Abstract
It has been established that insulin-dependent diabetes mellitus (IDDM) in nonobese diabetic (NOD) mice results from a CD4+ and CD8+ T cell–dependent autoimmune process directed against the pancreatic beta cells. The precise roles that beta cell–reactive CD8+ and CD4+ T cells play in the disease process, however, remain ill defined. Here we have investigated whether naive beta cell–specific CD8+ and CD4+ T cells can spontaneously accumulate in pancreatic islets, differentiate into effector cells, and destroy beta cells in the absence of other T cell specificities. This was done by introducing Kd– or I-Ag7–restricted beta cell–specific T cell receptor (TCR) transgenes that are highly diabetogenic in NOD mice (8.3- and 4.1-TCR, respectively), into recombination-activating gene (RAG)-2–deficient NOD mice, which cannot rearrange endogenous TCR genes and thus bear monoclonal TCR repertoires. We show that while RAG-2−/− 4.1-NOD mice, which only bear beta cell–specific CD4+ T cells, develop diabetes as early and as frequently as RAG-2+ 4.1-NOD mice, RAG-2−/− 8.3-NOD mice, which only bear beta cell–specific CD8+ T cells, develop diabetes less frequently and significantly later than RAG-2+ 8.3-NOD mice. The monoclonal CD8+ T cells of RAG-2−/− 8.3-NOD mice mature properly, proliferate vigorously in response to antigenic stimulation in vitro, and can differentiate into beta cell–cytotoxic T cells in vivo, but do not efficiently accumulate in islets in the absence of a CD4+ T cell–derived signal, which can be provided by splenic CD4+ T cells from nontransgenic NOD mice. These results demonstrate that naive beta cell– specific CD8+ and CD4+ T cells can trigger diabetes in the absence of other T or B cell specificities, but suggest that efficient recruitment of naive diabetogenic beta cell–reactive CD8+ T cells to islets requires the assistance of beta cell–reactive CD4+ T cells.
Insulin-dependent diabetes mellitus (IDDM)1 in nonobese diabetic (NOD) mice, which spontaneously develop a form of diabetes closely resembling human IDDM, is the result of a CD4+ and CD8+ T cell–dependent autoimmune process directed against the pancreatic beta cells (1, 2). Although the role of T cells as effectors of beta cell damage in IDDM is well established, the nature of the cells and immunological mechanisms that initiate diabetogenesis in genetically susceptible mice remain to be elucidated.
Adoptive T cell transfer studies using spleen cells from prediabetic NOD mice have demonstrated that transfer of IDDM into immunodeficient NOD mice requires both CD4+ and CD8+ T cells (3–6). However, splenic CD4+ T cells from NOD mice and some, though not all, preactivated I-Ag7–restricted, beta cell–specific CD4+ T cell clonotypes can home into pancreatic islets and destroy beta cells in the absence of CD8+ T cells (6–13). Since beta cells do not express MHC class II molecules (I-Ag7; references 14, 15), it has been proposed that naive autoreactive CD4+ T cells may differentiate into effector cells by engaging autoantigens that are purportedly shed from the beta cells by a yet to be determined initial insult, in the context of I-Ag7 molecules on local APCs (1, 16).
Studies of β2-microglobulin–deficient NOD mice (17– 19) and anti-CD8 mAb-treated NOD mice (20), which do not bear mature CD8+ T cells and develop neither diabetes nor insulitis, have suggested that development of insulitis in NOD mice is somehow regulated by CD8+ T cells. Although these studies could not rule out a general influence of MHC class I molecules and/or CD8+ T cells on the insulitogenic activity of naive autoreactive CD4+ T cells (20), they argued in favor of the hypothesis that the initial beta cell insult that triggers the shedding of beta cell autoantigens and subsequent CD4+ T cell activation may be mediated by beta cell–cytotoxic CD8+ T cells, which are consistently present in pancreatic islets of NOD mice (16, 21– 26). This view is compatible with two other observations: that restoring expression of MHC class I molecules on beta cells of β2-microglobulin–deficient NOD mice restores insulitis susceptibility (27), and that splenocytes from prediabetic NOD mice cannot efficiently transfer insulitis into β2-microglobulin–deficient NOD.scid mice (28).
This hypothesis, however, is in apparent conflict with other observations that support the alternative view that diabetogenesis is initiated by MHC class II–restricted autoreactive CD4+ T cells. For example, splenic CD4+ T cells from prediabetic NOD mice can transfer insulitis into NOD.scid mice without CD8+ T cell cotransfer, and splenic CD8+ T cells from diabetic NOD mice cannot home into pancreatic islets of irradiated NOD mice or NOD.scid mice in the absence of CD4+ T cells (6, 29). Furthermore, since beta cells do not express the costimulatory B7.1 or B7.2 molecules (30), naive beta cell–specific MHC class I–restricted CD8+ T cells are unlikely to differentiate into cells capable of effecting beta cell damage unless their target beta cell autoantigens are shed from the beta cell by an earlier insult, and later presented to them by APCs capable of processing exogenous antigens through the MHC class I pathway. Finally, and most importantly, genetic susceptibility and resistance to both insulitis and IDDM are profoundly affected by polymorphisms of MHC class II genes (2, 31), which control the development and function of CD4+, but not CD8+, T cells.
The autoreactive T cells that affect the initial beta cell insult in spontaneous IDDM should be able to home into pancreatic islets, differentiate into effector cells, and effect beta cell damage in the absence of other T cells. To investigate the independent insulitogenic and diabetogenic potential of naive beta cell–reactive CD4+ or CD8+ T cells in spontaneous IDDM, we have compared the ability of NOD mouse islet-derived, I-Ag7– or Kd–restricted, beta cell–specific TCRs to: (a) accelerate diabetogenesis in TCR-transgenic NOD mice, and (b) trigger insulitis and diabetes in TCR-transgenic, recombination-activating gene (RAG)- 2−/− NOD mice, which cannot rearrange endogenous TCR genes and thus bear monoclonal TCR repertoires. We show that unlike naive diabetogenic CD4+ T cells, which require neither mature B cells nor CD8+ T cells to accumulate in islets and destroy beta cells, naive diabetogenic CD8+ T cells are largely dependent on CD4+ T cells to efficiently accumulate in pancreatic islets and cause diabetes. On the basis of these findings, we propose that recruitment of the first autoreactive CD8+ T cells into pancreatic islets of NOD mice is preceded and/or accompanied by the recruitment of autoreactive CD4+ T cells.
Materials and Methods
Production of TCR-transgenic, RAG-2− /− NOD Mice.
The H-2Kd–restricted, beta cell–cytotoxic CD8+ T cell clone NY8.3 was chosen as donor of TCR-α (Vαn.1-Jα34) and TCR-β transgenes (Vβ8.1-Dβ2.1-Jβ2.4) to generate 8.3-NOD mice. This T cell clone was isolated from islets of an acutely diabetic NOD mouse (24), uses a TCR-α–CDR3 sequence homologous or identical to those used by many NOD islet-derived beta cell–cytotoxic CD8+ T cells (25, 26), and is diabetogenic in adoptive T cell transfer experiments (24, 32). 4.1-NOD mice (expressing the TCR-α and -β rearrangements of the I-Ag7–restricted beta cell–specific CD4+ T cell clone NY4.1) and 8.3–TCR-β–transgenic NOD mice (expressing the TCR-β rearrangement of NY8.3) are described elsewhere (26, 33). Transgenic constructs bearing the functional VDJβ and VJα rearrangements of NY8.3 (25), upstream regulatory sequences, and the TCR-β enhancer (for the TCR-β construct) or the TCR-α or IgH enhancers (for the TCR-α construct) were generated as described (26, 33). After removing prokaryotic sequences by digestion with PvuI and SalI (TCR-β), ClaI and SacII (TCR-α–IgHenh), or ClaI and SalI (TCR-α–αenh), the constructs (21.5, 12.7, or 14.5 kb, respectively) were coinjected into fertilized (SJL × C57BL/6) F2 eggs (DNX, Princeton, NJ). Offspring were screened for integration of the transgenes by Southern blotting using VDJβ and VJα cDNA probes. Two transgenic founder mice expressing the transgenes (8.3–AN6B7– TCR-α/β with the TCR-α–αenh construct, and 8.3–IgH3A– TCR-α/β with the TCR-α–IgHenh construct) were backcrossed with NOD/Lt mice (I-Ag7, I-E−, Kd, Db, from The Jackson Laboratory, Bar Harbor, ME) for three to six generations to generate 8.3–TCR-α/β–transgenic NOD (8.3-NOD) mice. Studies of mice derived from these two different founders yielded similar results.
RAG-2−/− NOD mice were generated by backcrossing the RAG-2 mutation of RAG-2−/− C57BL/6 mice (34; a gift from F. Alt, Boston Children's Hospital, Boston, MA) onto the NOD background for 10 generations, followed by intercrossing of N10 heterozygotes. RAG-2−/− 4.1-NOD and RAG-2−/− 8.3-NOD mice (also referred to as monoclonal T cell NOD mice) were generated by backcrossing the TCR transgenes of 4.1-NOD and 8.3-NOD mice onto RAG-2−/− NOD mice. Mice were screened for inheritance of the transgenes and mutated or wild-type RAG-2 alleles by PCR of tail DNA. All mice were housed in a specific pathogen-free facility at The University of Calgary.
Cell Lines, Antibodies, and Flow Cytometry.
