Abstract
Chemokines are a structurally related family of cytokines that are important for leukocyte trafficking. The C-C chemokine monocyte chemoattractant protein-1 (MCP-1) is a potent monocyte activator in vitro and has been associated with monocytic infiltration in several inflammatory diseases. One C-C chemokine receptor, CCR2, has been identified that mediates in vitro responses to MCP-1 and its close structural homologues. CCR2 has also recently been demonstrated to be a fusion cofactor for several HIV isolates. To investigate the normal physiological function of CCR2, we generated mice with a targeted disruption of the ccr2 gene. Mice deficient for CCR2 developed normally and had no hematopoietic abnormalities. However, ccr2 −/− mice failed to recruit macrophages in an experimental peritoneal inflammation model. In addition, these mice were unable to clear infection by the intracellular bacteria, Listeria monocytogenes. These results suggest that CCR2 has a nonredundant role as a major mediator of macrophage recruitment and host defense against bacterial pathogens and that MCP-1 and other CCR2 ligands are effectors of those functions.
Chemokines are a large family of structurally related secreted proteins that are important for leukocyte trafficking (1–3). The regulated interaction of chemokines with their respective receptors are thought to mediate the controlled recruitment of specific leukocyte subpopulations required during host defense and inflammation (4). The specific biological functions of chemokines and their receptors has been difficult to predict, since most chemokines bind more than one receptor and most chemokine receptors bind more than one chemokine ligand in vitro. The analysis of mutant mice lacking a single chemokine ligand or chemokine receptor gene has been useful for determining some of their specific physiological functions. Nonredundant roles in neutrophil and eosinophil recruitment (5–7), hematopoiesis (5–8), inflammatory response to viral infection (9), and neutrophil-mediated host defense, granuloma formation, and cytokine balance (6) have been demonstrated in mice lacking the chemokine receptors CXCR2 and CCR1 and the chemokine ligands macrophage inflammatory protein (MIP) -1α, stromal cell-derived factor-1 (SDF-1), and eotaxin. Although many chemokines including MIP-1α are chemotactic for monocytes in vitro and several chemokine receptors including CCR1 are expressed on monocytes and macrophages, no chemokines or receptors have been identified so far with a specific role in monocyte or macrophage function.
The chemokine ligand monocyte chemoattractant protein-1 (MCP-1) is a potent in vitro monocyte activator that has been associated with monocytic infiltration in several inflammatory diseases (10). Two related human receptors, CCR2A and CCR2B, have been identified that mediate in vitro responses to MCP-1, and one homologous murine receptor, CCR2, has been identified that mediates in vitro responses to the murine MCP-1 analogue, JE (11–13). Both human and murine CCR2 function as receptors for several other close structural homologues of MCP-1 (13–17), and human CCR2 can also function as a fusion coreceptor for several HIV isolates (18, 19). Transgenic mouse models have demonstrated monocyte/macrophage recruitment to sites of human MCP-1 or murine JE expression (20–22), and neutralizing antibody studies have implicated MCP-1 as a major mediator of macrophage recruitment in several inflammatory models (23–25). These studies have suggested that MCP-1 is important for monocyte/macrophage recruitment in vivo, and that CCR2 may mediate such in vivo responses. To determine if CCR2 may play a role in macrophage recruitment and function, we have generated mice with a targeted disruption of the ccr2 gene. Our studies indicate that CCR2 has a nonredundant function as a major mediator of macrophage recruitment and host defense to bacterial infection.
Materials and Methods
Targeted Disruption of the Murine ccr2 Gene.
