Abstract
During gram-negative bacterial infections, lipopolysaccharide (LPS) stimulates primed macrophages (Mφ) to release inflammatory mediators such as tumor necrosis factor (TNF)-α, which can cause hypotension, organ failure, and often death. Several different receptors on Mφ have been shown to bind LPS, including the type A scavenger receptor (SR-A). This receptor is able to bind a broad range of polyanionic ligands such as modified lipoproteins and lipoteichoic acid of gram-positive bacteria, which suggests that SR-A plays a role in host defense. In this study, we used mice lacking the SR-A (SRKO) to investigate the role of SR-A in acquired immunity using a viable bacillus Calmette Guérin (BCG) infection model. We show that activated Mφ express SR-A and that this molecule is functional in assays of adhesion and endocytic uptake. After BCG infection, SRKO mice are able to recruit Mφ to sites of granuloma formation where they become activated and restrict BCG replication. However, infected mice lacking the SR-A are more susceptible to endotoxic shock and produce more TNF-α and interleukin-6 in response to LPS. In addition, we show that an antibody which blocks TNF-α activity reduces LPS-induced mortality in these mice. Thus SR-A, expressed by activated Mφ, plays a protective role in host defense by scavenging LPS as well as by reducing the release by activated Mφ of proinflammatory cytokines. Modulation of SR-A may provide a novel therapeutic approach to control endotoxic shock.
The macrophage (Mφ)1 scavenger receptor type A (SR-A) is a trimeric integral membrane glycoprotein which exists in two forms, type I and II, generated by alternative splicing of a single gene product (1–4). This family of SRs has recently been extended through the discovery of several additional SR genes and now includes at least three independent SR classes (5). SRs are defined according to their ability to bind and mediate uptake of modified low density lipoproteins (LDL), such as acetylated (Ac) LDL. The recent detection of SR-A in atheromatous plaques, and its ability to mediate uptake of modified LDL by arterial wall Mφ, has implicated the molecule in the pathogenesis of atherosclerosis (6–8).
The range of ligands recognized by SR-A is wide, including LPS of gram-negative and lipoteichoic acid of gram-positive bacteria (9, 10). SR-A types I and II exhibit similar binding properties, specifically binding a large selection of polyanionic ligands with high affinity. This broad ligand specificity has suggested that SR-A may play a role in a wide range of Mφ-associated physiological and pathophysiological processes (11–13). For example, Janeway has suggested that such receptors may have arisen early in the evolution of host defense systems and could enable self/ nonself discrimination (14). SR-A is expressed on a wide range of tissue Mφ and also on the sinusoidal endothelium of the liver (15). This tissue distribution is consistent with a pattern recognition function for SR-A and also suggests that it may play a role in host defense by recognizing and mediating the clearance of pathogens (16).
Recently the repertoire of SR-A functions has been extended. Work in our laboratory, using a monoclonal antibody (2F8) which recognizes the mouse type I and II SR-A, has established that SR-A mediates a component of adhesion of Mφ in vitro (17). SR-A might therefore function as an adhesion molecule in vivo and act to retain Mφ within ligand-rich tissues. Support for this theory has come from observations, using physiological ligands, that SR-A can mediate in vitro adhesion of rodent microglia and human monocytes to β-amyloid fibril–coated surfaces, implicating SR-A in the pathogenesis of Alzheimers disease (18). An additional role for SR-A may be as a receptor used in the phagocytosis of apoptotic cells in the thymus (19).
This study was designed to further our understanding of the role of SR-A in host defense. In a model of cell-mediated immunity, we identify activated Mφ and examine whether SR-A is required for Mφ recruitment to sites of granuloma formation. Previous studies have shown that Mφ can bind, internalize, and partially break down LPS, lipid A, and its bioactive precursor lipid IVa (9). This binding and subsequent metabolism to a less active form by Mφ-like RAW 264.7 cells is mediated by the SR-A. SR-A ligands greatly inhibit uptake of lipid IVa in mice (9). Taken together, these observations suggested that SR-A may have a role in the uptake and degradation of endotoxin in animals. Using wild-type and SR-A–deficient (SRKO) mice, we investigate an in vivo role for SR-A in the body's response to LPS. These results provide the first evidence that SR-A acts to prevent the development of endotoxic shock.
Materials and Methods
Animals.
Mice deficient in type I and II SR-A were produced by disruption of exon 4 of the SR-A gene that codes for the α-helical coiled coil domain, which is essential for the formation of functional trimeric receptors (4). These mice were bred onto a 129/ICR background and are described here as SRKO. Wild-type 129 mice were cross-bred on to an identical 129/ICR background and are described here as 129 mice. Brother–sister matings were used to generate homozygous SRKO and 129 mice on an identical genetic background. Mice used in these experiments were housed at the Dunn School of Pathology and were used between 6 and 10 wk of age.
Media and Reagents.
RPMI 1640 (GIBCO BRL, Paisley, UK) was supplemented with 10% heat-inactivated fetal bovine serum (GIBCO BRL), 10 mM Hepes, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (R10). All chemicals were obtained from Sigma Chemical Co. (St. Louis, MO) unless otherwise specified.
Antibodies.
The following rat anti–mouse mAbs were prepared in our laboratory and were used as diluted hybridoma supernatants unless specified; F4/80, specific for an Mφ plasma membrane antigen (20, 21); FA11, which recognizes macrosialin (22, 23); 2F8, which recognizes class I and II SR-A (24); and TIB 120, which recognizes class II MHC (25). The hamster mAb TN3 19.12 used in vivo was a gift of Dr. R. Schreiber (Washington University, St. Louis, MO) and was used at 250 μg/mouse. The IgG2b isotype-matched controls, CAMPATH (CP)-1G (rat anti–human) and anti–Thy-1 (rat anti–canine) were a gift of Dr. S. Cobbold (University of Oxford, Oxford, UK; reference 26).
Microorganisms and Determination of Bacillus Calmette Guérin (BCG) CFU.
Live BCG (Pasteur strain) was provided by Dr. G. Milon (Pasteur Institut, Paris, France). BCG stocks were stored at −70°C, thawed, and sonicated immediately before use. Mice were inoculated with ∼107 CFU in 0.2 ml of PBS by intraperitoneal injection. Organs were removed from the mice at various time points after inoculation with BCG. A sample of the organ (0.2 g) was homogenized in 5 ml of sterile PBS containing 0.02% BSA and 0.05% Tween 20. 10-fold serial dilutions of the homogenate were prepared and 25 μl was plated onto a quadrant of a plate containing Middlebrook 7A10 agar with OADC enrichment (Difco, Detroit, MI). Plates were incubated at 37°C and colonies of BCG were counted 10–14 d later.
