Abstract
Human CD8+ memory- and effector-type T cells are poorly defined. We show here that, next to a naive compartment, two discrete primed subpopulations can be found within the circulating human CD8+ T cell subset. First, CD45RA−CD45R0+ cells are reminiscent of memory-type T cells in that they express elevated levels of CD95 (Fas) and the integrin family members CD11a, CD18, CD29, CD49d, and CD49e, compared to naive CD8+ T cells, and are able to secrete not only interleukin (IL) 2 but also interferon γ, tumor necrosis factor α, and IL-4. This subset does not exert cytolytic activity without prior in vitro stimulation but does contain virus-specific cytotoxic T lymphocyte (CTL) precursors. A second primed population is characterized by CD45RA expression with concomitant absence of expression of the costimulatory molecules CD27 and CD28. The CD8+CD45RA+CD27− population contains T cells expressing high levels of CD11a, CD11b, CD18, and CD49d, whereas CD62L (L-selectin) is not expressed. These T cells do not secrete IL-2 or -4 but can produce IFN-γ and TNF-α. In accordance with this finding, cells contained within this subpopulation depend for proliferation on exogenous growth factors such as IL-2 and -15. Interestingly, CD8+CD45RA+CD27− cells parallel effector CTLs, as they abundantly express Fas-ligand mRNA, contain perforin and granzyme B, and have high cytolytic activity without in vitro prestimulation. Based on both phenotypic and functional properties, we conclude that memory- and effector-type T cells can be separated as distinct entities within the human CD8+ T cell subset.
Recognition of antigen by the immune system evokes a coordinate number of changes in lymphocytes and lymphocyte subsets that allow the system (a) to eliminate or neutralize potential harmful agents and (b) to respond more rapidly and appropriately after renewed antigen encounter, a process referred to as immunological memory. Within the T cell compartment, unprimed (or naive) T cells (i.e., cells which have not yet encountered antigen), effector T cells (i.e., cells with specialized functions such as cytolysis), and memory T cells can be discerned. Functionally, memory T cells have a number of characteristics that distinguish them from unprimed cells. Not only do they respond efficiently to recall antigens, but, compared to naive cells, memory T cells also have less stringent requirements for activation and have the potential to secrete a more extensive set of cytokines (1–4). In addition, memory and naive T cells differ in the expression of several cell surface antigens (5, 6), although it is questioned whether these markers can be looked upon as true memory markers or if they reflect cellular activation (7, 8).
Recent studies by Zimmermann et al. (9) have documented phenotypic distinctions between naive, effector, and memory CD8+ T cells in a murine model of lymphocytic choriomeningitis virus infection and an adoptive transfer system with CD8+ T cells from TCR transgenic mice. Their data confirmed previous reports (10–14) by demonstrating that compared to naive T cells CTL effectors have strongly downregulated the lymph node homing receptor CD62L (L-selectin) while expression of CD44, CD11a/CD18, CD11b, and CD49d is enhanced. In contrast to effector T cells, memory T cells are heterogenous with respect to CD62L expression, have no CD11b on their surface, and have an intermediate CD49d expression. Finally, CD45 isoform expression could not be used as a reliable marker for memory-type T cells (9).
In contrast to the murine T cell subsets, description of human CD8+ naive, effector, and memory cells is still rather fragmentary. Cytolytic function of CD8+ cells has been linked to cells with a CD28−, a CD11b+, and/or a CD57+ phenotype (11, 15, 16). Moreover, memory-type CD8+ CD45RA−CD45R0+ T cell subsets that contain enhanced frequencies of antigen-reactive precursor cells (17), have low stringent activation requirements, and have an ability to secrete, apart from IL-2, IFN-γ and TNF-α (18), have been previously described. However, discrimination between naive and memory cells, based solely on CD45 isoform expression, has been proven unreliable, since the CD45RA+ population contains cells that share a number of phenotypic features with primed cells, such as low CD62L (19) and high CD11a/CD18 (20) expression. Moreover, part of the CD45RA+ cells have downregulated CD27 (21), a feature considered to result from persistent antigenic stimulation in vivo (22, 23).
In this study we used simultaneous staining with CD45RA and CD27 mAbs to separate functionally distinct subpopulations of human CD8+ T cells. Our observations demonstrate that, within the human CD8+ compartment, in accordance with data obtained in experimental animal models, naive, memory- and effector-type cells can be discriminated with coherent phenotypic and functional properties. This type of CD8+ T cell subset analysis could prove useful in monitoring the immune system in several clinical situations.
Materials and Methods
Reagents.
The mAbs CLB-T11.1/1, CLB-T11.2/1, CLB-HIK27 (all CD2), CLB-CD3/3 CLB-CD3/4.1, CLB-CD4/1, CLB-CD8/1, CLB-CD14/1, CLB-FcR gran1 (CD16), CLB-CD19/1, CLB-CD27/1, CLB-CD28, CLB-LFA-1/1 (CD18), CLB-CD49d (VLA-4), and CLB-CD49e (VLA-5), and FITC-conjugated goat anti–mouse Ig, were all produced at the Central Laboratory of the Netherlands Red Cross Blood Transfusion Service (CLB; Amsterdam, The Netherlands). CD95 mAb was provided by Dr. Yonehara (Pharmaceutical Basic Research, Tokyo, Japan) and CD58 mAb (TS2/9) was a gift from Dr. T.A. Springer (Center for Blood Research, Boston, MA). CD11b (OKM-1) mAb was purchased from Ortho Diagnostic Systems (Beerse, Belgium). FITC-conjugated–CD62L (Leu-8), –CD57, –CD16, –TCR-α/β, and –CD8 mAbs (Leu2a), and PE-conjugated– and PerCP-labeled CD8 mAb were from Becton Dickinson (San Jose, CA). CD29 (4B4) and PE-conjugated CD45RA mAbs (2H4) were obtained from Coulter Immunology (Hialeah, FL). FITC-labeled CD45RO mAb (UCHL1) was purchased from DAKO (Glostrup, Denmark). Streptavidin red 670 was obtained from GIBCO BRL (Gaithersburg, MD).
Biotinylated CLB-IL-4/1 mAb (anti–human IL-4, mouse IgG1; reference 24) was provided by Dr. T. van der Pouw-Kraan (CLB) and biotinylated MD-1 mAb (anti–human IFN-γ, mouse IgG1) was obtained from Dr. P. van der Meide (Netherlands Organisation for Applied Sciences TNO, Rijswijk, The Netherlands). Biotinylated anti–human IL-2, FITC-conjugated anti– human TNF-α, and FITC-conjugated antiperforin mAbs were purchased from Hölzel Diagnostika (Köln, Germany). Antigranzyme B mAb, provided by Dr. C.E. Hack (CLB), was obtained after immunization of BALB/c mice with native granzyme B.
The following recombinant human cytokines were used: IL-4 and IFN-γ (Genzyme Corp., Cambridge, MA), IL-15 (PeproTech, London, UK), IL-2 (gift of Sandoz, Vienna, Austria), IL-6 (provided by Professor L. Aarden, CLB), IL-10 (gift of Dr. J. de Vries, DNAX, Palo Alto, CA), and IL-12 (donated by Dr. S. Wolf, Genetic Institute, Cambridge, MA).
Cell Preparation and Flow Cytometry.
Human PBMCs were isolated from buffy coats of healthy donors by Ficoll-Paque density centrifugation (Pharmacia Biotech AB, Uppsala, Sweden). For phenotypic analysis (Table 1), CD8+ cells (>95% CD8+ CD3+TCR-α/β+CD16−) were purified by incubating PBMCs with saturating amounts of CD4, CD19, CD16, and CD14 mAbs followed by depletion of positive cells with Dynal beads (Dynal, Oslo, Norway) as previously described (18). Triple color immunofluorescence analysis was performed as previously described (23). In brief, purified CD8+ T cells were incubated with an unlabeled mAb followed by FITC-conjugated goat anti–mouse Ig staining. After blocking free binding sites on the goat anti–mouse conjugate with 10% normal mouse serum, cells were incubated with PE-conjugated CD45RA and biotinylated CD27 mAbs. The latter was then stained with Streptavidin red 670.
Table 1.
