Abstract
Merozoite surface protein–1 (MSP-1) of the human malaria parasite Plasmodium falciparum undergoes at least two endoproteolytic cleavage events during merozoite maturation and release, and erythrocyte invasion. We have previously demonstrated that mAbs which inhibit erythrocyte invasion and are specific for epitopes within a membrane-proximal, COOH-terminal domain of MSP-1 (MSP-119) prevent the critical secondary processing step which occurs on the surface of the extracellular merozoite at around the time of erythrocyte invasion. Certain other anti–MSP-119 mAbs, which themselves inhibit neither erythrocyte invasion nor MSP-1 secondary processing, block the processing-inhibitory activity of the first group of antibodies and are termed blocking antibodies. We have now directly quantitated antibody-mediated inhibition of MSP-1 secondary processing and invasion, and the effects on this of blocking antibodies. We show that blocking antibodies function by competing with the binding of processing-inhibitory antibodies to their epitopes on the merozoite. Polyclonal rabbit antibodies specific for certain MSP-1 sequences outside of MSP-119 also act as blocking antibodies. Most significantly, affinity-purified, naturally acquired human antibodies specific for epitopes within the NH2-terminal 83-kD domain of MSP-1 very effectively block the processing-inhibitory activity of the anti-MSP-119 mAb 12.8. The presence of these blocking antibodies also completely abrogates the inhibitory effect of mAb 12.8 on erythrocyte invasion by the parasite in vitro. Blocking antibodies therefore (a) are part of the human response to malarial infection; (b) can be induced by MSP-1 structures unrelated to the MSP-119 target of processing-inhibitory antibodies; and (c) have the potential to abolish protection mediated by anti–MSP-119 antibodies. Our results suggest that an effective MSP-119–based falciparum malaria vaccine should aim to induce an antibody response that prevents MSP-1 processing on the merozoite surface.
The development of an effective malaria vaccine has become a major public health challenge. The protozoan organisms responsible for the disease, members of the genus Plasmodium, have a complicated life cycle, and in the human host the parasite exists in at least four morphologically and antigenically distinct forms. As a result, in individuals exposed to malarial infection, the immune response against the parasite is complex, and several stages of the life cycle are being explored as potential targets for vaccine-mediated immune intervention. Acute clinical malaria, which is often life-threatening in the case of infection with Plasmodium falciparum, is associated with replication of the asexual blood-stage parasite in circulating erythrocytes. Human passive immunization studies using antibodies isolated from donors clinically immune to falciparum malaria have indicated that antimalarial IgG can prevent this replication (1–4), and surface components of the infected erythrocyte and invasive merozoite stage of the parasite have therefore been studied intensively for their ability to induce protective immune responses. The merozoite expresses a number of surface proteins, one or more of which are thought to mediate the initial interaction between parasite and host erythrocyte (5, 6). Recent work in this laboratory has focused on the proteolytic processing of merozoite surface protein–1 (MSP-1).1 Initially synthesized as a large (∼200 kD) precursor during intracellular merozoite development, MSP-1 is present on the surface of the released merozoite in the form of a multicomponent protein complex derived via proteolytic processing (7, 8). At some point between merozoite release and completion of erythrocyte invasion, the membrane-bound component (MSP-142) of this surface complex is further cleaved at a single site to form two fragments, MSP-133 and MSP-119. This results in the majority of the complex being shed from the parasite surface, leaving only MSP-119, which represents the extreme COOH-terminal end of the MSP-1 precursor and is comprised of two epidermal growth factor (EGF)-like motifs, to be taken into the invaded cell on the parasite surface (9–12). Significantly, this so-called secondary processing of MSP-1 is conserved across the genus (13–15) and invariably goes to completion when a merozoite successfully invades a red blood cell, suggesting that it is a necessary step in the invasion pathway.
Studies in the rodent Plasmodium chabaudi and Plasmodium yoelii malaria models have shown that passive immunization with certain anti–MSP-119 mAbs, or immunization with recombinant MSP-119, can afford an astonishing degree of protection against a blood-stage challenge infection (16–20). Consistent with this, a number of reports have shown that polyclonal antibodies (21, 22) or mAbs (9, 23, 24) specific for epitopes within the P. falciparum MSP-119 domain can prevent erythrocyte invasion by merozoites in vitro. To investigate the mechanisms involved in this invasion inhibition, we recently studied a panel of anti–MSP-119 mAbs, and found that those antibodies which most effectively prevent invasion can, upon binding to MSP-1 on the surface of intact P. falciparum merozoites, completely prevent secondary processing of the molecule. Furthermore, of those mAbs which do not affect the processing, some can interfere with the processing-inhibitory activity of the first group of antibodies (25). This second group of antibodies was referred to as blocking antibodies.
In this study we extend this work to show that blocking antibodies act by competing with processing-inhibitory mAbs for binding to the merozoite surface. We show that polyclonal antibodies raised against MSP-1 sequences outside of MSP-119 can also have blocking properties similar to those of the anti–MSP-119 mAbs previously identified. Of most significance, human antibodies specific to the NH2-terminal domain of MSP-1, affinity-purified from sera of individuals naturally exposed to falciparum malaria, are potent blocking antibodies which can completely abolish the activity of invasion-inhibitory antibodies in vitro. Our observations reveal a mechanism by which the parasite can avoid the action of a class of protective antibodies, and have important implications for the optimal design, evaluation, and administration of MSP-1–based malaria vaccines.
Materials and Methods
Polyclonal and Monoclonal Antibodies.
Murine anti–MSP-119 mAbs 2.2, 7.5, 12.8, 12.10, 111.4, 117.2, 1E1, 2F10, 7E5, 8A12, and 12D11; the anti–MSP-183 mAb 89.1 and the mAb 25.1, which is specific for P. yoelii MSP-1; and the human anti–MSP-133 mAb X509 have all been previously described (7, 9, 10, 25–27). All mAbs were purified by affinity chromatography on protein A– or protein G–Sepharose (Pharmacia Biotech, St. Albans, Hertfordshire, UK) before use (28). A panel of polyclonal anti–MSP-1 antisera was raised in rabbits against defined regions of MSP-1 expressed as fusion proteins in Escherichia coli (8); IgG was purified from these sera by ion exchange chromatography on DEAE Sephadex (Pharmacia Biotech) using standard methods (28). The polyclonal rabbit antiserum reactive with the MSP-133 fragment of the Wellcome MSP-1 (Rb anti–MSP-133) was raised against a recombinant protein expressing a 93–amino acid region from within the NH2-terminal half of MSP-142; therefore, the antibodies recognize both MSP-142 and MSP-133, and show absolutely no reactivity with MSP-119 (13). Pooled human serum obtained from adult Gambian donors clinically immune to falciparum malaria was a kind gift of Dr. Hilton Whittle (Medical Research Council Laboratories, Fajara, The Gambia, West Africa). Human serum from European donors who had never been exposed to malaria (nonimmune sera) was obtained from the Blood Transfusion Centre (Colindale, UK) and pooled.
Preparation of Recombinant Antigens.
Production of a recombinant pGEX-3X plasmid (29) to express the MSP-119 domain of the P. falciparum (Wellcome strain) MSP-1 fused to Schistosoma japonicum glutathione S-transferase has been described previously (26). Fusion protein was adsorbed to glutathione agarose (Sigma Chemical Co., St. Louis, MO), and the malarial portion (rMSP-119) cleaved in situ from the carrier protein (30) by overnight incubation with Factor Xa (Boehringer Mannheim, Mannheim, Germany) at 4°C. Eluted protein was further purified by gel filtration in PBS on Sephadex G50 Superfine (Pharmacia Biotech), and concentrated by ultrafiltration using a YM1 membrane (Amicon, Ltd., Stonehouse, Gloucs., UK).
Recombinant expression plasmid pME6 encodes Leu208 to Asp416 of the P. falciparum Wellcome strain MSP-1 gene (numbering according to reference 31), as an NH2-terminal fusion with β-galactosidase (8). Fusion protein (also referred to as pME6; reference 8) was purified by affinity chromatography on p-aminophenyl-β-d-thiogalactopyranoside-agarose (32) and stored as a precipitate in 50% (wt/vol) ammonium sulfate.
Radioiodination of Antibodies.
Protein G–purified mAbs 12.8, 12.10, and X509, and purified rabbit anti-IgG antibodies (Sera-Lab, Ltd., Sussex, UK) were labeled at 4°C with 125I by the Iodogen (Pierce Chemical Co., Rockford, IL) method (33). Labeled antibody was separated from free isotope by gel filtration on a PD-10 column (Pharmacia Biotech) preequilibrated with PBS containing 1% BSA and 0.02% (wt/vol) sodium azide. The specific activity of the labeled antibody was ∼3.1 × 106 cpm μg−1. Labeled antibody was stored at 4°C.
