lambda vector according to the instructions of the manufacturer (Stratagene, La Jolla, CA). After rescue of the cDNA inserts and appropriate restriction enzyme digests, it was determined that 96% of the plamids contained inserts, with an average size of 1.7 kB. 5 × 105 plaques were screened with 32P-labeled mouse CD81 cDNA probe. After hybridization, nitocellulose filters were washed one time with 2× SSC containing 0.1% SDS (room temperature) and three times with 0.5× SSC containing 0.1% SDS at 50°C. Filters were autoradiographed and plaques picked and eluted. Candidate plaques were subjected to three additional rounds of plaque purification before rescue the cDNA inserts into pBluescript. Sequencing was performed on eleven isolates and all were found to align with EMBL/GenBank/DDBJ accession number U19894 isolated from rat brain (29–31).
Transfections.
Rat CD81 cDNA from two isolates was subcloned into the pBJ1neo expression vector (4) and 20 μg of ethanol-precipitated DNA was used for electroporation of C1.MC/ C57.1 cells (1,050 μF, 270 V). Selection of stable transfectants was initiated 48 h later by replating at 500–10,000 cells/well with 2 mg/ml G418 (GIBCO BRL, Gaithersburg, MD).
Confocal Microscopy.
After overnight adherence and saturation of FcεRI with DNP-specific IgE, RBL-2H3 cells were washed with buffer and incubated with 3 μM fluo3/AM (Molecular Probes, Eugene, OR) and 0.2 mg/ml Pluronic (Molecular Probes) at 37°C for 30 min (5% CO2) in a buffer containing 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM glucose, and 1 mM Na-Hepes (pH 7.4). Dye-loaded cells were then washed once with the same buffer before preincubation (30 min, room temperature) with buffer (± 5D1, 1 μg/chamber/105 cells) and triggering with 100 ng/ml DNP–HSA. Ca2+ measurements in single cells were monitored using a laser-scanning confocal microscope (LSM4, Zeiss, New York) equipped with an argon/kryton laser to excite the dye at 488 nm. Fluorescence emission above 510 nm was measured after placing a long pass filter in front of the photomultiplier tube. The confocal system was used in slow scan mode and fluorescence images were collected every 5 s. Fluo-3 fluorescence measurements were normalized by dividing the average fluorescence intensity (F) occurring during the course of the experiment to the average fluorescence intensity determined at the beginning of the experiment (F0). All measurements were performed at 22–24°C.
Passive Cutaneous Analphylaxis in Rats.
Male Wistar rats (275– 300 g) were used in these experiments. Rats were first anesthetized with ether, back skin hair was shaved, and rats were injected intradermally with 50 μl containing 100 ng anti-DNP IgE or 25 ng anti-DNP–IgE mixed with 50 μg of MOPC 31c (mouse IgG1, specificity unknown) or 5D1 (mouse IgG1; anti–rat CD81). Control sites received buffer alone (PBS containing 10 μg/ml−1 mouse serum albumin; Sigma Chemical Co). Sites were marked on the skin for orientation and rats that received 100 ng anti-DNP injections received a second injection 21 h later with 50 μg of 5D1 or anti–rat LFA-1β (CD18; mouse IgG1) into previously injected sites. Sites receiving IgE and IgG1 were injected in triplicate on the same rat. 24 h after IgE injections animals received 1 ml of 1 mg/ml DNP–HSA containing 1% Evan's blue dye injected intravenously under ether anesthesia. 30 min after intravenous injection, rats were killed and punch biopsies (2.5 cm2) were obtained, minced, and extracted three times with hot formamide (80°C, 3 h) (32). Pooled samples from tissue sites were centrifuged and absorbance at 610 nm was measured. A610 values were converted to micrograms of Evan's blue dye based on a standard curve of dilutions of Evan's blue in formamide.
Results and Discussion
To identify membrane proteins capable of regulating FcεRI signaling, we produced mAbs to the rat basophilic leukemia (RBL-2H3) cell line and identified antibodies that inhibited FcεRI-mediated degranulation. As shown in Fig. 1 A, pretreatment of anti-DNP IgE-saturated RBL-2H3 cells with purified mAb 5D1 inhibited FcεRI-mediated degranulation by 75% as measured by release of granule-stored [3H] serotonin. Blockage of serotonin release was significant (*, P <0.05) even at subsaturating concentrations of 5D1 (2.5 ng mAb/105 cells; Fig. 1 A; data not shown). 5D1-mediated inhibition was specific for FcεRI signaling as degranulation induced by PMA and calcium ionophore ionomycin was unaffected (Fig. 1 B). Furthermore, maximal inhibition of FcεRI-mediated degranulation by mAb 5D1 required only brief periods of preincubation (Fig. 1 C) and inhibition was sustained for at least 1 h of antigen stimulation (Fig. 1 D).