The murine ccr2 gene was cloned from a 129/Sv embryonic stem cell genomic library using a 150-bp ccr2 cDNA fragment as probe. A 1.1-kb XbaI-BamHI fragment containing part of the 3′-UTR of ccr2 was inserted between the neomycin resistance cassette (PGK-neo) and herpes simplex virus thymidine kinase cassette (PGK-TK) of the pPNT vector. A 5.5-kb SpeI-BamHI fragment containing the amino-terminal coding region of ccr2 was then cloned into the opposite side of the PGK-neo cassette. A 4.2-kb SalI-XhoI fragment containing the lacZ gene was inserted 3′ of the 5.5-kb genomic fragment to encode an in-frame fusion of the 57 NH2-terminal residues of CCR2 and β-galactosidase. The resulting 18.4-kb targeting vector (pPNT-ccr2lacZ) was linearized with NotI and electroporated (240V/500 μF) into 129/Sv-derived CJ7 embryonic stem cells. After positive selection with G418 (375 μg/ml) and negative selection with FIAU, doubly resistant clones were screened for homologous recombination by Southern blotting with a 0.5-kb BamHI-SpeI external probe. Homologous recombination was detected at a frequency of 1 in 480 clones, and correctly targeted ES cells were injected into blastocysts or aggregated with morula from ICR mice. Male chimeras derived from both injection and aggregation were mated with ICR females to obtain germline transmission of the mutated allele.
Antibodies.
The region of ccr2 encoding the 49 amino-terminal amino acids was amplified by PCR using the primers: 5′-GCGGGAATTCGATGGAAGACAATAATATGTTACCT-3′ and 5′-GTAGGGATCCCTAACTGGTTTTATGACAAGGCTCACC-3′. The amplified fragment was digested with EcoRI and BamHI, cloned into the EcoRI/BamHI sites of the MS2 polymerase fusion vector pEX34c, and the fusion protein induced as described (26). The fusion protein was purified from MetaPhor agarose (FMC Corp., Rockland, ME) gels according to the manufacturer's instructions, and the purified protein injected into New Zealand white rabbits. IgG was purified from rabbit serum by MabTrapG (Pharmacia Biotech Inc., Piscataway, NJ).
Flow Cytometry and Immunoprecipitations.
Flow cytometry was performed with a flow cytometer (Coulter Epics Profile II; Coulter Corp., Hialeah, FL). Single-cell suspensions were prepared from femoral bone marrow and stained as previously described (27). 5 × 105 cells were first incubated with 1 μg of anti-CCR2 IgG and then incubated with PE-conjugated anti-rabbit IgG and FITC-F4/80. For immunoprecipitations, 6 × 106 bone marrow cells were labeled overnight with 1 mCi [35S]methionine. Cell extracts were prepared and immunoprecipitation performed as previously described (26), using 1 μg of anti-CCR2 IgG.
Thioglycollate-induced Peritonitis and Peritoneal Leukocyte Counts.
Peritoneal leukocytes were lavaged with 6 ml of RPMI1640/10% fetal bovine serum and total cell counts determined with a hemacytometer. 5 × 104 cells were cytospun, stained with Diff-Quik, and the percentage of macrophages, neutrophils, and eosinophils determined from a count of at least 300 cells. These percentages were multiplied by total cell number to obtain the number of peritoneal macrophages, neutrophils, and eosinophils. Thioglycollate-elicited cells were obtained by peritoneal lavage 72 h after i.p. injection of 2 ml of 3% Brewer's thioglycollate broth (Difco, Detroit, MI) in PBS.
Listeria Monocytogenes Infection.
Listeria monocytogenes (laboratory strain A25616) cultures were grown in Tryptic Soy Broth (GIBCO BRL, Gaithersburg, MD). Adult mice were infected by intravenous injection (via tail vein) with 2,500 CFU of L. monocytogenes. The lungs, liver, and spleen of infected animals were removed 5 d after injection or at autopsy for animals that died on day 4. The numbers of viable L. monocytogenes present in the liver, spleen, and lungs were determined by plating serial dilutions of organ homogenates on 5% sheep blood agar. A portion of the sampled organs were fixed by immersion in 10% neutral-buffered formalin for histopathological evaluation. Tissues were processed by routine methods, embedded in paraffin, and 5-μm sections were stained with either hematoxylin and eosin or Gram stain.
Results and Discussion
Generation of ccr2 Null Mice.
The murine ccr2 gene was inactivated by homologous recombination using 129/Sv-derived embryonic stem (ES) cells (Fig. 1 a). Correctly targeted ES cells were used to generate chimeras, two of which transmitted the mutant allele to their offspring. Heterozygous mice were intercrossed under pathogen-free conditions, and resulting litters were healthy and normal in size. Genotypic analysis of such intercrosses indicated that wild-type (+/+), heterozygous (+/−), and mutant (−/−) mice were born at the expected Mendelian ratio (Fig. 1 b). CCR2-deficient mice bred normally and were histopathologically unremarkable (data not shown).
