Abstract
Cysteine proteases of the CED-3 and ICE family have been recently proposed as the ultimate executioners in several mammalian cell death pathways. Among them, the cysteine protease CPP32 has been shown to participate in programmed cell death (PCD), or apoptosis, affecting lymphoid cells in vitro. In the thymus, negative selection is a mechanism through which developing thymocytes expressing a TcR with high affinity for self peptide–MHC complexes are eliminated by PCD. In order to investigate the role of CPP32 in thymic apoptosis, isolated thymocytes were submitted to cell surface CD3 crosslinking by immobilized anti-CD3 mAb or to dexamethasone treatment. Although apoptosis occurred in the absence or after crosslinking with anti-CD3 mAb, specific activation of CPP32, as assessed by the extent of proteolytic cleavage of the p32 zymogen, was only detected in thymocytes cultured in the presence of the immobilized antibody or dexamethasone. This activation was a very early event during apoptosis as it occurred before the exposure of phosphatidyl serine to the upper side of the cell membrane. This was observed both in anti–CD3- and dexamethasone-induced apoptosis. Moreover, using mice transgenic for pigeon cytochrome C (PCC)-specific TcR, we were able to show that, after injection of PCC, the activation of CPP32 and cleavage of its substrate occurred in thymocytes obtained from mice expressing a permissive MHC haplotype for PCC presentation (H-2k). Moreover, PCC induced apoptosis was blocked by the caspase inhibitor zVAD. While spontaneous apoptosis was not accompanied by detectable levels of CPP32 processing, it was characterized by the proteolysis of poly(ADP-ribose) polymerase (PARP) and was blocked by the cysteine protease inhibitor, zVAD-CH2F. Taken together, these results support the concept that CPP32 is among the earliest effectors of the pathway leading to negative selection of autoreactive thymocytes. Our results also suggest the involvement of a distinct CPP32-like cysteine protease in spontaneous apoptosis of thymocytes.
The basic cellular machinery carrying out programmed cell death (PCD),1 or apoptosis, is thought to involve the active participation of endogenous cellular proteases that act as direct effectors as well as regulatory factors of apoptosis (1–3). Among these proteases, the cysteine protease CPP32 (YAMA, apopain or caspase 3) has recently been shown to be a key player in PCD of mammalian cells (4–6). CPP32 is a 32-kD protein that, like other cysteine proteases, is synthesized as an inactive precursor that undergoes proteolytic conversion to eliminate a 3-kD NH2-terminal propeptide and to generate the active heterodimer enzyme composed of 12-kD (p12) and 17-kD (p17) subunits (5). Among its known substrates are key enzymes involved in cellular repair and maintenance. They include poly(ADP-ribose) polymerase (PARP) (5, 6), the 70-kD unit of the U1-ribonucleoprotein (U1-70kD) (7), the catalytic subunit of DNA-dependent protein kinase (DNA-PK) (7) as well as sterol-regulatory element binding proteins (SREBP) (8), the GDP dissociation inhibitor (D4-GDI) for the Ras-related Rho family GTPases (9), and huntingtin, a cystoplasmic protein involved in the neurodegenerative Huntington's disease (10). CPP32 proteolytic activity occurs at specific (P4)Asp-X-X-Asp(P1) motives and cleaves the substrates between their functional domains (5–9).
PCD takes an integral part in the development and homeostasis of the immune system. The involvement of CPP32 in PCD of lymphoid cells has been documented. For example, Fas-mediated apoptosis, which is thought to be essential for silencing immune responses as well as for the elimination of autoreactive cells in periphery, involves the activation of CPP32 (11–14). Moreover, cell-mediated death delivered by antigen-specific CTL involves the proteolytic activation of CPP32 by granzyme B (15). All these studies, however, have been performed on transformed cell lines, hybridomas and in cell free systems. Evidence for the proteolytic activation of CPP32 in in vivo models of apoptosis is still lacking. In the thymus, apoptosis is known to be a crucial step of thymic differentiation. It affects immature CD4+CD8+ thymocytes expressing antigen receptors with potentially harmful reactivity for self (negative selection) (16–19) as well as cells lacking receptors capable of binding peptide–MHC complexes (death by neglect) (20). Whether the cysteine protease CPP32 participates in negative selection in the thymus is not known. In this report, we describe the specific involvement of CPP32 in this critical step of T cell development.
Materials and Methods
Mice.
4–6-wk-old inbred C57Bl/6 and B10.D2 female mice were obtained from Charles River (Saint Constant, Canada) and Jackson Laboratories (Bar Harbor, Maine), respectively. AD10 H-2k mice transgenic for pigeon cytochrome C–specific TcR have been described previously (21). AD10 H-2b congenics were generated by successive back-crosses with C57BL/6 mice.
Assay for Apoptosis.
Thymocytes (2 × 106 cells per well) were cultured in 24-well culture plates in complete RPMI-1640 medium (GIBCO BRL, Burlington, Canada) (0.5 ml per well) supplemented with 10 mM Hepes, 5 mM l-glutamine and 10% FCS. Cells were incubated alone, or in the presence of immobilized affinity-purified 145-2C11 mAb (hamster anti–mouse CD3ε; 10 μg per ml per well), or with dexamethasone (10-6 M from a 35-mM stock solution prepared in ethanol). After incubation at 37°C, cells (5 × 105) were harvested and the presence of apoptotic cells was detected by multiparameter flow cytometry with propidium iodide (PI) and FITC-conjugated annexin V (Nexins Research B.V., Hoeven, Netherlands) (22). Cells were labeled with annexin V-FITC according to manufacturer's instructions. Immediately before analysis, PI was added to single-cell suspensions (2 μg per ml final dilution). Cell fluorescence was analyzed on a FACSCAN® (Becton Dickinson, Mississauga, Canada). Acquisition of data was performed without gating on forward light scatter. A minimum of 104 events were acquired for each sample.
For protease inhibitor experiments, thymocytes (4 × 106 cells per ml) were cultured with or without immobilized anti-CD3 mAb or dexamethasone in the presence of the ICE-family tripeptide inhibitor benzyloxycarbonyl-valinyl-alaninyl-aspartyl(o-methyl)- fluoromethylketone (zVAD-CH2F) (100 μM; Enzyme Systems Products, Dublin, CA). Levels of apoptosis were measured as described above.
In experiments where apoptosis was triggered in vivo, AD10.H-2k and AD10.H-2b mice were challenged by a single intraperitoneal injection of pigeon cytochrome C (1 mg per mouse) (Sigma Chemicals, Mississauga, Canada) in HBSS. Mice were killed 16 or 24 h after injection and thymocytes were isolated and cultured as described above with or without zVAD-CH2F as described above.
Cell Sorting.
After incubation for 24 h in the presence of immobilized anti-CD3, thymocytes were labeled with annexin V-FITC as described above. Annexin V+ and annexin V− cells were sorted on a FACStar Plus® (Becton Dickinson). Purity of each sorted population was verified and was always >99.5%.
Immunolabeling.
Specific staining was performed with PE-conjugated rat anti–mouse CD4 mAb (GIBCO BRL) and with biotin-conjugated rat anti–mouse CD8 mAb (GIBCO BRL) revealed by streptavidin-TRIColor® (Caltag, San Francisco, CA). Optimal antibody dilutions were determined before use.
Production of Antiserum Specific for CPP32.
