Abstract
Many biologically important macromolecules are internalized into cells by clathrin-coated pit endocytosis. The mechanism of clathrin-coated pit budding has been investigated intensively, and considerable progress has been made in characterizing the proteins involved in internalization. Membrane lipid composition and the lateral organization of lipids and proteins within membranes are believed to play an important role in the regulation of membrane-trafficking processes. Here we report that membrane cholesterol plays a critical role in clathrin-coated pit internalization. We show that acute cholesterol depletion, using β-methyl-cyclodextrin, specifically reduces the rate of internalization of transferrin receptor by more than 85%, without affecting intracellular receptor trafficking back to the cell surface. The effect on endocytosis is attributable to a failure of coated pits to detach from the plasma membrane, as visualized by using a green fluorescent protein–clathrin conjugate in living cells. Ultrastructural studies indicate that acute cholesterol depletion causes accumulation of flat-coated membranes and a corresponding decrease in deep-coated pits, consistent with the possibility that flat clathrin lattices are direct precursors of indented pits and endocytic vesicles in intact cells. We conclude that clathrin is unable to induce curvature in the membrane depleted of cholesterol.
Keywords: endocytosis, internalization
The uptake of membrane through clathrin-coated pits is one of the most important and intensively studied internalization mechanisms in cells (reviewed in ref. 1). Clathrin-mediated internalization involves the assembly of clathrin and its associated adaptin (AP-2) complex on the plasma membrane, concentration of membrane proteins in clathrin-coated pits, budding of the coated pits from the membrane, release of clathrin and AP-2 from the vesicles, and reformation of new coated pits. In addition to clathrin and the AP-2 complex, a number of proteins involved in clathrin-coated pit formation and budding have been identified, and progress has been made in characterizing their roles in internalization (2–4).
A number of studies have focused on the role of lipids in regulating membrane trafficking (reviewed in ref. 5). In regard to clathrin-mediated internalization, AP-2 binds phosphatidylinositides, and this binding may play a role in recruiting AP-2 to membranes as well as in affecting its function (e.g., refs. 6 and 7). Dynamin, a GTPase that is involved in the budding of clathrin-coated vesicles, binds acidic phospholipids, and this binding may play a role in recruiting or regulating the activity of dynamin (8, 9). Thus, the local lipid environment may be important in regulating the assembly and function of clathrin-coated pits.
There is evidence that a lateral organization of lipids in cholesterol- and sphingolipid-rich domains is involved in trafficking processes (10–15). Most direct evidence for the function of these domains in membrane traffic comes from studies of glycosylphosphatidylinositol (GPI)-modified proteins. For example, the concentration of GPI-linked proteins in these cholesterol- and sphingolipid-rich domains is believed to be a mechanism for targeting GPI-linked proteins to the apical membrane from the trans-Golgi network (e.g., ref. 16). One frequently used approach to examine the function of cholesterol has been to deplete or sequester cholesterol (14, 16–20). To examine the role of cholesterol in clathrin-mediated internalization, we studied the effect of acute cholesterol depletion, using β-methyl-cyclodextrin (CD), on the endocytic behavior of the transferrin receptor (TR). The trafficking of the TR has been well characterized in a number of different cell types, and it is used often as a marker of clathrin-mediated endocytosis. Here we report that acute cholesterol depletion results in a marked reduction in the rate of TR internalization. This effect is a result of a corresponding decrease in detachment of endocytic-coated pits, visualized by using a green fluorescent protein (GFP)–clathrin conjugate in living cells. Electron microscopy analysis indicates that cholesterol depletion acts by preventing coated pit budding.
METHODS
Cell Culture and Kinetic Analysis.
The Chinese hamster ovary (CHO) cell line and the kinetic assays used to characterize the rates of TR internalization and exocytosis have been described (21, 22). Cells were depleted of cholesterol by incubation for 30 min in 10 mM of CD (Sigma) at 37°C. To replete cholesterol, cells were incubated with CD preloaded with cholesterol (Sigma) for 10 min. The cholesterol content of cells was measured by an enzymatic method (Free cholesterol C; Wako Biochemicals, Osaka).
Fluorescence Microscopy.
