Abstract
Infection with HIV-1 requires expression of CD4 and the chemokine receptors CXCR4 or CCR5 at the target cell surface. Engagement of these receptors by the HIV-1 envelope glycoprotein is essential for membrane fusion, but may additionally activate intracellular signaling pathways. In this study, we demonstrate that chemokines and HIV-1 envelope glycoproteins from both T-tropic and macrophage-tropic strains rapidly induce tyrosine phosphorylation of the protein tyrosine kinase Pyk2. The response requires CXCR4 and CCR5 to be accessible on the cell surface. The results presented here provide the first evidence for activation of an intracellular signaling event that can initiate multiple signaling pathways as a consequence of contact between HIV-1 and chemokine receptors.
The chemokine receptors CCR5 and CXCR4 (also called fusin or LESTR) serve as entry cofactors for HIV (1). The viral strains that infect macrophages as well as primary T cells (macrophage [M]-tropic viruses) use CCR5 as an entry cofactor, whereas viral strains that infect transformed CD4+ cell lines as well as primary T cells (laboratory-adapted or T-tropic viruses) use CXCR4. HIV entry is mediated by CD4-dependent interactions between the surface subunit of the envelope glycoprotein (gp120) and the chemokine receptors. Such interactions are thought to result in exposure of the fusion domain of the transmembrane envelope glycoprotein subunit (gp41) and in subsequent viral entry (2). Viral preference for CCR5 versus CXCR4 undergoes a characteristic change during the course of infection and disease progression. Infection is initiated by M-tropic viruses and generally requires CCR5, as indicated by the resistance of CCR5-null individuals to HIV infection (3). The decline in immune system function coincides with the appearance of viruses with broader tropism, characterized by their ability to use CXCR4 and often additional chemokine receptors (4). The change in tropism from CCR5 to CXCR4 suggests that there is selection for usage of different coreceptors depending on the stage of disease. Numerous factors may contribute to this selection, including the distribution of chemokine receptors in different tissues, the regulation of receptor availability on the cell surface, and the effect of receptor engagement on the physiological state of the cell. To begin assessing the effect of envelope–receptor interactions on cell physiology and HIV dissemination, we evaluated the ability of HIV envelope glycoproteins to initiate intracellular signals through contact with CXCR4 and CCR5.
Materials and Methods
Cell Culture.
The growth medium for the cell lines was RPMI supplemented with 2 mM glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml), 1 mM sodium pyruvate, 1% nonessential amino acids (all from GIBCO BRL, Bethesda, MD), and 10% FCS (Hyclone, Logan, UT). For cultures of HL60, the growth medium was supplemented with 50 μM 2-mercaptoethanol (Fisher Scientific Co., Pittsburgh, PA). DU6 is a CD4+ T cell line specific for PPD (purified protein derivative of mycobacteria) that was established from an uninfected donor. The EU2 T cell line was generated from the PBMC of a CCR5-null HIV exposed uninfected individual (designated EU2 in Liu et al., reference 5). CD8+ T cells were removed by magnetic bead sorting (Dynal Inc., Lake Success, NY). The CD8-depleted lymphocytes were then activated with 5 μg/ml PHA (Sigma Chemical Co., St. Louis, MO) and 100 U/ml human IL-2 (Chiron Corp., Emeryville, CA). Both T cell lines were periodically restimulated every 12–16 d using 5 μg/ml PHA and allogeneic PBMC (106/ml) treated with 50 μg/ml mitomycin C (Sigma Chemical Co.) for 30 min at 37°C. Cells were then expanded and maintained in growth medium with 100 U/ml human IL-2. DU6 and EU2 were maintained in culture for 2–4 mo, and for each assay were used at least 13 d after the previous activation.
Reagents.
RANTES (regulated on activation normal T cell expressed and secreted) and macrophage inhibitory protein (MIP)-1β were purchased from R & D Systems (Minneapolis, MN). SDF (stromal cell–derived factor)-1α was synthesized by native chemical ligation as previously described (6) and has activity comparable to native SDF-1α (Siani, M.A., and D.A. Thompson, unpublished data). Soluble gp120 from the T-tropic BH10 and SF2 strains was a gift of R. Sweet (SmithKline Beecham, King of Prussia, PA; reference 7). Soluble gp120/gp41 JRFL oligomeric complexes were prepared as previously described (8). The anti-CXCR4 monoclonal antibody 12G5 (mouse IgG2a) was supplied by J. Hoxie (University of Pennsylvania, Philadelphia, PA) and used at 50 μg/ml. The anti-CD8 monoclonal antibody (Leu2, mouse IgG1) was purchased from Becton Dickinson (San Jose, CA), dialyzed against PBS overnight at 4°C to remove sodium azide, and used at saturation (1:3 dilution). Pertussis toxin was from List Biologicals (Campbell, CA).
