Abstract
Topical exposure of mice to chemical allergens results in the migration of epidermal Langerhans cells (LCs) from the skin and their accumulation as immunostimulatory dendritic cells (DCs) in draining lymph nodes. Epidermal cell–derived cytokines have been implicated in the maturation and migration of LCs, but the adhesion molecules that regulate LC migration have not been studied. We hypothesized that integrin-mediated interactions with extracellular matrix components of the skin and lymph node may regulate LC/DC migration. We found that α6 integrins and α4 integrins were differentially expressed by epidermal LCs and lymph node DCs. A majority of LCs (70%) expressed the α6 integrin subunit, whereas DCs did not express α6 integrins. In contrast, the α4 integrin subunit was expressed at high levels on DCs but at much lower levels on LCs. The anti-α6 integrin antibody, GoH3, which blocks binding to laminin, completely prevented the spontaneous migration of LCs from skin explants in vitro and the rapid migration of LCs from mouse ear skin induced after intradermal administration of TNF-α in vivo. GoH3 also reduced the accumulation of DCs in draining lymph nodes by a maximum of 70% after topical administration of the chemical allergen oxazolone. LCs remaining in the epidermis in the presence of GoH3 adopted a rounded morphology, rather than the interdigitating appearance typical of LCs in naive skin, suggesting that the cells had detached from neighboring keratinocytes and withdrawn cellular processes in preparation for migration, but were unable to leave the epidermis. The anti-α4 integrin antibody PS/2, which blocks binding to fibronectin, had no effect on LC migration from the epidermis either in vitro or in vivo, or on the accumulation of DCs in draining lymph nodes after oxazolone application. RGD-containing peptides were also without effect on LC migration from skin explants.
These results identify an important role for α6 integrins in the migration of LC from the epidermis to the draining lymph node by regulating access across the epidermal basement membrane. In contrast, α4 integrins, or other integrin-dependent interactions with fibronectin that are mediated by the RGD recognition sequence, did not influence LC migration from the epidermis. In addition, α4 integrins did not affect the accumulation of LCs as DCs in draining lymph nodes.
Mature lymphoid dendritic cells (DCs)1 are derived from immunologically immature precursors in nonlymphoid tissues. The best studied example of an immature DC is the epidermal Langerhans cell (LC). LCs play important roles in the induction of sensitization to contact allergens. After topical application of allergen, LCs are induced to migrate from the skin to the draining lymph node, where they interact with naive T cells migrating in from the blood. Accompanying this migration is their maturation from an antigen-uptake and processing phenotype, typical of immature DCs in nonlymphoid tissues (1, 2), to an antigen-presenting phenotype typical of DCs in lymphoid tissues (3, 4). Maturation and migration of LCs are central events in the initiation of cutaneous immune responses to chemical allergens. Epidermal cell–derived cytokines such as GM-CSF and IL-1 stimulate the maturation of LCs in vitro (5, 6). Recent studies have also identified a role for epidermal cell–derived cytokines in regulating LC migration from the skin to draining lymph nodes. Antibodies to TNF-α prevent the early migration of LCs from the epidermis, the accumulation of DCs in draining lymph nodes, and the development of optimum contact sensitization in response to chemical allergens. Conversely, intradermal injection of homologous recombinant TNF-α stimulates the rapid migration of LCs out of the epidermis and the accumulation of DCs in draining nodes (7–9). Other cytokines derived from epidermal cells, and in particular IL-1β, may act in concert with TNF-α to promote LC migration (10).
The migration of other types of leukocytes is regulated by specific cell adhesion molecules on the cell surface. The adhesion molecules that regulate LC migration from the epidermis to the draining lymph nodes are poorly understood. During migration, LCs dissociate from neighboring keratinocytes, cross the underlying basement membrane (BM) into the dermis, enter the afferent lymphatics and subcapsular sinus leading into the draining lymph node, and relocate in the paracortical or T cell area. In so doing, LCs interact with several different BMs as well as with extracellular matrix (ECM) components of the dermis and lymph nodes. We hypothesized that integrins on the LC surface may regulate LC migration from the epidermis to draining lymph nodes.
Integrins are noncovalently linked α/β heterodimers that form a large family of cell surface adhesion receptors (11). There are 8 β and 16 α subunits, divided into subfamilies that share β subunits, giving rise to 22 distinct heterodimers. Integrins are important receptors for adhesion to ECM proteins, although some integrins, such as the α4 and β2 subunit–containing integrins on leukocytes, mediate cell–cell adhesion. The epidermal BM comprises a complex mixture of ECM proteins, including laminin, type IV collagen, and proteoglycans (12). BMs surrounding lymphatics have a similar composition and the paracortical region of lymph nodes is rich in these ECM proteins as well as fibronectin (13). In this study, we have used blocking antibodies and peptides to determine the potential role of integrin-mediated interactions with two major components of BM and the ECM, laminin and fibronectin, in regulating LC migration from the epidermis to draining lymph nodes. We report that α6 integrins regulate the initial stages of LC migration out of the epidermis across the underlying BM. In contrast, the α4 integrin or Arg-Gly-Asp (RGD) peptide–mediated interactions with fibronectin are not required.
Materials and Methods
Mice.
Young adult, 6–8-wk-old BALB/c mice bred in the Specific Pathogen Free Units at either the National Institute for Medical Research or Zeneca Pharmaceuticals were used for these studies.
Antibodies.
The following antiintegrin antibodies were used for these studies: PS/2 (anti–murine α4 integrin subunit, rat IgG2b) from the American Type Culture Collection (Rockville, MD); GoH3 (anti–murine α6 integrin subunit, rat IgG2a), purchased as an affinity-purified antibody from Immunotech (Marseille, France) and obtained as hybridoma supernatant from Dr. A. Sonnenberg (Amsterdam University, Amsterdam, The Netherlands); EA-1 (anti–mouse α6 integrin subunit, rat IgG2a), a gift from Dr. B. Imhof (Geneva University, Switzerland); and 346-11A (anti–mouse β4 integrin, rat IgG2a), purchased from PharMingen (San Diego, CA). M5/114 (anti–I-Ad and anti–I-Ed, rat IgG2b; reference 14) and NLDC-145 (anti–DEC-205, rat IgG2a; reference 15) were used to identify LCs and DCs. MAC193 (antiovine placental lactogen, rat IgG2a), from Dr. G. Butcher (Babraham Institute, Cambridge, UK) and HRPN11/12a (anti– horseradish peroxidase [HRP], rat IgG2b) from Dr. S. Hobbs (Royal Marsden Hospital, London) were used as isotype-matched control antibodies. Antibodies were purified from tissue culture supernatants by affinity chromatography using protein G HiTrap columns (Pharmacia Biotech AB, Uppsala, Sweden). FITC- (Sigma Chemical Co., St. Louis, MO) and biotin- (Pierce Chemical Co., Rockford, IL) conjugated forms of M5/114 were also used in some experiments; 50 mg of FITC and 150 mg of biotin were used per milligram of antibody for conjugation. The following were used as secondary antibodies: PE-conjugated anti–rat Ig (Southern Biotechnology Associates, Birmingham, AL), FITC-conjugated anti–rat Ig (Sigma Chemical Co.), HRP-conjugated anti–rat Ig (DAKO Corp., Carpinteria, CA), and streptavidin (SA) conjugated to either HRP (DAKO Corp.) or PE (Southern Biotechnology Associates) were used to detect biotinylated reagents.
Peptides.
Gly-Arg-Gly-Asp-Ser (GRGDS) and the control peptide Gly-Arg-Asp-Gly-Ser (GRDGS) were synthesized on a model 430A peptide synthesizer (Perkin-Elmer Corp., Norwalk, CT) using FastMocTM chemistry.
Chemicals and Exposure.
The skin-sensitizing chemical oxazolone (4-ethoxymethylene-2-phenyloxazol-5-one; Sigma Chemical Co.) was dissolved in a 4:1 mixture of acetone/olive oil. Groups of mice received 25 μl of either 1 or 0.5% oxazolone on the dorsum of each ear.
Cytokine Administration.
Recombinant murine TNF-α (specific activity: 2 × 108 U/mg by L929 cytotoxicity assay) was obtained from Genzyme Corp. (Cambridge, MA) as a sterile solution in PBS containing 0.1% BSA as carrier protein. Preparations were diluted with sterile PBS containing 0.1% BSA and administered using 1-ml syringes with 30-gauge stainless steel needles. Mice received 30 μl (50 ng) intradermal injections of cytokine into both ear pinnae. Controls included untreated mice or mice which had received an equivalent volume of carrier protein alone.
Antibody Treatment.
