Abstract
Mice lacking the transcription factor interferon consensus sequence binding protein (ICSBP), a member of the interferon regulatory factor family of transcription proteins, were infected with the intracellular protozoan, Toxoplasma gondii. ICSBP-deficient mice exhibited unchecked parasite replication in vivo and rapidly succumbed within 14 d after inoculation with an avirulent Toxoplasma strain. In contrast, few intracellular parasites were observed in wild-type littermates and these animals survived for at least 60 d after infection. Analysis of cytokine synthesis in vitro and in vivo revealed a major deficiency in the expression of both interferon (IFN)-γ and interleukin (IL)-12 p40 in the T. gondii exposed ICSBP−/− animals. In related experiments, macrophages from uninfected ICSBP−/− mice were shown to display a selective impairment in the mRNA expression of IL-12 p40 but not IL-1α, IL-1β, IL-1Ra, IL-6, IL-10, or TNF-α in response to live parasites, parasite antigen, lipopolysaccharide, or Staphylococcus aureus. This selective defect in IL-12 p40 production was observed regardless of whether the macrophages had been primed with IFN-γ. We hypothesize that the impaired synthesis of IL-12 p40 in ICSBP−/− animals is the primary lesion responsible for the loss in resistance to T. gondii because IFN-γ–induced parasite killing was unimpaired in vitro and, more importantly, administration of exogenous IL-12 in vivo significantly prolonged survival of the infected mice. Together these findings implicate ICSBP as a major transcription factor which directly or indirectly regulates IL-12 p40 gene activation and, as a consequence, IFN-γ–dependent host resistance.
ICSBP is a transcription factor belonging to the interferon regulatory factor (IRF)1 family (1) which also includes the IRF-1, IRF-2, IRF-3, interferon-stimulated gene factor (ISGF)3γ, and Pip/IRF4 proteins (2–6). The gene for interferon consensus sequence binding protein (ICSBP) has been sequenced in both human and mouse, and in the latter species has been localized to chromosome 8 (7). Unlike the other members of the IRF family, ICSBP expression is limited to cells of the immune system including resting B cells (8) and activated macrophages (9) and T cells (8). Proteins of the IRF family, including ICSBP, bind to the interferon-stimulated response element (ISRE) and control activities of promoters carrying this element, which is present in many IFN-α/β–inducible genes (10). IRF-1 and ISGF3 are activators of IFN-α/β–inducible genes (2, 11), while IRF-2 and ICSBP are repressors of the same set of promoters (3, 8). Although the above observations link ICSBP to IFN-α/β–regulated gene activation, ICSBP expression is also stimulated by IFN-γ suggesting that the transcription factor is a component of the gamma-activated site signal transducer and activator of transcription pathway of transcription (GAS-STAT).
Analyses of knockout (ko) mice carrying disrupted IRF genes have begun to elucidate the distinct roles of ICSBP and other IRF family proteins in host defense against various infectious agents (12–18). It was recently noted that ICSBP-deficient mice display increased susceptibility to infection with vaccinia and lymphocytic choriomeningitis viruses but not vesicular stomatitis virus (13). This differential susceptibility points to a defect in IFN-γ– rather than IFN-α/β–dependent defense, since protection against vesicular stomatitis virus is primarily associated with type I interferon, whereas control of vaccinia virus and lymphocytic choriomeningitis viruses involves cooperation between both type I and II IFNs (19). Similarly, ICSBP-deficient mice were shown to be highly susceptible to infection with Listeria monocytogenes, a bacterium that is also controlled by IFN-γ– dependent host resistance mechanisms (16, 20). Nevertheless, the above studies did not address the possibility that induction of IFN-γ itself might be impaired in these animals, a defect that would be consistent with the observation that constitutive expression of mRNA for IFN-γ as well as IL-12 p40 is diminished in splenic tissue from naive ICSBP−/− mice (13). Since IL-12 plays a critical role in IFN-γ induction, the impaired IFN-γ–dependent resistance displayed by ICSBP−/− animals could thus result from a lesion in IL-12 responsiveness.
The IL-12 protein is a heterodimeric cytokine composed of heavy (40 kD) and light (35 kD) chain subunits that are encoded by two genes on separate chromosomes. Although p35 expression is constitutive, p40 expression is induced upon stimulation. Thus, p40 expression governs the production of the bioactive cytokine (reviewed in reference 21). The 70-kD IL-12 heterodimer is synthesized by macrophages, neutrophils, and dendritic cells and triggers effective immune responses indirectly by stimulating NK cells to produce IFN-γ and by inducing naive T cells to develop into IFN-γ–producing CD4+ T cells (Th1) (22–24). IFN-γ, in turn, induces expression of effector molecules that elicit potent antiviral, antibacterial, and antiparasitic activities (reviewed in reference 25). The IL-12 p40 gene is highly sensitive to IFN-γ priming, and this effect results in a positive feedback loop on IL-12 p40 mRNA and protein synthesis (26, 27).
In this paper, the role of ICSBP in regulating IL-12 function was examined using a model of Toxoplasma gondii infection in which the cytokine is known to be critical for host resistance (reviewed in reference 28). Infection with this apicomplexan protozoan is characterized by a brief acute stage during which rapidly dividing tachyzoites disseminate to peripheral host tissues followed by a chronic stage in which the latent bradyzoite form persists in muscle and CNS tissue. Studies of host immunity to the parasite have identified IFN-γ as the key cytokine that restricts T. gondii expansion early in infection and prevents reactivation of dormant parasite stages (reviewed in reference 29). Nonetheless, neither T nor NK cells are directly stimulated by the parasite to synthesize IFN-γ; rather, T. gondii triggers synthesis of IL-12 by cells belonging to the macrophage/ dendritic cell lineage, which in turn drives IFN-γ production by the former cell populations (reviewed in reference 28).
In the present study, we show that ICSPB−/− mice fail to develop early resistance to Toxoplasma infection due to a selective defect in IL-12 p40 production. We further demonstrate that ICSBP−/− cells display a generalized deficiency in IL-12 p40 mRNA and protein responses when stimulated with IFN-γ and parasitic or bacterial products. We conclude that ICSBP plays an essential role in the activation of the IL-12 gene and, as such, this transcription factor can directly regulate IFN-γ–dependent host defense.
Materials and Methods
Experimental Animals
Mice with a targeted disruption of the ICSBP gene (ICSBP−/−) were generated as previously described (13). In brief, a neomycin resistance gene (PGK-neo) was inserted into the second exon, and a reading frame disruption was introduced by multiple stop codons. ICSBP−/− animals were backcrossed six times to the C57BL/6 strain, and wild-type (wt), heterozygous and homozygous mice were generated by brother–sister mating of heterozygote mice. Young ICSBP−/− mice (6–12 wk old) were used in all experiments as they do not display the CML-like syndrome which can develop in aged animals. Breeding pairs of IL-12 p40−/− mice (30) were kindly provided by Dr. Jeanne Magram (Hoffman-LaRoche, Nutley, NJ). Animals carrying the IL-12 p40 mutant allele were backcrossed five times to the C57BL/6 genetic background followed by intercross of the heterozygotes to generate mice homozygous for the targeted mutation. Breeding pairs of mice with a targeted disruption of the IFN-γ gene were originally provided by Dyana Dalton and T. Stewart (Genentech, San Bruno, CA) (31). The IFN-γ ko mice used were at the seventh generation of backcrossing to the C57BL/6 strain. Animals were housed in specific pathogen-free conditions, and both male and female mice were used for experiments at 5–12 wk of age.
Parasites and Experimental Infection
Tachyzoites of the virulent RH strain were maintained in vitro by infection of human foreskin fibroblasts and biweekly passage in DMEM (GIBCO BRL, Gaithersburg, MD) supplemented with 1% fetal calf serum (Hyclone Laboratories, Logan UT), penicillin (100 U/ml), and streptomycin (100 μg/ml). Cysts of the avirulent ME49 strain (initially provided by Dr. J. Remington, Palo Alto Research Foundation, CA) were harvested from the brains of C57BL/6 mice that had been inoculated with ∼20 cysts i.p. 1 mo prior. For experimental infections, mice received 20 ME49 cysts by the i.p. route. Soluble tachyzoite antigen (STAg) was prepared from T. gondii (RH strain) tachyzoites by repeatedly sonicating the parasites, centrifuging the sonicate at 100,000 g, collecting the supernatant, and then determining the protein concentration.
In Vivo Assessment of Acute Infection
Acute tachyzoite growth was assessed by evaluating cytocentrifuge smears of cells from infected animals. Samples were prepared from 1.5 × 105 peritoneal exudate cells in a Cytospin (Shandon Lipshaw, Pittsburgh, PA) set for 5 min at 1,000 rpm. Slide preparations were fixed in absolute methanol for 5 min and then stained with Diff-Quik (Baxter Healthcare Corporation, McGaw Park, IL), a modified Wright-Giemsa stain, as specified by the manufacturer. Differential analyses, including assessment of the number of infected cells, were performed on 400–500 cells using an oil immersion (100× objective).
