Abstract
Negative selection is the process by which the developing lymphocyte receptor repertoire rids itself of autoreactive specificities. One mechanism of negative selection in developing T cells is the induction of apoptosis in immature CD4+CD8+ (DP) thymocytes, referred to as clonal deletion. Clonal deletion is necessarily T cell receptor (TCR) specific, but TCR signals alone are not lethal to purified DP thymocytes. Here, we identify two distinct mechanisms by which TCR-specific death of DP thymocytes can be induced. One mechanism requires simultaneous TCR and costimulatory signals initiated by CD28. The other mechanism is initiated by TCR signals in the absence of simultaneous costimulatory signals and is mediated by subsequent interaction with antigen-presenting cells. We propose that these mechanisms represent two distinct clonal deletion strategies that are differentially implemented during development depending on whether immature thymocytes encounter antigen in the thymic cortex or thymic medulla.
Acentral feature of the immune system is its ability to respond to foreign antigens while tolerating self-antigens. Burnet's model of self-nonself discrimination proposed that receptor engagements that were stimulatory for mature immune cells would induce immature immune cells to die, resulting in the removal of autoreactive receptor specificities from the developing repertoire (1–2). The removal of autoreactivities from the functional lymphocyte repertoire is known as negative selection. For T cells, negative selection can occur at different developmental stages in the thymus by a variety of mechanisms, including (a) clonal deletion, (b) developmental or clonal arrest, or (c) clonal inactivation or anergy (3–4). CD4+CD8+ double positive (DP)1 thymocytes in particular are susceptible to clonal deletion induced in response to TCR signals (5–7). Curiously, while clonal deletion of DP thymocytes must involve TCR engagement, TCR engagement by itself is insufficient to stimulate DP thymocyte apoptosis in vitro (8–12). Rather, DP thymocyte death has been found to require signals in addition to TCR, such as those provided by CD28 (9–11). This requirement for second signals parallels the requirement for second signals in TCR-mediated activation and proliferation of mature single positive (SP) T cells (13). Because CD28 is not a unique costimulatory molecule for mature T cells (14–22), it is likely that CD28 is not the only molecule capable of transducing second signals for TCR dependent DP thymocyte apoptosis (6, 14, 23–24). Indeed, other molecules have been implicated in clonal deletion of DP thymocytes, such as CD30 (21) and fas (25). The role of fas in negative selection is particularly controversial (26–29).
TCR-dependent apoptosis of mature T lymphocytes was not foreseen in Burnet's model of self-nonself discrimination but is now a recognized consequence of mature T cell activation (reviewed by 30–31). In mature T cells, TCR signals do not directly induce an apoptotic program, but rather act indirectly by upregulating surface expression of members of the TNF family, specifically fas ligand (fasL) and TNF (32–34). These proteins bind to fas or TNF receptor (TNFR) which mediate apoptosis through death domains expressed in their cytosolic regions (35). This mechanism of apoptosis necessarily results in death of both TCR-stimulated T cells and neighboring (or bystander) cells expressing fas or TNFR. In contrast, clonal deletion in the thymus is presumed not to involve bystander death since it should be limited to cells directly stimulated by TCR interactions.
In this study we have focused on identifying mechanisms of TCR-dependent clonal deletion of rigorously purified DP thymocytes to avoid confounding signals that can result from contact with other thymic elements. We identify two distinct mechanisms for generating second signals leading to TCR-induced DP thymocyte apoptosis: (a) a CD28-dependent mechanism that requires simultaneous engagement of TCR and CD28 surface molecules, and (b) a CD28-independent mechanism initiated by TCR signals but mediated subsequently by APC signals. While we found that fas signals can induce DP thymocyte death, they were not involved in either TCR-specific apoptotic mechanism. We propose that the two mechanisms of DP apoptosis revealed in this report represent two distinct TCR-specific clonal deletion strategies in the thymus: (a) a CD28-dependent mechanism specific for antigens on B7+ cells, i.e., APC and medullary epithelium, and (b) a CD28-independent mechanism specific for antigens on B7− cells in the thymic cortex.
Materials and Methods
Mice.
Young adult female C57BL/6 (B6) mice, gld/gld mice (B6-SMN C3H fasL), and lpr/lpr mice (B6 mrl lpr/lpr) were obtained from The Jackson Laboratory (Bar Harbor, ME) and young adult female B6 Ly5.2 mice were obtained from the Frederick Cancer Research Center (Frederick, MD). CD28-deficient mice (CD28 KO) (14) were bred to the C57BL/6 background and maintained at the Bethesda Naval Medical Research Institute. Mice transgenic for the human bcl-2 gene (driven by the lck proximal promoter [36] were generously provided by Dr. Stanley Korsmeyer and bred in our facility at the NIH. Mice deficient in either the p55 TNFR (p55) (37) or both p55 and p75 TNFR (38 and 38a) were obtained from Immunex (Seattle, WA).