Mice were injected intraperitoneally with 100 μl of anti-α6 integrin antibody (GoH3, at 100, 200, or 400 μg/ml) or anti-α4 integrin antibody (PS/2 at 200, 400, or 1,000 μg/ml). Animals treated with 100 μg anti-α4 antibody received a second intraperitoneal injection of 100 μg 8 h after oxazolone treatment. Control mice were injected intraperitoneally with equal amounts of isotype-matched control antibody (MAC193 or HRPN) diluted in sterile PBS. In some experiments both ear pinnae were injected intradermally with 12 μg of anti-α6 integrin antibody (GoH3) or control antibody (MAC193) in 30 μl of sterile PBS. In all experiments, one group of animals was left untreated.
Preparation of Epidermal Cell Suspensions.
Ears from naive mice were separated into dorsal and ventral halves with forceps. Dorsal ear halves were incubated in 0.5% trypsin 1:250 (Sigma Chemical Co.) in HBSS (GIBCO BRL, Bethesda, MD) for 20 min at 37°C. Epidermal sheets were removed with forceps and washed three times in RPMI 1640 growth medium (Sigma Chemical Co.), supplemented with 25 mM Hepes, 50 μg/ml streptomycin, 50 U/ml penicillin, 2 mM glutamine (RPMI), and 20% (vol/vol) heat-inactivated (56°C for 30 min) FCS. Single cell suspensions of epidermal cells were prepared by mechanical disaggregation of the sheets through a stainless steel gauze. The cells were washed twice and resuspended in RPMI containing 10% FCS (RPMI-FCS) for flow cytometry.
Isolation and Enrichment of Lymph Node DCs.
18 h after topical application of oxazolone, DCs were isolated from auricular lymph nodes as previously described (16). In brief, mice were killed by CO2 inhalation, lymph nodes were pooled for each experimental group, and a suspension of lymph node cells was prepared by mechanical disaggregation through a stainless steel gauze. Cells were washed with 10 ml RPMI-FCS, centrifuged for 5 min at 300 g, and then resuspended in RPMI-FCS at 5 × 106/ml. DCs were enriched by density gradient centrifugation. 2 ml of Metrizamide (Nycomed, Oslo, Norway) at 14.5% in RPMI-FCS was layered under 8 ml of lymph node cells and centrifuged (600 g) for 15 min at room temperature. Interface cells (the low buoyant density fraction) were collected, washed once, and resuspended in RPMI-FCS. DCs were analyzed by flow cytometry or were assessed by direct morphological examination using phase-contrast microscopy to determine the frequency of DCs in low buoyant density fractions. DC frequencies are expressed as number of DCs per node.
Flow Cytometric Analysis.
Epidermal LCs and lymph node DCs were identified and analyzed for the presence of various cell surface markers by dual color immunofluorescent staining. Cells (105) were resuspended in PBS containing 0.2% BSA and 0.3% sodium azide (PBA) and incubated on ice with primary antibody for 30 min. Cells were washed and centrifuged (300 g for 5 min) twice with 2 ml of PBA, resuspended in PBA containing either PE-conjugated anti–rat Ig plus 10% normal mouse serum or PE-SA for biotinylated antibodies, and then cells were incubated for 30 min on ice. Cells were washed once with 2 ml of PBA and then resuspended in PBA containing 10% normal rat serum for 10 min to block residual anti–rat Ig reactivity. After two washes to remove rat serum, cells were incubated for 30 min with FITC-conjugated M5/114 (to identify LCs or DCs) and washed twice, and then a minimum of 10,000 cells were analyzed on a FACStar® flow cytometer. Data were analyzed using FACSplot® software developed by John Green (Computing Laboratory, National Institute for Medical Research).
Skin Explant Assay.
Naive mice were killed by CO2 inhalation and the ears were cut at the base with scissors. The ears were washed twice with PBS and once with 70% ethanol. Under sterile conditions, ears were spread out on a petri dish, allowed to dry, and then split into dorsal and ventral halves with forceps. The dorsal halves were floated individually on 2 ml of RPMI-FCS, or on RPMI-FCS containing antibody or peptide in 16-mm-diameter wells of 24-well cluster trays (Costar Corp., Cambridge, MA). The explants were incubated at 37°C in a 5% CO2 incubator. At various times, explants were removed and epidermal sheets were prepared and analyzed for the presence of LCs as described below. In experiments designed to test the effect of peptide or antibody on LC migration, three skin explants were used for each treatment. Control explants were also established in triplicate.
Preparation and Analysis of Epidermal Sheets.
Epidermal sheets were prepared from naive mice, skin explants, or mice previously exposed to TNF-α by intradermal injection. Dorsal ear halves were incubated in 0.02 M EDTA in PBS for 1–1.5 h. Epidermal sheets were fixed in acetone for 20 min at −20°C, washed three times with PBS, and then incubated for 30 min at room temperature either with anti–MHC class II (M5/114) or biotinylated anti–MHC class II (M5/114) diluted in PBS containing 0.2% BSA. Sheets were washed three times with PBS and incubated for 30 min at room temperature with either HRP-conjugated rabbit anti–rat Ig, FITC-conjugated goat anti–rat Ig, or HRP conjugated to SA for biotinylated anti–MHC class II. Sheets were washed twice with PBS and mounted onto glass slides in Citifluor (Citifluor Ltd., London, UK) for fluorescence analysis. For immunocytochemical staining, sheets received a further wash with Tris-HCl buffer (50 mM, pH 7.4), and were developed with 1.5 mM diaminobenzidine (Sigma Chemical Co.) in Tris-HCl buffer for 10 min and washed for a minimum of 10 min with tap water. The sheets were then mounted onto glass microscope slides, left to dry for 2 h, dehydrated in alcohol, cleared in Histoclear (National Diagnostics, Atlanta, GA), and mounted in DPX.
LCs were enumerated by counting MHC class II positive cells in epidermal sheets. For immunohistochemical analysis, six areas were chosen at random for each of three sheets, photographed at a magnification of 200, and the number of MHC class II positive cells was counted per photograph, which corresponded to an area of 0.16 mm2. Cell frequency was converted to LC/mm2 and results expressed as mean ± SD. For fluorescence analyses, the frequency of stained cells was assessed at a magnification of 100 using an eyepiece with a calibrated grid of 0.068 mm2. 4 epidermal sheets were prepared from each experimental group, and for each sheet 10 random consecutive fields were examined. Cell frequency was converted to Lc/mm2 and results expressed as mean ± SD. The statistical significance of differences between experimental groups was calculated using Student's t test.
Results
Differential Expression of Integrin Subunits by LCs and DCs.
We hypothesized that LCs may use distinct integrin adhesion receptors to interact with the underlying BM and other ECM proteins in skin and/or lymph nodes during migration from the skin to draining lymph nodes. We therefore examined epidermal LCs and lymph node DCs for the expression of integrins that mediate adhesion to two of the major components of BM and ECM, laminin and fibronectin (Fig. 1).
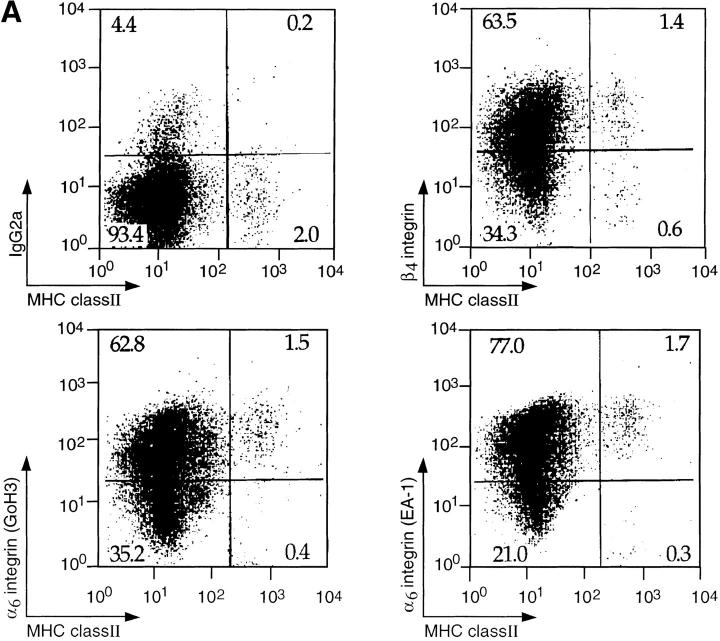
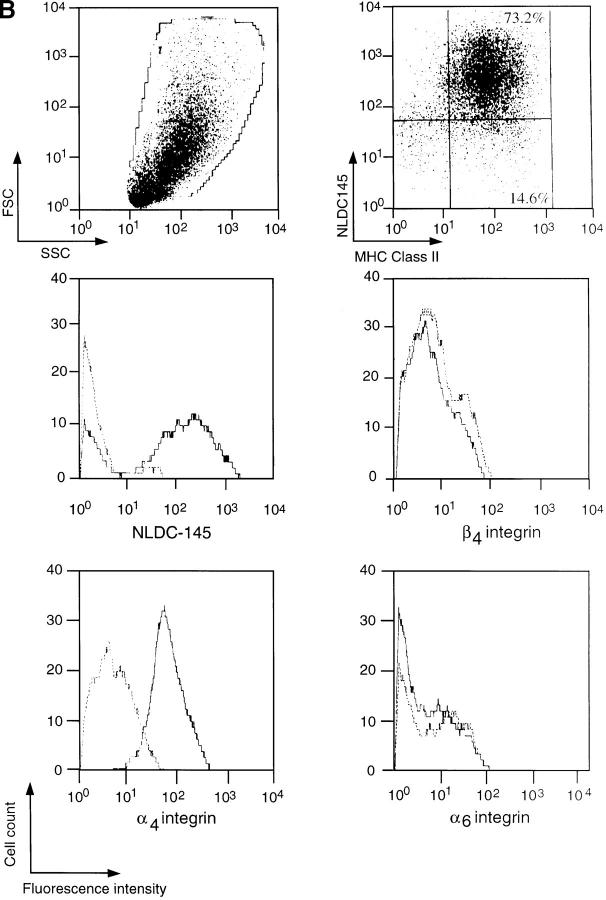
Figure 1.
