Abstract
Cytotoxic T lymphocyte antigen 4 (CTLA-4) is an important regulator of T cell homeostasis. Ligation of this receptor leads to prominent downregulation of T cell proliferation, mainly as a consequence of interference with IL-2 production. We here report that CTLA-4 engagement strikingly selectively shuts off activation of downstream T cell receptor (TCR)/CD28 signaling events, i.e., activation of the microtubule-associated protein kinase (MAPKs) ERK and JNK. In sharp contrast, proximal TCR signaling events such as ZAP70 and TCR-ζ chain phosphorylation are not affected by CTLA-4 engagement on activated T cells. Since activation of the ERK and JNK kinases is required for stimulation of interleukin (IL)-2 transcription, these data provide a molecular explanation for the block in IL-2 production imposed by CTLA-4.
Activated T cells are subject to a number of downregulatory mechanisms designed to maintain T cell homeostasis, and several receptors involved in this process have been identified. Among these are the CD95 and the TNF receptor which both trigger apoptotic pathways (1, 2). Recently, it has become clear that CTLA-4 also downregulates T cell responses (for reviews see references 3, 4). Antibodies to CTLA-4 can, when crosslinked, prevent proliferation and IL-2 production of T cells stimulated through the TCR, whereas (blocking) Fab fragments of anti–CTLA-4 can actually enhance T cell responses (5–8). The physiological relevance of these findings is dramatically demonstrated by the phenotype of mutant mice lacking CTLA-4; such mice develop severe lymphoproliferative disease and massive lymphocytic infiltration and tissue destruction that is lethal by 3–4 wk of age (9, 10). The potential of CTLA-4 as a negative regulatory receptor is also illustrated by the recent findings that in vivo blockage of CTLA-4 retards the growth of an immunogenic tumor, implying augmented T cell–mediated antitumor immunity (11). Moreover, similar in vivo blockage of CTLA-4 has been found to markedly exacerbate disease in mice induced to develop experimental allergic encephalytis (EAE)1 (12).
CTLA-4 function is regulated by engagement with its ligands CD80 and CD86 on antigen-presenting cells (13). These molecules also regulate the function of CD28, a receptor promoting T cell activation and persistence of T cell responses by enhancing IL-2 production and expression of survival factors (for review see reference 4). How the same ligands can induce such opposite processes in T cells depending on the receptor engaged may be explained by the different expression patterns of CD28 and CTLA-4. Although CD28 is expressed constitutively on all T cells, CTLA-4 is expressed only after activation, reaching its peak after 48 h (6, 14–16). These expression patterns suggest that during the initial phase of T cell activation, CD28 may dominate the response to CD80/CD86. At later times after activation, CD80/CD86 molecules might downregulate the response by engaging CTLA-4. However, CTLA-4 can already function during the first 24 h of activation as demonstrated by antibody cross-linking studies, suggesting that CTLA-4 may also play a role in setting a threshold for activation (7).
Two nonmutually exclusive models have been proposed for the mode of action of CTLA-4 (17). First, CTLA-4 may specifically antagonize CD28 function, either by competing for CD80/CD86 molecules and/or by actively blocking CD28 signal transduction. The finding that the inhibitory effects of cross-linked anti–CTLA-4 can be overcome, to some extent, by addition of high doses of anti-CD28 might be interpreted as support for this model (6). Alternatively, CTLA-4 might interfere with TCR signaling as suggested by the hyperactivity of kinases associated with the TCR such as Lck and Fyn, as well as hyperphosphorylation of TCR-ζ and ZAP70 in T cells from CTLA-4 knockout mice (18).
The present study was designed to gain insight into the mechanism(s) used by CTLA-4 for negative regulation of T cell responses by directly examining signal transduction associated with CTLA-4 triggering. Using preactivated T cells, we find that CTLA-4 coengagement with the antigen receptor and CD28 leads to a reduction in the activities of both jun NH2-terminal kinase (JNK) and extracellular signal-regulated-kinase 2 (ERK-2). Since ERK2 activity induced by TCR engagement alone (i.e., in the absence of CD28 triggering) was also blocked by CTLA-4 engagement, these data demonstrate that CTLA-4 interferes with TCR signal transduction independently of any possible effects on CD28-mediated events. However, anti-CD3–induced phosphorylation of TCR-ζ and of ZAP70 were found to be unaffected by CTLA-4 engagement. Thus, our data demonstrate that CTLA-4 imposes a block in TCR-mediated signal transduction downstream of these early events, but upstream of ERK2 and JNK. As these kinases play crucial roles in induction of IL-2 transcription (19–21), this finding provides a molecular explanation for the block in IL-2 production that results from CTLA-4 engagement.
Materials and Methods
Mice.
Lymph nodes were isolated from C57BL/6 mice (6–8-wk-old). The mice were bred at The Netherlands Cancer Institute (Amsterdam, The Netherlands) under specific pathogen-free conditions.
Media, Antibodies, and Other Reagents.
Iscove's modified Dulbecco's medium (GIBCO BRL, Paisley, UK) was supplemented with 10% FCS (BioWhittaker, Inc., Verviers, Belgium), 100 U/ml penicillin, 100 mg/ml streptomycin, and 2 × 10−5 M 2-mercaptoethanol (Merck, Darmstadt, Germany).
The following antibodies were used in this study: 145.2C11 (anti-CD3; reference 22) and M5/114 (anti-MHC II; American Type Culture Collection, Rockville, MD); 37.51 (anti-CD28; reference 23), UC10-4F10-11 (anti–CTLA-4; PharMingen, San Diego, CA); goat anti–hamster IgG (H + L; Pierce, Rockford, IL); 4G10 (antiphosphotyrosine; Upstate Biotechnology Inc., Lake Placid, NY); rabbit anti–mouse and swine anti–rabbit peroxidase-conjugated antibodies (DAKO, Glostrup, Denmark); rabbit polyclonal IgG anti–ERK2 and rabbit polyclonal IgG anti-JNK1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); 6B10.2.5 (anti–TCR-ζ–PO4) and 1E7.2 (anti–ZAP70) were provided by Drs. H. van Oers and A. Weiss.
Production and purification of glutathione-S-transferase (GST)– c-Jun (1–135) was performed as described (24). MBP was purchased from Sigma Chemical Co. (St. Louis, MO).
T Cell Stimulation.