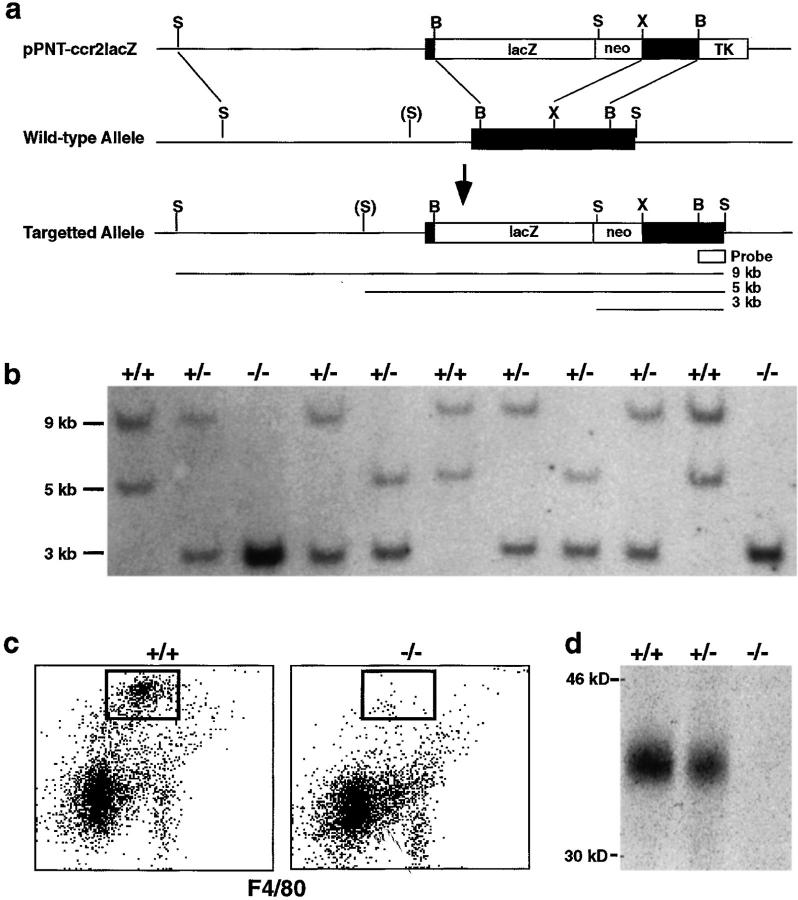
Figure 1.
Targeted disruption of the mouse ccr2 gene. (a) The wild-type ccr2 genomic DNA locus is depicted in the middle. Relevant restriction endonuclease sites are indicated: B, BamHI; S, SpeI; X, XbaI. S is a polymorphic SpeI site present in ICR and absent in 129/Sv DNA. The targeting vector pPNT-ccr2lacZ is shown at the top. Thick lines represent genomic sequences, and the thin lines represent plasmid DNA sequences. The black box indicates the ccr2 coding exon. The lacZ-, PGK-neo, and PGK-TK cassettes are shown as open boxes. The targeted allele created by homologous recombination of the targeting vector with wild-type genomic DNA is shown at the bottom. The 0.5-kb SpeI-BamHI genomic fragment used for Southern blot analyses is indicated along with the lengths of diagnostic restriction fragments. (b) Analysis of offspring from ccr2 +/− heterozygote intercrosses. Tail DNA was digested with SpeI and analyzed by Southern blotting. The 9-kb fragment indicates a wild-type 129/Sv allele, the 5-kb fragment indicates a wild type ICR allele, and the 3-kb fragment is specific for the recombined allele. (c) Flow cytometry analysis of femoral bone marrow cells isolated from wild-type (+/+) and mutant (−/−) mice. Cells were stained with anti-CCR2 IgG and FITC-F4/80 followed by PE-goat anti–rabbit IgG. The rectangle highlights a CCR2-staining cell population present in wild-type but not mutant mice. (d) Immunoprecipitation analysis of CCR2 expression in wild-type (+/+), heterozygous (+/−), and homozygous (−/−) mutant mice. Labeled protein extracts from femoral bone marrow cells was immunoprecipitated with anti-CCR2 IgG.