Inactivated Corynebacterium parvum (Propionibacterium acnes) whole cells were purchased from RIBI Immunochemical Research Inc. (Hamilton, MT). Organisms were washed in nonpyrogenic saline twice, resuspended in PBS, and sonicated before use. Mice were inoculated with 500 μg in 0.2 ml of PBS by intraperitoneal injection.
Flow Cytometry.
SRKO and 129 mice were injected intraperitoneally with 107 CFU of BCG. 5 d later, peritoneal cells were harvested, resuspended in R10, and plated onto tissue culture plastic (TCP) dishes. 5 h later nonadherent cells were removed by repeated washing with PBS. Lidocaine (4 mg/ml) and EDTA (5 mM) in PBS were used to lift the Mφ from the plate. The cells were fixed on ice for 40 min using 4% paraformaldehyde/250 mM Hepes in PBS. The cells were then permeabilized for 30 min at 4°C using saponin (0.1%) in PBS. Cells were resuspended in FACS® buffer (0.1% saponin, 0.1% BSA, 1% normal mouse serum, and 10 mM sodium azide) and incubated with primary antibodies (e.g., 10 μg/ml purified 2F8 or CP) for 1 h, washed three times, and incubated for 1 h with FITC-conjugated mouse anti–rat second Ab (Jackson ImmunoResearch Labs., West Grove, PA) at a 1:500 dilution. The cells were washed and analyzed on a FACScan® using Cellquest software.
Adhesion Assays.
BCG peritoneal cells were harvested as described for flow cytometry. Cells were plated in R10 at 3 × 105 Mφ/well in a 96-well plate in the presence of various antibodies and/or EDTA. Plates were incubated at 4°C for 30 min, then at 37°C for 90 min before washing to remove nonadherent cells. Adherent cells were fixed in methanol and stained with 40% Giemsa for 1 h. The level of adhesion was quantified by solubilizing the dye in methanol and reading the OD at 450 nm. Adhesion (mean ± SD) is represented as the percentage of that obtained in medium alone, and is the result of quadruplicate well assays.
Endocytic Uptake of DiIAcLDL. (Biogenesis, Bournemouth, UK).
SRKO and 129 mice were injected intraperitoneally with 107 CFU of BCG. 7 d later, peritoneal cells were harvested and resuspended in R10. Cells were plated at 3 × 105 Mφ/well of a 96-well plate and cultured overnight. Nonadherent cells were removed by washing with PBS. Medium (R10) was added containing either the 2F8 or isotype control (CP) antibodies, and the plate was placed on ice for 30 min. DiIAcLDL was added to each well and the plate was incubated at 37°C for 5 h. The cells were washed, resuspended using 5 mM EDTA/4 mg/ml lidocaine, and the dye was solubilized with Butan-1-ol. Fluorescence was read on a Fluoroskan II plate reader (Titertek; ICN, Costa Mesa, CA). Uptake of DiIAcLDL is expressed as units of fluorescence (mean ± SD) of four replicate wells.
Immunohistochemistry.
At day 25 of BCG infection, livers were harvested from 129 and SRKO mice, frozen in OCT compound (BDH-Merck, Dorset, UK), and cooled in isopentane over dry ice. Frozen sections were cut at 5 μm and fixed for 10 min in 2% paraformaldehyde before staining. Sections were washed in 0.1% Triton X-100 in PBS . Endogenous peroxidase activity was quenched by incubation of sections with 10−2 M glucose, 10−3 M sodium azide, and 40 U glucose oxidase in 100 ml PBS for 15 min at 37°C. Avidin/biotin blocking agents (Vector Labs., Peterborough, UK) were used according to the supplier's recommendation. Fetal bovine serum (10%) and normal rabbit serum (5%) were then used to block irrelevant binding sites and sections were incubated for 60 min in hybridoma supernatant or isotype-matched control Ab. Sections were washed, affinity-purified, and then biotinylated second Ab was added at 1% for 30 min. The sections were washed and avidin-biotin-peroxidase complex (ABC elite; Vector Labs.) was used for 30 min according to the supplier's recommendation. The presence of antigen was revealed by incubation with 0.5 mg/ml diaminobenzidine (Polysciences Inc., Northampton, UK) and 0.024% hydrogen peroxide in 10 mM PBS imidazole. Sections were counterstained with cresyl violet acetate and mounted in DPX (BDH-Merck).
Assessment of Biological Response to LPS.
Mice (age-matched wild-type and SRKO) were injected intraperitoneally with 107 CFU of BCG. At 14 d after infection, mice were injected with various doses of LPS from Salmonella typhimurium (Westphal strain; Difco) in nonpyrogenic saline intraperitoneally. To block the activity of TNF-α, 250 μg of TN3 19.12 mAb was given to the mice 10 h before administration of the LPS. The condition of the mice was monitored regularly over 5 d and those suffering significant morbidity were killed.
Cytokine Assays.
SRKO and 129 mice were injected intraperitoneally with ∼107 CFU of BCG. At 14 d after infection, mice were injected with 10 μg of LPS intraperitoneally. The concentration of TNF-α and IL-6 in serum at various time points after injection was determined using capture ELISAs. In brief, for the TNF-α assay, 50 μl of TN3 19.12 mAb (4 μg/ml) in carbonate buffer (pH 9.6) were added per well of a 96-well plate (Sterilin, Stone, UK) and left at 4°C overnight. Plates were washed twice with PBS/0.05% Tween 20 and twice with PBS alone, and then samples of the sera (1:10 dilution in R10) were added along with a dilution series of recombinant murine TNF-α (Serotec, Kidlington, Oxford, UK). Plates were incubated overnight at 4°C, washed as above, and then 100 μl/well of rabbit anti– murine TNF-α (Serotec; 1:1000 in PBS) was added and left at room temperature for 90 min. Plates were washed as above, donkey anti–rabbit horseradish peroxidase (Chemikon International, Inc., Temecula, CA) at 1:1,000 in PBS/Tween (0.05%)/BSA (0.1%) was added and incubated at 20°C for 1 h. Plates were washed and then 100 μl of reaction mix was added as recommended by supplier. The reaction was stopped by the addition of 50 μl of 3 M sulfuric acid and plates were read at 492 nM. A similar protocol was adopted for measurement of IL-6 using appropriate antibodies (PharMingen, San Diego, CA).