CPP32 cDNA, reverse-transcribed from whole RNA isolated from Jurkat cells, was amplified by polymerase chain reaction using sense and anti-sense oligonucleotides located on the coding sequence of CPP32 p20. The oligonucleotides were: GGGATCCATGGAGAACACTGAAAACTC (sense) and ATGAATTCCCTAGTCTGTCTCAATGCCACA (antisense). Amplified products were cloned in Blue Script PBS KS+ (Stratagene, La Jolla, CA) and sequenced. Soluble GST fusion protein was made using the pGEX 2Tk (Pharmacia, Baie d'Urfé, Canada). The protein was purified on Glutathione Sepharose 4B columns (Pharmacia) and cleaved with thrumbin. Rabbits were immunized intramuscularly and subcutaneously by five injections of purified protein (250 μg per injection at 4-wk intervals). First and second injections were made in CFA and IFA, respectively. Specificity of the antiserum was tested by Western blotting on Jurkat cells and mouse T cell hybridomas and compared with that of an anti-CPP32 mAb supplied by Transduction Laboratories (Mississauga, Canada). The anti-serum failed to show any signal when tested on ES cells homozygous for CPP32 null mutation (Hakem, R., personal communication). This confirmed that our anti-serum was specific to CPP32 and did not cross react with other murine caspases.
Western Blotting.
Cells were washed with PBS, sonicated for 20 s in sample buffer (62.5 mM Tris-HCl pH 6.8; 6 M urea; 10% glycerol; 2% SDS; 0.00125% bromophenol; 5% β-mercaptoethanol), and boiled for 3 min. Samples were resolved on SDS-PAGE (10% acrylamide for anti-PARP, 15% acrylamide for anti-CPP32) and transferred to nitrocellulose membrane (Hybond C Super; Amersham, Oakville, Canada). Blots were blocked for 1 h at room temperature in PBS containing 5% of nonfat dried milk. Membranes were then incubated overnight with rabbit anti-sera to human CPP32 (see above) or to mouse PARP (a generous gift from Dr. G. Poirier, Centre Hospitalier de l'Université Laval, Québec, Canada) in PBS with 5% of nonfat dried milk under gentle shaking. Membranes were washed three times in PBS containing urea (2 M) and 0.05% Tween and twice in PBS containing 0.05% Tween. For PARP antiserum, detection was achieved by incubating with goat anti–rabbit Ig antibodies conjugated to HRP (Jackson Laboratories) (1/2,500 in PBS with 0.05% Tween and 5% dried milk). Detection for CPP32 antiserum was done with HRP-conjugated protein A (Amersham) (1/1,000 in PBS containing 5% dried milk). After three washes in PBS containing 0.05% Tween, signals were revealed with the enhanced chemiluminescence ECL Western blotting kit (Amersham) and visualized by autoradiography.
Results
CPP32 Is Activated during Apoptosis Induced In Vitro by Anti-CD3 Crosslinking and by Dexamethasone.
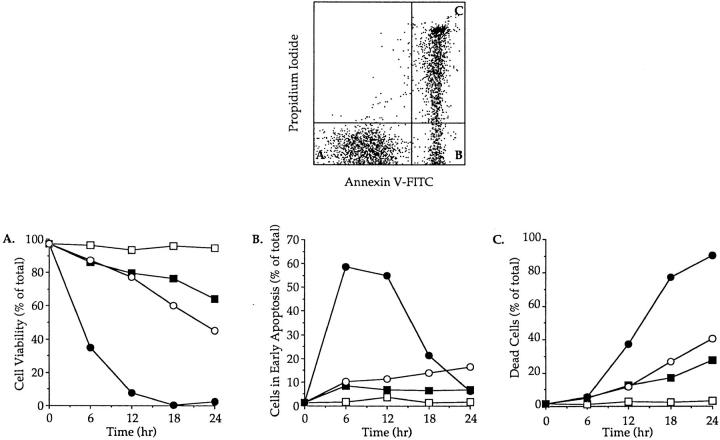
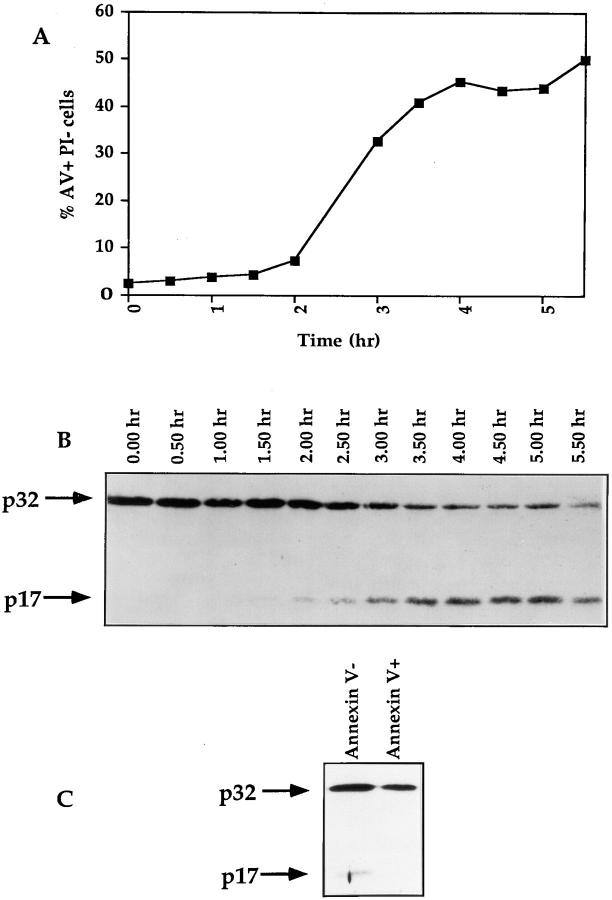
In four independent experiments, freshly isolated thymocytes were cultured in medium alone at 4 or 37°C in the presence of immobilized anti-CD3 mAb or dexamethasone. Cell viability was assessed by flow cytometry using PI and FITC-conjugated annexin V staining. Early apoptosis has been characterized by the loss of plasma membrane asymmetry and the subsequent exposure of phosphatidylserine at the cell surface (annexin V+) while cellular integrity is maintained as evidenced by exclusion of PI (22). The combined use of PI and FITC-annexin V therefore allows the simultaneous analysis of viable cells (PI− annexin V−), cells in early apoptosis (PI− annexin V+), and dead cells (PI+ annexin V+) (Fig. 1, top).
Figure 1.
Time course of apoptosis in isolated thymocytes cultured alone or in the presence of immobilized anti-CD3 mAbs or dexamethasone. The upper panel shows the flow cytometry measurement of annexin V binding (x axis) vs. propidium iodide uptake (y axis) on thymocytes cultured for 12 h in the presence of immobilized anti-CD3. Viable cells and cells in early apoptosis are annexin V− PI− (A) and annexin V+ PI− (B), respectively. Dead cells are annexin V+ PI+ (C). The lower panels display the evolution of cell viability (A), the percentage of cells in early apoptosis (B) and the mortality (C) in thymocyte populations incubated at 4°C (open square) or cultured at 37°C alone (closed squares), in the presence of immobilized anti-CD3 mAb (open circles) or with dexamethasone (closed circles). This experiment produced identical results in a total of four independent occasions.
