Abstract
The transporter associated with antigen presentation (TAP) complex shuttles cytosolic peptides into the exocytic compartment for association with nascent major histocompatibility complex class I molecules. Biochemical studies of murine and human TAP have established that substrate length and COOH-terminal residue identity are strong determinants of transport efficiency. However, the existence of these specificities in the intact cell and their influences on T cell responses have not been demonstrated. We have devised a method for studying TAP- mediated transport in intact cells, using T cell activation as a readout. The approach makes use of a panel of recombinant vaccinia viruses expressing peptides containing the Kd-restricted nonamer influenza nucleoprotein residues 147–155. The COOH terminus of each construct was appended with a dipeptide composed of an internal threonine residue followed by a varying amino acid. Synthetic peptide versions of these 11-mers exhibit vastly different transport capabilities in streptolysin O–permeabilized cells, in accordance with the predicted influence of the COOH-terminal residues. Presentation of the endogenously expressed version of each construct requires TAP-mediated transport and cooexpression with a vac-encoded exocytic COOH-terminal dipeptidase, angiotensin converting enzyme, to allow liberation of the minimal epitope. Recognition by epitope-specific CTLs therefore signifies TAP-mediated transport of a complete 11-mer within the target cell. Under normal assay conditions no influences of the COOH-terminal residue were revealed. However, when T cell recognition was limited, either by blocking CD8 coreceptor interactions or by decreasing the amount of transport substrate synthesized, significant COOH-terminal effects were revealed. Under such conditions, those peptides that transported poorly in biochemical assays were less efficiently presented. Therefore, TAP specificity operates in the intact cell, appears to reflect previously defined rules with regard to the influence of the COOH-terminal residue, and can strongly influence T cell responses.
CD8+ CTLs recognize short (8–10-amino acid) peptide portions of antigen (epitopes) complexed with major histocompatibility class I molecules (1–3). The initial processing of most antigens destined for recognition by class I–restricted CTLs occurs in the cytosol. The resultant fragments are then conveyed to the exocytic compartment by the transporter associated with antigen presentation (TAP)1 heterodimer where, perhaps after further processing, they become available for binding to nascent class I. Because of this function, the TAP complex, a member of the ABC family of transporters (4, 5), is critical for presentation of the vast majority of class I–restricted epitopes, as well as for the surface expression of class I molecules themselves (6).
Numerous potential epitopes are contained within an antigenic protein, but very few trigger CTL responses. Although much of this selectivity can be attributed to stringent haplotype-specific class I binding requirements (7), other factors determining epitope immunogenicity include the availability of appropriate T cell specificities and the ability of the proteolytic machinery to excise the epitope without destroying it too rapidly (8, 9). As understanding of the critical role of TAP in class I–restricted antigen presentation has grown, it has been intriguing to speculate that TAP substrate specificity may also have a significant hand in determining which epitopes are available for T cell recognition.
It was initially observed that polymorphism at the rat cim locus, the TAP homologue in that species, could be correlated with variability in the array of class I–associated peptides (10). This finding was of particular significance because it implied an influence of transport specificity in a relatively unmanipulated system. Although the cim effect could be shown to influence T cell responses in the rat, it is important to note that the cim locus exhibits far greater variability than does the TAP locus in either mice or humans (3). This approach, applied to a study of class I–associated peptides in mice and humans, has failed to identify a similar influence of allelic variation on TAP specificity (11, 12). However, in vitro biochemical assays, using either streptolysin O–permeabilized cells or isolated microsomes, have provided evidence for substrate preferences by all TAP alleles in both species (13–18). By these means, a size optimum of 8–12 residues has been established, and it has been found that mouse TAPs prefer peptides with hydrophobic COOH-terminal residues; human TAPs transport peptides with both hydrophobic and acidic COOH termini (19, 20). These preferences are consistent with MHC binding capabilities and this, along with the genetic linkage between TAP and MHC (3), has given rise to the speculation that the two have coevolved to enhance the efficiency of the class I–restricted response. To date, these biochemical assays provide the only evidence for murine or human TAP selectivity; as yet it is undemonstrated whether the measured preferences have significance for T cell recognition.
Some evidence suggests that murine TAP specificity may not play a large role in shaping T cell responses. It is clear that substrates showing little or no transport capability in in vitro transport assays can be presented to T cells and serve as potent immunogens in vivo. Shepherd et al. previously reported that the minimal epitope influenza nucleoprotein residues 147–155 (NP147–155) is not detectably transported into isolated microsomes, with a 50% inhibitory concentration (IC50) value of >50 μM (14). Although these authors suggested that a longer fragment containing the epitope may be transported and further processed in the exocytic compartment, a minigene expressing only the minimal epitope is a more efficient immunogen than full-length NP (21, 22). Furthermore, it has been reported that cells expressing murine TAP and the human HLA-A3 gene product can efficiently produce ligands for this class I molecule despite its preference for positively charged COOH termini (23), which are likely to be generated before transport (24). Given the high sensitivity of T cells, it is possible that TAP selectivity may not significantly impact their responses under physiological conditions. Additionally, as with in vitro nuclear transport assays (25), streptolysin O and microsome isolation may lead to a loss of molecules that influence transport in vivo. It is possible that such molecules physically interact with the TAP complex, processed fragments, or both, to influence transport specificity and/or rates. Therefore, we wished to test whether biochemically established transport capability was in fact correlated with TAP selectivity within intact cells and, if so, begin to elucidate conditions of antigen expression and CTL sensitivity under which selectivity might influence an immune response.