T cells were purified from lymph node cell suspensions as follows. Nylon wool passed (NWP) lymph node cells were incubated with anti–class II mAb (M5/114). The NWP lymph node cells were depleted of antibody binding cells through magnetic bead depletion using goat anti–mouse Ig beads (Advanced Magnetics, Cambridge, MA) and sheep anti–rat Ig beads (Dynal, Oslo, Norway). T cell preparations were typically >98% pure by anti-CD3 staining.
To induce CTLA-4 expression, purified T cells (4 × 106 cells/ well) were preactivated in 24-well plates coated with anti-CD3 and anti-CD28 mAbs (10 μg/ml each). After 40 h, cells were harvested and then rested for 5 h in 10% FCS–containing medium at 37°C. Purified naive (fresh cells rested in vitro for 5 h in 10% FCS–containing medium at 37°C) or preactivated T cells were incubated in medium with anti-CD3, anti-CD28, and anti– CTLA-4 (each at 10 μg/ml) at the indicated combinations for 30 min on ice. Cells were washed twice with cold medium and goat anti–hamster antibody was added at 10 μg/ml in warm (37°C) medium. After this, cells were incubated for the indicated times at 37°C. The reactions were stopped by addition of ice-cold PBS after which the cells were spun down. Subsequently, cells were lysed as described below.
ERK2 and JNK Kinase Assays.
Cells were lysed for 30 min on ice with whole cell extract lysis buffer (25 mM Hepes at pH 7.7, 0.3 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% Triton X-100, 0.5 mM dithiothreitol [DTT], 20 mM β-glycerolphosphate, 0.1 mM Na3VO4, 2 μg/ml of leupeptin, and 1 mM of PMSF) (25). Lysates were cleared by centrifugation at 14,000 g for 10 min at 4°C. Protein concentration was determined using the bicinchininic acid protein assay (Pierce), and 40 μg of total protein/lane was used for immunoprecipitation. Whole cell extracts were diluted to get as final concentration of the buffer: 20 mM Hepes at pH 7.7, 75 mM NaCl, 2.5 mM MgCl2, 0.1 mM EDTA, 0.05% Triton X-100, 0.5 mM DTT, 20 mM β-glycerol phosphate, 0.1 mM Na3VO4, 2 μg/ml of leupeptin, and 1 mM of PMSF. For JNK precipitation, cell extracts were incubated overnight (o/n) at 4°C with 10 μg of GST–c-Jun bound to glutathione-agarose beads (Sigma Chemical Co.). Precipitates were washed 4 times with Hepes binding buffer (20 mM Hepes at pH 7.7, 50 mM NaCl, 2.5 mM MgCl2, 0.1 mM EDTA, and 0.05% Triton X-100), and subsequently resuspended in 30 μl of kinase buffer (20 mM Hepes at pH 7.6, 20 mM MgCl2, 20 mM β-glycerolphosphate, 20 mM paranitro phenyl phosphate, 0.1 mM Na3VO4, and 2 mM DTT) containing 20 μM cold ATP and 5 μCi of γ-[32P]ATP. After 20 min at 37°C the reaction was stopped by washing the beads 3 times with Hepes binding buffer. GST–c-Jun was eluted and resolved on 10% SDS-PAGE gel.
For ERK2 immunoprecipitation, the cell extracts were incubated o/n at 4°C with 1 μg anti-ERK2 bound to protein A–Sepharose beads (Pharmacia Biotech, Uppsala, Sweden). Immunoprecipitates were washed 3 times with 50 mM Tris, pH 7.5, 100 mM NaCl, 1 mM EGTA, 20 mM β-glycerolphosphate, 0.1 mM Na3VO4, and once with 20 mM Hepes of pH 7.5, 0.5 mM MnCl2, 10 mM MgCl2, 1 mM EGTA, 20 mM β-glycerolphosphate, and 0.1 mM Na3VO4. Kinase reactions were performed at 37°C in 30 μl of kinase buffer containing 20 mM Hepes of pH 7.5, 0.5 mM MnCl2, 10 mM MgCl2, 1 mM EGTA, 1 mM DTT, 0.3 mg/ml MBP, 25 μM cold ATP, and 5 μCi of γ-[32P]ATP. After 20 min, the reaction was finished by adding 5 μl of 5× sample buffer. Samples were boiled and resolved on 12.5% SDS-PAGE. Phosphorylation of GST–c-Jun and MBP was visualized and the radioactivity of each band quantified using a phosphorimager.
For ERK2 and JNK immunoblots, proteins from total lysates were resolved on 10% SDS-PAGE and transferred onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). Membranes were blocked o/n at 4°C with 10 mM Tris-HCl of pH 8.0, 150 mM NaCl, 0.2% Tween 20, and 10% nonfat milk (Profitar, Nutricia, Holland). After blocking, membranes were blotted with polyclonal rabbit anti-ERK2 or anti-JNK antibodies, respectively, followed by a swine anti–rabbit peroxidase-conjugated antibody. Blots were developed using enhanced chemiluminescence (Amersham Intl., Buckinghamshire, UK).
TCR-ζ Chain and ZAP70 Immunoprecipitation and Immunoblotting.
Preactivated T cells were lysed in lysis buffer containing 20 mM Tris-HCl of pH 7.6, 150 mM NaCl, 2 mM EDTA, and protease and phosphatase inhibitors. After 30 min of incubation on ice, lysates were cleared by centrifugation and incubated o/n at 4°C with anti-TCR-ζ chain or anti-ZAP70 antibodies bound to Sepharose–protein A beads. Beads were washed 3 times with lysis buffer and proteins eluted by boiling in 2× sample buffer. Proteins were resolved in SDS-PAGE gels, transferred to polyvinylidene difluoride membranes (Millipore, Bedford, Massachusetts), and blocked o/n in PBS, 0.2% Tween 20, and 1% BSA. Tyrosine phosphorylation was detected with antiphosphotyrosine antibody followed by rabbit anti–mouse peroxidase-conjugated secondary antibody. Blots were developed using enhanced chemiluminescence.
Results
ERK2 Activity Is Reduced upon CTLA-4 Engagement.
Studying the individual contributions to T cell activation of the CD28 and CTLA-4 receptors is complicated by the fact that both receptors are triggered by the same ligands, the CD80/CD86 molecules. However, the availability of mAbs to CD28 and CTLA-4 has made it possible to separately investigate the functions of these receptors. The inhibitory role of CTLA-4 in T cell activation has now been visualized in several studies (5–8). The block imposed by CTLA-4 engagement on T cell activation predominantly results from interference with IL-2 production (6, 8), resulting in inhibition of cell cycle progression.