(A) Expression of α6 and β4 integrin subunits on LCs. Epidermal cell suspensions were prepared from the ears of naive mice by treatment with 0.5% trypsin and LCs were identified by the expression of MHC class II. The expression of integrin subunits was determined using antibodies to α6 (GoH3 or EA-1) or β4 subunits in comparison with an isotype-matched control antibody (IgG2a). Results show log fluorescence (0–104 channels) for MHC class II (x axis) and integrin subunit (y axis). The percentage of cells within each quadrant is given. (B) Expression of α4, α6, and β4 integrin subunits on lymph node DCs. DCs were enriched from the draining lymph nodes of oxazolone-treated mice by density gradient centrifugation on Metrizamide. DCs were distinguished from lymphocytes by forward scatter (FSC) versus side scatter (SSC) analysis and identified by high expression of MHC class II and dual staining for NLDC145 antigen. Histograms show the expression of α4, α6, and β4 integrin subunits on MHC class II positive DCs (solid lines) in comparison with isotype-matched control antibodies (dashed lines). The profiles show log fluorescence (0–104 channels) on the x axis and cell number (0–40) on the y axis.
LCs were isolated from the ears of naive mice and distinguished from other epidermal cells by MHC class II expression (5, 6). The majority (>70%) of LCs expressed α6 and β4 integrin subunits. The expression of α6 integrins was determined using two different mAbs, GoH3 and EA-1, which gave similar results. The staining pattern obtained with GoH3 was similar using either affinity-purified antibody or hybridoma supernatant. The majority of class II negative epidermal cells, which are primarily keratinocytes, expressed α6 and β4 integrin subunits, as previously reported (17). The expression of α4 integrin subunit was not detectable on isolated LCs, or on other epidermal cells. The expression of NLDC-145 antigen (DEC-205) was also not detectable on isolated LCs. DCs were isolated from draining (auricular) lymph nodes of oxazolone-sensitized mice and identified according to size, granularity, expression of NLDC-145, and by high levels of MHC class II expression (Fig. 1; references 14 and 18). In contrast to LCs, lymph node DCs expressed the α4 integrin subunit, but did not express α6 or β4 integrin subunits. Expression of the α4 integrin or NLDC-145 was no longer detectable on DCs after incubation in 0.5% trypsin for 20 min, thus, the lack of α4 integrin and NLDC-145 expression on LCs could reflect loss of these epitopes during the enzyme digestion used to isolate LCs. In fact, immunocytochemical staining of epidermal sheets showed that LCs in situ express high levels of NLDC-145 (data not shown). A comparison between LCs in situ and cytocentrifuged preparations of isolated DCs showed that LCs expressed much lower levels of the α4 integrin than did isolated lymph node DCs (data not shown).
In summary, the majority of LCs expressed the α6 and β4 integrin subunits whereas DCs did not express either α6 or β4 integrins. Lymph node DCs expressed the α4 integrin subunit, but much lower levels were found on LCs.
Antibodies to α6 but not to α4 Integrin Subunit Block the Migration of LCs from Skin Explants.
The differential expression of α6 and β4 integrin subunits by LCs and lymphoid DCs suggested that the α6β4 integrin may regulate the early stages of LC migration from the epidermis. Conversely, the higher level of expression of the α4 integrin subunit by DCs in comparison with LCs raises the possibility that α4 integrins may be involved in DC localization within the lymph node. To study the roles of these integrins in regulating the initial stages of LC migration from the epidermis, blocking antibodies to α6 and α4 integrin subunits were included in the culture medium of skin explants and their effects on LC migration were determined (Fig. 2). Affinity-purified anti-α6 (GoH3) and anti-α4 (PS/2) integrin antibodies were added to the culture medium at 10, 50, and 100 μg/ml. Control explants were incubated on culture medium containing isotype-matched control antibody at 100 μg/ml, or on culture medium alone. Explants were incubated for 72 h, the epidermis was removed, and the number of LCs/mm2 was determined. The number of LCs in fresh epidermis ranged from 1,000 to 1,200 LCs/mm2. After 72 h of incubation in culture medium, this number decreased by ∼70% to 300–350 LCs/mm2. Inclusion of 100 μg/ml MAC 193 (rat IgG2a) or HRPN (rat IgG2b) had no effect on the number of LCs remaining in the epidermis after 72 h and these antibodies were therefore used as isotype-matched controls.
Figure 2.
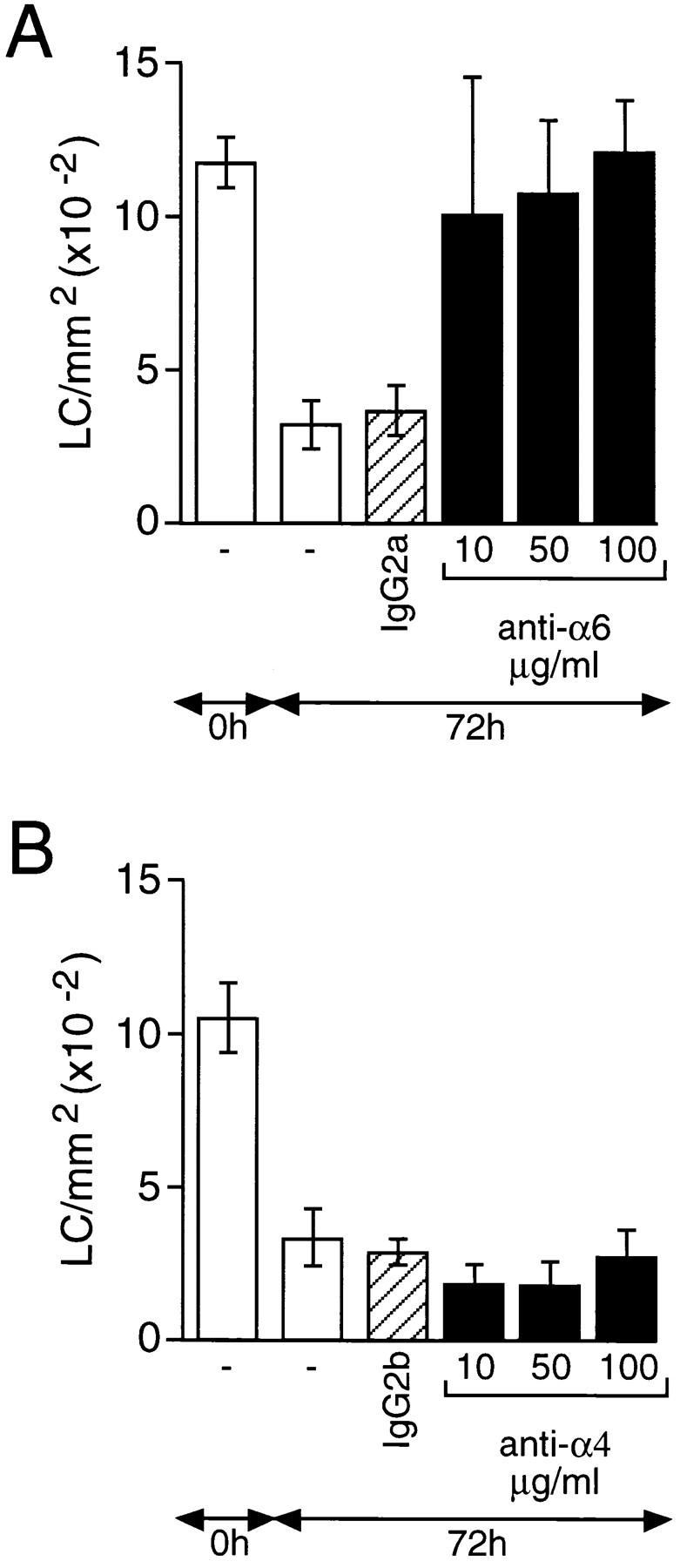
The effect of anti-α6 and anti-α4 integrin antibodies on the migration of epidermal LCs in vitro. Skin explants were derived from the ears of naive mice and incubated on medium containing either (A) anti-α6 (GoH3) or (B) anti-α4 (PS/2) integrin antibodies at 10, 50, or 100 μg/ml (solid bars). Controls included explants cultured on medium containing either no antibody (open bars) or isotype-matched control antibody at 100 μg/ml (hatched bars). Epidermal sheets were prepared from fresh ear skin (0 h) and explants after 72 h of incubation and the number of LCs/mm2 determined by immunohistochemistry. The results are expressed as means ± SD (n = 18). At all concentrations tested, in cultures containing anti-α6 antibodies, the frequency of LCs did not differ significantly from that found in fresh explants. These same values were significantly (P <0.0001) higher than those found in fresh explants cultured for 72 h in the absence of antibody, or with an isotype-matched control antibody (A). Similar treatment of explant cultures with anti-α4 antibody failed to result, at any concentration, in a significant increase in LC numbers compared with controls (B).