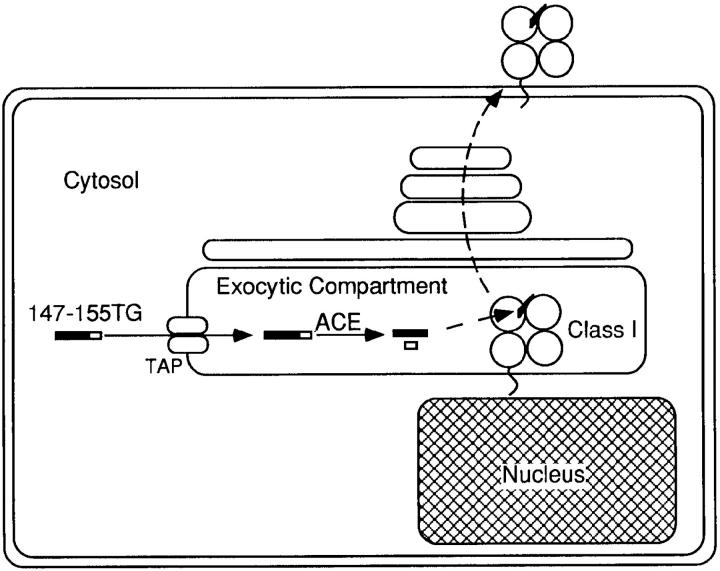
In this study, we describe a unique approach to measuring the influence of TAP specificity within live, intact cells, using the physiologically significant readout of CTL recognition. A previous study demonstrated that the Kd-restricted influenza nucleoprotein epitope, amino acids 147–155, cannot be processed and presented when appended by two COOH-terminal residues, threonine and glycine, due to the unavailability of appropriate proteolytic activity (21). Presentation of the epitope can take place when the fragment is coexpressed with the dipeptidyl carboxypeptidase angiotensin converting enzyme (ACE; references 26 and 27). As rescue is intracellular and TAP-dependent, and this enzyme is active only in the exocytic compartment, and ACE-mediated processing of the 11-mer is a reflection of TAP-mediated transport within intact target cells (see Fig. 1 for a schematic of this process). We have used this system to examine class I–restricted presentation of an array of substrates that differ significantly in their in vitro transport capabilities.
Figure 1.
Schematic diagram of transport-dependent ACE-mediated processing in the exocytic compartment.
Materials and Methods
Cell Lines.
The L-Kd (L929 transfected with the Kd gene) cell line was maintained in DMEM, 5% FCS. RMA and RMA-S cells were maintained in RPMI, 5% FCS.
Peptides and Antibodies.
Peptides were acquired from Research Genetics (Huntsville, AL) and either were obtained at >90% purity or were purified by reverse-phase HPLC. Purified anti-CD8 β subunit antibody 2.43 was provided by Dr. Y. Sykulev (Jefferson Medical College).
PCR-based Mutagenesis and Vaccinia Virus Generation.
Minigene s and minigene-encoding vaccinia (vac) recombinants were generated as previously described (21) with the following modification: minigenes were made by PCR of the NP/R − gene. The synthetic oligonucleotide primers used contained a SalI site in the upstream primer and a NotI site in the downstream primer to enable directional ligation. The downstream primer contained random bases at the triplet encoding the residue at the “X” position. All minigenes were preceded by Kozak's consensus sequence and an initiating methionine and followed by two stop codons to ensure efficient and accurate translation (28). After ligation into a modified pSC11 plasmid, DNA was isolated from random colonies and sequenced to determine the identity of the randomly mutated residue. ACE vac has been previously described (27). Cytosolic ACE was generated by PCR of the ACE gene. The synthetic oligonucleotide primers used contained a NotI site in the upstream primer and XhoI in the downstream primer to anneal to a unique XhoI site within ACE. The upstream primer annealed to the portion of ACE directly after the leader sequence. The PCR product was cloned into NotI-XhoI cut pBS-KS+-ACE and then shuttled into the modified pSC11 for recombination into vac. Radioimmunoprecipitation of cytosolic ACE vac-infected cells yielded two rapidly degraded anti-ACE–specific bands as previously observed (27). Generation of the pSC11 plasmid containing the original thermostable duplex barrier (HP20) construct has been previously described (29) and contained a KpnI site at the top of the hairpin loop structure. To generate constructs with two (HP18), four (HP16), or six (HP14) mismatched bps, synthetic oligonucleotide pairs encoding the desired changes and designed to ablate the KpnI site after successful ligation were inserted into KpnI-SalI cut vector. SalI/NotI-ended 147–155TX (TX) constructs were ligated into these vectors and the resulting plasmids were used to generate vac recombinants.
Target Cell Sensitization by Vaccinia Virus Infection.
L-Kd cells (L929 cells stably expressing the class I Kd molecule at the cell surface) were trypsinized, washed once with balanced salt solution with 0.1% BSA (BSS/BSA), placed at 2 × 107 cells/ml in BSS/BSA, and infected for 1 h with 5 PFU/cell vac. Cells were then diluted with Iscove's medium and infection proceeded for an additional 3 h.
CTL Assays.
CTL assays were performed as described elsewhere (21). Infected cells were pelleted, suspended at 4 × 107 cells/ml in Iscove's medium with 100 μCi 51Cr, and incubated for 1 h at 37°C. Cells were then washed three times with PBS, suspended in medium, and combined with NP-specific CTLs. Targets and CTLs were coincubated for 4 h at 37°C, then supernatants were collected and counted. For assays using the partially blocking anti-CD8 antibodies, CTLs, used at a constant effector/ target ratio of 10:1, were coincubated with the antibody dilutions for 30 min before addition of the chromium-labeled target cells.
Generation of CTL.