Endosomal compartments were labeled with Cy3-transferrin as described previously (23). Cells were stained with filipin as described elsewhere (24). Indirect immunofluorescence was performed on cells fixed in 3.7% formaldehyde and permeabilized in 0.05% Triton X-100. Images were collected by using a charge-coupled device (CCD) K1317 camera (Princeton Instruments, Trenton, NJ) with a KAF-1400 Kodak chip attached to a DM IRB inverted microscope (Leica, Deerfield, IL) by using a ×63 1.32 NA Plan Apochromat objective.
Analysis of Clathrin Dynamics by Using a GFP-Clathrin Light Chain Conjugate.
COS1 cells were transiently transfected with an enhanced GFP conjugated to the amino terminus of mouse placental clathrin light chain (GFP-LCa) and were used 48 hr later as described in detail elsewhere (25). Cells expressing the lowest levels of GFP fluorescence compatible with detection were imaged by using a Zeiss Axiovert S100TV microscope with a Plan-NEOFLUAR ×63 objective (1.25 NA) and a Quantix-cooled CCD camera (Photometrics, Tucson, AZ) with a KAF 1400 chip. GFP fluorescence was visualized with 1-sec exposures by using 430–490 excitation and a 500–550 emission filter with 495LP dichroic (Chroma Technology, Brattleboro, VT). iplab scientific imaging software (Scanalytics, Billerica, MA) was used for image acquisition and analysis.
Electron Microscopy.
For ultrastructural studies, samples were fixed and processed by using a standard protocol described previously (26). Pits were scored as deeply indented if their neck width was less than the pit depth.
RESULTS
Acute Cholesterol Depletion Increases the Number of TR on the Cell Surface.
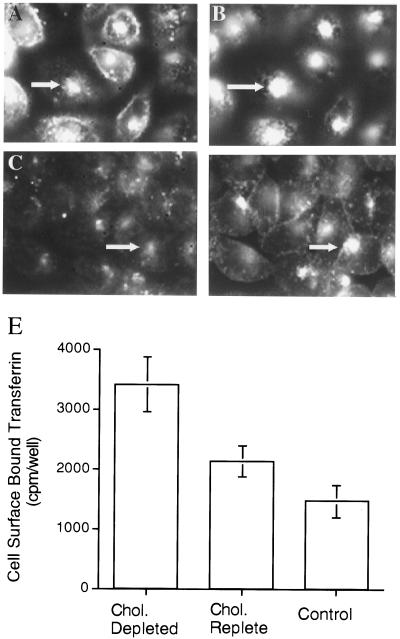
β-Cyclodextrins previously have been used to extract cholesterol from the plasma membrane of cells (e.g., refs. 16, 19, and 20). Incubation of CHO cells with 10 mM of CD for 30 min at 37°C reduced total cellular cholesterol by 46 ± 16% (±SD, n = 4). To assess the effect of CD treatment on cellular cholesterol distribution, we used the fluorescent polyene antibiotic, filipin, to visualize cholesterol. We used fluorescent transferrin internalized from the medium to determine the effect of cholesterol depletion on the morphology of endosomal compartments. In CHO cells, filipin stains both the plasma membrane and the pericentriolar region of the cells (Fig. 1A) (24). The latter compartment also contains transferrin internalized from the medium (Fig. 1B), which identifies it as the general endosomal recycling compartment (23, 24, 27). After CD treatment both the plasma membrane and intracellular filipin staining were reduced (Fig. 1C). The morphology of the transferrin-containing, pericentriolar endosomal recycling compartment was not altered significantly by CD treatment (Fig. 1D). However, in the cholesterol-depleted cells there appeared to be a pronounced increase in the amount of transferrin on the surface and a concomitant decrease in intracellular transferrin, suggesting that cholesterol depletion shifts the distribution of the TR from endosomes to the plasma membrane.
Figure 1.