Phenotypic Characterization of CD8+ T Cell Subsets*
| CD45RA+CD27+ | CD45RA+CD27− | CD45RA−CD27+ | CD45RA−CD27− | |||||
|---|---|---|---|---|---|---|---|---|
| Homing receptors | ||||||||
| CD62L | 76 ± 9‡ | 28 ± 13‖ | 20 ± 7‖ | 30 ± 3‖ | ||||
| Integrins | ||||||||
| CD49d | 25 ± 13 | 87 ± 2‖ | 41 ± 15‖ | 34 ± 20 | ||||
| CD49e | 25 ± 14 | 76 ± 12‖ | 56 ± 6‖ | 61 ± 12‖ | ||||
| CD29 | 27 ± 15 | 93 ± 2‖ | 73 ± 6‖ | 83 ± 3‖ | ||||
| CD11a | 290 ± 60§ | 716 ± 50‖ | 369 ± 100 | 317 ± 100 | ||||
| CD18bright | 21 ± 20 | 87 ± 7‖ | 47 ± 17 | 32 ± 18 | ||||
| CD11b | 10 ± 7 | 67 ± 13‖ | 9 ± 3 | 25 ± 3‖ | ||||
| Activation and costimulatory molecules | ||||||||
| CD58 | 18 ± 16 | 86 ± 8‖ | 80 ± 8‖ | 88 ± 10‖ | ||||
| CD28 | 77 ± 12 | 17 ± 4‖ | 83 ± 2 | 50 ± 7‖ | ||||
| CD57 | 1.0 ± 0.7 | 50 ± 13‖ | 10 ± 6‖ | 14 ± 9‖ | ||||
| CD95 | 10 ± 1§ | 29 ± 8‖ | 56 ± 2‖ | 58 ± 10‖ | ||||
CD8+ cells were >97% CD3+TCR-α/β+CD16− in all subsets.
Percentage of positive cells ± SD, n = 5.
Mean fluorescence intensity ± SD, n = 5.
Significant difference (P <0.05) compared to the CD45RA+CD27+ subset (Wilcoxon signed rank test).
For subset purification, CD8+ T cells were prepared by positive enrichment using the MACS system (Miltenyi Biotec, Bergisch-Gladbach, Germany). In brief, PBMCs (107 cells/80 μl incubation buffer; PBS/2% FCS/5 mM EDTA) were stained with CD8 microbeads (Miltenyi Biotec; 20 μl/107 positive cells) for 15 min at 8°C. After one washing, step cells were resuspended in incubation buffer (107 cells/400 μl) and enrichment was performed with BS columns (capacity: 108 cells) and the VarioMACS magnet according to manufacturer's instructions. The resulting CD8+ T cells were >98% CD8+CD3+TCR-α/β+CD16− as determined by immunofluorescence analysis with directly labeled mAb. Next, cells were stained with PE-conjugated CD45RA and FITC-conjugated CD27 and sorted into CD45RA+CD27+, CD45RA+ CD27−, and CD45RA−CD27+ populations (purity: >98%) on a FACStar®.
Detection of Fas-ligand mRNA.
Total RNA was prepared (TRIzol Reagent; Life Technologies, Paisley, UK) from 2.5 × 106 sorted T cell subsets (see above). Single-stranded cDNA was synthesized from RNA in a 20-μl reaction mixture containing 500 ng oligo(dT)12–18 and 200 U reverse transcriptase (RT).1 1 μl of the reaction mixture was diluted with 11.5 μl of PCR buffer containing 2 mM MgCl2 and 100 pmol of forward and reverse oligonucleotides. Two PCR reactions were set up for each cDNA, corresponding to Fas ligand (forward: 5′-GGGTCGACGGGATGTTTCAGCTCTTCCACCTAC - 3′ ; reverse: 5′-GCTCTAGAACATTCCTCGGTGCCTGTAAC-3′; reference 25) and HPRT (hypoxanthine-guanine phosphoribosyltransferase; forward: 5′-TATGGACAGGACTGAACGTCTTGC-3′; reverse: 5′-GACACAAACATGATTCAAATCCCTGA-3′; reference 26). PCR products were resolved on a 1% agarose gel containing ethidium bromide.
Flow Cytometric Detection of Cytokine Production and Intracellular Staining for Perforin and Granzyme B.
Flow cytometric measurement of cytokine production was performed as previously described (27, 28). In brief, 106 cells/ml were stimulated for 4 h (IL-4, IFN-γ, and TNF-α) or 8 h (IL-2) with 1 ng/ml PMA and 1 μM ionomycin in the presence of the protein-secretion inhibitor monensin (1 μM; all from Sigma Chemical Co., St. Louis, MO). This short-term stimulation did not alter the membrane phenotype with respect to CD45 or CD27 expression. After cell surface staining with PE-conjugated CD45RA and FITC-conjugated CD27 cells were washed twice with cold PBS and fixated with PBS/4% paraformaldehyde (at 4°C for 5 min). Fixation was followed by permeabilization with PBS/0.1% saponin (Sigma Chemical Co.)/10% human pooled serum (at 4°C for 10 min). For all subsequent incubation and washing steps, PBS/0.1% saponin/0.5% BSA was used. Staining of the cytoplasm with biotinylated cytokine mAbs (IL-2, IL-4, and IFN-γ; all 5 μg/ml) was followed by incubation with Streptavidin red 670 (both at 4°C for 20 min). Biotinylated or FITC-conjugated mouse IgG1 control mAbs (DAKO) were used as negative controls. Data acquisition was performed on a FACScan® and analysis was done using PC-Lysis software.
Intracellular content of perforin and granzyme B was measured in freshly isolated CD8+ cells without previous stimulation. A permeabilization and staining protocol identical to that described above for the cytokine analysis was used.
Cell Culture and Activation.
All cell culture experiments were performed in IMDM supplemented with 10% FCS and antibiotics. Sorted CD8+ T cell subsets (5 × 104 cells/well) were stimulated with a combination of three CD2 mAbs (CLB-T11.1/1, CLB-T11.2/1, and CLB-HIK27, all 5 μg/ml) in the presence or absence of the following recombinant human cytokines: IL-2 (50 U/ml), IL-4 (10 ng/ml), IL-6 (100 U/ml), IL-10 (100 U/ml), IL-12 (1 ng/ml), IL-15 (10 ng/ml), and IFN-γ (100 U/ml). Proliferation was measured on day 4 by adding 0.2 μCi/well of [3H]thymidine (Amersham, Buckinghamshire, UK) during the last 4 h of culture. All cultures were set up in triplicate.
To measure cytokine secretion, triplicate cultures of sorted cells (2.5 × 104) were stimulated with PMA (100 ng/ml) and ionomycin (1 μM). Supernatant was taken after 24 h for detection of IL-2 and on day 4 for measurement of IFN-γ, TNF-α, and IL-4 content. In our experience, the indicated time points have been proven optimal. IFN-γ, TNF-α, and IL-4 were detected with specific ELISAs (CLB). IL-2 production was measured in a bioassay using IL-2–dependent mouse CTLL cells (29).
Cytotoxicity Assay.
CTL activity was determined in a CD3 mAb–mediated cytotoxicity assay as previously described (30). In brief, FcR-bearing P815 target cells were radiolabeled with Na51CrO4 (Amersham) for 30 min at 37°C. Purified CD8+ T cell subsets were incubated with P815 target cells at varying effector/ target ratios in the presence or absence of 5 μg/ml CD3 mAb (CLB-CD3/4.1). After a 4 h incubation period at 37°C, the supernatants of triplicate cultures were collected and counted in a gamma counter. Specific cytotoxicity was determined according to the formula: percentage of specific lysis = 100 × [(cpm experimental release − cpm spontaneous release)/(cpm maximal release − cpm spontaneous release)]. The standard error of the mean percentage lysis did not exceed 5%.
Determination of HIV-1–derived Peptide-specific CTL Precursor Frequency.
HIV-1–specific CTL precursors (CTLp) were expanded in vitro by Ag-specific stimulation and frequencies of RT derived peptide aa 244-252 (IVLPEKDSW)–specific CTLp were determined as previously described (31, 32). In brief, cryopreserved PBMCs from an HIV-1 seropositive individual (L090; for case report see reference 32) were thawed and sorted into CD8+ CD45RA+CD27+ and CD8+CD45RA−CD27+ subsets. Sorted cells were cocultivated with stimulator cells (paraformaldehyde fixated autologous EBV-transformed B lymphoblastoid cell lines [B-LCL] infected with five multiplicity of infection of recombinant vaccinia virus expressing HIV-1Lai RT) and irradiated feeder cells (autologous PBMCs; references 31, 32). At days 2 and 9, microcultures were fed with rIL-2 (10 U/ml Proleukin; provided by Dr. R. Rombouts, Chiron Benelux B.V., Amsterdam, The Netherlands). Cultures were restimulated at day 7 by addition of fixated autologous stimulator cells in the presence of rIL-2 (10 U/ml). On day 15, wells were split and tested for cytotoxicity on 51Cr-labeled autologous B-LCL that were preincubated for 30 min at room temperature with synthetic peptide at a final concentration of 5 μg/ ml. Lysis of target cells was measured as described above. Wells were considered positive when 51Cr-release exceeded 10% of specific lysis. Frequency estimates were made using the “single-hit Poisson model” described by Strijbosch et al. (33).