CTL populations were generated as previously described (21). BALB/c mice (H-2d haplotype) were injected intraperitoneally with 107 PFU of vac recombinants expressing NPM147–155. After at least 2 wk, splenocytes were harvested. One-third of total spleen cells was infected with PR8, washed, and then mixed with the remaining cells in Iscove's medium. Cultures were harvested after 6 or 7 d and used in CTL assays.
Streptolysin O Peptide Transport Assay.
In vitro assay of TAP-mediated peptide transport was performed as previously described (17). RMA cells (3 × 106/sample) were permeabilized with streptolysin O (2 U/ml; Murex Diagnostics, Norcross, GA) and incubated for 15 min at 37°C with ∼100 ng radioiodinated reporter peptide (amino acid sequence TVNKTERAY, described in reference 17, iodinated by the Enzymobead method, Bio-Rad, Hercules, CA, to a specific activity of ∼20 μCi/μg), 10 μl 100 mM ATP, and indicated dilutions of competitor peptides. Glycosylated reporter peptide (indicating transport to the exocytic compartment) was recovered using Con A Sepharose (Pharmacia, Biotech AB, Uppsala, Sweden) and quantitated on a gamma counter. The peptides were tested without the initiating methionine due to the predicted action of methionyl aminopeptidase on the endogenously expressed substrates. Reporter peptide transport in TAP-minus RMA-S cells was always assessed as a negative control and typically yielded counts <2% of those seen with RMA + reporter peptide alone. Samples were done singly except for RMA cells with no competitor, which was done in duplicate. The experiment was repeated four times, and each time yielded similar results.
Measurement of the Kinetics of ACE-mediated Substrate Modification.
10 ng of synthetic peptide was coincubated with ACE (0.001 U; Sigma Chemical Co., St. Louis, MO) in Hepes-buffered saline, pH 7.3, in a 20-μl reaction. At the indicated time points, samples were frozen on ethanol/dry ice, and then dried and analyzed by reverse-phase HPLC. Reactions were monitored by the disappearance of a peak corresponding to starting substrate. Substrate concentrations at the various time points were calculated based on the peak area of 10 nM of starting substrate. Initially both reactions resulted in the appearance, at comparable kinetics, of a species which coeluted with synthetic 147–155. This species was eventually also destroyed by ACE.
Radioimmunoprecipitation.
106 L-Kd were infected for 1 h with 10 PFU/cell of the indicated vacs in BSS/BSA, followed by the addition of 1 ml of methionine-free DME containing 70 μCi [35S]methionine (Amersham Corp., Arlington Heights, IL). Infection proceeded for an additional 5 h and then cells were lysed in 140 mM NaCl, 10 mM Tris, pH 7.4, 0.5% NP-40, and 2 mM PMSF. Nuclei were removed at high speed centrifugation, lysates were precleared with protein A–Sepharose beads overnight, and were then incubated for 90 min with protein A–Sepharose beads coupled with the NP-specific antibodies HB65 and H19-S24 (provided by Dr. W. Gerhard, Wistar Institute, Philadelphia, PA). Washed pellets were boiled in reducing SDS-PAGE sample buffer, then 10-fold dilutions were made of the supernatant from the wild-type NP sample for comparison to undiluted supernatants from the HP vacs. Samples were analyzed on an SDS-PAGE 10% gel. Relative band intensities were determined by scanning the gel with a Personal Densitometer SI (Molecular Dynamics, Sunnyvale, CA) using a Windows workstation.
Results
ACE-mediated Rescue of a Panel of 11-mer Fragments.
A panel of vacs was generated containing an array of TX minigenes in which the residue at the COOH-terminal position was randomly changed. Eight constructs were made, with substitutions representing each of the amino acid types and, more importantly, representing examples of COOH-terminal residues previously shown to have either a positive or negative influence on transport (15, 17). All of the constructs studied contain an initiating methionine that is predicted to be removed by methionyl aminopeptidase (30), and which has therefore been eliminated from the nomenclature.
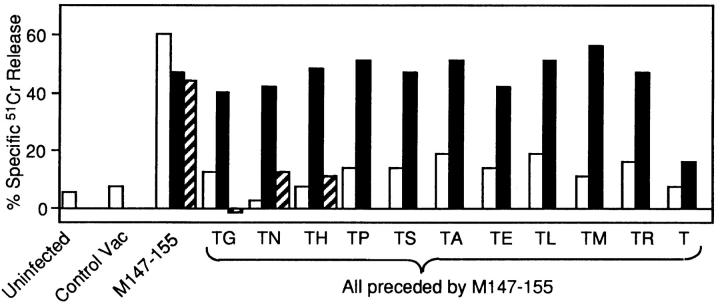
None of these constructs was presented in the absence of ACE, reiterating severe limitations on available mono- or dipeptidyl carboxypeptidase activity suggested by the TG block of presentation (Fig. 2). When coexpressed with ACE, every TX vac sensitized target cells for lysis by NP-specific CTLs. One trivial explanation for this phenomenon is that the COOH-terminal (X) residue may be cleaved in the cytosol, followed by transport and unconventional ACE-mediated removal of the remaining threonine. To address this possibility, we generated and tested a vac recombinant that expressed 147–155T (with no X residue). This construct failed to sensitize target cells for NP-specific lysis above background levels, and its presentation could not be rescued by ACE. Also shown in this figure, a form of ACE intentionally delivered to the cytosol failed to process TG and all other TX constructs tested. Taken with the previous finding that cytosolically delivered ACE is rapidly degraded and all detectable intracellular wild-type ACE activity segregates with the glycosylated form of the enzyme (27), this result indicates that the effects seen in our system are not due to anomalous expression of small amounts of ACE within the cytosol, and is consistent with a strict requirement for transport to the exocytic compartment. Thus, each member of the panel is transported sufficiently well within an intact cell to stimulate recognition and lysis by class I–restricted CTLs in an optimized chromium release assay.