In Vitro Responses of Cells from Infected Animals
Single cell suspensions were prepared from spleen and PECs harvested at various time points after infection. PECs were cultured at 4 × 105 cells and spleen cells at 8 × 105 per well in a total volume of 200 μl in a medium consisting of RPMI-1640 (BioWhittaker, Walkersville, MD) supplemented with 10% fetal calf serum, penicillin (100 U/ml), streptomycin (100 μg/ml), L-glutamine (2 mM), Hepes (10 mM), and 2-mercaptoethanol (5 × 10−5 M) in the presence or absence of STAg (5 μg/ml). Supernatants were harvested 72 h later for IFN-γ and IL-12 p40 measurements (see Cytokine Analysis below).
Serum Preparation
Blood was collected from mice at the time of death and allowed to clot at room temperature for 2 h. Sera were then separated from the individual samples after a 5-min centrifugation at 5,000 rpm and assayed for cytokine content.
Responses of Macrophage-Enriched Populations from Uninfected Animals
Resident macrophages and inflammatory macrophages were harvested from animals that were untreated or inoculated i.p. 4–5 d previously with 1.5 ml of 3% thioglycollate (Sigma Chemical Corporation, St. Louis, MO). Cells were harvested by injecting cold RPMI into the peritoneal cavity and plated at 2 × 106 per ml in 24- and 96-well plates for 2 h in the presence or absence of rMuIFN-γ 200 U/ml (generously provided by Genentech, Inc., South San Francisco, CA). In the experiments indicated, cells were incubated for 2 h at 37°C, and nonadherent cells were removed before the addition of IFN-γ. Cultures were then incubated in the presence of medium alone, STAg (5 μg/ml), RH tachyzoites (0.2 or 1.0 per cell), LPS from Escherichia coli K235 (200 ng/ml; Sigma) or Staphylococcus aureus Cowan strain 1 (SAC) (Pansorbin; Calbiochem-Gehring, La Jolla, CA; 0.0075%). In selected experiments, neutralizing mAb specific for IL-4 (32) and IL-10 (33) (Genzyme Diagnostics, Cambridge, MA) were included to assess the effects of these cytokines on the induction of monokine synthesis by cell cultures. 24-well plates were harvested 6 h after stimulation. A second set of cultures was incubated for 48 h and the supernatants from these cells used to measure the induction of IL-12 p40 protein and of nitrite (NO2).
Parasite killing was assessed in parallel cultures using a standard T. gondii proliferation assay (34, 35). In brief, 96-well plates containing peritoneal cells and media alone or media and live tachyzoites were pulsed with Uracil-[5,6-3H] (ICN Pharmaceuticals, Inc., Irvine CA) at 0.5 μCi/well from 24 to 36 h after culture initiation. T. gondii growth was assessed by measuring the incorporation of radioactive uracil by the parasites. The percentage of killing was determined by the following calculation (values are in cpm):
[1−({[IFN-γ(+parasites)]−[IFN-γ(−parasites)]}− {[media(+parasites)]−[media(−parasites)]})] × 100%
Cytokine Assays
ELISA Assay of Cytokine Proteins (IFN-γ, IL-12 p40).
Levels of IFN-γ and IL-12 p40 protein were assayed by two-site ELISA as previously described (36, 37). Cytokine levels were quantitated by reference to standard curves generated with rIFN-γ (donated by Genentech) or rIL-12 (provided by Genetics Institute, Cambridge, MA).
RT-PCR Measurement of Cytokine mRNAs.
RNA was prepared from spleens dissolved in RNAzol (Tel-Test, Inc., Friendswood, TX) by phenol-chloroform extraction. cDNAs were synthesized from total RNA using Molony murine leukemia virus reverse transcriptase (Gibco BRL) in a reaction mixture containing 75 mM KCl, 3 mM MgCl2, 50 mM Tris-HCl, pH 8.3, 0.25 mM dNTPs, 0.8 U of RNasin, and random hexamer primers (Promega, Madison, WI). cDNA was added to a solution containing 50 mM KCl, 3 mM MgCl2, 10 mM Tris-HCl, pH 9.0, 250 μM of dNTPs, and 1 U Taq DNA polymerase (Promega) and 80 μg of sense and antisense primers in a total volume of 25 or 50 μl. For detection of IL-12 p40, the sense and antisense primers 5′-GTGAAGCACCAAATTACTCCGG-3′ and 5′-GCTTCATCATCTGCAAGTTCTTGGG-3′ were used and probed with 5′-CAGTGTCCTGCCAGGAGGATGT-3′. For IL-12 p35, primers 5′-GGCTACTAGAGAGACTTCTTCC-3′ and 5′-GTGAAGCAGGATGCAGAGCTTC-3′ were used and probed with 5′-GATGACCAGACAGAGTTCCAG-3′. Oligomers for HPRT, IL-4, IL-10, and TNF-α have been described (38). Reactions for spleen RNAs was initiated by denaturation at 95°C for 4 min followed by 28 cycles of amplification reactions at 94°C for 1 min, 55°C for 1 min, and 72°C for 1.5 min for IL-12 p40, IL-12 p35, IL-4, IL-10, and TNF-α. Reactions for HPRT had 22 cycles. Reactions under these conditions were within the linear range of amplification (39). Samples were then separated on 1 or 1.5% agarose gel and analyzed by Southern blot hybridization with [32P]-labeled oligonucleotide probes.
RNAse Protection Assay of Cytokine Gene Expression.
Peritoneal macrophages were prepared from thioglycollate-elicited peritoneal exudates (see above). Total RNA (3 to 5 μg) was subjected to multi-probe RNAse protection assay by using a mouse cytokine/ chemokine template set (mck-2) supplied by PharMingen (San Diego, CA) as a DNA template. The antisense riboprobes were synthesized with the T7 RNA polymerase to an average specific activity of 1.5 × 109 dpm/μg in an in vitro transcription system provided by Ambion (Austin, TX). Total RNA (3–4 μg) was annealed to 3 × 105 dpm of riboprobes overnight at 55°C in 300 mM NaCl. The duplexes were digested with a cocktail containing 10 mM Tris-HCl, pH 7.5, 5 mM EDTA, 300 mM NaCl, 40 μg/ml RNase A, and 2 μg/ml RNase T1 (Boehringer Mannheim, Indianapolis, IN) for 1 h at 30°C. Samples were purified by phenol-chloroform extraction and resolved on a 6% denaturing polyacrylamide gel. The dried gels were autoradiographed on XAR-5 (Eastman-Kodak, Rochester, NY) with an intensifying screen (DuPont, Wilmington, DE).
Statistical Analyses
Statistical determinations of the difference between means of experimental groups was determined using an unpaired, two-tailed Student's t test.
Results
ICSBP− /− Mice Succumb to Acute Infection with T. gondii.
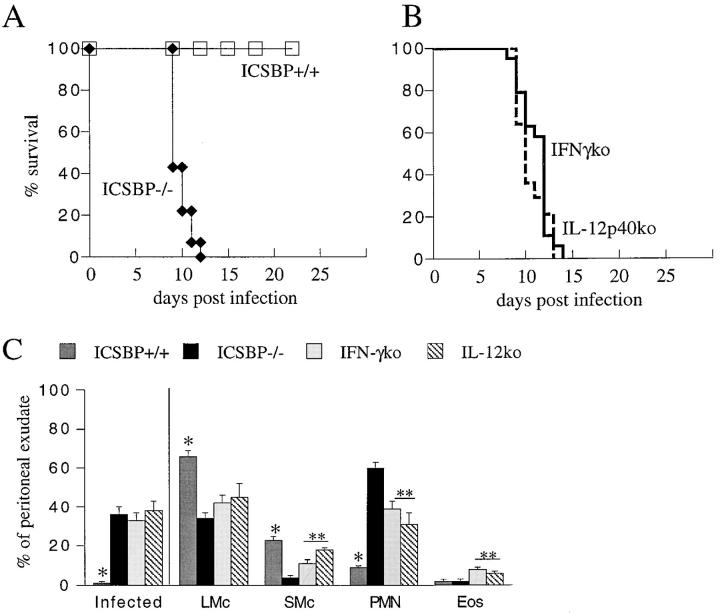
The observation of decreased constitutive levels of IL-12 p40 and IFN-γ mRNA in spleen cells from ICSBP−/− mice (13) suggested that these mice might display increased susceptibility to T. gondii infection. However, we and others have described an association between neutrophil responses and control of infection (40, 41). Since ICSBP−/− animals display a predilection toward neutrophilia (13), the mutation could potentially result in increased rather than decreased resistance to parasite exposure. To assess their susceptibility to T. gondii, ICSBP−/− mice were infected with 20 cysts of the ME49 strain and their survival compared with IFN-γ−/−, IL-12 p40−/−, and ICSBP wt littermates. In agreement with previous reported findings (27, 40), the IFN-γ−/− and IL-12 p40−/− strains succumbed to the infection within 14 d of parasite exposure, while the wt animals survived throughout the 30-d duration of the experiment (Fig. 1, A and B). In contrast to the wt controls, ICSBP−/− mice exhibited an acute mortality pattern comparable to that of IFN-γ−/− and IL-12 p40−/− mice. Enumeration of infected cells in cytospin smears of PECs taken 5 d after parasite exposure revealed numerous cells containing parasites in ICSBP−/− but not wt animals (> 35 versus < 1%, Fig. 1 C). PEC populations from wt controls, IFN-γ−/−, IL-12p40−/−, and ICSBP−/− mice were all similar in composition before infection consisting of 70– 80% large mononuclear cells (LMc), 20-30% small mononuclear cells (SMc), 1–3% PMN, 2–4% eosinophils (Eos), and 1–3% mast cells. At 5 d after infection the number of cells recovered increased from two- to fivefold and the composition of the exudate was significantly different amongst the groups of mice (Fig. 1 C). As compared with the wt animals, exudates from infected ICSBP−/− mice contained a lower percentage of LMc and SMc and the difference was largely attributable to a corresponding increase in PMNs. However, cells from infected IL-12 p40−/− and IFN-γ−/− mice were comparable and contained a greater percentage of SMc and Eos than the exudates from ICSBP−/− animals.