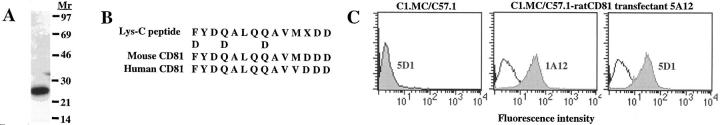
Next, we sought to identify the protein recognized by the degranulation–inhibitory 5D1 mAb. 5D1 and a second degranulation–inhibitory mAb (designated 1A12) recognized proteins of Mr 25 kD (Fig. 2 A; data not shown). 5D1 and 1A12 blocked each others' binding to RBL-2H3 cells, although neither mAb inhibited IgE binding and, conversely, saturation of FcεRI with IgE had no effect on 1A12 binding (data not shown), suggesting that (a) 1A12 and 5D1 recognized the same protein (see Fig. 2 C) and (b) FcεRI and the 1A12/5D1 antigen were not colocalized on the cell membrane. Because mAb 1A12 was more effective at immunoprecipitation and on Western blots, it was used for protein purification. Batch preparations of RBL-2H3 extracts were immunoprecipitated with mAb 1A12, resolved on preparative SDS-PAGE, and transferred to nitrocellulose for protein sequencing. In Fig. 2 B, peptide sequence obtained from Lys-C digests of 1A12 immunoprecipitates is shown aligned with homologous sequences from mouse and human CD81. Based on these data, we cloned rat CD81 from a RBL-2H3 cDNA library using mouse CD81 cDNA as a probe and expressed the cDNA in the mouse mast cell line C1.MC/C57.1 (25). As shown in Fig. 2 C, both degranulation–inhibitory mAbs 1A12 and 5D1 recognized rat CD81.
Figure 2.
Characterization and isolation of rat CD81. (A) Western blot of 5 × 105 cell equivalents from Triton X-100 extracted RBL-2H3 cells immunoblotted with 1 μg/ml 1A12 mAb. (B) 1A12 immunoprecipitates from 1010 RBL-2H3 cells were digested with Lys-C and purified by HPLC. 1A12 peptide sequence is shown aligned with homologous sequences from mouse and human CD81. (C) Expression of rat CD81 in mouse mast cell line C1.MC/C57.1 (25) and FACS® staining with 5D1 and 1A12 mAbs. Rat CD81 was isolate from a RBL-2H3 cDNA library screened with mouse CD81 cDNA (provided by Dr. S. Levy, Stanford University, Stanford, CA). Two rat CD81 isolates were cloned into the pBJ1neo expression vector (4) and transfected into C1.MC/C57.1 cells by electroporation with identical results. FACS® profiles of C1.MC/C57.1 transfectant 5A12 stained mAbs 1A12 and 5D1 are shown.
CD81 belongs to the transmembrane 4 superfamily (TM4SF) which includes CD9, CD53, CD63, and CD82 (33). It is broadly expressed on hematopoietic cells (T and B lymphocytes, granulocytes, monocytes) and on some nonlymphoid tumors. The function of CD81 (or other TM4SF proteins) is incompletely understood although CD81 appears to modulate the signaling of other membrane receptors. CD81 is found in the CD19–CD21 complex on B cells and mAbs to CD81 or CD19 have been reported to reduce the threshold for B cell receptor signaling (34) and enhance B cell adhesion via VLA4 (35). Consistent with a costimulatory role in B cell receptor signaling, CD81 −/− mice express lower levels of CD19 on B cells, which is proposed to contribute to a defect in humoral immunity (36). For T lineage cells, both stimulatory and inhibitory activities for anti-CD81 mAbs have been reported (37–40). CD81 ligation enhances IL-4 production from antigen-specific CD4+ T cells (37), and integrin activation and IL-2–dependent proliferation in human thymocytes (38). Alternatively, CD81 was originally called TAPA-1 (target of antiproliferative antibody) based on inhibition of proliferation in human T cell lines by CD81 antibodies (39). Some of these pleiotropic effects may stem from the potential signaling molecules with which CD81 has been reported to associate, including CD4, CD8, MHC class II, other TM4SF proteins, integrin VLA4, and phosphatidylinositol 4 kinase (33, 41–44).
To target the site of CD81 inhibition of degranulation, we next examined the effect of CD81 antibodies on the earliest events of FcεRI signal transduction, i.e., tyrosine phosphorylation of proteins by activated, nonreceptor tyrosine kinases including Lyn and Syk, and calcium mobilization (1–4). In these experiments, IgE-saturated RBL-2H3 cells were pretreated with purified anti-CD81 before triggering with DNP–HSA for the indicated periods of time, followed by extraction and immunoprecipitation of total tyrosine-phosphorylated proteins. As shown in Fig. 3 A, no major changes in the pattern of FcεRI-induced tyrosine phosphorylation were detected with anti-CD81 treatment before antigen triggering. Although not shown in Fig. 3 A, incubation of RBL-2H3 cells with 5D1 alone (no antigen triggering) did not induce detectable tyrosine phosphorylation. This observation in RBL-2H3 cells contrasts with anti-CD81–induced tyrosine phosphorylation induced in B cells, the latter probably signaling through the CD19–CD21 complex (45).
Figure 3.