Cells kept at 4°C showed little signs of apoptotic cell death even after 24 h of culture (Fig. 1, A–C). Crosslinking cell surface CD3 complexes decreased cell viability to ∼45% of viable cells after 24 h of culture (Fig. 1 A), while the rate of apoptosis, as determined by the percentage of cells in early apoptosis, increased progressively to reach ∼15% after 24 h (Fig. 1 B). As expected the number of dead cells increased proportionally after anti-CD3 treatment (Fig. 1 C). Differences in both magnitude and kinetics of apoptosis were observed between anti-CD3–mediated and spontaneous apoptosis. Indeed, and contrarily to what was observed with anti-CD3 treatment, the level of early apoptosis at 37°C increased during the first 6 h of culture to stabilize at 7–9% for the next 18 h (about twofold lower than with anti-CD3) (Fig. 1 B). Moreover in all our experiments the number of viable cells after 24 h was always significantly higher (>10–15%) in thymocyte cultures which had not been submitted to anti-CD3 treatment (Fig. 1 A). Conversely, the number of dead cells was reproducibly and significantly lower in the latter cultures than in anti-CD3–treated ones (Fig. 1 C). Taken together these results indicate that anti-CD3 crosslinking was more efficient than culturing at 37°C in inducing PCD and that this was due to a faster rate of apoptosis. Finally, dexamethasone induced massive cell death with a relative number of cells in early apoptosis peaking at 58% after 6 h (Fig. 1 B). This was accompanied by a quick drop in cell viability with a relative number of viable cells reaching <5% after 12 h of culture (Fig. 1 C).
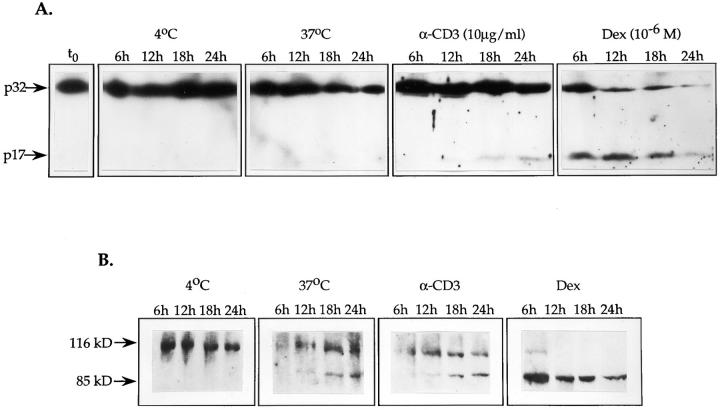
To determine whether the activation of CPP32 is a common feature of different apoptotic pathways in thymocytes, Western blots with anti-CPP32 rabbit serum were performed in two independent experiments on total cell lysates obtained from thymocyte cultures subjected to different apoptotic stimuli. As mentioned earlier, activation of CPP32 results from the proteolytic cleavage of the proenzyme form p32 into two subunits p17 and p12 (5). Results illustrated in Fig. 2 A show that anti-CD3 or dexamethasone treatments were able to activate CPP32. Indeed, the presence of the p17 subunit was noticed as early as after 12 h of culture with immobilized anti-CD3 and its abundance increased progressively thereafter. Dexamethasone induced a rapid and massive emergence of the p17 band as early as 6 h after the initiation of culture. A direct correlation between the appearance of the active form of CPP32 and the onset of apoptosis was observed for both anti-CD3 and dexamethasone treatments. In addition, the levels of activated CPP32 were directly proportional to the number of cells undergoing apoptosis (Fig. 1 and 2 A). Altogether these results show the activation of CPP32 as an integral component of these apoptotic pathways. In contrast, spontaneous cell death that occurred at 37°C did not induce detectable levels of CPP32 processing as assessed by the complete absence of the p17 subunit even upon 24 h of culture and overexposure of the autoradiogram (Fig. 2 A and data not shown). The lack of CPP32 processing during spontaneous apoptosis was not due to lower cell death than in cultures with anti-CD3. Indeed, even at equal levels of cell death for spontaneous apoptosis and for anti-CD3 treatment (after 24 and 18 h, respectively) (Fig. 1 C), CPP32 activation still distinguished anti-CD3–mediated apoptosis (Fig. 2 A).
Figure 2.
In isolated thymocytes, CPP32 is activated during apoptosis induced by crosslinking of CD3 or exposure to dexamethasone. Time course of CPP32 activation (A) and cleavage of PARP (B) in thymocytes cultured at 4°C, or at 37°C alone, with immobilized anti-CD3 mAb or with dexamethasone for the indicated time. Cell lysates were electrophoresed, and substrate cleavage was visualized by immunoblotting as described in Materials and Methods. The results presented in this figure are representative of two independent experiments.
Spontaneous Apoptosis Is Characterized by PARP Cleavage and Is Inhibited by zVAD-CH2F.
To determine whether CPP32-like activity could be detected in apoptosis stimulated by cell-surface crosslinking of CD3, by treatment with dexamethasone or during spontaneous apoptosis, cell lysates were blotted with rabbit antisera to mouse PARP. Cleavage of PARP by CPP32 and CPP32-like enzymes is known to yield products of ∼85 and 31 kD (5, 6). As shown in Fig. 2 B, both anti-CD3 and dexamethasone treatments induced the appearance of a specific 85-kD protein. This coincided with the activation of CPP32 (Fig. 2 B) and was observed as early as after 12 h of culture with anti-CD3 mAb and after 6 h with dexamethasone. Complete proteolysis of PARP could be seen after 12 h of treatment with dexamethasone. Surprisingly, although the activation of CPP32 was not detected in thymocytes cultured alone at 37°C (see above), the cleavage of PARP was observed after 12 h of culture under the same conditions (Fig. 2 B). Altogether, these observations imply that induction of cell death in thymocytes by anti-CD3 crosslinking or exposure to dexamethasone or during spontaneous apoptosis involves CPP32-like activity. They also suggest that spontaneous apoptosis of thymocytes activates (a) CPP32-like cysteine protease(s) that is (are) able to cleave PARP.
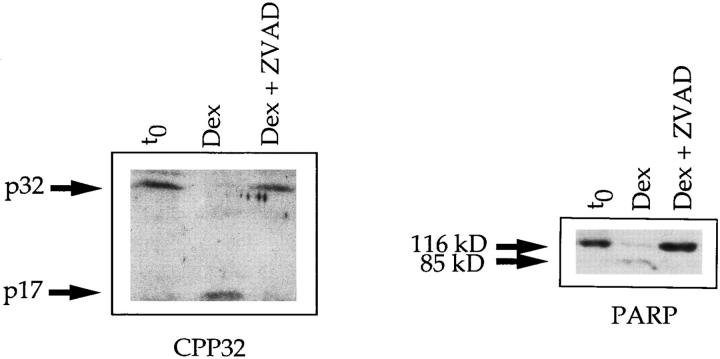
The tripeptide zVAD-CH2F is a well known inhibitor of cysteine proteases of ICE/CED-3 family, including CPP32 (reference 23 and Fig. 3). To confirm the involvement of CPP32-like activity in spontaneous apoptosis, the inhibition profile of the tripeptide on thymocyte cultures was determined. The results presented in Fig. 4 are representative of two independent experiments. Interestingly, zVAD-CH2F was a potent inhibitor of spontaneous apoptosis of thymocytes as well as apoptosis mediated either by anti-CD3 mAbs or dexamethasone. Altogether, these results confirm the direct involvement of CPP32 and CPP32-like proteases in thymocyte apoptosis.
