Abstract
CD4 and CD8 are thought to function as coreceptors by binding to the cognate major histocompatibility complex (MHC) molecules recognized by the T cell antigen receptor (TCR) and initiating the signal transduction cascade. We report that during T cell–antigen-presenting cell interaction, triggered TCRs and coreceptors are downregulated and degraded with identical kinetics. This coordinated disappearance takes place whenever the TCR is triggered, even when the coreceptor does not engage the cognate MHC molecule and is the consequence of binding of the coreceptor-associated Lck to ZAP-70. The interaction of coreceptor and cognate MHC molecules is dispensable when T cells are stimulated by optimal ligands, but becomes crucial when suboptimal ligands are used. In the latter case the coreceptor increases the efficiency of TCR triggering without changing the activation threshold or the quality of the T cell response.
Because of their capacity to bind to the same MHC molecule as that engaged by the TCR (1, 2), and because of their association with the tyrosine kinase Lck (3), CD4 and CD8 have been defined as coreceptors (4, 5). The binding of coreceptor to the cognate MHC molecule is thought to perform two functions: (a) to stabilize the TCR– peptide MHC interaction (6–8); and (b) to carry Lck in contact with the TCR to initiate phosphorylation events (9). Indeed, cross-linking of TCRs and coreceptors results in enhanced T cell response (10, 11), whereas interference with the coreceptor–MHC interaction inhibits T cell activation or changes the quality of the response (12–14).
However, there are clear cases where the interaction of coreceptors with cognate MHC molecules appears to be dispensable for full T cell activation (15). These findings raise the question of whether the contribution of the coreceptor to T cell activation is qualitative, in the sense that it provides signals additional to and different from those provided by TCR alone, or whether the coreceptor acts by facilitating the triggering of TCRs when the interaction has lower than optimal kinetics.
We report that during T cell–APC interaction, the coreceptors are recruited to triggered TCRs and are downregulated with identical kinetics, even when the coreceptor does not engage the cognate MHC molecule. This process is the consequence of the binding of coreceptor-associated Lck to ZAP-70/ζ and takes place whenever the TCR is triggered. We also show that the contribution of the coreceptors becomes crucial when suboptimal ligands are used. In this case, engagement of the coreceptor with the cognate MHC molecule increases the efficiency of serial TCR triggering without changing the activation threshold or the quality of the T cell response.
Materials and Methods
T Cell Clones.
HLA-DR1101–restricted (KS140, KS70, and KS164) and DR1302-restricted (AL15.1) CD4+ T cell clones specific for tetanus toxin (TT)830-843 peptide and HLA-A2– restricted CD8+clones (CER22, CER43) specific for the influenza matrix (M) 58–66 peptide were used. T cell clones were conjugated with EBV-transformed B (EBV-B) cells pulsed with various concentrations of either peptide or bacterial superantigens (toxic shock syndrome toxin or staphylococcal enterotoxin B) or monovalent anti-CD3 antibodies (W632/T3 or L243/T3) as described (16). CD4+CD8+ alloreactive T cell clones were isolated by sorting double positive cells from a primary MLR. CD8+ alloreactive T cell clones that recognize class II molecules on the class I− EBV-B .221 cells were generated, stimulating PBMC with irradiated .221 cells, followed by sorting and cloning of the CD8+CD4− cells. The class II specificity of the clones was verified using a panel of typed EBV-B cells as well as inhibition by anti–class II antibodies.
FACS® Analysis.
T cells were conjugated with autologous EBV-B cells pulsed with peptide, superantigen, or anti-CD3 for 5 h at 37°C. Downregulation and degradation of TCR/CD3 and coreceptors were measured by indirect immunofluorescence on intact and permeabilized cells as previously described (17, 18) using antibodies to CD3 (OKT3 or Leu3a), CD4 (6D10), and CD8 (OKT8). The absolute numbers of CD3, CD4, and CD8 molecules per cell were estimated by reference to a standard curve of beads coated with known amounts of mouse Ig (Flow Cytometry Standards Europe, Leiden, The Netherlands).