Cholesterol and TR codistribute in control and CD-treated CHO cells, and cholesterol depletion increases TR on the cell surface. Cholesterol distribution detected with filipin staining (A) and TR distribution detected with Cy3-transferrin (B) in the same field of cells. Filipin stains both the plasma membrane and the transferrin-containing, pericentriolar endocytic recycling compartment of CHO cells (arrows in A and B). Cholesterol distribution detected with filipin staining (C) and TR distribution detected with Cy3-transferrin (D) in the same field of cholesterol-depleted CHO cells. The endocytic recycling compartment (arrows in C and D) is diminished in intensity when labeled by both filipin and Cy3-transferrin, and the amount of Cy3-transferrin on the cell surface is increased. Cells were incubated with Cy3-transferrin for 1.5 hr at 37°C. Cholesterol was depleted by adding 10 mM of CD for the last 30 min of incubation. The cells were washed, fixed, and stained with filipin. The focal planes were chosen by using the filipin staining. (E) Cells were incubated in serum-free medium (Control) or in serum-free medium with 10 mM of CD for 30 min at 37°C and incubated in serum-free medium (Control and Cholesterol depleted) or serum-free medium with 10 mM of CD preloaded with cholesterol (Cholesterol replete) for 10 min. Cells were washed, incubated with iodinated transferrin in serum-free medium for 8 min at 37°C, placed on ice, and washed extensively with medium (4°C), and surface-bound transferrin was removed by incubation in a pH 2.0 buffer (24). The data are the averages of three determinations ±SD from a representative experiment.
The increase in surface TR induced by cholesterol depletion was quantified by measuring the amount of iodinated transferrin bound to cells at 4°C (Fig. 1E). After a 30-min incubation with 10 mM of CD there was an ≈2.5-fold increase in the amount of iodinated transferrin bound to the surface of cells. The effect of cholesterol depletion on the amount of TR on the surface was reversible, returning to near-control levels when cholesterol-depleted cells were incubated for 10 min in CD preloaded with cholesterol (Fig. 1E). The effect of CD treatment also was reversed by incubation in serum-containing growth medium for 12 hr, demonstrating that the CD treatment is not toxic. CD treatment also increased the amount of TR expressed on the surface of COS cells and 3T3-L1 fibroblast cells (not shown).
CD Treatment Inhibits Internalization but Not Recycling Back to the Cell Surface.
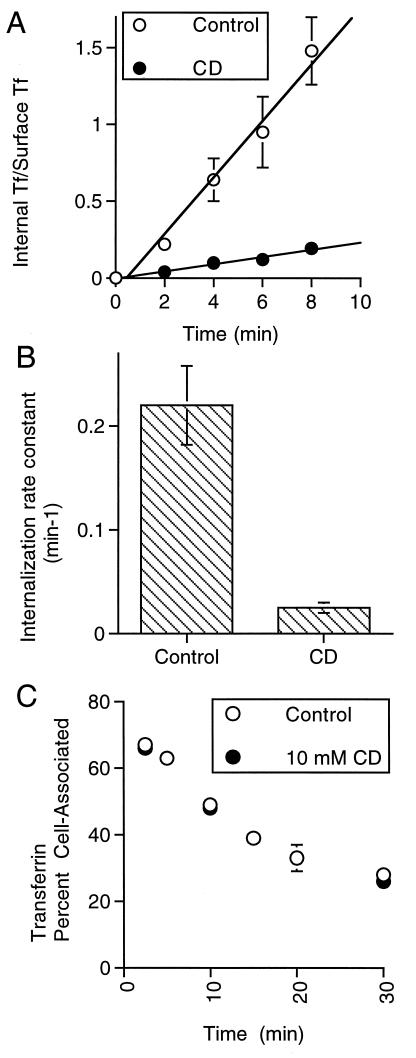
The TR is constitutively internalized and recycled back to the cell surface (28). Thus, the increase in TR on the surface of CD-treated cells could result from a block in internalization, an increase in recycling back to the surface, or changes in both parameters. To distinguish among these possibilities the effect of CD treatment on TR internalization was measured directly. Internalization of the TR was inhibited by CD treatment (Fig. 2 A and B), with a rate constant after cholesterol depletion of 13% ± 3 (±SD, n = 4) of the control rate.
Figure 2.
Cholesterol depletion inhibits internalization of the wild-type TR and does not affect the exocytosis of the TR. (A) A plot of the ratio of internalized transferrin to transferrin bound to the surface of cells as a function of incubation time with radioactive transferrin at 37°C. The open circles are data from control cells, and the solid circles are data from cells incubated with 10 mM of CD at 37° for 30 min. The slope of this line is the internalization rate constant (22). The data are from a representative experiment and are the averages of four measurements per time point ±SD. (B) A plot of the average internalization rate constants (±SD) determined in three independent experiments as those shown in A. (C) A plot of cell-associated transferrin in the absence (solid circles) and presence of 10 mM of CD (open circles). Cells were incubated with iodinated transferrin for 1.5 hr, and 10 mM of CD was added for the last 30 min of incubation. The cells were washed and incubated in medium without iodinated transferrin. At the times noted, the amount of transferrin released into the medium and that remaining cell-associated were determined. The transferrin remaining cell-associated is plotted as a percentage of the total. The data are from a representative experiment and are the averages ±SD of three measurements per time point.