Stimulation of Pyk2 Phosphorylation by Chemokine or HIV-1 Env.
20–48 h before the mixing experiments, HL60 cells were transferred to growth medium with 0.5% FCS to lower the basal level of Pyk2 activation. The T cell lines were kept in regular growth medium until the time of the experiment. For the pertussis toxin inhibition experiments, HL60 cells were treated with pertussis toxin at 100 ng/ml for 20 h before treatment with envelope or chemokine. 4 × 106 HL60 cells or 0.5–1.5 × 107 T cells were resuspended in 0.1 ml growth medium/0.5% FCS. The cells were kept at 37°C for 15 min before treatment. In the antibody blocking experiments, the cells were treated with antibody at the start of the 15 min incubation. Chemokines or 293T transfectants were added to the target cells in an equal volume of growth medium/0.5% FCS. Cell lysis, immunoprecipitation, and immunoblotting were performed as previously described (9).
Generation of 293T Cells Expressing HIV-1 Envelope Proteins.
293T cells were transiently transfected by calcium phosphate coprecipitation as previously described (10). Expression vectors encoding gp120/gp41 from HXB2 or JRFL strains, or an empty expression vector, were cotransfected with pRev, encoding HIV-1 Rev (11), and phGFP-S65T (Clontech, Palo Alto, CA), encoding green fluorescent protein. On average 30–40% of the transfected cells were green fluorescent protein–positive. The level of envelope expression was determined by flow cytometry or Western blotting (Davis, C.B., unpublished data). The transfected cells were harvested 48 h after transfection using 0.6 mM EDTA/PBS plus brief trypsinization, and resuspended at 2–4 × 107/ml. 1–4 × 106 cells in 0.1 ml were used for the mixing experiments.
Preparation of Pseudotyped HIV–luciferase Virus.
HIV–luciferase (HIV–luc) reporter virions pseudotyped with JRFL or vesicular stomatitis virus–G envelopes were generated according to Connor et al. (12). 293T cells were transfected by calcium phosphate coprecipitation with HIV–luc provirus and plasmids encoding one of the two envelopes. Virus was harvested 48–60 h after transfection. The JRFL-pseudotyped virions were concentrated fivefold on a Minitan®-S filtration system (Millipore Corp., Bedford, MA). p24 levels of the virion preparations were determined by ELISA (Cellular Products, Inc., Buffalo, NY). Pseudotyped virions were prepared at a final concentration of 5 μg p24/ml. 1 ml of virus was added to 5 × 106 cells in 50 μl and the mixture was incubated at 37°C for 90 s before lysis.
Results and Discussion
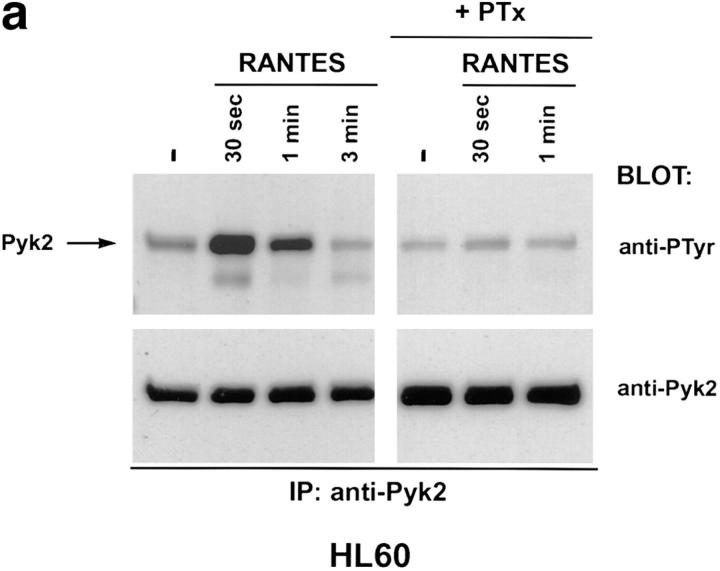
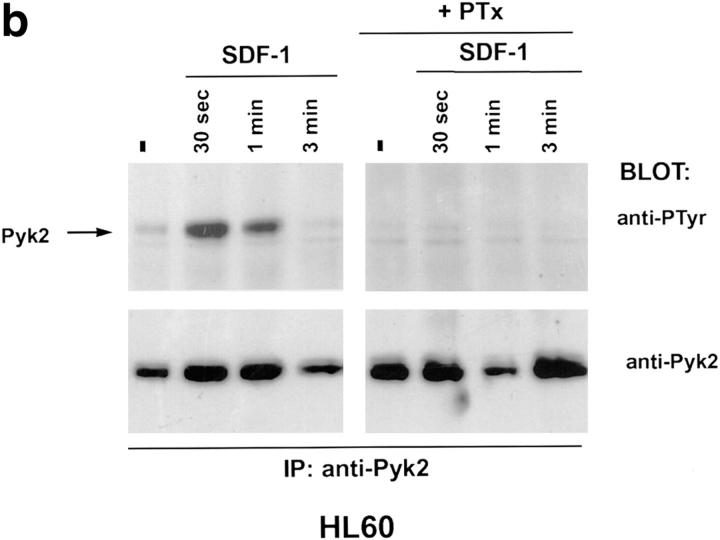
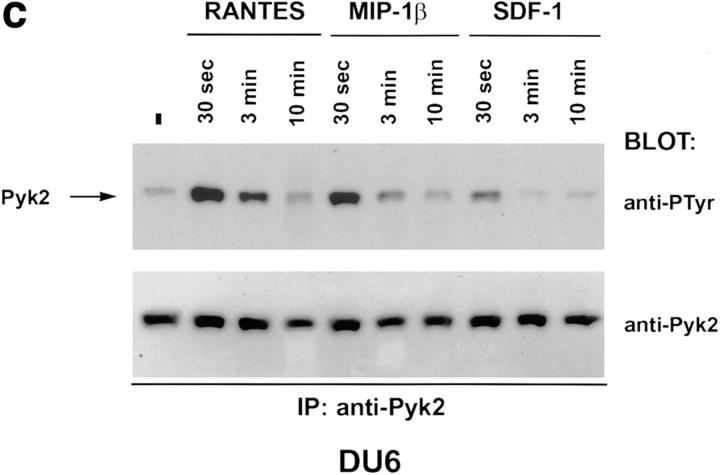