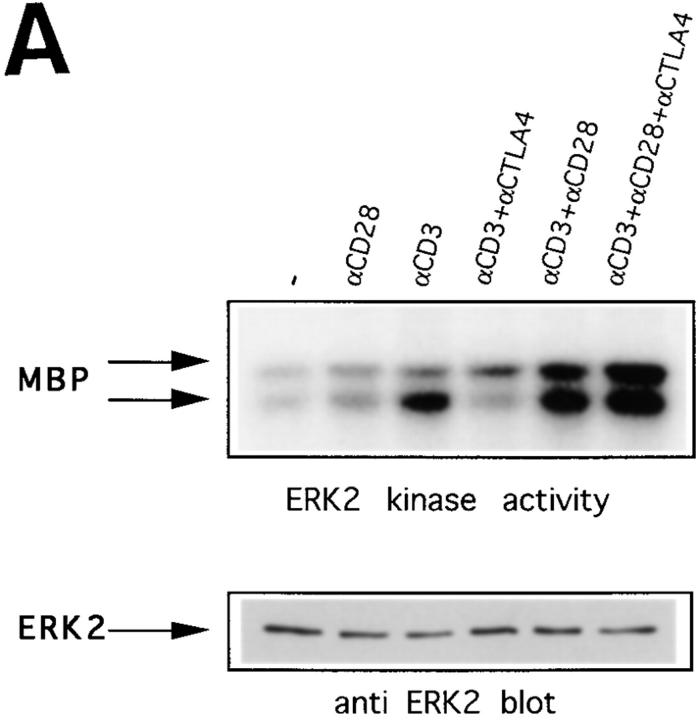
The ERK family of MAP kinases has been found to be required for stimulation of IL-2 transcription (19, 26). Therefore, we investigated whether the activity of ERK2 would be affected by CTLA-4 triggering. Because CTLA-4 has been reported to be expressed only after activation, peaking after 2 d (6), we first preactivated T cells purified from lymph nodes for 40 h on plates coated with anti-CD3 and anti-CD28. After harvesting, we incubated the preactivated cells in fresh medium for an additional 5 h, as we observed that immediately after the 40-h activation period, ERK2 and JNK were too strongly activated to allow visualization of induction by TCR triggering (data not shown). After this 5-h incubation, cells were stimulated with different combinations of antibodies as indicated, and lysed. In vitro kinase reactions were performed with anti-ERK2 immunoprecipitates from these lysates. As has been shown previously (20, 27), efficient ERK2 activation can be achieved by triggering the TCR alone (Fig. 1 A). However, in lysates derived from cells on which CTLA-4 is coengaged together with the antigen receptor, ERK2 activity is almost abrogated. Shown here are the results obtained with cells lysed 1 min after cross-linking the antibodies. In most experiments at this time point, anti-CD3–induced ERK2 activity has reached its maximum, being undetectable by 5 min after stimulation. However, in experiments in which anti-CD3– induced ERK2 activity persisted longer, this activity was still reduced by CTLA-4 engagement at 5 min after triggering (data not shown). The pronounced anti–CTLA-4–induced reduction in ERK2 activity in preactivated cells is contrasted with results in freshly isolated T cells in which no effect of addition of anti–CTLA-4 on ERK2 activity was observed (Fig. 1 B). These findings are consistent with the reported requirement for activation of CTLA-4 expression.
Figure 1.
CTLA-4 inhibits ERK2 activity induced by anti-CD3 in preactivated T cells, but not in naive T cells. (A) 2-d preactivated T cells were coated on ice with the indicated combinations of anti-CD3, anti-CD28, and anti–CTLA-4. 1 min after addition of cross-linking anti– hamster antibody (10 μg/ml) in warm (37°C) medium the cells were lysed, ERK2 was immunoprecipitated, and in vitro kinase reactions were performed using MBP as substrate in the presence of γ-[32P]ATP. Indicated by arrows are the bands representing phosphorylated MBP (top). The same lysates were tested for equal protein abundance on immunoblot (bottom). (B) Naive purified T cells were rested for 5 h and coated with the different combinations of antibodies as indicated. Cells were lysed 1 or 5 min after addition of warm cross-linking antibody and in vitro kinase reactions were performed as in A.
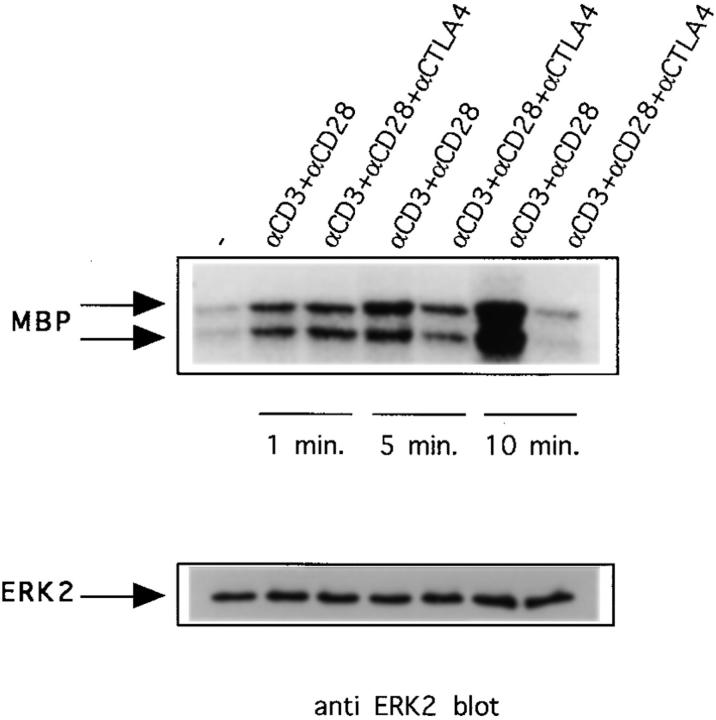
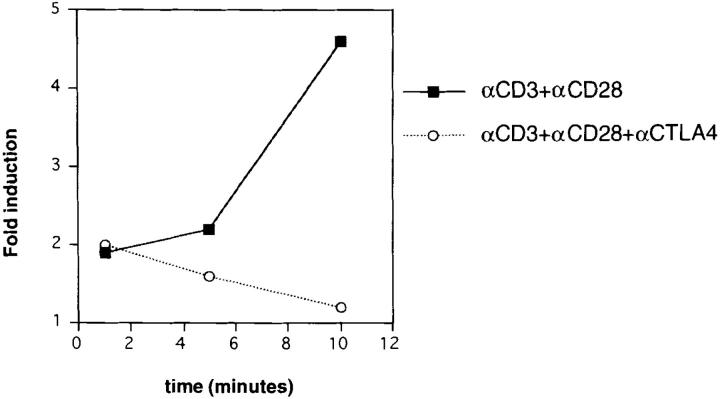
In contrast to earlier published results that suggest that ERK2 activity is not affected by CD28 triggering (20, 27), we consistently found that in cells stimulated with anti-CD3 and anti-CD28, ERK2 activity is somewhat higher (Fig. 1). Moreover, we found that ERK2 activity induced in the presence of anti-CD28 usually persisted longer than ERK2 activity induced by TCR triggering alone, reaching its peak by 10 min after activation (Fig. 2). Interestingly, an effect of CD28 signaling on ERK2 activity is also revealed by the finding that 1 min after stimulation the inhibition of ERK2 activity/activation by CTLA-4 is prevented by anti-CD28 (compare Figs. 1 A and 2). At later time points, however, anti-CD3– and anti-CD28–induced ERK2 activity is also downregulated by CTLA-4 (Fig. 2) with almost complete inhibition at 10 min. Together, these results demonstrate that CTLA-4 on preactivated T cells downregulates TCR-induced ERK2 activity, either by interfering with activation of this kinase or by directly modulating ERK2 kinase activity.