Figure 3.
zVAD-CH2F inhibits the processing of CPP32 and the cleavage of PARP. CPP32 activation and cleavage of PARP in thymocytes cultured with dexamethasone for 6 h in the presence or absence of 100 μM zVAD-CH2F. Cell lysates were electrophoresed, and substrate cleavage was visualized by immunoblotting as described in Materials and Methods.
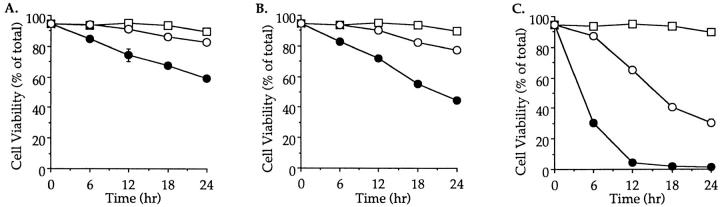
Figure 4.
Inhibition of thymocyte apoptosis by zVAD-CH2F. In vitro, apoptosis in thymocytes cultured for 18 h at 37°C alone (A), with immobilized anti-CD3 mAb (B) or with dexamethasone (C) is inhibited by zVAD-CH2F. Cell viability in the presence (open circles) or absence (closed circles) of zVAD-CH2F was determined as in Fig. 1. Cells incubated at 4°C were used as a negative control (open squares). These experiments were repeated on two separate occasions with identical results.
Activation of CPP32 Is a Very Early Event in Thymocyte Apoptosis.
As mentioned above, after 6 h of treatment with dexamethasone, usually 50–60% of thymocytes are in early apoptosis whereas 5–20% of cells are in late apoptosis. The massive activation of CPP32 at this time point (especially in the experiment presented in Fig. 1 C where only 6% of thymocytes are in late apoptosis), suggests that CPP32 activation occurs as soon as or before the exposure of the phosphatidylserine at the upper side of the cell membrane. The ability of zVAD-CH2F to inhibit annexin V staining and CPP32 activation in thymocytes (Fig. 4) further suggests that activation of cysteine proteases occurs before the loss of membrane asymmetry. To determine whether CPP32 activation is a very early event of thymocyte apoptosis, a time course of CPP32 activation was carried out on thymocytes stimulated with dexamethasone. As illustrated in Fig. 5 a, treatment with dexamethasone did not induce significant apoptosis, as evidenced by the absence of annexin V+ PI−, during the first 150 min. After this initial lag an abrupt increase in the relative number of cells in early apoptosis was noted. A plateau in percentage of annexin V+ PI− cells was reached at 4 h. Western blot analysis showed that activation of CPP32 (as indicated by the appearance of the p17 subunit) occurred 90 min after the addition of dexamethasone (Fig. 5 b). Moreover, this experiment confirmed that CPP32 activation is a very early event during dexamethasone-induced apoptosis since it takes place before the exposure of phosphatidylserine at the cell surface.
Figure 5.
The activation of CPP32 is a very early event of thymocyte apoptosis. (A and B) Thymocytes from C56BL/6 mice were isolated and incubated with dexamethasone. Samples were taken at 30-min intervals and analyzed for levels of apoptosis with annexin V-FITC and PI (A). Cell lysates were submitted to Western blotting analysis for the processing of CPP32 (B). (C) Thymocytes were cultured for 20 h in the presence of immobilized anti-CD3 mAb. Cells were then labeled with annexin V-FITC, and annexin V− and annexin V+ cells were sorted at 4°C with a FACStar® Plus. Sorted cells were lysed and extract contents were analyzed for processing of CPP32 by Western blotting.
Because induction of apoptosis with anti-CD3 is slower than that observed with dexamethasone (Fig. 1), the relative number of cells in apoptosis observed during this process results from the cumulative effect of spontaneous apoptosis that does not involve CPP32 (Fig. 2 a) and anti-CD3 induced apoptosis which induces the activation of CPP32 (Fig. 2 a). To circumvent this caveat we sorted annexin V+ and annexin V− cells and analyzed the activation of CPP32 in these populations. While p17 is present in viable cells (annexin V−) it is not detectable in apoptotic or dying cells (Fig. 5 c). It is likely that p17 is labile and degraded in apoptotic cells. Indeed, inhibition of the proteasome activates CPP32 suggesting that the proteasome tightly controls the turnover of CPP32 (24). Disappearance of P17 also appears to be a general phenomenon since it is also observed in dexamethasone-induced apoptosis. In Fig. 2 A, after 18 and 24 h of treatment of thymocytes with dexamethasone, we clearly show that the p17 does not accumulate in dying cells but rather progressively disappear. We also observed similar results in different cell types during anti-Fas- (Rhéaume et al., manuscript submitted for publication) or ceramide-induced apoptosis (Alam et al., manuscript in preparation). Altogether these results confirm that activation of CPP32 is a very early event during anti-CD3 induced apoptosis which occurs before the loss of membrane asymmetry.
Induction of Negative Selection in the Thymus Activates CPP32.
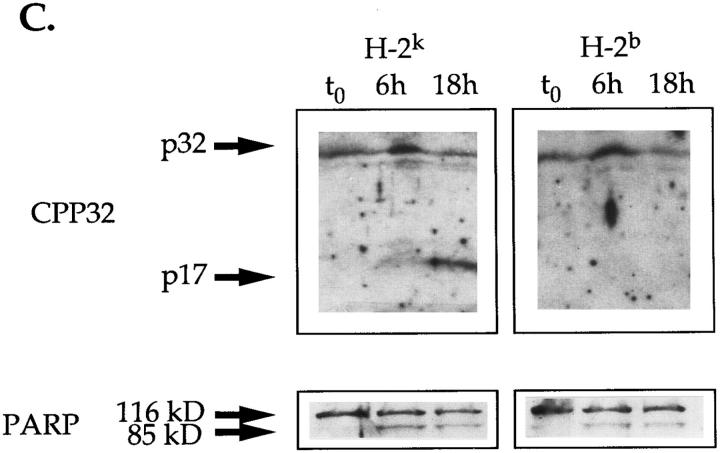
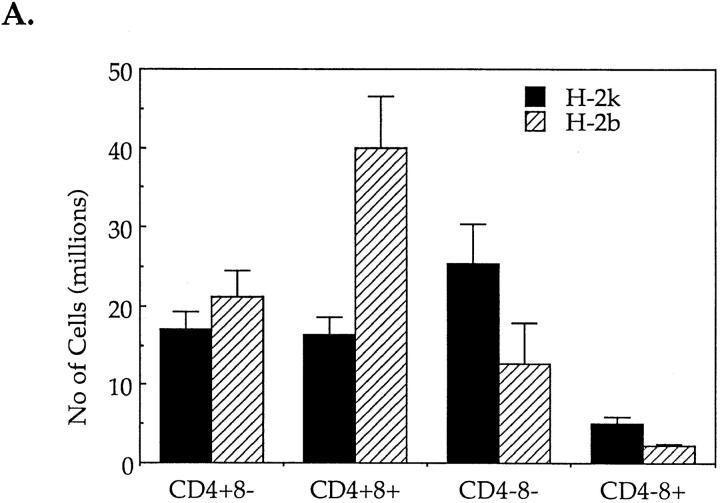
Exposure to CD3-specific antibodies, in vitro and in vivo, stimulates apoptosis predominantly in CD4+8+ thymocytes (26–30). Based on the above observations it was tempting to suggest that, in vivo, CPP32 could take an active part in TcR-induced negative selection of autoreactive CD4+8+ T cells in the thymus. In order to verify this hypothesis, mice expressing an IEk-restricted pigeon cytochrome C (PCC)-specific TcR transgene (AD10) were injected intraperitoneally with PCC. 24 h after PCC challenge, the expression of the early activation marker CD69 on isolated thymocytes was only observed in PCC-injected AD10 mice of the H-2k and not of the H-2b haplotype (data not shown). Moreover, the thymus cellularity in injected H-2k animals was greatly affected (Fig. 6 A). The absolute number of CD4+8+ cells was at least twice lower in H-2k mice than in H-2b mice. As the absolute number of CD4+8− cells did not differ significantly between the two types of animals, the drop of CD4+8+ cell number in H-2k mice was interpreted as the result of PCC-stimulated PCD rather than of PCC-induced maturation in CD4+8− cells. Altogether these observations indicated that H-2k but not H-2b thymocytes were stimulated in vivo by PCC and were undergoing negative selection. Interestingly, the number of CD4−8− cells was always two fold higher in H-2k– injected mice as compared with H-2b animals. It is known that, soon after expressing a functional preTcR, CD4−8− thymocytes undergo a step of proliferation leading to maturation into the CD4+8+ stage (31). As in these two types of mice immature CD4−8− thymocytes express the TcR transgene, it is possible that injection of PCC in H-2k mice stimulates these cells to proliferate.