Culture and Biosynthetic Radiolabeling of P. falciparum, and Merozoite Purification.
Highly synchronous blood-stage cultures of the FCB-1 and T9/96 isolates of P. falciparum were maintained in vitro in human A+ erythrocytes, and the naturally released merozoites were purified by filtration through 3 μm and 1.2 μm pore-size acrylic membrane filters as previously described (34). Merozoites were recovered from the filtrate by centrifugation and washed twice in ice-cold PBS, supplemented with the protease inhibitors leupeptin, antipain, and aprotinin, all at 10 μg ml−1 and tosyl-l-lysyl choromethyl ketone (TLCK) at 10 μM. Merozoites not immediately used were pelleted by centrifugation and stored in aliquots at −70°C. Merozoite preparations were consistently free of schizont contamination, as determined by microscopic analysis of Giemsa-stained samples.
When required, schizont-enriched cultures were metabolically radiolabeled with [35S]methionine and cysteine (Pro-mixTM; Amersham International, Little Chalfont, UK), placed back into culture in medium containing 0.5% (wt/vol) AlbumaxTM (GIBCO BRL, Paisley, UK), and allowed to undergo merozoite release in the presence of fresh erythrocytes as previously described (10). Labeled MSP-133 was immunoprecipitated from harvested culture medium using mAb X509 coupled to Sepharose, and analyzed by SDS-PAGE and fluorography as previously described (10, 11). When appropriate, ring-stage parasitemia in cultures after reinvasion was assessed by microscopic examination of Giemsa-stained thin blood films.
Quantitation of Antibody-mediated Inhibition of MSP-1 Secondary Processing.
Analysis and quantitation of secondary processing of MSP-1 in merozoite preparations was by modification of an assay described previously (13, 34). Washed merozoites were resuspended in ice-cold 50 mM Tris-HCl, pH 7.5, containing 10 mM CaCl2 and 2 mM MgCl2, supplemented with the following protease inhibitors: antipain, leupeptin, aprotinin, and TLCK (reaction buffer). Aliquots of ∼109 merozoites were dispensed into 1.4-ml Eppendorf tubes on ice, and the parasites were pelleted in a microfuge at 12,000 g for 2 min at 4°C. The buffer was aspirated, and individual merozoite pellets were resuspended on ice in 20 μl of reaction buffer further supplemented with protease inhibitors or antibodies as appropriate. Merozoites were maintained on ice for 15 min to allow antibody binding, then transferred to a 37°C water bath for 1 h to allow processing to proceed. Assays always included the following controls: a “positive processing” control sample of merozoites, resuspended in reaction buffer only; a negative “no processing” sample of merozoites, resuspended in reaction buffer plus 1 mM PMSF; and a zero time (0 h) control, in which processing was immediately stopped before the 37°C incubation step by the addition of an equal volume of 2% (vol/vol) NP-40 (BDH Chemicals, Ltd., Poole, UK; reference 13).
Processing was stopped by the addition of 20 μl of 2% NP-40. Samples were vortexed and extracted on ice for 1 h, then centrifuged for 15 min at 12,000 g. The supernatant was removed to a new tube containing an equal volume of 2 × SDS-PAGE sample buffer, and 5–20 μl of each sample was subjected to electrophoresis under nonreducing conditions on 12.5 or 15% polyacrylamide minigels (Pharmacia Biotech) before being transferred electrophoretically to nitrocellulose (Schleicher and Schuell, Inc., Dassel, Germany, 0.2 μm pore size). Blots were blocked in PBS containing 7% (wt/vol) BSA and probed with a 1:100 dilution of Rb anti–MSP-133. After washing three times in PBS containing 0.05% (vol/vol) Tween -20 (PBS/T), bound antibody was detected by further incubation with radioiodinated anti–rabbit IgG. Blots were washed for 1 h with several changes of PBS/T, and then dried. Bands on the blot corresponding to MSP-133 and MSP-142 were visualized by autoradiography; direct quantitation of the radioactivity associated with these bands was then performed by excising the appropriate regions from the blots and measuring the associated radioactivity (in cpm) in a gamma counter. Merozoite samples were routinely assayed in triplicate, and results were expressed as mean percentage MSP-142 processing, using the formula [(X − B)/(A − B) × 100], where A was the mean amount of MSP-133 (in cpm) in control samples incubated in reaction buffer alone; B was the mean amount of MSP-133 in the 0 h control (i.e., background levels of MSP-133 present at the start of the assay); and X was the mean amount of MSP-133 produced in the presence of the antibody under test or protease inhibitor.
Preparation of Merozoite Antigen Sonicate for Immunoassays.
This study investigated recognition by antibodies of MSP-1 in the form in which it exists on the surface of the free merozoite. Since the MSP-1 precursor undergoes proteolytic modification at or before merozoite release, possibly resulting in conformational differences between the precursor molecule and the merozoite surface complex, it was decided to avoid the use of detergent-solubilized precursor protein for experiments exploring the mechanisms involved in blocking antibody activity, and to use merozoite-derived, nondetergent-solubilized antigen instead. Purified merozoites were suspended on ice in 0.1 M carbonate/bicarbonate buffer, pH 9.6, 0.02% (wt/vol) sodium azide (coating buffer), containing the protease inhibitors leupeptin, antipain, TLCK, and 1 mM PMSF. The suspension was sonicated in a Kerry KS 1000 sonicating water bath (Kerry Ultrasonics, Hitchin, Herts., UK) for 1 min, centrifuged at 12,000 g for 15 min, and then the resulting supernatant was further diluted (usually 100-fold) in coating buffer before being used to coat ELISA or RIA plates.
ELISA.
An ELISA was used to titrate the binding of antibodies to native or recombinant MSP-1 and to determine saturating antibody concentrations under these conditions. Serially diluted mAbs, rabbit antibodies, or human antibodies were added to ELISA plates (Immulon 4; Dynatech Labs., Inc., Chantilly, VA) coated with an optimal concentration of purified rMSP-119 or merozoite antigen sonicate. Bound antibody was detected using horseradish peroxidase-conjugated (HRP) rabbit anti–mouse IgG or HRP mouse anti–rabbit IgG, or HRP rabbit anti–human IgG (Sigma Chemical Co., UK) as appropriate. Assays were otherwise performed and developed as previously described (35). In preliminary experiments, titration curves obtained using anti–MSP-119 mAbs in the two ELISA systems (rMSP-119 and merozoite sonicate) were indistinguishable.
Competitive RIA.
A competitive solid-phase RIA was used to determine whether or not anti–MSP-1 mAbs or rabbit antibodies could competitively block the binding of processing-inhibitory mAbs 12.8 and 12.10 to their epitopes. Wells of polyvinyl chloride microtiter plates (Falcon Labware, Becton Dickinson and Co., Oxnard, CA) were coated overnight at 4°C with 100 μl of merozoite antigen sonicate, or rMSP-119 at a final concentration of 10 μg ml−1 in coating buffer. Plates were then washed three times in PBS/T and treated overnight at 4°C with PBS/T containing 1% (wt/vol) bovine serum albumin (PBS/T/BSA). The plates were then washed and 50 μl PBS/T/BSA containing serum or purified antibody at a saturating concentration (predetermined by ELISA; see above) was added to wells in triplicate. Plates were incubated for 2 h at room temperature, then washed again, and 50 μl of optimally diluted radioiodinated mAb 12.8 or 12.10 was added in PBS/T/BSA. Optimal concentrations of radiolabeled mAbs were determined in preliminary radioimmune titration assays; the final concentration of radiolabeled mAbs used in the competitive RIAs corresponded to those in the linear part of the dose–response curve, so that changes in 12.8 and 12.10 binding in the presence of blocking antibodies would be readily apparent. Plates were incubated for a further 2 h at room temperature, then washed as before. Individual wells were excised and counted for 1 min in a gamma counter. Samples were routinely assayed in triplicate, and the binding of radiolabeled mAbs was expressed as a percentage of that obtained in the absence of pretreatment of wells.
Affinity Purification of Human Antibodies Reactive with pME6.