Cholesterol depletion did not affect the rate or the extent of transferrin recycling from endosomes back to the cell surface (Fig. 2C). The exocytic rate constants of the TR in the absence and in the presence of 10 mM of CD were: 0.045 min−1 ± 0.009 (±SD, n = 6) and 0.041 min−1 ± 0.004 (±SD, n = 6), respectively. This observation provides additional evidence that the effect of cholesterol depletion on internalization is not due to nonspecific effects (e.g., cellular ATP depletion) because recycling vesicles can form from the endocytic recycling compartment and fuse with the plasma membrane.
CD Treatment Does Not Affect the Steady-State Distribution of Clathrin.
We next examined whether the steady-state distributions of components of clathrin-coated pits were affected by cholesterol depletion. The overall steady-state distributions of clathrin and the plasma membrane clathrin adaptin complex, AP-2, were unaffected by CD treatment (not shown). The number of plasma membrane clathrin-coated pits on the plasma membrane (examined by immunofluorescence with the AP.6 anti-AP-2 mAb) were counted in control and CD-treated cells. In control cells there were 34 ± 11 pits per 70-μm2 area (±SD, n = 15), and in the cholesterol-depleted cells there were 35 ± 9 pits per 70-μm2 area (±SD, n = 13). Thus, the steady-state distribution of components of clathrin-coated pits on the plasma membrane was not altered dramatically by cholesterol depletion.
CD Treatment Inhibits the Budding of Clathrin-Coated Pits.
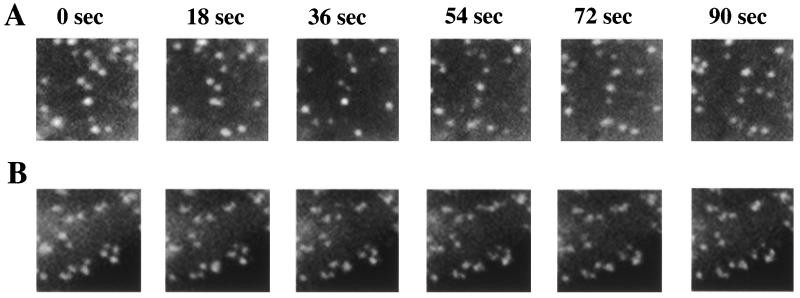
To examine the effect of cholesterol depletion on clathrin dynamics in living cells, we used COS cells transiently expressing a clathrin light chain-GFP construct (25). A time series of clathrin dynamics in control cells is shown Fig. 3A. The punctate distribution of clathrin on the membrane changes over time as clathrin-coated pits bud and detach (spots disappear) and new pits are formed (spots appear). The lifetime of clathrin-coated pits on the cell surface ranges from ≈30 to 80 sec, and in a typical experiment more than 80% of the clathrin-coated pits present initially bud over the course of a 90-sec incubation. Cholesterol depletion has a dramatic effect on the dynamics of clathrin-coated pits. In CD-treated cells the punctate distribution of clathrin on the membrane changes very little over the course of a 90-sec incubation (Fig. 3B). In a typical experiment only ≈5% of the clathrin-coated pits present initially bud over the course of 90 sec in cholesterol-depleted cells, indicating that cholesterol depletion increases the lifetime of clathrin-coated pits to more than 9 min. The greater than 7-fold increase in the lifetime of plasma membrane clathrin-coated pits correlates well with the 7-fold reduction in TR internalization. These data directly demonstrate that the inhibition of TR internalization by cholesterol depletion is a consequence of the decreased detachment of plasma membrane clathrin-coated pits to form endocytic vesicles. The inhibition of clathrin budding is reversed rapidly when the membrane is repleted with cholesterol. Clathrin dynamics are restored within 5 min of incubation of cholesterol-depleted cells with CD preloaded with cholesterol (not shown).
Figure 3.