Figure 2.
A panel of 147–155TX minigene-expressing vac recombinants containing random amino acid replacements at the COOH-terminal X position are all transported, processed by ACE, and presented by intact target cells. L-Kd cells were infected for 4 h with 5 PFU of the indicated vac with 5 PFU of control virus (open bars), or with 5 PFU ACE vac (solid bars), or cytosolically delivered ACE vac (hatched bars). 51Cr-labeled target cells were then assessed for recognition by NP147–155-specific CTL at an effector/target ratio of 50:1. Control virus in these experiments was NP296–498 vac.
In Vitro Transport of TX Mutant Constructs.
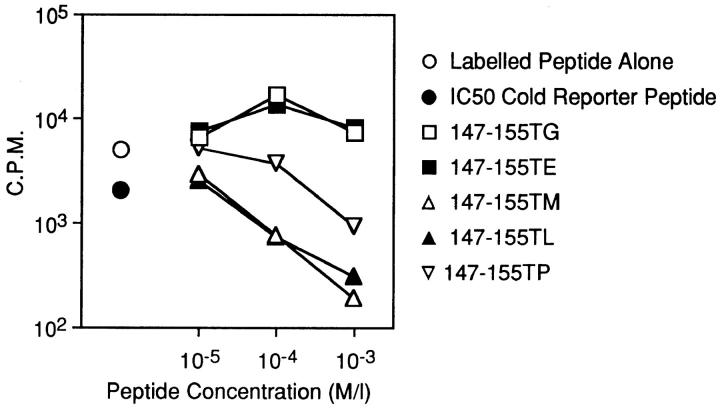
Because each construct sensitized target cells for recognition by CTLs, we considered the possibility that these substrates do not differ in their transport capabilities despite the predicted influence of the COOH-terminal replacements (15, 17). Synthetic versions of selected constructs predicted to be either transport-permissive (147–155TL, –TM) or -nonpermissive (147–155TP, –TE, –TG) were tested for their ability to inhibit TAP-dependent transport of the radiolabeled reporter peptide, TVNKTERAY (17), in streptolysin O–permeabilized RMA cells (Fig. 3). TL and TM were both well-transported, with IC50 values of ∼10 μM. TP was transported at a rate at least a log below that of the well-transported substrates, with inhibition of reporter peptide transport occurring only at substrate concentrations well above those normally tested in this type of assay. Unexpectedly, we frequently observed an enhancement rather than inhibition of reporter peptide transport in the presence of both TE and TG. We are presently investigating this intriguing phenomenon, but based on this data can only state that neither of these substrates appears to be transported under the conditions of this assay. This property was also exhibited by the unappended minimal epitope, generally in agreement with a previously reported IC50 value of >50 μM (14). Therefore, this panel of substrates exhibits a wide range of in vitro transport capabilities. Note that, although rules regarding the influence of COOH-terminal residues on transport have been established on a limited range of test peptides, predictions of transport capability based on these rules were completely borne out.
Figure 3.
In vitro transport of synthetic versions of the 147-155TX constructs. RMA cells were permeabilized with streptolysin O (2 U/ml) and incubated with radioiodinated reporter peptide (amino acid sequence TVNKTERAY) plus the indicated dilutions of test peptides. Reporter peptide transport in TAP-minus RMA-S cells was always assessed as a negative control and typically yielded counts <2% of those seen with RMA + reporter peptide alone. Samples were done singly except for RMA cells with no competitor, done in duplicate. The experiment was repeated four times, all yielding similar results.
Purified ACE-mediated Modification of the Synthetic Form of Each TX Construct.
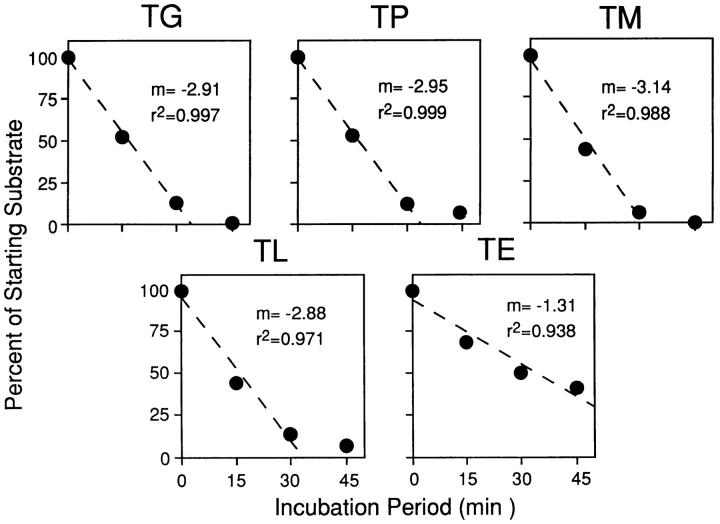
It was necessary to ensure that differences in ACE-mediated substrate modification would not confound our analysis, perhaps masking true transport differences. Purified ACE was incubated with a starting concentration of 10 nM of synthetic peptide. The reaction was stopped at various time points and the reaction products were analyzed by reverse-phase HPLC. The kinetics of ACE-mediated peptide modification were determined by following the rate of disappearance of the peak representing starting substrate (Fig. 4). Of the substrates tested, only ACE-mediated presentation of TE differed significantly from the other substrates, being modified more slowly. Therefore, this construct was excluded from further analysis. Consistent with this, note that this construct exhibited slightly but repeatably lower levels of presentation under standard assay conditions (Fig. 2).
Figure 4.
Kinetics of purified ACE modification of synthetic substrates. 10 ng synthetic peptide was coincubated with ACE (0.001 U) for the indicated times. Samples were then subjected to reverse-phase HPLC. Reaction rates were monitored by the disappearance of the peak corresponding to starting substrate.
