Abstract
Granzyme (gzm) A and gzmB have been implicated in Fas-independent nucleolytic and cytolytic processes exerted by cytotoxic T (Tc) cells, but the underlying mechanism(s) remains unclear. In this study, we compare the potential of Tc and natural killer (NK) cells of mice deficient in both gzmA and B (gzmA×B−/−) with those from single knockout mice deficient in gzmA (−/−), gzmB (−/−), or perforin (−/−) to induce nuclear damage and lysis in target cells. With the exception of perforin−/−, all in vitro– and ex vivo–derived Tc and NK cell populations from the mutant strains induced 51Cr-release in target cells at levels and with kinetics similar to those of normal mice. This contrasts with their capacity to induce apoptotic nuclear damage in target cells. In gzmA×B−/− mice, Tc/NK-mediated target cell DNA fragmentation was not observed, even after extended incubation periods (10 h), but was normal in gzmA-deficient and only impaired in gzmB-deficient mice in short-term (2–4 h), but not long-term (4–10 h), nucleolytic assays. This suggests that gzmA and B are critical for Tc/NK granule– mediated nucleolysis, with gzmB being the main contributor, while target cell lysis is due solely to perforin and independent of both proteases.
Cytotoxic T (Tc) cells mediate target cell lysis by two independent pathways, one involving exocytosis of preformed granules, the other requiring ligation of Fas ligand on the effector cell with the Fas receptor on target cells (1–7). Both processes lead to target cell apoptosis and lysis (7) by intracellular mechanisms which are still poorly understood. Perforin, gzmA, and gzmB have been implicated as main contributors to membrane and/or nuclear disintegration of target cells by the secretory pathway (7). Membrane damage of target cells, as measured by 51Cr-release, is mainly accounted for by perforin, which itself is capable of causing target cell cytolysis (7–9). However, gzmA and B have been shown to cause DNA fragmentation and apoptotic morphology of target cells, but only in the presence of perforin (10–12).
Controversy still remains as to the distinct contribution of each of the two proteases to Fas-independent Tc/NK cell– mediated DNA fragmentation of target cells and to what extent they affect perforin-mediated target cell lysis. gzmA and B seem to be able to cross the target cell membrane (7, 13) via a still undefined process, but their nucleolytic activities seem to follow distinct pathways. Whereas gzmB induces early DNA fragmentation by cleaving multiple cysteine proteases of the caspase cascade, including FLICE (14) and CPP32 (15–17), the nucleolytic activity of gzmA seems to be delayed and independent of caspases (12, 18, 19). Although indirect evidence suggested that Tc cell–mediated target cell lysis correlates with the expression of gzmA and/or B (11, 20, 21), no reduction of Fas-independent cytolytic activity was observed with Tc cell populations from gzmA (22, 23) or gzmB knockout (ko) mice (18). Here we use double ko mice deficient in both gzmA and B to investigate their role in cytotoxicity further.
Materials and Methods
Mouse Strains and Genetic Analysis.
gzmA×gzmB− ko mice (gzmA×B−/−) were generated by crossing gzmA−/− (22) with gzmB−/− (18) mice and by subsequent intercrossing of heterozygous F1 animals. C57BL/6 (B6), BALB/c (B/c), gzmA−/−, gzmB−/−, and two strains of perforin deficient mice, perforin−/− (B6 background; reference 2) and perforin−/− (129 × B6 background; reference 24), were maintained at the Max-Planck-Institut and the John Curtin School of Medical Research under pathogen-free conditions. No differences were found in any of the experiments between the two perforin−/− strains. Only mice of the same sex were used in individual experiments at 12–20 wk of age.
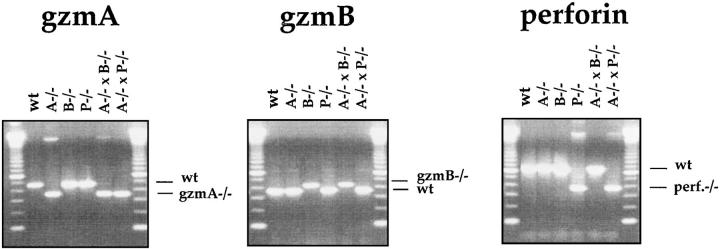
For detection of the respective mutations (Fig. 1), DNA was analyzed by PCR, as previously described (22), using the following primers: gzmA−/−: 5′-AGG AGC AAT ATA TAC CAA TGG-3′ and 5′-AGG TAG GTG AAG GAT AGC CAC-3′; neo-primer: 5′-CGG AGA ACC TGC GTG CAA TC-3′. gzmB−/−: 5′-CTG CTA CTG CTG ACC TTG TCT-3′ and 5′-TGA GGA CAG CAA TTC CAT CTA-3′; neo-primer: 5′-TTC CTC GTG CTT TAC GGT ATC-3′. Perforin−/−: 5′-CCA CTC CAC CTT GAC TTC AAA AAG GCG-3′ and 5′-TGG GCA GCA GTC CTG GTT GGT GAC CTT-3′; neo-primer: 5′-CGG AGA ACC TGC GTG CAA TC-3′. The genomic DNA was subjected to amplification by PCR and analyzed as previously described (22).