Inclusion of 100 μg/ml anti-α6 antibody (GoH3) in the medium completely prevented the emigration of LCs from skin explants over a 72-h period. The number of LCs/mm2 remaining in the epidermis (1,193 ± 174) was similar to that in fresh epidermis (1,168 ± 87). Lower doses of GoH3, down to 10 μg/ml, also substantially inhibited LC migration. The morphology of LCs remaining in the epidermis of anti-α6 integrin antibody–treated explants differed significantly from that of LCs in fresh epidermis (Fig. 3). LCs in α6 integrin antibody–treated skin explants were rounded in appearance and lacked the interdigitating cellular processes typical of LCs in naive skin. The few LCs remaining in skin explants incubated either in the complete absence of antibody or in the presence of isotype-matched control antibody showed similar morphologies; LCs were slightly larger than in naive skin and showed a reduced number of interdigitating processes. The staining for MHC class II on LCs in α6 integrin antibody–treated skin and in control skin explants was more intense than that on LCs in fresh epidermal sheets.
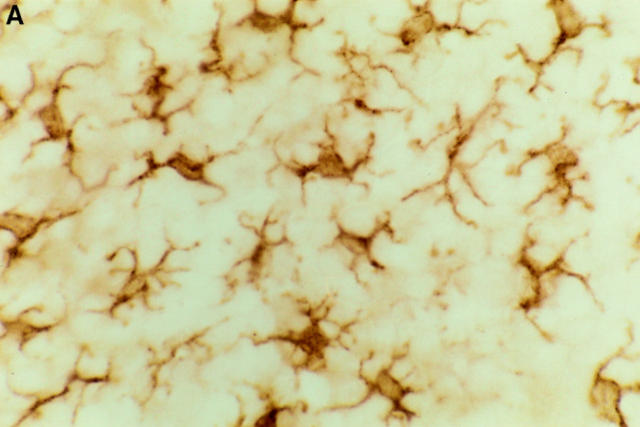
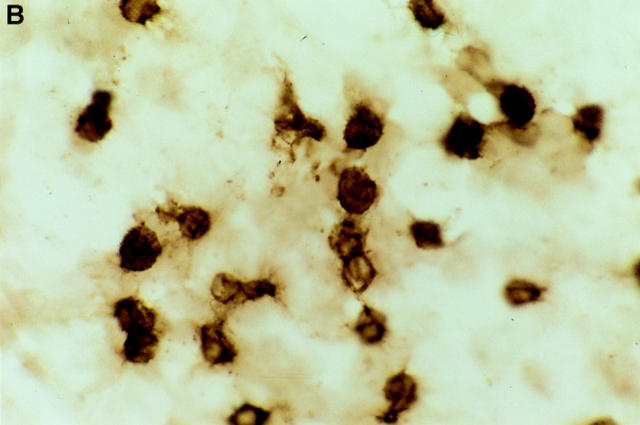
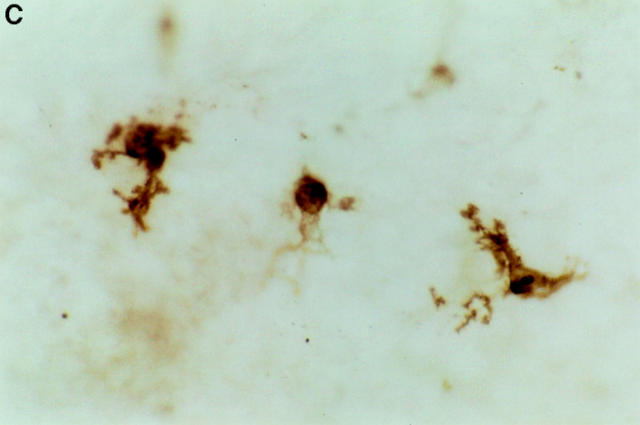
Figure 3.
Immunohistochemical staining of epidermal LCs in situ. Epidermal sheets were prepared either from (A) naive mice, or from skin explants that had been incubated for 72 h on (B) culture medium containing anti-α6 integrin antibody GoH3 at 50 μg/ml, or (C) culture medium alone. LC are stained for MHC class II using indirect immunoperoxidase staining. Note rounded morphology of LCs in anti-α6 integrin antibody– treated explants in comparison with interdigitating morphology of LCs in naive skin. Magnification, ×600.
In contrast to the α6 integrin antibody, the addition of up to 100 μg/ml antibody to the α4 integrin subunit had no significant effect on the migration of LCs from skin explants. The number of LCs/mm2 remaining in the epidermis after 72 h of incubation was 254 ± 85, which was not significantly different from epidermal sheets that had been incubated either in the complete absence of antibody or in the presence of the IgG2b control antibody (274 ± 46; Fig. 2). Microscopic examination revealed that the few LCs remaining in α4 integrin antibody–treated explants were morphologically similar to those in control explants incubated either in the absence of antibody or in the presence of isotype-matched control antibody. As described above, LCs were larger than in naive skin, showed reduced numbers of interdigitating processes, and had increased expression of MHC class II.
The Anti-α6 Integrin mAb EA-1 Does Not Affect LC Migration.
The mAb EA-1 recognizes the α6 integrin subunit and has been shown to inhibit α6 integrin–mediated binding of prothymocytes to thymic blood vessels in mice (19). However, unlike GoH3, it does not block the interaction of α6 integrins with laminin, suggesting that α6 integrins have an alternative ligand to laminin (20). Skin explants were incubated on culture medium containing ⩽50 μg/ml EA-1 and the number of LCs was determined after 24 and 72 h (Fig. 4). In contrast to the effects of GoH3, the EA-1 antibody had no effect on the migration of LCs from skin explants even after 72 h of incubation.
Figure 4.
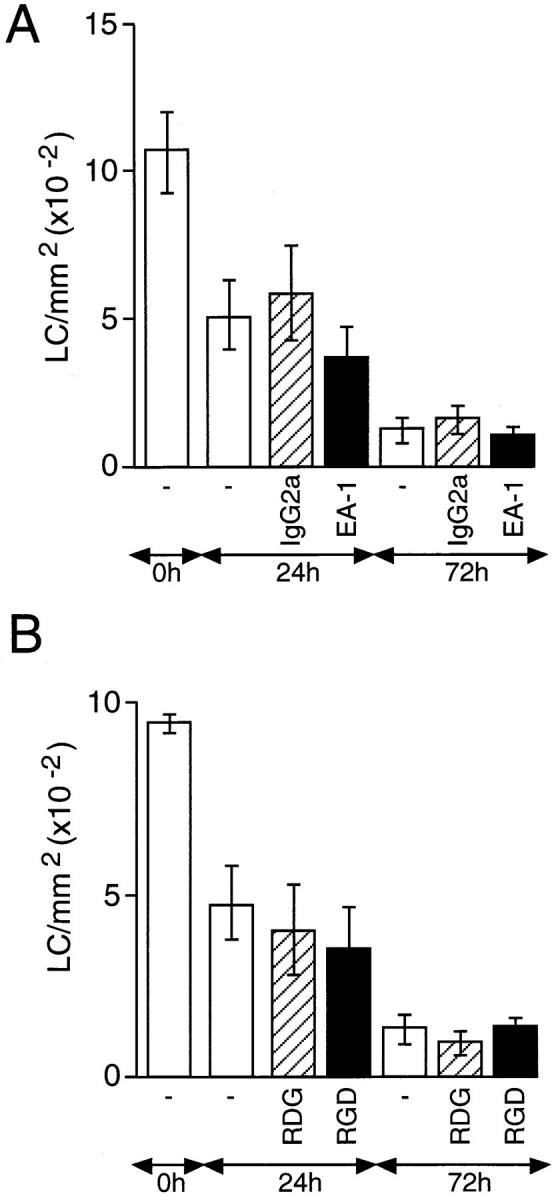
The effect of anti-α6 integrin antibody EA-1 and GRGDS peptide on the migration of epidermal LCs in vitro. Skin explants were incubated on culture medium containing: (A) 50 μg/ml EA-1 (solid bars) or control antibody (hatched bars); (B) 500 μM GRGDS (RGD; solid bars) or GRDGS (RDG; hatched bars). Additional controls included explants incubated on medium containing no antibody (open bars). The number of LCs/ mm2 was determined after 24 and 72 h of incubation and compared with fresh skin (0 h). Results are expressed as means ± SD (n = 18). Treatment of explant cultures with EA-1 antibody failed to cause a significant increase in the frequency of LCs at 24 or 72 h compared with cultures containing no antibody or an isotype-matched control antibody (A). Addition to explant cultures of GRGDS also failed to cause a significant increase in the frequency of LCs at 24 or 72 h compared with cultures containing no peptide or GRDGS.