Comparison of Presentation Efficiencies under Suboptimal Conditions.
It has been demonstrated that, under the conditions of vaccinia-driven antigen expression and the time course of the typical chromium release assays, it can be difficult to resolve significant differences in presentation efficiencies (9, 31). For example, if only 15 MHC–peptide complexes are required on the cell surface to stimulate optimal levels of T cell lysis, then any processing events that yield more than this relatively small number will stimulate similar lysis levels. To ensure the finest resolution, the assay must be performed under conditions that are suboptimal for T cell recognition. This may be accomplished by limiting the number of MHC–peptide complexes that reach the cell surface through the addition of the fungal metabolite brefeldin A (32–34), by limiting T cell receptor–ligand interaction through the use of partially blocking anti–T cell receptor or anti-CD8 antibodies, or by limiting the amount of starting substrate through genetic manipulations of the test constructs.
Partial T Cell Receptor Blocking with Anti-CD8 Antibodies.
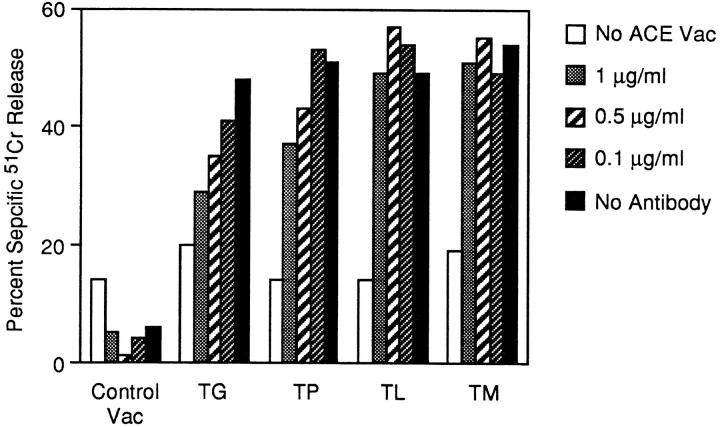
When we examined presentation of the epitope from the various TX contexts following the addition of brefeldin A at numerous time points after infection, we failed to distinguish presentation differences between them (data not shown). However, the two other approaches described above yielded more discriminating results. First, we examined ACE-mediated presentation of the constructs in the presence of increasing doses of purified 2.43, a monoclonal antibody directed to the β subunit of CD8. Although the results were somewhat variable between experiments, this antibody will completely block CTL recognition at doses of 5–10 μg/ml. As shown in Fig. 5, susceptibility to the blocking antibodies across the panel of constructs precisely mirrored the in vitro transport capability of the peptides. Presentation of TG, which exhibited no detectable transport in streptolysin O–permeabilized cells, was severely inhibited at 1 μg/ml of the antibody. This concentration had a lesser, but still significant, blocking effect on TP presentation, a poorly transported substrate. The very well transported substrates, TL and TM, showed only slightly suboptimal presentation levels under this condition. This pattern of differential susceptibility was maintained at decreasing antibody doses. Even at a concentration of 0.1 μg/ml, perhaps one-tenth the concentration of antibody required to block optimally expressed peptide complexes, the poorly transported TG suboptimally sensitized target cells for lysis. Therefore, under conditions of limited T cell stimulation, biochemically established rules of transport capability also appear to apply within the intact cell.
Figure 5.
ACE-rescued presentation under conditions of limited T cell receptor stimulation. L-Kd cells were coinfected for 4 h with 5 PFU of the indicated vac alone and 5 PFU ACE vac. 51Cr-labeled target cells were then assessed for recognition by NP147–155-specific CTLs with or without the indicated concentrations of anti-CD8 antibody 2.43. CTLs were used at a constant effector/target ratio of 10:1.
Limiting Starting Levels of Antigen Expression.
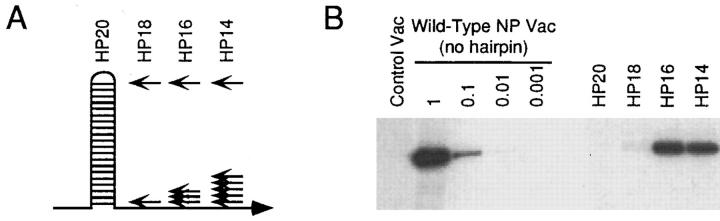
It is possible that levels of antigen expression resulting from in vitro vac infection of target cells are higher than those that occur in many physiological situations. This is particularly a concern with minigene products (31). We therefore wished to assess presentation when starting substrate expression was limited. To do this, we made use of a previously described thermostable duplex barrier, comprised of a hairpin structure containing 20 paired bases (HP20; reference 29). When placed between the P7.5 vac promoter and the initiating methionine of the vac-encoded NP, presentation of three NP epitopes was significantly reduced (29). We extended this approach to include three additional structures, denoted HP18, HP16, and HP14, each containing two, four, or six fewer base pairings than the original structure. This panel was used to try to ensure that we would achieve suboptimal conditions for each of the TX constructs tested.
To quantitatively ascertain the effects of these barrier structures on steady state substrate expression levels, the expression of vac-encoded full-length NP was assessed by NP-specific immunoprecipitation of metabolically labeled vac-infected cells. We found that the most thermostable of these, HP20, reduced NP expression to undetectable levels (>100-fold reduction) by this biochemical approach, while HP18 reduced expression by ∼100-fold. Interestingly, the HP16 and HP14 structures showed similar decreases of approximately two- to fivefold (Fig. 6).
Figure 6.