Figure 1.
Analysis of wild-type (C57BL/ 6) and the mutant mice gzmA−/−, gzmB−/−, gzmA×B−/−, and perforin−/− mice by PCR. Tail DNA from individual mice was analyzed by PCR amplification using the gzmA-, gzmB-, and perforin-specific primer pairs, as indicated in Materials and Methods.
All mutant and normal B6 mice (female and male) were analyzed for their gzmA, gzmB, and perforin genotypes (Fig. 1) before experimentation.
Target Cells.
P815 (H-2d), EL4.F15 (EL4), (H-2b), L1210.3 (H-2d), and L1210.Fas (EL4 transfected with Fas cDNA, H-2d; reference 1), TA-3 (H-2d), A1.1 (H-2d), and YAC-1 (H-2a) were maintained in culture as has been previously described (22, 25).
For detection of influenza virus–immune Tc cells, EL4 cells were pretreated with 10−5 M synthetic peptide (ASNENMETM) derived from the nucleoprotein of A/influenza virus specific for Db (NPP; reference 26) as has been previously described (25).
Generation of Tc and NK Cells In Vitro and In Vivo.
To generate alloreactive Tc cells in vitro, H-2b responder splenocytes from mutant and B6 mice (106/ml) were cultivated together with irradiated (3,000 rad) stimulator splenocytes from B/c mice (106/ml) at a ratio of 2:1 for 5–6 d. Restimulation for secondary MLC was performed by incubating in vitro–derived Tc cells (5 × 104/ml) with stimulator (2.5 × 106/ml) cells in medium supplemented with supernatant (10% final solution) from rat spleen cells desensitized with 5 μg/ml Con A in medium for 24 h (Con A–SN, supplemented with 20 mg/ml α-methyl-d-mannoside; Sigma Chemical Co., Munich, Germany).
To generate primary in vivo alloreactive Tc cells, mice were injected intraperitoneally with 107 PFU of recombinant vaccinia virus encoding the MHC class I heavy chain gene Kd, as has been previously described (27). Effector splenocytes were used as ex vivo alloreactive Tc cells 6 d after immunization.
The generation of influenza-immune Tc cells has been previously described (28). In brief, B6 mice were immunized intravenously with 103 hemagglutination units of influenza virus A/WSN (H1N1). The cytolytic potential of their spleen cells was tested at day 6 after infection on NPP-modified or control EL4 target cells.
In vivo NK effector cells were splenocytes from mice injected intraperitoneally with either 100 μg poly (I:C) 20 h (29) or immunized with 108 PFU Semliki forest virus (SFV) 2 d earlier. Effector cells were phenotyped as described (30).
Cytotoxicity Assays.
All cytotoxicity assays were performed in cell culture medium, in which FCS was replaced by BSA (2 mg/ml). The 51Cr-release assay was performed for 2–10 h, as previously described (22). The 125I-DNA–release assay was performed essentially as previously described (31), with some modifications (22). Target cells (2 × 106) were labeled with 10 μCi 125I-deoxyuridine (125I-UDR; Amersham Corp., Arlington Heights, IL) in 400 μl in polystyrol tubes for 3 h. Effector cells were mixed with 2 × 103–2 × 104 labeled target cells in triplicates at the indicated effector/target ratio (e/t) in 200 μl IMDM, complemented with 12 mM Hepes and 2 mg/ml BSA. The plates were centrifuged at 500 rpm for 5 min and incubated for 1 h. After centrifugation (1,200 rpm, 10 min) 100 μl of supernatant was removed and 100 μl of lysis buffer (10 mM Tris/HCl, pH 7.5, 1 mM EDTA, 0.4% Triton- X-100) was added. After 10 min of incubation at room temperature, the plates were centrifuged (1,200 rpm, 10 min). 100 μl of supernatant was collected and released radioactivity was measured. In some experiments, 3 mM EGTA plus 4.5 mM Mg2+ (EGTA-Mg2+) was added to the assay to exclusively assess Fas-based cytotoxicity (32).
Results
Role of gzmA, gzmB, and Perforin in Target Cell Lysis.