Clathrin dynamics in control and cholesterol-depleted cells. (A) Images of an 8.6-μm × 8.6-μm region from control cells at 18-sec intervals reveal substantial change in clathrin-coated pit patterns. In a typical experiment, a set of 60 images taken at intervals of 1.5 sec were collected, and analyses of these data indicate that more than 80% of the clathrin-coated pits present disappear initially (bud) within 90 sec. (B) Comparable images from cholesterol-depleted cells show almost no change in the clathrin-coated pit patterns during this period. Similarly, fewer than 5% of the coated pits present disappeared initially within 90 sec.
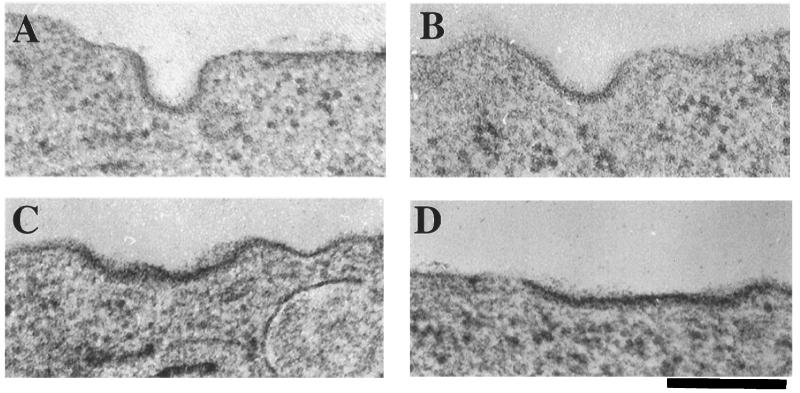
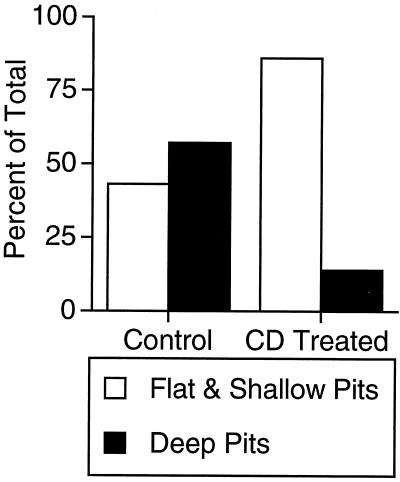
The morphology of clathrin-coated plasma membrane structures was examined to determine at which step in the budding and detachment process cholesterol is required (Fig. 4). In control cells the proportion of deeply indented coated pits was approximately equal to that of shallow or relatively flat coated membrane segments (Fig. 5). However, in cholesterol-depleted cells the frequency of detection of deep pits was reduced to ≈15%, and the occurrence of only slightly curved or distinctly flat coated membranes correspondingly was increased. Many of these coated segments appeared to be quite large, as though they correspond to multiple, adjacent coated pit regions (i.e., Fig. 4 C and D). These data suggest that cholesterol is required to permit the formation of deeply invaginated clathrin structures and that the inability to convert flat to deeply invaginated structures in CD-treated cells is responsible for the inhibition of endocytosis.
Figure 4.
In CD-treated cells the majority of clathrin-coated structures are flat to moderately indented. Representative electron micrographs of the morphologies of the predominant clathrin-coated structures in control (A and B) and CD-treated cells (C and D) are shown. Examples of a deeply indented pit (A) and shallow pit (B) in control cells. Examples of a shallow pit (C) and a flat pit (D) in CD-treated CHO cells. (Bar = 200 nm.) Coated structures were identified visually by the presence of the electron-dense coating of the membrane.
Figure 5.
Quantification of clathrin-coated pit ultrastructural morphology. The values are a percentage of the total clathrin-coated structures counted. The results for the CD-treated cells are from 45 cells and a total of 212 clathrin-coated structures. The results for the control cells are from 45 cells and a total of 162 clathrin-coated structures. Structures whose depth was less than the width of the opening of the pit were scored as flat or shallow.