Immunoprecipitations and Kinase Assays.
T cells were stimulated or not with the appropriate APC for 2 min at 37°C and lysed for 30 min at 4°C in 1% Brij96 buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM MgCl2, and 1 mM EGTA) in the presence of protease and phosphatase inhibitors (10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM Pefabloc-SC, 50 mM NaF, 10 mM Na4P2O7, and 1 mM NaVO4). CD4 immunoprecipitation, in vitro kinase assay, and reimmunoprecipitation with anti–ZAP-70 or anti-ζ were performed as previously described (19, 20) using the following antibodies: 19Thy-5D7 (IgG2a, anti-CD4), 21Thy-2D3 (IgG1, anti-CD8), and rabbit polyclonal antibodies to ZAP-70 and ζ.
Drugs and Transfection.
Genistein and herbimycin A were purchased from Calbiochem (La Jolla, CA) and used as previously described (21). After overnight treatment with herbimycin, which is required to deplete Lck, the expression of coreceptor was reduced by ∼50%. cDNAs encoding wild-type human CD4 and the mutant CD4.401 molecule that does not associate with Lck were a gift of Dr. Dan Littman (Skirball Institute of Biomolecular Medicine, NYU Medical Center, New York; reference 22). The cDNA was subcloned in the pSAM-EN retroviral vector (23) using the SalI–XhoI restriction sites. Plasmid DNA was purified and transfected in the amphotropic packaging cell line PA317 as previously described (22). The transduction of Jurkat cells was done as previously described (22).
Substituted Peptides and Anticoreceptor Antibodies.
Several substituted analogs of TT830-843 were tested for their capacity to trigger T cell proliferation, and peptides with unaltered capacity to bind to DR molecules but weaker T cell stimulatory capacity were selected. Blocking anti-CD4 antibodies (M-T310, M-T413, M-T414, and M-T435) and anti-CD8 (733) were a gift of Dr. E.P. Rieber (Institute of Immunology, Technical University Dresden, Germany) and Dr. E. Roosnek (University Hospital, Geneva, Switzerland). Antibodies were used in the culture at 10 μg/ml.
Results
Parallel Downregulation and Degradation of the Coreceptor and TCR.
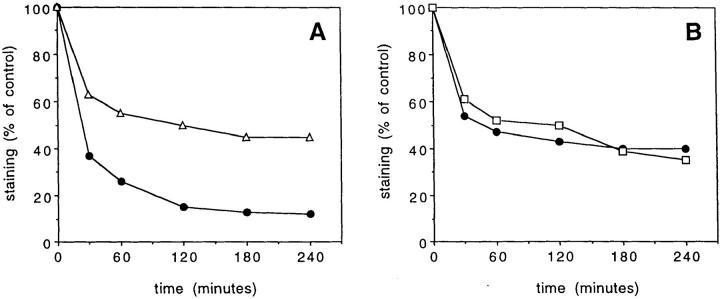
To understand the contribution of the coreceptor to TCR triggering and T cell activation, we studied the TCR–coreceptor interaction in T cells stimulated by a specific ligand. It has been shown that, after triggering by agonists, TCRs are downregulated and degraded and this downregulation can be used to measure the number of TCRs triggered (17, 18). Therefore, we investigated whether the CD4 or CD8 coreceptors would also be downregulated together with the TCR. We observed that in specific T–APC conjugates, TCRs and coreceptors are downregulated with the same kinetics and with fixed stoichiometry (Fig. 1, A and B). In addition, downregulation is followed by rapid degradation of both coreceptor and TCR (Fig. 1 C). By reference to a standard curve of Ig-coated beads, we estimated that approximately two CD4 or four CD8 molecules are downregulated for each TCR (data not shown), an estimate which is consistent with that reported for CD4 by Saizawa and Janeway (24).