Since ccr2 mRNA is detected in mononuclear cells (11–13), CCR2 protein expression was evaluated in bone marrow cells using a CCR2 polyclonal antibody. Flow cytometry analysis demonstrated CCR2 costaining with the macrophage marker F4/80 on bone marrow cells from ccr2 +/+ mice (Fig. 1 c). The same F4/80-positive population was present in bone marrow cells from ccr2 −/− mice but surface expression of CCR2 was not detected. To confirm the lack of CCR2 protein, radiolabeled bone marrow cell extracts were immunoprecipitated with anti-CCR2. A 40-kD protein corresponding to CCR2 was detected in extracts from both ccr2 +/+ and ccr2 +/− mice but not from ccr2 −/− mice (Fig. 1 d). The presence of similar F4/80-positive populations in the bone marrow of wild-type and homozygous mutant mice suggests that CCR2 is not essential for development of the mononuclear cell lineage. Flow cytometry of thymocytes, splenocytes, lymph node, and bone marrow cells with macrophage, T cell, and B cell markers also revealed no differences between ccr2 +/+ and ccr2 −/− mice (data not shown). Thus, unlike mice deficient for the chemokine ligands SDF-1 (8) and eotaxin (7) or the chemokine receptors CXCR2 (5) and CCR1 (6), CCR2-deficient mice have no obvious defect in hematopoietic development.
Macrophage Recruitment Defect in CCR2-Mutant Mice.
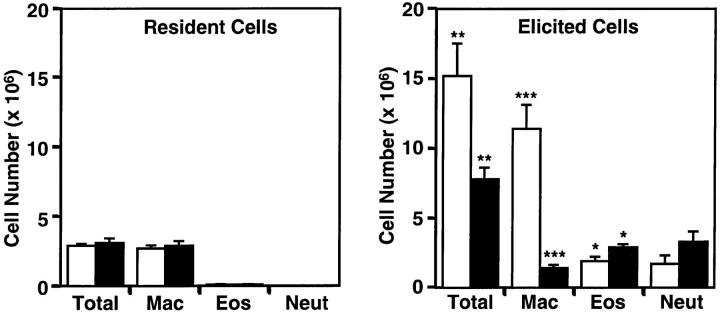
MCP-1, a high-affinity ligand for CCR2, has been associated with monocytic infiltrates in several inflammatory diseases (10), and transgenic mouse models have demonstrated that monocytes and macrophages are recruited to sites of MCP-1 expression (20–22). These results suggested that CCR2 may be involved in monocyte trafficking during inflammation. To assess the role of CCR2 in macrophage recruitment in response to a nonspecific inflammatory stimulus, thioglycollate was injected into the peritoneum of wild-type and homozygous mutant mice. There were no differences in the total number of cells and macrophages in the peritoneum of uninjected ccr2 +/+ and ccr2 −/− mice (Fig. 2). At 72 h after thioglycollate injection, there were significantly fewer cells recovered from the peritoneum of ccr2 −/− mice compared with ccr2 +/+ mice (−/−, 7.8 ± 0.8 × 106 cells, mean ± SEM, n = 11; +/+, 15.2 ± 2.3 × 106 cells, mean ± SEM, n = 11; P <0.01). The number of macrophages in the peritoneal exudate of CCR2-deficient mice was only one-eighth that of wild-type mice (−/−, 1.4 ± 0.2 × 106 macrophages, n = 11; +/+, 11.4 ± 1.7 × 106 macrophages, mean ± SEM, n = 11; P <0.00005). Since there was no increase in the number of peritoneal macrophages in the ccr2 −/− mice after thioglycollate injection the data indicates that CCR2 is essential for new macrophage recruitment in response to this non-specific inflammatory stimulus. The recruitment defect was specific to macrophages, as neutrophil and eosinophil infiltration was not impaired in mutant animals. Interestingly, there were a significantly greater number of recruited eosinophils in ccr2 −/− mice compared with ccr2 +/+ mice. This increase may represent an enhanced eosinophil recruitment in response to a defect in macrophage recruitment. The inability of CCR2-deficient mice to recruit macrophages in response to thioglycollate suggests that CCR2 and its ligands are specific mediators of macrophage trafficking during inflammation.