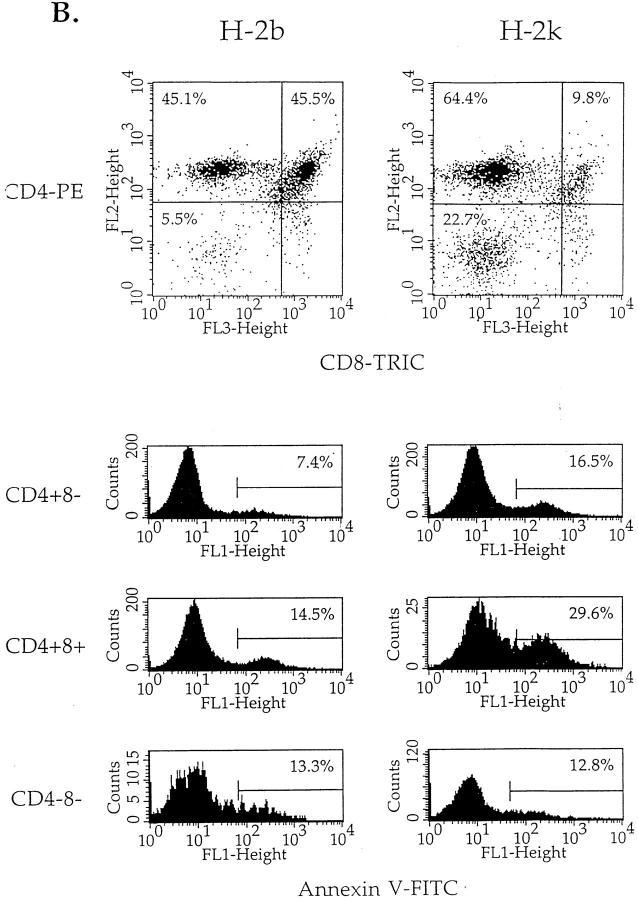
Figure 6.
Injection of PCC induce negative selection and activation of CPP32 in thymocytes of PCC-specific TcR transgenic mice of the H-2k, but not H-2b, haplotype. (A) Absolute number of cells in the different cell populations of thymuses isolated from H-2k and H-2b TcR transgenic mice 24 h after PCC injection. (B, top) Percentages of expression of CD4 and CD8 molecules by viable thymocytes (annexin V−) isolated from PCC-injected H-2k and H-2b TcR transgenic mice after 18 h of culture at 37°C. Thymuses from noninjected H-2k TcR transgenic mice contain 34.3 ± 2.5% of CD4+8− cells, 48.4 ± 5.7% of CD4+8+ cells and 15.0 ± 5.6% of CD4−8− cells. Thymuses from noninjected H-2b TcR transgenic mice contain 37.0 ± 1.8% of CD4+8− cells, 46.0 ± 4.1% of CD4+8+ cells and 14.8 ± 3.9% of CD4−8− cells. (B, bottom) Percentages of annexin V+ cells in CD4+8−, CD4+8+ and CD4−8− thymocytes isolated from PCC-injected H-2k and H-2b TcR transgenic mice after 18 h of culture at 37°C. (C) Proteolytic cleavage of CPP32 and PARP in thymocytes isolated from PCC-injected H-2k and H-2b TcR transgenic mice. After 6 or 18 h of culture at 37°C, cells were lysed and the lysates were electrophoresed. The substrate cleavage was visualized by immunoblotting as described in Materials and Methods.
Thymocytes isolated from injected mice were placed in culture medium and incubated at 37°C. 18 h later, cell aliquots were taken and analyzed by flow cytometry for their phenotype as well as for evidence of apoptosis. As seen in Fig. 6 B, among viable cells (annexin V−), the relative percentage of CD4+8+ cells was five times lower in populations isolated from H-2k mice than in those issued from H-2b mice, indicating that PCC injection had induced specific cell death in CD4+8+ H-2k thymocytes. The effect of PCC injection on thymocyte apoptosis was, however, not restricted to the CD4+8+ cell subset. Indeed, a twofold increase in the percentage of annexin V+ cells could be observed for CD4+8+ and CD4+8− cells isolated from H-2k mice, while CD4−8− cell populations from H-2k and H-2b animals included the same percentage of annexin V+ cells (Fig. 6 C). Higher levels of apoptosis in CD4+8+ and CD4+8− cells from PCC-injected H-2k mice was also noticed in DNA degradation assays using TUNEL® staining (data not shown). Similar results were obtained in two independent experiments including groups of three mice.
Cell lysates from cultures isolated from all H-2k or H-2b transgenic mice used in our experiments were immunoblotted for the detection of CPP32 activation and PARP cleavage. Freshly isolated thymocytes from mice injected with PCC did not contain cells expressing activated CPP32 nor were they annexin V+ (Fig. 6 C and data not shown). This situation may find its explanation in the capacity of macrophages in vivo to eliminate by phagocytosis cells in early apoptosis as soon as or before they expose phosphatidylserine residues at their surface (32). In contrast, upon in vitro incubation, cleavage of the 32-kD proenzyme occurred only in thymocytes isolated from H-2k transgenic animals previously injected with soluble PCC (Fig. 6 C). This was seen as early as after 6 h of culture in all PCC-injected H-2k transgenic animals. However, thymocytes from PCC-injected H-2b mice, although undergoing spontaneous apoptosis (Fig. 6 B), did not show any evidence for CPP32 processing at any time point. This confirmed our previous observation that spontaneous apoptosis of thymocytes did not involve proteolytic cleavage of CPP32. The lack of detection of activated CPP32 in H-2b thymocytes could not be attributed to a lower number of apoptotic cells than in H-2k cells. Indeed, at identical number of annexin V+ cells (after 6 h and 18 h of incubation at 37°C for H-2k and H-2b transgenic mice, respectively (data not shown), no processing of CPP32 could be seen in thymocytes isolated from H-2b transgenic mice while it was readily detectable in cells taken from H-2k animals (Fig. 6 C). In contrast, cleavage of PARP was seen in both PCC-mediated apoptosis of H-2k thymocytes and during spontaneous apoptosis of H-2b thymocytes (Fig. 6 C). Taken together these results support the hypothesis that TcR engagement during negative selection induced by PCC injection activates CPP32 in H-2k transgenic mice. They also associate the cleavage of PARP during spontaneous apoptosis of thymocytes from H-2b mice with the activation of a cysteine protease distinct from CPP32, but having similar substrate specificity.