Figure 1.
Parallel downregulation and degradation of CD4 and CD8 together with CD3 in T cell clones stimulated by specific antigen. (A) Time course of CD3 (•) and CD4 (▵) downregulation in clone KS140 stimulated with APCs pulsed with 10 nM TT830-842. (B) CD3 (•) and CD8 (□) downregulation in clone CER22 stimulated with APCs pulsed with 100 nM M58-66. (C) Surface and total levels of CD3 and CD4 as determined by staining before and after permeabilization in KS70 cells conjugated for 5 h with APCs unpulsed (empty bars) or pulsed with 0.1 μM (hatched bars) or 10 μM (filled bars) TT830-842.
Intracellular Recruitment of Coreceptor to Triggered TCRs in the Absence of an Interaction with Cognate or Noncognate MHC Molecules.
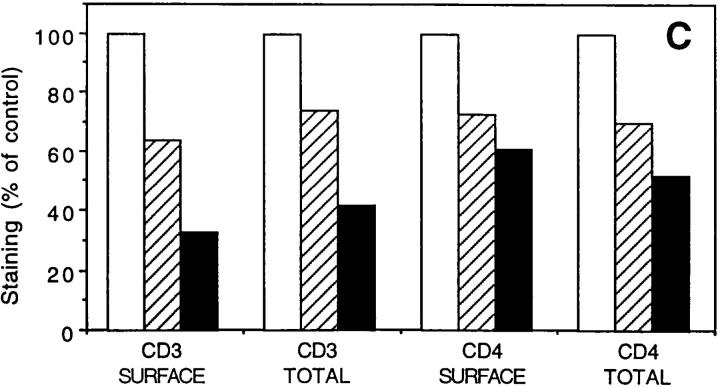
We investigated whether the codownregulation of TCR and coreceptor would require the interaction between coreceptor and cognate MHC molecule or whether it would simply be a consequence of TCR triggering. Three lines of evidence indicate that coreceptor downregulation can occur in the absence of interaction with the cognate MHC molecule. First, a parallel downregulation of TCR and coreceptor was induced not only by specific peptide–MHC complexes, but also by bacterial superantigens or monovalent anti-CD3 antibodies (Fig. 2, A and B). Second, class I–restricted T cell clones expressing both CD4 and CD8 downregulated both coreceptors to the same extent (Fig. 2 C). Third, class II–alloreactive CD8+ T cell clones, when stimulated by class II+ APCs lacking class I molecules, downregulated CD8 together with TCR (Fig. 2 D).
Figure 2.
Downregulation of coreceptors can occur in the absence of an interaction with cognate or noncognate MHC molecules. Downregulation of CD3 and coreceptor in CD4+ (KS70; A) or CD8+ (CER43; B) T cell clones stimulated by specific peptide–MHC (•), superantigens (□), or monovalent anti-CD3 antibodies: w632/T3 (▵), L243/T3 (○). (C) Downregulation of CD3 versus CD4 (▴) and CD8 (○) in alloreactive CD4+ CD8+ T cell clones stimulated by specific alloantigen (D). Downregulation of CD3 and CD8 in CD8+, class II–alloreactive T cell clones stimulated with class I− APCs (.221) expressing the relevant class II alloantigen.