Statistics.
Parametric data for cytokine assays and DiIAcLDL endocytic uptake were compared using Student's t test with the Welch modification to compensate for different levels of variance for data sets. Survival data were compared using a likelihood ratio test (27).
Results
Expression and Function of SR-A on BCG-activated Mφ.
4–6 d after intraperitoneal injection of BCG, a mixed leukocyte population which is rich in Mφ can be harvested (28). These Mφ have undergone a process of activation mediated by IFN-γ, resulting in changes in both secreted products and cell surface receptor expression. Using immunostaining with FA11, a pan-Mφ marker which recognizes the intracellular protein macrosialin, the Mφ in the peritoneal population were identified (Fig. 1, c and d; reference 22).
Figure 1.
FACS® staining of isolated BCG-activated peritoneal cells. Peritoneal cells were recruited after intraperitoneal inoculation of 107 CFU of BCG. Cells were harvested and stained with a range of primary antibodies. a, c, e: wild-type (129) cells. b, d, f : SRKO cells. (a and b) 2F8, which recognizes the type I and II SR-A; (c and d) FA11, recognizes macrosialin, a macrophage specific marker; (e and f) TIB 120, recognizes MHC class II antigen. Filled histograms show the indicated surface markers, and unfilled histograms represent isotype-matched control antibodies. The results shown are representative of three independent experiments.
Similar numbers of peritoneal Mφ were harvested from wild-type and SRKO mice (∼2 × 107 Mφ/mouse). Using an electronic gate to select FA11 positive cells, BCG-activated Mφ from the wild-type mice were shown to express SR-A, whereas BCG-activated peritoneal Mφ from the SRKO mouse lacked the SR-A (Fig. 1, a and b). The TIB 120 mAb was used to demonstrate that despite lacking SR-A, BCG-primed Mφ from SRKO mice were activated and expressed MHC class II (Fig. 1, e and f). Supporting evidence for activation was provided for wild-type and SRKO BCG peritoneal Mφ since both produce high levels of nitric oxide spontaneously when harvested after day 4 of intraperitoneal infection (results not shown).
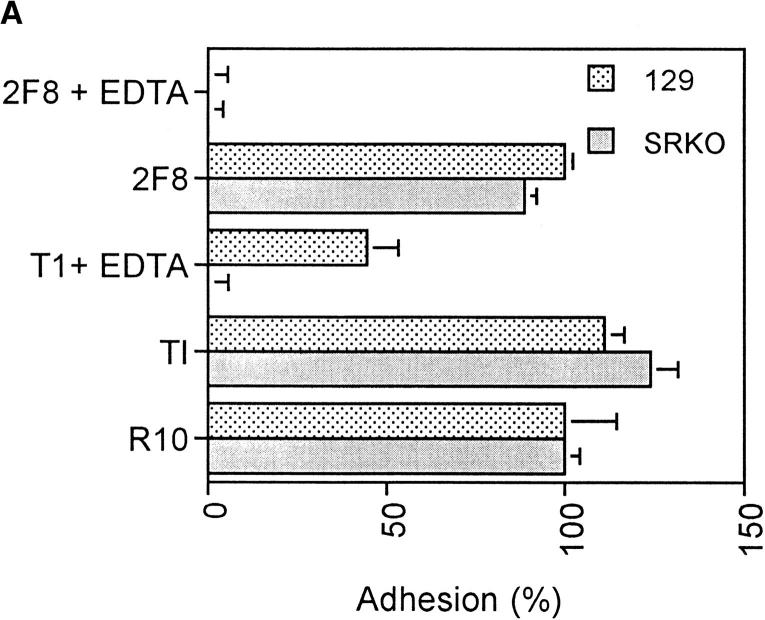
Previous work has demonstrated a role for SR-A in mediating cation-independent adhesion of thioglycollate broth– elicited peritoneal Mφ (29) to TCP in the presence of serum (17). To examine whether activated Mφ from the SRKO mouse showed an altered adhesion phenotype in vitro, BCG-elicited peritoneal cells were harvested and their adhesion to TCP was examined in the presence of serum (Fig. 2 A). In the presence of medium (R10) alone or control antibody (anti–Thy-1), the adhesion of wild-type and SRKO cells was identical. However, in the presence of EDTA, which chelates divalent cations, significant adhesion of the wild-type cells remained, whereas adhesion of the SRKO cells was completely inhibited. In the additional presence of the anti–SR-A blocking mAb 2F8, wild-type adhesion was further reduced to the level of the SRKO cells.
Figure 2.
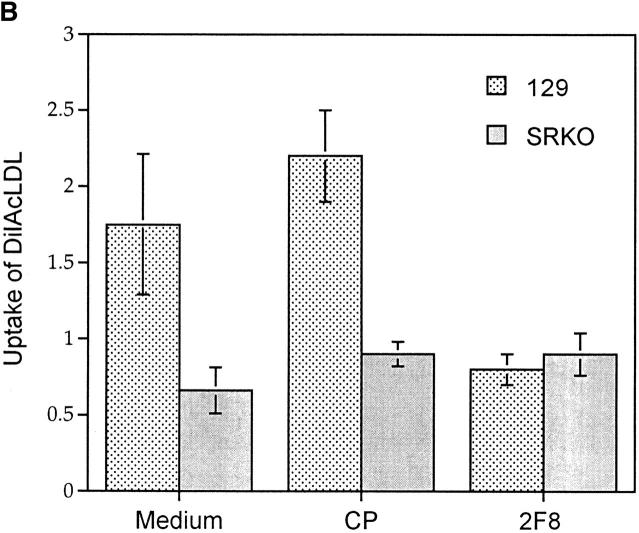
Functional phenotype of BCG-activated SRKO and wild-type Mφ. (A) Adhesion of activated Mφ to FBS-coated TCP. Cells were plated at 3 × 105 macrophages/well of a 96-well plate in the presence of various mAbs and/or EDTA. BCG-recruited peritoneal cells from SRKO and wild-type (129) mice show similar adhesion in medium (R10) alone, but in the presence of both EDTA and isotype-matched control mAb (T1), adhesion of SRKO Mφ is completely inhibited (compare with wild-type 129), showing that SR-A accounts for all EDTA-resistant adhesion. Addition of 2F8 mAb and EDTA is required to completely inhibit the remaining adhesion of 129 activated Mφ. Adhesion is represented as the percentage of that obtained in medium alone, where 100% represents an absorbance (OD) at 450 nm of 0.15. Results (mean ± SD) are derived from a single experiment (n = 4) and are representative of three similar experiments. (B) The uptake of DiIAcLDL by SRKO and wild-type (129) BCG-recruited peritoneal cells. Cells were harvested as in A and the uptake of DiIAcLDL measured as described in Materials and Methods. SRKO endocytose only 40% of the DiIAcLDL taken up by 129 cells. 2F8 mAb reduces the uptake by 129 cells to that of SRKO cells, whereas an isotype-matched control Ab (CP) has no effect. Uptake of DiIAcLDL is expressed by units of fluorescence (mean ± SD) of four replicate wells and is relative to a blank well of cells without the addition of DiIAcLDL. Results are derived from a single experiment and are representative of at least three similar experiments. Uptake of DiIAcLDL by 129 Mφ in the presence of medium or CP is significantly greater than uptake by SRKO Mφ (P <0.005).