Figure 2.
Macrophage recruitment defect in ccr2 −/− mice. Resident and thioglycollate-elicited (72 h post-i.p. injection) peritoneal cells were lavaged from groups of wild-type (open bars) and ccr2 −/− (black bars) mice. Macrophage (Mac), eosinophil (Eos), and neutrophil (Neut) cell numbers were determined from total cell numbers after differential staining with Diff-Quik. For resident cell counts, values represent mean ± SEM of 5 wild-type or ccr2 −/− mice. For elicited cell counts, values represent mean ± SEM of 11 wild-type or ccr2 −/− mice. * P <0.05, ** P <0.006, ***P <0.00001; probabilities were determined by the unpaired Student's t-test.
CCR2-Deficient Mice are Unable to Clear Listeria monocytogenes Infection.
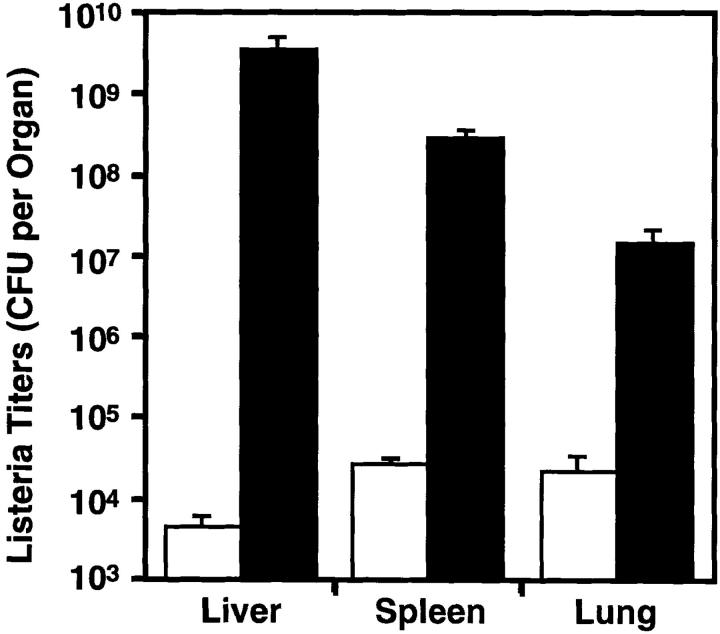
Since ccr2 −/− mice are defective in the recruitment of new macrophages to the peritoneum, we determined if macrophage-dependent immunity to intracellular pathogens was also compromised in these mice. During the early phase of infection with the bacterial pathogen L. monocytogenes, the accumulation of blood-derived macrophages to the foci of infection are critical for controlling bacterial growth in infected organs (28). Groups of wild-type and homozygous mutant mice were challenged intravenously with 2,500 CFU of L. monocytogenes. After 5 d, when macrophages are essential for control of L. monocytogenes infection, all ccr2 +/+ mice appeared active and healthy. In contrast, 33% of ccr2 −/− mice died 4 d after infection and the remainder were moribund at 5 d. The bacterial burdens in the spleen, liver, and lungs were determined at day 5 (or at autopsy for ccr2 −/− mice that died on day 4), and mutant mice had between 3–7 log–fold higher listerial titers than wild-type animals in all tissues (Fig. 3), confirming that CCR2 is required for listerial clearance. The level of sensitivity of CCR2-deficient mice to L. monocytogenes was similar to that seen in mice lacking the 55-kD tumor necrosis factor receptor (29, 30) or the interferon-γ receptor (31). At autopsy, livers and spleens from wild-type animals were of normal color but enlarged, while livers and spleens from mutant mice were of normal size but appeared mottled and off color. Histopathological analyses revealed minimal inflammatory foci comprised of macrophages, neutrophils, and individual necrotic hepatocytes in the livers of wild-type mice (Fig. 4, a and c). In contrast, severe, multifocal inflammation and necrosis were observed in the livers of ccr2 −/− mice (Fig. 4, b and d). These lesions were comprised of a central core of coagulative necrosis containing neutrophils and cellular debris and a rim of degenerating and necrotic hepatocytes containing abundant intracellular bacteria. Severe, multifocal inflammation and necrosis was also observed in the spleens of ccr2 −/− but not wild-type mice (data not shown).