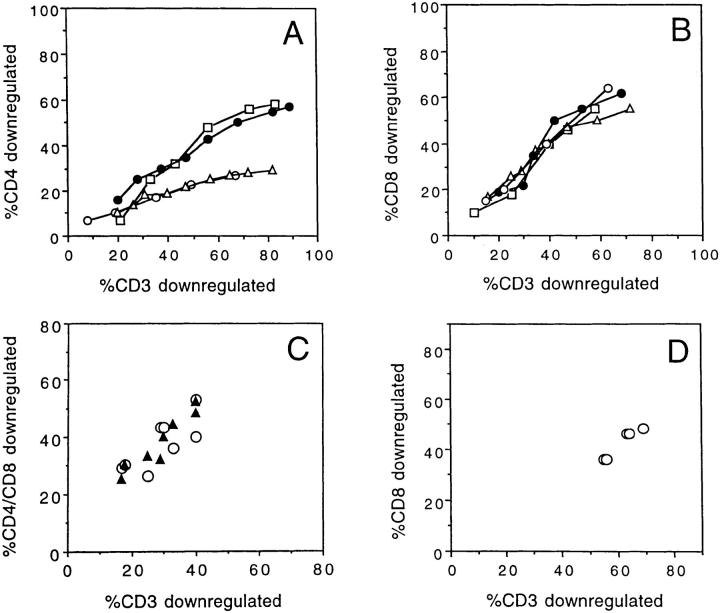
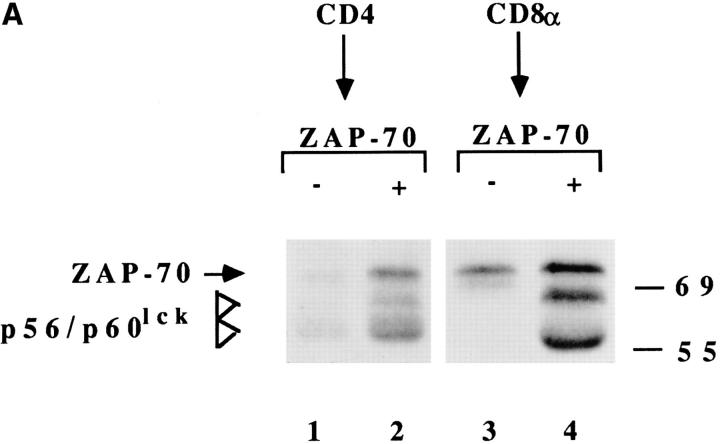
The mechanism responsible for the downregulation of the coreceptor was next investigated. Three lines of evidence indicate that the downregulation of the coreceptor was due to an intracellular association with triggered TCRs. First, immunoprecipitation experiments showed that, as previously demonstrated in Jurkat cells activated by anti-CD3 antibodies (19, 20), a complex-containing coreceptor, Lck and ZAP-70, is formed after T cell activation by a specific ligand (Fig. 3 A). Comparable results were obtained when the reimmunoprecipitation was performed with either anti–ZAP-70 or anti-ζ. Second, treatment with the Lck inhibitors genistein or herbimycin A does not interfere with TCR downregulation, but completely inhibits coreceptor downregulation (data not shown). Third, truncated CD4 molecules that fail to associate with Lck are not downregulated with triggered TCRs (Fig. 3 B).
Figure 3.
Intracellular association of coreceptor with triggered TCRs. (A) T cell clones were stimulated (+) or not (−) with APCs, lysed, and the CD4 or CD8 immunoprecipitates were subjected to an in vitro kinase assay, followed by reimmunoprecipitation with anti–ZAP-70 antiserum. CD4+ clone KS140 conjugated with peptide-pulsed or unpulsed APCs (lanes 1 and 2). CD8+ clone MS3 either untreated or conjugated with allogeneic class I− APC (lanes 3 and 4). (B) Downregulation of CD3 (○, •) and CD4 (▵, ▴) in CD4− Jurkat cells transfected with wild-type CD4 (▵, ○) or with mutant CD4.401 (▴, •).
Quantitative Contribution of Coreceptor to TCR Triggering and T Cell Activation.
Taken together, the above results demonstrate that coreceptors become associated to, and are downregulated together with, triggered TCRs, and that this process can occur after TCR triggering, even in the absence of interaction of the coreceptor with the cognate MHC molecules. However, these results do not explain how coreceptors contribute to T cell antigen recognition in most cases. Considering the serial engagement model (25), it is conceivable that the requirement for coreceptor might vary with the kinetics of the TCR–ligand interaction. Although ligands with optimal kinetics might efficiently trigger TCRs even in the absence of coreceptor, ligands with higher off-rates may require the extracellular interaction of the coreceptor with the cognate molecule to increase the stability of the complex and, consequently, the rate of triggering.