SR-A mediates endocytosis of a wide range of ligands (9). The uptake of DiIAcLDL in vitro has provided a relatively convenient assay for this activity (30). The SRKO mouse now provides us with a model system in which to investigate both the role of SR-A and the contribution of other potential scavenger molecules in this uptake. We observed that BCG-activated Mφ from the SRKO mouse, when plated in vitro, endocytosed only 30–40% of the amount of DiIAcLDL taken up by control cells (Fig. 2 B). When the 2F8 mAb was added to the medium, the uptake by the wild-type cells was reduced to the level obtained with the SRKO cells, whereas addition of an isotype-matched control mAb (CP) had no effect. Thus the 2F8 mAb can completely inhibit the component of DiIAcLDL uptake attributable to SR-A. However, this SR-A independent uptake can be reduced further in the presence of the polyanionic inhibitor Poly G, suggesting the involvement of other cell surface molecules in the endocytic uptake of modified lipoproteins (not shown).
Recruitment of Mφ to BCG Granulomata.
To investigate whether SR-A is involved in the adhesion and recruitment of Mφ to sites of infection in vivo, wild-type and SRKO mice were injected intraperitoneally with BCG. In this model of cellular immunity, Mφ are recruited to sites of infection (granulomata) and undergo the process of activation mediated by IFN-γ. Using immunohistochemistry of the liver, we discovered that SR-A is expressed not only on resident Kupffer cells and endothelium but also on activated Mφ in granulomata of wild-type mice (Fig. 3). In addition, staining with F4/80 and FA11 established that, despite lacking SR-A, Mφ are efficiently recruited to sites of infection in vivo (Fig. 3, b and d). These Mφ fail to stain with 2F8 since they lack SR-A, but become activated and upregulate MHC class II (Fig. 3, f and h). Examination of BCG CFU for up to 2 wk of infection in liver, spleen, and lung showed that SRKO mice were able to limit mycobacterial replication (e.g., liver BCG CFU at day 8 of infection: wild-type, 6.20 ± 1.23 × 107; SRKO, 6.26 ± 3.19 × 107).
Figure 3.

Immunohistochemistry of BCG granulomata in SRKO and wild-type (129) mice. Livers were collected from SRKO and wild-type (129) mice at day 25 of BCG infection and frozen in OCT embedding medium. Sections of liver were cut and immunostained with a range of primary antibodies. a, c, e, and g: control sections. b, d, f, and h: SRKO sections. (a and b) F4/80; resident macrophages (Kupffer cells) and newly recruited activated macrophages forming granulomata are stained. (c and d) FA11; macrosialin is found in all macrophages and here shows the similar morphology of granulomas in wild-type and SRKO mice. (e and f) TIB 120; both 129 and SRKO Kupffer cells and granuloma macrophages express high levels of MHC class II. (g and h) 2F8, which recognizes the type I and II SR-A. (g) Shows that SR-A is expressed on Kupffer cells, freshly recruited macrophages, and the hepatic endothelium, whereas h shows the absence of SR-A in the SRKO sections. Original magnification: ×500.
Examination of a Role for SR-A in a Model of Endotoxic Shock.
BCG-infected mice contain large numbers of activated Mφ which can produce high levels of proinflammatory cytokines (e.g., TNF-α, IL-1) in response to LPS challenge (31). Unlike signaling caused by ligation of the CD14 receptor, SR-A is thought to be a neutral receptor for LPS (9). SRKO mice provide a powerful tool to test whether SR-A binding to LPS in vivo reduces the risk of endotoxic shock and were used in the following model of endotoxic shock.
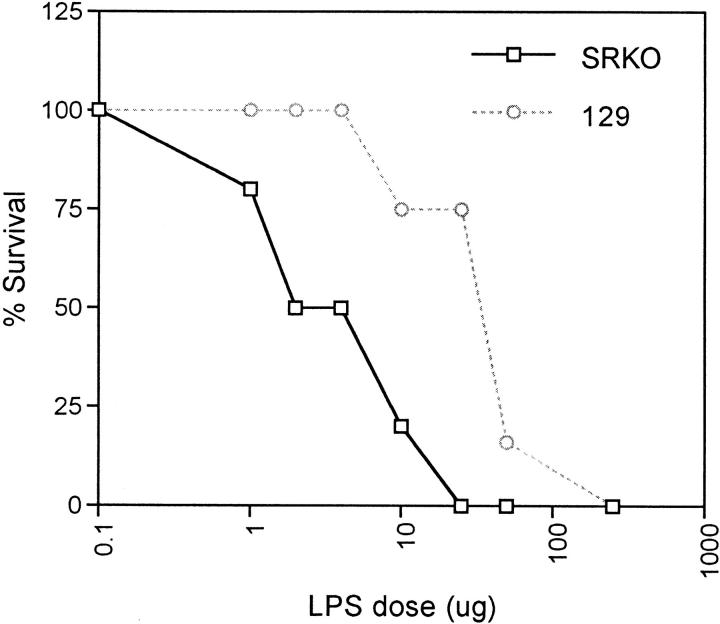
Wild-type and SRKO mice were infected with BCG, resulting in the production of numerous Mφ-rich granulomas in parenchymatous organs. Groups of BCG-infected mice were challenged with a range of LPS doses by intraperitoneal injection at days 12–14 of infection. The significant discovery was that the SRKO mice showed approximately sevenfold higher morbidity and mortality than wild-type mice (Fig. 4). In preliminary studies, similar results were obtained when an inactivated preparation of C. parvum was used to prime the mice before LPS challenge (e.g., LPS dose 10 μg: mortality of 129, 0%; of SRKO, 75%; n = 4). In addition, increased susceptibility to LPS challenge of the SRKO mice can be demonstrated even in uninfected mice which have received no stimulus for IFN-γ priming of Mφ, although doses of LPS required are two orders of magnitude higher (e.g., LPS dose 500 μg: mortality of 129, 0%; of SRKO, 66%; n = 3).