Helper and Suppressor Activity for Ig Production.
CD8+ T cells and CD8+ subsets were prepared as described above. The CD8− fraction obtained after purification of CD8+ cells was either depleted of monocytes, NK cells, the remaining CD8+ T cells, and B cells with CD14, CD16, CD8, and CD19 mAbs and magnetic beads (Dynal) to generate CD4+ T cells (>95% CD4+) or else depleted of monocytes, NK cells, and T cells with CD14, CD16, and CD3 mAbs to prepare B cells (>90% CD19+). To determine helper capacity, cells of the different T cell subsets (5 × 104/well) were cultured with autologous B cells (5 × 104/well) in 96-well flat-bottomed microtiter plates (Greiner, Nürnberg, Germany) coated with 5 μg/ml CD3 mAb (CLB-CD3/3). Suppressor activity was determined by adding cells of the different CD8+ T cell subsets (5 × 104/well) to a coculture of autologous CD4+ T and B cells. All cultures were set up in triplicate and supernatants were harvested after 14 d and tested for Ig content. IgM and IgG production was measured by ELISA.
Results
Phenotypic Heterogeneity of the CD8+ T Cell Subset.
As has been previously noted (21), in contrast to CD4+CD27− T lymphocytes, which are exclusively CD45RA−, within the circulating CD8+ compartment CD27− T cells can be found within both CD45RA− and CD45RA+ fractions (Fig. 1). These CD8+CD45RA+CD27− cells are, like CD8+ CD45RA−CD45R0+ cells, absent from cord blood (Fig. 1). Therefore, we hypothesize that in response to antigen two separate primed CD8+ populations may develop in humans: (a) a population of CD45RA− T cells, and (b) a population of CD45RA+CD27− T cells. This study was performed to analyze whether these phenotypes discriminate between functionally distinct CD8+ subsets.
Figure 1.
Triple color immunofluorescence analysis of cord blood lymphocytes and PBMCs from an adult donor. Cells were simultaneously stained with either CD4-PerCP or PerCP-labeled CD8 in combination with PE-conjugated CD45RA and FITC-conjugated CD27 mAbs. Cells were gated on forward scatter/side scatter and PerCP-fluorescence. Four distinct populations can be defined within CD8+ cells from adult blood: (1) CD45RA+CD27+ cells, (2) CD45RA−CD27+ cells, (3) CD45RA+ CD27− cells, and (4) CD45RA−CD27− cells. Note that there is also a transitional population with dull CD45RA expression. As in the CD4+ subset (23), these cells have an intermediate phenotype for the studied surface markers (data not shown). Functionally, this subset was not analyzed.
In a population of healthy adult donors (n = 30), the distribution of the CD8+ T cell subsets defined by simultaneous CD45RA and CD27 staining was as follows (for definition of the subsets see Fig. 1): (1) CD45RA+CD27+ cells 55 ± 17% (range 28–83%); (2) CD45RA−CD27+ cells 25 ± 11% (range 9–48%); (3) CD45RA+CD27− cells 13 ± 13% (range 1–50%); and (4) CD45RA−CD27− cells 4 ± 3% (range 0–15%).
To further define the cell surface phenotype of these subsets, CD8+ T cells were purified from peripheral blood and analyzed in triple color immunofluorescence. As expected on the basis of the observation that these were the only cells present in cord blood, CD45RA+CD27+ cells showed characteristics of unprimed cells since they were CD62L+ and had low CD49d, CD49e, CD29, CD11a/ CD18, and CD58 expression. The majority of cells were CD28+ and did not express CD45R0 (Table 1 and data not shown). The CD45RA− population had features of antigen-primed cells. CD62L was downregulated, and CD49e, CD29, and CD58 were upregulated when compared to the unprimed population. The main difference between CD27+ and CD27− cells within the CD45RA− population was the expression of CD28. Although the majority of CD27+ cells expressed the antigen, only half of the CD27− cells were CD28+.
Although CD45R0 was absent from CD45RA+CD27− cells, these T cells had a number of features compatible with a primed phenotype. CD62L was found to be downregulated, whereas CD49d, CD49e, CD29, CD11a/CD18, and CD58 were highly expressed. In comparison to the CD45RA− population, expression levels of CD49d and CD11a/CD18 were even higher on CD45RA+CD27− cells. Remarkably, CD11b and CD57 were present on >50% of the cells and the majority did not express CD28.
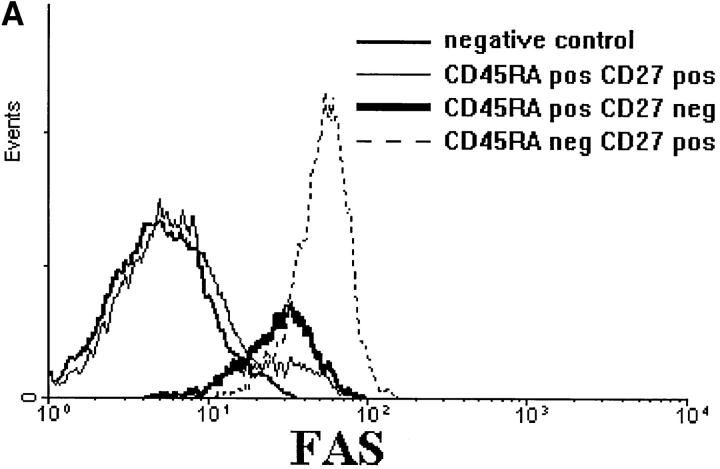
Fas (CD95) was not expressed on CD8+CD45RA+ CD27+ lymphocytes but could be detected on the majority of CD45RA+CD27− T cells. It should be noted that the antigen density on the latter population was markedly lower than on CD8+CD45RA− T cells (Table 1 and Fig. 2 A). Interestingly, Fas ligand mRNA was not present in CD8+CD45RA+CD27+ T cells but was strongly expressed in CD8+CD45RA+CD27− lymphocytes (Fig. 2 B, lanes B and C). In contrast, only trace amounts of Fas ligand mRNA were detected in CD8+CD45RA−CD27+ cells (Fig. 2 B, lane D).
Figure 2.
(A) CD8+ T cell subsets differ in Fas expression. (B) Fas ligand (FasL) and HPRT (hypoxanthine-guanine phosphoribosyltransferase) mRNA expression in sorted CD8+ T cell subsets as assessed by RT-PCR. Lane A, CD8 total; lane B, CD8+ CD45RA+CD27+; lane C, CD8+ CD45RA+CD27−; lane D, CD8+ CD45RA−CD27+.
Finally, it should be noted that a CD8+CD27+ population expressing low levels of CD45RA can be found in most donors (Fig. 1). Analogous to similar cells within the CD4+ T cell compartment (34), these cells coexpress CD45R0 and were found to have an intermediate membrane phenotype between CD45RA+CD27+ and CD45RA− CD27+ T cells (data not shown).
Cytokine Production Capacity of CD8+ T Cell Subsets.
In contrast to naive T cells, which primarily secrete IL-2, the ability to produce a variety of cytokines is a typical feature of primed T cells (35). Cytokine production capacity of CD8+ T cell subsets was measured after stimulation for 4 h with PMA and ionomycin at a single cell level (Fig. 3 and Table 2). CD8+CD45RA+CD27+ cells paralleled naive CD4+ cells in that IL-2 was the main cytokine produced by this subset. In agreement with what would be expected from primed cells, CD8+CD45RA− cells were able to secrete IL-2, IL-4, IFN-γ, and TNF-α. IL-4+ cells were exclusively found within this population with both CD27+ and CD27− cells contributing to the total IL-4 secretion. Also with respect to cytokine expression, the CD45RA+ CD27− subset showed characteristics of antigen-experienced cells, in that a high percentage of IFN-γ and TNF-α producers could be detected. Remarkably, in contrast to CD45RA− cells, neither IL-2 nor IL-4 production was found. Since IL-2 production in CD45RA+ cells has been described to reach its maximum later than in CD45RA− cells (36), we also analyzed 8-h stimulated cells. Indeed, the frequency of IL-2 producers increased in the CD45RA+ CD27+ subset. However, also at this time point, CD45RA+ CD27− cells did not produce any IL-2. From Fig. 3 it should be noted that, with respect to the cytokine secretion pattern, cells with a CD27dull expression behave as CD27− T cells in this assay. Furthermore, expression of surface markers on these cells was also comparable to CD27− cells (data not shown).