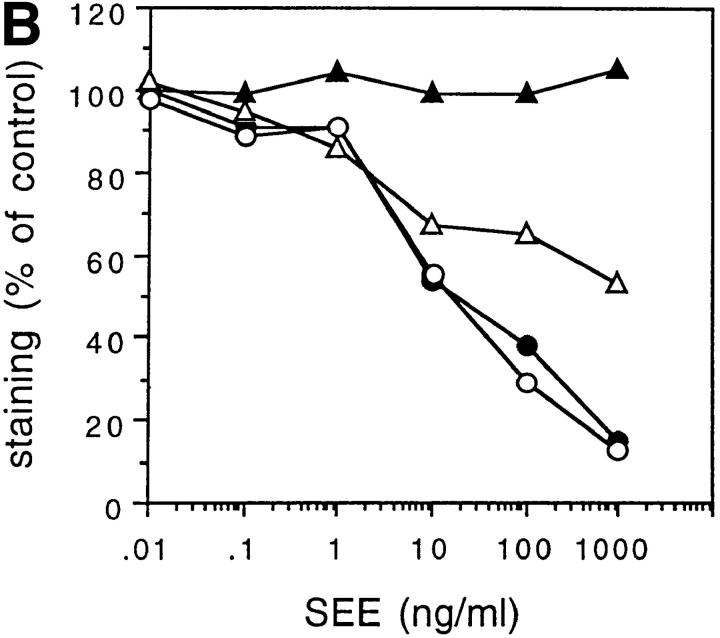
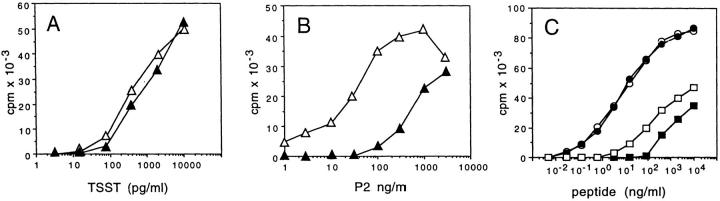
To address this point, we stimulated T cell clones with ligands of different potency and tested the effect of CD4 or CD8 antibodies on the extent of TCR triggering, the T cell activation threshold, and the quality of the response. As shown in Fig. 4, the proliferative response of T cell clone KS70 was not affected by anti-CD4 antibody when the clone was triggered by the bacterial superantigen TSST, but was inhibited by anti-CD4 when the clone was triggered by peptide–MHC complexes. Interestingly, clone KS164, which displayed a better dose–response curve to the same peptide–DR complex, was not inhibited by anti-CD4. However, this clone became sensitive to inhibition when a modified peptide with weaker agonistic properties was used. Comparable results were obtained with two additional CD4+ and two additional CD8+ clones, indicating that the sensitivity to inhibition by anticoreceptor antibodies is inversely correlated to the efficiency of the TCR–ligand interaction.
Figure 4.
The sensitivity to inhibition by anti-CD4 depends on the nature of the ligand. (A and B) Proliferative response of clone KS70 to various doses of TSST or TT830-843 in the presence (▴) or absence (▵) of anti-CD4. (C) Proliferative response of clone KS164 to TT830-843 (•, ○) or to TT830-843 Ala839-substituted peptide that behaves as a weak agonist (▪, □) in the presence (•, ▪) or absence (○, □) of anti-CD4.