Purified pME6 protein was bound to cyanogen bromide–activated Sepharose 4B (Pharmacia Biotech) at 5 mg ml−1 swollen gel according to the manufacturer's instructions. 30 ml of pooled serum derived from adult Gambian donors was diluted 1:4 in 50 mM Tris-HCl, pH 8.0, containing 0.02% (wt/vol) sodium azide, clarified by passage through a 0.45-μm filter, then passed over a 5-ml affinity column at a flow rate of 10 ml h−1. The column was washed extensively in 50 mM Tris-HCl, pH 8.0, and bound Ig was eluted in the same buffer containing 8 M urea. Samples of eluate fractions were subjected to SDS-PAGE under reducing conditions, and assessed for the presence and purity of IgG by examination of Coomassie blue–stained gels. Peak fractions were pooled, dialyzed exhaustively against PBS, concentrated in an ultrafiltration cell using an XM10 membrane (Amicon, Inc.), and stored at 4°C. Yield of IgG was quantified by spectrometry assuming an A280 for human IgG of 1.4 at 1.0 mg ml−1 (1-cm path length).
Results
Development and Validation of an Assay to Quantitate Antibody-mediated Inhibition of MSP-1 Processing.
In previous work, a panel of MSP-119–specific mAbs was tested for their ability to interfere with secondary processing of MSP-1 (25) using a Western blot–based procedure that allowed only a semiquantitative estimate of processing inhibition. To improve the assay for this study, a radioiodinated, affinity-purified anti–rabbit IgG was used. Autoradiography of the probed blots allowed visualization of bands corresponding to MSP-142 and its processed product MSP-133, and the amount of antibody bound to each was determined by direct counting in a gamma counter. When an extract of incubated merozoites was analyzed by this method, the radioactivity associated with each of the MSP-133 and MSP-142 bands on the blot was, within limits imposed by the protein binding capacity of the blotting membrane, directly proportional to the volume of merozoite extract loaded on the gel (not shown). This linear relationship did not hold if an extract of more than ∼2 × 108 merozoites was loaded per track, and in all subsequent experiments this limit was not exceeded. During a 1-h incubation of merozoites, the observed decrease over time in the number of counts associated with MSP-142 (due to processing of the polypeptide) was concomitant with a corresponding increase in the number of counts associated with MSP-133, and at least 50% of the MSP-142 underwent processing in this period (data not shown; see reference 13). These results are in accordance with previous data showing stoichiometric conversion of MSP-142 to MSP-133 (13), and indicated that accurate quantitation of MSP-1 processing is possible with this assay. In a typical assay, the number of cpm associated with the MSP-133 band in the zero time (0 h) control and the positive processing control sample (incubated for 1 h in reaction buffer only; see Materials and Methods) was 20 and 1,300 cpm, respectively (data not shown).
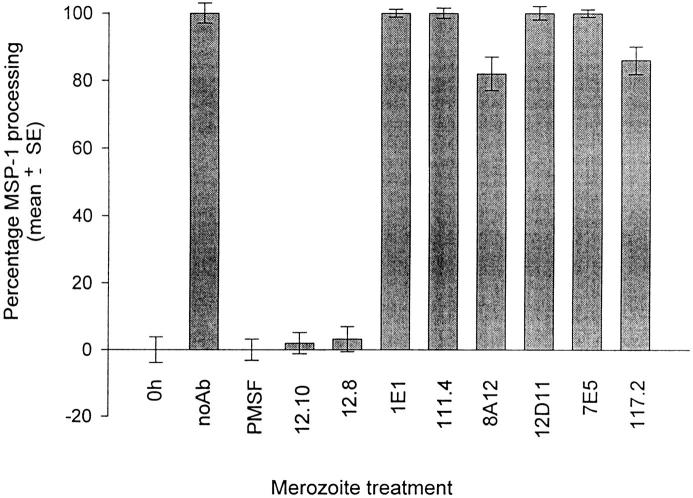
The assay was used to quantify MSP-1 secondary processing and its inhibition by a panel of anti–MSP-119 mAbs. Washed FCB-1 merozoites were incubated on ice in the presence of individual purified mAbs, then transferred to 37°C for 1 h to allow processing to occur. MSP-142 processing in the individual samples was then assessed using the above protocol. Fig. 1 shows that mAb 12.8, which recognizes a conserved epitope in the first EGF-like motif of MSP-119 (36, 37), inhibited processing by 96% of the control value, whereas mAb 12.10, which recognizes an epitope formed by the two EGF-like motifs together (37), inhibited processing by 98%. Monoclonal antibody 1E1 showed no processing-inhibitory activity in this assay system. Interestingly, our earlier data obtained using a semiquantitative Western blot–based assay indicated that mAb 1E1 appeared to induce abnormal processing rather than preventing the processing; in addition, mAb 1E1 does not prevent erythrocyte invasion in in vitro cultures of P. falciparum (25). Antibodies 8A12 and 117.2 inhibited MSP-142 processing by 18 and 12%, respectively, whereas mAbs 111.4, 12D11, and 7E5 did not detectably prevent processing. Neither mAb 89.1, which recognizes an epitope within the NH2-terminal domain of MSP-1 (MSP-183), nor the anti– P. yoelii MSP-1 mAb 25.1, had any effect on the processing (data not shown). These results confirm that mAbs 12.8 and 12.10 are potent inhibitors of MSP-142 processing. In similar assays using merozoites of the P. falciparum clone T9/96, which expresses the alternative dimorphic form of MSP-1 (31), but retains the nonpolymorphic epitopes recognized by mAbs 12.8 and 12.10 (38), both mAbs showed similarly potent processing inhibition activity (data not shown).
Figure 1.
Inhibition of MSP-142 processing by anti–MSP119 mAbs. Washed FCB-1 merozoites were either immediately detergent solubilized (0h) or incubated for 1 h at 37°C in the presence of no antibodies (noAb), 1 mM PMSF as inhibitor control, or purified mAbs 12.10, 12.8, 1E1, 111.4, 8A12, 12D11, 7E5 or 117.2, all at a final concentration of 300 μg ml−1. MSP-1 secondary processing in the samples was then quantified as described. All samples were tested in triplicate, and percentage of processing was calculated as described in Materials and Methods.
Blocking Antibodies Act by Competitively Preventing the Binding of Processing-inhibitory mAbs to Merozoites.
Previous work (25) has indicated that a number of anti–MSP-119 mAbs, which themselves do not inhibit MSP-1 processing, can block the ability of mAbs 12.8 and 12.10 to interfere with the processing. Although the mechanism of this blocking activity was not elucidated, the most likely explanation is that a blocking antibody can compete with a processing-inhibitory antibody for binding to MSP-1 on the merozoite surface. In this study, this hypothesis was directly tested using a competitive RIA to investigate the effects of known blocking antibodies on binding of processing-inhibitory antibodies to native, merozoite-derived MSP-1.
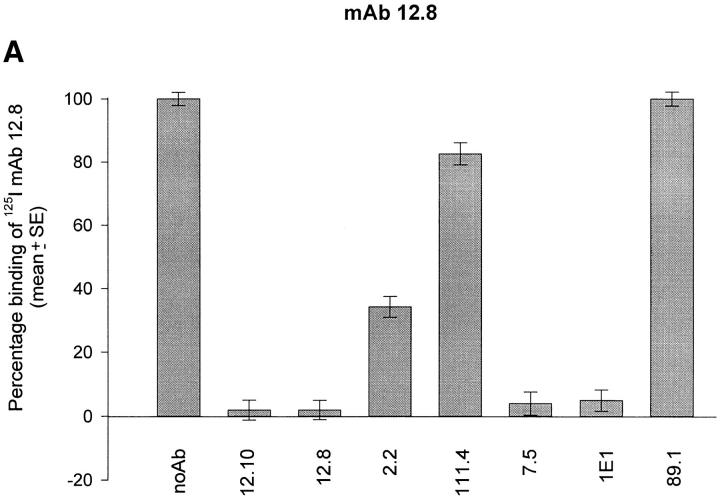
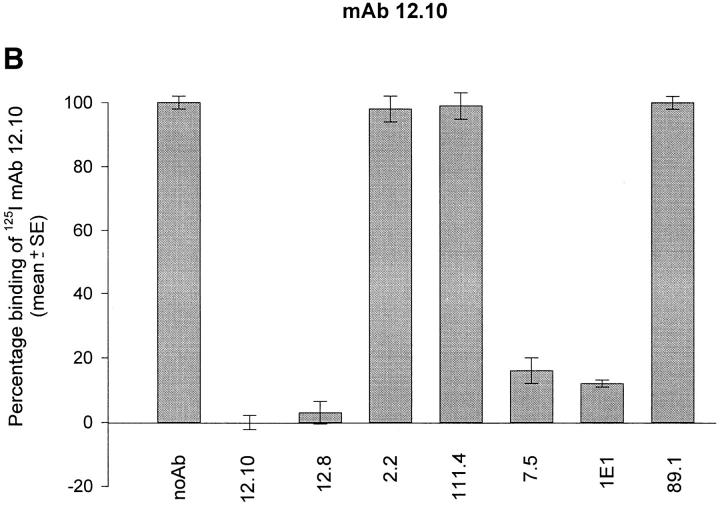
Wells of 96-well polyvinyl chloride plates coated with merozoite antigen extract were incubated with anti–MSP-119 mAbs at saturating concentrations. The plates were then washed and an optimal concentration of radioiodinated mAb 12.8 or 12.10 was added. After further incubation, plates were washed and individual wells were counted directly in a gamma counter. Fig. 2 shows that antibodies known to interfere with the processing activity of mAbs 12.8 and 12.10 prevented these mAbs from binding to immobilized antigen. Although mAbs 7.5 and 1E1 prevented binding of both radiolabeled mAbs, mAb 2.2 only significantly prevented binding of mAb 12.8, consistent with its ability to interfere with the processing-inhibitory activity of mAb 12.8 but not 12.10 (25). Preincubation with mAb 111.4 had little or no effect on binding of the radiolabeled mAbs, consistent with its lack of blocking activity (25); mAb 89.1 was similarly ineffective in competing with 12.8 or 12.10 binding. Identical results were obtained when rMSP-119 was used to coat RIA plates (data not shown).