DISCUSSION
In this report we demonstrate that acute cholesterol depletion results in a 7-fold reduction in the rate of TR internalization. The decreased internalization correlates with an increase in the residence time of clathrin-coated pits on the cell surface and a decrease in the proportion of deeply invaginated clathrin-coated pits. From these results we conclude that plasma membrane cholesterol is required for invagination of clathrin-coated pits. Occasional flat clathrin lattices predominantly composed of hexagonal polygons have been observed previously in intact cells (29, 30). Based on studies with broken cell systems (31–33), these flat lattices are proposed to be functional precursors of deeply indented coated pits and vesicles, although this concept has been controversial because the mechanism for conversion of hexagons to the pentagonal facets present in curved lattices is unknown (29, 34). Nonetheless, the compensatory changes in coated membrane shapes on cholesterol depletion in intact cells that we report here are consistent with the interpretation that flat lattices indeed are transient precursors of deep coated pits. Interestingly, the effects of cholesterol depletion on endocytic trafficking of the TR are specific for budding from the plasma membrane because intracellular trafficking of the TR through the endosomal system back to the cell surface is unaffected.
The seemingly contradictory findings that cholesterol depletion inhibits clathrin-coated pit budding without increasing the number of coated pits provides compelling evidence for the proposal that there are specialized clathrin assembly-budding sites on the membrane (26, 35). If clathrin-coated pits can only assemble and bud from a limited number of specialized sites, then an inhibition of budding would inhibit the formation of new pits by blocking the assembly sites. This interpretation is also consistent with the tendency of coated pits to occur in clusters or large patches in cholesterol-depleted cells, in agreement with our previous studies, suggesting that these sites are localized and function in proximity to each other (25).
A couple of reports have examined the role of cholesterol in clathrin-mediated endocytosis. It has been reported that filipin, a cholesterol-sequestering drug, does not block diphtheria toxin inhibition of protein synthesis (36), nor does it inhibit α2-macroglobulin degradation (37). These findings are not consistent with ours because both diphtheria toxin and α2-macroglobulin are internalized by clathrin-coated pits (38–40). One potential explanation is that in the other studies, unlike in ours, internalization was not measured directly but postinternalization processes were examined. For example, because a single molecule of diphtheria toxin can kill a cell, it is possible that sufficient toxin is internalized to inhibit protein synthesis even when internalization through clathrin-coated pits is inhibited by CD. Another important consideration is the method used for cholesterol depletion. It is possible that filipin sequestering of cholesterol has different effects than the CD removal of cholesterol from membranes.
The role of membrane cholesterol in trafficking has been studied most intensively for GPI-modified proteins. It is proposed that cholesterol- and sphingolipid-rich domains provide a lateral organization of lipids and proteins within membranes and that this organization is involved in some membrane-trafficking processes (e.g., refs. 15 and 41). GPI-linked proteins are internalized from the cell surface, although the internalization pathway used (clathrin-coated pits or caveolae) remains an area of considerable controversy (18, 42–44). Two previous studies have shown that cholesterol depletion results in no change or a small increase in the internalization of GPI-linked proteins. In light of our results, it could be concluded that GPI-linked proteins are internalized by a nonclathrin-coated pit pathway. It is important to note that in the studies of the GPI-linked proteins noted above, cholesterol was depleted by growing cells (for 4–5 days) in low density lipoprotein (LDL)-depleted medium, whereas in our study cholesterol was acutely depleted. Cells grown in LDL-deficient medium may compensate for reduced cholesterol by altering lipid desaturase activity or by changing lipid composition (45). It is unlikely that cells could compensate when cholesterol is acutely depleted with CD.
Cholesterol depletion has significant effects on the intracellular trafficking of some transmembrane and GPI-linked proteins. Cholesterol- and sphingolipid-rich domains are involved in the intracellular trafficking of GPI proteins and some transmembrane proteins (16–18, 45). Although the exact function of these domains is not known, these domains are postulated to concentrate (or exclude) proteins based on direct interactions with these lipid microdomains (15, 41). None of the proteins known to be involved in clathrin-coated pit internalization are GPI-linked; therefore, other models for the role of cholesterol in clathrin-mediated internalization must be considered. A cholesterol-dependent lateral organization of the plasma membrane may provide the specific local lipid (and/or protein) environment required for budding. There is evidence that phospholipids play a role in the recruitment and/or anchoring of dynamin, AP-2, and arrestins (4, 7, 25). Our finding that the budding of clathrin from the membrane is affected by CD treatment indicates that internalization is inhibited at a step after recruitment of coat proteins. However, it remains possible that cholesterol depletion affects the ability of these proteins to participate in the lattice rearrangements necessary to induce invagination of clathrin-coated membrane.