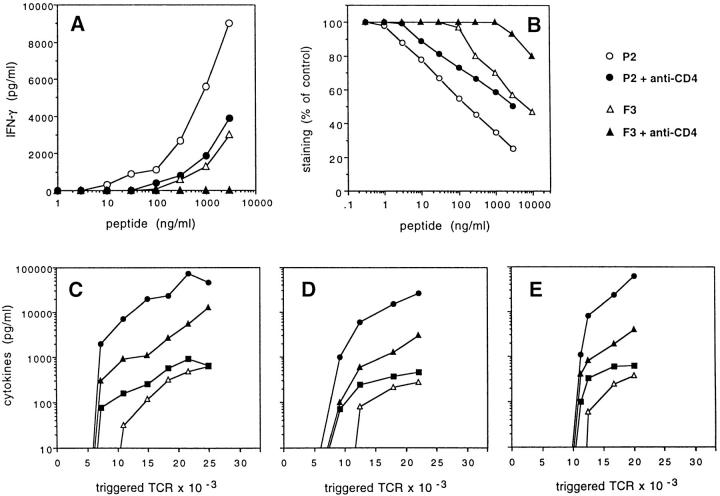
To investigate whether the lower response of T cells in the presence of anti-CD4 antibodies reflects a lower extent of TCR triggering or, rather, an altered signal leading to qualitatively different responses, we correlated TCR downregulation and cytokine production in T cells stimulated by strong or weak agonists in the presence or absence of anti-CD4 antibodies. As shown in Fig. 5, the inhibition of T cell response by anti-CD4 antibodies precisely correlated with a reduced level of TCR downregulation both when strong and weak agonists were used. These results indicate that the anti-CD4 antibody mimics the effect of a weak agonist, i.e., results in a decreased efficiency of serial triggering. However, in spite of a decreased efficiency of TCR triggering, the threshold of T cell activation and the type of cytokines produced were not affected by anti-CD4. Indeed, T cells produced IFN-γ, IL-2, and TNF-α when ∼20–30% of TCRs were triggered, irrespective of the strength of the agonist and of the presence or absence of anti-CD4. IL-3 production consistently required higher levels of TCR occupancy, which were comparable in all experimental conditions.
Figure 5.
Quantitative contribution of CD4 to TCR triggering and T cell activation. (A) IFN-γ production by T cell clone AL15.1 stimulated by TT830-843 (•, ○) or by TT830-843 Gln839 (▴, ▵) in the presence (•,▴) or absence (○, ▵) of anti-CD4 antibody. (B) CD3 downregulation in the same experiment. (C–E) Levels of IFN-γ (▴), IL-2 (•), TNF-α (▪), and IL-3 (▵) as a function of the number of TCRs downregulated in cultures stimulated by wild-type agonist (C), wild-type agonist + anti-CD4 (D), or weak agonist (E). No cytokine production was observed in cultures stimulated by weak agonist + anti-CD4.
Discussion
Our results reconcile several apparently contradictory observations concerning coreceptor dependency and the role of extracellular and intracellular interactions in coreceptor function. We have shown that in T cells activated by peptide–MHC, superantigens, or anti-CD3 antibodies the coreceptors are recruited to triggered TCRs and are downregulated and degraded together with them. This process does not necessarily require the interaction of the coreceptor with the cognate MHC molecule, but takes place whenever the TCR is triggered via the intracellular association of Lck and ZAP-70/ζ.
It is interesting that even in unstimulated T cell clones ζ and ZAP-70 can be immunoprecipitated by anti-CD4 or anti-CD8 antibodies, although the complexes contain only low levels of kinase activity. This finding suggests that in human as well as mouse T cells a fraction of TCRs is constitutively associated with the coreceptor (26). This association may be responsible for the constitutive association of phospho-ζ and ZAP-70 observed in thymocytes and lymph node T cells (27). It is tempting to speculate that the constitutive association of TCR and coreceptors might play a role in inducing positive selection by self-ligands in the thymus as well as in facilitating the response to low-affinity ligands in periphery.