Figure 2.
The binding of processing-inhibitory mAbs 12.8 and 12.10 to FCB-1 merozoite-derived MSP-1 is competitively prevented by certain other anti–MSP-1 mAbs. Plates coated with a merozoite antigen extract were preincubated in triplicate with either no antibody (noAb; control wells), or with predetermined saturating concentrations of mAbs 12.10, 12.8, 2.2, 111.4, 7.5, 1E1, or 89.1. The effects of this pretreatment on binding of radioiodinated mAbs 12.8 (A) or 12.10 (B) to the immobilized antigen was then assessed. All samples were tested in triplicate. Blocking activity of individual mAbs was calculated as described in Materials and Methods.
Antibodies Against the NH2-terminal Region of MSP-1 Can Block the Binding of Processing-inhibitory mAbs Directed against Epitopes within MSP-119.
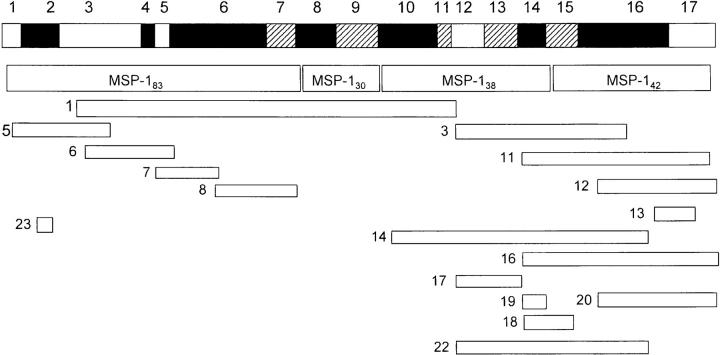
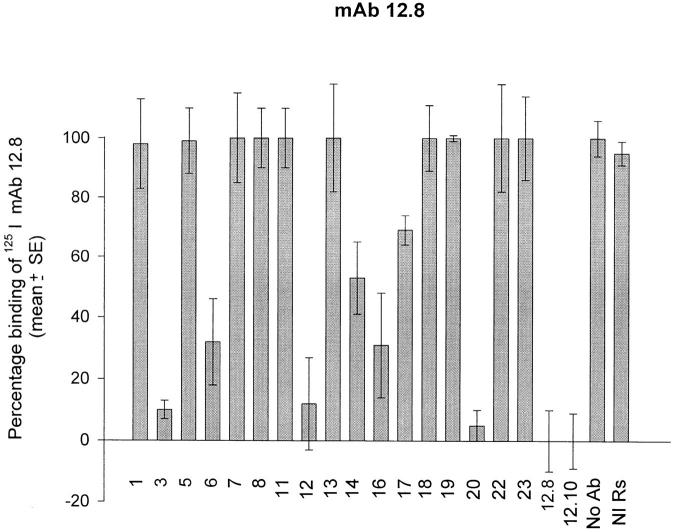
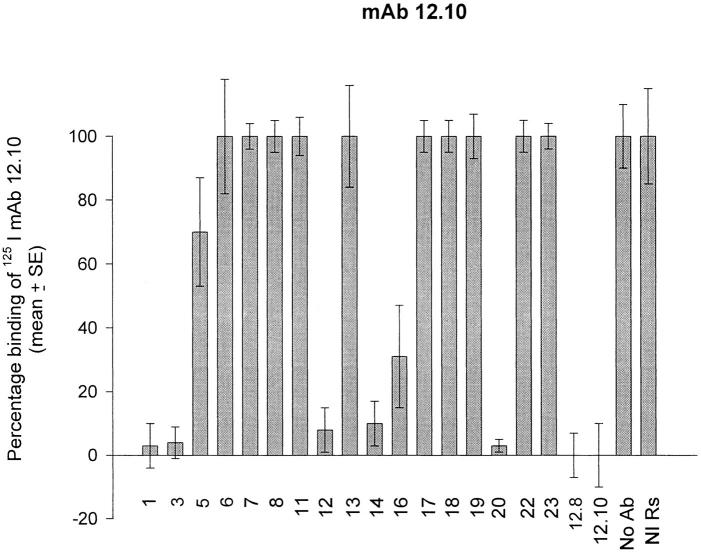
The above results showed that the binding of processing-inhibitory antibodies to MSP-119 can be specifically prevented by the interaction of other antibodies with the same polypeptide, and explained how blocking antibodies interfere with the processing-inhibitory activity of mAbs 12.8 and 12.10. Interestingly, Wilson et al. (38) found that mAb 13.2, which recognizes an epitope within the NH2-terminal domain of MSP-1, prevents the binding of mAb 12.8 to intact MSP-1, raising the possibility that antibodies specific to other components of the MSP-1–derived, merozoite surface protein complex might have blocking activity. To investigate this possibility, a series of rabbit antibodies, raised against recombinant proteins corresponding to regions covering all of MSP-1 (reference 8; Fig. 3) were tested for their ability to competitively prevent recognition of merozoite-derived MSP-1 by mAbs 12.8 and 12.10. Fig. 4 shows that binding of radioiodinated mAbs 12.8 and 12.10 to the merozoite antigen was significantly blocked by some but not all of the polyclonal antibodies. The fact that rabbit antibodies raised against pME12, 16, and 20 were able effectively to block binding was not unexpected, due to the presence of the 12.8 and 12.10 epitopes within the sequence of the recombinant proteins used to raise these rabbit sera. However, it was found that antibodies raised against constructs corresponding to domains of MSP-1 outside the COOH-terminal region also showed potent blocking activity; in particular, the anti-pME6, anti-pME14, and anti-pME3 sera inhibited binding of mAb 12.8 to the immobilized antigen by 68, 48, and 91%, respectively, and the rabbit anti-pME14, anti-pME1, and anti-pME3, but not the anti-pME6 antibodies, significantly prevented binding of mAb 12.10. These results show that polyclonal antibodies specific for fragments of the MSP-1 complex other than MSP-119 can act as blocking antibodies.
Figure 3.
Schematic of recombinant (pME) MSP-1 constructs relative to the MSP-1 gene and its products. Shown is a diagrammatic representation of the complete MSP-1 gene, divided into conserved (open) blocks 1, 3, 5, 12, and 17, semiconserved (hatched) blocks 7, 9, 11, 13, and 15, and poorly conserved or polymorphic (filled) blocks 2, 4, 6, 8, 10, 14, and 16, as defined by Tanabe et al. (48). The positions of the MSP-1 primary processing products (MSP-183, MSP-130, MSP-138 and MSP-142) are shown relative to the gene, as are the relative positions of the pME series of recombinant expression constructs against which polyclonal rabbit antisera have been raised (8). IgG purified from the anti-pME rabbit sera was used in this study.
Figure 4.
Rabbit polyclonal antibodies raised against defined domains of MSP-1 have blocking activity. Rabbit antibodies raised against recombinant MSP-1 expression constructs pME1, 3, 5, 6, 7, 8, 11, 12, 13, 14, 16, 17, 18, 19, 20, 22, and 23 were assayed at predetermined saturating concentrations for their ability to prevent binding of radioiodinated mAb 12.8 or 12.10 to immobilized FCB-1 merozoite antigen. Control wells were pretreated either with mAbs 12.8 or 12.10, or with buffer alone (No Ab) or with a nonimmune rabbit serum (NI Rs) at a final dilution of 1: 100. All samples were assayed in triplicate, and SE bars are indicated.
Naturally Acquired Human Antibodies Specific for Epitopes within the NH2-terminal Domain of MSP-1 Block the Activity of Processing-inhibitory Anti–MSP-119 Antibodies.