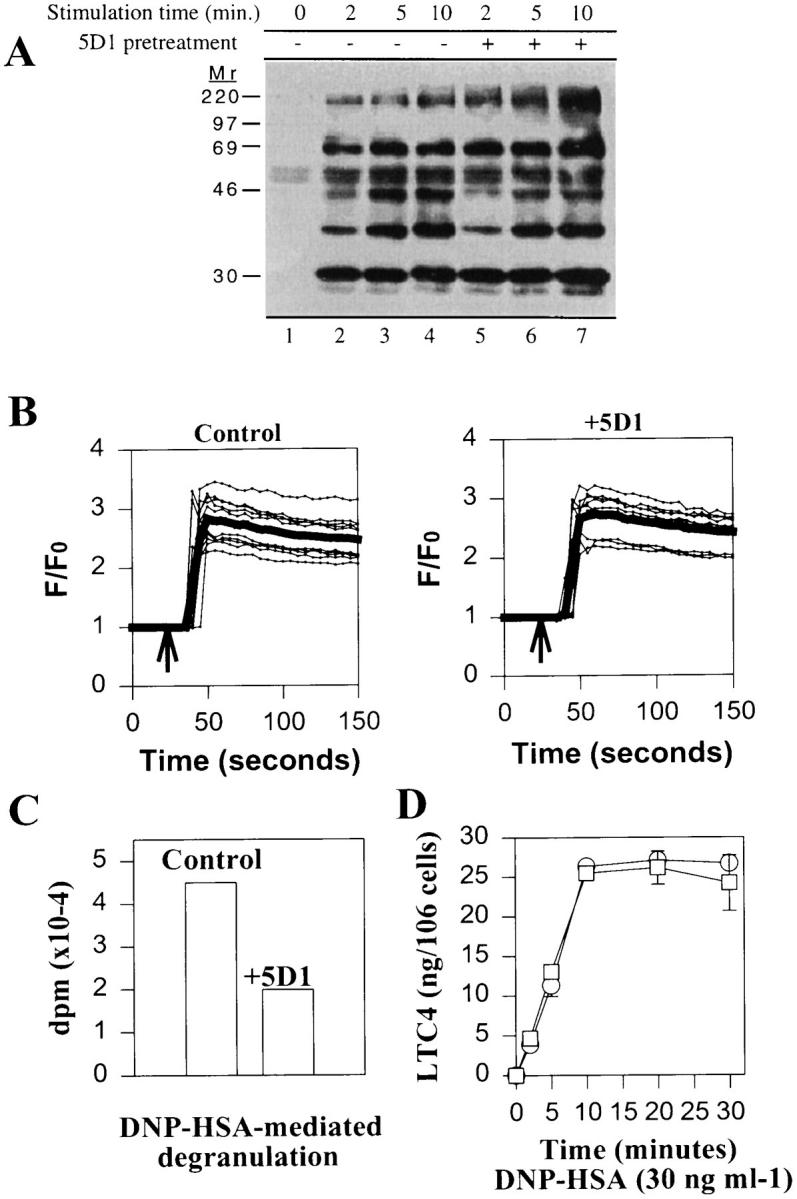
CD81 mAbs fail to inhibit FcεRI-induced tyrosine phosphorylation, calcium mobilization, and leukotriene synthesis. (A) Phosphotyrosine Western blot of antiphosphotyrosine immunoprecipitates from FcεRI-triggered RBL-2H3 cells without (lanes 2–4) or with (lanes 5–7) pretreatment with anti-CD81 mAb 5D1. (B) Effect of anti-CD81 on calcium mobilization of fura-2–loaded RBL-2H3 cells triggered through FcεRI as measured by confocal microscopy. Fluo-3 fluorescence measurements were normalized by dividing the average fluorescence intensity (F) occurring during the course of the experiment to the average fluorescence intensity at the beginning of the experiment (F 0) and expressed as F/F 0. Traces are shown of 10 individual cells (thin lines) together with mean values for these cells (thick lines) and represent typical results obtained from five separate experiments. (C) [3H]serotonin release from RBL-2H3 cells prepared as in confocal microscopy measurements except that 3 μCi/ml [3H]serotonin was added to cultures. Release was measured as in Fig. 1. (D) LTC4 measurements from 106 anti-DNP IgE saturated RBL-2H3 treated with 1 μg 5D1 (open squares) or buffer (open circles) before triggering with 30 ng/ml DNP–HSA for the indicated periods of time.
The effect of anti-CD81 on FcεRI-induced calcium mobilization was monitored on individual, adherent RBL-2H3 cells by confocal microscopy in cells loaded with calcium dye fluo-3. As shown in Fig. 3 B, no inhibition of FcεRI-induced calcium mobilization in anti-CD81–treated versus controls was observed by confocal microscopy, despite inhibition of degranulation under these conditions (Fig. 3 C). Anti-CD81 pretreatment had no effect on calcium release from intracellular stores in cells triggered in Ca2+-free buffer containing 0.5 mM EGTA or on pretriggering baseline values (data not shown). Similar results were also obtained with RBL-2H3 triggered through FcεRI in suspension using a spectrophotometer (data not shown). In separate experiments, anti-CD81 mAb 5D1 did not inhibit leukotriene C4 (LTC4) production induced by DNP–HSA/IgE stimulation (Fig. 3 D). LTC4 production is dependent on activation of phospholipase A2 (tyrosine kinase and calcium dependent) and is regulated by PMA-sensitive, protein kinase C isozymes (46, 47). These data suggest that CD81 acts independently of early tyrosine phosphorylation and calcium mobilization events that are critical for mast cell degranulation.