The Caspase Inhibitor zVAD-CH2F Blocks Early Events in Apoptosis Leading to Negative Selection.
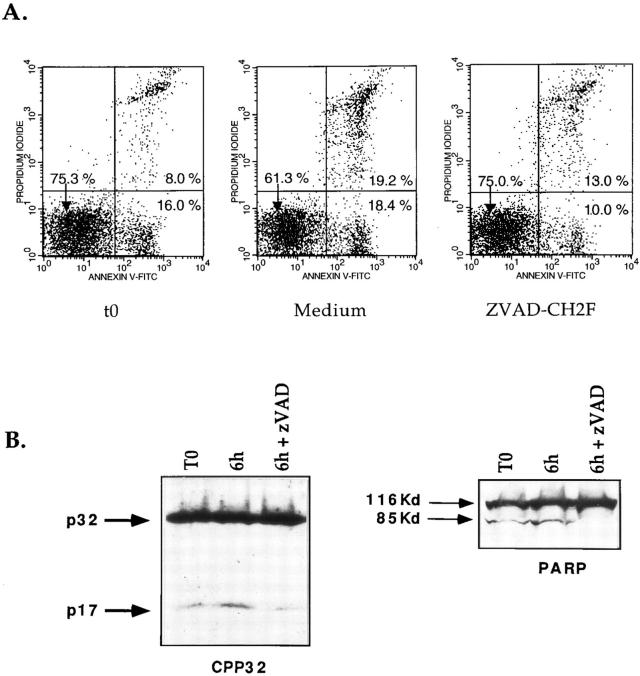
To confirm the involvement of CPP32 in negative selection we obtained apoptotic cells ex vivo after injection of PCC. Parameters were modified including the duration of PCC treatment (16 vs. 24 h) and the age of injected mice (3 vs. 5–6 wk). Under these conditions, we were able to detect substantial levels of apoptosis ex vivo in thymocytes of transgenic H-2k mice (13.0 ± 0.5% annexin V+ PI−, 12.0 ± 3.3% annexin V+ PI+ for H-2k mice) (Fig. 7 A). Control H-2b mice were injected and studied for levels of apoptotic cells using the same protocol (1.6 ± 0.9% annexin V+ PI−, 1.3 ± 0.5% annexin V+ PI+ for H-2b mice). In view of these results and together with the observation that CPP32 cleavage is a very early event of anti-CD3–induced apoptosis, experiments were carried out to determine if inhibition of cysteine protease activity could block death of cells already committed to apoptosis. Thymocytes isolated from PCC-injected H-2k transgenic mice were then incubated at 37°C in the presence or absence of zVAD-CH2F. As depicted in Fig. 7 A, zVAD was able to inhibit completely viable cells (annexin V− PI−) from undergoing apoptosis. Indeed the percentage of annexin V− PI− remained constant throughout the experiment (75.0%; Fig. 7 A) while the relative number of annexin V− PI− cells decreased in untreated cells (61.3%; Fig. 7 A). In contrast this inhibitor did not prevent the passage of cells from early apoptosis (annexin V+ PI−) to late apoptosis (annexin V+ PI+) (Fig. 7 A). This suggests that once thymocytes express phosphatidylserine at the upper face of the cell membrane, these are condemned to die. Moreover these results confirm that cysteine proteases play a major role during apoptosis before the loss of membrane asymmetry.
Figure 7.
zVAD-CH2F blocks early apoptosis and CPP32 activity in thymocytes undergoing negative selection. 3 wk-old H-2k transgenic mice were injected with PCC. 16 h later thymocytes were isolated and cultured with or without zVAD-CH2F. After 6 h, cells were compared for levels of apoptosis with annexin V-FITC and PI. Cell extracts were analyzed by Western blotting for processing of CPP32 and cleavage of PARP.
Analysis of CPP32 and PARP confirmed that CPP32 is activated during negative selection in vivo as we were able to detect the p17 of CPP32 and the cleaved 85-K d form of PARP in ex vivo isolated thymocytes from PCC injected H-2k mice (Fig. 7 B). Incubation of the cells at 37°C increased the processing of CPP32 and the cleavage of PARP during the first 6 h. zVAD not only inhibited the processing of cpp32 but it also decreased the intensity of the p17. Moreover the complete absence of the 85Kd fragment of PARP clearly confirm that zVAD-CH2F inhibits CPP32 activation. This phenomenon was observed in three different animals, confirming that processed forms of these proteins were subjected to further proteolysis.
Discussion
In the present paper the activation of the cysteine protease CPP32 is shown to be one of the earlier and initiating events in thymocytes undergoing apoptosis which results from the crosslinking of cell surface CD3/TcR complexes. Indeed, we observed that the processing of CPP32 occurs before the loss of membrane asymmetry in dexamethasone- and anti-CD3–mediated apoptosis. The ability of caspase inhibitors to block the loss of membrane asymmetry, clearly indicates that caspases are part of the enzymatic machinery which is upstream of cell surface–related events. Moreover, CPP32 activation and cell death are also observed in thymocytes of PCC-specific TcR transgenic mice previously injected with PCC. These results indicate that CPP32 is involved in the molecular pathway leading to the clonal deletion of autoreactive cells in the thymus. By contrast, during apoptosis occurring spontaneously in cultures of thymocytes at 37°C we were unable to detect processing of CPP32. However, apoptosis at 37°C as well as cell death induced by anti-CD3/TcR ligation were both accompanied by cleavage of PARP and were both sensitive to the ICE protease family inhibitor, zVAD-CH2F. This would suggest the involvement of another CPP32-like cysteine protease in spontaneous apoptosis of thymocytes.
Our study is the first to show direct activation of the cysteine protease, CPP32, in thymocytes during negative selection. Recently, it has been reported that spontaneous apoptosis and anti-CD3-mediated apoptosis of isolated thymocytes are both sensitive to inhibitors specific to CPP32-like proteases but not to ICE (33). Moreover, ICE-deficient thymocytes have been shown to undergo normal negative selection in the thymus (34). These observations would suggest that only CPP32, or CPP32-like enzymes, but not ICE-like proteases, are key components of the molecular processes leading to negative selection of thymocytes. Recently, activation of an unidentified cysteine protease during the process of negative selection has been reported (35). Finally with the knowledge that DNA fragmentation is the hallmark of apoptosis, the recent observation that CPP32 activate the DNA fragmentation factor demonstrate the importance of this caspase in the executioner phase of programmed cell death (36).