Effect of a panel of thermostable duplex barriers on NP expression. (A) Schematic diagram of the hairpin structures. Arrows indicate the sites of mismatched bps. (B) L-Kd cells were infected for 1 h with 10 PFU of the indicated vacs, followed by an additional 5 h in the presence of [35S]methionine. Cells were lysed and protein A–Sepharose–cleared lysates were subjected to immunoprecipitation by NP-specific antibodies.
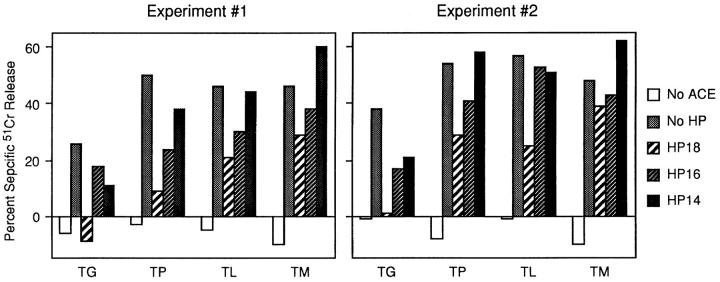
The panel of TX constructs was placed behind each of these structures, recombined into vaccinia, and tested for ACE-mediated presentation. We failed to see significant presentation of any of the constructs when placed behind HP20 and thus concentrated our analysis on the three less stringent barrier hairpins. As shown in Fig. 7, presentation efficiencies of the constructs across the range of barriers were consistent with the biochemical and anti-CD8 analyses. For example, the well-transported constructs, TM and TL, sensitized targets suboptimally when placed behind both the HP18 and the HP16 barriers. Both achieved optimal presentation when placed behind HP14, the least thermostable of the hairpin structures. TP, a poorly transported substrate in vitro, exhibited significantly lower presentation levels in all of the hairpin contexts. In experiment 2, presentation was still suboptimal in the HP14 context. Presentation of the substrate exhibiting no detectable transport in vitro, TG, was significantly lower in all contexts; presentation was still significantly suboptimal when placed behind HP14. In fact, in the experiments shown, even wild-type TG presentation was somewhat lower than the other constructs, although this was not consistently observed (see Fig. 2).
Figure 7.
ACE-mediated rescue under conditions of limited starting substrate. L-Kd cells were coinfected for 4 h with 5 PFU of the indicated vacs alone and 5 PFU ACE vac. 51Cr-labeled target cells were then assessed for recognition by NP147–155-specific CTLs. Shown are two independent experiments. Effector/target ratios were 30:1 for experiment 1 and 28:1 for experiment 2.
Discussion
We have used ACE-mediated processing in the exocytic compartment, followed by recognition of the processed peptide by CTLs, as a way of gauging TAP function within intact cells. Several endogenously expressed substrates differing only at the COOH-terminal residues were generated and their presentation efficiencies were compared to the transport capabilities of synthetic correlates. This approach allowed comparison of substrates that differed in transport capability but all contained the same epitope. The analysis was confined to substrates that could be modified by ACE with similar kinetics. This eliminated differences in processing, MHC binding, or T cell recognition as confounding factors.
We found that TAP selectivity within the intact cell can strongly influence T cell responses and that, at least with regard to the contribution of the COOH-terminal residue, this selectivity follows previously established rules. However, the in vitro assays do not entirely predict the effects of transport selectivity. 147-155, TG, and TE (reference 14 and data not shown), despite exhibiting no detectable transport in vitro, can sensitize target cells for CTL lysis. Whether this is due entirely to the exquisite sensitivity of T cells, or reflects the presence in the intact cell of factors that enhance transport efficiency, is not known.
It will be of interest to determine whether established biochemical results consistently predict effects of TAP specificity upon T cell responses. Basic and acidic residues at the COOH terminus, as well as proline and glycine, studied in this paper, have been shown to have strong negative effects upon transport in vitro (15, 16). However, as shown in Fig. 4, we were unable to evaluate glutamic acid due to slower modification of TE by ACE. It may be that our approach is not feasible for this group of amino acids. Identity of internal residues and peptide length are also strong determinants of in vitro transport efficiency. Investigation of the internal residues using the system described here would likely be challenging. First, changes within the epitope itself will alter T cell responses, either by modifying binding to class I or altering contact with T cell receptor, limiting the number of possible internal changes. Second, the assay depends upon the absence of antigen processing in the cytosol and precise processing by a coexpressed protease after transport. With increasing peptide length, these conditions may be difficult to achieve.
A subtle reduction in the amount of starting substrate, as small as a twofold reduction in TX minigene expression levels, allowed for discernment of the effects of TAP selectivity. This is of particular importance given the findings of Antón et al. (31) that endogenous expression of the NP147–155 minigene results in a 1,000-fold greater number of epitopes produced than when full-length NP is the starting substrate. This is presumably due to more molecules being synthesized in a given time frame and to a lack of the requirement for processing in the cytosol. We assume that expression of the TX minigenes gives rise to roughly the same peptide numbers as the minigene-encoded minimal epitope. If this is true, then the least stringent hairpin, HP14, where expression levels are reduced only 2-fold, may still give rise to substrate levels ∼500-fold greater than those normally seen after cytosolic generation of transportable peptides from full-length NP. Yet TAP selectivity is clearly evident in this case, becoming even more so when substrate levels dip to ∼10-fold more than what would be predicted for full-length NP. This suggests that in the physiologic situation, where antigen is cytosolically processed before transport, TAP preferences can be a powerful influence on the T cell response. Similarly, by using as little as a 100-fold less anti-CD8 antibody than is required to completely block T cell receptor–ligand interactions, one can discern differences between well- and poorly transported substrates. This suggests that TAP selectivity may allow for qualitatively different responses of low versus high affinity T cells.