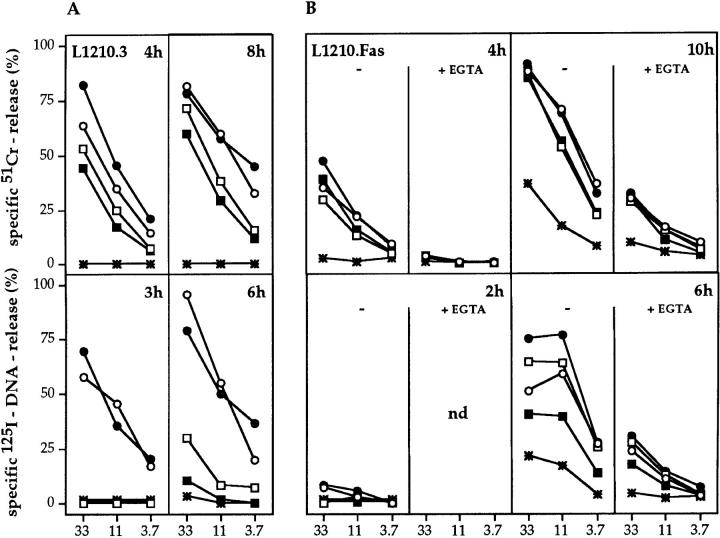
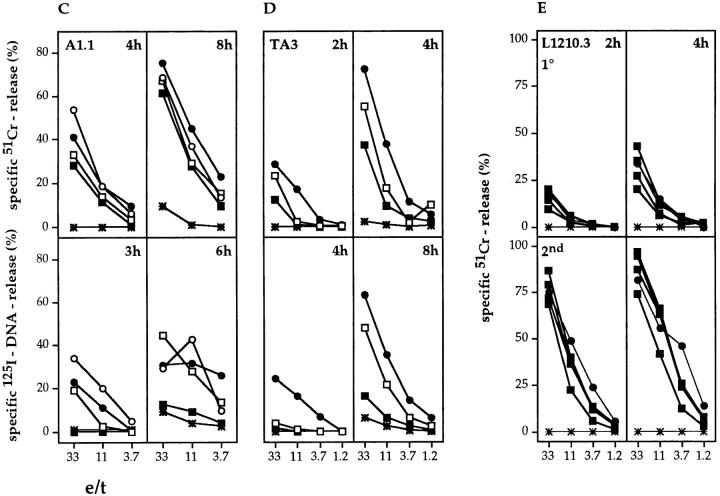
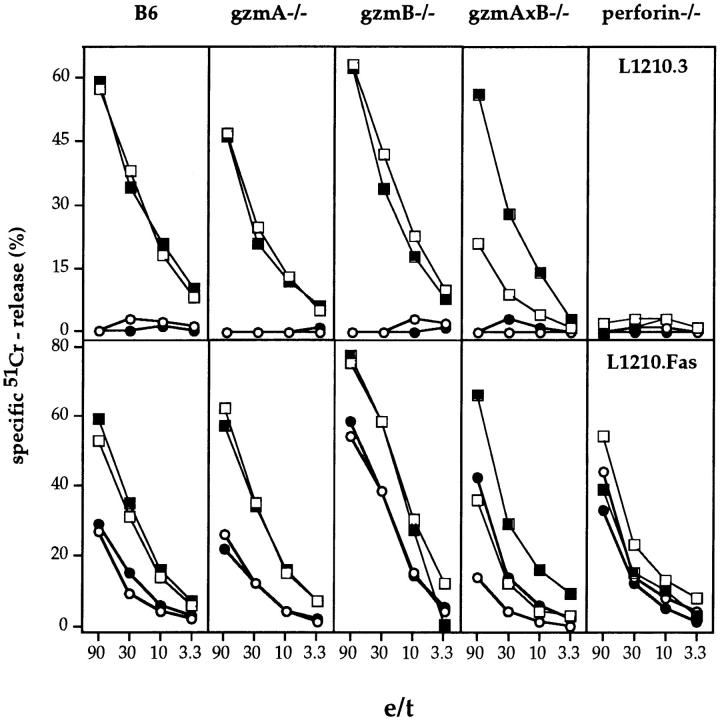
In vitro–derived H-2d–reactive Tc cells from gzmA−/−, gzmB−/−, gzmA×B−/−, perforin−/−, and B6 mice were tested for their ability to induce 51Cr-release in various target cell populations in 2–10-h cytotoxicity assays. Representative experiments are shown in Fig. 2, A–E. Tc cells from all gzm ko mice, including gzmA×B−/−, expressed cytolytic activities with kinetics and levels comparable to those seen with B6 Tc, when tested on either L1210.3, L1210.Fas, A1.1, or TA3 lymphoma target cells (Fig. 2, A–D, top panels) and P815 mastocytoma targets (data not shown). In addition, Tc cells from gzmA−/−, gzmB−/−, gzmA×B−/−, and B6 also lysed L1210.Fas target cells in the presence of EGTA-Mg2+ to the same extent, when tested at 10 h, indicating the involvement of the Fas pathway (1). Tc cells from perforin−/− mice did not express any cytolytic activity on the respective target cells with the exception that 51Cr-release was observed with L1210.Fas target cells in a 10-h assay, which was reduced in the presence of EGTA-Mg2+. As slight variations in lytic activity of gzmA×B−/− T cells occurred between individual sets of experiments, we tested the cytolytic activity of alloreactive Tc cell populations from eight (four male, four female) individual gzmA×B−/− mice and compared them with B6 alloreactive Tc cells in a primary and secondary MLC (Fig. 2 E, only shown for male mice). No differences in lytic activity could be discerned. Similar results were obtained with in vivo–derived H-2d reactive splenocytes from two individual mice of B6, gzmA−/−, gzmB−/−, gzmA×B−/−, and perforin−/− strains (Fig. 3). This was true for both target cells, L1210.3 and L1210.Fas, with the exception of one gzmA×B−/− mouse, which showed reduced cytolytic activity on both target cells. However, in vivo–generated alloreactive Tc cells from perforin−/− mice lysed L1210.Fas (Fig. 3) and P815 (data not shown) targets at levels comparable to those from B6 and gzm−/− mice. Lysis in the presence of EGTA-Mg2+ was only marginally reduced. The phenotype of the in vitro– and ex vivo–derived cytolytic cells from all mutant and B6 mice was CD4−CD8+ and no cytolytic activity was observed on H-2–matched EL4 target cells (data not shown). The differences observed in the cytolytic activity of in vitro– versus ex vivo–derived alloreactive Tc cells from perforin−/− mice may reflect differential regulation of Fas ligand and Fas expression during ex vivo induction and in vitro culture.