Figure 3.
CCR2-deficient mice cannot clear Listeria infection. Wild-type (open bars) and ccr2 −/− (black bars) mice were injected intravenously with 2,500 CFU of L. monocytogenes. Listerial titers in liver, spleen, and lung were determined from mice killed 5 d after infection or at autopsy for mice that died after 4 d. Values represent mean ± SEM of 15 wild-type or ccr2 −/− mice. For all organs, the difference between wild-type and mutant mice is significant (P <0.0002 by Mann-Whitney two sample nonparametric analysis).
Figure 4.
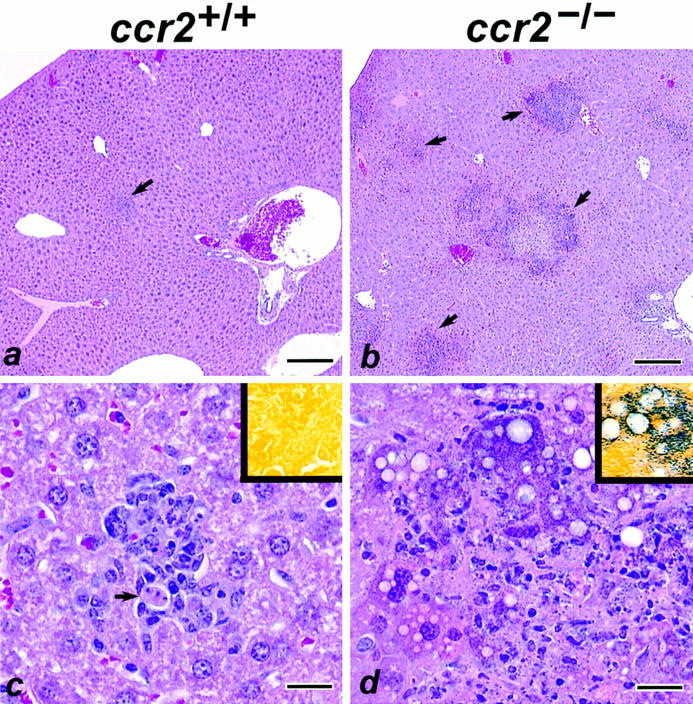
Histopathology of Liver from Listeria-infected mice. (a) Minimal, focal inflammation in a wild-type ccr2 +/+ mouse (arrow). (b) Severe, multifocal inflammation and necrosis in a ccr2 −/− mouse (arrows). (c) Higher magnification of a. The localized inflammatory focus contains macrophages and neutrophils with necrosis of individual hepatocytes (arrow). (d) Higher magnification of b. The lesion is characterized by a central core of coagulative necrosis containing neutrophils and cellular debris. A rim of degenerating and necrotic hepatocytes that contain abundant intracellular Gram-positive bacteria are located at the peripheral margin of the lesion. Insets are sections stained with Gram stain. Bars: (a, b) 200 μm; (c, d) 20 μm.