Several CPP32-like proteases, such as caspase 6 (Mch2) (36), caspase 7 (Mch3, ICE-LAP3, CMH-1) (38–40), have been identified. Whether activation of these proteases constitutes a component of the molecular pathway leading to thymic negative selection remain to be determined. This participation could take place either by an activation cascade of different proteases, as proposed by others using cell-free system (12), or by a redundant action of different proteases. The latter proposition finds its substance in the recent observation made by Flavell and coworkers (41) that thymocytes isolated from CPP32-deficient mice would retain normal susceptibility to apoptosis induced by combined anti-CD3 and anti-CD28 mAb treatments. This would imply that CPP32 is not the only cysteine protease involved in negative selection of thymocytes. However, whether in vitro combined anti-CD3 and anti-CD28 crosslinkings induce the same molecular events as those involved in negative selection of thymocytes in vivo can be questioned. This is particularly relevant when one considers that negative selection in CD28-deficient mice appears to be normal (42). In this context and in line with the observation presented in the present study, it would be interesting to know whether CPP32-deficient thymocytes display normal sensitivity to negative selection when challenged with specific antigen or endogenous superantigen in vivo. The use of TcR-transgenic CPP32-deficient animals should provide a direct answer to this important question.
We also observed that CPP32 processing occurs during apoptosis induced by dexamethasone. Glucocorticoids are known to affect thymocyte development in several ways. On one hand they stimulate apoptosis in immature thymocytes and on the other hand they promote the survival of TcR stimulated cells (43–45). Our observation shows that the pathway used by glucocorticoids in thymocyte apoptosis involves the activation of CPP32. However, the redundant action of other CPP32-like cysteine proteases in this pathway cannot be ruled out. This is supported by the observation that thymocytes from CPP32-deficient mice would exhibit normal sensitivity to dexamethasone-induced cell death (41).
Finally, we observed that not only CD4+8+ thymocytes but also CD4+8− thymocytes in H-2k TcR-transgenic mice were sensitive to PCD induced by injection of PCC. These results would indicate that CD4+8− thymocytes are also susceptible to the process of negative selection as demonstrated for superantigens (46). This idea is also supported by the recent observation made by Kishimoto and Sprent that CD4+8− thymocytes expressing an immature phenotype (HSAhigh) are sensitive to TcR-mediated apoptosis in vivo and in vitro (47).
The findings presented in the present paper make two important points: (a) they correlate for the first time the activation of a cysteine protease, CPP32, with the phenomenon of negative selection of T cells in the thymus and (b) they show that different types of apoptosis of thymocytes use pathways involving the activation of different cysteine proteases. Future studies involving new reagents specific for these proteases should lead to a more exact definition of these different death pathways in developing T cells.
Acknowledgments
We thank Dr. G.G. Poirier for kindly providing us with rabbit anti-sera specific for mouse PARP. We also thank N. Tessier for her expertise in cell sorting.
This work was supported by grants from the Medical Research Council (MRC) of Canada to R.-P. Sekaly (MRC Industry, grant no. UI12373), P. Hugo (grant no. MT12637) and F. Denis (National Health Research and Development Program, grant no. 6605-4847-AIDS). R.-P. Sekaly holds a MRC scientist award. P. Hugo is a MRC scholar. S. Lesage is recipient of a studentship from the Fond de la Recherche en Santé du Québec.
Abbreviations used in this paper
- PARP
proteolysis of poly(ADP-ribose) polymerase
- PCC
pigeon cytochrome C
- PCD
programmed cell death
- PI
propidium iodide
- SREBP
sterol-regulatory element binding proteins
Footnotes
The first two authors contributed equally to this work.
References
- 1.Steller H. Mechanisms and genes of cellular suicide. Science (Wash DC) 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 2.Martin SJ, Green DR. Protease activation during apoptosis: death by a thousand cuts? . Cell. 1995;82:349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- 3.Fraser A, Evan G. A license to kill. Cell. 1996;85:781–784. doi: 10.1016/s0092-8674(00)81005-3. [DOI] [PubMed] [Google Scholar]
- 4.Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caenorhabditis eleganscell death protein Ced-3 and mammalian interleukin-1 β-converting enzyme. J Biol Chem. 1994;269:30761–30764. [PubMed] [Google Scholar]
- 5.Tewari M, Quan LT, O'Rourke K, Desnoyers S, Zheng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM. YAMA/CPP32β, a mammalian homolog of CED-3, is a Crma-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 6.Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnick YA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature (Lond) 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 7.Casciola-Rosen L, Nicholson DW, Chong T, Rowan KR, Thornberry NA, Miller DK, Rosen A. Apopain/CPP32 cleaves proteins that are essential for cellular repair: a fundamental principle of apoptotic death. J Exp Med. 1996;183:1957–1964. doi: 10.1084/jem.183.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Zelenski NG, Yang J, Sakai J, Brown MS, Goldstein JS. Cleavage of sterol regulatory element binding proteins (SREBPs) by CPP32 during apoptosis. EMBO (Eur Mol Biol Organ) J. 1996;15:1021–1027. [PMC free article] [PubMed] [Google Scholar]
- 9.Na S, Chuang T-H, Cunningham A, Turi TG, Hanke JH, Bokoch GM, Danley DE. D4-GDI, a substrate for CPP32, is proteolysed during Fas-induced apoptosis. J Biol Chem. 1996;271:11209–11213. doi: 10.1074/jbc.271.19.11209. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg YP, Nicholson DW, Rasper DM, Kalchman MA, Koide HB, Graham RK, Bromm M, Kazemi-Esfarjani P, Thornberry NA, Vaillancourt JP, Hayden MR. Cleavage of huntingtin by apopain, a proapoptotic cysteine protease, is modulated by the polyglutamine tract. Nature Genet. 1996;13:442–449. doi: 10.1038/ng0896-442. [DOI] [PubMed] [Google Scholar]
- 11.Schlegel J, Peters I, Orrenius S, Miller DK, Thornberry NA, Yamin TT, Nicholson DW. CPP32/apopain is a key interleukin 1 β converting enzyme-like protease involved in Fas-mediated apoptosis. J Biol Chem. 1996;271:1841–1844. doi: 10.1074/jbc.271.4.1841. [DOI] [PubMed] [Google Scholar]
- 12.Enari M, Talanian RV, Wong WW, Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature (Lond) 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa J, Kamada S, Kamiike W, Shimizu S, Imazu T, Matsuda H, Tsujimoto Y. Involvement of CPP32/ YAMA(-like) proteases in Fas-mediated apoptosis. Cancer Research. 1996;56:1713–1718. [PubMed] [Google Scholar]
- 14.Erhardt P, Cooper GM. Activation of the CPP32 apoptotic protease by distinct pathways with differential sensitivity to Bcl-XL. J Biol Chem. 1996;271:17601–17604. doi: 10.1074/jbc.271.30.17601. [DOI] [PubMed] [Google Scholar]