Antibodies which prevent MSP-1 processing and erythrocyte invasion may be involved in mediating protection against blood-stage parasitemia. If antibodies induced to other domains of MSP-1 can block the activity of processing-inhibitory antibodies specific for MSP-119, their presence in human sera may be disadvantageous to the host. In light of the above data, it was decided to investigate the ability of naturally acquired antibodies, specific for the region of MSP-1 corresponding to pME6, to block the processing-inhibitory activity of mAbs 12.8 and 12.10. This particular construct was chosen because pME6 is readily soluble (8), and the E. coli clone which expresses pME6 does so at very high levels. Human antibodies reactive with pME6 were isolated from pooled Gambian adult immune serum by affinity chromatography on immobilized pME6 fusion protein. The eluted Ig was judged to be >98% pure as assessed by SDS-PAGE under reducing conditions (data not shown). The Ig was concentrated by ultrafiltration and assayed by immunoblot for reactivity with FCB-1 merozoite polypeptides. Strong reactivity was observed with only two merozoite polypeptides of ∼83 and 195 kD (Fig. 5); these most likely correspond to MSP-183 and the residual MSP-1 precursor protein. Note that the purified antibodies showed no reactivity with the MSP-142 and MSP-119 species (Fig. 5, arrows). In confirmation of this, analysis of the affinity-purified Ig by indirect immunofluorescence showed strong reactivity with acetone-fixed FCB-1 or T9/96 schizonts, but none with newly invaded ring stage parasites, which contain only MSP-119 (9–12) (data not shown). Note that since the pME6 construct covers much of the highly conserved MSP-1 block 3 domain, as well as all of the conserved block 5 (see Fig. 3), antibodies against pME6 would be expected to recognize both allelic forms of MSP-1.
Figure 5.
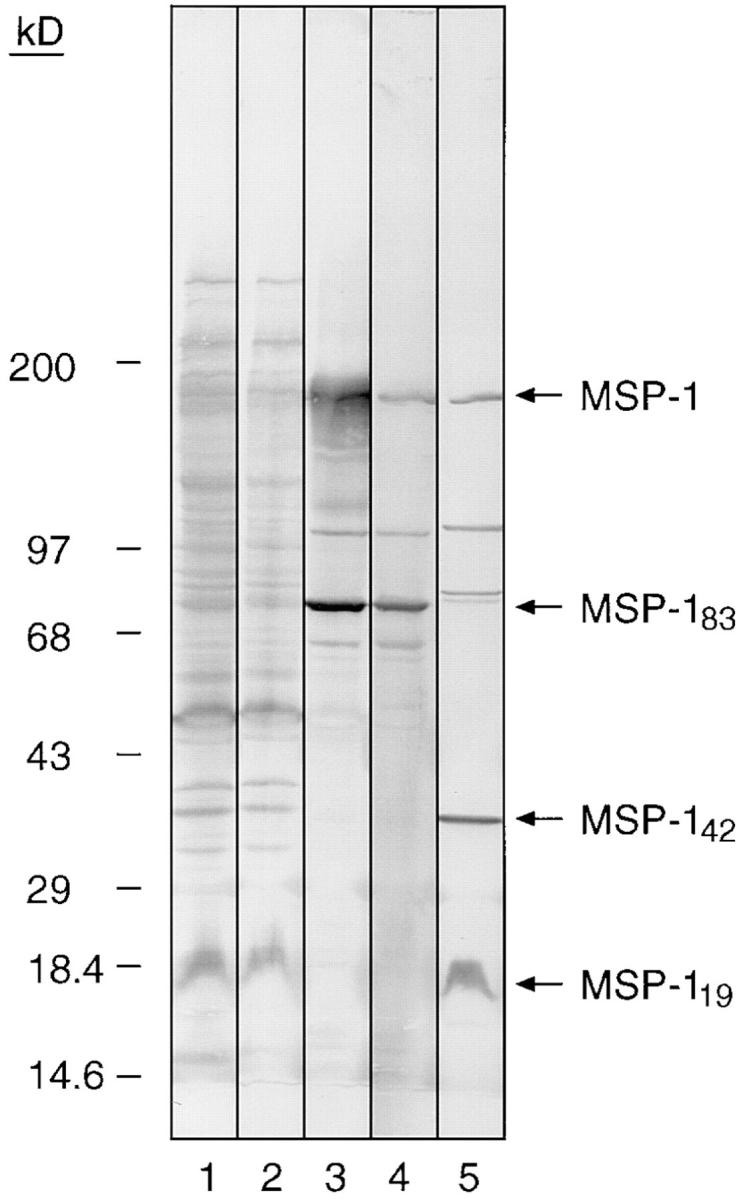
Specificity of affinity-purified human anti-pME6 antibodies shown by Western blot analysis. An SDS extract of FCB-1 merozoites was subjected to SDS-PAGE under nonreducing conditions on a 12.5% gel, transferred to nitrocellulose, then probed with a sample of pooled human immune serum taken before chromatography over the pME6 affinity column (lane 1); serum taken after passage over the column (lane 2); affinity-purified anti-pME6 antibodies (lane 3); mAb 89.1 (specific for MSP-183; lane 4); and mAb 111.4 (specific for MSP-142 and MSP-119; lane 5). Positions of molecular mass marker proteins are indicated, and bands corresponding to the MSP-1 precursor, MSP-183, MSP-142, and MSP-119 are arrowed.
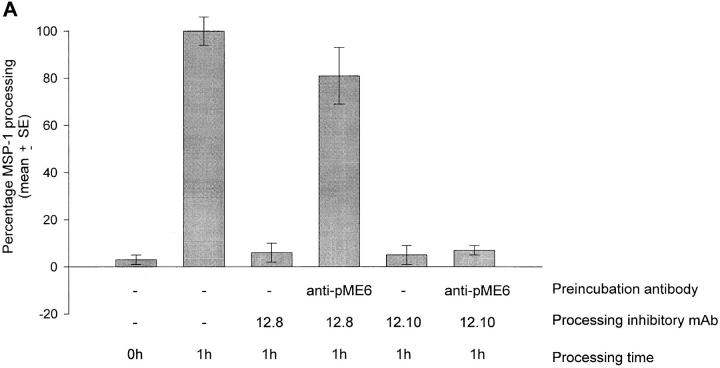
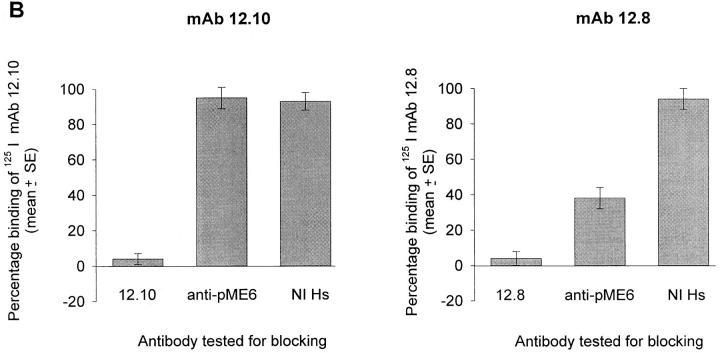
The ability of the affinity-purified human antibodies to block the processing-inhibitory effects of mAbs 12.8 and 12.10 was then assessed. Merozoites were incubated on ice in the presence or absence of the human anti-pME6 antibodies, and then mAb 12.8 or 12.10 was added and the samples were incubated for 20 min on ice before transfer to 37°C for 1 h to allow processing to take place. Fig. 6 A shows that pretreatment with the anti-pME6 antibodies virtually abolished the processing-inhibitory activity of mAb 12.8, but interestingly had no effect on the inhibitory activity of mAb 12.10. In parallel binding assays (Fig. 6 B), the anti-pME6 antibodies competed effectively with binding of mAb 12.8, but not mAb 12.10, to immobilized merozoite-derived antigen.
Figure 6.
Affinity-purified, naturally acquired human anti-pME6 antibodies are potent blocking antibodies. (A) Equal aliquots of washed FCB-1 merozoites were solubilized directly into detergent (0 h control), or preincubated either with reaction buffer only or with affinity-purified human anti-pME6 antibodies at a final concentration of 300 μg ml−1. An equal concentration of mAb 12.10 or 12.8 was then added to some samples as shown, and processing was allowed to proceed for 1 h in all but the 0 h control. Inhibition of MSP-1 processing mediated by mAb 12.8 alone (96%) was almost completely reversed by preincubation with the anti-pME6 antibodies, whereas the inhibition of processing mediated by mAb 12.10 alone (97%) was completely unaffected by preincubation with anti-pME6 antibodies. (B) RIA plates coated with merozoite antigen were pretreated with nonradioactive mAb 12.10 or 12.8 at a saturating concentration (100 μg ml−1), or affinity-purified anti-pME6 antibodies at a saturating concentration (300 μg ml−1), or nonimmune human serum (NI Hs) at an equivalent final antibody concentration, before assessing the ability of radioiodinated mAb 12.8 or 12.10 to bind. All samples were assayed in triplicate, and SE bars are shown.