Figure 1.
Progression of T. gondii infection in ICSBP−/− versus control animals. Mice were infected by the i.p. route of infection with 20 ME49 cysts and cumulative mortality monitored. The data shown in (A and B) are pooled from three independent experiments and involve a total of 14 ICSBP−/−, 15 ICSBP+/+, 14 IL-12 p40 ko and 19 IFN-γ ko animals. (C) Qualitative analysis of the PEC present in wt (dark grey bars), ICSBP−/− (black bars), IFN-γ ko (pale grey bars) and IL-12 p40 ko (hatched bars) animals at 5 d after infection with ME49. Cytospin smears of PEC were prepared and stained with Diff-Quik reagent, as described in Materials and Methods. The percentage of infected cells, large mononuclear cells (LMc; macrophages, monocytes, and blasting lymphocytes), small mononuclear cells (SMc; resting lymphocytes), polymorphonuclear cells (PMN) and eosinophils (Eos) were calculated. Uninfected mice of each of the four strains did not differ significantly in the composition of their PECs. Values shown are the mean ± SE of 6–12 infected animals per group. Statistically significant differences between the values observed in ICSBP−/− and wt (*) or ICSBP−/− and both IFN-γ ko−/− and IL-12 p40 ko (**) samples are indicated (P ⩽0.05).
Toxoplasma gondii-Infected, ICSBP−/− Mice Fail to Develop IFN-γ and IL-12 p40 Responses to the Parasite.
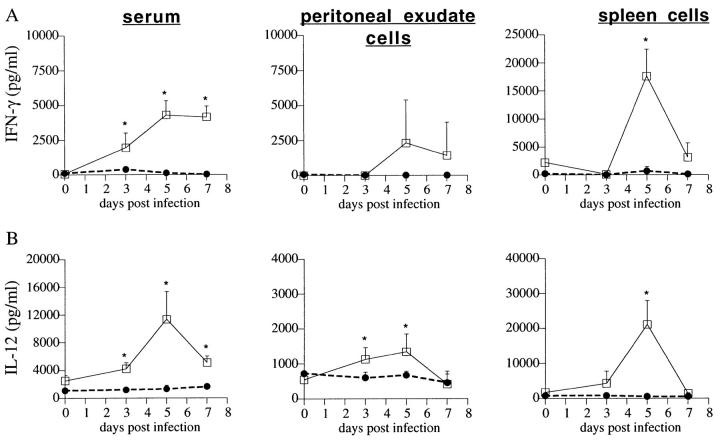
The rapid development of fulminant infection in ICSBP−/− mice and their subsequent mortality within the first 2 wk of parasite exposure suggested that induction of IFN-γ synthesis might be defective in these animals. To investigate this hypothesis, IFN-γ levels were compared in ICSBP−/− and ICSBP+/+ mice at different times during the first week of T. gondii infection. Wt mice displayed a characteristic elevation in IFN-γ protein as measured directly in the serum and in cultures of cells from the peritoneum and spleen (Fig. 2 A). In contrast, IFN-γ levels did not increase in ICSBP−/− mice at any of the time points analyzed (Fig. 2 A), and addition of STAg to the cultures failed to amplify the production of cytokine (data not shown).
Figure 2.
Kinetics of cytokine synthesis in infected wt (open squares) and ko (solid circle) animals. Serum, PECs, and spleen tissue were harvested from ICSBP−/− and ICSBP+/+ mice on days 0, 3, 5, and 7 after i.p. infection with ME49. IFN-γ (A) and IL-12 p40 (B) levels were measured by ELISA in diluted sera or in 72-h culture supernatants of single-cell suspensions as described in Materials and Methods. Data points are the mean ± SE for four individual mice. An asterisk indicates a statistically significant difference (P <0.05 by Student's t test) between the values observed in ICSBP−/− and ICSBP+/+ samples. The experiment shown is representative of three performed.
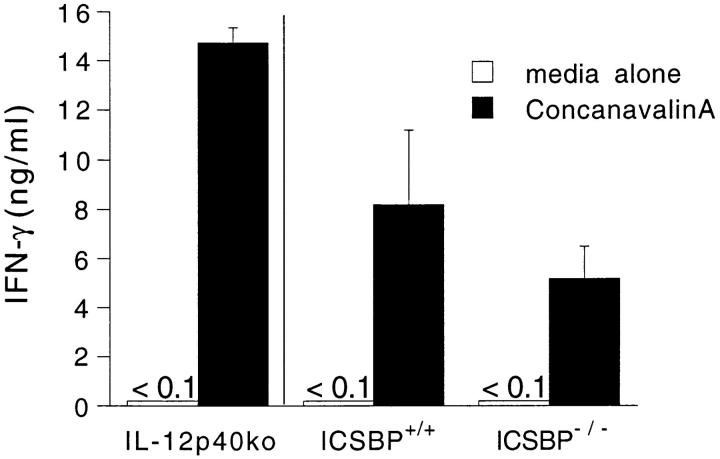
Since IL-12 triggers early IFN-γ synthesis in T. gondii-infected mice (reviewed in reference 28), we asked whether the diminished IFN-γ expression might result from defective IL-12 activity in the ICSBP−/− mice. Whereas IL-12 p40 increased dramatically in the serum and in cultures of peritoneal and spleen cells from wt mice, these responses were essentially absent in the mutant animals (Fig. 2 B). Levels of IL-12 p40 protein were also two- to threefold lower in serum and splenic cell cultures from naive ICSBP−/− compared with ICSBP+/+ mice. Notably, however, spleen cells from ICSBP−/− mice produced substantial amounts of IFN-γ when stimulated with concanavalin A, a response which is IL-12 p40 independent (Fig. 3).
Figure 3.
Con A–induced IFN-γ production by spleen cells from uninfected ICSBP+/+, ICSBP−/−, and IL-12 p40−/− mice. Spleen cells were cultured with media alone (white bars) or Con A (black bars) at 5 μg/ml and the supernatants assayed for IFN-γ 72 h later. Cytokine synthesis (mean ± SE) is shown for four individual animals per group.
Defective IL-12 p40 mRNA Response in T. gondii-infected, ICSBP− /− Splenic Tissue.
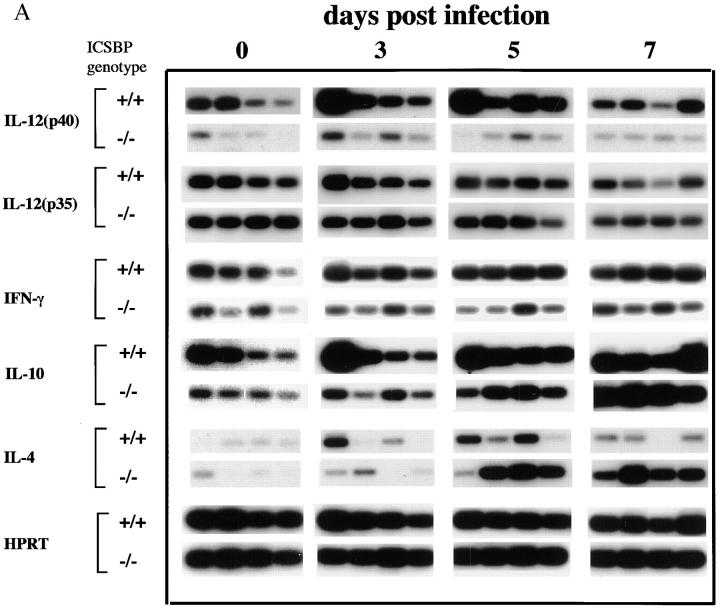
To evaluate whether the lack of IL-12 p40 production in infected ICSBP−/− mice is attributable to a deficiency in the mRNA expression, semiquantitative RT-PCR was performed to measure levels of transcripts for IL-12 p40 and p35 during parasite infection (Fig. 4). After parasite inoculation, IL-12 p40 mRNA levels remained low in ICSBP−/− mice throughout the 7 d of the experiment, with only minor variation among animals. In contrast, IL-12 p40 mRNA levels were markedly increased in wt mice after infection, reaching maximum levels on days 3 and 5 and declining by day 7. Of note, the levels of mRNA encoding the constitutively expressed IL-12 p35 subunit were comparable between ICSBP−/− and ICSBP+/+ mice before parasite exposure and did not significantly change for 7 d after infection. Consistent with the reduced production of IFN-γ protein (Fig. 2), the IFN-γ transcript levels remained low in the ICSBP−/− spleens, while clearly increasing in tissues from +/+ animals.
Figure 4.