There is clear evidence that coreceptor can stabilize the TCR/peptide–MHC interaction (6–8). We have shown here that this stabilization may actually result in an increased rate of TCR triggering. Indeed, the coreceptor appears to be dispensable when the ligands have optimal kinetics, allowing efficient serial TCR triggering. However, the binding of coreceptor to cognate MHC molecules becomes critical in the case of low-affinity ligands because in this case the coreceptor can initially stabilize the TCR–ligand interaction, thus increasing the probability that an engagement event will result in triggering.
Our results also show that coreceptors play a quantitative role in T cell activation. Indeed the reduced response observed in cultures stimulated with suboptimal ligands or in the presence of anticoreceptor antibodies can be fully accounted for by a reduced level of TCR triggering. The fact that the T cell activation thresholds and the profile of cytokines produced are comparable in all conditions of stimulation is not surprising. Indeed, in all cases the triggered TCRs have been shown to be complexed with coreceptors, suggesting similar composition and transduction capacity of the signaling complexes.
Acknowledgments
We thank E.P. Rieber and E. Roosnek for providing blocking anti-CD4 and anti-CD8 antibodies, D. Littmann for providing CD4 constructs, and K. Karjalainen and M. Cella for critical reading and comments.
Footnotes
The Basel Institute for Immunology was founded and is supported by F. Hoffmann La Roche Ltd., Basel, Switzerland.
References
- 1.Doyle C, Strominger JL. Interaction between CD4 and class II MHC molecules mediated cell adhesion. Nature (Lond) 1987;330:256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- 2.Norment AM, Salter RD, Parham P, Engelhard VH, Littman DR. Cell–cell adhesion mediated by CD8 and MHC class I molecules. Nature (Lond) 1988;336:79–81. doi: 10.1038/336079a0. [DOI] [PubMed] [Google Scholar]
- 3.Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 4.Janeway CA., Jr T cell development. Accessories or coreceptors? . Nature (Lond) 1988;335:208–210. doi: 10.1038/335208a0. [DOI] [PubMed] [Google Scholar]
- 5.Janeway CA., Jr The co-receptor function of CD4. Semin Immunol. 1991;3:153–160. [PubMed] [Google Scholar]
- 6.Luescher IF, Vivier E, Layer A, Mahiou J, Godeau F, Malissen B, Romero P. CD8 modulation of T cell antigen receptor–ligand interactions on living cytotoxic T lymphocytes. Nature (Lond) 1995;373:353–356. doi: 10.1038/373353a0. [DOI] [PubMed] [Google Scholar]
- 7.Renard V, Romero P, Vivier E, Malissen B, Luescher IF. CD8β increases CD8 coreceptor function and participation in TCR–ligand binding. J Exp Med. 1996;184:2439–2444. doi: 10.1084/jem.184.6.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia KC, Scott CA, Brunmark A, Carbone FR, Peterson PA, Wilson IA, Teyton L. CD8 enhances formation of stable T-cell receptor/MHC class I molecule complexes [see comments] Nature (Lond) 1996;384:577–581. doi: 10.1038/384577a0. [DOI] [PubMed] [Google Scholar]
- 9.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 10.Emmrich F, Strittmatter U, Eichmann K. Synergism in the activation of human CD8 T cells by cross-linking the T-cell receptor complex with the CD8 differentiation antigen. Proc Natl Acad Sci USA. 1986;83:8298–8302. doi: 10.1073/pnas.83.21.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janeway CA, Jr, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275–285. doi: 10.1016/0092-8674(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 12.Madrenas J, Chau LA, Smith J, Bluestone JA, Germain RN. The efficiency of CD4 recruitment to ligand-engaged TCR controls the agonist/partial agonist properties of peptide–MHC molecule ligands. J Exp Med. 1997;185:219–229. doi: 10.1084/jem.185.