Figure 2.
Combined triggering of CD3 and CD28 delays downregulation of ERK2 activity by CTLA-4. Preactivated T cells were coated with different combinations of antibodies as in Fig. 1 and lysed 1, 5, or 10 min after addition of warm cross-linking antibody. ERK2 was immunoprecipitated and in vitro kinase reactions were performed (top). The same lysates were tested for ERK2 protein abundance (middle). ERK2 activities were quantified using a phosphorimager and represented as relative values compared to ERK2 activities from unstimulated cells (unstimulated = 1; bottom).
JNK Activity Is Reduced upon CTLA-4 Engagement.
Like the ERKs, the JNK family of MAP kinases have been implicated in induction of IL-2 gene transcription, as these enzymes phosphorylate the NH2 terminus of c-Jun and thereby enhance its transcriptional activity (20, 21, 25, 28, 29). We therefore tested whether the activity of JNK might also be regulated by CTLA-4. JNKs bind with high affinity to c-Jun, such that these kinases can be precipitated from cell lysates with immobilized GST–c-Jun coupled to beads (25), after which in vitro kinase reactions can be performed. It has been shown in both Jurkat T cells and in T cell clones that activation of JNKs by TCR triggering is dependent on costimulation (20, 27). Indeed, we find that also in these preactivated primary T cells, JNK activity is only induced if CD3 triggering is accompanied by CD28 engagement (Fig. 3). Cocross-linking with anti–CTLA-4 results in a clear reduction in JNK activity, demonstrating that CTLA-4 also interferes with the activation or activity of these kinases involved in induction of IL-2 transcription. Whether this reflects a direct effect of CTLA-4 on JNK pathway components or is a consequence of CTLA-4– induced abrogation of TCR signaling remains to be investigated.
Figure 3.
CTLA-4 reduces JNK activity induced by CD3 and CD28 triggering. Preactivated T cells were coated with different combinations of antibodies and lysed 10 min after addition of warm cross-linking antibody. Lysates were tested for JNK activity by precipitation with GST–c-jun precoupled to glutathione beads followed by in vitro kinase reactions as described in Materials and Methods. Phosphorylated GST–c-jun is indicated by the arrow (top). The same lysates were tested for JNK protein abundance on immunoblot (middle). JNK activities were quantified using a phosphorimager and represented as relative values compared to JNK activity from unstimulated cells (unstimulated = 1; bottom). Data for the 10-min time point are shown because no JNK activity could be measured at earlier times.
CTLA-4 Does Not Affect Early Parameters of TCR Triggering.
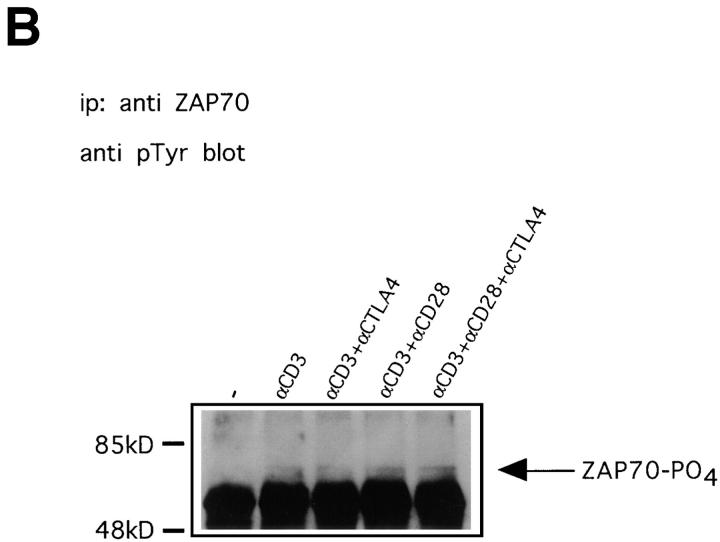
The data presented above demonstrate that CTLA-4 imposes a block in signal transduction upstream or at the level of ERK2 and JNK. Furthermore, these findings document that CTLA-4 has an effect on signal transduction events induced by TCR triggering independent of CD28 engagement, as ERK2 activity induced by anti-CD3 alone was reduced upon coengagement of CTLA-4 (Fig. 1 A). A role for CTLA-4 counteracting early activation events associated with TCR signal transduction is also suggested by findings in T cells derived from CTLA-4–deficient mice. Thus, in CTLA-4−/− cells, proteins such as TCR-ζ and ZAP70 are constitutively hyperphosphorylated (18). For these reasons, we directly investigated whether anti-CD3– induced TCR-ζ phosphorylation and ZAP70 phosphorylation are affected by CTLA-4 triggering.