Binding of chemokines to their cognate receptors is often assayed by measuring calcium mobilization (13). Treatment of several CD4+ cell lines with HIV-1 envelope failed to trigger a calcium response, even though such responses could be elicited by chemokines (Davis, C.B., unpublished data). Since chemokine receptor stimulation can have biological effects in the absence of measurable calcium mobilization (14), we searched for other signaling molecules that could be positioned downstream of the chemokine receptors. Pyk2 is a protein tyrosine kinase, related to p125-FAK, and is expressed primarily in cells of the neuronal and hematopoietic lineages (15). Pyk2 is phosphorylated on tyrosine after ligand binding to G protein–coupled receptors or treatment with agents that elevate intracellular Ca2+ concentration (9, 15). Tyrosine phosphorylation of Pyk2 in turn leads to stimulation of kinase activity and phosphorylation of exogenous substrates (9, 15). Since CXCR4 and CCR5 are G protein–coupled receptors, we examined the possibility that their ligands could activate Pyk2. Human promyelocytic leukemia cells (HL60) or CD4+ T cells (DU6) were treated with the following chemokines: RANTES, which binds primarily to CCR1, CCR4, and CCR5; MIP-1β, which binds to CCR5; and SDF-1α, which binds to CXCR4 (13). RANTES and SDF-1α, but not MIP-1β, rapidly induced Pyk2 phosphorylation in HL60 cells (Fig. 1, a and b, and Dikic, I., unpublished data). The response peaked at ∼30 s and had declined to basal levels by 5 min. Pretreatment of HL60 cells with pertussis toxin ablated the Pyk2 response, suggesting that Pyk2 phosphorylation is mediated via Gi proteins (Fig. 1, a and b) (16). The RANTES effect is probably mediated through CCR1 or CCR4, since HL60 cells failed to mobilize calcium to MIP-1β (Davis, C.B., unpublished data), consistent with the lack of CCR5. In contrast, the DU6 cell line did mobilize calcium in response to MIP-1β (Davis, C.B., unpublished data), indicating expression of CCR5. Indeed, MIP-1β, as well as RANTES and SDF-1α, induced Pyk2 phosphorylation in DU6 cells with kinetics comparable to those in HL60 cells (Fig. 1 c).
Figure 1.
Tyrosine phosphorylation of Pyk2 in response to chemokines. (a and b) HL60 promyelocytic leukemia cells were incubated with 500 nM RANTES (a) or SDF-1α (b) at 37°C for the indicated times before lysis. (+PTx) Cells were pretreated with pertussis toxin before incubation with chemokines. Pyk2 was immunoprecipitated (IP) with rabbit antibodies against Pyk2 and blotted with antiphosphotyrosine (anti-PTyr) or anti-Pyk2 antibodies. (c) DU6 CD4+ T cells were incubated with RANTES, MIP-1β, or SDF-1α (500 nM each) for the indicated times before lysis and processing as in a and b.