H-2Kd –transfected L929 cells (L929-Kd) were obtained from J. Yewdell (National Institutes of Health, Bethesda, MD). NOD mouse–derived NIT-1 and MIN6N8a insulinoma cells were provided by E.H. Leiter (The Jackson Laboratory) and J.-I. Miyazaki (University of Tokyo, Tokyo, Japan), respectively. WEHI-164 clone 13 cells were provided by D. Remick (University of Michigan, Ann Arbor, MI). A hybridoma secreting the Vβ8.1/8.2-specific mAb KJ16 was a gift from P. Marrack (National Jewish Center for Immunology, Denver, CO). Hybridomas secreting mAbs 1411-2C11 (anti-CD3), GK1.5 (anti-CD4), RA3-6B2 (anti-CD45RA/B220), F4/80 (antimacrophage), and M1/70.15.11.5.HL (anti–Mac-1) were obtained from the American Type Culture Collection (Rockville, MD). Anti-Lyt-2 (CD8α)-PE (53-6.7), anti-L3T4-FITC or anti-L3T4-biotin (CD4) (H129.19), anti-CD2-biotin (RM2-5), anti-CD5 (53-7.3)-biotin, anti-CD11a-biotin (M17/4), anti-CD24- biotin (M1/69), anti-CD28-biotin (37.51), anti-CD44-FITC (IM7), anti-CD45RB-FITC (23G2), anti-L-selectin-biotin (CD62L) (MEL-14), anti-CD69-biotin (H1.2F3), anti-Vβ8.1/8.2-FITC (MR5-2), and anti-H-2Kd-FITC (SF1-1.1) were purchased from PharMingen (San Diego, CA). Anti-IL-2R-FITC (CD25) (AMT13) was purchased from Cedarlane Laboratories (Hornby, Ontario, Canada). Mouse IgG-absorbed FITC-conjugated goat anti–rat IgG and FITC-conjugated goat anti–mouse IgG were obtained from CALTAG (San Francisco, CA) and Becton Dickinson (San Jose, CA), respectively. Streptavidin-PerCP was obtained from Becton Dickinson. Thymi, spleens, and islet-derived T cell lines were analyzed by three-color flow cytometry using a FACScan® (Becton Dickinson) as described elsewhere (26).
TNF-α Secretion.
Splenocytes from 8.3-NOD mice were depleted of CD4+ T cells using anti-CD4 mAb (GK-1.5)–coated magnetic beads, as described (26). The remaining cells were analyzed by flow cytometry and adjusted to 104 CD8+ T cells/100 μl of complete medium (CM; RPMI 1640 media containing 10% heat-inactivated fetal bovine serum [GIBCO BRL, Gaithersburg, MD], 50 U/ml penicillin, 50 μg/ml streptomycin (Flow Laboratories, McLean, VA), and 50 μM 2-ME [Sigma Chemical Co., St. Louis, MO]) and challenged with beta cells (NIT-1 or MIN6N8a) or control target cells (L929-Kd) (104 cells/100 μl) for 24 h. The culture supernatants (100 μl) were assayed for the presence of TNF-α by measuring their cytolytic effect on WEHI-164 clone 13 cells (35) using a colorimetric assay (Cell Proliferation Kit II, Boehringer Mannheim, Mannheim, Germany). rTNF-α (R&D Systems, Minneapolis, MN) was used to generate the standard curves. In some experiments, splenic CD8+ T cells were preactivated with plate-bound anti-CD3 mAb (1411-2C11, 10 μg/ml) for 3 d at 37°C in 5% CO2 (26) and expanded with 0.5 U/ml of rIL-2 (Takeda, Osaka, Japan) for 7 d before being assayed for their ability to secrete TNF-α in response to beta cells.
Proliferation Assays.
Pancreatic islet cells were prepared from 5–8-wk-old NOD mice as described (26). Splenic CD8+ T cells (2 × 104) were incubated, in triplicate, with γ-irradiated (3,000 rad) islet cells (3–100 × 103/well) in 96–round-bottomed well tissue culture plates for 3 d at 37°C in 5% CO2. Cultures were pulsed with 1 μCi of [3H]thymidine during the last 18 h of culture and harvested. Thymidine incorporation was measured by scintillation counting. Specific proliferation was calculated by substracting background proliferation (cpm of cultures containing islet cells alone and cpm of cultures of T cells alone) from islet cell–induced proliferation (cpm of cultures containing T cells and islet cells). For anti–TCR-β stimulation, CD8+ T cells (2 × 104) were added, in triplicate, to ELISA-grade wells precoated with 10-fold serial dilutions of KJ16 mAb (anti-Vβ8.1/8.2)–containing ascites (1:10–1:10,000; reference 26), incubated for 48 h, pulsed with [3H]thymidine for 18 h, harvested, and analyzed by scintillation counting.
Limiting Dilution Analyses.
To determine the peripheral frequency of beta cell–reactive CD8+ T cells, 12 replicate cultures of 10-fold serial dilutions of splenocytes (101–105 cells/well) were stimulated with irradiated (2,500 rads) NOD islets (8/well) for 4 d, expanded in rIL-2 (0.5 U/ml) for 10 d, and restimulated with islets and rIL-2. Control plates received rIL-2, but not islets. The resulting cultures were split, challenged with 104 NIT-1 cells or L929-Kd cells for 24 h, and the supernatants were collected to measure the contents of TNF-α. Cultures that secreted TNF-α in response to MIN6N8a cells (absorbance values two standard deviations below the average values of control cultures) but not L929-Kd cells were considered to contain beta cell–reactive CD8+ T cells. Frequencies were calculated by Poisson statistics.
Generation of Spleen- and Islet-derived CD8+ T Cell Lines and Clones.
CD4+ T cell–depleted spleen cells (104 cells/well in 96-well plates) were stimulated with irradiated NOD islets for 3 d, and the activated cells expanded with 0.5 U/ml rIL-2 for 10–14 d. Growing cultures were split and individual subcultures assayed at this point for serine esterase content (see below) or beta cell reactivity.
Islet-infiltrating CD8+ T cell lines were isolated from prediabetic or acutely diabetic mice as described previously (26). In brief, isolated islets were cultured in CM containing 0.5 U/ml of rIL-2, and the lymphocytes obtained after 4–5 d were analyzed by flow cytometry to determine their phenotype, depleted of CD4+ T cells by negative selection with GK1.5 mAb-coated immunobeads, used for messenger RNA extraction, cloned in 96-well plates under limiting dilution conditions (1–5 cells/well), and stimulated once with irradiated NOD islets in the presence of rIL-2. Growing cultures were split into three subcultures and assayed within 10 d of cloning for beta cell specificity (TNF-α release in response to NIT-1 or L929-Kd cells) and serine esterase content, or expanded further to obtain sufficient cells for cytotoxicity assays.
Determination of Serine Esterase Content.
Confluent 96-well plate subcultures were lysed with 1% Triton X-100 and the lysates analyzed for serine esterase activity (26, 36). Absorbance readings two standard deviations above the mean of negative cultures (nonstimulated splenocytes) were considered positive.
Cytokine Reverse Transcription-PCR.
Total cellular RNA was extracted from islet-derived T cell lines depleted of CD4+ T cells (>98% CD8+) by the guanidium isothiocyanate-phenol-chloroform method, and 2 μg used in a reverse transcription reaction using oligo(dT)12–18 (GIBCO BRL) as a primer. The cDNAs were amplified by PCR using previously described primers specific for several cytokines, including IL-2, IFN-γ, IL-4, IL-10, TNF-α, and for hypoxanthine phosphoribosyl transferase (HPRT) as internal control (37). The PCR products were electrophoresed in a 1.5% agarose gel and detected by ethidium bromide staining.
51Cr–Release Assays.
Single islet cells (5 × 105) from NOD (H-2g7: I-Ag7, Kd, Db) and C57BL/6 (H-2b: I-Ab, Kb, Db) mice were labelled with [51Cr]sodium chromate (DuPont New England Nuclear, Boston, MA), resuspended at 105 cells/ml, and seeded at 104 cells/100 μl/well. Effector cells (islet-derived CD8+ T cell clones) were added to each well, in duplicate, at several target/effector ratios. Plain medium was added to a set of target cells for examination of spontaneous cell lysis. Total cell lysis was determined by lysing the cells with 1% Triton X-100. The plates were centrifuged at 100 g for 3 min and incubated at 37°C for 8 h. After incubation, the plates were centrifuged and the supernatants (100 μl) harvested. The radioactivity of the supernatants was measured in a gamma counter (LKB-Wallac, Turku, Finland). Specific 51Cr release was calculated as follows: percent lysis = 100 × (test cpm − spontaneous cpm)/(total cpm − spontaneous cpm).
Adoptive T cell Transfer.
Splenocytes from 4–5-wk-old nontransgenic female NOD mice were depleted of CD8+ T cells using anti-CD8 mAb (53-6.7)–coated magnetic beads (26). The remaining cells (containing <0.5% CD8+ T cells) were injected (8 × 106 cells/mouse) into 5–8-wk-old female RAG-2−/− 8.3-NOD mice. Recipient mice were monitored for diabetes or killed 10 wk after transfer for histology. Some RAG-2−/− 8.3-NOD mice were transfused with splenic T cells from nontransgenic NOD mice (1.5 × 107 cells/mouse) enriched for CD4+ T cells (>92%) and depleted of CD8+ T cells and B220+, F4/80+, Mac-1+, and 33D1+ cells (<0.4%) using mAb-coated immunobeads and affinity columns (R&D Systems).
Diabetes.
Diabetes was assessed by measuring urine glucose levels with Diastix strips (Miles, Ontario, Canada) twice a week. Animals were considered diabetic after two consecutive readings ⩾3+.
Histopathology.
Submandibular salivary gland, thyroid, adrenal gland, kidney, liver, stomach, small intestine, colon, pancreas (one half), and muscle were fixed in formalin, embedded in paraffin, sectioned at 4.5 μM, stained with hematoxylin and eosin, and examined for inflammation. The degree of insulitis in the pancreas was evaluated by scoring 15–30 islets/mouse in a blinded fashion using the following criteria: 0, normal islet; 1, periinsulitis; 2, mononuclear cell infiltration in <25% of the islet; 3, mononuclear cell infiltration in 25–50% of the islet; 4, >50% of the islet infiltrated. A second half of each pancreas was snap frozen in liquid nitrogen, immersed in OCT, sectioned at 6–7 μM, fixed in cold acetone for 10 min, and stained with anti-CD4 (GK1.5) and anti-CD8 (53-6.7) mAbs followed by anti–rat IgG-FITC, or with anti–rat IgG-FITC alone, and observed under a fluorescence microscope (26).
Statistical Analyses.
Statistical analyses were performed using Mann-Whitney U and χ2 tests.
Results
Expression of the 8.3-TCR in 8.3-NOD Mice.