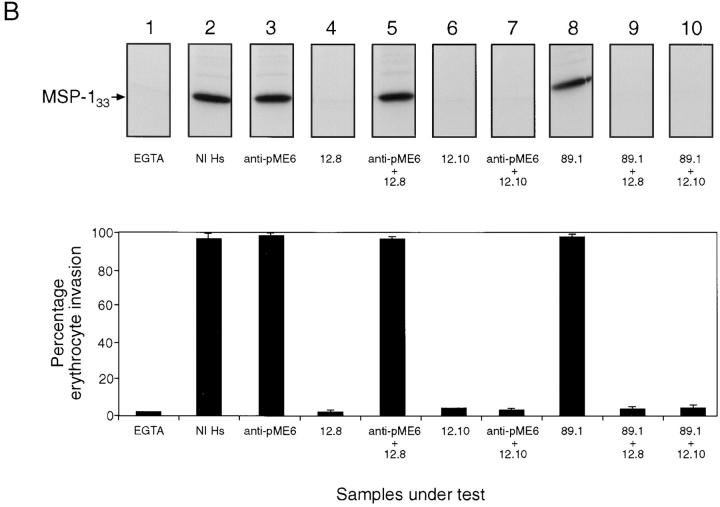
These data clearly show that the binding of antibodies specific to one component of the MSP-1–derived merozoite surface complex can interfere with the binding of antibodies to another component of the complex. Erythrocyte invasion by the malaria merozoite is rapid, going to completion within seconds of the initial interaction between parasite and red cell surface (39). Over such a short time span, could the presence of blocking antibodies interfere with the ability of processing-inhibitory antibodies to bind the merozoite surface and prevent both processing and invasion? To address this question directly in an in vitro system, a series of invasion experiments was performed. Mature, biosynthetically radiolabeled T9/96 schizonts were washed and placed in culture with fresh red cells. Merozoite release and red cell invasion were then allowed to proceed in the presence or absence of mAbs 12.8 and 12.10, with or without the additional presence of affinity-purified anti-pME6 human antibodies. The overall efficiency of invasion was assessed by counting the number of new ring stage parasites formed over the course of the experiment; MSP-1 processing in individual samples was subsequently assessed by direct immunoprecipitation of MSP-133 from the culture supernatants using mAb X509 coupled to Sepharose. In preliminary dose–response experiments, a concentration of ⩾400 μg ml−1 of either mAb 12.10 (Fig. 7 A) or mAb 12.8 (data not shown) was sufficient to reduce the amount of MSP-133 release to a level of inhibition seen in the presence of 5 mM EGTA, a potent inhibitor of MSP-1 secondary processing (11). The results of a typical experiment (of a total of three independent experiments) investigating the effects of the anti-pME6 blocking antibodies on the activity of mAbs 12.8 and 12.10 are presented in Fig. 7 B. In isolation, mAbs 12.8 and 12.10 virtually abolished both invasion (Fig. 7 B, bottom) and MSP-133 release (Fig. 7 B, top). However, in the presence of equal concentrations of the anti-pME6 human antibodies, the effects of mAb 12.8, but not of 12.10, were completely reversed (Fig. 7 B, lanes 5 and 7). Neither the anti-pME6 antibodies alone nor mAb 89.1 alone had any effect on either processing or invasion (Fig. 7 B, lanes 3 and 8), and mAb 89.1 exhibited no blocking activity (Fig. 7 B, lanes 9 and 10). These results unambiguously demonstrate that, under conditions of active release of viable merozoites, mAbs 12.8 and 12.10 effectively prevent both MSP-1 processing and erythrocyte invasion, and this activity can be efficiently abrogated by the presence of human blocking antibodies.
Figure 7.
Processing-inhibitory anti–MSP119 mAbs can prevent MSP-1 and erythrocyte invasion in in vitro culture, and can be rendered ineffective by the simultaneous presence of anti-pME6 blocking antibodies. (A) Dose–response effect of mAb 12.10 on MSP-1 secondary processing. Metabolically radiolabeled T9/96 schizonts were supplemented with fresh erythrocytes and medium to obtain a parasitemia of ∼2% and a hematocrit of 1%. The culture was then divided into equal aliquots and incubated at 37°C in the presence of 5 mM EGTA as control inhibitor (lane 1), or mAb 12.10 at a final concentration of 2 μg ml−1 (lane 2), 1 μg ml−1 (lane 3), 500 μg ml−1 (lane 4), 400 μg ml−1 (lane 5), 300 μg ml−1 (lane 6), 200 μg ml−1 (lane 7), 100 μg ml−1 (lane 8), or no antibody (lane 9). Schizont rupture and merozoite release were then allowed to proceed for 6 h, and culture supernatants were analyzed by immunoprecipitation using mAb X509 coupled to Sepharose for the presence of MSP-133. (B) Blocking anti-pME6 antibodies reverse the processing-inhibitory (top) and invasion-inhibitory (bottom) activity of mAb 12.8. Cultures containing metabolically radiolabeled T9/96 schizonts prepared as described above were incubated in the presence of 5 mM EGTA (lane 1), 10% (vol/ vol) nonimmune human serum (lane 2), anti-pME6 antibodies (lane 3), mAb 12.8 (lane 4), mAb 12.8 plus anti-pME6 antibodies (lane 5), mAb 12.10 (lane 6), mAb 12.10 plus anti-pME6 antibodies (lane 7), mAb 89.1 (lane 8), mAb 89.1 plus mAb 12.8 (lane 9) and mAb 89.1 plus mAb 12.10 (lane 10). In this case all antibodies were added to a final concentration of 400 μg ml−1. Analysis of 6-h culture supernatants by immunoprecipitation with mAb X509 (B, top) was as above, and in addition erythrocyte invasion in individual cultures was assessed by counting the number of ring-stage parasites in 5,000 red cells, in triplicate (B, bottom). Invasion is expressed as a percentage of the ring-stage parasitemia (10%) obtained in a control culture with no additions (data not shown).
Discussion
Four major conclusions can be drawn from this study. First, blocking antibodies function by competitively preventing the binding of processing-inhibitory antibodies to the merozoite surface, and can be effective under conditions of active merozoite release and erythrocyte invasion. Second, blocking activity can be mediated not only by antibodies specific for the MSP-119 domain, but also by antibodies binding to polypeptides other than the MSP-119 target of processing-inhibitory antibodies; here we have shown that antibodies reactive with a region within MSP-183, a polypeptide derived from the NH2-terminal domain of the MSP-1 precursor, possess potent blocking activity. Antibodies against other fragments of the merozoite surface complex, possibly including the non–MSP-derived components of it (12, 40), may also mediate blocking activity; indeed, our present data suggest that antibodies against the region of MSP-1 represented by pME14 possess significant blocking activity (Fig. 4). Third, human blocking antibodies can be induced by natural exposure to malarial infection. Fourth, if prevention of MSP-1 processing is a major mechanism by which anti–MSP-119 antibodies exert their effect on erythrocyte invasion by the P. falciparum merozoite, then the protective potential of inducing such antibodies by vaccination could be impaired by a preexisting or simultaneously induced blocking antibody response directed against MSP-119 itself, or other components of the MSP-1 protein complex.
MSP-1 is receiving increasing interest as a candidate antigen for a blood-stage malaria vaccine. Experimental passive immunization and direct immunization-challenge studies focusing on the protective capacity of anti–MSP-119 antibody responses have been substantiated by epidemiological studies in malaria-endemic areas showing a significant positive association between levels of serum antibodies against MSP-119 and resistance to morbidity associated with falciparum malaria (41, 42). However, the seroepidemiological data are ambiguous. For example, there is not a simple relationship between seropositivity and clinical immunity, and there is extensive evidence that parasite replication can take place in vivo in the presence of substantial levels of circulating anti–MSP-1 antibody (41–43). With no clear consensus on either the mechanism(s) by which anti–MSP-1 antibodies control replication of the parasite or the biological function of MSP-1 on the merozoite surface (6, 44), the effector mechanisms required of an optimally protective anti– MSP-1 immune response have been unclear. Given the imminent availability of first generation MSP-119-based vaccines for clinical evaluation, there is a pressing need to define indicators of a protective anti–MSP-1 response which are amenable to quantitative serological assay (45).