These results were unexpected in light of the reported modes of action of other inhibitory receptors. These proteins fall into two major classes: type I, transmembrane proteins that are members of the Ig superfamily (FcγRIIb1, KIR, CTLA-4, CD22, gp49b1, paired Ig-like receptors, signal-regulatory proteins) and type II, transmembrane, C-type lectins (e.g., Ly-49, NKG2A, mast cell function– associated protein) (21–23, 48–55). Inhibitory receptors share a cytoplasmic motif, the immunoreceptor tyrosine-based inhibitory motif (ITIM consensus sequence V/Ix2Yx2I/L; references 20, 48), which is a target for tyrosine phosphorylation during receptor activation. Phosphorylated ITIMs bind the phosphatases SHP-1, SHP-2, or SHIP, and physical associations with these phosphatases and/or functional evidence of these associations (tyrosine dephosphorylation and/or decreased calcium mobilization) have been demonstrated in all inhibitory receptors that have been characterized (21–23, 48, 49, 52–54).
CD81 differs from these inhibitory receptors in three important ways. First, unlike other inhibitory receptors, CD81 inhibits FcεRI-mediated degranulation while leaving both tyrosine phosphorylation and calcium mobilization apparently unaffected. Although these results cannot exclude a very selective inhibition of kinase activity by CD81 antibodies, it is clear that no detectable effect is found on tyrosine kinase–sensitive calcium mobilization or LTC4 production. Second, CD81 belongs to a different structural class of proteins than the other inhibitory receptors. CD81 is a TM4SF protein with four transmembrane spanning segments, two extracellular loops, two short cytoplasmic tails, and a short intracellular loop between transmembrane segments 2 and 3 (33). Third, the cytoplasmic tails of CD81 lack ITIM motifs. Although there is an ITIM-like sequence (GCYGAI) in the short intracellular loop between transmembrane segments 2 and 3, there is no evidence that this site is phosphorylated by tyrosine kinases or capable of binding to SH2 domains.
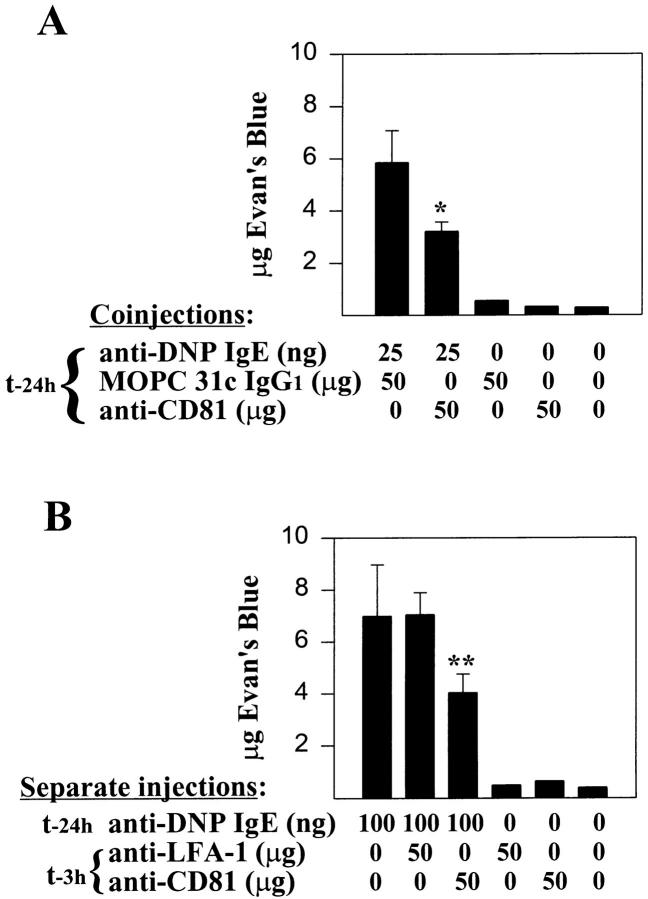
To assess the activity of anti-CD81 in FcεRI signaling in normal mast cells, we chose the PCA model, a classical system for studying mast cell activation in vivo (32, 56). In these experiments, rats were injected intradermally with anti-DNP IgE mixed with anti-CD81 mAb 5D1 (IgG1) or with class-matched mouse IgG1 as controls (Fig. 4 A). Additional rats received anti-DNP IgE alone into the skin at time 0, followed by a second injection (buffer, 5D1, or anti–rat LFA-1β [IgG1]) (Fig. 4 B) into IgE-injected sites 21 h after IgE injections. 24 h after IgE priming, rats received 1 mg of antigen intravenously (DNP–HSA containing 1% Evan's blue dye). Mast cell activation through FcεRI in PCA results in the release of several vasoactive substances, which act to increase vascular permeability, a property that is quantified by local accumulation of the Evan's blue dye from the vasculature into the sites of IgE injections. These results are expressed as micrograms of Evan's blue dye converted from A610 measurements of formamide-extracted tissue biopsies (32). As shown in Fig. 4 A, coinjection of anti-CD81 mAb 5D1 during IgE priming significantly inhibited IgE-dependent PCA reactions (P = 0.024) compared with class-matched controls. To limit the possibility of nonspecific suppression of PCA reactions due to tissue deposition of IgG1 mAbs, we repeated these experiments by injecting anti-CD81 mAb 5D1 or anti-LFA-1β (CD18) into the IgE-injected sites 3 h before antigen administration. LFA-1β is expressed on mast cell lines including RBL-2H3, whereas anti-LFA-1β has no effect on FcεRI-mediated degranulation in RBL-2H3 cells (57; data not shown). Similar to coinjection of IgE and IgG1 mAbs, separate injections of anti-CD81 yielded significant inhibition of PCA reactions compared with anti-LFA-1β controls (Fig. 4 B).