Figure 3.
Intracellular measurement of cytokines in CD8+ T cells. Purified CD8+ cells were stimulated for 4 h (IFN-γ and IL-4) or 8 h (IL-2) with PMA and ionomycin in the presence of the protein secretion inhibitor monensin. After surface staining with CD45 and CD27 mAbs, cells were fixated and permeabilized and intracellular accumulated cytokines were detected with specific mAbs. The histograms show the cytokine staining. Dotted lines indicate the negative controls; the positive cell fraction is painted black; and the dot-plots show the distribution of positive cells (black) among the total cell population (gray).
Table 2.
Intracellular Measurement of Cytokine Production in CD8+ T Cell Subsets
| Cells | IL-2* | IL-2‡ | IFN-γ≳ | TNF-α≳ | IL-4* | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total CD8+ | 17 ± 2 | 16 | 51 ± 15 | 48 ± 14 | 3 ± 1 | |||||
| CD45RA+CD27+ | 9 ± 4 | 27 | 6 ± 2 | 12 ± 4 | <1 | |||||
| CD45RA+CD27− | 1 ± 1 | <1 | 77 ± 16 | 69 ± 10 | <1 | |||||
| CD45RA−CD27+ | 43 ± 17 | 31 | 76 ± 9 | 78 ± 8 | 6 ± 4 | |||||
| CD45RA−CD27− | 33 ± 14 | 16 | 76 ± 12 | 69 ± 6 | 9 ± 8 |
Cells were stimulated for 4 h, mean percentage of positive cells ± SD, n = 6.
Cells were stimulated for 8 h, mean percentage of positive cells, n = 2.
The results obtained by intracellular cytokine staining were confirmed by measuring the cytokine content of the supernatant after 4 d of culture in the presence of PMA and ionomycin (Table 3). Again, CD45RA+CD27+ T cells mainly produced IL-2. CD45RA− cells secreted all measured cytokines, whereas CD45RA+CD27− cells were only capable of IFN-γ and TNF-α production.
Table 3.
Cytokine Secretion by CD8+ T Cell Subsets
| Cells* | IL-2 | IFN-γ | TNF-α | IL-4 | ||||
|---|---|---|---|---|---|---|---|---|
| U/ml | pg/ml | pg/ml | pg/ml | |||||
| CD45RA+CD27+ | 100 | 2,860 | 370 | <1 | ||||
| CD45RA+CD27− | <10 | 9,310 | 2,220 | <1 | ||||
| CD45RA−CD27+ | 100 | 11,940 | 1,660 | 10 |
Sorted cells (2.5 × 104) were stimulated with PMA (100 ng/ml) and ionomycin (1 μM) for 4 d. Cytokine content of the supernatants was tested with a bioassay for IL-2 and ELISAs for IFN-γ, TNF-α, and IL-4.
To test if the distinction in cytokine production profiles between the two primed CD8+ T cell subpopulations was reflected in their proliferation capacities, three fractions were sorted from purified CD8+ T cells, i.e., CD45RA+CD27+, CD45RA+CD27−, and CD45RA−CD27+ cells, and stimulated with a combination of CD2 mAbs supplemented with various cytokines (Fig. 4). The CD45RA−CD27− population could not be analyzed because of the low number of these cells in most donors (see above). Culture of the sorted cells with the indicated cytokines alone did not result in proliferation (data not shown). In accordance with a previous report (18) on stringent activation requirements of unprimed CD8+ T cells, CD45RA+CD27+ cells were not able to proliferate in response to CD2 mAbs alone, but addition of IL-2, -15, -4, and, to a lesser extent, -12 resulted in a vigorous proliferative response, whereas IL-6 and -10 and IFN-γ had no effect on proliferation. In contrast, CD2 mAbs alone were sufficient to induce proliferation of CD45RA−CD27+ cells. IL-2, -15, and -12 further increased proliferation, but IL-4, -6, and -10, and IFN-γ only marginally influenced the CD2-induced response. Proliferation of CD45RA+CD27− cells was only observed in the presence of either IL-2 or -15. However, the magnitude of the response was lower than that of the other two populations.
Figure 4.
Proliferative capacity of CD8+ T cell subsets. Purified CD8+ T cells were sorted into the indicated subsets and stimulated with a combination of three CD2 mAbs in the presence of different cytokines. Proliferation was measured on day 4 by adding [3H]thymidine during the last 4 h of the culture. Results from one out of three experiments are shown.
CD45RA+CD27−, but not CD45RA−CD27+ T Cells, Have Cytolytic Activity without Previous In Vitro Stimulation.
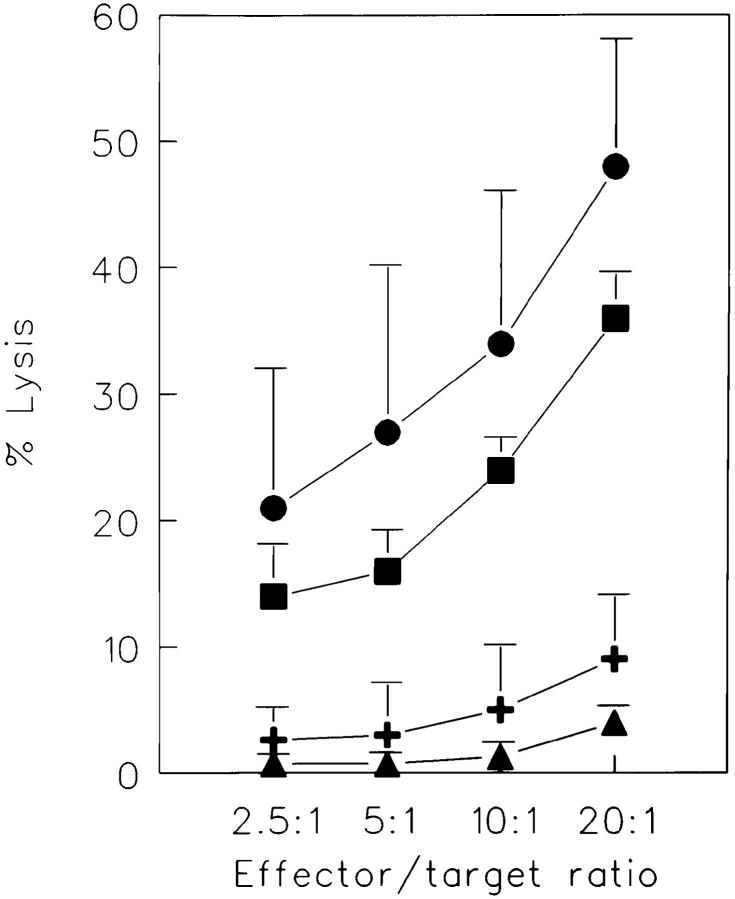
Our data thus far have shown that CD45RA+ CD27− cells possess a number of phenotypic characteristics that have been associated with murine (9) and human (11, 15, 16, 37) cytolytic T cells and that these cells secrete IFN-γ and TNF-α, two lymphokines known to play a role in controlling virus infections (38). Therefore, we addressed the question of whether these cells behave as CTLs ex vivo. Purified CD8+ cells were sorted into the distinct subsets and their cytotoxic capacity was tested in a CD3 mAb–mediated redirected cytotoxicity assay (Fig. 5). As expected, unprimed CD45RA+CD27+ cells were not able to lyse the target cells. CD45RA−CD27+ cells had a low but detectable cytolytic activity at relatively high effector/target ratios. Strikingly, however, the CD45RA+CD27− population had potent cytolytic activity.
Figure 5.
Cytotoxic capacities of CD8+ T cell subsets. Purified CD8+ T cells were sorted into the indicated subsets and cytotoxicity was directly analyzed against P815 target cells in the presence of CD3 mAb in a 4-h 51Cr-release assay. All effector populations used were unable to lyse P815 cells in the absence of CD3 mAb (lysis <3%), thus excluding NK cell–like activity in this system (data not shown). Results are the mean ± SD of three independent experiments. The distribution of cells in the different subsets was as follows: CD45RA+CD27+, 18–37%; CD45RA+ CD27−, 7–23%; and CD45RA− CD27+, 30–38%. (CD8+ T cells, squares; CD8+CD45RA+CD27+, triangles; CD8+CD45RA−CD27+; plus; CD8+ CD45RA+CD27−, circles.)