Addition of GRGDS Peptide to Skin Explants Does Not Affect LC Migration.
We were unable to determine the expression of other integrins that may be involved in fibronectin binding, such as the α5 and β1 integrin subunits, due to lack of available antibodies. Therefore, we have studied the potential role of the α5β1 integrin in regulating LC migration using a pentapeptide containing the RGD sequence which blocks α5β1 integrin–fibronectin interactions (21). Skin explants were incubated on culture medium alone or medium containing either the active pentapeptide GRGDS or the inactive peptide GRDGS (Fig. 4). A maximum dose of peptide (500 μM) was chosen based on previous studies (22) and this completely inhibited the binding of EL-4 lymphoma cells to immobilized fibronectin (data not shown). The number of LCs in the epidermis was determined in fresh epidermal sheets and after 24 and 72 h of incubation. Neither GRGDS nor the control peptide had any effect on the migration of LCs from the epidermis over a 72-h period.
In Vivo Administration of Antibodies to α6 Integrins, but not α4 Integrins, Blocks LC Migration from the Epidermis.
To determine whether α6 integrins play a role in the migration of LCs from the epidermis in vivo, affinity-purified α6 integrin antibody was administered systemically and its effect on LC migration was determined after administration of mouse recombinant TNF-α (Fig. 5). In mice pretreated with 40 μg of isotype-matched control antibody (MAC 193) the number of LCs/mm2 was reduced from 826 ± 31 in naive animals to 671 ± 6 after administration of TNF-α, which is similar to previously reported results (8). However, mice pretreated with 40 μg anti-α6 integrin antibody GoH3 showed no reduction in the frequency of LCs; the number of LCs/mm2 at 833 ± 37 was similar to that in naive ear skin. The morphology of LCs remaining in the epidermis of α6 integrin antibody–treated mice was different to that of LCs in naive skin (Fig. 6). A proportion of LCs adopted a rounded morphology, similar to that of LCs in α6 integrin antibody–treated skin explants, as opposed to the interdigitating appearance typical of LCs. Systemic administration of GoH3 had no effect on the morphology of resident LCs in untreated skin.
Figure 5.
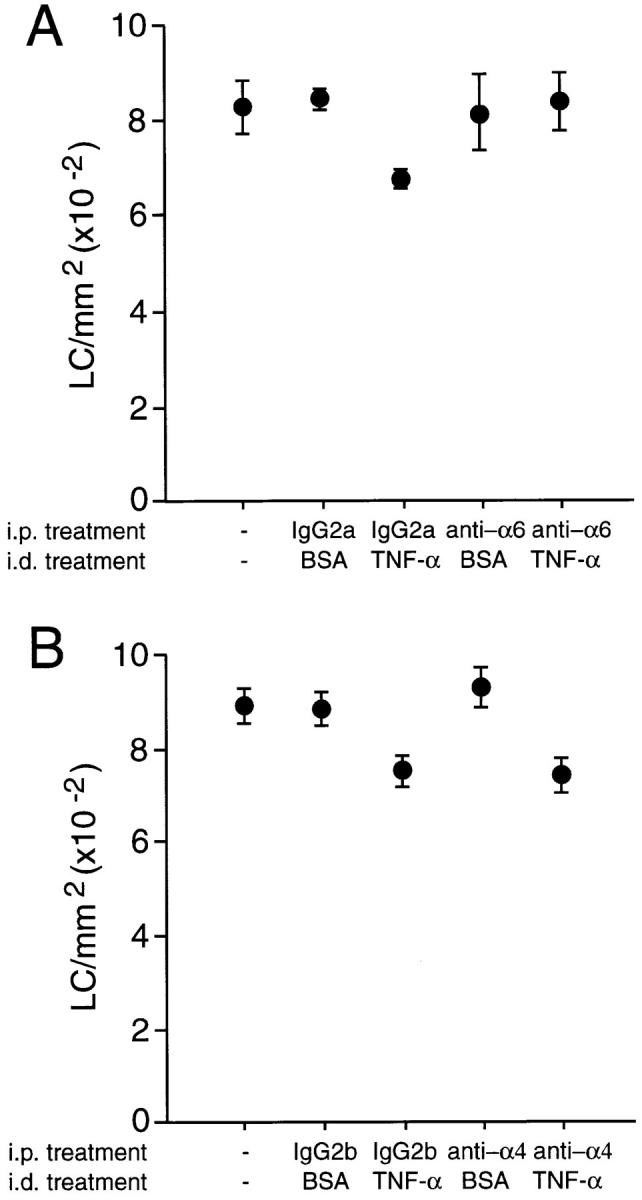
The effect of anti-α6 and anti-α4 integrin antibodies on TNF-α–induced epidermal LC migration in vivo. Groups of four mice received 40 μg of (A) anti-α6 integrin (GoH3) or (B) anti-α4 integrin (PS/2) antibody intraperitoneally. Controls either received isotype-matched control antibody or were left untreated. 2 h after administration of antibody, two mice per group received 30 μl injection intradermally into both ear pinnae of 50 ng murine TNF-α in 0.1% BSA. The remaining two mice received 30 μl intradermal injection of 0.1% BSA. Ears were removed 30 min later, epidermal sheets were prepared, and the number of LCs/mm2 was determined by immunofluorescence analysis. Results are means ± SD (n = 40). Treatment of mice with TNF-α (in both A and B) caused a significant decrease in the frequency of LCs compared with either untreated controls or mice exposed to BSA alone (P <0.005). Treatment with anti-α6 resulted in a significantly higher frequency of LC compared with mice exposed to TNF-α together with an isotype-matched control antibody (P <0.005; A). In contrast, anti-α4 failed to affect significantly LC frequency compared with isotype controls.
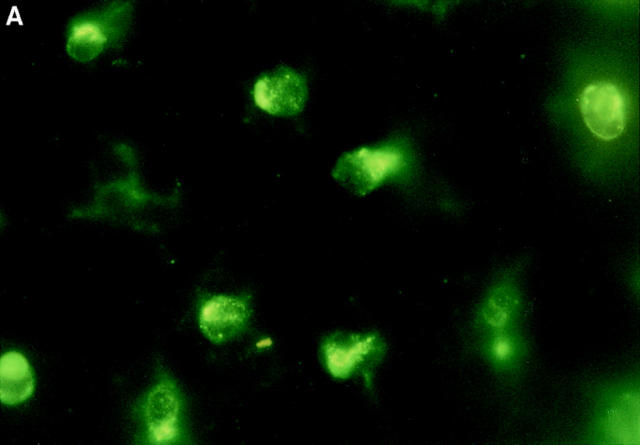
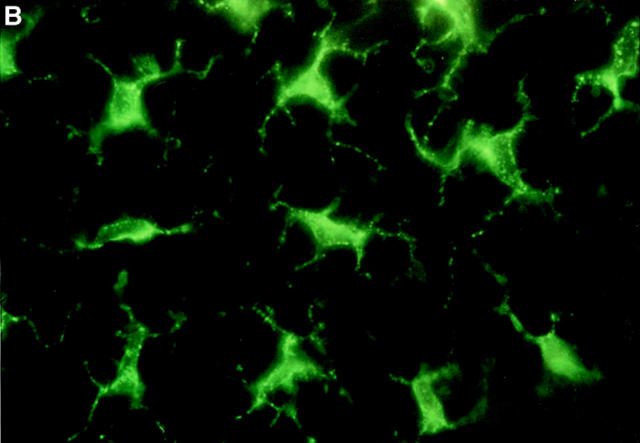
Figure 6.