Figure 4.
Inhibition of passive cutaneous anaphylaxis in Wistar rats by anti-CD81. Male Wistar rats were injected intradermally with (A) 25 ng DNP-specific IgE mixed with 50 μg anti-CD81 mAb 5D1 (mouse IgG1) or control mouse IgG1 mAb (MOPC 31c; specificity unknown) or (B) 100 ng DNP-specific IgE alone. Mice receiving 100 ng IgE alone were reinjected 21 h later with buffer, 5D1, or a mouse IgG1 control mAb to LFA-1β chain (anti-CD18; WT.3). Control sites (no IgE) received buffer or 50 μg of IgG1 subclass mAb. 24 h after IgE injections, 1 mg of antigen (DNP–HSA; 1 mg/ml in PBS containing 1% Evan's blue dye) was injected intravenously and rats were killed 30 min later. Extravasation of Evan's blue dye into the skin was quantified by A610 measurements of serial hot formamide extractions (54) of minced punch biopsies (2.5 cm2/ site). Sample A610 values were converted to micrograms of Evan's blue dye based on standard curves of Evan's blue A610 absorbance in formamide. Results taken from three injected sites per condition (IgE plus buffer or antibody) or a single site (IgG1 controls without IgE) from a single rat and expressed as mean micrograms of Evan's blue dye ± standard deviation. Comparable results were obtained on two additional rats for both experiments shown. Statistical significance was determined using an unpaired Student's t test: *, P <0.05; **, P <0.01 (actual values: A, P = 0.024 versus MOPC 31c controls; B, P = 0.009 versus anti-LFA-1β controls).
In conclusion, we have demonstrated that CD81 is a novel inhibitory receptor for FcεRI. The observation that CD81 acts on calcium-independent events required for mast cell degranulation distinguishes CD81 from previously described inhibitory receptors, such as FcγRIIb1 and KIR, that act upstream of calcium influx. Anti-CD81 mAbs also inhibited IgE-dependent PCA reactions, which suggests the CD81 pathway is present in normal mast cells and capable of being engaged to inhibit mast cell responses in vivo. Therefore, the pathway engaged by CD81 is a candidate for therapeutic strategies aimed at intervention of allergic responses.
Acknowledgments
We thank Dr. Shoshana Levy for mouse CD81 cDNA, Dr. Lan Bo Chen for use of the confocal microscope, and Dr. Marie-Hélène Jouvin for critical reading of the manuscript.
This work was supported in part by USPHS grants CA/AI-72074 and AI/CA-23990 (S.J. Galli) and GM-53950 (J.-P. Kinet, T.J. Fleming, by fellowships from the Human Frontier Science Program (E. Donnadieu), the Korea Science and Engineering Foundation (C.H. Song), and the Beth Israel Hospital Pathology Foundation.
Footnotes
Address all correspondence to Dr. Jean-Pierre Kinet, Department of Pathology, Laboratory of Allergy and Immunology, Beth Israel Deaconess Medical Center and Harvard Medical School, 330 Brookline Avenue, Boston, MA 02215. Phone: 617-667-1324; FAX: 617-667-3616; E-mail: jkinet@mercury.bidmc.harvard.edu. The present address of F. Van Laethem is Free University of Brussels, 1180 Brussels, Belgium.
Abbreviations used in this paper: DNP–HSA, DNP–human serum albumin; ER, endoplasmic reticulum; FcεRI, high affinity receptor for IgE; FcγRIIbl, low affinity receptor for IgG; ITAM, immunoreceptor tyrosine-based activation motif; ITIM, immunoreceptor tyrosine-based inhibitory motif; KIR, killer cell inhibitory receptor; LTC4, leukotriene C4; PCA, passive cutaneous anaphylaxis; PTK, protein tyrosine kinase; RBL-2H3, rat basophilic leukemia cell line; SH, Src homology; SHIP, SH2-domain–containing polyphosphatidylinositol (3,4,5) 5′ phosphatase; SHP-1/SHP-2, SH2-domain– containing phosphatase-1 or -2; TM4SF, transmembrane 4 superfamily.