The TCR-ζ chain contains three so-called ITAMs (immunoreceptor tyrosine based activation motif) sequences that are necessary for coupling the TCR to the intracellular signaling machinery (30–32). Upon TCR activation, these motifs become phosphorylated on tyrosine residues as a consequence of which the electrophoretic mobility of the protein changes (33, 34). We immunoprecipitated TCR-ζ from lysates obtained as described above using an antibody specific for phosphorylated TCR-ζ. After immunoblotting the precipitates with an antibody against phosphotyrosine, bands of 21 kD and p23 kD were visualized, representing different phosphorylated isoforms of TCR-ζ (Fig. 4 A; reference 34). Although the 21-kD band was also present in unstimulated cells as previously reported for fresh lymph node T cells (35, 36), the p23 band specifically appeared after CD3 triggering. Importantly, at none of the time points examined was the intensity of either one of these two phospho-ζ bands altered upon coengagement of CTLA-4 with CD3 (Fig. 4 A). Yet, in these same cells, ERK2 activity was again abrogated by CTLA-4 engagement (data not shown). We conclude that ζ-phosphorylation is not affected by CTLA-4.
Figure 4.
Anti-CD3–induced phosphorylation of TCR-ζ and ZAP70 is not affected by CTLA-4. (A) Preactivated T cells were coated with different combinations of antibodies and lysed 1, 5, or 10 min after addition of warm cross-linking antibody. TCR-ζ was immunoprecipitated and immunoblotted with antiphosphotyrosine antibody 4G10. Indicated with arrows are the p21 and p23 isoforms of tyrosine phosphorylated TCR-ζ. (B) Lysates prepared as in A from cells stimulated for 1 min were immunoprecipitated with anti-ZAP70 antibody and immunoblotted with antiphosphotyrosine antibody 4G10. Indicated with an arrow is phosphorylated ZAP70. Similar results were also obtained on lysates from 5 and 10 min–stimulated cells (not shown).
Phosphorylation of TCR-ζ allows binding of ZAP70 via its SH2 domains (37). Moreover, the recruited ZAP70 molecules become phosphorylated on tyrosine residues shortly after TCR triggering, leading to activation of this kinase (36, 38, 39). To investigate potential effects of CTLA-4 on ZAP70 phosphorylation, we immunoprecipitated this kinase and immunoblotted with an antiphosphotyrosine antibody. As shown in Fig. 4 B, phosphorylation of ZAP70 at 1 min after stimulation was not affected by CTLA-4 engagement. Similarly, we found no changes in the phosphorylation state of this kinase at later time points (data not shown). Together, these data strongly suggest that the block imposed by CTLA-4 resulting in decreased ERK and JNK activity lies downstream of the TCR proximal events that result in phosphorylation of TCR-ζ and ZAP70.
Discussion
CTLA-4 is an important regulator of peripheral T cell homeostasis by virtue of its ability to negatively regulate T cell expansion. Although the functional consequences of CTLA-4 engagement have been studied extensively in vitro and in vivo (3), very little is known about the signaling events resulting from ligation of this receptor. Some investigators have reported a receptor-ligation–inducible association of PI3 kinase with the cytoplasmic tail of CTLA-4 (40). However, as the same kinase also binds to the cytoplasmic tail of CD28 (41), it seems unlikely that association of this kinase with CTLA-4 would explain its inhibitory effects on induction of T cell proliferation. It has also been suggested that the role of PI3 kinase binding to CTLA-4 may be to regulate transport of this receptor, most of which is localized in intracellular vesicles, to the cell surface (42– 44). Other investigators have found that a phosphatase termed Syp (also known as SHP2 or PTP1D) can be coimmunoprecipitated with CTLA-4 (18). Furthermore, it was shown that these precipitates could dephosphorylate the adapter protein Shc (see below) in vitro. In apparent agreement with a role for a phosphatase in the function of CTLA-4, these same investigators showed that in T cells derived from CTLA-4–deficient mice, the kinases Fyn, Lck, and ZAP70 were hyperactivated (18). Also, other proteins such as TCR-ζ and Shc, were constitutively hyperphosphorylated in CTLA-4−/− mice (18). However, rather than being a direct consequence of the absence of CTLA-4, such observations might also result from the hyperactivated state of T cells in CTLA-4–deficient mice (9, 10).
In the present study, we used preactivated T cells to directly investigate how CTLA-4 engagement influences signal transduction events associated with T cell activation. We found that antigen receptor and CD28-mediated activation of the MAP kinases ERK2 and JNK is impeded upon coengagement of CTLA-4. In sharp contrast, phosphorylation of TCR-ζ and ZAP70 were unaffected by CTLA-4 triggering. CTLA-4–induced T cell inactivation is thus linked to defective ERK and JNK activation, and occurs downstream of immediate TCR signaling events.
It has been suggested that one way in which CTLA-4 may function might be by counteracting the consequences of CD28 signaling (17). This proposal resulted from the observation that high doses of an antibody to CD28 can, at least to some extent, rescue T cell proliferation from anti– CTLA-4–induced inhibition (6). The present data, however, unequivocally demonstrate that CTLA-4 interferes with signal transduction events induced by the TCR independent of CD28 triggering. These findings are therefore consistent with the hypothesis that CTLA-4 interferes directly with downstream TCR signaling events. In addition, CTLA-4 may affect signal transduction through CD28, as suggested by the reduction in JNK activity induced by anti– CTLA-4. Whether this reflects a direct or indirect effect remains to be investigated. The “rescuing” effects of anti-CD28 on T cell proliferation may in fact be the result of modulation of TCR signaling by CD28. In support of this possibility, we found anti-CD28 to affect both the strength and the persistence of ERK2 activity induced by TCR signaling. Indeed, it also prevented the blockade of TCR-induced ERK2 activity by CTLA-4 at early time points after activation. Although at later time points the CTLA-4–induced block in ERK2 activity did manifest itself despite CD28 engagement, it is conceivable that at lower concentrations of anti–CTLA-4 or higher concentrations of anti-CD28, rescuing effects from CD28 became dominant. A dominance of CD28 over CTLA-4 might be relevant in situations in which CTLA-4 expression on T cells is still low and CD80/CD86 expression is relatively high.
In view of a recent suggestion (45) that CTLA-4 can actually also provide positive costimulatory signals in T cell activation, it will be interesting to compare the signaling pathways emanating from TCR and CTLA-4 under conditions that visualize either positive or negative effects of CTLA-4 engagement. Our experiments were performed under conditions in which CTLA-4 engagement results in an abrogation of IL-2 production, and such findings have been documented by many laboratories (for review see references 3, 17). A role for CTLA-4 in blocking IL-2 production is also consistent with the phenotype of CTLA-4−/− mice (9, 10). Nevertheless, a positive regulatory effect of CTLA-4 may, under other conditions (45), dominate the response, although the latter studies (45) did not exclude an adhesive interaction between CTLA-4 and CD80, allowing other costimulatory receptors to exert their effect.