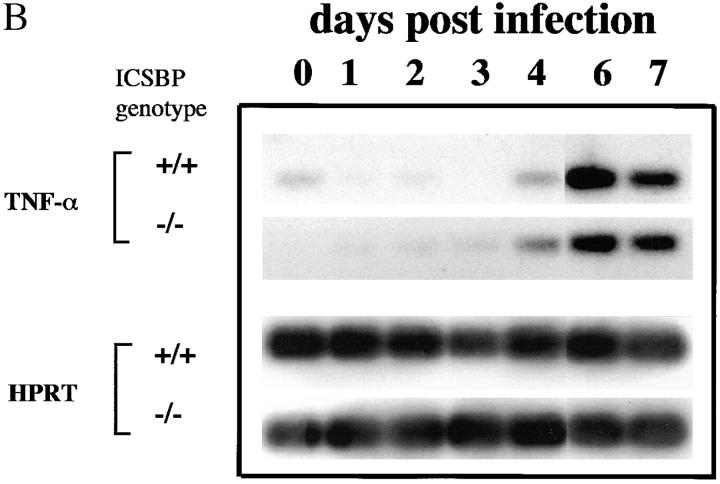
Kinetics of cytokine mRNA expression in splenic tissue from T. gondii-infected ICSBP+/+ and ICSBP−/− mice. (A) Splenic tissue was harvested from 4 individual mice at days 0, 3, 5, and 7 after parasite exposure from the same animals studied in Fig. 2. Extracted RNAs were subjected to quantitative RT-PCR analysis using primers specific for IL-12 p40, IL-12 p35, IFN-γ, IL-10, IL-4, and HPRT genes. (B) RNA from four individual mice were pooled and levels of TNFα expression were determined by RT-PCR as performed in (A). Similar results were attained in a second experiment.
We also assayed expression of IL-4, a signature cytokine for Th2 responses. Before infection, IL-4 mRNA was undetectable in both ICSBP−/− and ICSBP+/+ mice. On days 5 and 7, IL-4 transcripts were induced in all ICSBP−/− mice and, with the exception of one sample, these animals displayed uniformly higher expression of IL-4 mRNA than did wt mice. IL-10 mRNA was also detected in both wt and ICSBP−/− mice before and after infection. On days 3 and 5, the levels were somewhat higher in ICSBP+/+ mice than in ICSBP−/− mice, although they became similar by day 7. Levels of HPRT mRNA, measured as a housekeeping gene control, were comparable for all samples tested. Expression of TNF-α transcripts was also investigated since IL-12 and TNF-α are co-induced in macrophages exposed to T. gondii (42). As shown in Fig. 4 B, TNF-α mRNA was clearly induced in both wt and ICSBP−/− mice at comparable levels.
Peritoneal Macrophages from ICSBP− /− Mice Exhibit a Global Impairment in IL-12 p40 Responses.
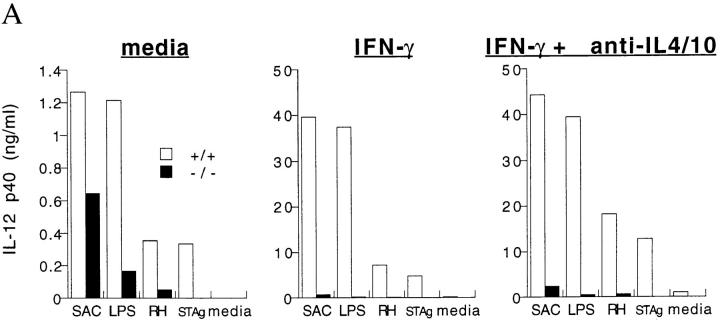
Having demonstrated that mice deficient in ICSBP express negligible amounts of IL-12 p40 after exposure to T. gondii in vivo, we next asked whether cells from these animals are also defective in their IL-12 response to other stimuli both in the absence and presence of an IFN-γ priming signal. The ability of adherent, thioglycollate-elicited peritoneal macrophages from uninfected mice to produce IL-12 p40 protein after stimulation in vitro was assessed. In both +/+ and −/− cultures, >85% of the adherent population were macrophages as determined by their expression of the Mac-1 but not GR-1 cell surface markers (data not shown). Exposure of wt cells to live tachyzoites, STAg, LPS, or heat-killed SAC resulted in increased IL-12 p40 levels. In contrast, while cells from ICSBP−/− mice also responded to these stimuli, the relative increase in IL-12 p40 production was markedly less (Fig. 5 A, left). In the four individual experiments performed, the most dramatic reduction in IL-12 synthesis resulting from the ICSBP mutation was observed in the response to live tachyzoites or STAg (always >80%), followed by LPS (>70%) and then SAC (>50%). This hierarchy was consistently observed in each experiment.
Figure 5.
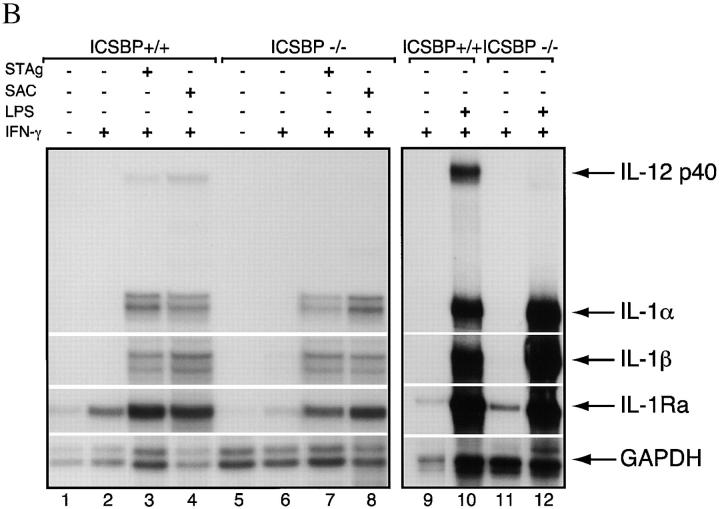
Defective IL-12 p40 protein and mRNA response in thioglycollate-elicited macrophage-enriched populations from ICSBP−/− and ICSBP+/+ mice. (A) IL-12 p40 protein synthesis in cultures of −/− (black bars) and +/+ (white bars) cells. (B) IL-12 p40 mRNA induction in peritoneal macrophages stimulated in vitro. PECs were collected from five mice that had been injected with thioglycollate 5 d earlier. Pooled adherent cells (macrophage enriched) were cultured in triplicate in the presence of SAC (1:1,000), LPS (200 ng/ml), RH (0.2 tachyzoites/cell), STAg (5 μg/ml), or media alone. Where indicated, cells were incubated with IFN-γ (200 U/ml) and/or anti–IL-4/10 mAbs (10 μg/ml each) for 2 h before the addition of stimuli and throughout the culture period. Cells were collected at 6 h for RNase protection assay and supernatants at 48 h for protein measurement by ELISA. Three μg of RNA were subjected to RNase protection assay to detect transcripts for IL-12 p40, IL-1α, IL-1β, IL-1Ra, and GAPDH. The experiment shown is representative of three performed.
Adherent, thioglycollate-elicited cell populations produce significant levels of IFN-γ upon stimulation with STAg (Sher, A., unpublished findings) and thus the influence of ICSBP on IL-12 p40 production by these cells could reflect an indirect effect on IFN-γ priming. To rule out this possibility, we evaluated the response of spleen cells from uninfected mice to STAg stimulation, a system in which IL-12 p40 responses are induced de novo in the absence of endogenous IFN-γ (55). Spleen cells from wt and IFN-γ−/− animals produced high levels of IL-12 p40 when exposed to STAg in vitro (Table 1). The latter response was totally absent in the ICSBP−/− cultures. Moreover, addition of an excess of neutralizing mAb against IFN-γ failed to reduce IL-12 p40 production by the wt cells, confirming the IFN-γ–independent nature of that response.
Table 1.
Synthesis of IL-12 p40 by ICSBP− /− Spleen Cells
| IL-12p40 (pg/ml) | ||||||
|---|---|---|---|---|---|---|
| Media | STAg | STAg + anti–IFN-γ | ||||
| ICSBP+/+ | 315 ± 27 | 1166 ± 423 | 1064 ± 457 | |||
| ICSBP−/− | ⩽100 | ⩽100 | ⩽100 | |||
| IFN-γ ko | ⩽100 | 2864 ± 632 | 2940 ± 619 | |||
Spleen cells from individual animals were cultured with media, STAg, or STAg + IFN-γ as described in Materials and Methods. Values are the IL-12 p40 measurements (mean ± SD) for three individual mice.
Activation of IL-12 p40 gene transcription is greatly enhanced when macrophages are preexposed to IFN-γ (26). To assess the role of ICSBP in this second level of IL-12 triggering, we measured its synthesis in IFN-γ–primed, thioglycollate-elicited macrophage populations (Fig. 5 A, middle). Addition of rIFN-γ to the wt cell cultures significantly increased IL-12 p40 levels in response to STAg, live tachyzoites, LPS, or SAC. In striking contrast, in 4 of 4 experiments, the level of IL-12 p40 synthesis by ICSBP−/− cells was not affected by the addition of rIFN-γ to the cultures. Similar results were obtained in experiments in which IFN-γ–primed resident PEC populations from ICSBP−/− or ICSBP+/+ mice were used (data not shown).