References
- 1.Jouvin M-H, Adamczewski M, Numerof R, Letourneur O, Valle A, Kinet J-P. Differential control of the tyrosine kinases Lyn and Syk by the two signaling chains of the high affinity immunoglobulin E receptor. J Biol Chem. 1994;269:5918–5925. [PubMed] [Google Scholar]
- 2.Penhallow RC, Class K, Sonoda H, Bolen JB, Rowley RB. Temporal activation of nontransmembrane protein–tyrosine kinases following mast cell Fc epsilonRI engagement. J Biol Chem. 1995;270:23362–23365. doi: 10.1074/jbc.270.40.23362. [DOI] [PubMed] [Google Scholar]
- 3.Scharenberg AM, Lin S, Cuenod B, Yamamura H, Kinet J-P. Reconstitution of interactions between tyrosine kinases and the high affinity IgE receptor are controlled by receptor clustering. EMBO (Eur Mol Biol Organ) J. 1995;14:3385–3394. doi: 10.1002/j.1460-2075.1995.tb07344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin S, Cicala C, Scharenberg AM, Kinet J-P. The FcεRIβ subunit functions as an amplifier of FcεRIγ-mediated cell activation signals. Cell. 1996;85:985–995. doi: 10.1016/s0092-8674(00)81300-8. [DOI] [PubMed] [Google Scholar]
- 5.Paul WE, Seder RA, Plaut M. Lymphokine and cytokine production by FcεRI+ cells. Adv Immunol. 1993;53:1–29. [PubMed] [Google Scholar]
- 6.Kawakami Y, Yao L, Mirua T, Tsukada S, Witte ON, Kawakami T. Tyrosine phosphorylation and activation of Bruton tyrosine kinase upon FcεRI cross-linking. Mol Cell Biol. 1994;14:5108–5113. doi: 10.1128/mcb.14.8.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawakami Y, Yao L, Tashiro M, Gibson S, Mills GB, Kawakami T. Activation and interaction with protein kinase C of a cytoplasmic tyrosine kinase, Itk/Tsk/ Emt, on Fc epsilon RI cross-linking on mast cells. J Immunol. 1995;155:3556–3562. [PubMed] [Google Scholar]
- 8.Hamawy MM, Mergenhagen SE, Siraganian RP. Tyrosine phosphorylation of pp125FAK by the aggregation of high affinity immunoglobulin E receptors requires cell adherence. J Biol Chem. 1993;268:6851–6854. [PubMed] [Google Scholar]
- 9.Ozawa K, Szallasi Z, Kazanietz MG, Blumberg PM, Mischak H, Mushinski JF, Beaven MA. Ca2+-dependent and Ca2+-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells. Reconstitution of secretory responses with Ca2+and purified isozymes in washed permeabilized cells. J Biol Chem. 1993;268:1749–1756. [PubMed] [Google Scholar]
- 10.Hirasawa N, Scharenberg A, Yamamura H, Beaven MA, Kinet J-P. A requirement for syk in the activation of the microtubule-associated protein kinase/phospholipase A2 pathway by Fc epsilon R1 is not shared by a G protein– coupled receptor. J Biol Chem. 1995;270:10960–10967. doi: 10.1074/jbc.270.18.10960. [DOI] [PubMed] [Google Scholar]
- 11.Ota Y, Beitz LO, Scharenberg AM, Donovan JA, Kinet J-P, Samelson LE. Characterization of cbl tyrosine phosphorylation and a cbl–syk complex in RBL–2H3 cells. J Exp Med. 1996;184:1713–1723. doi: 10.1084/jem.184.5.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jabril-Cuenod B, Zhang C, Scharenberg AM, Paolini R, Numerof R, Beaven MA, Kinet J-P. Syk-dependent phosphorylation of Shc. A potential link between FcεRI and the ras/mitogen-activated protein kinase signaling pathway through Sos and Grb2. J Biol Chem. 1996;271:16268–16272. doi: 10.1074/jbc.271.27.16268. [DOI] [PubMed] [Google Scholar]
- 13.Flaswinkel H, Barner M, Reth M. The tyrosine actrivation motif as a target of protein tyrosine kinases and SH2 domains. Semin Immunol. 1995;7:21–27. doi: 10.1016/1044-5323(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 14.Cambier JC. Antigen and Fc receptor signaling. The awesome power of the immunoreceptor tyrosine-based activation motif (ITAM) J Immunol. 1995;155:3281–3285. [PubMed] [Google Scholar]
- 15.Johnson SA, Pleiman CM, Pao L, Schneringer J, Hippen K, Cambier JC. Phosphorylated immunoreceptor signaling motifs (ITAMs) exhibit unique abilities to bind and activate Lyn and Syk tyrosine kinases. J Immunol. 1995;155:4596–4603. [PubMed] [Google Scholar]
- 16.Kimura T, Kihara H, Bhattacharyya S, Sakamoto H, Appella E, Siraganian RP. Downstream signaling molecules bind to different phosphorylated immunoreceptor tyrosine-based activation motif (ITAM) peptides of the high affinity IgE receptor. J Biol Chem. 1996;271:27962–27968. doi: 10.1074/jbc.271.44.27962. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Berenstein EH, Evans RL, Siraganian RP. Transfection of Syk protein tyrosine kinase reconstitutes high affinity IgE receptor-mediated degranulation in a Syk-negative variant of rat basophilic leukemia RBL–2H3 cells. J Exp Med. 1996;184:71–79. doi: 10.1084/jem.184.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor JA, Karas JL, Ram MK, Green OM, Seidel-Dugan C. Activation of the high affinity immunoglobulin E receptor FcεRI in RBL–2H3 cells is inhibited by syk SH2 domains. Mol Cell Biol. 1995;15:4149–4157. doi: 10.1128/mcb.15.8.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scharenberg AM, Kinet J-P. The emerging field of receptor mediated inhibitory signaling: SHP or SHIP? . Cell. 1996;87:961–964. doi: 10.1016/s0092-8674(00)81790-0. [DOI] [PubMed] [Google Scholar]