Figure 2.
51Cr-release (top) and 125I-DNA release (bottom) of L1210 (A and E), L1210.Fas (B), A1.1 (C), and TA3 (D) target cells induced by in vitro– derived alloreactive Tc cells. Splenocytes from B6 (•), gzmA−/− (○), gzmB−/− (□), gzmA×B−/− (▪), and perforin−/− (*) mice (pools of two spleens per mouse strain, A–D; (E) spleens of four individual gzmA×B−/− (▪), one B6 mouse (•), and one perforin−/− mouse (*) were activated in primary MLC (A–D, E, top) or secondary MLC (E, bottom), and tested for cytolytic (top) and nucleolytic (bottom) activities for the indicated time periods. All values are the mean lysis of triplicate samples at three e/t values given. SEM never exceeded 3%.
Figure 3.
51Cr-release from L1210.3 (top) and L1210.Fas (bottom) target cells induced by in vivo–derived alloreactive splenic effector cells from two individual (open and closed symbols) mutant or B6 mice in the absence (squares) and presence (circles) of EGTA. Mice were immunized intraperitoneally with 107 PFU of vaccinia virus encoding Kd 6 d before removal of spleen. All assays were harvested after 6 h. All values are the mean lysis of triplicate samples at the four e/t values given. SEM never exceeded 3%.
To determine if ex vivo–derived virus-specific Tc cells possess cytolytic phenotypes similar to those of in vitro– or ex vivo–derived alloreactive Tc cells, we tested ex vivo– derived influenza virus A/WSN–immune Tc cell for 51Cr-release of NPP-modified EL4 target cells (Fig. 4). Effector populations of all three infected gzm mutant mouse strains, but not perforin−/− mice, showed specific cytolytic activities in 2- and 4-h assays, at levels and with kinetics comparable to those of B6 mice. No 51Cr-release was seen with any of the Tc populations on untreated EL4 cells (data not shown).
Figure 4.
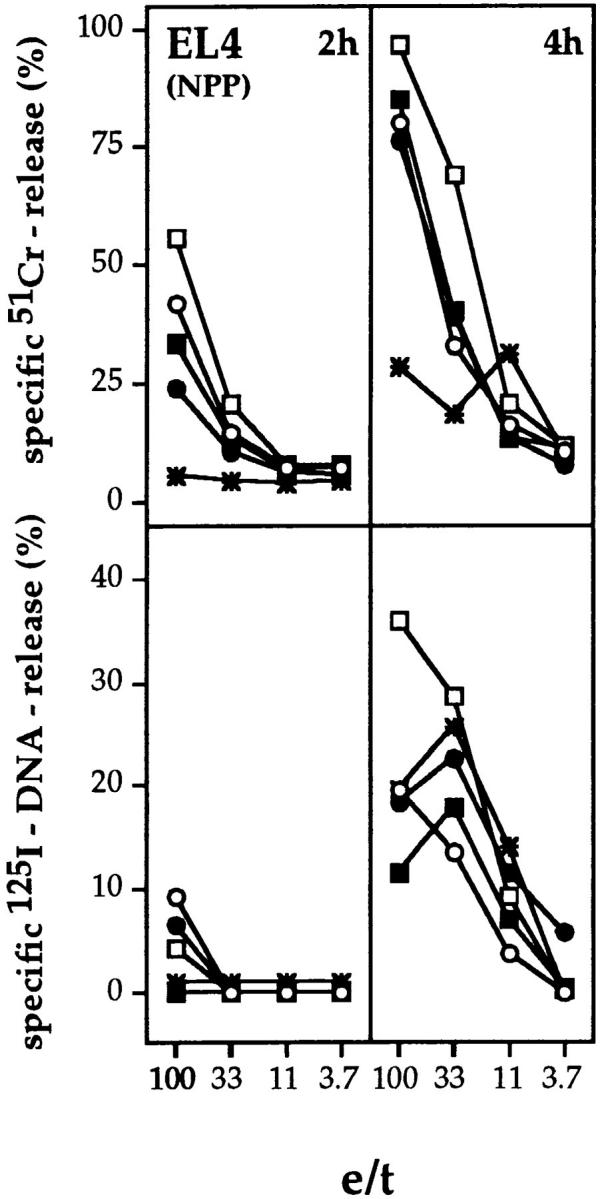
51Cr-release (top) and 125I-DNA release (bottom) from NPP-modified EL4 target cells by ex vivo–derived influenza virus A/WSN–immune Tc from mutant or B6 mice. B6 (•), gzmA−/− (○), gzmB−/− (□), gzmA×B−/− (▪), and perforin−/− (*) mice were infected intravenously with influenza virus A/WSN and their splenocytes (pool of three mice) were tested after 6 d for cytolytic (top) and nucleolytic (bottom) activities on NPP-modified EL4 target cells. Assay times were 2 and 4 h. All values are the mean lysis of triplicate samples at the four e/t values given. SEM never exceeded 3%.