Since IL-12 p40 synthesis by macrophages is known to be suppressed by IL-10 or IL-4 (43, 44), the decreased IL-12 p40 mRNA expression in infected ICSBP−/− as compared with ICSBP+/+ mice could conceivably result from the effects of these downregulatory cytokines. Indeed, as noted earlier (Fig. 4), overproduction of IL-4 mRNA was observed in spleen cells from infected ICSBP−/− animals. Addition of neutralizing mAbs against both IL-4 and IL-10 resulted in a two- to threefold increase in the IL-12 p40 response in both ICSBP−/− and ICSBP+/+ cultures (Fig. 5 A, right). Nonetheless, even in cultures containing the anti–IL-4/10 mAbs, the IL-12 p40 mRNA and protein levels in ICSBP−/− cells were 20–40-fold less than those of comparably treated wt cells.
The Induction of IL-12 p40 Transcripts is Selectively Impaired in ICSBP− /− Macrophages.
To confirm that the observed impairment of IL-12 p40 expression occurs at the RNA level, RNAse protection and RT-PCR assays were performed on thioglycollate elicited macrophage populations exposed to T. gondii or bacterial stimuli (Fig. 5 B and 6). The issue of whether the above stimuli induce other cytokine genes in ICSBP−/− macrophages was also addressed. RNAse protection results (Fig. 5 B) demonstrate that IFN-γ primed ICSBP+/+ macrophages express IL-12 p40 transcripts when stimulated by SAC, STAg or LPS. In contrast, none of these agents induced detectable IL-12 p40 mRNA in ICSBP−/− macrophages. Although IFN-γ alone did not stimulate IL-12 p40 even in the wt cells, it stimulated expression of IL-1 receptor antagonist (IL-1Ra) transcripts in cells from both mouse strains. Furthermore, ICSBP−/− macrophages expressed IL-1α and IL-1β transcripts when stimulated by SAC, STAg, or LPS at levels comparable to ICSBP+/+ cells.
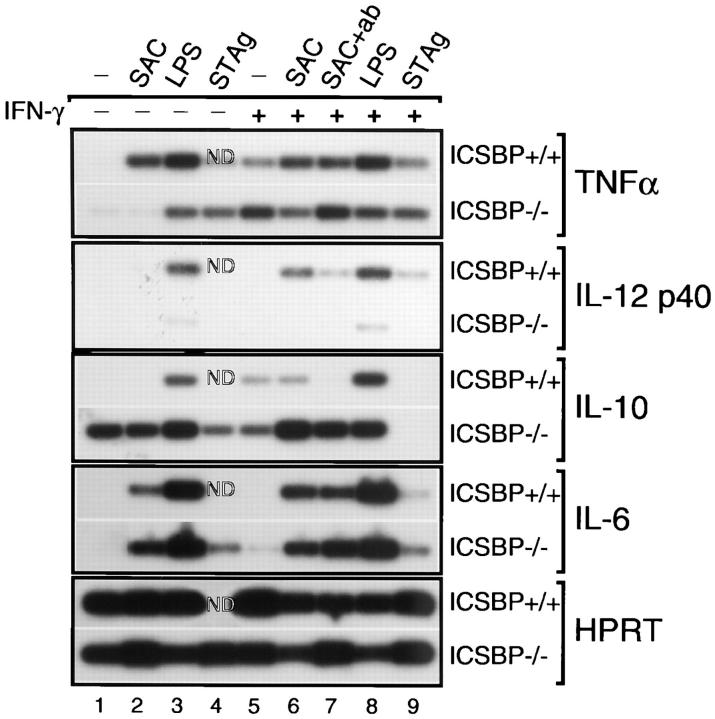
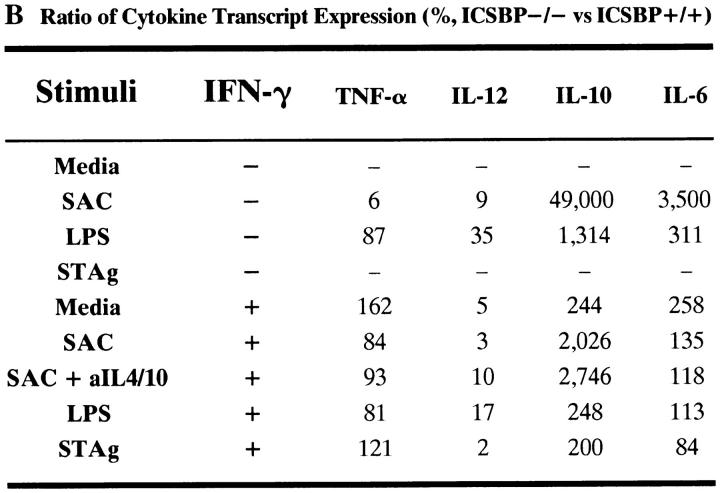
To further address the selectivity of the ICSBP macrophage deficiency the expression of three additional cytokines, TNFα, IL-6, and IL-10, was analyzed by RT-PCR (Fig. 6). IL-12 p40 mRNA induction was negligible in ICSBP−/− macrophages in response to SAC, LPS, or STAg even after priming with IFN-γ. Addition of anti–IL-4 and anti–IL-10 antibodies failed to reveal IL-12 p40 mRNA expression in SAC stimulated ICSBP macrophages suggesting that the defect observed is not due to IL-4−/− IL-10– mediated suppression. In contrast to the impaired IL-12 p40 mRNA induction, ICSBP−/− macrophages expressed TNFα mRNA in response to IFN-γ, STAg and LPS at levels comparable with ICSBP+/+ cells. Nevertheless, TNFα mRNAs induced by SAC alone, but not SAC + IFN-γ, were reduced in macrophages from ICSBP−/− mice. Macrophages from both strains of mice also synthesized comparable levels of IL-6 transcripts in response to all of the agents tested. Interestingly, ICSBP−/− macrophages constitutively expressed IL-10 mRNA and higher levels of this cytokine transcript were induced in ICSBP−/− as compared with ICSBP+/+ macrophages in response to most of the stimuli. Quantitative analysis of the cytokine transcript data in Fig. 6 A is provided in Fig. 6 B.
Figure 6.
Survey of cytokine expression (TNF-α, IL-12 p40, IL-10, and IL-6) by RT-PCR analysis in thioglycollate elicited macrophage populations after stimulation with IFN-γ and/or SAC, STAg or LPS. (A) Two to four million peritoneal macrophages were cultured with (+) or without (−) pretreatment with IFN-γ at 200 U/ml for 2 h followed by the addition of STAg (5 μg/ml), SAC (1:1,000) or LPS (200 ng/ml) for 6 h. RNA was extracted as described in Materials and Methods and samples were subjected to quantitative RT-PCR as in Fig. 4. In lane 7, anti–IL-4 and anti–IL-10 mAbs (10 μg/ml each) were included in the cultures. ND indicates that the cDNA sample could not be evaluated. (B) Relative levels of mRNA induction were calculated by first normalizing the densitometry units for each cytokine to the densitometry units of the housekeeping gene (HPRT) for each sample. Second, a ratio of the values determined for ICSBP−/− and ICSBP+/+ samples was calculated. Values of 100% indicate equal expression between the two groups, those <100% signify ICSBP−/− <ICSBP+/+ and those >100% that ICSBP−/− >ICSBP+/+. (−) indicate that a value could not be determined because below the level of detection (see Media −IFN) or because the HPRT value for at least 1 sample was not available (see STAg–IFNγ). The experiment shown is representative of three performed.
ICSBP− /− Mice Retain the Ability to Control Parasite Infection in Response to Exogenous IL-12 or IFN-γ.
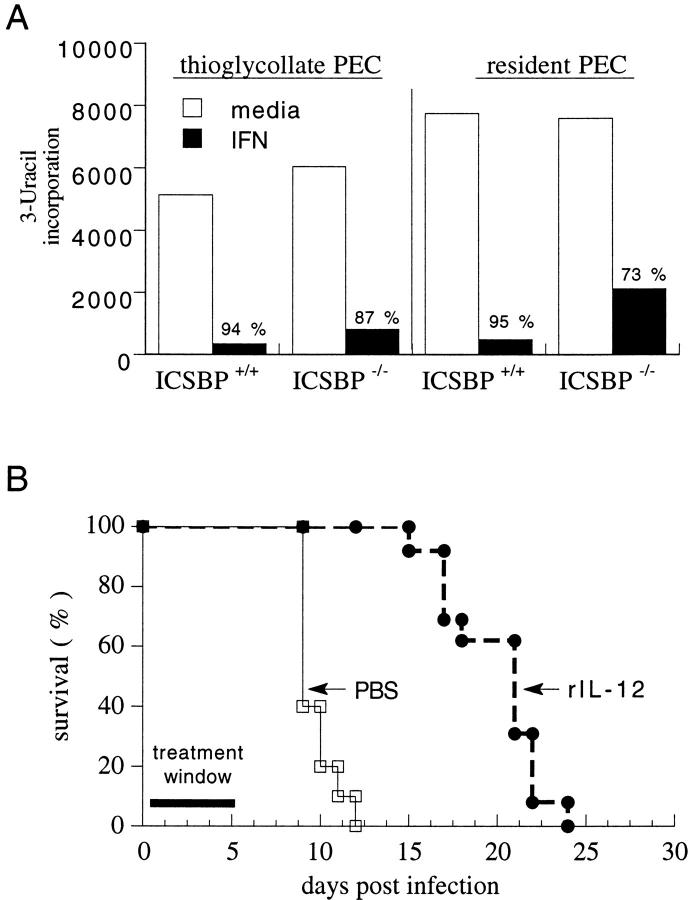
To determine whether the ICSBP−/− lesion also influences effector functions downstream from the defect in IL-12 p40 induction, we assessed the ability of IFN-γ–activated macrophage populations from ICSBP mutant animals to restrict the growth of T. gondii in vitro as measured by incorporation of [3H]uracil, a nucleotide preferentially used by the parasite. Unactivated cells from either wt or ICSBP−/− mice exhibited a 50–80-fold increase in nucleotide incorporation after infection with tachyzoites (0.2/cell) and addition of IFN-γ dramatically reduced tachyzoite proliferation in cultures from both mouse strains. Similar results were obtained using thioglycollate-elicited or resident macrophage-enriched populations (Fig. 7 A).