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vidal K, Hsu BL, Williams CB, Allen PM. Endogenous altered peptide ligands can affect peripheral T cell responses. J Exp Med. 1996;183:1311–1321. doi: 10.1084/jem.183.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mannie MD, Rosser JM, White GA. Autologous rat myelin basic protein is a partial agonist that is converted into a full antagonist upon blockade of CD4. Evidence for the integration of efficacious and nonefficacious signals during T cell antigen recognition. J Immunol. 1995;154:2642–2654. [PubMed] [Google Scholar]
- 15.Cerundolo V, Tse AG, Salter RD, Parham P, Townsend A. CD8 independence and specificity of cytotoxic T lymphocytes restricted by HLA-Aw68.1. Proc R Soc Lond B Biol Sci. 1991;244:169–177. doi: 10.1098/rspb.1991.0066. [DOI] [PubMed] [Google Scholar]
- 16.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds [see comments] Science (Wash DC) 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 17.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T cell receptors by a few peptide–MHC complexes [see comments] Nature (Lond) 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 18.Valitutti S, Muller S, Salio M, Lanzavecchia A. Degradation of T cell receptor (TCR)–CD3-ζ complexes after antigenic stimulation. J Exp Med. 1997;185:1859–1864. doi: 10.1084/jem.185.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thome M, Duplay P, Guttinger M, Acuto O. Syk and ZAP-70 mediate recruitment of p56lck/CD4 to the activated T cell receptor–CD3/ζ complex. J Exp Med. 1995;181:1997–2006. doi: 10.1084/jem.181.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thome M, Germain V, DiSanto JP, Acuto O. The p56lck SH2 domain mediates recruitment of CD8/ p56lck to the activated T cell receptor/CD3/ζ complex. Eur J Immunol. 1996;26:2093–2100. doi: 10.1002/eji.1830260920. [DOI] [PubMed] [Google Scholar]
- 21.Salio M, Valitutti S, Lanzavecchia A. Agonist-induced TCR downregulation: molecular requirements and dissociation from T cell activation. Eur J Immunol. 1997;27:1769–1773. doi: 10.1002/eji.1830270726. [DOI] [PubMed] [Google Scholar]
- 22.Bedinger P, Moriarty A, von Borstel RC, II, Donovan NJ, Steimer KS, Littman DR. Internalization of the human immunodeficiency virus does not require the cytoplasmic domain of CD4. Nature (Lond) 1988;334:162–165. doi: 10.1038/334162a0. [DOI] [PubMed] [Google Scholar]
- 23.Candotti F, Oakes SA, Johnston JA, Notarangelo LD, O'Shea JJ, Blaese RM. In vitro correction of JAK3-deficient severe combined immunodeficiency by retroviral-mediated gene transduction. J Exp Med. 1996;183:2687–2692. doi: 10.1084/jem.183.6.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saizawa K, Janeway CA., Jr Evidence for a physical association of CD4 and the CD3:α:β T-cell receptor. Nature (Lond) 1987;328:260–263. doi: 10.1038/328260a0. [DOI] [PubMed] [Google Scholar]
- 25.Valitutti S, Lanzavecchia A. Serial triggering of T-cell receptors: a basis for the sensitivity and specificity of T cell antigen recognition. Immunol Today. 1997;18:299–304. [PubMed] [Google Scholar]
- 26.Diez-Orejas R, Ballester S, Feito MJ, Ojeda G, Criado G, Ronda M, Portoles P, Rojo JM. Genetic and immunochemical evidence for CD4-dependent association of p56lck with the αβ T-cell receptor (TCR): regulation of TCR-induced activation. EMBO (Eur Mol Biol Organ) J. 1994;13:90–99. doi: 10.1002/j.1460-2075.1994.tb06238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Oers NSC, Killeen N, Weiss A. ZAP-70 is constitutively associated with tyrosine-phosphorylated TCR ζ in murine thymocytes and lymph node T cells. Immunity. 1994;1:675–685. doi: 10.1016/1074-7613(94)90038-8. [DOI] [PubMed] [Google Scholar]