Immunofluorescence staining of epidermal LCs in situ. Groups of mice (n = 2) received a single 100 μl injection intraperitoneally of 40 μg anti-α6 integrin antibody (GoH3) 2 h before intradermal injection into both ear pinnae of (A) 50 ng murine recombinant TNF-α or (B) 0.1% BSA alone. Ears were removed 30 min later, epidermal sheets were prepared, and the morphology of LCs was assessed after indirect immunofluorescence staining for MHC class II expression. Note the rounded morphology of LCs in TNF-α–treated ears in comparison with interdigitating morphology of LCs in controls. Magnification, ×800.
In contrast to the effect of anti-α6 integrin antibody, systemic administration of 40 μg anti-α4 integrin antibody PS/2 had no effect on TNF-α–induced LC migration from the epidermis (Fig. 5). The morphology of LCs remaining in the epidermis of anti-α4 integrin antibody–treated mice was indistinguishable from that of LCs in either naive skin or skin from mice treated with control antibody (data not shown).
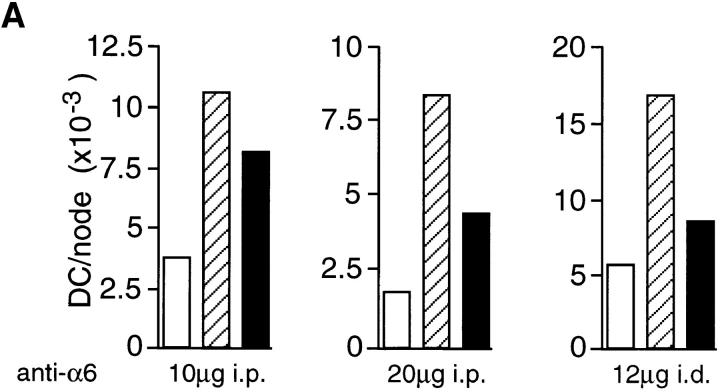
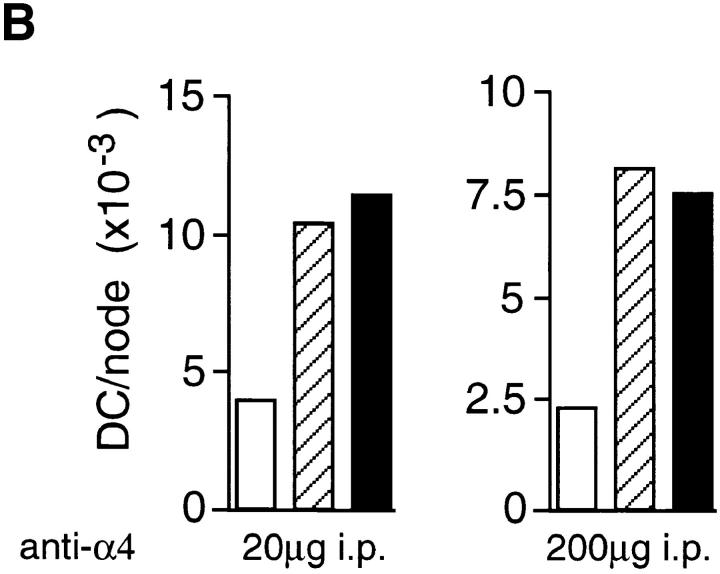
If α6 integrins are required for LC migration from the epidermis, in vivo administration of anti-α6 integrin antibody should also inhibit the accumulation of DCs in draining lymph nodes. Anti-α6 integrin antibody was administered either systemically, or directly into the dermis of ear skin, 2 h before topical application of oxazolone. 18 h after exposure to oxazolone, draining auricular lymph nodes were removed and the number of DCs per node was determined. The results from three separate experiments are presented in Fig. 7. In individual experiments, the number of DCs in untreated, naive mice ranged from 2,000 to 5,000/ node. Topical application of oxazolone consistently increased the number of DCs per lymph node by three- to fourfold. Administration of the anti-α6 antibody, GoH3, significantly reduced the number of DCs per lymph node. For example, oxazolone increased the number of DCs per node from 1,675 to 8,275 in mice treated with control antibody, and intraperitoneal injection of 20 μg of GoH3 reduced DC accumulation to 4,353 per node, representing a 59% inhibition of DC accumulation. 10 μg of GoH3 gave slightly less inhibition at 37% and the higher dose of 40 μg did not further inhibit DC accumulation (data not shown). However, 12 μg of antibody administered intradermally was more effective, inhibiting DC accumulation by 74% (Fig. 7).
Figure 7.
The effect of (A) anti-α6 and (B) anti-α4 integrin antibodies on oxazolone-induced DC accumulation in lymph nodes in vivo. Antibody was administered to groups of 10 mice in single 100 μl injections intraperitoneally for the 10, 20, and 200 μg doses or in single 30 μl injections intradermally into the dorsum of both ears for 12 μg dose. The 200 μg dose of anti-α4 integrin antibody was given in 2 × 100 μg doses 2 h before and 8 h after oxazolone treatment. Each graph represents a separate experiment and the amount of antibody received per mouse, and the method of administration is indicated below each graph. 2 h after antibody administration, mice received 25 μl of 0.5% oxazolone on the dorsum of both ears. Draining auricular lymph nodes were excised 18 h later and the number of DCs per lymph node was determined. Results for isotype-matched control antibody (hatched bars) and integrin antibody (solid bars) are compared with untreated, naive mice (open bars).
Anti-α4 antibody had no effect on LC migration from the epidermis of skin explants, yet this integrin was expressed on lymph node DCs (Fig. 1). The possibility exists that, rather than being necessary for migration of LCs from the epidermis, α4 integrins are required for DC migration into the draining lymph nodes. Therefore, it was of interest to test the effect of the anti-α4 antibody on lymph node DC accumulation in vivo.
A single intraperitoneal injection of 20 μg of anti-α4 antibody, PS/2, had no effect on oxazolone-induced DC accumulation; the number of DCs per lymph node at 11,322 was similar to that in animals treated with control antibody (10,355). In the second experiment, a higher dose of antibody was used. A total of 200 μg of anti-α4 antibody was administered to each animal in two 100 μg doses at 2 h before and 8 h after oxazolone treatment. The numbers of DCs in the draining lymph nodes 18 h after oxazolone treatment were similar in control and anti-α4 antibody– treated mice at 8,130 and 7,480, respectively. This lack of effect was not due to insufficient amounts of functionally blocking antibody, as lymphocytes taken from these animals were inhibited from binding to immobilized recombinant vascular cell adhesion molecule (VCAM)–1 protein by 70% using standard adhesion assays (reference 23 and data not shown). Analysis of DCs from anti-α4 antibody–treated mice showed that the cells were saturated with antibody, such that the staining profile of DCs was similar to that shown in Fig. 1 (data not shown).
Discussion
In marked contrast to other types of leukocytes, LCs migrate away from, not towards, a wide range of inflammatory stimuli. After stimulation, LCs dissociate from neighboring keratinocytes, leave their position in the epidermis, pass through the underlying BM into the dermis, enter the afferent lymphatics, and relocate in the T lymphocyte–rich areas of the draining lymph node as mature DCs (24, 25). LCs need to migrate across BMs other than the epidermal BM, including those in the lymphatics and subcapsular sinus that drain into nodes, and we predicted that LCs may use distinct cell adhesion molecules to interact with these BMs during migration from the skin. Therefore, we have studied the expression and function of different integrin receptors for components of the BM on epidermal LCs and on lymph node DCs into which LCs mature.
We identified differential expression by LCs and DCs of α6 and β4 integrin subunits, which dimerize to form a receptor for laminin, and α4 subunit–containing integrins, which bind fibronectin as well as cell surface molecules such as VCAM-1 (11). The α6 and β4 integrin subunits were expressed by >70% of LCs, but neither subunit could be detected on lymph node DCs. The α4 integrin subunit was expressed by all lymph node DCs. It was not possible to quantitate α4 integrin expression by LCs after isolation, since the α4 integrin epitope was degraded by trypsin. However, immunocytochemical analysis of LCs in epidermal sheets indicated much lower levels of expression than on lymph node DCs, which agrees with Aiba et al. (26) who reported low level of α4 integrins on murine LCs. Other authors have reported the expression of several integrins on LCs, including α4 and β1 integrins (26–28), but to date, the function of these integrins on LCs has not been determined. Since α6 and α4 integrins bind to the ECM proteins laminin and fibronectin, respectively (11), we considered that these integrins may regulate the migration of LCs across the epidermal BM into the underlying dermis and/or the subsequent localization of LC within the draining lymph nodes.