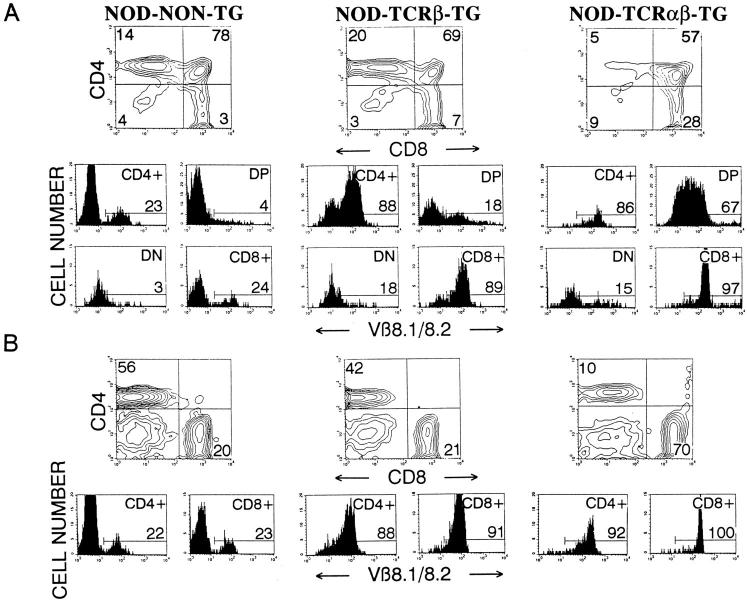
Three-color cytofluorometric studies showed that >97% CD4−CD8+ thymocytes (Fig. 1 A) and lymph node CD8+ T cells (Fig. 1 B) from 8.3-NOD mice expressed Vβ8.1/8.2+ TCRs, as compared with 18% of the CD4−CD8+ thymocytes and lymph node CD8+ T cells from nontransgenic NOD mice, indicating TCR-β transgene expression. Although we do not have a TCR-α–transgenic chain–specific mAb, and thus cannot directly quantitate its expression, three different lines of evidence indicate that the transgenic TCR-α chain is expressed on a significant fraction of thymocytes and peripheral T cells from 8.3-NOD mice. First, ∼67% of CD4+CD8+ thymocytes from 8.3-NOD mice expressed high levels of the transgenic TCR-β chain, as compared to only 18% of CD4+CD8+ thymocytes from TCR-β–transgenic NOD mice, suggesting early TCR-α chain expression and early upregulation of TCR-α/β heterodimers on immature thymocytes (38–42). Second, thymocyte development in 8.3-NOD mice, but not 8.3–TCR-β–transgenic NOD mice, was skewed towards the CD4−CD8+ subset (Fig. 1 A), suggesting TCR-α–transgene–dependent positive selection of 8.3-CD8+ thymocytes as in other MHC class I–restricted TCR-α/β–transgenic models (38, 41, 43). As a result, the lymph nodes of 8.3-NOD mice consistently had many more CD8+ T cells (>65%) and fewer CD4+ T cells (<15%) than the lymph nodes of 8.3– TCR-β–transgenic and nontransgenic NOD mice (with <25% CD8+ T cells and >40% CD4+ T cells) (Fig. 1 B). Finally, introduction of the 8.3–TCR-α and –TCR-β transgenes into RAG-2−/− NOD mice, which cannot rearrange endogenous TCR genes, resulted in the positive selection of CD8+, but not CD4+, Vβ8.1/8.2+ T cells (see below). Taken together, these data indicate that the 8.3-TCR is expressed on a significant fraction of thymocytes and peripheral T cells from 8.3-NOD mice, and that 8.3– TCR-α/β–transgene expression fosters the positive selection of CD8+ T cells.
Figure 1.
Expression of the TCR-α/β transgenes in 8.3-NOD mice. CD4, CD8, and Vβ8.1/8.2 profiles of thymocytes (A) and lymph node cells (B) from nontransgenic NOD, 8.3–TCR-β–transgenic NOD, and 8.3-NOD mice. (Top) CD4 versus CD8 contour plots of cell suspensions stained with anti-CD8-PE, anti-V β8.1/8.2-FITC, and anti-CD4-biotin plus Streptavidin-PerCP. The lower panels show the Vβ8.1/8.2 fluorescence histograms of each T cell subset after electronic gating. Numbers indicate the average percentage of cells (top) or the average number of Vβ8.1/8.2+ cells (bottom) in each subset. Data correspond to 3–9 mice/group. DP, double-positive cells; DN, double-negative cells.
Functional Responsiveness and Activation State of Peripheral CD8+ T Cells in 8.3-NOD Mice.
To investigate whether peripheral T cells displaying the 8.3-TCR were functionally responsive to beta cells, we compared the proliferative activity of CD4+ T cell–depleted splenic CD8+ T cells from 8.3-NOD and 8.3–TCR-β–transgenic NOD mice in response to irradiated NOD islet cells. As shown in Fig. 2 A, CD8+ T cells from 8.3-NOD, but not 8.3–TCR-β–transgenic NOD mice, proliferated vigorously in these assays, both in the presence and absence of exogenous rIL-2. The increased proliferative activity of splenic CD8+ T cells from 8.3-NOD versus 8.3–TCR-β–transgenic NOD mice resulted from an increased frequency of beta cell–reactive CD8+ T cells (measured at ∼1/17 versus 1/4,000 splenic CD8+ T cells, respectively; Fig. 2 B), rather than from differences in their general proliferative activity, since CD8+ T cells from both types of mice proliferated equally well in response to a plate-bound anti-Vβ8.1/8.2 mAb (KJ16; Fig. 2 C). Therefore, 8.3-NOD mice export numerous beta cell–specific CD8+ T cells to the periphery, and these autoreactive CD8+ T cells are not tolerant to antigen stimulation in vitro.
Figure 2.
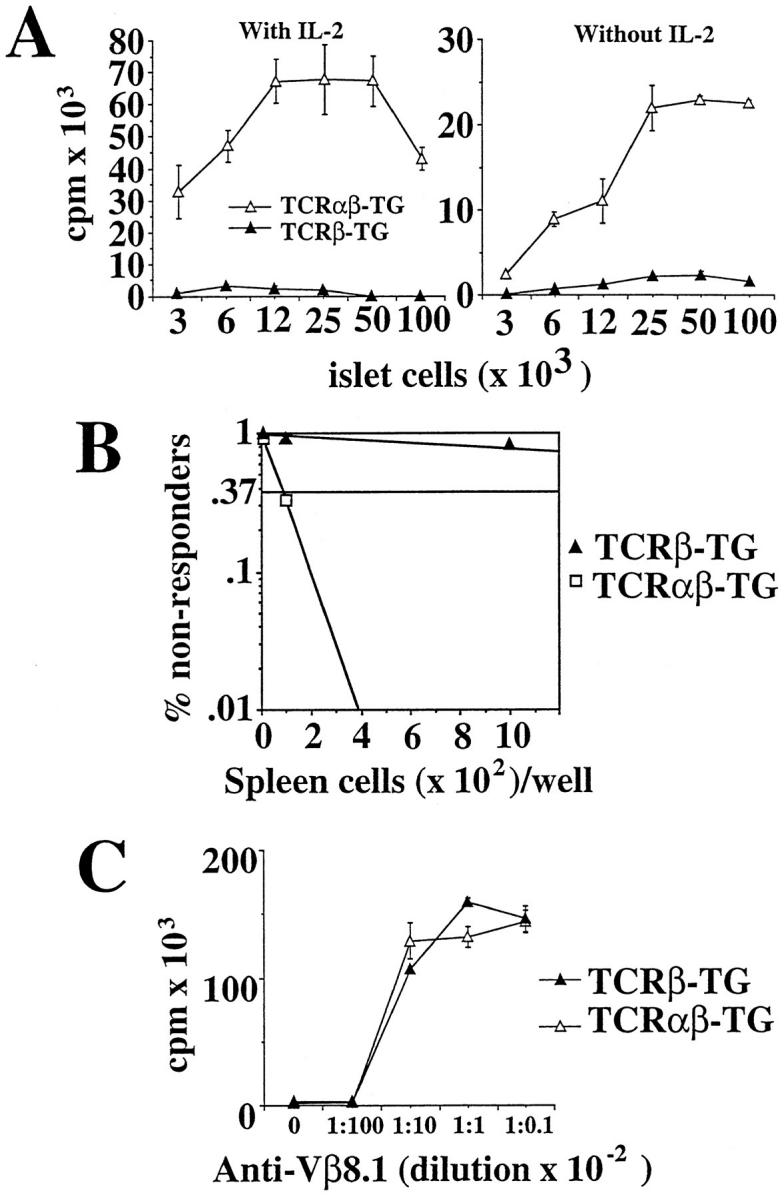
Responsiveness and peripheral frequency of beta cell–specific CD8+ T cells in 8.3-NOD mice. (A) Proliferation of splenic CD8+ T cells from 8.3-NOD and 8.3–TCR-β–transgenic NOD mice to islet cells. 2 × 104 splenic CD8+ T cells were incubated with γ-irradiated islet cells for 3 d, pulsed with [3H]thymidine, harvested, and counted. Bars show the standard error of the means. (B) Peripheral frequency of beta cell–reactive CD8+ T cells in 8.3-NOD and 8.3–TCR-β–transgenic NOD mice. 12 replicate cultures of serial dilutions of splenocytes (101– 105 cells/well) were stimulated with irradiated NOD islets (8/well) for 4 d, expanded in rIL-2 (0.5 U/ml) for 10 d and restimulated once with islets and rIL-2. The cultures were then challenged with 104 NIT-1 or L929-Kd cells for 24 h, and the supernatants collected to measure their TNF-α content. Cultures that secreted TNF-α in response to NIT-1, but not L929-Kd, cells were considered to contain beta cell–reactive CD8+ T cells. (C) General proliferative activity of splenic CD8+ T cells of 8.3-NOD and 8.3–TCR-β–transgenic NOD mice. 2 × 104 splenic CD8+ T cells were incubated with 10-fold serial dilutions of plate-bound KJ16 in rIL-2–containing CM for 3 d, pulsed with [3H]thymidine, harvested, and counted.