We propose that antibodies specific for the P. falciparum MSP-119 domain prevent merozoites from invading erythrocytes primarily by interfering with MSP-1 secondary processing. This hypothesis is supported by the apparently absolute correlation between antibody-mediated processing-inhibitory activity and invasion inhibitory activity; of a total of 11 distinct anti–P. falciparum MSP-119 mAbs tested to date, only mAbs 12.8 and 12.10 exhibit either activity (this study, reference 25, and our unpublished data). The hypothesis would explain the observed absence of a straightforward correlation between total serum anti–MSP-119 antibody levels, and immunity to blood-stage parasitemia in individuals naturally exposed to malaria; since many anti– MSP-119 antibody specificities clearly have no effect on MSP-1 processing, and indeed can block the activity of antibodies with “protective,” processing-inhibitory specificities, a simple evaluation of total anti–MSP-119 serum antibody titers in a naturally exposed individual may never provide a clear measure of the protective capacity of that antibody response. The additional fact, highlighted in this study, that blocking activity may also be mediated by naturally acquired antibodies against MSP-1–derived components other than MSP-119, further complicates attempts to predict the protective capacity of an antibody response to MSP-119 in the presence of a polyclonal response against the total MSP-1. Therefore, the validity of the continued use of simple ELISA-based assays in epidemiological studies may be questionable. We tentatively conclude that only a functional assay, such as one measuring MSP-1 processing inhibition, or the effect on invasion of affinity-purified antibodies (43), can provide an assessment of the overall protective capacity of an anti–MSP-1 antibody response. The critical test of our hypothesis will be the predictive power of the assay; opportunities to evaluate this will arise from immunization trials in naive primates or humans with MSP-119 or MSP-142– based vaccines in which significant protection is achieved (46, 47). This is a major priority, and work towards it is in progress. A further implication of our hypothesis is that, for an MSP-119–based vaccine to be effective, its design or mode of administration should be such that the overall balance of the induced antibody response is towards processing-inhibitory antibody specificities, rather than blocking specificities. Selectively inducing this type of functional antibody response may be the major challenge in MSP-119–based vaccine development.
How do antibodies specific for the NH2-terminal domain of MSP-1 (MSP-183) exert blocking activity? There are no published structural data on the merozoite surface complex. However, treatment of intact merozoites with the bifunctional, cleavable cross-linker 3, 3′-dithiobis(sulfosuccinimidylpropionate) results in almost quantitative cross-linking of the MSP-183 and MSP-142 components of the complex (Blackman, M.J., unpublished data), suggesting that at least in the conformation adopted by the membrane-bound form of the complex, these two polypeptides are spatially close. Given the additional fact that the molecular mass of an IgG molecule is not much less than that of the monomeric MSP-1 complex, the observation of steric competition between anti–MSP-183 and anti–MSP-119 antibodies is perhaps unsurprising. However, it is not clear why polyclonal antibodies reactive with the part of MSP-183 represented by pME6 should selectively block binding of mAb 12.8, but not 12.10; presumably the two processing-inhibitory mAbs adopt quite distinct orientations on binding. Whatever the case, this work has provided the first experimental evidence that antibodies against one part of a merozoite surface protein can “shield” the parasite from the potentially harmful effects of antibodies directed against another part of the same surface protein. MSP-183 is known to be immunogenic in human populations exposed to malaria (35, 41); it is conceivable that it is advantageous to the parasite to evoke an antibody response to this part of MSP-1, and this may provide a selective pressure to prevent sequence variation in the conserved parts of the molecule.
The physiological function of the proteolytic processing of MSP-1, and the identity of the protease which mediates it, are unknown. However, these results reemphasize the importance of the processing step, and the potential of the relevant enzyme as a novel target for development of protease inhibitor–based antimalarial drugs.
Acknowledgments
We are grateful to Muni Grainger and Terry Scott-Finnigan for excellent technical assistance with malaria parasite culture and monoclonal antibody production.
Footnotes
J.A. Guevara Patiño is in receipt of a studentship grant from the Venezuelan Government. J.S. McBride is supported by the Wellcome Trust. This investigation received financial support from the Medical Research Council (UK), and from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR).
Abbreviations used in this paper: EGF, epidermal growth factor; HRP, horseradish peroxidase–conjugated; MSP-1, merozoite surface protein–1; T, Tween 20; TLCK, tosyl-l-lysyl choromethyl ketone.
References
- 1.Cohen S, McGregor IA, Carrington SC. Gamma globulin and acquired immunity to human malaria. Nature (Lond) 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 2.Edozien JC, Gilles HM, Udeozo IOK. Adult and cord-blood gamma-globulin and immunity to malaria in Nigerians. Lancet. 1962;2:951–955. [Google Scholar]
- 3.McGregor IA, Carrington SP, Cohen S. Treatment of East African Plasmodium falciparummalaria with West African human gamma globulin. Trans R Soc Trop Med Hyg. 1963;57:170–175. [Google Scholar]
- 4.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparumblood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holder AA. Proteins on the surface of the malaria parasite and cell invasion. Parasitology. 1994;108:S5–S18. doi: 10.1017/s0031182000075673. [DOI] [PubMed] [Google Scholar]
- 6.Barnwell JW, Galinski MR. The adhesion of malaria merozoite proteins to erythrocytes: a reflection of function? . Res Immunol. 1991;142:666–672. doi: 10.1016/0923-2494(91)90147-b. [DOI] [PubMed] [Google Scholar]
- 7.McBride JS, Heidrich H-G. Fragments of the polymorphic Mr 185,000 glycoprotein from the surface of isolated Plasmodium falciparummerozoites form an antigenic complex. Mol Biochem Parasitol. 1987;23:71–84. doi: 10.1016/0166-6851(87)90189-7. [DOI] [PubMed] [Google Scholar]
- 8.Holder AA, Sandhu JS, Hillman Y, Davey LS, Nicholls SC, Cooper H, Lockyer MJ. Processing of the precursor to the major merozoite antigens of Plasmodium falciparum. . Parasitology. 1987;94:199–208. doi: 10.1017/s0031182000053889. [DOI] [PubMed] [Google Scholar]
- 9.Blackman MJ, Heidrich H-G, Donachie S, McBride JS, Holder AA. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990;172:379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackman MJ, Whittle H, Holder AA. Processing of the Plasmodium falciparummajor merozoite surface protein–1: identification of a 33-kilodalton secondary processing product which is shed prior to erythrocyte invasion. Mol Biochem Parasitol. 1991;49:35–44. doi: 10.1016/0166-6851(91)90128-s. [DOI] [PubMed] [Google Scholar]
- 11.Blackman MJ, Holder AA. Secondary processing of the Plasmodium falciparum merozoite surface protein–1 (MSP1) by a calcium-dependent membrane-bound serine protease: shedding of MSP133as a noncovalently associated complex with other fragments of the MSP1. Mol Biochem Parasitol. 1992;50:307–316. doi: 10.1016/0166-6851(92)90228-c. [DOI] [PubMed] [Google Scholar]
- 12.Stafford WHL, Blackman MJ, Harris A, Shai S, Grainger M, Holder AA. N-terminal amino acid sequence of the Plasmodium falciparummerozoite surface protein–1 (MSP-1) polypeptides. Mol Biochem Parasitol. 1994;66:157–160. doi: 10.1016/0166-6851(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 13.Blackman MJ, Chappel JA, Shai S, Holder AA. A conserved parasite serine protease processes the Plasmodium falciparummerozoite surface protein–1 (MSP1) Mol Biochem Parasitol. 1993;62:103–114. doi: 10.1016/0166-6851(93)90182-w. [DOI] [PubMed] [Google Scholar]
- 14.O'Dea KP, McKean PG, Harris A, Brown KN. Processing of the Plasmodium chabaudi chabaudiAS merozoite surface protein 1 in vivo and in vitro. Mol Biochem Parasitol. 1995;72:111–119. doi: 10.1016/0166-6851(95)00090-n. [DOI] [PubMed] [Google Scholar]