Another issue that remains to be resolved is to which extent the antibody-mediated cross-linking of TCR/CD28/ CTLA-4 used in this study truly reflect physiological, ligand-triggered events. An example of the different outcome between antibody- and ligand-mediated triggering was revealed some years ago by the Cantrell laboratory (ICRF, London, UK); although antibody-mediated triggering of CD28 resulted in p21ras activation, CD80-mediated triggering did not (46). An alternative explanation for those observations, however, might be that the CD80 ligand triggered both CD28 and CTLA-4, and actively abrogated p21ras activation. Antibodies to CD28, on the other hand, would allow activation of p21ras to be revealed. Until reciprocal mutants of CD80 or CD86 become available that selectively bind to either CD28 or CTLA-4, the issue of whether the anti–CTLA-4 triggered inhibitory effects on downstream TCR signaling events mimic ligand-induced events remains to be tested. It should be kept in mind, however, that the observed modulatory effect of anti–CTLA-4 on the MAPKs are consistent with the results of earlier functional studies (5–12) both in vitro and in vivo.
The inhibition of T cell proliferation by CTLA-4 engagement can be overcome by addition of IL-2 (8; data not shown). This suggests that the blockade of proliferation by CTLA-4 is predominantly a consequence of its interference with IL-2 production. The data presented here provide a molecular explanation for this interference, since both the ERKs and the JNKs are involved in induction of IL-2 gene transcription. A well documented role for the ERKs is phosphorylation of ternary complex factor (TCF)/Elk-1 (47–49), stimulating it to activate c-fos transcription. JNKs, on the other hand, enhance the transcriptional activity of c-Jun by NH2-terminal phosphorylation (25). Together, Fos and Jun proteins form AP1 complexes that bind to a functionally important AP1 site in the IL-2 promotor and, in addition, participate in formation of NFAT and NF–IL-2 (50–53). Transfection studies have established that, at least in transformed T cell lines, ERK activity is indeed required for IL-2 promotor–driven gene transcription (19). Furthermore, JNK activity also appears to be required, since transfected Jun mutants that cannot be phosphorylated inhibit IL-2 promotor activity in luciferase reporter assays (20).
Although in theory inhibition of either pathway alone might be sufficient to severely restrict IL-2 production, inhibition of both pathways probably results in a more pronounced block. The simplest explanation for how both of these kinases are inhibited would be that CTLA-4 inhibits a target that is commonly involved in turning on both cascades. As TCR triggering is required for activation of ERKs as well as of JNKs (20, 27, and this paper), this target would have to be activated by TCR triggering alone and lie downstream of TCR-ζ phosphorylation. In this respect, it would be attractive to speculate that CTLA-4 inhibits TCR signal transduction at the level of or upstream of p21ras. It is well established that in many cell types, including T cells, p21ras activates and is required for activation of ERK1 and ERK2 (54–56). Moreover, although Ras activity alone is insufficient to activate JNK in T cells, it has been reported that overexpression of a gene encoding dominant negative Ras does inhibit JNK activation (21). Consistent with an effect on Ras, others have observed that anti-Syp as well as anti– CTLA-4 immunoprecipitates could, in vitro, dephosphorylate the adaptor protein Shc (18), a protein that is used by various receptor tyrosine kinases for activation of p21ras via its association with Grb2–Sos complexes (57–60). Although the in vivo relevance of this in vitro dephosphorylation activity still remains to be established, these data might provide an explanation for the results presented here by us. However, there is some controversy about whether Shc is in fact involved in TCR-mediated Ras activation. Thus, although T cell hybridomas complexes of Shc-Grb2-SOS complexes were found after TCR cross-linking (61), such complexes could not be detected in TCR-activated peripheral blood T cells (62). Instead, in these latter cells, Grb-2 and Sos, were found complexed with another adapter of ∼36–38 kD, possibly the recently cloned p36lnk (63, 64). Furthermore, additional mechanisms, not involving Grb2– Sos, exist for TCR-mediated Ras activation in T cells, including inhibition of Ras GTPase–activating proteins (Ras-GAP) as well as PKC-mediated regulation (65, 66). Finally, in signaling from several receptor tyrosine kinases, the phosphatase Syp has actually been reported to stimulate rather than inhibit growth factor–induced ras and MAPK activation (67, 68). Thus, it is unclear whether possible effects from CTLA-4–associated Syp would provide a satisfactory explanation for the inhibition of ERK and JNK activation we here report. Nevertheless, involvement of a phosphatase seems conceptually appealing, given the role of phosphatases in inhibitory signaling by NK receptors and FcγRIIb (69).
Modulation of CTLA-4 activity promises to be a powerful option for manipulation of immune responses. The possibilities for this modulation are currently confined to the use of anti–CTLA-4 antibodies. Therefore, a more detailed understanding is required of the mechanisms used by this receptor for its downregulation of IL-2 production. Possibly a molecular understanding of CTLA-4 action may offer novel opportunities for downregulating undesired responses or stimulating useful ones. In the present study, we have shown that the molecular target(s) for CTLA-4–mediated interference with IL-2 production lie(s) upstream of ERK and JNK, but downstream of TCR-ζ. Identification of this target will be a major focus for the future.
Acknowledgments
We gratefully acknowledge Dr. N.S.C. van Oers and Dr. A. Weiss for providing anti–TCR-ζ and ZAP70 antibodies. Furthermore, we thank Dr. W. Li for providing technical advise on JNK and ERK kinase assays, Dr. P.L. Hordijk for GST–c-jun transformed bacteria, Drs. K.A. Reedquist, J.L. Bos, and J. Sen for advice, and Drs. J. Borst, T.N.M. Schumacher, and L. Smit for critically reading the manuscript. Finally, we thank Ms. Marie Anne van Halem for preparing the manuscript.
Footnotes
C.R. Calvo was supported by a grant from the Coordinacion de la Investigacíon, nr. 94/5735, Ministerio Sanidad y Consuma, Madrid, Spain.
C.R. Calvo and D. Amsen contributed equally to this work.
Abbreviations used in this paper: EAE, experimental allergic encephalytis; ERK2, extracellular-signal-regulated kinase 2; GST, glutathione-S-transferase; JNK, jun NH2-terminal kinase; o/n, overnight.