Figure 7.
ICSBP−/− mice can be stimulated exogenously to control T. gondii infection in vitro (A) and in vivo (B). (A) PECs were collected from naive mice (resident) or from animals injected i.p. with thioglycollate 5 d prior (thioglycollate elicited). Cells were pretreated with murine IFN-γ for 2 h. Cultures were infected with 0.2 RH tachyzoites per cell, pulsed 24 h later with [3H]uracil, and cultured overnight. Incorporated radioactivity was determined and expressed as mean CPM for triplicate cultures. Percentage killing is indicated above the bar for IFN-γ–treated cultures and was calculated using the formula indicated in Materials and Methods. Comparable results were observed in two additional experiments. (B) Survival was compared in ICSBP−/− mice that were treated with PBS (10 mice per group) or rIL-12 (0.5 μg, 13 mice per group) over the first 5 d of infection. The results shown are pooled from two experiments.
The above data indicate that IFN-γ–dependent effector functions downstream from IL-12 p40 synthesis are not directly affected by the ICSBP mutation. To evaluate this question in vivo, ICSBP−/− mice were injected with rIL-12 and control of infection assessed by monitoring mortality (Fig. 7 B). Animals were infected i.p. with 20 cysts of ME49 at day 0 and treated with 0.5 μg of rIL-12 i.p. on days 0, 1, 2, 3, and 4 after infection. Transient administration of rIL-12 prolonged the mean survival time of ICSBP−/− mice from 10 d in the PBS group to 20 d in the cytokine-treated group (Fig. 7 B). Because we have been unable to demonstrate any IFN-γ–independent mechanisms of IL-12–stimulated host resistance against T. gondii (27), it is likely that the enhanced survival of rIL-12–treated ICSBP−/− mice is due to induction of the former cytokine. Consistent with this argument, ICSBP−/− spleen cells were able to produce an IFN-γ response when stimulated in vitro with rIL-12, although the levels of cytokine were markedly less than that observed in ICSBP+/+ cultures (Table 2).
Table 2.
Induction of IFN-γ protein synthesis in ICSBP− /− and ICSBP+ /+ spleen cells after treatment with rIL-12
| ICSBP phenotype | Media | IL-12 | Fold induction | |||
|---|---|---|---|---|---|---|
| IFN-γ | ||||||
| ng/ml | ||||||
| −/− (1) | ⩽0.03 | 1.01 | 34 | |||
| −/− (2) | ⩽0.03 | 1.76 | 59 | |||
| +/+ (1) | ⩽0.03 | 5.00 | 167 | |||
| +/+ (2) | ⩽0.03 | 7.40 | 247 | |||
| −/− (3) | ⩽0.03 | 13.73 | 458 | |||
Spleen cells from individual, uninfectaed ICSBP−/− (n = 2) and ICSBP+/+ (n = 3) animals were cultured for 48 h in medium alone or rIL-12 (10 ng/ml). Supernatants were then assayed by ELISA for IFN-γ as described in Materials and Methods. Fold induction was determined by dividing the level of IFN-γ in the IL-12 treated samples by the limit of detection (0.03 pg/ml).
Discussion
In this study, we show that ICSBP−/− mice rapidly succumb to infection with the intracellular pathogen T. gondii. This enhanced susceptibility appears to be due to the inability of the mutant animals to produce IL-12 p40 and hence to stimulate IFN-γ during parasitic infection. Notably, however, cytokine expression and host defense pathways that are either unrelated to or downstream from IL-12 do not appear to be affected by ICSBP since T cells from ko mice synthesized IFN-γ when triggered with Con A (Fig. 3) and administration of rIL-12 to infected ICSBP−/− mice prolonged their survival (Fig. 7 B). In support of this argument, macrophage populations from ICSBP−/− animals inhibited parasite replication in vitro when exposed to IFN-γ (Fig. 7 A) and synthesized significant amounts of nitric oxide (data not shown), a known correlate of macrophage activation (45). Moreover, it has been previously shown that splenic T cells from ICSBP−/− animals are capable of restoring IFN-γ–dependent resistance against the bacterium Listeria monocytogenes when transferred to lymphocyte-deficient mice, indicating that these cells retain the ability to respond to antigen after the appropriate stimulatory signals (16). Indeed, we found that treatment of spleen cells with IL-12 induced significant quantities of IFN-γ (Table 2). Nonetheless, the response observed in cells from ICSBP−/− mice was less than that of cultured ICSBP+/+ cells. One interpretation of these data is that the expression of IL-12R is diminished in ICSBP−/− cells, a finding which may result from diminished levels of endogenous IL-12 p40 and IFN-γ in the uninfected ICSBP-deficient animals (13, 46).
In addition to their inability to produce IL-12 p40 in response to T. gondii infection, ICSBP−/− mice displayed a generalized impairment in macrophage production of the cytokine when stimulated with IFN-γ, LPS, SAC, or T. gondii itself. This defect appears to be selective for IL-12 p40 induction, since ICSBP−/− cells expressed normal levels of IL-1Ra, IL-1α, and IL-1β mRNA as assessed by RNAse protection (Fig. 5 B) and IL-6, IL-10, and TNFα by RT-PCR (Fig. 6) when exposed to the same stimuli with the possible exception of a defect in the TNFα response to SAC alone. Moreover, spleen cells from T. gondii-infected ICSBP−/− animals produced IL-10 and TNFα mRNA in quantities comparable to splenocytes from wt control mice (Fig. 4). Since ICSBP−/− macrophages are clearly capable of responding to IFN-γ, signal transduction events immediately downstream from IFN-γ–IFN-γ receptor interaction, including STAT1 activation, are likely to be intact. In further support of this argument, we recently observed that ICSBP−/− cells respond to IFN-γ to express IRF-1 a gene controlled by STAT1 (Masumi, A., and Ozato, K., unpublished observation). Based on these observations and findings that ICSBP−/− macrophages produce nitric oxide (16), a STAT1-regulated molecule, we suggest that ICSBP acts downstream in the STAT1 signal transduction pathway and that it affects only a subset of IFN-γ– inducible genes, which includes the IL-12 p40 gene. In this regard, naive ICSBP-deficient mice are known to have a number of hematologic abnormalities which are unlikely to result solely from the IL-12 p40 defect since animals with a targeted disruption in the latter gene are phenotypically normal (13, 30).
Regulation of IL-12 p40 synthesis is known to occur primarily at the level of transcription (26, 47) and to involve both IFN-γ–dependent and –independent mechanisms of induction in vivo (48). In the case of T. gondii– stimulated responses, IFN-γ, while dramatically enhancing production of IL-12 p40 by macrophages in vitro, is clearly not necessary for induction of the monokine in infected animals (27). The data presented here indicate that ICSBP regulates both the IFN-γ–dependent and –independent pathways of IL-12 p40 synthesis (Table 1; Fig. 5). The effects of ICSBP deficiency were most pronounced in the case of IFN-γ–primed macrophages where reductions of >95% in IL-12 p40 were observed in the responses to SAC, LPS, live RH tachyzoites, or STAg. The same defect was apparent when IL-12 p40 responses of macrophages to STAg were measured in the cultures initiated in the absence of IFN-γ priming (Fig. 5). Nevertheless, cultures of unprimed ICSBP−/− cells did produce detectable, although reduced levels of cytokine in response to SAC, LPS, or RH.
Since STAg-stimulated adherent thioglycollate exudate populations produce detectable levels of IFN-γ (unpublished observations), it was possible that the unresponsiveness of ICSBP−/− macrophages to that agent reflects an indirect effect on endogenous IFN-γ priming rather than a direct effect on p40 gene expression. This explanation was ruled out by showing that spleen cells from uninfected ICSBP−/− mice are totally defective in IL-12 p40 production when exposed to STAg. As confirmed here (Table 1), the IL-12 response of splenocytes from ICSBP+/+ mice to STAg is independent of endogenous priming by IFN-γ and, therefore, the defective response of the mutant cells cannot be explained by a priming deficiency. Taken together these findings argue that ICSBP plays a major role in regulating both IFN-γ–dependent and –independent IL-12 p40 synthesis, a conclusion consistent with the failure of ICSBP−/− mice to develop an IL-12 response to T. gondii infection in vivo. Preliminary results suggest that Toxoplasma itself can induce ICSBP expression in macrophages in the absence of IFN-γ priming, a finding that reinforces the concept that IFN-γ signaling may not be essential for ICSBP function (Scharton-Kersten, T., unpublished results).