The CCR5 ligands RANTES, MIP-1α, and MIP-1β can block infection by M-tropic HIV-1 strains (17), and the CXCR4 ligand SDF-1α can block infection by T-tropic strains (18, 19). Conversely, HIV envelopes can compete with the corresponding chemokines for binding to their receptors (8, 20, 21). The overlap between binding sites for chemokine and envelope suggests that envelope engagement of the chemokine receptor may mediate a signal similar to that of the physiological ligand. We therefore tested whether tyrosine phosphorylation of Pyk2 could be triggered by HIV-1 envelope glycoproteins.
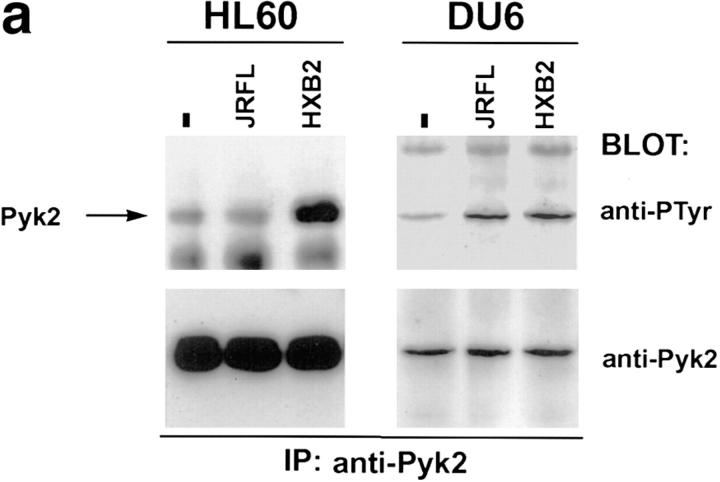
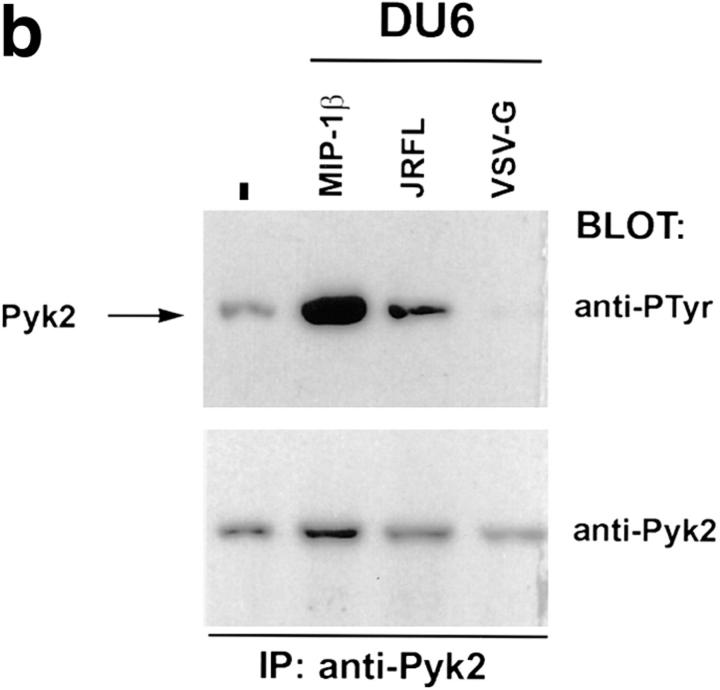
We first determined whether HIV-1 envelope glycoprotein expressed on the surface of 293T cells could trigger Pyk2 phosphorylation in cells expressing the appropriate chemokine receptor. The 293T cells, which do not express Pyk2 (9, 15), were transfected with HIV-1 envelope expression constructs encoding the T-tropic HXB2 or the M-tropic JRFL envelope glycoproteins (22, 23). These cells were then mixed with HL60 cells, which can fuse with envelopes from T-tropic but not M-tropic HIV-1 strains (24), or DU6, which can be infected by both T-tropic and M-tropic HIV-1 strains (Davis, C.B., unpublished data). Tyrosine phosphorylation of Pyk2 was observed when HL60 cells were mixed with 293T transfectants expressing the HXB2, but not the JRFL, envelope glycoprotein (Fig. 2 a, left). In contrast, DU6 cells showed increased tyrosine phosphorylated Pyk2 when mixed with transfectants expressing either HXB2 or JRFL envelope glycoprotein (Fig. 2 a, right). To test whether cell contact with HIV-1 virions triggers phosphorylation of Pyk2, we made use of a system in which envelope-deficient HIV–luc reporter viruses can be coated with different viral envelopes (12). HIV–luc particles coated with JRFL envelope glycoprotein were mixed with DU6 T cells and analyzed for the status of Pyk2 phosphorylation (Fig. 2 b).As a control for nonspecific effects mediated by HIV–luc viral particles, DU6 cells were also mixed with HIV–luc coated with the VSV-G envelope, which mediates infection of cells via binding to sialic acid residues (25). HIV–luc coated with JRFL, but not with VSV-G, could induce tyrosine phosphorylation of Pyk2 (Fig. 2 b). Overall, these results indicate that Pyk2 is tyrosine phosphorylated when uninfected cells encounter envelope on the surface of virions or infected cells.