Figure 4.
Role of SR-A in a model of endotoxic shock. SRKO and 129 mice were injected intraperitoneally with 107 CFU of BCG. At days 12–14 of infection, the mice were injected with various doses of LPS (μg). The mice were observed for 5 d and mortality was noted. The percentage of survival for each dose point represents the mean mortality of a group of at least five mice. The dose of LPS producing 50% mortality (LD50; ng/ml) of the 129 mice is: 34.1 ± 6.6 (standard error), which is significantly different from the SRKO LD50: 4.7 ± 1.5; P <0.0001.
In Vivo Cytokine Production After LPS Challenge.
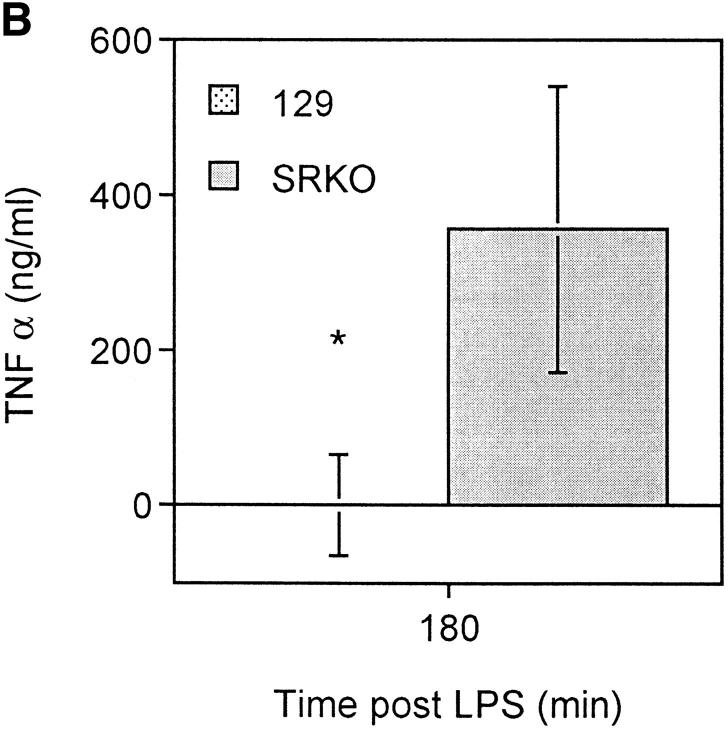
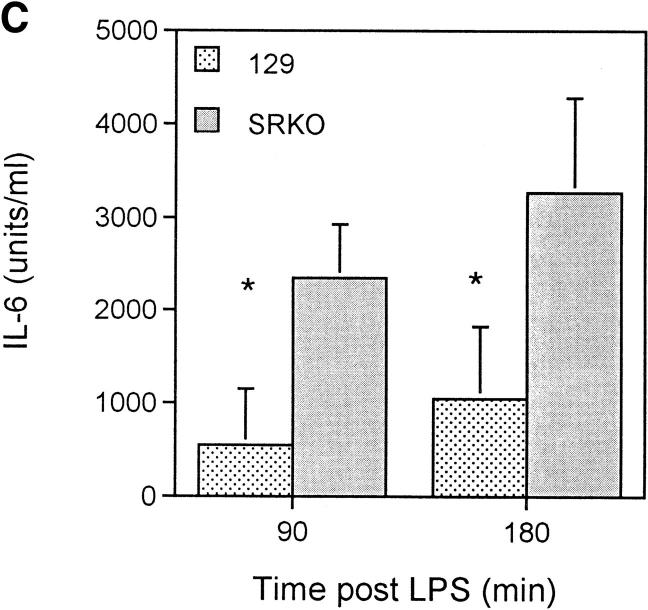
During the course of serious gram-negative bacterial infections, LPS induces stimulation of macrophages, which results in the release of inflammatory mediators (32). The activity of these mediators, which include prostaglandins and cytokines (e.g., TNF-α, IL-6, and IL-1β) is, in turn, responsible for hypotension, organ failure, and often death. TNF-α is produced in large amounts by activated Mφ in response to LPS (33). We reasoned that this molecule was a candidate mediator of the difference in mortality observed between wild-type and SRKO mice. Therefore, we assayed plasma cytokine levels in BCG infected mice after LPS challenge. Significantly higher levels of TNF-α were present in the plasma of SRKO mice relative to wild-type mice (Fig. 5 A). To confirm that the measured differences in this cytokine were not confounded by differences in response to viable microorganisms, mice were injected with inactivated C. parvum and subsequently challenged with LPS. When serum was assayed 3 h after LPS challenge, levels of TNF-α were also higher in those samples from mice deficient in SR-A (Fig. 5 B). In addition, levels of serum IL-6 were assayed at the same time points and similar differences between the wild-type and SRKO were observed (Fig. 5 C). In contrast, serum levels of IL-10 showed no significant difference at these time points (results not shown).
Figure 5.
Production of TNF-α after LPS challenge of SRKO and wild-type (129) mice. (A and B). SRKO and 129 mice were injected intraperitoneally with 107 CFU of BCG, or (C) inactivated C. parvum. At 14 d after infection, mice were injected with 10 μg of LPS intraperitoneally. The concentration of (A and C) TNF-α or (B) IL-6 in the serum was determined using capture ELISAs as described in Materials and Methods. Results at each time point represent the mean concentration ± SD of a group of five mice. *P <0.05 compared to SRKO serum cytokine levels.