The mechanism(s) by which ICSBP regulates IL-12 p40 gene transcription appear to be complex. Thus, although a consensus ISRE sequence is present in the p40 promoter we have so far been unable to demonstrate a function for this element in activation of the gene. It is thus possible that ICSBP binds to a previously unidentified cis regulatory element in the IL-12 promoter. Alternatively, ICSBP may act indirectly by interacting with other transcriptional regulators of the IL-12 p40 gene. Murphy et al. (47) have reported that NFκB plays a major role in the induction of the mouse IL-12 p40 promoter by IFN-γ/LPS. Since cytokine genes known to be induced by NFκB, such as IL-1α, IL-1β, and TNF-α are induced normally in ICSBP−/− cells, the defect is not likely to be dependent on NFκB. In addition, Ma et al. (26) have identified an upstream ets sequence, distinct from the NFκB site which they propose is as an essential regulator of the human IL-12 p40 promoter. One hypothesis bridging this and our observations is that ICSBP acts by forming a complex with ets binding transcription factors thereby modifying their activity. Finally Screpanti et al. (49) have suggested that the C/EBPβ (NF-IL-6) (50) influences IL-12 expression in macrophages, perhaps through its regulation of IL-6 synthesis. As IL-6, a direct target of C/ EBPβ is induced normally in ICSBP−/− macrophages (Fig. 6) we consider it highly unlikely that ICSBP acts through this transcription factor. An additional implication of our findings is that ICSBP can act as a positive transcriptional regulator in addition to its known negative activities on promoters carrying the ISRE sequence (51). Indeed, preliminary data from transfection experiments support the concept that ICSBP enhances, rather than suppresses, IL-12 p40 promoter activity (Wang, I.-M., and K. Ozato, unpublished observation). This dichotomy in ICSBP function is not completely unexpected since other transcription factors have been shown to act positively or negatively depending on the promoter context (52).
The impaired IL-12 expression in ICSBP−/− mice predicts that these animals might display enhanced susceptibility to other intracellular pathogens, particularly those agents against which IFN-γ is required for protection. In accordance with this view, ICSBP−/− mice have recently been demonstrated to display enhanced susceptibility to infection with another protozoan parasite, Leishmania major (Morse, H.C., personal communication) and were previously reported to show impaired resistance to the intracellular bacterium Listeria monocytogenes (16). Although the latter authors did not evaluate the molecular basis for the increased susceptibility of the ICSBP−/− mice to listeriosis, resistance to this pathogen is highly IL-12 dependent (53) and thus, the sensitivity of the animals to Listeria infection is likely to be related to defective IL-12 p40 induction. Of interest, these authors noted that listeriosis is much less severe in IRF-1−/− or IRF-2−/− mice than in ICSBP−/− mice. Based on the finding that IRF-2 expression is reduced in ICSB−/− spleen (13), Fehr et al. (16) suggested that the mutant mice manifest a “double ko phenotype.” One implication of this suggestion might be that both IRF-2 and ICSBP are required for IL-12 gene activation; however, recent experiments (data not shown) indicate that the expression of IRF-2 protein is unimpaired in macrophage populations or macrophage-like cell lines from ICSBP−/− mice. Thus, we favor the concept that ICSBP plays a central role in triggering IL-12 p40 expression that cannot be substituted for by other members of the IRF family.
Although the mechanism of action of ICSBP remains to be elucidated, the discovery of a transcription factor with highly specific effects on IL-12 p40 gene induction and, in turn, on IFN-γ–dependent immunity has important implications for our understanding of the regulation of host defense. In the future, it will be important to define the precise microbial signals leading to the induction of ICSBP as well as the properties of different pathogens that determine their selective triggering of the transcription factor. Such information could be useful in defining new approaches for regulating IL-12 expression to both enhance host resistance and, in the case of IL-12–dependent immunopathology, to prevent disease.
Acknowledgments
We are grateful to Dr. J. Magram (Hoffmann-LaRoche) for providing breeding pairs of IL-12 p40−/− animals; Dr. S. Wolf (Genetics Institute) for provision of rIL-12; Dr. J. Lou for his guidance on RT-PCR; Mr. S. Khan for genotyping the ICSBP−/− mice; and Ms. S. Hieny and Ms. P. Caspar for technical assistance. We also thank Drs. W. Leonard, D. Singer, and G. Yap for critical reading of the manuscript and Ms. Brenda Marshall for editorial assistance.
Footnotes
Abbreviations used in this paper: ICSBP, interferon consensus sequence binding protein; IRF, interferon regulatory factor; ISGF, interferon-stimulated gene factor; ISRE, interferon-stimulated response element; ko, knockout; LMc, large mononuclear cell; PEC, peritoneal cells; SAC, Staphylococcus aureus Cowan strain 1; SMc, small mononuclear cell; STAg, soluble tachyzoite antigen; STAT1, signal transducer and activator of transcription; wt, wild-type.
References
- 1.Driggers PH, Ennist DL, Gleason SL, Mak W-H, Marks MS, Levi B-Z, Flanagan JR, Appella E, Ozato K. An interferon-γ–regulated protein that binds the interferon-inducible enhancer element of major histocompatibility complex class I genes. Proc Natl Acad Sci USA. 1990;87:3743–3747. doi: 10.1073/pnas.87.10.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyamoto M, Fujita T, Kimura Y, Maruyama M, Harada H, Sudo Y, Miyata T, Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-β gene regulatory elements. Cell. 1988;54:903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- 3.Harada H, Fujita T, Miyamoto M, Kimura Y, Maruyama M, Fuira A, Miyata T, Taniguchi T. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989;58:729–739. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- 4.Veals SA, Schindler C, Leonard D, Fu X, Aebersold R, Darnell JE, Jr, Levy DE. Subunit of γ-interferon-responsive transcription factor is related to interferon regulatory factor and Myb families of DNA binding proteins. Mol Cell Biol. 1992;12:3315–3324. doi: 10.1128/mcb.12.8.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Au WC, Moore PA, Lowther W, Juang YT, Pitha PM. Identification of a member of the interferon regulatory factor family that binds to the interferon-stimulated response element and activates expression of interferon- induced genes. Proc Natl Acad Sci USA. 1995;92:11657–11661. doi: 10.1073/pnas.92.25.11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenbeis CF, Singh H, Storb U. Pip, a novel IRF family member, is a lymphoid-specific, PU.1-dependent transcriptional activator. Genes Dev. 1995;9:1377–1387. doi: 10.1101/gad.9.11.1377. [DOI] [PubMed] [Google Scholar]
- 7.Kanno Y, Kozak CA, Schindler C, Driggers PH, Ennist DL, Gleason SL, Darnell JE, Ozato K. The genomic structure of the murine ICSBP reveals the presence of the gamma interferon-responsive element, to which an ISGF3α subunit or similar molecule binds. Mol Cell Biol. 1993;13:3951–3963. doi: 10.1128/mcb.13.7.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson N, Kanno Y, Hong C, Contursi C, Fujita T, Fowlkes BJ, O'Connel E, Hu-Li J, Paul WE, Jankovic D, et al. Expression of interferon regulatory factor family proteins in lymphocytes. Induction of Stat1 and ICSBP expression by T cell activation. J Immunol. 1996;156:3711–3720. [PubMed] [Google Scholar]
- 9.Politis AD, Ozato K, Coligan JE, Vogel SN. Regulation of induced nuclear expression of IFN consensus sequence binding protein in murine peritoneal macrophages. J Immunol. 1994;152:2270–2278. [PubMed] [Google Scholar]
- 10.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science (Wash DC) 1994;14:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 11.Levy DE, Lew DJ, Decker T, Kessler DS, Darnell JE., Jr Synergistic interaction between interferon-γ and interferon-α through induced synthesis of one subunit of the transcription factor ISGF3. EMBO J. 1990;9:1105–1111. doi: 10.1002/j.1460-2075.1990.tb08216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuyama T, Kimura T, Kitagawa M, Pfeffer K, Kawakami T, Watanabe N, Kündig T M, Amakawa R, Kishihara K, Wakeham A, et al. Targeted disruption of IRF-1 or IRF-2 results in abnormal type I IFN gene induction and aberrant lymphocyte development. Cell. 1993;75:83–97. [PubMed] [Google Scholar]
- 13.Holtschke T, Löhler J, Kanno Y, Fehr T, Giese N, Rosenbauer R, Lou J, Knobeloch K-P, Gabriele L, Waring JF, et al. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell. 1996;87:307–317. doi: 10.1016/s0092-8674(00)81348-3. [DOI] [PubMed] [Google Scholar]
- 14.Khan IA, Matsuura T, Fonseka S, Kasper LH. Production of nitric oxide (NO) is not essential for protection against acute Toxoplasma gondii infection in IRF-1−/−mice. J Immunol. 1996;156:636–643. [PubMed] [Google Scholar]
- 15.Mittrücker HW, Matsuyama T, Grossman A, Kündig T, Potter J, Shahinan A, Wakeham A, Patterson B, Ohashi P, Mak TW. Requirement for the transcription factor LSIRF/IRF4 for mature B and T lymphocyte function. Science (Wash DC) 1997;275:540–543. doi: 10.1126/science.275.5299.540. [DOI] [PubMed] [Google Scholar]