Figure 2.
Tyrosine phosphorylation of Pyk2 after contact with HIV-1 envelope glycoprotein on the surface of transfected 293T cells and virions. (a) HL60 or DU6 cells were lysed 30 s after being mixed with 293T cells expressing M-tropic (JRFL), T-tropic (HXB2), or no (−) envelope. (b) DU6 cells were mixed with HIV–luc particles pseudotyped with either JRFL or VSV-G envelopes and lysed after 90 s. As a positive control, MIP-1β was incubated with DU6 cells for 30 s before lysis.
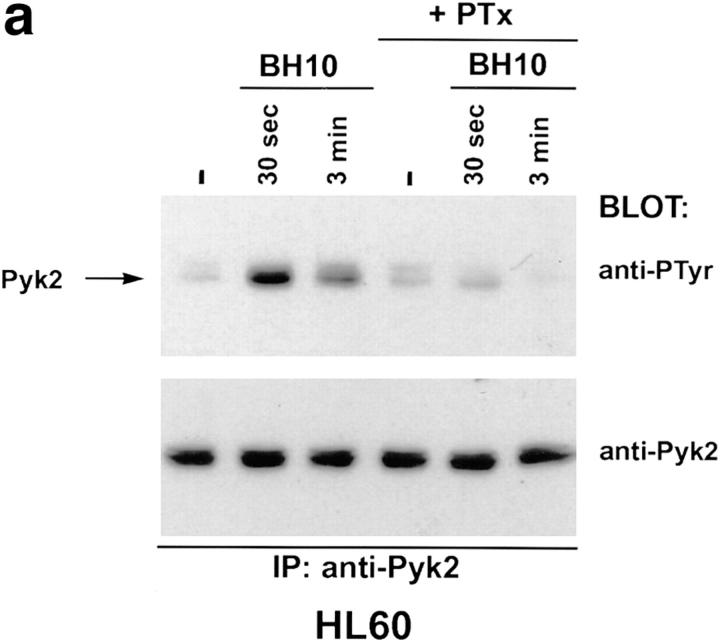
To further characterize the pathway leading to Pyk2 tyrosine phosphorylation after binding of T-tropic envelope to HL60, these cells were treated with a soluble monomeric form of the gp120 subunit from the BH10 clone of the T-tropic IIIB strain (26). Tyrosine phosphorylation of Pyk2 was readily detected after addition of BH10 gp120, with kinetics similar to those observed after treatment with the chemokines SDF-1α and RANTES (Fig. 3 a, compare with Fig. 1, a and b). Gp120 is thus sufficient to stimulate Pyk2 phosphorylation, and other molecules contributed by the 293T transfectants are not required. In addition, the Pyk2 response to monomeric gp120 suggests that receptor cross-linking is not required. The Pyk2 response to BH10 gp120 was inhibitable with pertussis toxin (Fig. 3 a), implicating Gi-linked pathways in the response to either SDF-1α or HIV envelope glycoprotein in these cells.
Figure 3.