Many investigators have questioned the adaptive value of limiting the array of epitopes that can access the exocytic compartment and become available for class I–restricted T cell recognition. It has been suggested that restrictions imposed by TAP specificity may represent a balance between the need to generate a variety of epitopes and the necessity of preventing excessive clonal deletion during the process of negative selection in the thymus (35). Alternatively, selectivity has been proposed to enhance efficiency of the processing pathway by minimizing the need to cycle non– MHC-binding fragments back into the cytosol for further destruction (36). To experimentally explore such questions, it is critical that we employ assay methods that can most finely discriminate the influences of such processes on T cell recognition.
Acknowledgments
We thank the Kimmel Cancer Institute Nucleic Acid Facility for synthetic oligonucleotide synthesis and DNA sequence analysis, Dr. Steven Carrithers for radioiodination of the transport assay reporter peptide, and Laura Franlin and Kim Lundgren for technical assistance.
Supported by the American Cancer Society (IM-726) and the Life Sciences Research Foundation (LSRF). A.J. Yellen-Shaw was a fellow of the LSRF during the course of this work.
Footnotes
Abbreviations used in this paper: ACE, angiotensin converting enzyme; IC50, 50% inhibitory concentration; NP, influenza nucleoprotein; TAP, transporter associated with antigen presentation; TE, 147–155TE; TG, 147–155TG; TL, 147–155TL; TM, 147–155TM; TP, 147–155TP; TX, 147–155TX; vac, vaccinia.
References
- 1.Yewdell JW, Bennink JR. Cell biology of antigen processing and presentation to major histocompatibility complex class I molecule–restricted T lymphocytes. Adv Immunol. 1992;52:1–123. doi: 10.1016/s0065-2776(08)60875-5. [DOI] [PubMed] [Google Scholar]
- 2.Germain RN, Margulies DH. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- 3.Heemels M-T, Ploegh H. Generation, translocation, and presentation of MHC class I–restricted peptides. Annu Rev Biochem. 1995;64:463–491. doi: 10.1146/annurev.bi.64.070195.002335. [DOI] [PubMed] [Google Scholar]
- 4.Spies T, Bresnahan M, Bahram S, Arnold D, Blanck G, Mellins E, Pious D, DeMars R. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature (Lond) 1990;348:744–747. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- 5.Attaya M, Jameson S, Martinez CK, Hermel E, Aldrich C, Forman J, Fischer K, Lindahl, Bevan MJ, Monaco JJ. Ham-2corrects the class I antigen–processing defect in RMA-S cells. Nature (Lond) 1992;355:647–649. doi: 10.1038/355647a0. [DOI] [PubMed] [Google Scholar]
- 6.Townsend A, Öhlen C, Bastin J, Ljunggren H-G, Foster L, Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature (Lond) 1989;340:443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- 7.Engelhard VH. Structure of peptides associated with class I and class II molecules. Annu Rev Immunol. 1994;12:181–207. doi: 10.1146/annurev.iy.12.040194.001145. [DOI] [PubMed] [Google Scholar]
- 8.Niedermann G, Butz S, Ihlenfeldt HG, Grimm R, Lucchiari M, Hoschützky H, Jung G, Maier B, Eichmann K. Contribution of proteasome-mediated proteolysis to the hierarchy of epitopes presented by major histocompatibility complex class I molecules. Immunity. 1995;2:289–299. doi: 10.1016/1074-7613(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 9.Yellen-Shaw AJ, Wherry EJ, Dubois GC, Eisenlohr LC. Point mutation flanking a CTL epitope ablates in vitro and in vivorecognition of a full-length viral protein. J Immunol. 1997;158:3227–3234. [PubMed] [Google Scholar]
- 10.Powis SJ, Deverson EV, Coadwell WJ, Ciruela A, Huskisson NS, Smith H, Butcher GW, Howard JC. Effect of polymorphism of an MHC-linked transporter on the peptides assembled in a class I molecule. Nature (Lond) 1992;357:211–215. doi: 10.1038/357211a0. [DOI] [PubMed] [Google Scholar]
- 11.Schumacher TNM, Kantesaria DV, Serreze DV, Roopenian DC, Ploegh HL. Transporters from the H-2b, H-2d, H-2s, H-2k, and H-2g7(NOD/Lt) haplotype translocate similar sets of peptides. Proc Natl Acad Sci USA. 1994;91:13004–13008. doi: 10.1073/pnas.91.26.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obst R, Armandola EA, Nijenhuis M, Momburg F, Hämmerling GJ. TAP polymorphism does not influence transport of peptide variants in mice and humans. Eur J Immunol. 1995;25:2170–2176. doi: 10.1002/eji.1830250808. [DOI] [PubMed] [Google Scholar]
- 13.Neefjes JJ, Momburg F, Hämmerling GJ. Selective and ATP-dependent translocation of peptides by the MHC-encoded transporter. Science (Wash DC) 1993;261:769–771. doi: 10.1126/science.8342042. [DOI] [PubMed] [Google Scholar]
- 14.Shepherd JC, Schumacher TNM, Ashton-Rickardt PG, Imaeda S, Ploegh HL, Janeway CA, Jr, Tonegawa S. TAP1-dependent peptide translocation in vitro is ATP dependent and peptide selective. Cell. 1993;74:577–584. doi: 10.1016/0092-8674(93)80058-m. [DOI] [PubMed] [Google Scholar]