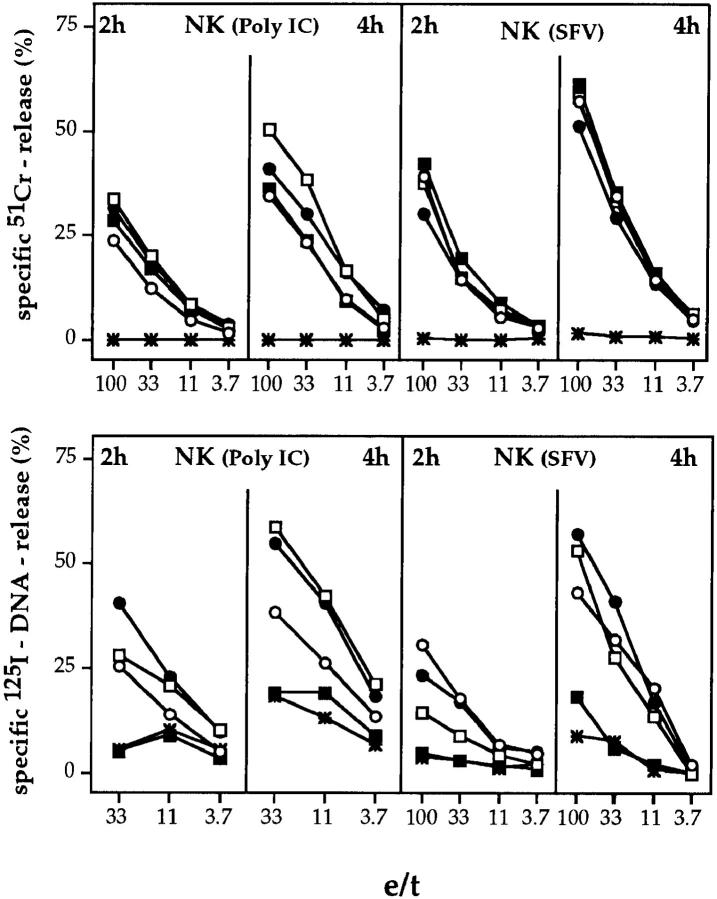
To assess the cytolytic activities of ex vivo–derived NK cell populations of mutant and B6 mice, recipients were treated with either poly I:C (29) or SFV (30). Splenocytes were tested for lytic activity on 51Cr-labeled YAC-1 target cells. Similar levels of cytolytic activity were observed with effector cell populations from gzmA−/−, gzmB−/−, gzmA× B−/−, and B6 mice, when assayed between 2 and 4 h (Fig. 5, top). The occasional reduction in the activity of NK cells from gzm single or double ko mice as compared to B6 mice to induce 51Cr-release was neither significant nor reproducible in three additional experiments (data not shown). As shown in previous studies (2), marginal or no NK cell– mediated lytic activity was obtained with splenocytes from perforin−/− mice.
Figure 5.
51Cr-release (top) and 125I-DNA release (bottom) from YAC-1 target cells by ex vivo–derived NK cells from mutant or B6 mice. B6 (•), gzmA−/− (○), gzmB−/− (□), gzmA×B−/− (▪), and perforin−/− (*) mice were either treated with poly I:C (20 h) or infected with SFV (2 d) and their splenocytes (poly I:C, pool of two spleens; SFV, pool of three spleens) were tested for cytolytic (top) or nucleolytic (bottom) activities on YAC-1 target cells. Assay times were 2 and 4 h. All values are the mean lysis of triplicate samples at three or four e/t values given. SEM never exceeded 3%.
Role of gzmA, gzmB, and Perforin in Target Cell DNA Fragmentation.