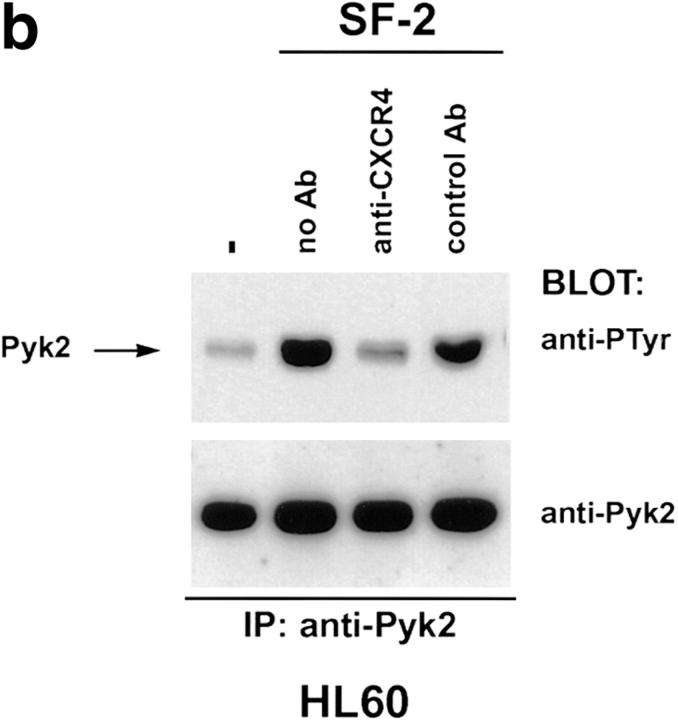
Tyrosine phosphorylation of Pyk2 mediated by T-tropic gp120 can be inhibited by pertussis toxin or a monoclonal antibody against CXCR4. (a) HL60 cells were pretreated with pertussis toxin or left untreated. Treated and untreated cells were incubated with T-tropic gp120 (BH10, 5 μg/ml) for the indicated times before lysis. (b) HL60 cells were resuspended in growth media/0.5% FCS either without antibody (no Ab) or containing monoclonal antibodies specific for CXCR4 (anti-CXCR4) or human CD8 (control Ab). Cells were incubated with antibody at 37°C for 15 min, then mixed with T-tropic SF-2 envelope for 30 s before lysis.
T-tropic HIV gp120 binds to both CD4 (7) and CXCR4 (21, 27). HIV gp120 binding to CD4 has been reported to transduce signals that are dependent on the CD4 cytoplasmic domain (28, 29). Therefore, Pyk2 phosphorylation could be triggered by gp120 binding to CXCR4, CD4, or both. To confirm that Pyk2 phosphorylation induced by T-tropic envelope requires CXCR4, HL60 cells were pretreated with a monoclonal antibody against CXCR4 that blocks T-tropic gp120 binding to CXCR4 and inhibits infection by CXCR4-tropic strains of HIV-1 and HIV-2 (21, 30). Pretreatment with this monoclonal antibody attenuated the Pyk2 response to gp120 from BH10 and a T-tropic virus, SF-2 (31; Fig. 3 b; and our unpublished data). CXCR4 thus must be accessible to gp120 and associated with functional G protein for Pyk2 to be activated and tyrosine phosphorylated.
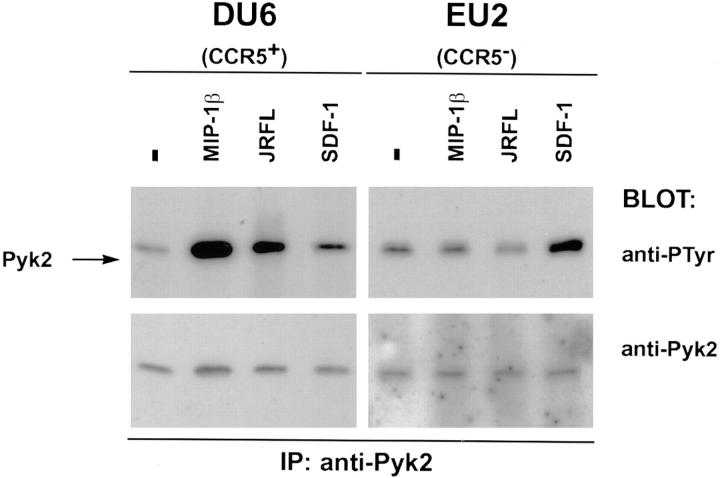
To determine whether CCR5 is required for signaling after binding of M-tropic envelope glycoprotein, Pyk2 tyrosine phosphorylation was compared in wild-type and CCR5− T cell lines. The mutant cell line was derived from an HIV-1 exposed uninfected patient (EU2) who is homozygous for a 32-bp deletion in the CCR5 coding sequence and lacks cell surface expression of CCR5. The EU2 cell line fails to display calcium mobilization or chemotaxis in response to MIP-1β treatment, and is resistant to M-tropic HIV-1 infection (5; Davis, C.B., unpublished data). DU6 and EU2 cells were treated with MIP-1β, SDF-1α, or soluble oligomerized JRFL gp120/gp41. Pyk2 was strongly phosphorylated in DU6 cells treated with either MIP-1β or JRFL envelope glycoprotein (Fig. 4, left). EU2 cells, in contrast, failed to respond to either stimulus, but responded to SDF-1α, indicating that the signaling pathway between CXCR4 and Pyk2 is intact (Fig. 4, right). Overall, these data indicate that CD4 engagement alone is insufficient for activation of Pyk2 via M-tropic envelope and that CCR5 must be available on the cell surface.
Figure 4.
Tyrosine phosphorylation of Pyk2 in response to M-tropic gp120/gp41 requires expression of CCR5 on target cells. DU6 cells (5 × 106) or the equivalent number of CD4+CCR5− T cells (EU2) were incubated with MIP-1β (500 nM), SDF-1α (500 nM), or JRFL gp120/gp41 (5 μg/ml) for 30 s before lysis.