References
- 1.Abbas AK. Die and let live: eliminating dangerous lymphocytes. Cell. 1996;84:655–658. doi: 10.1016/s0092-8674(00)81042-9. [DOI] [PubMed] [Google Scholar]
- 2.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumour necrosis. Nature (Lond) 1995;377:348–352. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 3.Tivol EA, Schweitzer AN, Sharpe AH. Costimulation and autoimmunity. Curr Opin Immunol. 1996;8:822–830. doi: 10.1016/s0952-7915(96)80011-2. [DOI] [PubMed] [Google Scholar]
- 4.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 5.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 6.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tivol EA, Borriello F, Schweitzer AN, Lunch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 10.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Riesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science (Wash DC) 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 11.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science (Wash DC) 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 12.Perrin PJ, Maldonado H, Davis TA, June CH, Racke MK. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J Immunol. 1996;157:1333–1336. [PubMed] [Google Scholar]
- 13.Linsley PS, Greene JL, Brady W, Bayorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 14.Linsley PS, Greene JL, Tan P, Bradshaw J, Ledbetter JA, Anasetti C, Damle NK. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med. 1992;176:1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindsten T, Lee KP, Harris ES, Petryniak B, Craighead N, Reynolds PJ, Lombard DB, Freeman GJ, Nadler LM, Gray GS, Thompson CB, June CH. Characterization of CTLA-4 structure and expression on human T cells. J Immunol. 1993;15:3489–3499. [PubMed] [Google Scholar]
- 16.Perkins D, Wang Z, Donovan C, He H, Mark D, Guan G, Want Y, Walunas T, Bluestone J, Listman J, Finn PW. Regulation of CTLA-4 expression during T cell activation. J Immunol. 1996;156:4154–4159. [PubMed] [Google Scholar]
- 17.Bluestone JA. Is CTLA-4 a master switch for peripheral T cell tolerance? . J Immunol. 1997;158:1989–1993. [PubMed] [Google Scholar]
- 18.Marengère LEM, Waterhouse P, Duncan GS, Mittrücker H-W, Feng G-S, Mak TW. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science (Wash DC) 1996;272:1170–1174. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- 19.Whitehurst CE, Geppert TD. MEK1 and the extracellular signal–regulated kinases are required for the stimulation of IL-2 gene transcription in T cells. J Immunol. 1996;156:1020–1029. [PubMed] [Google Scholar]
- 20.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y , JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 21.Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996;8:402–411. doi: 10.1016/s0952-7915(96)80131-2. [DOI] [PubMed] [Google Scholar]
- 22.Leo O, Foo M, Sachs DH, Samelson LE. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci USA. 1987;84:1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gross JA, Callas E, Allison JP. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992;149:380–382. [PubMed] [Google Scholar]
- 24.Smith DB, Johnson KS. Single step purification of polypeptides expressed in Escherichia colias fusions with glutathione S–transferase. Gene (Amst) 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 25.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 26.Owaki H, Varma R, Gillis B, Bruder JT, Rapp UR, Davis LS, Geppert TD. Raf-1 is required for T cell IL-2 production. EMBO (Eur Mol Biol Organ) J. 1993;12:4367–4373. doi: 10.1002/j.1460-2075.1993.tb06121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Wahley CD, Mondino A, Mueller DL. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+T cells. Science (Wash DC) 1996;271:1272–1275. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 28.Derijard BB, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1, a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1027. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 29.Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of c-Jun kinases. Nature (Lond) 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 30.Reth M. Antigen receptor tail clue. Nature (Lond) 1989;338:383–384. [PubMed] [Google Scholar]
- 31.Weiss A. T cell antigen receptor signal transduction: a tale of tails and cytoplasmic protein–tyrosine kinases. Cell. 1993;73:209–212. doi: 10.1016/0092-8674(93)90221-b. [DOI] [PubMed] [Google Scholar]
- 32.Borst J, Brouns GS, de Vries E, Verschuren MCM, Mason DY, van Dongen JJM. Composition and function of T-cell receptor and B-cell receptor complexes on precursor lymphocytes. Immunol Rev. 1996;8:181–190. doi: 10.1016/s0952-7915(96)80056-2. [DOI] [PubMed] [Google Scholar]
- 33.Koyasu S, McConkey DJ, Clayton LK, Abraham S, Yandava B, Katagriri T, Moingeon P, Yamamoto P, Reinherz EL. Phosphorylation of multiple CD3ζ tyrosine residues leads to formation of pp21 in vitro and in vivo. . J Biol Chem. 1992;267:3375–3381. [PubMed] [Google Scholar]
- 34.Qian D, Griswold-Prenner I, Rosner MR, Fitch FW. Multiple components of the T cell antigen receptor complex become tyrosine-phosphorylated upon activation. J Biol Chem. 1993;268:4488–4493. [PubMed] [Google Scholar]
- 35.Van Oers NSC, Tao W, Watts JD, Johnson P, Aebersold R, Teh H-S. Constitutive tyrosine phosphorylation of the T-cell receptor (TCR) ζ subunit: regulation of TCR-associated protein tyrosine kinase activity by TCR ζ. Mol Cell Biol. 1993;13:5771–5780. doi: 10.1128/mcb.13.9.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Oers NSC, Killeen N, Weiss A. ZAP-70 is constitutively associated with tyrosine-phosphorylated TCR ζ in murine thymocytes and lymph node T cells. Immunity. 1994;1:675–685. doi: 10.1016/1074-7613(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 37.Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: A 70 kd protein–tyrosine kinase that associates with the TCR ζ chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 38.Chan AC, Dalton M, Johnson R, Kong GH, Wang T, Thoma R, Kurosaki T. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO (Eur Mol Biol Organ) J. 1995;14:2499–2508. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wange RL, Guitián R, Isakov N, Watts JD, Aebersold R, Samelson LE. Activating and inhibitory mutations in adjacent tyrosines in the kinase domain of ZAP-70. J Biol Chem. 1995;270:18730–18733. doi: 10.1074/jbc.270.32.18730. [DOI] [PubMed] [Google Scholar]