In vitro–derived H-2d–reactive Tc cells from gzmA−/− and B6 mice were indistinguishable in their ability to induce DNA fragmentation in L1210.3, L1210.Fas, A1.1, and TA3 target cells when tested between 2 and 8 h of incubation (Fig. 2, bottom panels). In contrast, Tc cells from gzmB−/− mice were defective in their nucleolytic potential on the same targets in short term assays, i.e., between 2 and 4 h. However, after incubation of 6–8 h, this defect was either partially (L1210.3, 6 h) or completely (L1210.Fas, 6 h; A1.1, 6 h; TA3, 8 h) abrogated. Most importantly, the absence of nucleolytic activity was more pronounced in Tc cells from gzmA×B−/− as compared to gzmB−/− mice and only marginal nuclear damage was seen in long-term assays with gzmA×B−/− Tc cells. Although gzmA×B−/− Tc cells occasionally induced some DNA fragmentation in L1210.Fas and P815 target cells (data not shown), the level of nucleolytic activity was always lower than that seen with gzmB−/− Tc cells. Marginal or no nucleolytic activity was seen with Tc cells from perforin−/− mice. No 125I-release in H-2–matched control EL4 cells was seen with any of the five Tc cell populations (data not shown).
The analysis of the specific nucleolytic potential of ex vivo–derived influenza virus–immune Tc cell populations revealed that in a 2-h assay, perforin−/−, and gzmA×B−/− mice were defective, whereas Tc cell populations from gzmA−/−, gzmB−/−, and B6 mice each induced significant and similar amounts of 125I-release in NPP-modified EL4 target cells (Fig. 4, bottom) at high e/t ratio. Interestingly, in 4-h assays similar levels of DNA fragmentation were obtained on NPP-modified EL4 target cells with Tc cells from all mice tested, including gzmA×B−/− and perforin−/−. Since EL4 cells express Fas (data not shown), Fas-dependent nucleolytic activities of ex vivo–derived influenza virus immune Tc cells may contribute to DNA fragmentation of target cells at later stages of the assay.
When tested for their nucleolytic activity on YAC-1 target cells in 2- and 4-h assays, ex vivo–derived NK cells from perforin−/− and gzmA×B−/− mice were defective and caused, if any, only marginal DNA fragmentation. In contrast, NK cells from gzmA−/− and gzmB−/− mice expressed nucleolytic activities which were already apparent in 2-h assays and increased in 4-h assays, comparable with those of B6 mice.
Discussion
The results of this study show that perforin-mediated target cell lysis by in vitro– and ex vivo–derived Tc and NK cells is independent of gzmA and B, but that DNA fragmentation induced by this pathway may involve both enzymes. gzmB appears to be the main effector molecule acting early during leukocyte-mediated apoptosis, whereas gzmA nucleolytic activity acts with delayed kinetics that may complement or substitute for a gzmB deficiency.
The data obtained with gzmA−/− and gzmB−/− single ko and gzmA×B−/− double ko mice clarify previous conflicting results regarding the role of the two enzymes in Tc/NK cell–induced 51Cr-release from target cells. The fact that a noncytotoxic rat basophilic leukemia mast cell tumor line, transfected with perforin cDNA, only conferred optimal cytolytic activity upon coexpression of gzmA and B, was taken as evidence for a synergistic role of the two enzymes in perforin-mediated target cell death (11). On the other hand, the recent findings that Tc/NK cell–induced 51Cr-release was not altered in gzmA−/− or gzmB−/− single ko mice (18, 22, 23) suggested that neither of the two proteases alone is critical for perforin-induced target cell membrane damage. The present demonstration that in vitro– and ex vivo– derived Tc and NK cell populations from gzmA×B−/− mice induced lysis in various target cells, at levels and with kinetics similar to those of single mutant or B6 mice, unequivocally demonstrates that the cytolytic pathway leading to 51Cr-release is independent of gzmA and gzmB. However, the participation of other, as yet undefined, proteases in perforin-mediated lysis cannot be ruled out. At present, there is no obvious explanation for the discrepancy between the results presented here and a previous report demonstrating a role for gzmB in NK- but not Tc- or LAK cell–mediated 51Cr-release (33). It is particularly confounding that comparable ex vivo–derived NK cell populations (poly I:C) and target cells (YAC-1) were used in both studies.
Several recent reports have already provided evidence for the involvement of gzms in perforin-initiated nuclear damage (10–12), the absence of early DNA fragmentation in leukocyte-mediated killing of gzmB−/− mice being the most definitive (18). However, no such involvement of gzmA in these processes could be established using gzmA−/− mice (22, 23). The evidence suggests that the biological functions of gzmA and B are not merely redundant. This was deduced from the distinct substrate specificities of gzmA and B (34, 35) and because gzmA is not critical during early events of DNA fragmentation (22, 23). However, an involvement of gzmA in later stages of this pathway could not be excluded. This is also supported by the recent demonstration that inhibitors of caspases, in particular CPP32, prevent the expression of early gzmB-based but not late gzmA-based nucleolytic activity by Tc cells (19). This study provides definitive evidence for such an interpretation and presents additional information in that in vitro– and ex vivo–derived gzmA×B−/− Tc/NK cells are distinct from those of gzmB−/− mice in their perforin-based nucleolytic activity. Impairment of nucleolytic activity of gzmA×B−/− Tc/NK cells was shown in short-term and, in most cases, also in long-term assays.