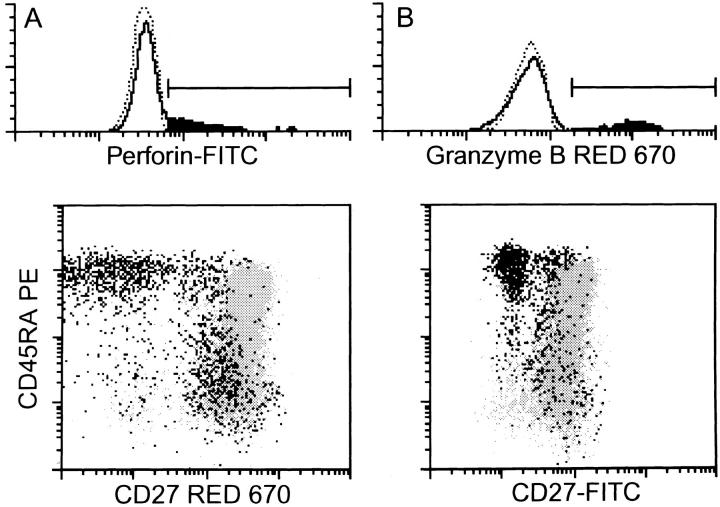
Fas–Fas ligand interaction is an important mechanism used by CTLs for cell-mediated cytotoxicity (39, 40). Next to this, CTL effectors can kill target cells by releasing a pore-forming protein (perforin) and serine proteases (granzymes), which are stored in intracellular granules, into the vicinity of target cell membranes (41, 42). We analyzed freshly isolated CD8+ cells for the presence of perforin and granzyme B. In accordance with the cytolytic capacities of the different subsets, nearly all cells of the CD45RA+CD27− population contained perforin and granzyme B. Unprimed CD45RA+CD27+ cells did not contain these enzymes, whereas low staining could be found within the CD45RA− population (Fig. 6 and Table 4). As already seen in the analysis of cytokine production, cells with dull CD27 expression showed a similar granzyme B and perforin expression pattern to CD27− cells.
Figure 6.
Expression of mediators of cytotoxicity in CD8+ T cell subsets. After surface staining of freshly isolated CD8+ cells with CD45RA and CD27 mAbs, cells were fixated and permeabilized and intracellularly present perforin and granzyme B were detected with specific mAbs. The histograms show the staining for the intracellular proteins. Dotted lines indicate the negative controls. The positive cell fraction is painted black. The dot-plots show the distribution of positive cells (black) among the total cell population (gray). Note that the different dot-plot staining is due to the use of either biotinylated CD27 mAb in combination with red 670 or FITC-labeled CD27 mAb.
Table 4.
Expression of Cytotoxic Mediators by CD8+ T Cell Subsets
| Cells | Perforin | Granzyme B | ||
|---|---|---|---|---|
| Total CD8+ | 24 ± 7* | 27 ± 13 | ||
| CD45RA+CD27+ | <1 | 3 ± 1 | ||
| CD45RA+CD27− | 91 ± 6 | 89 ± 9 | ||
| CD45RA−CD27+ | 16 ± 11 | 16 ± 6 | ||
| CD45RA−CD27− | 54 ± 16 | 36 ± 32 |
Percentage of positive cells ± SD (n = 4).
The CD45RA−CD27+ Subset Contains Antigen-specific Precursor CTL.
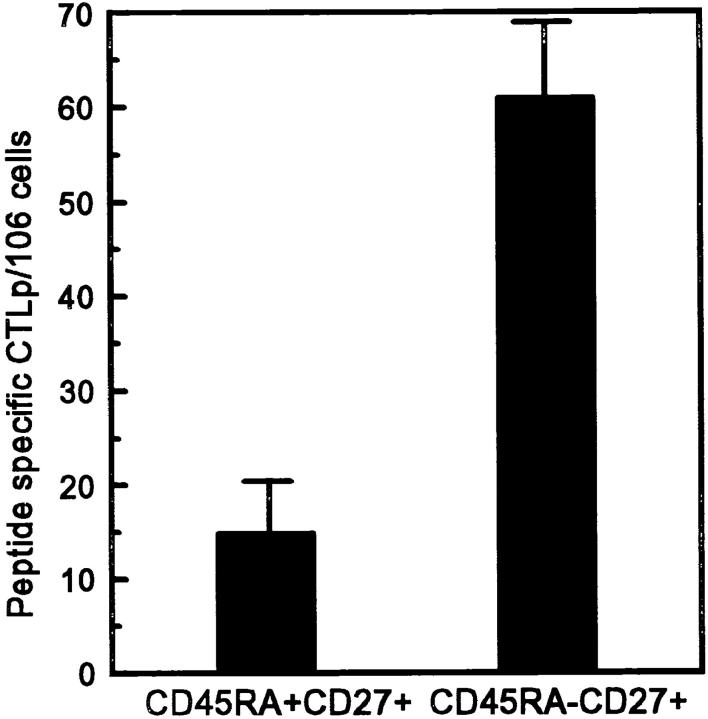
CTL effectors exert direct ex vivo cytolytic activity, whereas memory cells need restimulation with antigen to acquire cytotoxic function. Since CD45RA− CD27+ cells, which resemble memory T cells with respect to membrane phenotype and cytokine secretion ability, showed no direct ex vivo cytolytic activity, we studied whether cytotoxic effectors could be generated from this population. To this purpose, PBMCs from an HIV-1 seropositive individual were sorted into CD8+CD45RA+CD27+ and CD8+ CD45RA−CD27+ subsets. CTLs from this individual had been previously found to recognize an HLA-B57 restricted epitope of the HIV-1LAI RT aa 244-252 (Klein, M.R., unpublished results). Neither CD45RA+CD27+ nor CD45RA− CD27+ cells showed direct ex vivo cytotoxicity as measured in a redirected kill assay as described above. To determine the frequency of peptide-specific CTLp within the distinct subsets, sorted cells were seeded in serial dilutions and stimulated with autologous B-LCL infected with recombinant vaccinia virus expressing the HIV-1LAI RT gene. On day 15, effector cells were tested for cytotoxicity on autologous B-LCL pulsed with the HIV-1LAI reverse transcriptase–derived peptide aa 244-252 (Fig. 7). Indeed, cytotoxic effector cells could be generated from the CD45RA− CD27+ subset, which had an enhanced frequency of peptide-specific CTLps (61/106 cells) as compared to the CD45RA+CD27+ subset (15/106 cells).
Figure 7.
Using standard limiting dilution analysis, the CTLp frequencies for the HIV-1LAI RT-derived peptide aa 244-252 (IVLPEKDSW) were quantified in CD8+ T cell subsets from an HIV-infected individual. Peptide-specific CTLp frequencies were calculated by subtracting CTLp frequencies determined with targets without peptide from CTLp frequencies determined with targets pulsed with 5 μg/ml of RTLAI peptide aa 244-252. Mean + SD of three separate experiments.
CD45RA−CD27+ but not CD45RA+CD27− Support Ig Secretion In Vitro.
Apart from their cytolytic capacities, CD8+ cells have been shown to exert suppressor activity as well as helper activity for Ig synthesis (43). Helper capacity of CD8+ T cell subsets was tested in a CD3 mAb–driven T cell–dependent B cell differentiation assay (44). When total CD4+ and CD8+ cells were compared, helper activity was preferentially found in the CD4+ fraction (Fig. 8 A). However, when CD8+ cells were further separated into the indicated subsets, it was found that the CD45RA−CD27+ cells were also able to support B cell differentiation, whereas both the CD45RA+CD27+ and the CD45RA+CD27− cells showed no helper activity.
Figure 8.
Helper and suppressor capacities of CD8+ T cell subsets. B cells were cocultured with the indicated T cell subsets in CD3-coated wells for 14 d. Ig content of the supernatant was measured with specific ELISAs. (A) Cells of the different T cell populations were cultured with B cells alone to determine their helper capacities. (B) Cells of the distinct CD8+ subsets were added to the coculture of autologous B and CD4+ T cells to measure their suppressor activity. One out of two comparable experiments is shown. The distribution of cells in the different subsets was as follows: CD45RA+CD27+, 50%; CD45RA+CD27−, 5%; and CD45RA− CD27+, 35%.
To test suppressor capacity, sorted CD8+ subsets were added to the autologous coculture of CD4+ and B cells (Fig. 8 B). In accordance with a previous study (43), total CD8+ T cells markedly reduced IgM and IgG synthesis in this system. When the CD8+ cells were further separated into the different subsets, suppressor activity was primarily found in the CD45RA+CD27+ fraction. This effect was not due to enhanced IL-4 consumption of CD8+CD45RA+ CD27+ cells, as addition of exogenous IL-4 did not overcome suppression of the Ig synthesis.