Three-color cytofluorometric analyses of splenic CD8+ T cells from diabetic 8.3-NOD and nontransgenic NOD mice with mAbs against memory and activation markers (CD25, CD44, CD69, and CD62L2) did not reveal any significant differences (data not shown), suggesting that the numerous beta cell–reactive CD8+ T cells of 8.3-NOD mice are quiescent. This was further investigated by comparing the ability of naive splenic CD8+ T cells from 8.3-NOD and 8.3–TCR-β–transgenic NOD mice to secrete TNF-α in response to stimulation with beta cells. As shown in Table 1, splenic CD8+ T cells from 8.3-NOD mice did not secrete TNF-α when challenged with NOD-derived beta cells unless they had been previously activated in vitro with plate-bound anti-CD3 mAb and rIL-2. As expected, islet-derived CD8+ T cells from both 8.3-NOD and 8.3– TCR-β–transgenic NOD mice (enriched for in vivo–activated beta cell–specific CD8+ T cells) secreted high levels of TNF-α when challenged with NOD beta cells, but not L929-Kd fibroblasts. It thus appears that the beta cell–reactive CD8+ T cells of 8.3-NOD mice do not undergo spontaneous activation in the periphery.
Table 1.
Secretion of TNF-α by Splenic- and Islet-derived CD8+ T Cells from 8.3-TCR-transgenic NOD Mice (pg/ml)
| Effector | Target | |||
|---|---|---|---|---|
| MIN6N8a | L929-Kd | |||
| pg/ml | pg/ml | |||
| Naive | ||||
| TCR-αβ–TG | <1 | <1 | ||
| TCR-β–TG | <1 | <1 | ||
| α-CD3–activated* | ||||
| TCR-αβ–TG | 300 | <10 | ||
| TCR-β–TG | <1 | <10 | ||
| Islet-derived‡ | ||||
| TCR-αβ–TG | 1,000 | <1 | ||
| TCR-β–TG | 250 | <1 | ||
CD8+ T cells (104) were cultured with NOD-derived insulinoma cells (MIN6N8a) or H-2Kd–transfected L929 cells (L929-Kd) (104) for 24 h. The concentration of TNF-α in the supernatants was determined with a bioassay using WEHI clone 14 cells as indicators. TG, transgenic.
Activated with plate-bound anti-CD3 mAb (10 μg/ml) for 3 d and expanded with rIL-2 for 7 d.
From diabetic mice within 3 d of diabetes onset.
Acceleration of Diabetes in 8.3-NOD Mice.
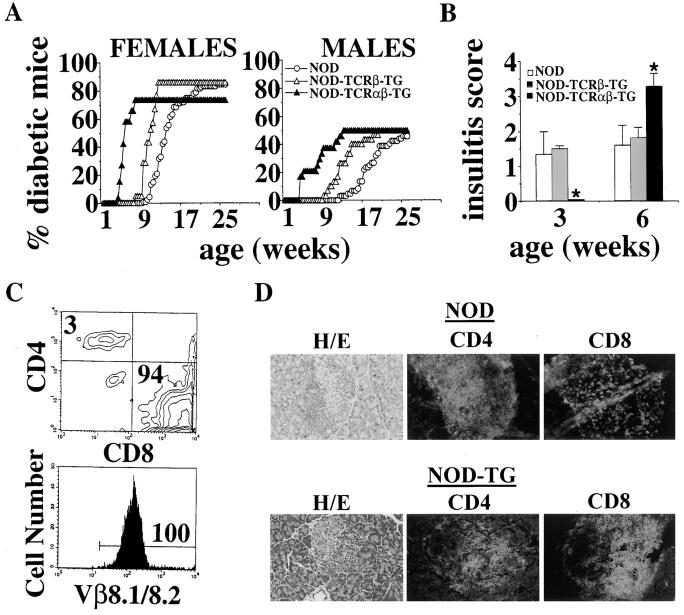
We next investigated whether positive selection of the beta cell–specific 8.3-TCR in 8.3-NOD mice had any pathogenic significance. We therefore followed 8.3-NOD mice of the N3-N6 backcrosses of the founder mice onto the NOD background for development of diabetes, and compared the cumulative incidence curves of these mice with those of nontransgenic NOD mice and 8.3–TCR-β–transgenic NOD mice (26). As shown in Fig. 3 A, female and male 8.3-NOD mice developed diabetes much earlier than female and male 8.3–TCR-β–transgenic (N4-N7), nontransgenic NOD littermates (N3-N5) or NOD/Lt mice (ages at onset: 43 ± 10 versus 88 ± 12, 120 ± 19 and 119 ± 26 d, respectively, for females, P <0.0001; and 66 ± 15 versus 109 ± 33, 173 ± 35, and 157 ± 28 d, respectively, for males, P <0.0001). Despite the dramatic acceleration of diabetes onset in 8.3-NOD mice, the cumulative incidence of diabetes and the kinetics of disease penetrance in the 8.3-NOD, 8.3–TCR-β–transgenic, nontransgenic NOD littermates, and NOD/ Lt mouse populations were remarkably similar (females: 73.1% versus 86, 78, and 84%, respectively; males: 50% versus 50, 31, and 46%, respectively; Fig. 3 A). No major differences in disease penetrance were noted among 8.3-NOD mice of the N3-N6 generations. In females, for example, the incidence and age at onset of diabetes for each generation were: N3, 6/8 mice at 40 ± 4 d; N4, 5/6 mice at 45 ± 6 d; N5, 2/3 mice at 37 ± 3 d; and N6, 6/9 mice at 51 ± 13 d. The same was true for 8.3–TCR-β–transgenic NOD mice (26), and for nontransgenic NOD littermates (N3 females: 3/3 mice at 140 ± 5 d; N4 females: 6/ 8 mice at 117 ± 46 d; and N5 females: 5/7 mice at 117 ± 14 d). Taken together, these data indicate that the 8.3-TCR is highly diabetogenic in the NOD background, and suggest that the events that trigger diabetes in 8.3-NOD mice are similar to those that trigger diabetes in nontransgenic NOD mice.
Figure 3.
8.3–TCR-α/β– transgene expression and diabetogenesis. (A) Incidence of IDDM in female (26 8.3–, 21 8.3–TCR-β–transgenic, and 114 nontransgenic) and male (24 8.3–, 30 8.3–TCR-β–transgenic, and 59 nontransgenic) NOD mice. (B) Progression of insulitis in nontransgenic, 8.3– TCR-β–transgenic, and 8.3-NOD mice (4–7 mice/age group; 15–30 islets/mouse). Bars show the standard deviation of the means. *, P <0.0001 (Mann-Whitney U test). (C) Flow cytometry profile of islet-derived T cells from diabetic 8.3-NOD mice. Islets from acutely diabetic 8.3-NOD mice were cultured in rIL-2–containing CM for 3–5 d and the resulting cells studied by flow cytometry as in Fig. 1. (D) Phenotype of islet-infiltrating T cells in 8.3-NOD versus nontransgenic NOD mice. Pancreas sections were stained with anti-CD8 (53.6-7) or anti-CD4 (GK1.5) mAbs and FITC-labeled anti–rat IgG, and observed under a fluorescence microscope. Original magnification: 200.
Pathogenic Basis of Disease Acceleration in 8.3-NOD Mice.
To elucidate the mechanisms underlying disease acceleration in 8.3-NOD mice, we followed the progression of insulitis in 3–6-wk-old 8.3-NOD, 8.3–TCR-β–transgenic and nontransgenic NOD mice. Unexpectedly, the insulitis scores of 3-wk-old 8.3-NOD mice were significantly lower than the insulitis scores of age-matched 8.3–TCR-β–transgenic and nontransgenic NOD mice (Fig. 3 B). In contrast, the insulitis scores of 6-wk-old 8.3-NOD mice were significantly more severe than those of 8.3–TCR-β–transgenic and nontransgenic NOD mice (Fig. 3 B). It thus appears that insulitis in 8.3-NOD mice starts later, but progresses much faster, than in 8.3–TCR-β–transgenic and nontransgenic NOD mice. As expected, most of the islet-derived T cells of acutely diabetic 8.3-NOD mice were CD8+ and expressed the transgene-encoded Vβ8.1 element (Fig. 3 C). Immunopathological studies of pancreata from these mice confirmed that the insulitis lesions of 8.3-NOD mice contained many more CD8+ T cells, and fewer CD4+ T cells, than the lesions of nontransgenic (Fig. 3 D) and 8.3– TCR-β–transgenic NOD mice (26). Between 3 and 27% of all the islet-derived, beta cell–reactive CD8+ T cell clones from these mice (but 0% of the spleen-derived, beta cell–reactive CD8+ T cell clones) contained serine esterase activity and were cytotoxic to NOD (H-2Kd, Db), but not C57BL/6 (H-2Kb, Db) islet cells in vitro (data not shown). We thus conclude that acceleration of diabetes in 8.3-NOD mice results from massive recruitment of highly diabetogenic, beta cell–cytotoxic CD8+ T cells (and CD4+ T cells) to pancreatic islets early in the disease process.
Spontaneous Diabetes in Monoclonal 8.3-NOD and 4.1-NOD Mice.
Comparison of the natural history of diabetes in 8.3-NOD mice to that in mice expressing the TCR rearrangements of a beta cell–specific, I-Ag7–restricted CD4+ T cell clone (4.1-NOD mice) (33), revealed a remarkable similarity (i.e., similar acceleration of the disease). To investigate whether the highly diabetogenic CD8+ and CD4+ T cells of these mice could spontaneously accumulate in pancreatic islets, differentiate into beta cell–cytotoxic effectors, and destroy beta cells in the absence of T cells displaying endogenous TCRs, we introduced the 8.3- and 4.1-TCR transgenes into RAG-2−/− NOD mice, which cannot rearrange endogenous TCR or Ig genes (34). As expected, virtually all of the lymph node T cells of RAG-2−/− 4.1-NOD mice and RAG-2−/− 8.3-NOD mice were CD4+ Vβ11+ or CD8+Vβ8.1/8.2+ T cells, respectively (Fig. 4 A).
Figure 4.