Use of a Blocking mAb to TNF-α.
To test whether TNF-α played a key role in the pathogenesis of endotoxic shock in this model, we injected SRKO mice with a blocking antibody to TNF-α (TN3 19.12) before LPS challenge. In contrast to mice pretreated with PBS, those receiving TN3 19.12 showed lower and delayed levels of mortality (Table 1). However, this treatment offered only partial protection. Therefore other proinflammatory cytokines, such as IL-1β, IL-12, and IL-6, probably also play a role in this mechanism.
Table 1.
A Blocking Antibody to TNF-α (TN3 19.12) Provides Partial Protection in a Model of Endotoxic Shock
| Time after LPS challenge | Survival | |||||
|---|---|---|---|---|---|---|
| PBS | TN3 19.12 | |||||
| h | ||||||
| Expt. 1 | 12 | 2/5 | 5/5 | |||
| 36 | 1/5 | 3/5 | ||||
| Expt. 2 | 12 | 3/5 | 5/5 | |||
| 36 | 0/5 | 4/5 | ||||
SRKO mice were injected intraperitoneally with 107 CFU of BCG. At day 14 of infection, groups of five mice were given either 250 μg of TN3 19.12 in 200 μl of PBS, or 200 μl of PBS alone. 10 h later mice were challenged with 25 μg of LPS. Results represent mortality at 12 h and 36 h after LPS challenge. Results from two independent experiments are shown.
Discussion
Our data provide the first direct evidence that SR-A acts to protect the host against endotoxin. Specifically, we show that SRKO mice suffer higher mortality in a model of endotoxic shock. After challenge with LPS, serum levels of proinflammatory cytokines are higher in SRKO relative to wild-type mice. We establish that TNF-α is an important mediator in the mechanism of shock in SRKO mice, through use of a blocking antibody which provides partial protection.
In addition, we demonstrate, for the first time, that activated Mφ express high levels of SR-A, which is functional in mediating both cell adhesion and endocytic uptake. Thus, activated Mφ from SRKO mice have an altered adhesion phenotype and express no compensatory mechanism for mediating adhesion in the presence of EDTA. In contrast, activated Mφ from SRKO mice are still able to mediate uptake of significant amounts of the ligand DiIAcLDL. One of the candidate molecules that may account for this remaining endocytic function is MARCO, a third class of SR-A, which has previously been shown to mediate binding of DiIAcLDL (34).
The development of granulomata in response to mycobacterial infection presents a model system for investigating molecules involved in cell adhesion and leukocyte recruitment. Recently, cytokines such as TNF-α (35) and IFN-γ (36) have been demonstrated to play a role in the development of granulomata. However, studying the key cell surface molecules involved in the adhesion and recruitment of Mφ to granulomata has proved more difficult. For example, blockade of the type 3 complement receptor (CR3) prevents formation of Mφ-rich granulomata in response to Listeria, but has no effect on the recruitment of Mφ to sites of BCG infection (37). In this laboratory, frozen section assays have been used to show that SR-A can mediate adhesion of Mφ to tissue ligands (15). Thus, SR-A is a candidate adhesion receptor involved in the Mφ recruitment process. Previous in vivo studies examining this hypothesis have been frustrated by the short plasma half-life of the 2F8 mAb (our unpublished results). In the model of BCG infection investigated here, Mφ lacking SR-A were able to migrate to sites of granuloma formation and become activated. Therefore, to evaluate any adhesion component attributable to SR-A, further studies will need to involve the blockade of additional adhesion receptors on SRKO and wild-type cells.
This model of mycobacterial infection also enabled us to test the immunocompetence of SRKO mice. Recent work has suggested that SRKO mice are more susceptible to Listeria monocytogenes and Herpes simplex virus, but the mechanism remains unclear (4). However, we found that levels of mycobacteria within the liver, lung, and spleen of wild-type and SRKO mice were not significantly different in this study. This result may reflect that Mφ are known to use a wide range of receptors to bind mycobacteria, including CR3 and the mannose receptor (38). Therefore, additional experiments whereby other cell surface receptors are blocked on Mφ already lacking SR-A are required to determine if SR-A plays a role in the binding and uptake of mycobacteria.
In addition to its potential role in adhesion, SR-A binds the gram negative cell wall component, LPS, with high affinity (39). SR-A is expressed widely by many Mφ popuations, such as those in the gut and Kupffer cells in the liver, and thus is well placed to mediate uptake of LPS in both physiological and pathological conditions. Results presented in this paper establish that mice lacking the SR-A are more susceptible to endotoxic shock. Thus, SR-A plays a protective role in the uptake and cellular response to LPS. Furthermore, we demonstrate that SRKO mice produce more TNF-α and IL-6 in response to LPS challenge than do wild-type mice. Therefore, to explain why mice deficient in SR-A are more susceptible to endotoxic shock, one must explore the mechanism that gives rise to higher levels of proinflammatory cytokines in these mice.
There are at least three possible explanations for these findings. The first is that there are simply greater numbers of activated Mφ in BCG-infected SRKO compared to wild-type mice. This could result in increased plasma levels of TNF-α after LPS challenge simply by summation of secreted products from a larger number of Mφ. However, our results indicate that similar numbers of activated Mφ are recruited to the peritoneal cavity in the SRKO and wild-type mice, and also to sites of granuloma formation (Fig. 3). Also, the differential response to LPS challenge occurs whether the mice are infected with BCG, injected with inactivated C. parvum, or left uninfected. Therefore, differences in numbers of Mφ cannot explain these results.
A second explanation is that SRKO and wild-type mice respond differently to LPS at the single cell level. For example, this might result from differences in relative levels of those receptors which trigger release of cytokines upon LPS binding, e.g., CD14, and those which bind LPS but are not thought to trigger a response, e.g., SR-A. Several receptors, such as CD14 and CD11c/CD18, are involved in the recognition and signaling of events after LPS binding (40, 41). Our unpublished observations indicate no difference in the level of expression of CD14 on wild type and SRKO BCG-elicited peritoneal cells. The data support the hypothesis that the response of activated Mφ to LPS depends to some extent on the relative levels of surface expression of SR-A and CD14. The smaller difference in LPS susceptibility between wild-type and SRKO mice compared with the extensive resistance of CD14 KO mice may reflect the fact that other scavenger receptors may also be involved in this protective role (42).