The anti-α6 integrin antibody, GoH3, completely inhibited the spontaneous migration of LCs from the epidermis of skin explants and the rapid migration of LCs from the epidermis stimulated by TNF-α in vivo. LCs that remained in the epidermis of α6 integrin antibody–treated skin explants had an altered morphology in comparison with LCs in naive skin in that the cells were rounded in appearance rather than interdigitating between keratinocytes. LCs in the epidermis of α6 integrin antibody/TNF-α–treated mice displayed two distinct morphologies, either a morphology typical of LCs in unstimulated epidermis, i.e., interdigitating among keratinocytes, or a round morphology. From these results it can be concluded that α6 integrins are necessary for the migration of LCs from the epidermis. Since α6 integrins are also expressed on keratinocytes, we cannot formally exclude an indirect role for α6 integrins on keratinocytes in regulating LC migration. Upon receiving a stimulus to migrate, LCs may dissociate from surrounding keratinocytes, for example by downregulating E-cadherin expression (29), and move towards the BM, but be prevented from traversing it by the anti-α6 integrin antibody GoH3, which blocks binding to laminin. The LCs therefore become trapped in the epidermis, but nevertheless free from keratinocytes. Ultrastructural analyses will be required to determine the precise location of these rounded LCs with respect to the BM. The increased expression of MHC class II on α6 integrin– blocked LCs suggests that they may mature in this location. Preliminary experiments (not shown) indicate that other markers associated with the maturation of LCs are upregulated on α6 integrin–blocked LCs. The findings in this study suggest that interactions with laminin in the BM may not be required for LC maturation.
The mAb GoH3 blocks the interaction of α6β1 and α6β4 integrins with laminin (30). The only defined ligands to date for α6 subunit–containing integrins are laminin (31), although recent studies with the EA-1 antibody suggest the existence of an alternative ligand. EA-1 recognizes the α6 subunit of both α6β1 and α6β4 integrins and inhibits α6 integrin–mediated migration of prothymocytes from the bloodstream into the thymus of mice. However, EA-1 does not inhibit α6β1 or α6β4 integrin–dependent binding to laminin (19, 20). In contrast to GoH3, the EA-1 antibody did not inhibit LC migration from the epidermis, suggesting that α6 integrin interactions with laminin, as opposed to other ligands, may regulate this step.
The α6 integrin subunit has two splice variant forms, α6A and α6B, which differ in their cytoplasmic domains (32). Both α6A and α6B associate with the β1 and β4 integrin subunits to form α6Aβ1, α6Bβ1, α6Aβ4, and α6Bβ4 integrins (30). All four integrins bind to laminin and differences in the ligand binding specificities of the α6A and α6B variants have not been detected (30). However, it is of considerable interest which β subunit is used to form the α6 integrin heterodimer on LCs. The results presented here demonstrate that >70% of LCs express the α6 integrin and >70% also express the β4 integrin. Based on this finding, it can be speculated that the α6β4 integrin is found on >70% of LCs, particularly as there are currently no other α subunits known to dimerize with the β4 subunit. However, expression of the β1 integrin subunit on LCs also needs to be determined. Antibodies to the mouse β1 integrin subunit were not available for such analyses and therefore, we cannot conclude which α6 integrin is used by LCs for migration. There has been one report demonstrating that all human epidermal LCs express β1 integrin (28), however, the same group reported a complete absence of β4 integrin on these cells (33).
In epithelial tissues, the α6β4 integrin is a key structural component of hemidesmosomes (34) which mediate stable interactions between epithelial cells and the underlying BM and are responsible, in part, for maintaining the functional integrity of the tissue (35). The α6β4 integrin has also been implicated in migratory events, including the migration of keratinocytes during wound healing (36). The apparently contradictory roles of the α6β4 integrin in the stable adhesion and in the migration of keratinocytes may be explained by the finding that epidermal growth factor receptor signaling in keratinocytes results in phosphorylation of multiple tyrosine residues on the β4 cytoplasmic tail, which causes the disruption of hemidesmosomes and α6β4 integrin–mediated migration (37). A change in the activation status of α6 integrins on LCs may regulate migration in that the integrin is normally held in an inactive conformation on resident LCs but after stimulation is converted to an active conformation allowing binding to laminin, thus facilitating migration across the BM. Alternatively, α6 integrins could be expressed in the active form on resident LCs but this is of no biological relevance as LCs are trapped within a keratinocyte matrix.
Since we were unable to determine the expression of other integrin receptors on LCs for fibronectin (such as α5β1), we used RGD peptides to study their potential role in regulating LC migration. The addition of a synthetic RGD-containing peptide to the culture medium of skin explants had no effect on LC migration. RGD is a consensus binding sequence in many integrin ligands, including fibronectin, fibrinogen, vitronectin, von Willebrand factor, and thrombospondin, but not laminin, and binding of α3β1, α5β1, αvβ1, αvβ3, αvβ5, αvβ6, and αIIbβ3 integrins to their ligands is dependent on this sequence (11, 21). The lack of effect of RGD peptides suggests that the α5β1 integrin, as well as other RGD-dependent β1 and β3 integrins, does not regulate LC migration from the epidermis.
Addition of antibodies against the α4 integrin also had no effect on LC migration either in vitro or in vivo. Although we could not quantitate α4 integrin expression on LCs, it was substantially lower than on lymph node DCs. We did not identify which of the two α4 subunit–containing integrins is expressed by DC (i.e., α4β1/very late antigen (VLA)–4, or α4β7) but the PS/2 antibody is an effective inhibitor of both (38, 39) and blocks binding to all ligands identified, including fibronectin, VCAM-1, mucosal address in cell adhesion molecule (MAdCAM)–1, and the α4 integrin subunit itself (39–41). The PS/2 antibody is also an effective inhibitor of α4 integrins on mouse leukocytes in vivo (42). The lack of effect of this antibody indicates that α4 integrin–mediated interactions with fibronectin or with any other ligand are not required for the initial stages of LC migration from the epidermis. However, the skin explant model used here does not study the subsequent stages of migration of LCs into the draining lymph nodes, and for this reason we studied the effect of integrin antibodies on the accumulation of DCs in draining lymph nodes in vivo.
Previous studies have shown that systemic administration of antibodies to TNF-α 2 h before sensitization with oxazolone inhibited the accumulation of DCs in draining lymph nodes in response to the chemical allergen (9). Therefore, we used a similar model to determine the effects of anti-α6 integrin and anti-α4 integrin antibodies on DC accumulation. Intraperitoneal administration of 40 μg of anti-α6 integrin antibody GoH3 maximally inhibited DC accumulation by 60%. Intradermal injection of 12 μg of GoH3 directly into the ear gave a slightly greater inhibition of 74%. The incomplete inhibition may be explained by the fact that some DCs may come directly from the dermis and/or the bloodstream. Although lymph node DCs do not express α6 integrins, it is not possible to conclude that α6 integrin–mediated interactions are not involved in the migration process once the cells have left the epidermis, since we do not know precisely when α6 integrin is downregulated during maturation into DCs.
In contrast, saturating doses of anti-α4 integrin antibody had no effect on DC accumulation. It is possible that this antibody affects the precise localization of DCs within the lymph node paracortex and further experiments are required to test this hypothesis.
In summary, this study has reported the expression of the α6 and β4 integrin subunits on murine LCs and has demonstrated, for the first time, the direct involvement of α6 integrins in regulating LC migration from the epidermis.
Footnotes
We would like to acknowledge the excellent technical assistance of Chris Atkins for flow cytometry, John Asante for histology, and Joe Brock for the figures.
This work was funded by the Medical Research Council (MRC, UK). Abigail Price was the recipient of an MRC Collaborative Studentship in conjunction with Zeneca.
Abbreviations used in this paper: BM, basement membrane; DC, dendritic cell; ECM, extracellular matrix; GRDGS, Gly-Arg-Asp-Gly-Ser; GRGDS, Gly-Arg-Gly-Asp-Ser; HRP, horseradish peroxidase; LC, Langerhans cell; RGD, Arg-Gly-Asp; SA, streptavidin; VCAM, vascular cell adhesion molecule.