- 16.Fehr T, Schoedon G, Odermatt B, Holtschke T, Schneemann M, Bachmann M F, Mak T W, Horak I, Zinkernagel RM. Crucial role of interferon consensus sequence binding protein, but neither of interferon regulator factor 1 nor of nitric oxide synthesis for protection against murine listeriosis. J Exp Med. 1997;183:147–157. doi: 10.1084/jem.185.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lohoff M, Ferrick D, Mittrucker H-W, Duncan GS, Bischof S, Rollinghoff M, Mak TW. Interferon regulatory factor-1 is required for a T helper 1 immune response in vivo. Immunity. 1997;6:681–689. doi: 10.1016/s1074-7613(00)80444-6. [DOI] [PubMed] [Google Scholar]
- 18.Taki S, Sato T, Ogasawara K, Fukuda T, Sato M, Hida S, Suauki G, Mitsuyama M, Shin E-H, Kojima S, Taniguchi T, Asano Y. Multistage regulation of Th1-type immune responses by the transcription factor IRF-1. Immunity. 1997;6:673–679. doi: 10.1016/s1074-7613(00)80443-4. [DOI] [PubMed] [Google Scholar]
- 19.Müller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science (Wash DC) 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 20.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann G, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-γ receptor. Science (Wash DC) 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 21.Ma X, Aste-Amezaga M, Trinchieri G. Regulation of interleukin-12 production. Ann NY Acad Sci. 1996;795:12–25. doi: 10.1111/j.1749-6632.1996.tb52651.x. [DOI] [PubMed] [Google Scholar]
- 22.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-γ) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh C, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Listeria-induced Th1 development in αβ-TCR transgenic CD4+T cells occurs through macrophage production of IL-12. Science (Wash DC) 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 24.Manetti R, Gerosa F, Giudizi MG, Biagiotti R, Parronchi P, Piccinni MP, Sampognaro S, Maggi E, Romagnani S, Trinchieri G. Interleukin-12 induces stable priming for interferon-γ (IFN-γ) production during differentiation of human T helper (Th) cells and transient IFN-γ production in established Th2 cell clones. J Exp Med. 1994;179:1273–1283. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farrar MA, Schreiber RD. The molecular cell biology of interferon-γ and its receptor. Ann Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 26.Ma X, Chow J, Gri G, Carra G, Gerosa F, Wolf S, Dzialo R, Trinchieri G. The interleukin 12 p40 gene promoter is primed by interferon-γ in monocytic cells. J Exp Med. 1996;183:147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scharton-Kersten TM, Wynn TA, Denkers EY, Bala S, Grunvald E, Hieny S, Gazzinelli RT, Sher A. In the absence of endogenous interferon-γ mice develop unimpaired IL-12 responses to Toxoplasma gondiiwhile failing to control acute infection. J Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- 28.Scharton-Kersten T, Denkers EY, Gazzinelli R, Sher A. Role of IL-12 in induction of cell-mediated immunity to Toxoplasma gondii. . Res Immunol. 1996;146:539–545. doi: 10.1016/0923-2494(96)83029-x. [DOI] [PubMed] [Google Scholar]
- 29.Subauste CS, Remington JS. Role of gamma interferon in Toxoplasma gondiiinfection. Eur J Clin Microbiol Infect Dis. 1991;10:58–67. doi: 10.1007/BF01964408. [DOI] [PubMed] [Google Scholar]
- 30.Magram J, Connaughton SE, Warrier RR, Carvajal DM, Wu C-Y, Ferrante J, Stewart C, Sarmiento U, Faherty DA, Gately MK. IL-12-deficient mice are defective in IFN-γ production and type I cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 31.Dalton KK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science (Wash DC) 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 32.Leiby DA, Fortier AH, Crawford RM, Schreiber RD, Nacy CA. In vivo modulation of the murine response to Francisella tularensisLVS by administration of anticytokine antibodies. Infect Immun. 1992;60:84–89. doi: 10.1128/iai.60.1.84-89.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powrie F, Menon S, Coffman RL. Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol. 1993;23:3043–3049. doi: 10.1002/eji.1830231147. [DOI] [PubMed] [Google Scholar]
- 34.Pfefferkorn ER, Pfefferkorn LC. Specific labeling of intracellular Toxoplasma gondiiwith uracil. J Protozool. 1977;24:449–453. doi: 10.1111/j.1550-7408.1977.tb04774.x. [DOI] [PubMed] [Google Scholar]
- 35.McLeod R, Remington JS. A method to evaluate the capacity of monocytes and macrophages to inhibit multiplication of an intracellular pathogen. J Immunol Methods. 1979;27:19–29. doi: 10.1016/0022-1759(79)90235-7. [DOI] [PubMed] [Google Scholar]
- 36.Curry RC, Keiner PA, Spitalny GL. A sensitive immunochemical assay for biologically active muIFN-γ. J Immunol Methods. 1987;104:137–142. doi: 10.1016/0022-1759(87)90497-2. [DOI] [PubMed] [Google Scholar]
- 37.Vieira LQ, Hondowicz BD, Afonso LC, Wysocka M, Trinchieri G, Scott P. Infection with Leishmania majorinduces IL-12 production in vivo. Immunol Lett. 1994;40:157–161. doi: 10.1016/0165-2478(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 38.Gazzinelli RT, Eltoum I, Wynn TA, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-α and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993;151:1672–1682. [PubMed] [Google Scholar]
- 39.Wang, A.M., and D.F. Mark. 1990. Quantitative PCR. In PCR Protocols. M.A. Innis, D.H. Gelfand, J.J. Sninsky, and T.J. White, editors. Academic Press, New York. 70–75.
- 40.Scharton-Kersten TM, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host defense against chronic but not acute infection with the intracellular pathogen, Toxoplasma gondii. . J Exp Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sayles, P.C., and L.L. Johnson. 1997. Exacerbation of Toxoplasmosis in neutrophil depleted mice. Nat. Immunity. In press. [PubMed]
- 42.Grunvald E, Chiramonte M, Hieny S, Wysocka M, Trinchieri G, Vogel SN, Gazzinelli RT, Sher A. Biochemical characterization and protein kinase C dependency of monokine-inducing activities of Toxoplasma gondii. . Infect Immun. 1996;64:2010–2018. doi: 10.1128/iai.64.6.2010-2018.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D'Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin-10 (IL-10) inhibits human lymphocyte interferon-γ production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D'Andrea A, Ma X, Aste-Amezaga M, Paganin C, Trinchieri G. Stimulatory and inhibitory effects of interleukin (IL)-4 and IL-13 on the production of cytokines by human peripheral blood mononuclear cells: priming for IL-12 and tumor necrosis factor-α production. J Exp Med. 1995;181:537–546. doi: 10.1084/jem.181.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nathan C. Natural resistance and nitric oxide. Cell. 1995;82:873–876. doi: 10.1016/0092-8674(95)90019-5. [DOI] [PubMed] [Google Scholar]
- 46.Gollob JA, Kawasaki H, Ritz J. Interferon-γ and interleukin-4 regulate T cell interleukin-12 responsiveness through the differential modulation of high affinity interleukin-12 receptor expression. Eur J Immunol. 1997;27:647–652. doi: 10.1002/eji.1830270311. [DOI] [PubMed] [Google Scholar]
- 47.Murphy T, Cleveland M, Kulesza P, Magram J, Murphy K. Regulation of interleukin 12 p40 expression through an NF-κB half-site. Mol Cell Biol. 1995;15:5258–5267. doi: 10.1128/mcb.15.10.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flesch IE, Kaufmann SH. Differential induction of IL12 synthesis by Mycobacterium bovis BCG and Listeria monocytogenes. . Res Immunol. 1995;146:520–526. doi: 10.1016/0923-2494(96)83026-4. [DOI] [PubMed] [Google Scholar]
- 49.Screpanti I, Romani L, Musiani P, Modesti A, Fattori E, Lazzaro D, Sellitto C, Scarpa S, Bellavia D, Lattanzio G, et al. Lymphoproliferative disorder and imbalanced T-helper response in C/EBPβ-deficient mice. EMBO (Eur Mol Biol Organ) J. 1995;14:1932–1941. doi: 10.1002/j.1460-2075.1995.tb07185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Isshiki H, Akira S, Tanabe O, Nakajima T, Shimamoto T, Hirano T, Kishimoto T. Constitutive and IL-1 inducible factors interact with the IL-1 responsive element in the IL-6 gene. Mol Cell Biol. 1990;10:2757–2764. doi: 10.1128/mcb.10.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson NJ, Marks MS, Driggers PH, Ozato K. ICSBP, a member of the IRF family, suppresses IFN induced gene transcription. Mol Cell Biol. 1993;21:1066–1081. doi: 10.1128/mcb.13.1.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi Y, Seto E, Chang L, Shenk T. Transcriptional repression by YY1, a human GLI-druppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991;67:377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- 53.Tripp CS, Gately MK, Hakimi J, Ling P, Unanue ER. Neutralization of IL-12 decreases resistance to Listeriain SCID and CB-17 mice: reversal by IFN. J Immunol. 1994;152:1883–1887. [PubMed] [Google Scholar]
- 54.Li Z-Y, Manthey CL, Perera PY, Sher A, Vogel SN. Toxoplasma gondiisoluble antigen induces a subset of lps-inducible genes and tyrosine phosphoproteins in peritoneal macrophages. Infect Immun. 1994;62:3434–3440. doi: 10.1128/iai.62.8.3434-3440.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reis e Sousa, C., S. Hieny, T. Scharton-Kersten, D. Jankovic, H. Charest, R.N. Germain, and A. Sher. In vivo microbial stimulation induces rapid CD40L-independent production of IL-12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. In press. [DOI] [PMC free article] [PubMed]