- 40.Schneider H, Prasad KVS, Shoelson SE, Rudd CE. CTLA-4 binding to the lipid kinase phosphatidylinositol 3-kinase in T cells. J Exp Med. 1995;181:351–356. doi: 10.1084/jem.181.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudd CE. Upstream-downstream: CD28 cosignaling pathways and T cell function. Immunity. 1996;4:527–634. doi: 10.1016/s1074-7613(00)80479-3. [DOI] [PubMed] [Google Scholar]
- 42.Leung HT, Bradshaw J, Cleaveland JS, Linsley PS. Cytotoxic T lymphocyte–associated molecule-4, a high-avidity receptor for CD80 and CD86, contains an intracellular localization motif in its cytoplasmic tail. J Biol Chem. 1995;270:25107–25114. doi: 10.1074/jbc.270.42.25107. [DOI] [PubMed] [Google Scholar]
- 43.Linsley PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4:535–543. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- 44.Hutchcroft JE, Bierer BE. Signaling through CD28/CTLA-2 family receptors. Puzzling participation of phosphatidylinositol-3 kinase. J Immunol. 1996;156:4071–4074. [PubMed] [Google Scholar]
- 45.Wu Y, Guo Y, Huang A, Zheng P, Liu Y. CTLA-4-B7 interaction is sufficient to costimulate T cell clonal expansion. J Exp Med. 1997;185:1327–1335. doi: 10.1084/jem.185.7.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nunès JA, Collete Y, Truneh A, Olive D, Cantrell DA. The role of p21ras in CD28 signal transduction: triggering of CD28 with antibodies, but not the ligand B7-1, activates p21ras . J Exp Med. 1994;180:1067–1076. doi: 10.1084/jem.180.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 48.Hipskind RA, Büscher D, Nordheim A, Baccarini M. Ras/MAP kinase-dependent and -independent signaling pathways target distinct ternary complex factors. Genes Dev. 1994;8:1803–1816. doi: 10.1101/gad.8.15.1803. [DOI] [PubMed] [Google Scholar]
- 49.Hill CS, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- 50.Crabtree GR. Contingent genetic regulatory events in T lymphocyte activation. Science (Wash DC) 1989;243:355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- 51.Jain J, McCaffrey PG, Valge-Archer VE, Rao A. Nuclear factor of activated T cells contains Fos and Jun. Nature (Lond) 1992;356:801–804. doi: 10.1038/356801a0. [DOI] [PubMed] [Google Scholar]
- 52.Ullman KS, Northrop JP, Admon A, Crabtree GR. Jun family members are controlled by a calcium-regulated, cyclosporin A–sensitive signaling pathway in activated T lymphocytes. Genes Dev. 1993;7:188–196. doi: 10.1101/gad.7.2.188. [DOI] [PubMed] [Google Scholar]
- 53.Northrop JP, Ullman KS, Crabtree GR. Characterization of the nuclear and cytoplasmic components of the lymphoid-specific nuclear factor of activated T cells (NF-AT) complex. J Biol Chem. 1993;268:2917–2923. [PubMed] [Google Scholar]
- 54.Rayter SI, Woodrow M, Lucas SC, Cantrell DA, Downward J. P21ras mediates control of IL-2gene promoter function in T cell activation. EMBO (Eur Mol Biol Organ) J. 1992;11:4549–4556. doi: 10.1002/j.1460-2075.1992.tb05556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Izquierdo M, Leevers SJ, Marshall CJ, Cantrell D. p21rascouples the T cell antigen receptor to extracellular signal-regulated kinase 2 in T lymphocytes. J Exp Med. 1993;178:1199–1208. doi: 10.1084/jem.178.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baldari CT, Heguy A, Telford JL. Ras protein activity is essential for T-cell antigen receptor signal transduction. J Biol Chem. 1993;268:2693–2695. [PubMed] [Google Scholar]
- 57.Rozakis-Adcock M, McGlade J, Mbamalu G, Pelicci G, Daly R, Li W, Batzer A, Thomas S, Brugge J, Pelicci PG, et al. Association of the Shc and Grb2/SEM5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature (Lond) 1992;360:689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- 58.Buday L, Downward J. Epidermal growth factor regulates p21rasthrough the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993;73:611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- 59.Egan SE, Giddings BW, Brooks MW, Buday L, Sizeland AM, Weinberg RA. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature (Lond) 1993;363:45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- 60.Li N, Batzer A, Daly R, Yajnik Y, Skolnik E, Chardin P, Bar-Sagi D, Margolis B, Schlessinger J. Guanine-nucleotide–releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to ras signaling. Nature (Lond) 1993;363:85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- 61.Ravichandran KS, Lee KK, Songyang Z, Cantley LC, Burn P, Burakoff SJ. Interaction of Shc with the zeta chain of the T cell receptor upon T cell activation. Science (Wash DC) 1993;262:902–905. doi: 10.1126/science.8235613. [DOI] [PubMed] [Google Scholar]
- 62.Osman N, Lucas S, Turner CH, Cantrell D. A comparison of the interaction of Shc and tyrosine kinase ZAP-70 with the T cell antigen receptor ζ chain tyrosine-based activation motif. J Biol Chem. 1995;270:13981–13986. doi: 10.1074/jbc.270.23.13981. [DOI] [PubMed] [Google Scholar]
- 63.Buday L, Egan SE, Viciana PR, Cantrell DA, Downward J. A complex of Grb2 adaptor protein. Sox exchange factor and a 36 kDa membrane bound tyrosine phosphoprotein is implicated in Ras activation in T cells. J Biol Chem. 1994;269:9019–9023. [PubMed] [Google Scholar]
- 64.Huang X, Li Y, Tanaka K, Moore KG, Hayashi JI. Cloning and characterization of Lnk, a signal transduction protein that links T-cell receptor activation signal to phospholipase Cγ1, Grb2, and phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1995;92:11618–11622. doi: 10.1073/pnas.92.25.11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Izquierdo M, Downward J, Graves JD, Cantrell DA. Role of protein kinase C in T-cell antigen receptor regulation of p21ras: evidence that two p21ras regulatory pathways coexist in T cells. Mol Cell Biol. 1992;12:3305–3312. doi: 10.1128/mcb.12.7.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cantrell DA. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 67.Tang TL, Freeman RM, Jr, O'Reilly AM, Neel BG, Sokol SY. The SH2-containing protein-tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early xenopus development. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- 68.Roche S, McGlade J, Jones M, Gish GD, Pawson T, Courneidge SA. Requirement of phospholipase Cγ, the tyrosine phosphatase Syp and the adaptor proteins Shc and Nck for PDGF-induced DNA synthesis: evidence for the existence of Ras-dependent and Ras-independent pathways. EMBO (Eur Mol Biol Organ) J. 1996;15:4940–4946. [PMC free article] [PubMed] [Google Scholar]
- 69.Scharenberg AM, Kinet J-P. The emerging field of receptor-mediated inhibitory signaling: SHP or SHIP? . Cell. 1996;87:961–964. doi: 10.1016/s0092-8674(00)81790-0. [DOI] [PubMed] [Google Scholar]