Cholesterol depletion may affect internalization because of a change in the biophysical properties of the bilayer, such as alterations in membrane tension, bending stiffness, or thickness. For example, it has been shown that high membrane tensions are correlated with an inhibition of endocytosis (46). If, however, the effects of cholesterol depletion on endocytosis are due to changes in the biophysical properties of the membrane, then budding from the plasma membrane apparently is more susceptible to these changes than endosomal membranes (which are also cholesterol-rich) because cholesterol depletion did not affect intracellular trafficking of the TR back to the cell surface.
Although future work is required to elucidate the exact functions of membrane cholesterol in regulating clathrin-coated pit budding, our data clearly demonstrate that cholesterol plays a major role in plasma membrane clathrin dynamics.
Acknowledgments
We thank William Mallet, Fred Maxfield, and Tim Ryan for their thoughtful comments. We thank Neelima Shah of the Diabetes and Endocrinology Research Center and the Biomedical Imaging Core Laboratory of the University of Pennsylvania for electron microscopy services. This work was supported by National Institutes of Health Grants DK52852 (T.E.M.) and GM28526 (J.H.K.) and by a fellowship program sponsored by the Charles H. Revson Foundation (A.S.).
ABBREVIATIONS
- TR
transferrin receptor
- CD
β-methyl-cyclodextrin
- GPI
glycosylphosphatidylinositol
- GFP
green fluorescent protein, CHO, Chinese hamster ovary
- AP
adaptin
Note Added in Proof
While this manuscript was in review, another paper presenting similar results was published (47).
References
- 1.Mukherjee S, Ghosh R N, Maxfield F R. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 2.Schmid S L. Trends Cell Biol. 1993;3:145–148. doi: 10.1016/0962-8924(93)90129-o. [DOI] [PubMed] [Google Scholar]
- 3.Moore M S, Mahaffey D T, Brodsky F M, Anderson R G. Science. 1987;236:558–563. doi: 10.1126/science.2883727. [DOI] [PubMed] [Google Scholar]
- 4.Takei K, Haucke V, Slepnev V, Farsad K, Salazar M, Chen H, De Camilli P. Cell. 1998;94:131–141. doi: 10.1016/s0092-8674(00)81228-3. [DOI] [PubMed] [Google Scholar]
- 5.De Camilli P, Emr S D, McPherson P S, Novick P. Science. 1996;271:1533–1539. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- 6.Gaidarov I, Chen Q, Falck J R, Reddy K K, Keen J H. J Biol Chem. 1996;271:20922–20929. doi: 10.1074/jbc.271.34.20922. [DOI] [PubMed] [Google Scholar]
- 7.Rapoport I, Miyazaki M, Boll W, Duckworth B, Cantley L C, Shoelson S, Kirchhausen T. EMBO J. 1997;16:2240–2250. doi: 10.1093/emboj/16.9.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuma P L, Stachniak M C, Collins C A. J Biol Chem. 1993;268:17240–17246. [PubMed] [Google Scholar]
- 9.Liu J P, Powell K A, Sudhof T C, Robinson P J. J Biol Chem. 1994;269:21043–21050. [PubMed] [Google Scholar]
- 10.Rothblat G H, Mahlberg F H, Johnson W J, Phillips M C. J Lipid Res. 1992;33:1091–1097. [PubMed] [Google Scholar]
- 11.Schroeder F, Jefferson J R, Kier A B, Knittel J, Scallen T J, Wood W G, Hapala I. Proc Soc Exp Biol Med. 1991;196:235–252. doi: 10.3181/00379727-196-43185. [DOI] [PubMed] [Google Scholar]
- 12.Schroeder F, Woodford J K, Kavecansky J, Wood W G, Joiner C. Mol Membr Biol. 1995;12:113–119. doi: 10.3109/09687689509038505. [DOI] [PubMed] [Google Scholar]
- 13.Simons K, van Meer G. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- 14.Anderson R G, Kamen B A, Rothberg K G, Lacey S W. Science. 1992;255:410–411. doi: 10.1126/science.1310359. [DOI] [PubMed] [Google Scholar]
- 15.Simons K, Ikonen E. Nature (London) 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 16.Scheiffele P, Roth M G, Simons K. EMBO J. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hannan L A, Edidin M. J Cell Biol. 1996;133:1265–1276. doi: 10.1083/jcb.133.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayor S, Sabharanjak S, Maxfield F R. EMBO J. 1998;17:4626–4638. doi: 10.1093/emboj/17.16.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilsdonk E P, Yancey P G, Stoudt G W, Bangerter F W, Johnson W J, Phillips M C, Rothblat G H. J Biol Chem. 1995;270:17250–17256. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- 20.Neufeld E B, Cooney A M, Pitha J, Dawidowicz E A, Dwyer N K, Pentchev P G, Blanchette-Mackie E J. J Biol Chem. 1996;271:21604–21613. doi: 10.1074/jbc.271.35.21604. [DOI] [PubMed] [Google Scholar]