In summary, within the CD8+ compartment, helper and suppressor activity could be ascribed to CD45RA−CD27+ and CD45RA+CD27+ T cells, respectively, whereas the CD45RA+CD27− does not appear to directly influence B cell differentiation.
Discussion
In this study, we define discrete subsets (summarized in Table 5) within the CD8+ T cell population based on expression patterns of CD45RA and CD27. First, similar to the CD4+ T cell subpopulation, CD45RA+CD27+ T cells are the only CD8+ lymphocytes present in cord blood, and the analysis of phenotype and function showed that this population primarily contains unprimed (naive) cells. CD8+CD45RA−CD27+ lymphocytes are T cells that have several features of antigen-experienced cells. In comparison to phenotypic data obtained in the murine system, these CD8+ cells most closely resemble memory-type cells, as they express intermediate levels of CD11a/CD18 and CD49d, but have no membrane expression of CD11b (9). Indeed, this CD8+CD45RA−CD27+ population from an HIV-1–infected individual contained, compared to CD8+ CD45RA+CD27+ T cells, an enhanced frequency of CTLp specific for an HIV-1 RT-derived peptide. In line with this, a study by Merkenschlager and Beverley (17) documented an elevated precursor frequency to recall antigens within the CD8+CD45RA−CD45R0+ fraction. A second population that has features of past antigen stimulation has a CD8+CD45RA+CD27− phenotype. Not only with respect to cytolytic potential, but also regarding surface expression of high levels of CD11b and CD49d and absence of the lymph node homing receptor CD62L, these cells fulfill the criteria of CTL effector-type cells that have been defined in murine models. Further characterization of the CD8+CD45RA+CD27− population allowed us to determine the functional abilities of human CTL-type effector cells as they occur in vivo. The data show that (a) on a per cell basis the vast majority of effector cells are able to produce both IFN-γ and TNF-α, (b) these cells contain high amounts of perforin and granzyme B, (c) Fas ligand mRNA is abundantly expressed in freshly isolated effector cells, and (d), that these cells exert potent cytotoxic activity without previous in vitro stimulation. Finally, a fourth population constituted of CD45RA−CD27− T cells can be found in the CD8+ fraction of all donors. The frequency of these cells in healthy donors is very low (usually <4%) and therefore not accessible for assays that depend on vast quantities of purified cells. However, based on membrane phenotype, cytokine production pattern, and expression of perforin and granzyme B, it is suggested that this population may contain both memory- and effector-type cells.
Table 5.
Properties of CD8+ T Cell Subsets
| Naive | Memory | Effector | ||||
|---|---|---|---|---|---|---|
| Genes expressed | ||||||
| CD45RA | + | − | + | |||
| CD27 | + | + | − | |||
| CD28 | + | + | − | |||
| CD11a | ± | + | ++ | |||
| CD11b | − | − | ++ | |||
| CD49d | − | + | ++ | |||
| CD57 | − | − | ++ | |||
| CD95 | − | ++ | + | |||
| Fas ligand | − | ± | ++ | |||
| IL-2 | + | ++ | − | |||
| IL-4 | − | + | − | |||
| IFN-γ | − | ++ | ++ | |||
| TNF-α | − | ++ | ++ | |||
| Perforin | − | ± | ++ | |||
| Granzyme B | − | ± | ++ | |||
| Functions | ||||||
| Cytoxicity | − | ± | ++ | |||
| B cell differentiation | ↓ | ↑ | − |
Based on the finding that the majority of CD8+CD45RA+ CD27− T cells lack CD28 expression, it was not surprising to find that this subset shares a number of features that have been previously reported for CD28− lymphocytes (15, 45, 46). Azuma et al. (15) showed that CD8+CD28− T cells are able to lyse FcR-bearing targets cells in the presence of CD3 mAb. Moreover, Berthou et al. recently reported that most of the perforin containing CD8+ lymphocytes lack the CD28 molecule (47). The current data confirm and extend these observations by showing that not only perforin but also granzyme B and Fas ligand are expressed within this T cell subset, which is likely to contribute to the lysis of target cells (39, 40). It is of interest to note that two major costimulatory receptors for T cell growth and cytokine production, CD28 and CD27, are absent from this effector-type T cell. The absence of CD28 implies that this cell type is unable to interact productively (in terms of induction of clonal expansion) with professional antigen-presenting cells. This inability is underscored by the observation that, in marked contrast to both the CD45RA+CD27+ and CD45RA−CD27+ subsets, CD45RA+CD27− T cells do not proliferate in response to IL-12, one of the major T cell stimulatory cytokines secreted by activated antigen-presenting cells (48). Finally, absence of CD27 on these cells also precludes costimulatory interactions with other, activated lymphocytes (49). Analysis of the proliferation requirements showed that CD45RA+CD27− T cells responded poorly to most stimuli, which is in agreement with functional studies on the CD8+CD28− T cell subset (15, 46). However, CD45RA+CD27− T cells do respond to IL-2 and to a lesser extent to IL-15, which suggests that in vivo this subset might be influenced by Th-derived signals.
In mice, the expression of CD49d nicely separates naive (low), memory (intermediate), and effector (high expression) T cells, and a study by Christensen et al. has shown that CD49d plays a critical role in efficient homing of effector T cells during virus infections (13). Also, in humans CD49d expression correlates well with the distinct CD8+ subsets, CD45RA+CD27− T cells being the ones with the highest expression. Apart from CD49d, a β1 integrin interacting with vascular cellular adhesion molecule–1 on activated endothelium and also the high expression of the β2 integrins CD11a and CD11b (Table 1) will probably influence the homing properties of these effector-type cells, which may be different from those of naive or memory cells. Indeed, CD8+CD45RA+CD27− cells were conspicuously absent from tonsils, whereas CD45RA−CD27+ cells could readily be demonstrated in this lymphoid tissue (Hamann, D., unpublished data). In line with this observation, it was reported that CD11b+ cells are present in blood, liver, and spleen, but absent from tonsil, lymph node, and thymus (45).
Thus, it would seem that in response to antigen, two types of primed CD8+ populations may develop. First, an effector-type population with high levels of granzyme, perforin, and Fas ligand expression that exerts cytolytic activity, which has lost the ability for autocrine proliferation but has retained the ability to synthesize IFN-γ and TNF-α, two cytokines implicated in the neutralization of viruses. Second, a population of memory-type cells, which is not cytolytic without further activation, produces a wide range of cytokines and is able to provide helper activity for B cell differentiation. Kinetic studies in mice showed that effector T cells can be found relatively early after virus infection (8 d), and decline thereafter. In contrast, memory cells become prominent at a later point (>50 d), and it has been suggested that effector cells seed the memory pool (9, 50). However, the relation between these subpopulations in humans is unclear at this moment. In major contrast to data obtained in the mouse system, effector-type T cell numbers in humans are relatively stable over time (>18 months, Rep, M.H.G., unpublished data). In addition, it has been reported that CD8+CD45RA+CD11abright, CD11b+, and CD28− T cells increase with age (45, 51, 52) and, in agreement with this (see Table 5 for phenotypic similarities), we found a similar relationship between age and the percentage of CD8+CD45RA+CD27− T cells (Rep, M.H.G., unpublished data). The reason for the discrepancy in the kinetics of effector-type T cells between mouse and human is not clear at this moment but it is possible that persistence of antigen, cross-reactive epitopes (53), and/or stimulatory cytokines plays a role (54). It can be imagined that these factors will more readily influence the maintenance of a polyclonal effector T cell population in a natural environment than that of the monoclonal T cell population present in laboratory animals housed under pathogen-free conditions. It is questionable, based on the in vitro experiments, whether effector T cells that are present in the human circulation have a sizable clonogenic potential. Still, it can be envisaged that this type of cell contributes to the inhibition of pathogen spread early in secondary infection through immediate lysis of infected cells.