- 15.Schumacher TNM, Kantesaria DV, Heemels M-T, Ashton-Rickardt PG, Shepherd JC, Fruh K, Yang Y, Peterson PA, Tonegawa S, Ploegh HL. Peptide length and sequence specificity of the mouse TAP1/TAP2 translocator. J Exp Med. 1994;179:533–540. doi: 10.1084/jem.179.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Momburg F, Roelse J, Howard JC, Butcher GW, Hämmerling GJ, Neefjes JJ. Selectivity of MHC-encoded peptide transporters from human, mouse and rat. Nature (Lond) 1994;367:648–651. doi: 10.1038/367648a0. [DOI] [PubMed] [Google Scholar]
- 17.Neisig A, Roelse J, Sijts AJAM, Ossendorp F, Feltkamp MCW, Kast M, Melief CJM, Neefjes JJ. Major differences in transporter associated with antigen presentation (TAP)–dependent translocation of MHC class I–presentable peptides and the effect of flanking sequences. J Immunol. 1995;154:1273–1279. [PubMed] [Google Scholar]
- 18.Momburg F, Roelse J, Hämmerling GJ, Neefjes JJ. Peptide size selection by the major histocompatibility complex–encoded peptide transporter. J Exp Med. 1994;179:1613–1623. doi: 10.1084/jem.179.5.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Androlewicz MJ, Cresswell P. How selective is the transporter associated with antigen processing? . Immunity. 1996;5:1–5. doi: 10.1016/s1074-7613(00)80304-0. [DOI] [PubMed] [Google Scholar]
- 20.Koopmann J-O, Post M, Neefjes JJ, Hämmerling GJ, Momburg F. Translocation of long peptides by transporters associated with antigen processing (TAP) Eur J Immunol. 1996;26:1720–1728. doi: 10.1002/eji.1830260809. [DOI] [PubMed] [Google Scholar]
- 21.Eisenlohr LC, Yewdell JW, Bennink JR. Flanking sequences influence the presentation of an endogenously synthesized peptide to cytotoxic T lymphocytes. J Exp Med. 1992;175:481–487. doi: 10.1084/jem.175.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Restifo NP, Bacik I, Irvine KR, Yewdell JW, McFarland BJ, Anderson RW, Eisenlohr LC, Rosenberg SA, Bennink JR. Antigen processing in vivoand the elicitation of primary cytotoxic T lymphocyte responses. J Immunol. 1995;154:4414–4422. [PMC free article] [PubMed] [Google Scholar]
- 23.Maier R, Rötzschke O, Maier B, Gnau V, Stevanovic S, Jung G, Rammensee HG, Meyerhans A. Peptide motifs of HLA-A3, -A24, and -B7 molecules as determined by pool sequencing. Immunogenetics. 1994;40:306–308. doi: 10.1007/BF00189978. [DOI] [PubMed] [Google Scholar]
- 24.Snyder HL, Yewdell JW, Bennink JR. Trimming of antigenic peptides in an early secretory compartment. J Exp Med. 1994;180:2389–2394. doi: 10.1084/jem.180.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore MS, Blobel G. A G protein involved in nucleocytoplasmic transport: the role of Ran. Trends Biochem Sci. 1994;19:211–216. doi: 10.1016/0968-0004(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 26.Sherman LA, Burke TA, Biggs JA. Extracellular processing of antigens that bind class I major histocompatibility molecules. J Exp Med. 1992;175:1221–1226. doi: 10.1084/jem.175.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenlohr LC, Bacik I, Bennink JR, Bernstein K, Yewdell JW. Expression of a membrane protease enhances presentation of endogenous antigens to MHC class I–restricted T lymphocytes. Cell. 1992;71:963–972. doi: 10.1016/0092-8674(92)90392-p. [DOI] [PubMed] [Google Scholar]
- 28.Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991;266:19867–19870. [PubMed] [Google Scholar]
- 29.Bullock TNJ, Eisenlohr LC. Ribosomal scanning past the primary initiation codon as a mechanism for expression of CTL epitopes encoded in alternative reading frames. J Exp Med. 1996;184:1319–1330. doi: 10.1084/jem.184.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moerschell RP, Hosokawa Y, Tsunasawa S, Sherman F. The specificities of yeast methionine aminopeptidase and acetylation of amino-terminal methionine in vivo. . J Biol Chem. 1990;265:19638–19643. [PubMed] [Google Scholar]
- 31.Antón LC, Yewdell JW, Bennink JR. MHC class I–associated peptides produced from endogenous gene products with vastly different efficiencies. J Immunol. 1997;158:2535–2542. [PubMed] [Google Scholar]
- 32.Nuchtern JG, Bonifacino JS, Biddison WE, Klausner RD. Brefeldin A implicates egress from endoplasmic reticulum in class I restricted antigen presentation. Nature (Lond) 1989;339:223–226. doi: 10.1038/339223a0. [DOI] [PubMed] [Google Scholar]
- 33.Yewdell JW, Bennink JR. Brefeldin A specifically inhibits presentation of protein antigens to cytotoxic T lymphocytes. Science (Wash DC) 1989;244:1072–1075. doi: 10.1126/science.2471266. [DOI] [PubMed] [Google Scholar]
- 34.Cox JH, Galardy P, Bennink JR, Yewdell JW. Presentation of endogenous and exogenous antigens is not affected by inactivation of E1ubiquitin-activating enzyme in temperature-sensitive cell lines. J Immunol. 1995;154:511–519. [PubMed] [Google Scholar]
- 35.Hill A, Ploegh H. Getting the inside out: the transporter associated with antigen processing (TAP) and the presentation of viral antigen. Proc Natl Acad Sci USA. 1995;92:341–343. doi: 10.1073/pnas.92.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roelse J, Grommé M, Momburg F, Hämmerling G, Neefjes J. Trimming of TAP-translocated peptides in the endoplasmic reticulum and in the cytosol during recycling. J Exp Med. 1994;180:1591–1597. doi: 10.1084/jem.180.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]