- 21.McGraw T E, Greenfield L, Maxfield F R. J Cell Biol. 1987;105:207–214. doi: 10.1083/jcb.105.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson L S, Dunn K W, Pytowski B, McGraw T E. Mol Biol Cell. 1993;4:1251–1266. doi: 10.1091/mbc.4.12.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson A O, Ghosh R N, Dunn K W, Garippa R, Park J, Mayor S, Maxfield F R, McGraw T E. J Cell Biol. 1996;135:1749–1762. doi: 10.1083/jcb.135.6.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukherjee S, Zha X, Tabas I, Maxfield F R. Biophys J. 1998;75:1915–1925. doi: 10.1016/S0006-3495(98)77632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaidarov I, Santini F, Warren R A, Keen J H. Nat Cell Biol. 1999;1:1–7. doi: 10.1038/8971. [DOI] [PubMed] [Google Scholar]
- 26.Santini F, Marks M S, Keen J H. Mol Biol Cell. 1998;9:1177–1194. doi: 10.1091/mbc.9.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashiro D J, Tycko B, Fluss S R, Maxfield F R. Cell. 1984;37:789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- 28.Dautry-Varsat A, Ciechanover A, Lodish H F. Proc Natl Acad Sci USA. 1983;80:2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heuser J. J Cell Biol. 1980;84:560–583. doi: 10.1083/jcb.84.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maupin P, Pollard T D. J Cell Biol. 1983;96:51–62. doi: 10.1083/jcb.96.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larkin J M, Brown M S, Goldstein J L, Anderson R G. Cell. 1983;33:273–285. doi: 10.1016/0092-8674(83)90356-2. [DOI] [PubMed] [Google Scholar]
- 32.Smythe E, Pypaert M, Lucocq J, Warren G. J Cell Biol. 1989;108:843–853. doi: 10.1083/jcb.108.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin H C, Moore M S, Sanan D A, Anderson R G. J Cell Biol. 1991;114:881–891. doi: 10.1083/jcb.114.5.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahaffey D T, Moore M S, Brodsky F M, Anderson R G. J Cell Biol. 1989;108:1615–1624. doi: 10.1083/jcb.108.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santini F, Keen J H. J Cell Biol. 1996;132:1025–1036. doi: 10.1083/jcb.132.6.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orlandi P A, Fishman P H. J Cell Biol. 1998;141:905–915. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schnitzer J E, Oh P, Pinney E, Allard J. J Cell Biol. 1994;127:1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keen J H, Maxfield F R, Hardegree M C, Habig W H. Proc Natl Acad Sci USA. 1982;79:2912–2916. doi: 10.1073/pnas.79.9.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moya M, Dautry-Varsat A, Goud B, Louvard D, Boquet P. J Cell Biol. 1985;101:548–559. doi: 10.1083/jcb.101.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran D, Carpentier J L, Sawano F, Gorden P, Orci L. Proc Natl Acad Sci USA. 1987;84:7957–7961. doi: 10.1073/pnas.84.22.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown R E. J Cell Sci. 1998;111:1–9. doi: 10.1242/jcs.111.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson R G. Proc Natl Acad Sci USA. 1993;90:10909–10913. doi: 10.1073/pnas.90.23.10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turek J J, Leamon C P, Low P S. J Cell Sci. 1993;106:423–430. doi: 10.1242/jcs.106.1.423. [DOI] [PubMed] [Google Scholar]
- 44.Smart E J, Mineo C, Anderson R G. J Cell Biol. 1996;134:1169–1177. doi: 10.1083/jcb.134.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keller P, Simons K. J Cell Biol. 1998;140:1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai J, Ting-Beall H P, Sheetz M P. J Gen Physiol. 1997;110:1–10. doi: 10.1085/jgp.110.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodal S K, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. Mol Biol Cell. 1999;10:961–974. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]