In agreement with recent data on CD28 subsets by Monteiro et al. (55), we found shortened telomers in CD8+ CD45RA+CD27− compared to CD8+CD45RA+CD27+ in some donors (Hamann, D., K.C. Wolthers, S.A. Otto, P.A. Baars, F.M. Miedema, and R.A.W. van Lier, manuscript submitted for publication). This finding shows that transition to the CD45RA+CD27− subset is accompanied by cellular division. Cell cycle analysis showed that, analogous to the CD4+ population (23, 56), the CD8+ cells that are in G2M can only be found in the minute fraction of CD8+CD45RAbrightCD45R0bright cells and not in CD45RA+CD45R0−CD27+ T cells (Baars, P.A., unpublished data). This finding implicates that CD45RA+CD27− cells could only develop via a CD45RA+CD45R0+ and a subsequent CD45RA−CD45R0+ stage. However, at this moment we cannot exclude that the transition from CD27+ to CD27− takes place in compartments, such as solid tissue, which are not readily available for cell-biological analysis. Finally, similar to what has been described for CD28− (57) and CD57+ (16, 37) T cells, we found a limited usage of Vβ elements by CD8+CD45RA+CD27− cells (Hamann, D., K.C. Wolthers, S.A. Otto, P.A. Baars, F.M. Miedema, and R.A.W. van Lier, manuscript submitted for publication). These findings, combined with the data on telomeric repeat length, infer that these circulating effector-type cells in humans are generated through antigen-dependent differentiation and proliferation processes.
Analysis of naive, memory, and CTL effector-type CD8+ T cells seems relevant not only in patients with chronic viral infections such as CMV, hepatitis B, or HIV-1, but also in patients actively immunized with tumor antigens and after having received an allotransplantation. Patients infected with CMV have been shown to have an increased percentage of CD57+ cells within the CD8+ population that contains virus-specific CTLs and can be oligoclonal (16). In HIV-1 infected individuals, an expanded CD28− population within the CD8+ T cell subset has been reported (46). This population has been found to be primarily CD57+, exerts potent cytolytic activity, and expresses perforin (46, 58). Interestingly, in contrast to healthy donors where CD27− and CD28− cells form highly overlapping subsets, a large population of CD45RA−CD28− cells that still express the CD27 antigen emerges during acute and persistent viral infections (Roos, M.T.L., manuscript submitted for publication), which may point to a sequential downregulation of costimulatory molecules during postthymic T cell differentiation.
Based on the data presented in this paper, we conclude that unidimensional analysis of CD8+ T cells, using either CD45RA, CD27, or CD28 mAbs, underestimates the complexity of this subset in humans. Therefore, we propose that combined staining with CD27 and CD45RA mAbs provides a tool not only for distinguishing unprimed CD45RA+CD27+ from primed CD45RA− cells, but also for following the dynamics and expansion of a CD45RA+ CD27− cytotoxic effector cell population in CD8+ T cells in different disease states.
Acknowledgments
We would like to thank our colleagues Susanne Lens, Kiki Tesselaar, Wiebo van der Graaff, Marijke Roos, Peter Schellekens, and Frank Miedema for stimulating discussions and their help in preparing this manuscript.
Footnotes
D. Hamann was supported by the Human Capital and Mobility Programme of the European Community, and P.A. Baars and M.H.G. Rep were supported by the Dutch Society for Support of Research on Multiple Sclerosis.
Abbreviations used in this paper: B-LCL, B lymphoblastoid cell lines; p, precursors; RT, reverse transcriptase.
References
- 1.Merkenschlager M, Terry L, Edwards R, Beverley PC. Limiting dilution analysis of proliferative responses in human lymphocyte populations defined by the monoclonal antibody UCHL1: implications for differential CD45 expression in T cell memory formation. Eur J Immunol. 1988;18:1653–1661. doi: 10.1002/eji.1830181102. [DOI] [PubMed] [Google Scholar]
- 2.Byrne JA, Butler JL, Cooper MD. Differential activation requirements for virgin and memory T cells. J Immunol. 1988;141:3249–3257. [PubMed] [Google Scholar]
- 3.Sanders ME, Makgoba MW, June CH, Young HA, Shaw S. Enhanced responsiveness of human memory T cells to CD2 and CD3 receptor-mediated activation. Eur J Immunol. 1989;19:803–808. doi: 10.1002/eji.1830190504. [DOI] [PubMed] [Google Scholar]
- 4.Picker LJ, Singh MK, Zdraveski Z, Treer JR, Waldrop SL, Bergstresser PR, Maino VC. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995;86:1408–1419. [PubMed] [Google Scholar]
- 5.Sanders ME, Makgoba MW, Sharrow SO, Stephany D, Springer TA, Young HA, Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL-1, CDw29, and Pgp-1) and have enhanced IFN-γ production. J Immunol. 1988;140:1401–1407. [PubMed] [Google Scholar]
- 6.Akbar AN, Terry L, Timms A, Beverley PC, Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988;140:2171–2178. [PubMed] [Google Scholar]
- 7.Michie CA, McLean A, Alcock C, Beverley PC. Life span of human lymphocyte subsets defined by CD45 isoforms. Nature (Lond) 1992;360:264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- 8.Bell EB, Sparshott SM. Interconversion of CD45R subsets of CD4 T cells in vivo. Nature (Lond) 1990;348:163–166. doi: 10.1038/348163a0. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann C, Brduscha-Riem K, Blaser C, Zinkernagel RM, Pircher H. Visualization, characterization, and turnover of CD8+memory T cells in virus-infected hosts. J Exp Med. 1996;183:1367–1375. doi: 10.1084/jem.183.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mobley JL, Dailey MO. Regulation of adhesion molecule expression by CD8 T cells in vivo. I. Differential regulation of gp90MEL-14 (LECAM-1), Pgp-1, LFA-1, and VLA-4 α during the differentiation of cytotoxic T lymphocytes induced by allografts. J Immunol. 1992;148:2348–2356. [PubMed] [Google Scholar]
- 11.McFarland HI, Nahill SR, Maciaszek JW, Welsh RM. CD11b (Mac-1): a marker for CD8+cytotoxic T cell activation and memory in virus infection. J Immunol. 1992;149:1326–1333. [PubMed] [Google Scholar]
- 12.Andersson EC, Christensen JP, Marker O, Thomsen AR. Changes in cell adhesion molecule expression on T cells associated with systemic virus infection. J Immunol. 1994;152:1237–1245. [PubMed] [Google Scholar]
- 13.Christensen JP, Andersson EC, Scheynius A, Marker O, Thomsen AR. α4 integrin directs virus-activated CD8+T cells to sites of infection. J Immunol. 1995;154:5293–5301. [PubMed] [Google Scholar]
- 14.Walker PR, Ohteki T, Lopez JA, MacDonald HR, Maryanski JL. Distinct phenotypes of antigen-selected CD8 T cells emerge at different stages of an in vivo immune response. J Immunol. 1995;155:3443–3452. [PubMed] [Google Scholar]
- 15.Azuma M, Phillips JH, Lanier LL. CD28−T lymphocytes. Antigenic and functional properties. J Immunol. 1993;150:1147–1159. [PubMed] [Google Scholar]
- 16.Wang ECY, Moss PA, Frodsham P, Lehner PJ, Bell JI, Borysiewicz LK. CD8highCD57+T lymphocytes in normal, healthy individuals are oligoclonal and respond to human cytomegalovirus. J Immunol. 1995;155:5046–5056. [PubMed] [Google Scholar]
- 17.Merkenschlager M, Beverley PC. Evidence for differential expression of CD45 isoforms by precursor for memory-dependent and independent cytotoxic responses: human CD8 memory CTLp selectively express CD45R0 (UCHL1) Int Immunol. 1989;1:450–459. doi: 10.1093/intimm/1.4.450. [DOI] [PubMed] [Google Scholar]
- 18.De Jong R, Brouwer M, Miedema F, van Lier RAW. Human CD8+ T lymphocytes can be divided into CD45RA+ and CD45R0+cells with different requirements for activation and differentiation. J Immunol. 1991;146:2088–2094. [PubMed] [Google Scholar]
- 19.Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995;95:2061–2066. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okumura M, Fujii Y, Inada K, Nakahara K, Matsuda H. Both CD45RA+ and CD45RA− subpopulations of CD8+T cells contain cells with high levels of lymphocyte function-associated antigen-1 expression, a phenotype of primed T cells. J Immunol. 1993;150:429–437. [PubMed] [Google Scholar]
- 21.Hintzen RQ, De Jong R, Lens SMA, Brouwer M, Baars P, van Lier RAW. Regulation of CD27 expression on subsets of mature T-lymphocytes. J Immunol. 1993;151:2426–2435. [PubMed] [Google Scholar]