References
- 1.Reis e. Sousa C, Stahl PD, Austyn JM. Phagocytosis of antigens by Langerhans cells in vitro. J Exp Med. 1993;178:509–519. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romani N, Lenz A, Glassel H, Stossel H, Stanzl U, Majdic O, Fritsch P, Schuler G. Cultured human Langerhans cells resemble lymphoid dendritic cells in phenotype and function. J Invest Dermatol. 1989;93:600–609. doi: 10.1111/1523-1747.ep12319727. [DOI] [PubMed] [Google Scholar]
- 3.Austyn J. New insights into the mobilization and phagocytic activity of dendritic cells. J Exp Med. 1996;183:1287–1292. doi: 10.1084/jem.183.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 5.Witmer-Pack M, Oliver W, Valinsky J, Schuler G, Steinman RM. Granulocyte-macrophage colony-stimulating factor is essential for viability and function of cultured murine epidermal Langerhans cells. J Exp Med. 1987;166:1484–1498. doi: 10.1084/jem.166.5.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heufler C, Koch F, Schuler G. Granulocyte/ macrophage colony–stimulating factor and interleukin 1 mediate the maturation of murine epidermal Langerhans cells into potent immunostimulatory dendritic cells. J Exp Med. 1988;167:700–705. doi: 10.1084/jem.167.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cumberbatch M, Kimber I. Dermal tumour necrosis factor–α induces dendritic cell migration to draining lymph nodes, and possibly provides one stimulus for Langerhans' cell migration. Immunology. 1992;75:257–263. [PMC free article] [PubMed] [Google Scholar]
- 8.Cumberbatch M, Fielding I, Kimber I. Modulation of epidermal Langerhans' cell frequency by tumour necrosis factor–α. Immunology. 1994;81:395–401. [PMC free article] [PubMed] [Google Scholar]
- 9.Cumberbatch M, Kimber I. Tumour necrosis factor–α is required for accumulation of dendritic cells in draining lymph nodes and for optimal contact sensitization. Immunology. 1995;84:31–35. [PMC free article] [PubMed] [Google Scholar]
- 10.Cumberbatch M, Dearman RJ, Kimber I. Interleukin-1β and the stimulation of Langerhans cell migration: comparison with tumour necrosis factor-α. Arch Dermatol Res. 1997;289:277–284. doi: 10.1007/s004030050193. [DOI] [PubMed] [Google Scholar]
- 11.Hynes RO. Integrins: versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 12.Martin GR, Timpl R. Laminin and other basement membrane components. Annu Rev Cell Biol. 1987;3:57–85. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- 13.van den Berg TK, van der Ende M, Dopp EA, Kraal G, Dijkstra CD. Localization of β1integrins and their ligands in human lymphoid tissues. Am J Pathol. 1993;143:1098–1110. [PMC free article] [PubMed] [Google Scholar]
- 14.Bhattacharya A, Dorf ME, Springer T. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981;127:2488–2495. [PubMed] [Google Scholar]
- 15.Kraal G, Breel M, Janse M, Bruin G. Langerhans cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986;163:981–997. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cumberbatch M, Illingworth I, Kimber I. Antigen-bearing dendritic cells in the draining lymph nodes of contact sensitized mice: cluster formation with lymphocytes. Immunology. 1991;74:139–145. [PMC free article] [PubMed] [Google Scholar]
- 17.Sonnenberg A, Calafat J, Janssen H, Daams H, van der Raaij-Helmer LM, Falcioni R, Kennel SJ, Aplin JD, Baker J, Loizidou M, Garrod D. Integrin α6β4complex is located in hemidesmosomes, suggesting a major role in epidermal cell–basement membrane adhesion. J Cell Biol. 1991;113:907–917. doi: 10.1083/jcb.113.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nussenzweig MC, Steinman RM, Unkeless JC, Witmer MD, Gutchinov B, Cohn ZA. Studies of the cell surface of mouse dendritic cells and other leukocytes. J Exp Med. 1981;154:168–187. doi: 10.1084/jem.154.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imhof BA, Ruiz P, Hesse B, Palacios R, Dunon D. EA-1, a novel adhesion molecule involved in the homing of progenitor T lymphocytes to the thymus. J Cell Biol. 1991;114:1069–1078. doi: 10.1083/jcb.114.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz P, Wiles MV, Imhof BA. α6integrins participate in pro–T cell homing to the thymus. Eur J Immunol. 1995;25:2034–2041. doi: 10.1002/eji.1830250735. [DOI] [PubMed] [Google Scholar]
- 21.Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science (Wash DC) 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 22.Ager A, Humphries MJ. Use of synthetic peptides to probe lymphocyte–high endothelial cell interactions. Lymphocytes recognize a ligand on the endothelial surface which contains the CS1 adhesion motif. Int Immunol. 1990;2:921–928. doi: 10.1093/intimm/2.10.921. [DOI] [PubMed] [Google Scholar]
- 23.May MJ, Entwistle G, Humphries MJ, Ager A. VCAM-1 is a CS1 peptide–inhibitable adhesion molecule expressed by lymph node high endothelium. J Cell Sci. 1993;106:109–119. doi: 10.1242/jcs.106.1.109. [DOI] [PubMed] [Google Scholar]
- 24.Larsen CP, Steinman RM, Witmer PM, Hankins DF, Morris PJ, Austyn JM. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990;172:1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fossum S. Lymph-borne dendritic leukocytes do not recirculate, but enter the lymph node paracortex to become interdigitating cells. Scand J Immunol. 1988;27:97–105. doi: 10.1111/j.1365-3083.1988.tb02326.x. [DOI] [PubMed] [Google Scholar]
- 26.Aiba S, Nakagawa S, Ozawa H, Miyake K, Yagita H, Tagami H. Up-regulation of α4integrin on activated Langerhans cells: analysis of adhesion molecules on Langerhans cells relating to their migration from skin to draining lymph nodes. J Invest Dermatol. 1993;100:143–147. doi: 10.1111/1523-1747.ep12462783. [DOI] [PubMed] [Google Scholar]
- 27.Teunissen MB, Rongen HA, Bos JD. Function of adhesion molecules lymphocyte function-associated antigen–3 and intercellular adhesion molecule–1 on human epidermal Langerhans cells in antigen-specific T cell activation. J Immunol. 1994;152:3400–3409. [PubMed] [Google Scholar]
- 28.Le Varlet B, Dezutter DC, Staquet MJ, Delorme P, Schmitt D. Human epidermal Langerhans cells express integrins of the β 1 subfamily. J Invest Dermatol. 1991;96:518–522. doi: 10.1111/1523-1747.ep12470229. [DOI] [PubMed] [Google Scholar]
- 29.Schwarzenberger K, Udey MC. Contact allergens and epidermal proinflammatory cytokines modulate Langerhans cell E-cadherin expression in situ. . J Invest Dermatol. 1996;106:553–558. doi: 10.1111/1523-1747.ep12344019. [DOI] [PubMed] [Google Scholar]
- 30.Niessen CM, Hogervorst F, Jaspars LH, de Melker AA, Delwel GO, Hulsman EH, Kuikman I, Sonnenberg A. The α6β4integrin is a receptor for both laminin and kalinin. Exp Cell Res. 1994;211:360–367. doi: 10.1006/excr.1994.1099. [DOI] [PubMed] [Google Scholar]
- 31.Mercurio AM. Laminin receptors: achieving specificity through cooperation. Trends Cell Biol. 1995;5:419–423. doi: 10.1016/s0962-8924(00)89100-x. [DOI] [PubMed] [Google Scholar]
- 32.Shaw LM, Turner C, Mercurio AM. The α6Aβ1 integrin and α6Bβ1integrin variants: signal differences in the tyrosine phosphorylation of paxillin and other proteins. J Biol Chem. 1995;270:23648–23652. doi: 10.1074/jbc.270.40.23648. [DOI] [PubMed] [Google Scholar]
- 33.Le Varlet B, Staquet MJ, Dezutter-Dambuyant C, Delorme P, Schmitt D. In vitro adhesion of human epidermal Langerhans cells to laminin and fibronectin occurs through β 1 integrin receptors. J Leukocyte Biol. 1992;51:415–420. doi: 10.1002/jlb.51.4.415. [DOI] [PubMed] [Google Scholar]
- 34.Dowling J, Yu QC, Fuchs E. β4integrin is required for hemidesmosome formation, cell adhesion, and cell survival. J Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green KJ, Jones JC. Desmosomes and hemidesmosomes: structure and function of molecular components. FASEB (Fed Am Soc Exp Biol) J. 1996;10:871–881. doi: 10.1096/fasebj.10.8.8666164. [DOI] [PubMed] [Google Scholar]
- 36.Kurpakus MA, Quaranta V, Jones JCR. Surface relocation of α6β4integrins and assembly of hemidesmosomes in an in vitro model of wound healing. J Cell Biol. 1991;115:1737–1750. doi: 10.1083/jcb.115.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mainiero F, Pepe A, Yeon M, Ren Y, Giancotti FG. The intracellular functions of α6β4integrin are regulated by EGF. J Cell Biol. 1996;134:241–253. doi: 10.1083/jcb.134.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyake K, Weissman IL, Greenberger JS, Kincade PW. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991;173:599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC. α4β7integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 40.Ruegg C, Postigo AA, Sikorski EE, Butcher EC, Pytela R, Erle DJ. Role of integrin α4β7/α4βpin lymphocyte adherence to fibronectin and VCAM-1 and in homotypic cell clustering. J Cell Biol. 1992;117:179–189. doi: 10.1083/jcb.117.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altevogt P, Hubbe M, Ruppert M, Lohr J, von Hoegen P, Sammar M, Andrew DP, McEvoy L, Humphries MJ, Butcher EC. The α4 integrin chain is a ligand for α4β7 and α4β1 . J Exp Med. 1995;182:345–355. doi: 10.1084/jem.182.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of α4 integrins in lymphocyte homing to mucosal tissues in vivo. . J Immunol. 1994;152:3283–3293. [PubMed] [Google Scholar]