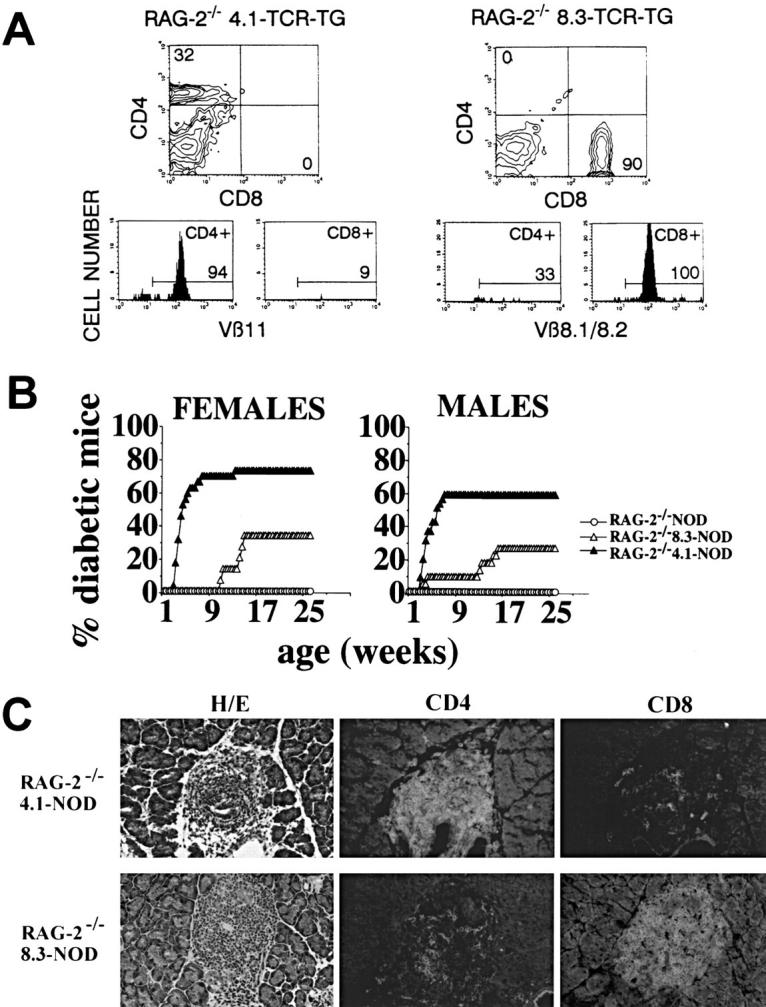
Diabetogenesis in TCR-transgenic RAG-2−/− NOD mice. (A) Flow cytometry profiles of lymph node cells from RAG-2−/− 4.1-NOD mice (left) and RAG-2−/− 8.3-NOD mice (right). (B) Incidence of diabetes in RAG-2−/− 4.1-NOD (n = 29 females and 35 males), RAG-2−/− 8.3-NOD (n = 12 females and 20 males), and RAG-2−/− NOD mice (n = 20 females and 20 males). The few cells of the opposite T cell subset that appear in the flow cytometry profiles of these mice are the result of nonspecific staining of dead cells. (C) Phenotype of islet-infiltrating T cells in RAG-2−/− 4.1-NOD and RAG-2−/− 8.3-NOD mice. Most of the few CD8+ T cells in RAG-2−/− 4.1-NOD mice, and the few CD4+ T cells in RAG-2−/− 8.3-NOD mice were the result of background staining, as they were also seen in anti–rat IgG-FITC–stained tissue.
Surprisingly, RAG-2−/− 4.1-NOD mice developed diabetes slightly earlier than, and almost as frequently as, RAG-2+ 4.1-NOD mice (ages at onset: 36 ± 10 versus 44 ± 13 d for females; and 39 ± 13 versus 56 ± 27 d for males; incidences of 72 versus 76% for females, and 60 versus 73% for males, respectively). In contrast, RAG-2−/− 8.3-NOD mice developed diabetes much less frequently (33 versus 73% for females, P <0.0001; and 26 versus 50% for males, P <0.0001), and significantly later than RAG-2+ 8.3-NOD mice (ages at onset: 111 ± 16 versus 43 ± 10 d for females, P <0.002; and 94 ± 50 versus 66 ± 15 d for males, P <0.05). We are confident that insulitis and diabetes in RAG-2−/− 4.1-NOD and RAG-2−/− 8.3-NOD mice were triggered by T cells expressing the transgenic TCRs, for two reasons. First, nontransgenic RAG-2−/− NOD mice developed neither diabetes (Fig. 4 B) nor insulitis (data not shown). Second, the pancreatic islets of diabetic RAG-2−/− 4.1-NOD and RAG-2−/− 8.3-NOD mice almost exclusively contained CD4+ or CD8+ T cells, respectively (Fig. 4 C). A few cells of the opposite T cell subset were also seen in these mice (Fig. 4 C); however, these cells were probably the result of background staining since they were also seen in sections stained with anti–rat IgG-FITC alone (data not shown). These results demonstrate that (a) 4.1-CD4+ T cells can accumulate efficiently in pancreatic islets and trigger diabetes in the absence of mature B cells and T cells expressing endogenous TCRs, and (b) although 8.3-CD8+ T cells can also cause diabetes in the absence of B cells and T cells expressing endogenous TCRs, they do so much less efficiently than in their presence.
Absence of Extra-pancreatic Pathology in TCR-transgenic RAG-2− /− NOD Mice.
Since nontransgenic NOD mice can also develop inflammation of other organs, notably the submandibular salivary glands and the thyroid (44), we investigated the presence of sialitis in diabetic TCR-transgenic RAG-2−/− NOD mice. Sialitis was invariably present in all the 8.3-NOD, 4.1-NOD, and nontransgenic NOD mice that were investigated (n = 2–7 mice/group), but completely absent in RAG-2−/− NOD, RAG-2−/− 4.1-NOD, and RAG-2−/− 8.3-NOD mice (n = 7–10 mice/group). Inflammation of the thyroid, adrenal glands, kidney, liver, gut, stomach, or muscle was also absent in all these mice. It thus appears that the inflammatory potential of 4.1-CD4+ and 8.3-CD8+ T cells is tissue specific.
Normal CD8+ T Cell Development but Slow Progression of Insulitis in RAG-2−/− 8.3-NOD Mice.
Experiments were next performed to elucidate the mechanisms underlying disease deceleration in RAG-2−/− 8.3-NOD versus RAG-2+ 8.3-NOD mice. Three-color cytofluorometric studies using mAbs against several differentiation markers, including the transgenic TCR, MHC class I (Kd), CD5, CD24, CD44, and CD69, revealed that the CD4+CD8+ and CD4−CD8+ thymocytes of RAG-2−/− 8.3-NOD mice and RAG-2+ 8.3-NOD mice were phenotypically similar (Fig. 5 A). Likewise, no phenotypic differences were noted between the peripheral CD8+ T cells of RAG-2−/− 8.3-NOD mice and those of RAG-2+ 8.3-NOD mice with respect to several markers, including Vβ8.1/8.2, CD2, CD11a, CD28, CD44, CD45RB, CD62L and CD69 (Fig. 5 B). Furthermore, splenic CD8+ T cells from both types of mice proliferated equally well in response to islet cells in vitro (Fig. 5 C). It thus appears that the 8.3-CD8+ T cells of RAG-2−/− 8.3-NOD mice are phenotypically and functionally similar to those of RAG-2+ 8.3-NOD mice.
Figure 5.
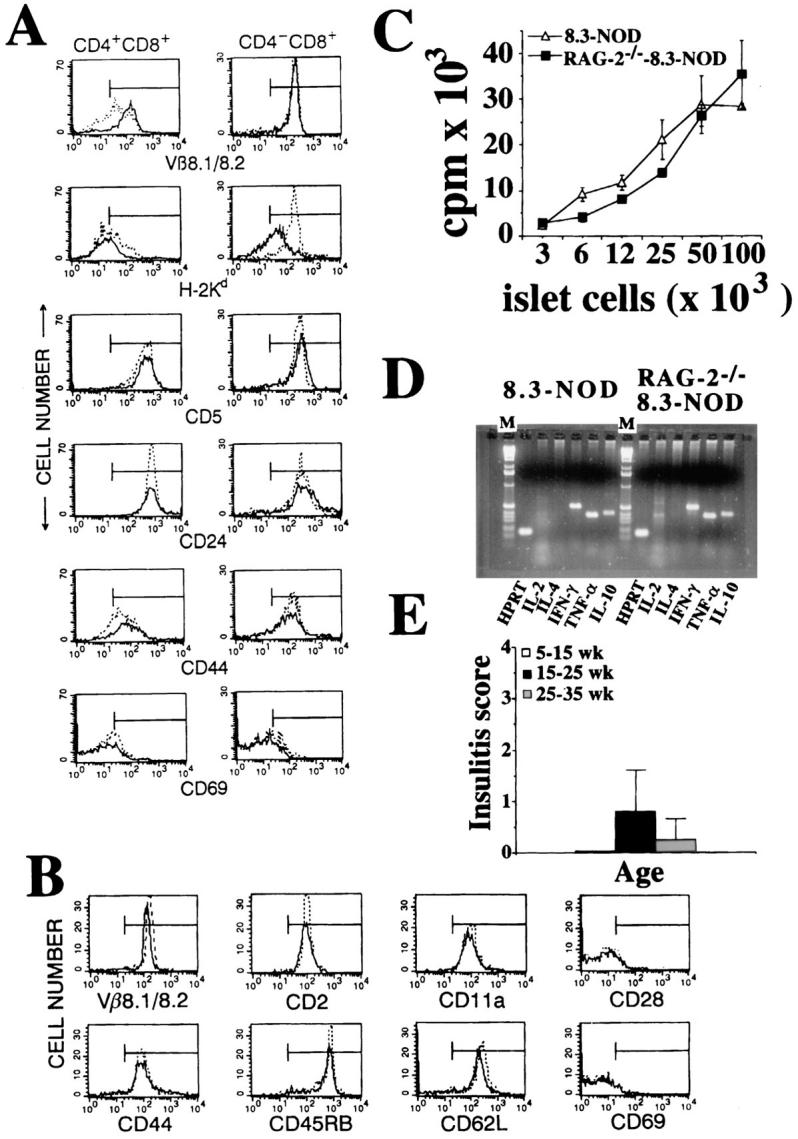
Phenotype and functional activity of CD8+ T cells from RAG-2−/− versus RAG-2+ 8.3-NOD mice. (A) Flow cytometry profiles of CD4+CD8+ (left) and CD4−CD8+ thymocytes (right) from 8.3-NOD mice (dotted line) and RAG-2−/− 8.3-NOD mice (solid line) for maturation markers. Thymocytes were analyzed by three-color cytofluorometry as in Fig. 1. Panels show the fluorescence histograms of each marker on gated CD4+CD8+ and CD4−CD8+ thymocytes. (B) Flow cytometry profiles of splenic CD8+ T cells from 8.3-NOD mice (dotted line) and RAG-2−/− 8.3-NOD mice (solid line) for activation and memory markers. (C) Proliferative activity of splenic CD8+ T cells from 8.3-NOD mice and RAG-2−/− 8.3-NOD mice in response to islet cells. (D) Reverse transcription-PCR analysis for cytokine gene expression of islet-derived CD8+ T cells from 8.3-NOD mice and RAG-2−/− 8.3-NOD mice. M, 1Kb ladder. (E) Kinetics of insulitis in RAG-2−/− 8.3-NOD mice.