- 20.Cambier JC. Inhibitory receptors abound? . Proc Natl Acad Sci USA. 1997;94:5993–5995. doi: 10.1073/pnas.94.12.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(gamma)RIIB. Nature (Lond) 1996;383:263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- 22.Gupta N, Scharenberg AM, Burshtyn DN, Wagtmann N, Lioubin MN, Rorhschneider LR, Kinet J-P, Long EO. Negative signaling pathways of the killer cell inhibitory receptor and FcγRIIb1 require distinct phosphatases. J Exp Med. 1997;186:473–478. doi: 10.1084/jem.186.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burshtyn DN, Scharenberg AM, Wagtmann N, Rajagopalan S, Berrada K, Yi T, Kinet J-P, Long EO. Recruitment of tyrosine phosphatase HCP by the killer cell inhibitory receptor. Immunity. 1996;4:77–85. doi: 10.1016/s1074-7613(00)80300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Binstadt BA, Brumbaugh KM, Dick CJ, Scharenberg AM, Williams BL, Colonna M, Lanier LL, Kinet J-P, Abraham RT, Leibson PJ. Sequential involvement of Lck and SHP-1 with MHC-recognizing receptors on NK cells inhibits FcR-initiated tyrosine kinase activation. Immunity. 1996;5:629–638. doi: 10.1016/s1074-7613(00)80276-9. [DOI] [PubMed] [Google Scholar]
- 25.Young JD-E, Liu C-C, Butler G, Cohn ZA, Galli SJ. Identification, purification, and characterization of a mast cell–associated cytolytic factor related to tumor necrosis factor. Proc Natl Acad Sci USA. 1987;84:9175–9179. doi: 10.1073/pnas.84.24.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paolini R, Jouvin M-H, Kinet J-P. Phosphorylation and dephosphorylation of the high-affinity receptor for immunoglobulin E immediately after receptor engagement and disengagement. Nature (Lond) 1991;353:855–858. doi: 10.1038/353855a0. [DOI] [PubMed] [Google Scholar]
- 27.Daëron M, Bonnerot C, Latour S, Fridman WH. Murine recombinant FcγRIII, but not FcγRII, trigger serotonin release in rat basophilic leukemia cells. J Immunol. 1992;149:1365–1373. [PubMed] [Google Scholar]
- 28.Ortega Soto, E., and I. Pecht. A monoclonal antibody that inhibits secretion from rat basophilic leukemia cells and binds to a novel membrane component. J Immunol. 1988;141:4324–4332. [PubMed] [Google Scholar]
- 29.Geisert EE, Jr, Murphy TP, Irwin MH, Larjava H. A novel cell adhesion molecule, G-CAM, found on cultured rat glia. Neurosci Lett. 1991;133:262–266. doi: 10.1016/0304-3940(91)90584-g. [DOI] [PubMed] [Google Scholar]
- 30.Irwin MH, Geisert EE., Jr The upregulation of a glial cell surface antigen at the astrocytic scar in the rat. Neurosci Lett. 1993;154:57–60. doi: 10.1016/0304-3940(93)90170-p. [DOI] [PubMed] [Google Scholar]
- 31.Geisert EE, Jr, Yang L, Irwin MH. Astrocyte growth, reactivity, and the target of the antiproliferative antibody, TAPA. J Neurosci. 1996;16:5478–5487. doi: 10.1523/JNEUROSCI.16-17-05478.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dombrowicz D, Flamand V, Miyajima I, Ravetch JV, Galli SJ, Kinet J-P. Absence of FcεRI alpha chain results in upregulation of FcγRIII-dependent mast cell degranulation and anaphylaxis. Evidence of competition between FcεRI and FcγRIII for limiting amounts of FcR β and γ chains. J Clin Invest. 1997;99:915–925. doi: 10.1172/JCI119256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright MD, Tomlinson MG. The ins and outs of the transmembrane 4 superfamily. Immunol Today. 1994;15:588–594. doi: 10.1016/0167-5699(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 34.Fearon DT, Carter RH. The CD19/CD2/ TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu Rev Immunol. 1995;13:127–149. doi: 10.1146/annurev.iy.13.040195.001015. [DOI] [PubMed] [Google Scholar]
- 35.Behr S, Schriever F. Engaging CD19 or target of antiproliferative antibody 1 on human B lymphocytes induces binding of B cells to the interfollicular stroma of human tonsils via integrin α4/β1 and fibronectin. J Exp Med. 1995;182:1191–1199. doi: 10.1084/jem.182.5.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maecker HT, Levy S. Normal lymphocyte development but delayed humoral immune response in CD81-null mice. J Exp Med. 1997;185:1505–1510. doi: 10.1084/jem.185.8.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Secrist H, Levy S, DeKruyff RH, Umetsu DT. Ligation of TAPA-1 (CD81) or major histocompatibility complex class II in co-cultures of human B and T lymphocytes enhances interleukin-4 synthesis by antigen-specific CD4+ T cells. Eur J Immunol. 1996;26:1435–1442. doi: 10.1002/eji.1830260706. [DOI] [PubMed] [Google Scholar]