A third possibility is that SR-A expressed on Mφ and hepatic endothelium clears the LPS more rapidly from the plasma after challenge. If this theory is correct, then one might predict a longer plasma half-life of LPS in SRKO mice, resulting in continued stimulation of primed Mφ through CD14, and higher levels of proinflammatory cytokines. Recent studies have reported that in uninfected wild-type and SRKO mice, there is no detectable difference in the plasma half-life of LPS (43). However, if the mice are pretreated with liposomes to eliminate Kupffer cells and other Mφ populations, then the investigators are able to demonstrate a longer plasma half-life for LPS in the SRKO mice. This result may indicate that SR-A expressed on hepatic endothelium plays a key role in LPS clearance. In addition, recent work failed to show a difference in the clearance of modified LDL (DiIAcLDL) from the circulation of SRKO relative to wild-type mice (4).
However, there are several important differences from the BCG infection model described here. Previously we established, using in situ hybridization, that the activated Mφ in the granuloma are the main sources of both TNF-α and IL-1β after LPS challenge (44). Thus, activated Mφ in this model are not only able to secrete cytokines in response to LPS binding to CD14, but may also bind and mediate uptake of LPS through SR-A without signaling. This raises the intriguing possibility of a built-in defense mechanism whereby, under appropriate circumstances, the activated Mφ may bind and take up LPS through SR-A, and thus reduce the amount of TNF-α produced from the same cell through LPS-mediated CD14 signaling. Indeed, this system may be more complex, since the net result of exposure of an activated Mφ to LPS is likely to depend not only on the relative expression levels of CD14, SR-A, and other receptors, but also on interactions between signaling pathways within the cell. Binding of LPS–LBP complexes to certain domains of CD14 has been shown to result in activation of c-Jun NH2-terminal kinase, which results in transcription of genes encoding for proinflammatory regulators of the immune response (45). By comparison, the downstream events after engagement of SR-A with its ligands remain to be established.
Further studies are required to determine the relative importance of SR-A in both plasma clearance and in the cellular response to LPS. However, it seems clear that this work establishes SR-A as an effective protective mechanism in the host response to endotoxin. Our results indicate that the modulation of SR-A may provide a novel therapeutic approach to control of endotoxic shock in humans.
Acknowledgments
We thank Dr. S. Cobbold for help with the FACS® equipment, Dr. G. Milon for generously providing the BCG organisms, and Mr. L. Tomlinson for photography. Dr. M. Cortina-Borja provided helpful advice on statistical analysis.
This work was supported by grants from the Medical Research Council, UK. Richard Haworth is supported by a Veterinary Research Training Scholarship, awarded by the Wellcome Trust.
Footnotes
Abbreviations used in this paper: Ac, acetylated; BCG, bacillus Calmette-Guérin; CP, CAMPATH; DiI, 1,1-dioctadecyl-1,3,3,3′,3′-tetramethylindocarbocyanineperchlorate; LDL, low density lipoproteins; Mφ, macrophages; SR, scavenger receptor; SRKO, SR-A–deficient; TCP, tissue culture plastic.
References
- 1.Kodama T, Freeman M, Rohrer L, Zabrecky J, Matsudaira P, Krieger M. Type I macrophage scavenger receptor contains α-helical and collagen-like coiled coils. Nature (Lond) 1990;343:531–535. doi: 10.1038/343531a0. [DOI] [PubMed] [Google Scholar]
- 2.Rohrer L, Freeman M, Kodama T, Penman M, Krieger M. Coiled-coil fibrous domains mediate ligand binding by macrophage scavenger receptor type II. Nature (Lond) 1990;343:570–572. doi: 10.1038/343570a0. [DOI] [PubMed] [Google Scholar]
- 3.Freeman M, Ashkenas J, Rees DJD, Kingsley DM, Copeland NG, Jenkins NA, Krieger M. An ancient, highly conserved family of cysteine-rich protein domains revealed by cloning type I and type II murine macrophage scavenger receptors. Proc Natl Acad Sci USA. 1990;87:8810–8814. doi: 10.1073/pnas.87.22.8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature (Lond) 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 5.Acton S, Scherer P, Lodish H, Krieger M. Expression cloning of SR-B1, a CD36-related class B scavenger receptor. J Biol Chem. 1994;269:21005–21009. [PubMed] [Google Scholar]
- 6.Freeman M, Ekkel Y, Rohrer L, Penman M, Freedman NJ, Chisolm GM, Krieger M. Expression of type I and type II bovine scavenger receptors in Chinese hamster ovary cells: lipid droplet accumulation and nonreciprocal cross competition by acetylated and oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1991;88:4931–4935. doi: 10.1073/pnas.88.11.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 8.Krieger M. Molecular flypaper and atherosclerosis: structure of the macrophage scavenger receptor. Trends Biochem Sci. 1992;17:141–146. doi: 10.1016/0968-0004(92)90322-z. [DOI] [PubMed] [Google Scholar]
- 9.Hampton RY, Golenbock DT, Penman M, Krieger M, Raetz CRH. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature (Lond) 1991;352:342–344. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- 10.Dunne WD, Resnick D, Greenburg J, Krieger M, Joiner KA. The type I macrophage scavenger receptor binds to Gram-positive bacteria and recognizes lipoteichoic acid. Proc Natl Acad Sci USA. 1994;91:1863–1867. doi: 10.1073/pnas.91.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman J. Innate immunity of insects. Curr Opin Immunol. 1995;7:4–10. doi: 10.1016/0952-7915(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 12.Abrams JM, Lux A, Steller H, Krieger M. Macrophages in Drosophilaembryos and L2 cells exhibit scavenger receptor–mediated endocytosis. Proc Natl Acad Sci USA. 1992;89:10375–10379. doi: 10.1073/pnas.89.21.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearson A, Lux A, Krieger M. Expression cloning of dSR-C1, a class C macrophage–specific scavenger receptor from Drosophila melanogaster. . Proc Natl Acad Sci USA. 1995;92:4056–4060. doi: 10.1073/pnas.92.9.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janeway CA. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 15.Hughes DA, Fraser IP, Gordon S. Murine macrophage scavenger receptor: in vivo expression and function as receptor for macrophage adhesion in lymphoid and non-lymphoid organs. Eur J Immunol. 1995;25:466–473. doi: 10.1002/eji.1830250224. [DOI] [PubMed] [Google Scholar]