The activation of signal transduction pathways by HIV-1 envelope glycoprotein binding to chemokine receptors raises several interesting questions regarding the role of signaling in HIV pathogenesis. Viral entry does not depend on Gi-coupled signaling (32), and mutations in CCR5 that ablate signal transduction do not affect coreceptor activity (33). G protein–linked signaling thus may be simply a by-product of envelope engagement of chemokine receptors. However, triggering of chemokine signaling cascades in vivo may prepare the target cells for viral replication and may be responsible for some of the cellular responses to the virus. For example, specific sequences in the envelope glycoprotein have been shown to direct not only envelope fusion, but also events in the intracellular viral life cycle after fusion (34). Differences in signaling pathways may also explain the differential use of chemokine receptors by strains of HIV-1 during the course of infection. It has been shown that Pyk2 can be activated in response to a variety of extracellular stimuli and that activated Pyk2 can feed into the MAP kinase or JUN kinase signaling pathways in different cell types (9, 15, 35). Moreover, activation of Pyk2 by extracellular stimulation also leads to tyrosine phosphorylation and modulation of ion channel function (15). Pyk2 is therefore able to provide a potential link between HIV binding to chemokine receptors and several intracellular pathways that regulate cell growth, cell survival, and cell differentiation. In addition, ligand binding to chemokine receptors has been shown to induce alterations in cytoskeletal structure and cell adhesion (13) that, in the case of HIV infection, may facilitate viral transmission from infected to uninfected cells. Elucidation of the role of these intracellular events will require in vivo models in which candidate signaling pathways can be specifically manipulated over the course of infection.
Acknowledgments
The authors thank M. Emmerman for the VSV-G expression plasmid, R. Sweet for the purified BH10 and SF-2 envelopes, J. Hoxie for the anti-CXCR4 antibody, and W. Paxton and R. Koup for the EU2 PBL. We also thank E. Skolnick for helpful comments, and V. KewalRamani for many valuable discussions concerning experimental design as well as preparation of the manuscript.
Footnotes
D.R. Littman is an Investigator of the Howard Hughes Medical Institute. This work was supported by grants from the National Institutes of Health (D.R. Littman) and Sugen, Inc. (J. Schlessinger).
C.B. Davis and I. Dikic contributed equally to this study.
References
- 1.Clapham PR, Weiss RA. Spoilt for choice of co-receptors. Nature (Lond) 1997;388:230–231. doi: 10.1038/40758. [DOI] [PubMed] [Google Scholar]
- 2.Wain-Hobson S. HIV. One on one meets two. Nature (Lond) 1996;384:117–118. doi: 10.1038/384117a0. [DOI] [PubMed] [Google Scholar]
- 3.Hill CM, Littman DR. Natural resistance to HIV? . Nature (Lond) 1996;382:668–669. doi: 10.1038/382668a0. [DOI] [PubMed] [Google Scholar]
- 4.Fauci AS. Host factors and the pathogenesis of HIV-induced disease. Nature (Lond) 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 5.Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 6.Dawson PE, Muir TW, Clarke-Lewis I, Kent SBH. Synthesis of proteins by native chemical ligation. Science (Wash DC) 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 7.Ivey-Hoyle M, Culp JS, Chaikin MA, Hellmig BD, Matthews TJ, Sweet RW, Rosenberg M. Envelope glycoproteins from biologically diverse isolates of immunodeficiency viruses have widely different affinities for CD4. Proc Natl Acad Sci USA. 1991;88:512–516. doi: 10.1073/pnas.88.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trkola A, Dragic T, Arthos J, Binley JM, Olson WC, Allaway GP, Cheng-Mayer C, Robinson J, Maddon PJ, Moore JP. CD4-dependent, antibody-sensitive interactions between HIV-1 and its coreceptor CCR-5. Nature (Lond) 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 9.Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein–coupled receptors with MAP kinase activation. Nature (Lond) 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 10.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature (Lond) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 11.Hope TJ, McDonald D, Huang XJ, Low J, Parslow TG. Mutational analysis of the human immunodeficiency virus type 1 Rev transactivator: essential residues near the amino terminus. J Virol. 1990;64:5360–5366. doi: 10.1128/jvi.64.11.5360-5366.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type–1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 13.Murphy PM. Chemokine receptors: structure, function, and role in microbial pathogenesis. Cytokine Growth Factor Rev. 1996;7:47–64. doi: 10.1016/1359-6101(96)00009-3. [DOI] [PubMed] [Google Scholar]