In contrast to cytolytic and nucleolytic activities observed with in vitro–generated Tc cells from B6 and mutant mice, data obtained with ex vivo–derived influenza immune Tc cells were variable. This may be due to the distinct genotypes of gzmA−/− (B6; reference 22) versus gzmB−/− (129 × B6; reference 18) and gzmA×B−/− (129 × B6) mice. It is well established that genes outside the MHC play a crucial role in the recovery from ectromelia, a natural viral pathogen, and from influenza infection (reference 36 and Simon, M.M., and A. Müllbacher, unpublished data). Thus, the various responses to the pathogens by non-MHC genes may influence the kinetics of NK/Tc cell development in gzmA−/− and gzmB−/− mice, thereby masking the actual role of gzms in cytolysis/nucleolysis. To alleviate this problem we are currently breeding the gzmB−/− gene locus onto the B6 background. This will provide us with the ability to reveal the biological roles of gzmA, gzmB, and perforin not only in target cell cytolysis/nucleolysis, but also in immunopathology and in the control of infections.
Acknowledgments
We would like to thank Ian Haidl for reading the manuscript and for valuable suggestions. We also thank the laboratory of R. Zinkernagel (Zürich, Switzerland) for the generous gift of perforin (B6) ko mice and T. Ley (St. Louis, MO) for providing the gzmB ko mouse.
This work was supported in part by a grant from the Deutsche Forschungsgemeinschaft, Si 214/7-1.
References
- 1.Rouvier R, Luciani M-F, Golstein P. Fas involvement in Ca2+-independent T cell–mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kägi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature (Lond) 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 3.Kägi D, Vignaux F, Ledermann B, Bürki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell–mediated cytotoxicity. Science (Wash DC) 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 4.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature (Lond) 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 5.Walsh CM, Matloubian M, Liu C-C, Ueda R, Kurahara CG, Christensen JL, Huang MTF, Young JD-E, Ahmed R, Clark WR. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kojima H, Shimohara N, Hanaoka S, Someya-Shirota Y, Takagaki Y, Ohnoa H, Saito T, Katayama T, Yagita H, Okumura K, et al. Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity. 1994;1:357–364. doi: 10.1016/1074-7613(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 7.Henkart PA. Lymphocyte-mediated cytotoxicity: two pathways and multiple effector molecules. Immunity. 1994;1:343–346. doi: 10.1016/1074-7613(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 8.Henkart PA, Millard PJ, Reynolds CW, Henkart MP. Cytolytic activity of purified cytoplasmic granules from cytotoxic rat large granular lymphocyte tumors. J Exp Med. 1984;160:75–93. doi: 10.1084/jem.160.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Podack ER, Hengartner H, Lichtenheld MG. A central role of perforin in cytolysis? . Annu Rev Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- 10.Hayes MP, Berrebi GA, Henkart PA. Induction of target cell DNA release by the cytotoxic T lymphocyte granule protease granzyme A. J Exp Med. 1989;170:933–947. doi: 10.1084/jem.170.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakajima H, Park HL, Henkart PA. Synergistic roles of granzymes A and B in mediating target cell death by rat basophilic leukemia mast cell tumors also expressing cytolysin/perforin. J Exp Med. 1995;181:1037–1046. doi: 10.1084/jem.181.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi L, Kam C-M, Powers JC, Aebersold R, Greenberg AH. Purification of three cytotoxic lymphocyte granule serine proteases that induce apoptosis through distinct substrate and target cell interactions. J Exp Med. 1992;176:1521–1529. doi: 10.1084/jem.176.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi LF, Mai S, Israels S, Browne K, Trapani JA, Greenberg AH. Granzyme B (graB) autonomously crosses the cell membrane and perforin initiates apoptosis and GraB nuclear localization. J Exp Med. 1997;185:855–866. doi: 10.1084/jem.185.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/ FADD-interacting protease, in Fas/APO-1- and TNF receptor–induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 15.Darmon AJ, Nicholson DW, Bleackley RC. Activation of the apoptotic protease CPP32 by cytotoxic T-cell-derived granzyme B. Nature (Lond) 1995;377:446–448. doi: 10.1038/377446a0. [DOI] [PubMed] [Google Scholar]