We then investigated whether the islet-derived CD8+ T cells of RAG-2−/− 8.3-NOD mice had differentiated into Th1-like CTLs (Tc1) in situ. We first isolated CD8+ T cells from the pancreatic islets of acutely diabetic RAG-2+ 8.3-NOD and RAG-2−/− 8.3-NOD mice and determined their cytokine profile by reverse transcription-PCR. As shown in Fig. 5 D, the islet-derived CD8+ T cells of both types of mice transcribed messenger RNA for IL-2, IFN-γ, IL-10, and TNF-α, but not IL-4. We then determined the percentage of serine esterase–containing T cell clones among those capable of secreting TNF-α specifically in response to NOD beta cells shortly after their isolation from islets. Interestingly, the percentage of serine esterase–positive clones per mouse was, on average, greater than that observed in 8.3-NOD mice (43 versus 13%; data not shown). Thus, lack of CTL generation does not appear to account for the relative diabetes resistance of RAG-2−/− 8.3-NOD mice.
Finally, to determine whether deceleration of diabetes in RAG-2−/− 8.3-NOD mice resulted from slow progression of insulitis, we scored the degree of insulitis in cohorts of 5–35-wk-old nondiabetic RAG-2−/− 8.3-NOD mice. We found that most of these mice had very mild or no insulitis and that, when present, it progressed much slower than in RAG-2+ 8.3-NOD mice (compare Figs. 5 E and 3 B). It thus appears that the rate-limiting factor of diabetogenesis in RAG-2−/− 8.3-NOD mice is the ability of these mice to develop insulitis.
CD4+ T Cells Can Trigger the Recruitment of Naive CD8+ T Cells in RAG-2− /− 8.3-NOD Mice.
To investigate whether nontransgenic splenocytes other than CD8+ T cells could foster the recruitment of the diabetogenic 8.3-CD8+ T cells of RAG-2−/− 8.3-NOD mice into islets, we transfused CD8+ T cell–depleted splenocytes from young nontransgenic NOD mice into RAG-2−/− 8.3-NOD mice or RAG-2−/− NOD mice. As shown in Table 2, spleen cell–transferred RAG-2−/− 8.3-NOD mice developed severe insulitis, owing to massive infiltration of islets by B cells and CD4+ and CD8+ T cells, and four of five mice developed diabetes shortly after transfer (at 36 ± 16 d). In contrast, none of the seven spleen cell–transfused RAG-2−/− NOD mice had developed diabetes or insulitis by 8–10 wk after transfer. To ascertain whether diabetes in RAG-2−/− 8.3-NOD mice was triggered by CD4+ T cells, we injected purified splenic CD4+ T cells from young nontransgenic NOD mice into two RAG-2−/− 8.3-NOD mice. As shown in Table 2, both mice developed severe insulitis (containing CD4+ and CD8+ T cells, but not B cells) and diabetes shortly after transfer (35 ± 22 d). These results demonstrate that the peripheral naive CD8+ T cells of RAG-2−/− 8.3-NOD mice can be induced to migrate into pancreatic islets by splenic CD4+ T cells from nontransgenic NOD mice.
Table 2.
Nontransgenic CD4+ T Cells Can Trigger the Recruitment and Activation of Autoreactive CD8+ T Cells in RAG-2− /− 8.3-NOD Mice
| Cells | Recipient | n | IDDM‡ (n) | Insulitis (n) | Cells in Lesion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B220+ | CD4+ | CD8+ | ||||||||||||
| CD8+ depleted* | RAG-2−/− NOD | 7 | 0/7 | 0 (3) | ND | |||||||||
| RAG-2−/− 8.3-NOD | 5 | 4/5 | 3.2 ± 0.3 (4) | +++ | ++ | +++ | ||||||||
| CD4+§ | RAG-2−/− 8.3-NOD | 2 | 2/2 | 3.4 ± 0.2 (2) | +/− | +++ | +++ | |||||||
Splenocytes from 4–5-wk-old nontransgenic female NOD mice were depleted of CD8+ T cells using anti-CD8 mAb (53-6.7)–coated magnetic beads, as described (26). 8 × 106 cells (containing <0.5% CD8+ T cells) were injected into 5–8-wk-old female mice.
8–10 wk after transfer.
Splenic T cells from nontransgenic female NOD mice were enriched for CD4+ T cells (>92%) and depleted of CD8+ T cells and B220+, F4/80+, Mac-1+, and 33D1+ cells (<0.4%) using mAb-coated immunobeads and affinity columns. Each mouse received 1.5 × 107 cells.
Discussion
Although it is well established that IDDM is a T lymphocyte–dependent autoimmune disease, the role of particular T cell subsets in the disease process remains a matter of intense debate. The emerging consensus, however, seems to be that diabetogenesis is initiated by autoreactive CD8+ T cells, that its progression requires the recruitment of autoreactive CD4+ T cells, and that clinically relevant beta cell destruction is effected by both CD4+ and CD8+ T cells recruited to the site later on in the process (45). This postulate would predict that naive, but potentially diabetogenic, autoreactive CD8+ T cells would be able to home into pancreatic islets and affect beta cell damage in the absence of other T cell specificities. Here we have tested this hypothesis by comparing the ability of TCR transgenes derived from beta cell–specific CD8+ or CD4+ T cells to trigger and/or to accelerate diabetogenesis in RAG-2+ and/or RAG-2−/− TCR-transgenic NOD mice. We have shown that both TCR transgenes have similar diabetogenic potential when expressed in wild-type NOD mice, but not when expressed in RAG-2−/− NOD mice; although CD4+ T cells expressing the 4.1-TCR remained highly diabetogenic when maturing in the absence of endogenous T cells, CD8+ T cells expressing the 8.3-TCR did not. We have also shown that these autoreactive CD8+ T cells do not require CD4+ T cell help to mature properly, to proliferate in response to antigenic stimulation, or to differentiate into CTLs, but that they require a CD4+ T cell–derived signal to efficiently home into pancreatic islets and effect clinically relevant beta cell damage.
Expression of the TCR-α and -β rearrangements of the beta cell–cytotoxic CD8+ T cell clone NY8.3 in NOD mice skewed T cell development towards the CD8+ T cell subset, as expected, and accelerated the onset of IDDM by several months, as a result of massive recruitment of naive CD8+ (and CD4+) T cells into pancreatic islets. Since the 8.3-TCR uses a TCR-α–CDR3 sequence that is highly homologous to that of TCRs used by many NOD islet-derived beta cell–cytotoxic CD8+ T cells (25), we are confident that this TCR is representative of a large fraction of MHC class I–restricted autoreactive TCRs recruited to pancreatic islets in spontaneous IDDM. The dramatic acceleration of diabetes in 8.3-NOD mice was not surprising, since it was consistent with the less dramatic, but significant, acceleration of diabetes that we had seen in transgenic NOD mice expressing only the TCR-β rearrangement of NY8.3 (26). The observation that disease acceleration occurred in 8.3-NOD and 8.3–TCR-β–transgenic NOD mice seemed, at first, to support the hypothesis that autoreactive CD8+ T cells play a critical role in disease initiation. This hypothesis, which is largely based on the observation that β2-microglobulin–deficient (17–19) and anti-CD8 mAb-treated NOD mice (20) develop neither diabetes nor insulitis, proposes that CD8+ T cells affect the initial beta cell insult that exposes beta cell autoantigens to APCs and diabetogenic CD4+ T cells in autoimmune diabetes. Two other observations in 8.3-NOD and 8.3–TCR-β–transgenic NOD mice, however, argued against this scenario. First, the age at onset of insulitis in 8.3-NOD mice was delayed when compared to the age at onset of insulitis in 8.3–TCR-β–transgenic and nontransgenic NOD mice, which have many fewer beta cell–reactive CD8+ T cells than 8.3-NOD mice. Second, despite the large abundance of beta cell–reactive CD8+ T cells in the peripheral lymphoid organs of 8.3-NOD mice, and the dramatic acceleration of diabetes onset, the overall incidence of diabetes in these mice was virtually identical to that seen in 8.3–TCR-β–transgenic and nontransgenic NOD mice. These observations, which are reminiscent of the inability of splenic CD8+ T cells from diabetic NOD mice to transfer insulitis into NOD.scid mice (6), suggested that autoreactive (8.3-like) CD8+ T cells might function more as effectors of beta cell damage, than as initiators of diabetogenesis. The invariable presence of beta cell–cytotoxic CD8+ T cells in pancreatic islets of prediabetic and acutely diabetic nontransgenic NOD mice (21–25), and the ability of some islet-derived CD8+ CTL clones to effect beta cell destruction in vivo in the absence of CD4+ T cells (16, 32), are compatible with this view.