- 14.Turner L, Ward SG, Westwick J. RANTES-activated human T lymphocytes. A role for phosphoinositide 3-kinase. J Immunol. 1995;155:2437–2444. [PubMed] [Google Scholar]
- 15.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature (Lond) 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 16.Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science (Wash DC) 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- 17.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+T cells. Science (Wash DC) 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 18.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature (Lond) 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 19.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, Schwartz O, Heard JM, Clark-Lewis I, Legler DF, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line–adapted HIV-1. Nature (Lond) 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 20.Wu L, Gerard NP, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso AA, Desjardin E, Newman W, et al. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature (Lond) 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 21.Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper SC, Hoxie J, Kolson DL, Taub D, Horuk R. CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Curr Biol. 1997;7:112–121. doi: 10.1016/s0960-9822(06)00055-8. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien WA, Koyanagi Y, Namazie A, Zhao JQ, Diagne A, Idler K, Zack JA, Chen IS. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature (Lond) 1990;348:69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- 23.Carrillo A, Trowbridge DB, Westervelt P, Ratner L. Identification of HIV-1 determinants for T lymphoid cell line infection. Virology. 1993;197:817–824. doi: 10.1006/viro.1993.1664. [DOI] [PubMed] [Google Scholar]
- 24.Alkhatib G, Broder CC, Berger EA. Cell type-specific fusion cofactors determine human immunodeficiency virus type 1 tropism for T-cell lines versus primary macrophages. J Virol. 1996;70:5487–5494. doi: 10.1128/jvi.70.8.5487-5494.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mastromarino P, Conti C, Ciuffarella MG, Orsi N. Involvement of carbohydrates in vesicular stomatitis virus-cell early interaction. Acta Virol. 1989;33:513–520. [PubMed] [Google Scholar]
- 26.Ratner L, Fisher A, Jagodzinski LL, Mitsuya H, Liou RS, Gallo RC, Wong-Staal F. Complete nucleotide sequences of functional clones of the AIDS virus. AIDS Res Hum Retroviruses. 1987;3:57–69. doi: 10.1089/aid.1987.3.57. [DOI] [PubMed] [Google Scholar]
- 27.Lapham CK, Ouyang J, Chandrasekhar B, Nguyen NY, Dimitrov DS, Golding H. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell lines. Science (Wash DC) 1996;274:602–605. doi: 10.1126/science.274.5287.602. [DOI] [PubMed] [Google Scholar]
- 28.Benkirane M, Schmid-Antomarchi H, Littman DR, Hirn M, Rossi B, Devaux C. The cytoplasmic tail of CD4 is required for inhibition of human immunodeficiency virus type 1 replication by antibodies that bind to the immunoglobulin CDR3–like region in domain 1 of CD4. J Virol. 1995;69:6904–6910. doi: 10.1128/jvi.69.11.6904-6910.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berube P, Barbeau B, Cantin R, Sekaly RP, Tremblay M. Repression of human immunodeficiency virus type 1 long terminal repeat–driven gene expression by binding of the virus to its primary cellular receptor, the CD4 molecule. J Virol. 1996;70:4009–4016. doi: 10.1128/jvi.70.6.4009-4016.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Endres MJ, Clapham PR, Marsh M, Ahuja M, Turner JD, McKnight A, Thomas JF, Stoebenau-Haggarty B, Choe S, Vance PJ, et al. CD4-Independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 31.McKeating JA, McKnight A, Moore JP. Differential loss of envelope glycoprotein gp120 from virions of human immunodeficiency virus type 1 isolates: effects on infectivity and neutralization. J Virol. 1991;65:852–860. doi: 10.1128/jvi.65.2.852-860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cocchi F, DeVico AL, Garzino-Demo A, Cara A, Gallo RC, Lusso P. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 33.Farzan M, Choe H, Martin KA, Sun Y, Sidelko M, Mackay CR, Gerard NP, Sodroski J, Gerard C. HIV-1 entry and macrophage inflammatory protein– 1β–mediated signaling are independent functions of the chemokine receptor CCR5. J Biol Chem. 1997;272:6854–6857. doi: 10.1074/jbc.272.11.6854. [DOI] [PubMed] [Google Scholar]
- 34.Mori K, Ringler DJ, Desrosiers RC. Restricted replication of simian immunodeficiency virus strain 239 in macrophages is determined by Env but is not due to restricted entry. J Virol. 1993;67:2807–2814. doi: 10.1128/jvi.67.5.2807-2814.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tokiwa G, Dikic I, Lev S, Schlessinger J. Activation of Pyk2 by stress signals and coupling with JNK signaling pathway. Science (Wash DC) 1996;273:792–794. doi: 10.1126/science.273.5276.792. [DOI] [PubMed] [Google Scholar]