- 15.Darmon AJ, Nicholson DW, Beackley RC. Activation of the apoptotic protease CPP32 by cytotoxic T-cell derived granzyme B. Nature (Lond) 1995;377:446–448. doi: 10.1038/377446a0. [DOI] [PubMed] [Google Scholar]
- 16.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 17.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell receptor transgenic mice involves deletion of nonmature CD4+8+thymocytes. Nature (Lond) 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 18.Sha WC, Nelson CA, Newberry RD, Kranz DM, Russel JH, Loh DY. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature (Lond) 1988;336:73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- 19.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+8+ TcRlo thymocytes in vivo. Science (Wash DC) 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 20.Teh HS, Kisielow P, Scott B, Kishi H, Uematsu Y, Blüthmann H, von Boehmer H. Thymic major histocompatibility complex antigens and the αβ T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature (Lond) 1988;335:229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- 21.Kaye J, Vasquez NJ, Hedrick SM. Involvement of the same region of the T cell antigen receptor in thymic selection and foreign peptide recognition. J Immunol. 1992;148:3342–3353. [PubMed] [Google Scholar]
- 22.van Engeland M, Ramaekers FCS, Schutte B, Reutelingsperger CPM. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry. 1996;24:131–139. doi: 10.1002/(SICI)1097-0320(19960601)24:2<131::AID-CYTO5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 23.Slee EA, Zhu H, Chow SC, Mac M, Farlane, Nicholson DW, Cohen GM. Benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethyl ketone (Z-VAD.FMK) inhibits apoptosis by blocking the processing of CPP32. Biochem J. 1996;35:21–24. doi: 10.1042/bj3150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drexler H C. Activation of the cell death program by inhibition of proteasome function. Proc Natl Acad Sci USA. 1996;94:855–860. doi: 10.1073/pnas.94.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhéaume, E., L.Y. Cohen, F. Uhlman, C. Lazure, A. Alam, J. Hurwitz, R.P. Sékaly, and F. Denis. 1997. The large subunit of replication factor C is a substrate for caspase-3 in vitro and is cleaved by a caspase-3-like protease during Fas induced apoptosis. EMBO (Eur. Mol. Biol. Organ.) J. In press. [DOI] [PMC free article] [PubMed]
- 26.Smith SA, Williams GT, Kingston R, Jenkinson EJ, Owen JT. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature (Lond) 1989;337:181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- 27.Finkel TH, Cambier JC, Kubo RT, Born WK, Marrack P, Kappler JW. The thymus has two functionally distinct populations of immature αβ+ T cells: one population is deleted by ligation of αβ TCR. Cell. 1989;58:1047–1054. doi: 10.1016/0092-8674(89)90503-5. [DOI] [PubMed] [Google Scholar]
- 28.MacConkey DJ, Hartzell P, Amador-Perez JF, Orenius S, Jondal M. Calcium-dependent killing of immature thymocytes by stimulation via CD3/T cell receptor complex. J Immunol. 1989;143:1801–1806. [PubMed] [Google Scholar]
- 29.Tadakuma T, Kizaki H, Odaka C, Kubota R, Ishimura Y, Yagita H, Okumura K. CD4+CD8+ thymocytes are susceptible to DNA fragmentation induced by ester phorbol, calcium ionophore, and anti-CD3. Eur J Immunol. 1990;20:779–784. doi: 10.1002/eji.1830200411. [DOI] [PubMed] [Google Scholar]
- 30.Shi Y, Bissonnette RP, Parfrey N, Szalay M, Kubo RT, Green D. In vivo administration of monoclonal antibodies to the CD3 T cell receptor complex induces cell death (apoptosis) in immature thymocytes. J Immunol. 1991;146:3340–3346. [PubMed] [Google Scholar]
- 31.Penit C, Vasseur F. Cell proliferation and differentiation in the fetal and the early postnatal mouse thymus. J Immunol. 1989;142:3369–3377. [PubMed] [Google Scholar]
- 32.Fadock VA, Voekler DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 33.Sarin A, Wu M-L, Henkart PA. Different interleukin-1β converting enzyme (ICE) family protease requirements for the apoptotic death of T lymphocytes triggered by diverse stimuli. J Exp Med. 1996;184:2445–2450. doi: 10.1084/jem.184.6.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science (Wash DC) 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 35.Clayton LK, Ghendler Y, Mizoguchi E, Patch RJ, Ocain TD, Orth K, Bhan AK, Dixit VM, Reinherz EL. T-cell receptor ligation by peptide/MHC induces activation of a caspase in immature thymocytes: the molecular basis of negative selection. EMBO (Eur Mol Biol Organ) J. 1997;16:2282–2293. doi: 10.1093/emboj/16.9.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, X., H. Zou, C. Slaughter, and X. Wang. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 89:175–184. [DOI] [PubMed]
- 37.Fernandes-Alnemri T, Litwack G, Alnemri ES. Mch-2, a new member of the apoptotic Ced-3/Ice cysteine protease gene family. Cancer Res. 1995;55:2737–2742. [PubMed] [Google Scholar]
- 38.Fernandes-Alnemri T, Takahashi A, Amstrong R, Krebs J, Fritz L, Tomaselli KJ, Wang L, Yu Z, Croce CM, Salveson G, et al. Mch3, a novel human apoptotic cysteine protease highly related to CPP32. Cancer Res. 1995;55:6045–6052. [PubMed] [Google Scholar]
- 39.Duan H, Orth K, Chinnaiyan AM, Poirier GG, Froelich CJ, He WW, Dixit VM. ICE-LAP6, a novel member of the ICE/Ced-3 gene family, is activated by the cytotoxic T cell protease granzyme B. J Biol Chem. 1996;271:16720–16724. doi: 10.1074/jbc.271.28.16720. [DOI] [PubMed] [Google Scholar]
- 40.Lippke JA, Gu Y, Sarnecki C, Caron PR, Su MS. Identification and characterization of CPP32/Mch2 homolog 1, a novel cysteine protease similar to CPP32. J Biol Chem. 1996;271:1825–1828. doi: 10.1074/jbc.271.4.1825. [DOI] [PubMed] [Google Scholar]
- 41.Kuida K, Zheng TS, Na S, Kuan C-Y, Yang D, Karasuyama H, Rakic P, Flavell R. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature (Lond) 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 42.Walunas TL, Sperling AI, Thompson CB, Bluestone JA. CD28 expression is not essential for positive and negative selection of thymocytes or peripheral T cell tolerance. J Immunol. 1996;156:1006–1013. [PubMed] [Google Scholar]
- 43.Cohen JJ. Glucocorticoid-induced apoptosis in the thymus. Seminars in Immunol. 1992;4:363–369. [PubMed] [Google Scholar]
- 44.Iseki R, Mukai M, Iwata M. Regulation of T lymphocyte apoptosis. Signals for the antagonism between activation- and glucocorticoid-induced death. J Immunol. 1991;147:4286–4292. [PubMed] [Google Scholar]
- 45.King LB, Vacchio MS, Dixon K, Hunziker R, Marguiles DH, Ashwell JD. A targeted glucocorticoid receptor antisense transgene increases thymocyte apoptosis and alters thymocyte development. Immunity. 1995;3:647–656. doi: 10.1016/1074-7613(95)90135-3. [DOI] [PubMed] [Google Scholar]
- 46.Ohashi PS, Pircher H, Burki K, Zinkernagel RM, Hentgartner H. Distinct sequence of negative or positive selection implied by thymocyte T-cell receptor densities. Nature (Lond) 1990;346:861–863. doi: 10.1038/346861a0. [DOI] [PubMed] [Google Scholar]
- 47.Kishimoto H, Sprent J. Negative selection in the thymus includes semimature T cells. J Exp Med. 1997;185:263–271. doi: 10.1084/jem.185.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]