Studies of RAG-2−/− 8.3-NOD mice provided additional support for this interpretation of the data; these mice developed diabetes less frequently and significantly later than 8.3-NOD mice, thus indicating that 8.3-CD8+ T cells had lost most of their diabetogenic potential in the absence of B cells and endogenous T cells. Cytofluorometric and functional studies of thymic and peripheral 8.3-CD8+ T cells of RAG-2−/− 8.3-NOD and 8.3-NOD mice indicated that deceleration of diabetes in RAG-2−/− 8.3-NOD mice was not due to abnormal maturation of 8.3-CD8+ T cells, to their inability to proliferate in response to beta cell autoantigen in vitro, or to their inability to differentiate into beta cell–cytotoxic effectors in the absence of CD4+ Th cells. The fact that 8.3-CD8+ T cells are not dependent on CD4+ Th cells for proliferation and differentiation into CTLs is surprising, but not unprecedented. MHC class II–deficient (46) or CD4-deficient (47) mice, for example, have decreased helper activity for antibody responses, but have unimpaired CTL responses against viruses. Alternatively, the in vitro beta cell–cytotoxic potential of the islet-derived CD8+ T cells of these mice is not acquired in vivo and is an artifact of in vitro manipulation, a possibility that we cannot rule out at present. Whether this is the case or not, our data suggest that decelerated diabetogenesis in RAG-2−/− 8.3-NOD mice results from a difficulty of naive 8.3-CD8+ T cells to home into and/or accumulate in pancreatic islets in the absence of CD4+ T cells; nondiabetic mice did not have, or only had a low degree of, insulitis, and splenic CD4+ T cells from nontransgenic NOD mice were able to trigger the recruitment of diabetogenic 8.3-CD8+ T cells into pancreatic islets when transfused into RAG-2−/− 8.3-NOD mice.
There are several possible mechanisms by which CD4+ T cells may foster the recuitment and/or accumulation of 8.3-CD8+ T cells in islets. CD4+ T cells, and/or the numerous macrophages that are recruited to the site by CD4+ T cells (our unpublished observation), may secrete cytokines and/or chemokines that are essential for the recruitment of naive CD8+ T cells to the site (48). The observation that certain activated, beta cell–reactive CD8+ clones can transfer diabetes into NOD.scid mice in the absence of CD4+ T cells (16) is not incompatible with this view; the homing potential of lymphocytes is a function of their activation state (49). An alternative, but not mutually exclusive, possibility is that since beta cells do not express B7 costimulatory molecules (30), which are essential in T cell activation and differentiation, accumulation of 8.3-CD8+ T cells in islets may require the shedding of their target beta cell autoantigen by a CD4+ T cell–mediated beta cell insult followed by its presentation by APCs capable of diverting exogenous antigens to the MHC class I antigen-processing pathway (e.g., dendritic cells; reference 50). The fact that some RAG-2−/− 8.3-NOD mice do develop diabetes does not necessarily argue against this scenario; because of their unnaturally high frequency, some of the beta cell–reactive CD8+ T cells of these mice may undergo activation in response to cross-reactive antigens or to autoantigenic molecules that are occasionally shed from beta cells even in the absence of any autoimmune-mediated beta cell damage.
The nature and specificity of the CD4+ T cells that may affect the initial beta cell insult that results in the recruitment and activation of naive MHC class I–restricted CD8+ T cells (8.3-like) and other beta cell–cytotoxic effectors in spontaneous IDDM is unknown. Although studies of individual TCR specificities do not allow to draw definitive conclusions about the relative roles of CD4+ versus CD8+ T cells in the initiation of autoimmune diabetes, four different lines of evidence suggest that 4.1-like CD4+ T cells may be capable of playing such a role. First, insulitis in 4.1-NOD mice appeared earlier than in 8.3-NOD mice (33). Second, 4.1-NOD developed diabetes more frequently than, but as early as, 8.3-NOD mice (33). Third, RAG-2−/− 4.1-NOD mice developed diabetes as frequently and as early as 4.1-NOD mice, demonstrating that, unlike 8.3-CD8+ T cells, 4.1-CD4+ T cells can efficiently home into and accumulate in pancreatic islets, differentiate into effector cells, and cause diabetes without the assistance of B cells or other T cells. Finally, and most importantly, the 4.1-TCR has the unique ability of engaging several distinct MHC class II molecules on thymic bone marrow–derived APCs with consequences that are highly reminiscent of the MHC class II–linked resistance of nontransgenic mice to insulitis and diabetes; antidiabetogenic MHC class II molecules can prevent the development of insulitis and diabetes by abrogating the development of 4.1-like CD4+ T cells (33). The above hypothesis that diabetogenesis might be initiated by 4.1-like CD4+ T cells is not incompatible with the large body of evidence indicating that development of insulitis in nontransgenic NOD mice requires CD8+ T cells and/or MHC class I molecules on beta cells (3–6, 17– 20, 27, 28). Development of full-blown insulitis in nontransgenic NOD mice may require both an initial beta cell insult by 4.1-like CD4+ T cells and the immediate recruitment and activation of MHC class I–restricted CD8+ T cells. As pointed out above for RAG-2−/− 8.3-NOD mice, the fact that diabetogenesis in RAG-2−/− 4.1-NOD mice bypasses the need for CD8+ T cells does not contradict this view; the high frequency of beta cell–reactive CD4+ T cells in 4.1-NOD mice may overwhelm the mechanisms that, in nontransgenic NOD mice, would prevent these cells from reaching an insulitogenic mass upon activation.
We do not yet know why 4.1-like CD4+ T cells are so highly diabetogenic; however, since beta cells do not normally express MHC class II molecules (14, 15), it would not be unreasonable to speculate that the diabetogenic potential of 4.1-CD4+ T cells stems from their ability to strongly engage autoantigen/I-Ag7 complexes that are already present on the surface of local APCs before any previous beta cell insult (e.g., secreted proteins). The fact that RAG-2−/− 4.1-NOD mice do not develop sialoadenitis, which is invariably present in nontransgenic NOD mice, does indeed suggest that both the recruitment and activation of 4.1-CD4+ T cells are driven by local APCs. Such a strong interaction between local APCs and 4.1-CD4+ T cells may trigger the upregulation of Fas ligand on the T cells, endowing them with the ability to kill bystander beta cells that have been induced (perhaps by cytokines) to express Fas (51). In fact, islet-derived 4.1-CD4+ T cells from 4.1-NOD mice are efficient killers of Fas cDNA-transfected fibroblasts in vitro (our unpublished observations). These activated CD4+ T cells may also be able to damage beta cells by activating the cytocidal activity (free radical production and cytokine secretion) of the numerous macrophages that they recruit to the site.
Our results do not imply that diabetogenesis in nontransgenic NOD mice is initiated by cells expressing the 4.1-TCR, nor do they rule out the possibility that there might be CD8+ T cell specificities with an independent ability to home into pancreatic islets and affect the initial beta cell insult before the recruitment of CD4+ T cells into pancreatic islets (i.e., those recruited to islets early in the disease process; reference 16). However, the strikingly different natural histories of diabetes in RAG-2−/− 8.3-NOD versus RAG-2−/− 4.1-NOD mice, and the ability of antidiabetogenic MHC class II molecules to prevent diabetogenesis in 4.1-NOD mice by interfering with the developmental biology of the highly diabetogenic 4.1-TCR, lend support to our hypothesis that diabetogenesis in nontransgenic NOD mice may be initiated by 4.1-like CD4+ T cells, rather than by 8.3-like CD8+ T cells.
Acknowledgments
We thank M. Davis, S. Hedrick, E.H. Leiter, P. Marrack, J.-I. Miyazaki, D. Remick, T. Utsugi, J. Yewdell, and J.-W. Yoon for reagents; K. Hirasawa for advice on immunopathology; R. Sangha and R. Sparkes for help with histology; R. Dawson and L. Mock for animal care; L. Bryant for assistance with flow cytometry; and H. Kominek for editorial assistance.
Footnotes
J. Verdaguer was supported by a postdoctoral fellowship from CIRIT (Comissió Interdepartamental de Reçerca i Innovació Tecnològica) Generalitat de Catalunya, Barcelona, Spain. P. Santamaria is a scholar of the Medical Research Council of Canada. This research was supported by grants from the Medical Research Council of Canada, the Natural Sciences and Engineering Council of Canada, and the Juvenile Diabetes Foundation International.
Abbreviations used in this paper: CM, complete medium; HPRT, hypoxanthine phosphoribosyl transferase; IDDM, insulin-dependent diabetes mellitus; NOD, nonobese diabetic; RAG, recombination-activating gene.