- 15.Blackman MJ, Dennis ED, Hirst EMA, Kocken CH, Scott-Finnigan TJ, Thomas AW. Plasmodium knowlesi: secondary processing of the malaria merozoite surface protein-1. Exp Parasitol. 1996;83:229–239. doi: 10.1006/expr.1996.0069. [DOI] [PubMed] [Google Scholar]
- 16.McKean PG, O'Dea K, Brown KN. A single amino acid determines the specificity of a monoclonal antibody which inhibits Plasmodium chabaudiAS in vivo. Mol Biochem Parasitol. 1993;62:211–222. doi: 10.1016/0166-6851(93)90110-j. [DOI] [PubMed] [Google Scholar]
- 17.Majarian WR, Daly TM, Weidanz WP, Long CA. Passive immunization against murine malaria with an IgG3 monoclonal antibody. J Immunol. 1984;132:3131–3137. [PubMed] [Google Scholar]
- 18.Ling IT, Ogun S, Holder AA. Immunization against malaria with a recombinant protein. Parasite Immunol (Oxf) 1993;16:63–67. doi: 10.1111/j.1365-3024.1994.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 19.Daly TM, Long CA. A recombinant 15-kilodalton carboxyl-terminal fragment of Plasmodium yoelii yoelii17XL merozoite surface protein 1 induces a protective immune response in mice. Infect Immun. 1993;61:2462–2467. doi: 10.1128/iai.61.6.2462-2467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian J-H, Miller LH, Kaslow DC, Ahlers J, Good MF, Alling DW, Berzofsky JA, Kumar S. Genetic regulation of protective immune response in congenic strains of mice vaccinated with a subunit malaria vaccine. J Immunol. 1996;125:1176–1183. [PubMed] [Google Scholar]
- 21.Hui GSN, Siddiqui WA. Serum from Pf195 protected Aotus monkeys inhibit Plasmodium falciparum growth in vitro. . Exp Parasitol. 1987;64:519–522. doi: 10.1016/0014-4894(87)90068-3. [DOI] [PubMed] [Google Scholar]
- 22.Chang SP, Gibson HL, Lee-Ng CT, Barr PJ, Hui GSN. A carboxyl-terminal fragment of Plasmodium falciparumgp195 expressed by a recombinant baculovirus induces antibodies that completely inhibit parasite growth. J Immunol. 1992;149:548–555. [PubMed] [Google Scholar]
- 23.Pirson PJ, Perkins ME. Characterization with monoclonal antibodies of a surface antigen of Plasmodium falciparummerozoites. J Immunol. 1985;134:1946–1951. [PubMed] [Google Scholar]
- 24.Cooper JA, Cooper LT, Saul AJ. Mapping of the region predominantly recognized by antibodies to the Plasmodium falciparummerozoite surface antigen MSA 1. Mol Biochem Parasitol. 1992;51:301–312. doi: 10.1016/0166-6851(92)90080-4. [DOI] [PubMed] [Google Scholar]
- 25.Blackman MJ, Scott-Finnigan TJ, Shai S, Holder AA. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J Exp Med. 1994;180:389–393. doi: 10.1084/jem.180.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burghaus P, Holder AA. Expression of the 19-kilodalton carboxy-terminal fragment of the Plasmodium falciparum merozoite surface protein–1 in Escherichia coliproduces a correctly folded protein that is recognized by protective monoclonal antibodies. Mol Biochem Parasitol. 1994;64:165–169. doi: 10.1016/0166-6851(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 27.Holder AA, Freeman RR. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature (Lond) 1981;294:361–366. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- 28.Antibodies. A Laboratory Manual. 1988. E. Harlow and D. Lane, editors. Cold Spring Harbor Laboratory, New York.
- 29.Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia colias fusions with glutathione S–transferase. Gene (Amst) 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 30.Abath FGC, Simpson AJG. A simple method for the recovery of purified recombinant peptides cleaved from glutathione S–transferase fusion proteins. Peptide Res. 1990;3:167–168. [PubMed] [Google Scholar]
- 31.Miller LH, Roberts T, Shahabuddin M, McCutchan TF. Analysis of sequence diversity in the Plasmodium falciparummerozoite surface protein-1 (MSP-1) Mol Biochem Parasitol. 1993;59:1–14. doi: 10.1016/0166-6851(93)90002-f. [DOI] [PubMed] [Google Scholar]
- 32.Steers E, Cuatrecasas P, Pollard HB. The purification of β-galactosidase from E. coliby affinity chromatography. J Biol Chem. 1971;246:196–200. [PubMed] [Google Scholar]
- 33.Fraker JP, Speck JC. Protein and cell membrane iodination with a sparingly soluble chloramide 1,3,4,6,-tetrachloro-3α,6α-diphenylglycoluril. Biochem Biophys Res Commun. 1978;80:849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- 34.Blackman MJ. Purification of Plasmodium falciparummerozoites for analysis of the processing of merozoite surface protein–1. Methods Cell Biol. 1994;45:213–220. doi: 10.1016/s0091-679x(08)61853-1. [DOI] [PubMed] [Google Scholar]
- 35.Blackman MJ, Holder AA. Use of a recombinant baculovirus product to measure naturally-acquired human antibodies to disulphide-constrained epitopes on the P. falciparummerozoite surface protein–1 (MSP1) FEMS Immunol Med Microbiol. 1993;6:307–316. doi: 10.1111/j.1574-695X.1993.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 36.Blackman MJ, Ling IT, Nicholls SC, Holder AA. Proteolytic processing of the Plasmodium falciparummerozoite surface protein–1 produces a membrane-bound fragment containing two epidermal growth factor–like domains. Mol Biochem Parasitol. 1991;49:29–34. doi: 10.1016/0166-6851(91)90127-r. [DOI] [PubMed] [Google Scholar]
- 37.Chappel JA, Holder AA. Monoclonal antibodies that inhibit Plasmodium falciparum invasion in vitrorecognise the first growth factor–like domain of merozoite surface protein–1. Mol Biochem Parasitol. 1993;60:303–312. doi: 10.1016/0166-6851(93)90141-j. [DOI] [PubMed] [Google Scholar]
- 38.Wilson CF, Anand R, Clark JT, McBride JS. Topography of epitopes on a polymorphic antigen of Plasmodium falciparumdetermined by the binding of monoclonal antibodies in a two-site radioimmunoassay. Parasite Immunol (Oxf) 1987;9:737–746. doi: 10.1111/j.1365-3024.1987.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 39.Dvorak JA, Miller LH, Whitehouse WC, Shiroishi T. Invasion of erythrocytes by malaria merozoites. Science (Wash DC) 1975;187:748–750. doi: 10.1126/science.803712. [DOI] [PubMed] [Google Scholar]
- 40.Stafford WH, Günder B, Harris A, Heidrich H-G, Holder AA, Blackman MJ. A 22 kDa protein associated with the Plasmodium falciparummerozoite surface protein–1 complex. Mol Biochem Parasitol. 1996;80:159–169. doi: 10.1016/0166-6851(96)02696-5. [DOI] [PubMed] [Google Scholar]
- 41.Riley EM, Allen SJ, Wheeler JG, Blackman MJ, Bennett S, Takacs B, Schonfelds H-J, Holder AA, Greenwood BM. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen of Plasmodium falciparumare associated with reduced malaria morbidity. Parasite Immunol (Oxf) 1992;14:321–337. doi: 10.1111/j.1365-3024.1992.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 42.Egan AF, Morris J, Barnish G, Allen S, Greenwood BM, Kaslow DC, Holder AA, Riley EM. Clinical immunity to Plasmodium falciparummalaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J Infect Dis. 1996;173:765–769. doi: 10.1093/infdis/173.3.765. [DOI] [PubMed] [Google Scholar]
- 43.Chappel JA, Egan AF, Riley EM, Druilhe P, Holder AA. Naturally acquired human antibodies which recognize the first epidermal growth factor–like module in the Plasmodium falciparummerozoite surface protein 1 do not inhibit parasite growth in vitro. Infect Immun. 1994;62:4488–4494. doi: 10.1128/iai.62.10.4488-4494.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holder AA, Blackman MJ. What is the function of MSP-1 on the malaria merozoite? . Parasitol Today. 1994;10:182–184. doi: 10.1016/0169-4758(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 45.Miller LH, Good MF, Kaslow DC. The need for assays predictive of protection in development of malaria bloodstage vaccines. Parasitol Today. 1997;13:46–47. doi: 10.1016/s0169-4758(96)20063-8. [DOI] [PubMed] [Google Scholar]
- 46.Kumar S, Yadava A, Keister DB, Tian JH, Ohl M, Perdue-Greenfield KA, Miller LH, Kaslow DC. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein–1 in Aotusmonkeys. Mol Med. 1995;1:325–332. [PMC free article] [PubMed] [Google Scholar]
- 47.Chang SP, Case SE, Gosnell WL, Hashimoto A, Kramer KJ, Tam LQ, Hashiro CQ, Nikaido CM, Gibson HL, Lee-Ng CT, et al. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotusmonkeys against malaria. Infect Immun. 1996;64:253–261. doi: 10.1128/iai.64.1.253-261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanabe K, Mackay M, Goman M, Scaife JG. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. . J Mol Biol. 1987;195:273–287. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]