Our results demonstrate that CCR2 is a key mediator of inflammatory and immune responses and that other chemokine receptors, although functionally redundant with CCR2 in vitro, cannot substitute for CCR2 function in vivo. The failure of CCR2-deficient mice to recruit macrophages in response to intraperitoneal thioglycollate injection is the first genetic evidence for the requirement of a chemokine receptor in macrophage trafficking and identifies CCR2 as a potential therapeutic target for inflammatory diseases. Neutralizing antibody studies have implicated MCP-1 as a major mediator of macrophage recruitment in several inflammatory models (23–25), and the ccr2 −/− mice will provide a useful genetic system to test the potential role of MCP-1/ CCR2 interactions in these models. The extreme sensitivity of CCR2-deficient mice to L. monocytogenes suggests that CCR2-mediated responses can be as important for host resistance as TNF-α receptor– and IFN-γ receptor–mediated responses. Paradoxically, transgenic mice expressing high serum levels of MCP-1 also have increased sensitivity to L. monocytogenes (32). We hypothesize that high levels of MCP-1 may have partially desensitized CCR2 on blood monocytes in these transgenic mice, causing a similar, but less severe, phenotype than in the CCR2-deficient animals. Genetic linkage between sensitivity to L. monocytogenes and low thioglycollate-induced peritoneal macrophage recruitment has previously been identified in several inbred mouse strains (33). The identification of the same defects in the ccr2 −/− mice raises the possibility that CCR2 function may be impaired in some of those strains. It is unclear from these studies if defects in ccr2 −/− mice result specifically from the lack of MCP-1 signaling, since other ligands (MCP-2, 3, 4, and 5) for CCR2 have been identified (13–17). Targeted disruption of each of the CCR2 ligands will be informative for determining which ligands contribute to the macrophage recruitment and host defense functions mediated by CCR2.
Acknowledgments
We thank Ken Class for flow cytometry, Sergio Lira and Alice Lee for blastocyst injections, Anne Lewin for histology, and Cheryl Rizzo for cell culture assistance. We also thank Mark Kowala for valuable discussions and the staff of Veterinary Sciences at Bristol-Myers Squibb for their excellent support.
References
- 1.Oppenheim JJ, Zachariae COC, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Ann Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 2.Miller MD, Krangel MS. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12:17–46. [PubMed] [Google Scholar]
- 3.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 4.Premack BA, Schall TJ. Chemokine receptors: gateways to inflammation and infection. Nature Med. 1996;2:1174–1178. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- 5.Cacalano G, Lee J, Kikly K, Ryan AM, Pitts-Meek S, Hultgren B, Wood WI, Moore MW. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science (Wash DC) 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 6.Gao J-L, Wynn TA, Chang Y, Lee EJ, Broxmeyer HE, Cooper S, Tiffany HL, Westphal H, Kwon-Chung J, Murphy PM. Impaired host defense, hematopoiesis, granulomatous inflammation and type 1-type 2 cytokine balance in mice lacking CC chemokine receptor 1. J Exp Med. 1997;185:1959–1968. doi: 10.1084/jem.185.11.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothenberg ME, MacLean JA, Pearlman E, Luster AD, Leder P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J Exp Med. 1997;185:785–790. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S-I, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature (Lond) 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 9.Cook DN, Beck MA, Coffmann TM, Kirby SL, Sheridan JF, Pragnell IB, Smithies O. Requirement of MIP-1α for an inflammatory response to viral infection. Science (Wash DC) 1995;269:1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 10.Rollins BJ. Monocyte chemoattractant protein 1: a potential regulator of monocyte recruitment in inflammatory disease. Mol Med Today. 1996;2:198–204. doi: 10.1016/1357-4310(96)88772-7. [DOI] [PubMed] [Google Scholar]
- 11.Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci USA. 1994;91:2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boring L, Gosling J, Monteclaro FS, Lusis AJ, Tsou C-L, Charo IF. Molecular cloning and functional expression of murine JE (monocyte chemoattractant protein 1) and murine macrophage inflammatory protein 1 alpha receptors: evidence for two closely linked C-C chemokine receptors on chromosome 9. J Biol Chem. 1996;271:7551–7558. doi: 10.1074/jbc.271.13.7551. [DOI] [PubMed] [Google Scholar]