- 16.Quan LT, Tewari M, O'Rourke K, Dixit V, Snipas SJ, Poirier GG, Ray C, Pickup DJ, Salvesen GS. Proteolytic activation of the cell death protease Yama/CPP32 by granzyme B. Proc Natl Acad Sci USA. 1996;93:1972–1976. doi: 10.1073/pnas.93.5.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandes-Alnemri T, Armstrong RC, Krebs J, Srinivasula SM, Wang L, Bullrich F, Fritz LC, Trapani JA, Tomaselli KJ, Litwack G, Alnemri ES. In vitro activation of Cpp32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc Natl Acad Sci USA. 1996;93:7464–7469. doi: 10.1073/pnas.93.15.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heusel JW, Wesselschmidt RL, Shresta S, Russell JH, Ley TJ. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76:977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- 19.Anel A, Gamen S, Alava MA, Schmitt-Verhulst AM, Pineiro A, Naval J. Inhibition of CPP32-like proteases prevents granzyme B– and Fas-, but not granzyme A–based cytotoxicity exerted by CTL clones. J of Immunol. 1997;158:1999–2006. [PubMed] [Google Scholar]
- 20.Shiver JW, Su L, Henkart PA. Cytotoxicity with target DNA breakdown by rat basophilic leukemia cells expressing both cytolysin and granzyme A. Cell. 1992;71:315–322. doi: 10.1016/0092-8674(92)90359-k. [DOI] [PubMed] [Google Scholar]
- 21.Macen JL, Garner RS, Musy PY, Brooks MA, Turner PC, Moyer RW, McFadden G, Bleackley RC. Differential inhibition of the Fas- and granule-mediated cytolysis pathways by the orthopoxvirus cytokine response modifier A/SPI-2 and SPI-1 protein. Proc Natl Acad Sci USA. 1996;93:9108–9113. doi: 10.1073/pnas.93.17.9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebnet K, Hausmann M, Lehmann-Grube F, Müllbacher A, Kopf M, Lamers M, Simon MM. Granzyme A–deficient mice retain potent cell-mediated cytotoxicity. EMBO (Eur Mol Biol Organ) J. 1995;14:4230–4239. doi: 10.1002/j.1460-2075.1995.tb00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müllbacher A, Ebnet K, Blanden RV, Stehle T, Museteanu C, Simon MM. Granzyme A is essential for recovery of mice from infection with the natural cytopathic viral pathogen, ectromelia. Proc Natl Acad Sci USA. 1996;93:5783–5787. doi: 10.1073/pnas.93.12.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowin B, Beermann F, Schmidt A, Tschopp J. A null mutation in the perforin gene impairs cytolytic T lymphocyte– and natural killer cell–mediated cytotoxicity. Proc Natl Acad Sci USA. 1994;91:11571–11575. doi: 10.1073/pnas.91.24.11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müllbacher A, Hill AB, Blanden RV, Cowden WB, King NJ, Hla RT. Alloreactive cytotoxic T cells recognize MHC class I antigen without peptide specificity. J Immunol. 1991;147:1765–1772. [PubMed] [Google Scholar]
- 26.Rammensee HG, Friede T, Stevanoviic S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;414:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 27.Flynn K, Mullbacher A. Memory alloreactive cytotoxic T cells do not require costimulation for activation in vitro. Immunol Cell Biol. 1996;74:413–420. doi: 10.1038/icb.1996.71. [DOI] [PubMed] [Google Scholar]
- 28.Yap KL, Ada GL. Cytotoxic T cells specific for influenza virus-infected target cells. Immunology. 1977;32:151–159. [PMC free article] [PubMed] [Google Scholar]
- 29.Morelli L, Lusigman Y, Lemieux S. Heterogeneity of natural killer cell subsets in NK-1.1+ and NK-1.1-inbred mouse strains and their progeny. Cell Immunol. 1992;141:148–160. doi: 10.1016/0008-8749(92)90134-b. [DOI] [PubMed] [Google Scholar]
- 30.Müllbacher A, King NJC. Target cell lysis by natural killer cells is influenced by β2-microglobulin expression . Scand J Immunol. 1989;30:21–29. doi: 10.1111/j.1365-3083.1989.tb01184.x. [DOI] [PubMed] [Google Scholar]
- 31.Russell JH. Internal disintegration model of cytotoxic lymphocyte-induced target damage. Immunol Rev. 1983;72:97–118. doi: 10.1111/j.1600-065x.1983.tb01074.x. [DOI] [PubMed] [Google Scholar]
- 32.Vignaux F, Vivier E, Malissen B, Depraetere V, Nagata S, Golstein P. TCR/CD3 coupling to Fas-based cytotoxicity. J Exp Med. 1995;181:781–786. doi: 10.1084/jem.181.2.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shresta S, MacIvor DM, Heusel JW, Russell JH, Ley TJ. Natural killer and lymphokine-activated killer cells require granzyme B for the rapid induction of apoptosis in susceptible target cells. Proc Natl Acad Sci USA. 1995;92:5679–5683. doi: 10.1073/pnas.92.12.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon, M.M., K. Ebnet, and M.D. Kramer. 1993. Molecular analysis and possible pleiotropic function(s) of the T cell–specific serine proteinase-1 (TSP-1). In Cytotoxic Cells: Effector Function, Generation, Methods. M. Sitkowsky and P.A. Henkart, editors. Birkhäuser, Boston. 278–294.
- 35.Haddad, P., D.E. Jenne, O. Kraehenbuehl, and J. Tschopp. 1993. Structure and possible functions of lymphocyte granzymes. In Cytotoxic cells: Effector Function, Generation, Methods. M.V. Sitkovsky and P.A. Henkart, editors. Birkhäuser, Boston. 251–262.
- 36.Blanden, R.V., B.D. Deak, and H.O. McDevitt. 1989. Strategies in virus-host interactions. In Immunology of Virus Diseases. R.V. Blanden, editor. Brolga Press, Curtin, Australia.