- 22.De Jong R, Brouwer M, Hooibrink B, van der Pouw-Kraan T, Miedema F, van Lier RAW. The CD27− subset of peripheral blood memory CD4+lymphocytes contains functionally differentiated T lymphocytes that develop by persistent antigenic stimulation in vivo. Eur J Immunol. 1992;22:993–999. doi: 10.1002/eji.1830220418. [DOI] [PubMed] [Google Scholar]
- 23.Baars PA, Maurice MM, Rep M, Hooibrink B, van Lier RAW. Heterogeneity of the circulating human CD4+ T cell population: further evidence that the CD4+ CD45RA−CD27−T cell subset contains specialized primed T cells. J Immunol. 1995;154:17–25. [PubMed] [Google Scholar]
- 24.van der Pouw-Kraan T, van Kooten C, Rensink I, Aarden L. IL-4 production by human T cells. Differential regulation of IL-4 versus IL-2 production. Eur J Immunol. 1992;22:1237–1241. doi: 10.1002/eji.1830220519. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi T, Tanaka M, Inazawa J, Abe T, Suda T, Nagata S. Human Fas ligand: gene structure, chromosomal localization and species specificity. Int Immunol. 1994;6:1567–1572. doi: 10.1093/intimm/6.10.1567. [DOI] [PubMed] [Google Scholar]
- 26.Jolly DJ, Okayama H, Berg P, Esty AC, Filpula D, Bohlen P, Johson GG, Shivelly JE, Hunkapillar T, Friedman T. Isolation and characterization of a full-length expressible cDNA for human hypoxanthine phosphoribosyltransferase. Proc Natl Acad Sci USA. 1983;80:477–482. doi: 10.1073/pnas.80.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung T, Schauer U, Heusser C, Neuman C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 28.Hamann D, Hilkens CMU, Grogan JL, Lens SMA, Kapsenberg ML, Yazdanbakhsh M, van Lier RAW. CD30 expression does not discriminate between human Th- and Th-2 type T cells. J Immunol. 1996;156:1387–1391. [PubMed] [Google Scholar]
- 29.Gillis S, Ferm MM, Ou W, Smith KA. T-cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978;120:2027–2033. [PubMed] [Google Scholar]
- 30.De Jong R, Brouwer M, Rebel VI, Van Seventer GA, Miedema F, van Lier RAW. Generation of alloreactive cytolytic T lymphocytes by immobilized anti-CD3 monoclonal antibodies. Analysis of requirements for human cytolytic T-lymphocyte differentiation. Immunology. 1990;70:357–364. [PMC free article] [PubMed] [Google Scholar]
- 31.van Baalen CA, Klein MR, Geretti AM, Keet RIPM, Miedema F, Van Els CACM, Osterhaus ADME. Selective in vitro expansion of HLA class I–restricted HIV-1 Gag-specific CD8+T cells: cytotoxic T-lymphocyte epitopes and precursor frequencies. AIDS (Phila) 1993;7:781–786. [PubMed] [Google Scholar]
- 32.Klein MR, Holwerda AM, van Baalen CA, Bende RJ, Kerkhof SR, Osterhaus ADME, Keet IPM, Eeftinck JKM, Scattenkerk, Schuitemaker H, Miedema F. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strijbosch LWG, Does RJMM, Buurman WA. Computer-aided design and evaluation of limiting and serial dilution experiments. Int J Bio-Med Comput. 1988;23:279–290. doi: 10.1016/0020-7101(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 34.Hamann D, Baars PA, Hooibrink B, van Lier RAW. Heterogeneity of the human CD4+ T-cell population: two distinct CD4+T-cell subsets characterized by coexpression of CD45RA and CD45R0 isoforms. Blood. 1996;9:3513–3521. [PubMed] [Google Scholar]
- 35.Salmon M, Kitas GD, Bacon PA. Production of lymphokine mRNA by CD45R+ and CD45R− helper T cells from human peripheral blood and by human CD4+T cell clones. J Immunol. 1989;143:907–912. [PubMed] [Google Scholar]
- 36.Adamthwaite D, Cooley MA. CD8+T-cell subsets defined by expression of CD45 isoforms differ in their capacity to produce Il-2, IFN-γ and TNF-β. Immunology. 1994;81:253–260. [PMC free article] [PubMed] [Google Scholar]
- 37.Wang ECY, Taylor-Wiedeman J, Perera P, Fisher J, Borysiewicz LK. Subsets of CD8+, CD57+cells in normal, healthy individuals: correlation with human cytomegalovirus (HCMV) carrier status, phenotypic and functional analyses. Clin Exp Immunol. 1993;94:297–305. doi: 10.1111/j.1365-2249.1993.tb03447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 39.Kägi D, Vignaux F, Lederman B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T-cell mediated cytotoxicity. Science (Wash DC) 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 40.Lowin B, Hahne M, Mattman C, Tshopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature (Lond) 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 41.Smyth MJ, Trapani JA. Granzymes: exogenous proteinases that induce target cell apoptosis. Immunol Today. 1995;16:202–206. doi: 10.1016/0167-5699(95)80122-7. [DOI] [PubMed] [Google Scholar]
- 42.Liu CC, Walsh CM, Young JD. Perforin: structure and function. Immunol Today. 1995;16:194–201. doi: 10.1016/0167-5699(95)80121-9. [DOI] [PubMed] [Google Scholar]
- 43.Rieber EP, Rank G. CDw60: a marker for human CD8+T helper cells. J Exp Med. 1994;179:1385–1390. doi: 10.1084/jem.179.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Lier RAW, Brouwer M, Rebel VI, van Noesel CJ, Aarden LA. Immobilized anti-CD3 monoclonal antibodies induce accessory cell–independent lymphokine production, proliferation and helper activity in human T lymphocytes. Immunology. 1989;68:45–50. [PMC free article] [PubMed] [Google Scholar]
- 45.Hoshino T, Yamada A, Honda J, Imai Y, Nakao M, Inoue M, Sagawa K, Yokoyama MM, Oizumi K, Itoh K. Tissue-specific distribution and age-dependent increase of human CD11b+T cells. J Immunol. 1993;151:2237–2246. [PubMed] [Google Scholar]
- 46.Borthwick NJ, Bofill M, Gombert WM, Akbar AN, Medina E, Sagawa K, Lipman MC, Johnson MA, Janossy G. Lymphocyte activation in HIV-1 infection. II. Functional defects of CD28−T cells. AIDS (Phila) 1994;8:431–441. doi: 10.1097/00002030-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Berthou C, Legros-Maida S, Soulie A, Wargnier A, Guillet J, Rabian C, Gluckman E, Sasportes M. Cord blood T lymphocytes lack constitutive perforin expression in contrast to adult peripheral blood T lymphocytes. Blood. 1995;85:1540–1546. [PubMed] [Google Scholar]
- 48.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–4027. [PubMed] [Google Scholar]
- 49.Hintzen RQ, De Jong R, Lens SMA, van Lier RAW. CD27: marker and mediator of T-cell activation. Immunol Today. 1994;15:307–310. doi: 10.1016/0167-5699(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 50.Bruno L, Kirberg J, Von Boehmer H. On the cellular basis of immunological T cell memory. Immunity. 1995;2:37–43. doi: 10.1016/1074-7613(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 51.Okumura M, Fujii Y, Takeuchi Y, Inada K, Nakahara K, Matsuda H. Age-related accumulation of LFA-1high cells in a CD8+CD45RAhighT cell population. Eur J Immunol. 1993;23:1057–1063. doi: 10.1002/eji.1830230512. [DOI] [PubMed] [Google Scholar]
- 52.Fagnoni FF, Vescovini R, Mazzola M, Bologna G, Nigro E, Lavagetto G, Franceshi C, Passeri M, Sansoni P. Expansion of CD8+CD28−T cells in healthy ageing people, including centenarians. Immunology. 1996;88:501–507. doi: 10.1046/j.1365-2567.1996.d01-689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beverley PC. Is T-cell memory maintained by crossreactive stimulation? . Immunol Today. 1990;11:203–205. doi: 10.1016/0167-5699(90)90083-l. [DOI] [PubMed] [Google Scholar]
- 54.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo [see comments] Science (Wash DC) 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 55.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28− CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+counterparts. J Immunol. 1996;156:3587–3590. [PubMed] [Google Scholar]
- 56.Picker LJ, Treer JR, Ferguson-Darnell B, Collins PA, Buck D, Terstappen LWMM. Control of lymphocyte regulation in man. I. Differential regulation of the peripheral lymph node homing receptor L-selectin on T cells during the virgin to memory transition. J Immunol. 1993;150:1105–1121. [PubMed] [Google Scholar]
- 57.Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy.” . J Exp Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vingerhoets JH, Vanham GL, Kestens LL, Penne GG, Colebunders RL, Vandenbruaene MJ, Goeman J, Gigase PL, De Boer M, Ceuppens JL. Increased cytolytic T lymphocyte activity and decreased B7 responsiveness are associated with CD28 down-regulation on CD8+T cells from HIV-infected subjects. Clin Exp Immunol. 1995;100:425–433. doi: 10.1111/j.1365-2249.1995.tb03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]