- 38.Todd SC, Lipps SG, Crisa L, Saloman DR, Tsoukas CD. CD81 expressed on human thymocytes mediates integrin activation and interleukin-2–dependent proliferation. J Exp Med. 1996;184:2055–2060. doi: 10.1084/jem.184.5.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oren R, Takahashi S, Doss C, Levy R, Levy S. TAPA-1, the target of an antiproliferative antibody, defines a new family of transmembrane proteins. Mol Cell Biol. 1990;10:4007–4015. doi: 10.1128/mcb.10.8.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boismenu R, Rhein M, Fischer WH, Havran WL. A role for CD81 in early T cell development. Science (Wash DC) 1996;271:198–200. doi: 10.1126/science.271.5246.198. [DOI] [PubMed] [Google Scholar]
- 41.Imai T, Kakizaki M, Nishimura M, Yoshie O. Molecular analysis of the association of CD4 with two members of the transmembrane 4 superfamily, CD81 and CD82. J Immunol. 1995;155:1229–1239. [PubMed] [Google Scholar]
- 42.Angelisova P, Hilgert I, Horejsi V. Association of four antigens of the tetraspan family (CD37, CD53, TAPA-1, and R2/C33) with MHC class II glycoproteins. Immunogenetics. 1994;39:249–256. doi: 10.1007/BF00188787. [DOI] [PubMed] [Google Scholar]
- 43.Mannion BA, Berditchevski F, Kraeft SK, Chen LB, Hemler ME. Transmembrane-4 superfamily proteins CD81 (TAPA-1), CD82, CD63, and CD53 specifically associated with integrin α4 β1 (CD49d/CD29) J Immunol. 1996;157:2039–2047. [PubMed] [Google Scholar]
- 44.Berditchevski F, Tolias KF, Wong K, Carpenter CL, Hemler ME. A novel link between integrins, transmembrane-4 superfamily proteins (CD63 and CD81), and phosphatidylinositol 4-kinase. J Biol Chem. 1997;272:2595–2598. doi: 10.1074/jbc.272.5.2595. [DOI] [PubMed] [Google Scholar]
- 45.Schick MR, Nguyen VQ, Levy S. Anti-TAPA-1 antibodies induce protein tyrosine phosphorylation that is prevented by increasing intracellular thiol levels. J Immunol. 1993;151:1918–1925. [PubMed] [Google Scholar]
- 46.Currie S, Roberts EF, Spaethe SM, Roehnm NW, Kramer RM. Phosphorylation and activation of Ca2+-sensitive cytosolic phospholipase A2 in MCII mast cells mediated by high-affinity Fc receptor for IgE. Biochem J. 1994;304:923–928. doi: 10.1042/bj3040923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ali A, Ford-Hutchinson AW, Nicholson DW. Activation of protein kinase C down-regulates and attenuates cysteinyl leukotriene production in an eosinophilic substrain of HL-60 cells. J Immunol. 1994;153:776–788. [PubMed] [Google Scholar]
- 48.Marengere LE, Waterhouse P, Duncan GS, Mittrucker HW, Feng GS, Mak TW. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science (Wash DC) 1996;272:1170–1173. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- 49.Law CL, Sidorenko SP, Chandran KA, Zhao Z, Shen SH, Fischer EH, Clark EA. CD22 associates with protein tyrosine phosphatase 1C, Syk, and phospholipase C–gamma(1) upon B cell activation. J Exp Med. 1996;183:547–560. doi: 10.1084/jem.183.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katz HR, Austen KF. A newly recognized pathway for the negative regulation of mast cell–dependent hypersensitivity and inflammation mediated by an endogenous cell surface receptor of the gp49 family. J Immunol. 1997;158:5065–5070. [PubMed] [Google Scholar]
- 51.Kubagawa H, Burrows PD, Cooper MD. A novel pair of immunoglobulin-like receptors expressed by B cells and myeloid cells. Proc Natl Acad Sci USA. 1997;94:5261–5266. doi: 10.1073/pnas.94.10.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature (Lond) 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 53.Nakamura MC, Niemi EC, Fisher MJ, Shultz LD, Seaman WE, Ryan JC. Mouse Ly-49A interrupts early signaling events in natural killer cell cytotoxicity with the SHP-1 tyrosine phosphatase. J Exp Med. 1997;185:673–684. doi: 10.1084/jem.185.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Houchins JP, Lanier LL, Niemi EC, Phillips JH, Ryan JC. Natural killer cell cytolytic activity is inhibited by NKG2-A and activated by NKG2-C. J Immunol. 1997;158:3603–3609. [PubMed] [Google Scholar]
- 55.Guthmann MD, Tal M, Pecht I. A secretion inhibitory signal transduction molecule on mast cells is another C-type lectin. Proc Natl Acad Sci USA. 1995;92:9397–9401. doi: 10.1073/pnas.92.20.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wershil BK, Furuta GT, Lavigne JA, Choudhury AR, Wang ZS, Galli SJ. Dexamethasone or cyclosporin A suppress mast cell–leukocyte cytokine cascades. Multiple mechanisms of inhibition of IgE- and mast cell–dependent cutaneous inflammation in the mouse. J Immunol. 1995;154:1391–1398. [PubMed] [Google Scholar]
- 57.Weber S, Babina M, Feller G, Henz BM. Human leukaemic (HMC-1) and normal skin mast cells express beta 2–integrins: characterization of beta 2–integrins and ICAM-1 on HMC-1 cells. Scand J Immunol. 1997;45:471–481. doi: 10.1046/j.1365-3083.1997.d01-420.x. [DOI] [PubMed] [Google Scholar]