- 16.Pearson AM. Scavenger receptors in innate immunity. Curr Opin Immunol. 1996;8:20–28. doi: 10.1016/s0952-7915(96)80100-2. [DOI] [PubMed] [Google Scholar]
- 17.Fraser I, Hughes D, Gordon S. Divalent cation-independent macrophage adhesion inhibited by monoclonal antibody to murine scavenger receptor. Nature (Lond) 1993;364:343–346. doi: 10.1038/364343a0. [DOI] [PubMed] [Google Scholar]
- 18.Khoury JE, Thomas CA, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD. Scavenger receptor mediated adhesion of microglia to β-amyloid fibrils. Nature (Lond) 1996;382:716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- 19.Platt N, Suzuki H, Kurihara Y, Kodama T, Gordon S. Role for the class a macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc Natl Acad Sci USA. 1996;93:12456–12460. doi: 10.1073/pnas.93.22.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 21.McKnight AJ, MacFarlane AJ, Dri P, Turley L, Gordon S. Molecular cloning of F4/80, a murine macrophage–restricted cell-surface glycoprotein with homology to the G-protein–linked transmembrane 7 hormone receptor family. J Biol Chem. 1996;271:486–489. doi: 10.1074/jbc.271.1.486. [DOI] [PubMed] [Google Scholar]
- 22.Rabinowitz S, Gordon S. Macrosialin, a macrophage-restricted membrane sialoprotein differentially glycosylated in response to inflammatory stimuli. J Exp Med. 1991;174:827–836. doi: 10.1084/jem.174.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith MJ, Koch GLE. Differential expression of murine macrophage surface glycoprotein antigens in intracellular membranes. J Cell Sci. 1987;87:113–119. doi: 10.1242/jcs.87.1.113. [DOI] [PubMed] [Google Scholar]
- 24.Fraser I, Hughes D, Gordon S. Divalent cation-independent macrophage adhesion inhibited by monoclonal antibody to murine scavenger receptor. Nature (Lond) 1993;364:343–346. doi: 10.1038/364343a0. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharya A, Dorf ME, Springer TA. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981;127:2488–2495. [PubMed] [Google Scholar]
- 26.Hale G, Cobbold SP, Waldmann H, Easter G, Matejtschuk P, Coombs RR. Isolation of low-frequency class-switch variants from rat hybrid myelomas. J Immunol Methods. 1987;103:59–67. doi: 10.1016/0022-1759(87)90242-0. [DOI] [PubMed] [Google Scholar]
- 27.Snedecor, G.W., and W.G. Cochran. 1980. Statistical Methods. 7th ed. Iowa State University Press, Ames, Iowa.
- 28.Gordon S, Keshav S, Stein M. BCG-induced granuloma formation in murine tissues. Immunobiology. 1994;1994:369–377. doi: 10.1016/S0171-2985(11)80442-0. [DOI] [PubMed] [Google Scholar]
- 29.Johnson RBJ, Godzik CA, Cohn ZA. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978;148:115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penman M, Lux A, Freedman NJ, Rohrer L, Ekkel Y, Mckinstry H, Resnick D, Krieger M. The type I and type II bovine scavenger receptors expressed in Chinese hamster ovary cells are trimeric proteins with collagenous triple helical domains comprising noncovalently associated monomers and Cys83-disulfide–linked dimers. J Biol Chem. 1991;266:23985–23993. [PubMed] [Google Scholar]
- 31.Wysocka M, Kubin M, Trinchieri G. Interleukin 12 is required for γ-IFN production and lethality in LPS-induced shock in mice. Eur J Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]
- 32.Raetz CRH. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- 33.Kusunoki T, Hailman E, Wright SD. Molecules from Staphlococcus aureusthat bind CD14 and stimulate innate immune responses. J Exp Med. 1995;182:1673–1682. doi: 10.1084/jem.182.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elomaa O, Kangas M, Tryggvason K. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell. 1995;80:603–609. doi: 10.1016/0092-8674(95)90514-6. [DOI] [PubMed] [Google Scholar]
- 35.Kindler V, Sappino A-P, Grau GE, Piguet P-F, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 36.Kamijo R, Le J, Shapiro D, Havell EA, Huang S, Aguet M, Bosland M, Vilcek J. Mice that lack the interferon-γ receptor have profoundly altered responses to infection with Bacillus Calmette-Guérin and subsequent challenge with lipopolysaccharide. J Exp Med. 1993;178:1435–1440. doi: 10.1084/jem.178.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosen H, Gordon S, North RJ. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. J Exp Med. 1989;170:27–37. doi: 10.1084/jem.170.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlesinger LS, Bellinger-Kawahara CG, Payne NR, Horwitz MA. Phagocytosis of Mycobacterium tuberculosisis mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2711–2780. [PubMed] [Google Scholar]
- 39.Krieger M, Acton S, Ashkenas J, Pearson A, Penman M, Resnick D. Molecular flypaper, host defense, and atherosclerosis. J Biol Chem. 1993;268:4569–4572. [PubMed] [Google Scholar]
- 40.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science (Wash DC) 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 41.Ingalls RR, Golenbock DT. CD11c/CD18, a transmembrane signaling receptor for lipopolysaccharide. J Exp Med. 1995;181:1473–1479. doi: 10.1084/jem.181.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haziot A, Ferrero E, Goyert SM. Resistance to endotoxin shock and reduced dissemination of gram negative bacteria in CD-14 deficient mice. Immunity. 1996;4:407–414. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 43.Amersfoort, E.S.V., T.J.C. Van Berkel, and J. Kuiper. 1996. Clearance of lipopolysaccharide in scavenger receptor knock out mice. J. Leukocyte Biol. 210(Suppl):48.
- 44.Keshav, S., M. Stein, L.P. Chung, and S. Gordon. 1992. Cytokine gene expression in situ: differential expression of lysozyme, IL-1, and TNF mRNA in murine liver during BCG infection. In Mononuclear Phagocytes. R.V. Furth, editor. Kluwer Academic, Dordrecht, The Netherlands. 366–374.
- 45.Hambleton J, Weinstein SL, Defranco AL. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci USA. 1996;93:2774–2778. doi: 10.1073/pnas.93.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]