References
- 1.Serreze DV, Leiter EH. Genetic and pathogenic basis of autoimmune diabetes in NOD mice. Curr Opin Immunol. 1994;6:900–906. doi: 10.1016/0952-7915(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 2.Tisch R, McDevitt HO. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–297. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 3.Bendelac A, Carnaud C, Boitard C, Bach J-F. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+T cells. J Exp Med. 1987;166:823–832. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller BJ, Appel MC, O'Neil JJ, Wicker LS. Both the Lyt-2+ and L3T4+T cell subsets are required for the transfer of diabetes in non-obese diabetic mice. J Immunol. 1988;140:52–58. [PubMed] [Google Scholar]
- 5.Yagi H, Matsumoto M, Kunimoto K, Kawaguchi J, Makino S, Harada M. Analysis of the roles of CD4+ and CD8+ T cells in autoimmune diabetes of NOD mice using transfer to NOD athymic nude mice. Eur J Immunol. 1992;22:2387–2393. doi: 10.1002/eji.1830220931. [DOI] [PubMed] [Google Scholar]
- 6.Christianson SW, Shultz LD, Leiter EH. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD.NON-thy-1a donors. Diabetes. 1993;42:44–55. doi: 10.2337/diab.42.1.44. [DOI] [PubMed] [Google Scholar]
- 7.Reich EP, Sherwin RS, Kanagawa O, Janeway CA., Jr An explanation for the protective effect of the MHC class II I-E molecule in murine diabetes. Nature (Lond) 1989;341:326–328. doi: 10.1038/341326a0. [DOI] [PubMed] [Google Scholar]
- 8.Haskins K, McDuffie M. Acceleration of diabetes in young NOD mice with a CD4+ islet-specific T cell clone. Science (Wash DC) 1990;249:1433–1436. doi: 10.1126/science.2205920. [DOI] [PubMed] [Google Scholar]
- 9.Nakano N, Kikutani H, Nishimoto H, Kishimoto T. T cell receptor V gene usage of islet beta cell–reactive T cells is not restricted in non-obese diabetic mice. J Exp Med. 1991;173:1091–1097. doi: 10.1084/jem.173.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley BJ, Haskins K, LaRosa FG, Lafferty K. CD8+ T cells are not required for islet destruction induced by a CD4+ islet-specific T-cell clone. Diabetes. 1992;41:1603–1608. doi: 10.2337/diab.41.12.1603. [DOI] [PubMed] [Google Scholar]
- 11.Daniel D, Gill RG, Schloot N, Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from the NOD mouse. Eur J Immunol. 1994;25:1056–1062. doi: 10.1002/eji.1830250430. [DOI] [PubMed] [Google Scholar]
- 12.Katz JD, Benoist C, Mathis D. T helper cell subsets in insulin dependent diabetes. Science (Wash DC) 1995;268:1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 13.Peterson JD, Haskins K. Transfer of diabetes in the NOD-scid mouse by CD4 T cell clones. Differential requirements for CD8 T cells. Diabetes. 1996;45:328–336. doi: 10.2337/diab.45.3.328. [DOI] [PubMed] [Google Scholar]
- 14.Signore A, Pozzilli P, Gale EAM, Andreani D, Beverley PCL. The natural history of lymphocyte subsets infiltrating the pancreas of NOD mice. Diabetologia. 1989;32:282–289. doi: 10.1007/BF00265543. [DOI] [PubMed] [Google Scholar]
- 15.McInerney MF, Rath S, Janeway CA., Jr Exclusive expression of MHC class II proteins on CD45+ cells in pancreatic islets of NOD mice. Diabetes. 1991;40:648–651. doi: 10.2337/diab.40.5.648. [DOI] [PubMed] [Google Scholar]
- 16.Wong FS, Visintin I, Wen L, Flavell RA, Janeway CA., Jr CD8 T cell clones from young NOD islets can transfer rapid onset of diabetes in NOD mice in the absence of CD4 T cells. J Exp Med. 1996;183:67–76. doi: 10.1084/jem.183.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz J, Benoist C, Mathis D. Major histocompatibility complex class I molecules are required for the development of insulitis in non-obese diabetic mice. Eur J Immunol. 1993;23:3358–3360. doi: 10.1002/eji.1830231244. [DOI] [PubMed] [Google Scholar]
- 18.Wicker LS, Leiter EH, Todd JA, Renjilian RJ, Peterson E, Fisher PA, Podolin PL, Zijlstra M, Jaenisch R, Peterson LB. β2-microglobulin–deficient NOD mice do not develop insulitis or diabetes. Diabetes. 1994;43:500–504. doi: 10.2337/diab.43.3.500. [DOI] [PubMed] [Google Scholar]
- 19.Serreze DV, Leiter EH, Christianson GJ, Greiner D, Roopenian DC. Major histocompatibility complex class I–deficient NOD-β2mnullmice are diabetes and insulitis resistant. Diabetes. 1994;43:505–508. doi: 10.2337/diab.43.3.505. [DOI] [PubMed] [Google Scholar]
- 20.Wang B, Gonzalez A, Benoist C, Mathis D. The role of CD8+ T cells in initiation of insulin-dependent diabetes mellitus. Eur J Immunol. 1996;26:1762–1769. doi: 10.1002/eji.1830260815. [DOI] [PubMed] [Google Scholar]
- 21.Hayakawa M, Yokono K, Nagata M, Hatamori N, Ogawa W, Miki A, Mizoguti H, Baba S. Morphological analysis of selective destruction of pancreatic beta cells by cytotoxic T lymphocytes in NOD mice. Diabetes. 1991;40:1210–1217. doi: 10.2337/diab.40.9.1210. [DOI] [PubMed] [Google Scholar]
- 22.Nagata M, Yoon J-W. Studies on autoimmunity for T-cell–mediated beta cell destruction. Diabetes. 1992;41:998–1008. doi: 10.2337/diab.41.8.998. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu J, Kanagawa O, Unanue E. Presentation of beta cell antigens to CD4+ and CD8+ T cells of non-obese diabetic mice. J Immunol. 1993;151:1723–1730. [PubMed] [Google Scholar]
- 24.Nagata M, Santamaria P, Kawamura T, Utsugi T, Yoon J-W. Evidence for the role of CD8+ cytotoxic T cells in the destruction of pancreatic beta cells in NOD mice. J Immunol. 1994;152:2042–2050. [PubMed] [Google Scholar]
- 25.Santamaria P, Utsugi T, Park B-J, Averill N, Yoon J-W. Beta cell–cytotoxic CD8+ T-cells from nonobese diabetic mice use highly homologous T-cell receptor α-chain CDR3 sequences. J Immunol. 1995;154:2494–2503. [PubMed] [Google Scholar]
- 26.Verdaguer J, Yoon J-W, Anderson B, Averill N, Utsugi T, Park B-J, Santamaria P. Acceleration of spontaneous diabetes in TCRβ-transgenic nonobese diabetic mice by beta cell–cytotoxic CD8+ T-cells expressing identical endogenous TCR α chains. J Immunol. 1996;157:4726–4735. [PubMed] [Google Scholar]
- 27.Kay TWH, Parker JL, Stephens LA, Thomas HE, Allison J. RIP-β2-microglobulin transgene expression restores insulitis, but not diabetes, in β2-microglobulinnullnonobese diabetic mice. J Immunol. 1996;157:3688–3693. [PubMed] [Google Scholar]
- 28.Serreze DV, Chapman HD, Varnum DS, Gerling I, Leiter EH, Shultz LD. Initiation of autoimmune diabetes in NOD/Lt mice is MHC class I-dependent. J Immunol. 1997;157:3978–3986. [PubMed] [Google Scholar]
- 29.Thivolet C, Bendelac A, Bedossa P, Bach JF, Carnaud C. CD8+ T cell homing to the pancreas in the nonobese diabetic mouse is CD4+ T cell-dependent. J Immunol. 1991;146:85–93. [PubMed] [Google Scholar]
- 30.Stephens LA, Kay TWH. Pancreatic expression of B7 co-stimulatory molecules in the non-obese diabetic mouse. Int Immunol. 1995;7:1885–1895. doi: 10.1093/intimm/7.12.1885. [DOI] [PubMed] [Google Scholar]
- 31.Vyse TJ, Todd JA. Genetic analysis of autoimmune disease. Cell. 1996;85:311–318. doi: 10.1016/s0092-8674(00)81110-1. [DOI] [PubMed] [Google Scholar]
- 32.Utsugi T, Yoon J-W, Park B-J, Imamura M, Averill N, Kawazu S, Santamaria P. Major histocompatibility complex class I–restricted infiltration and destruction of pancreatic islets by NOD mouse-derived β-cell cytotoxic CD8+ T-cell clones in vivo. Diabetes. 1996;45:1121–1131. doi: 10.2337/diab.45.8.1121. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt D, Verdaguer J, Averill N, Santamaria P. A mechanism for the MHC-linked resistance to autoimmunity. J Exp Med. 1997;186:1059–1075. doi: 10.1084/jem.186.7.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinkai Y, Rathbun G, Lam K-P, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. RAG-2–deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 35.Spevik T, Niessen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–106. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 36.Taffs, R., and M. Sitkovsky. 1992. Granule enzyme exocytosis assay for cytotoxic T lymphocyte activation. In Current Protocols in Immunology. J.E. Coligan, A.M. Krusbeek, D.H. Margulies, E.M. Shevach, and W. Strober, editors. John Wiley and Sons, New York. 3.16.1–3.16.8. [DOI] [PubMed]
- 37.Wesselingh SL, Levine B, Fox RJ, Choi S, Griffin DE. Intracerebral cytokine mRNA expression during fatal and non-fatal alpha-virus encephalitis suggests a predominant type 2 T cell response. J Immunol. 1994;152:1289–1297. [PubMed] [Google Scholar]
- 38.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T cell receptor transgenic mice involves deletion of nonmature CD4+CD8+ thymocytes. Nature (Lond) 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 39.Berg LJ, Fazekas de St B, Groth, Pullen AM, Davis MM. Phenotypic differences between αβ vs β T-cell receptor transgenic mice undergoing negative selection. Nature (Lond) 1989;340:559–562. doi: 10.1038/340559a0. [DOI] [PubMed] [Google Scholar]
- 40.Berg LJ, Pullen AM, Fazekas de St B, Groth, Mathis D, Benoist C, Davis MM. Antigen/MHC-specific T-cells are preferentially exported from the thymus in the presence of their MHC ligand. Cell. 1989;58:1035–1046. doi: 10.1016/0092-8674(89)90502-3. [DOI] [PubMed] [Google Scholar]
- 41.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature (Lond) 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 42.Ohashi PS, Pircher H, Bürki K, Zinkernagel RM, Hengartner H. Distinct sequence of negative or positive selection implied by thymocyte T-cell receptor densities. Nature (Lond) 1990;346:861–863. doi: 10.1038/346861a0. [DOI] [PubMed] [Google Scholar]
- 43.Sha W, Nelson C, Newberry R, Kranz D, Russell J, Loh D. Positive and negative selection of an antigen receptor on T-cells in transgenic mice. Nature (Lond) 1988;336:73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- 44.Wicker LS, Appel MC, Dotta F, Pressey A, Miller BJ, DeLarato NH, Fischer PA, Boltz RC, Jr, Peterson LB. Autoimmune syndromes in major histocompatibility complex (MHC) congenic strains of non-obese diabetic (NOD) mice. The NOD MHC is dominant for insulitis and cyclophosphamide-induced diabetes. J Exp Med. 1992;176:67–77. doi: 10.1084/jem.176.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benoist C, Mathis D. Cell death mediators in autoimmune diabetes: no shortage of suspects. Cell. 1997;89:1–3. doi: 10.1016/s0092-8674(00)80174-9. [DOI] [PubMed] [Google Scholar]
- 46.Grusby MJ, Johnson RS, Papaioannou VE, Glimcher LH. Depletion of CD4+cells in major histocompatibility complex class-II–deficient mice. Science (Wash DC) 1991;253:1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- 47.Rahemtulla A, Fung-Leung WP, Schillam MW, Kundig TM, Sambhara SR, Narendran A, Arabian A, Wakeham A, Paige CJ, Zinkernagel RM, et al. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature (Lond) 1991;353:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 48.Mackay CR. Chemokine receptors and T cell chemotaxis. J Exp Med. 1996;184:799–802. doi: 10.1084/jem.184.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science (Wash DC) 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 50.Germain R. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 51.Chervonsky AV, Wang Y, Wong FS, Visintin I, Flavell RA, Janeway CA, Jr, Matis LA. The role of Fas in autoimmune diabetes. Cell. 1997;89:17–24. doi: 10.1016/s0092-8674(00)80178-6. [DOI] [PubMed] [Google Scholar]