- 13.Kurihara T, Bravo R. Cloning and functional expression of mCCR2, a murine receptor for the C-C chemokines JE and FIC. J Biol Chem. 1996;271:11603–11606. doi: 10.1074/jbc.271.20.11603. [DOI] [PubMed] [Google Scholar]
- 14.Yamagami S, Tanaka H, Endo N. Monocyte chemoattractant protein-2 can exert its effects through the MCP-1 receptor (CC CKR2B) FEBS Lett. 1997;400:329–332. doi: 10.1016/s0014-5793(96)01411-1. [DOI] [PubMed] [Google Scholar]
- 15.Franci C, Wong LM, Van Damme J, Proost P, Charo IF. Monocyte chemoattractant protein-3, but not monocyte chemoattractant protein-2, is a functional ligand of the human monocyte chemoattractant protein-1 receptor. J Immunol. 1995;154:6511–6517. [PubMed] [Google Scholar]
- 16.Garcia-Zepeda EA, Combadiere C, Rothenberg ME, Sarafi MN, Lavigne F, Hamid Q, Murphy PM, Luster AD. Human monocyte chemoattractant protein (MCP)-4 is a novel CC chemokine with activities on monocytes, eosinophils, and basophils induced in allergic and nonallergic inflammation that signals through the CC chemokine receptors (CCR)-2 and -3. J Immunol. 1996;157:5613–5626. [PubMed] [Google Scholar]
- 17.Sarafi MN, Garcia-Zepeda EA, MacLean JA, Charo IF, Luster AD. Murine monocyte chemoattractant protein (MCP)-5: a novel CC chemokine that is a structural and functional homologue of human MCP-1. J Exp Med. 1997;185:99–109. doi: 10.1084/jem.185.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doranz BJ, Rucker J, Yi Y, Smyth RJ, Samson M, Peiper SC, Parmentier M, Collman RG, Doms RW. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 19.Connor RI, Sheridan KE, Ceradini D, Choe S, Landau NR. Change in coreceptor use correlates with disease progression in HIV-1–infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuentes ME, Durham SK, Swerdel MR, Lewin AC, Barton DS, Megill JR, Bravo R, Lira S. Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemattractant protein-1. J Immunol. 1995;155:5769–5776. [PubMed] [Google Scholar]
- 21.Gunn MD, Nelken NA, Liao X, Williams LT. Monocyte chemoattractant protein-1 is sufficient for the chemotaxis of monocytes and lymphocytes in transgenic mice but requires an additional stimulus for inflammatory activation. J Immunol. 1997;158:376–383. [PubMed] [Google Scholar]
- 22.Grewal I, Rutledge BJ, Fiorillo JA, Gu L, Gladue RP, Flavell RA, Rollins BJ. Transgenic monocyte chemoattractant protein-1 (MCP-1) in pancreatic islets produces monocyte-rich insulitis without diabetes: abrogation by a second transgene expressing systemic MCP-1. J Immunol. 1997;159:401–408. [PubMed] [Google Scholar]
- 23.Flory CM, Jones ML, Warren JS. Pulmonary granuloma formation in the rat is partially dependent on monocyte chemoattractant protein 1. Lab Invest. 1993;69:396–404. [PubMed] [Google Scholar]
- 24.Huffnagle GB, Strieter RM, Standiford TJ, McDonald RA, Burdick MD, Kunkel SL, Toews GB. The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4+ T cells during a pulmonary cryptococcus neoformans infection. J Immunol. 1995;155:4790–4797. [PubMed] [Google Scholar]
- 25.Chensue SW, Warmington KS, Ruth JH, Sanghi PS, Lincoln P, Kunkel SL. Role of monocyte chemoattractant protein-1 (MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen-induced granuloma formation: relationship to local inflammation, Th cell expression, and IL-12 production. J Immunol. 1996;157:4602–4608. [PubMed] [Google Scholar]
- 26.Heinrich JN, Ryseck R-P, Macdonald-Bravo H, Bravo R. The product of a novel growth factor-activated gene, fic, is a biologically active “C-C”-type cytokine. Mol Cell Biol. 1993;13:2020–2030. doi: 10.1128/mcb.13.4.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weih F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck R-P, Lira S, Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 28.North RJ. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970;132:521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfeffer K, Matsuyama T, Kundig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Kronke M, Mak TW. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 30.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. . Nature (Lond) 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 31.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science (Wash DC) 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 32.Rutledge BJ, Rayburn H, Rosenberg R, North RJ, Gladue RP, Corless CL, Rollins BJ. High level monocyte chemoattractant protein-1 expression in transgenic mice increases their susceptibility to intracellular pathogens. J Immunol. 1995;155:4838–4843. [PubMed] [Google Scholar]
- 33.Stevenson MM, Kongshavn PAL, Skamene E. Genetic linkage of resistance to Listeria monocytogeneswith macrophage inflammatory responses. J Immunol. 1981;127:402–407. [PubMed] [Google Scholar]