Cell Preparation.
CD4+CD8+ thymocytes were purified from 4–6-wk-old female mice either by panning on anti-CD8 (83-12-5) coated plates (39) or by Percoll density fractionation (40). Similar experimental results were obtained with cells isolated by either procedure. In each case >95% of isolated cells were CD4+CD8+. APC were prepared by treating splenocytes with anti-CD4 (RL-172), anti-CD8 (3-155), anti-Thy-1 (30H12), and rabbit complement as previously described (3). Viable cells were isolated by centrifugation over Lymphocyte-M (Cedarlane Laboratory, Ltd., Ontario, CA). These cell preparations were free of T cells as determined by CD3 staining and FACS® analysis. Lymph node T cells were prepared from B6 and gld/gld mice by treating single-cell suspensions of cells isolated from a pool of popliteal, inguinal, axillary, brachial, and submandibular lymph nodes with a combination of anti-class II (M5114), anti-NK1.1 (PK136), anti-HSA (J11d) culture supernatants, and rabbit complement.
Antibodies.
Anti-CD28 (37.51 [41]) and anti–TCR-β (H57-597 [42]) were affinity purified in our laboratory from hybridoma culture supernatant on columns of protein G– and protein A–Sepharose (Pharmacia LKB Nuclear, Gaithersburg, MD), respectively. Anti-CD2 (RM2-5), anti-CD43 (S7), anti-CD27 (LG.3A10), anti-41BB (1AH2), neutralizing anti–TNF-α (G281-2626), anti-CD80 (1G10), anti-FcR (2.4G2), anti-fas (Jo2), and FITC-conjugated anti-HSA, FITC anti-Ly5.2 (CD45.1) and FITC anti-Ly5.1 (CD45.2) were purchased from Pharmingen laboratories. FITC-conjugated anti-CD5 (Ly-1) was purchased from Beckton Dickinson (San Jose, CA). Anti-CD81 was the product of hybridoma 2F7 (43). Anti-CD9 (9D3 [20]), anti-CD24 (20C9 [44]), anti-CD30 (mCD30.1 [45]), anti-CD30L (M15 [46]), and anti-B7-2 (GL1 [47]), were generously provided by Drs. Hiromi Fujiwara (Osaka University, Osaka, Japan), Charles Janeway (VCI, NIH, Bethesda, MD), Eckhardt Podack (University of Miami School of Medicine, FL), Phil Morrissey (Immunex, Seattle, WA), and Richard Hodes (NIH, Bethesda, MD), respectively.
Reagents.
Murine recombinant TNF-α (R&D Labs., Minneapolis, MN) was used at a final concentration of 100 ng/ml. CD30-Ig was generously provided by Dr. Eckhardt Podack. Cyclosporine A was purchased from Calbiochem-Novabiochem (La Jolla, CA) and cycloheximide, wortmannin, and GF109203X were purchased from Sigma Chem. Co. (St. Louis, MO). The caspase inhibitor Cbz-Val-Ala-Asp-(Ome)-fluoromethyl ketone (ZVAD-FMK) was purchased from Enzyme Systems Products (Dublin, CA).
Culture and Stimulation Conditions.
Purified cell populations were cultured for 16–20 h in a 7% CO2 humidified incubator in RPMI 1640 supplemented with 5 × 10−5 M 2-ME and 10% FCS at 37°C. Single-cell suspensions of DP thymocytes were plated in 24-well tissue culture plates (Corning Glass, Corning, NY) at a cell density of 2 × 106/ml in a total of 500 μl per well. When mixed culture experiments were performed, DP cells from CD28-deficient mice were incubated with DP cells from Ly5.2 mice at a 1:1 ratio and with LN T from Ly5.2 mice at a 1:2 or 1:3 ratio. APC were mixed with DP thymocytes at a 2:1 or 3:1 ratio and 3 × 106 total cells were plated per well. For stimulation, wells in a 24-well plate were coated with antibody combinations by incubating them overnight at 4°C with 350 μl of a 10 μg/ml (most antibodies) or 50 μg/ml (anti-CD28) of each affinity-purified antibody specified in PBS.
Staining and Flow Cytometry.
Cell death was assayed as previously described (9, 48). In brief, 5 × 105 cultured cells were incubated with saturating concentrations of the FITC conjugated antibodies specified in staining medium (HBSS, 0.5% BSA, 0.5% NaN3) for 30 min at 4°C, washed, and then incubated with a 1 μg/ml ethidium bromide (EtBr; Sigma) for another 30 minutes at 4°C. Cells were washed again and analyzed using CellQuest software on a FACScan®. In mixed cell culture experiments, DP thymocytes were identified by expression of Ly5.1, Ly5.2, HSA, and Thy-1, as indicated.
Measures of Cell Death.
Specific cell death (% cell death) was determined by EtBr staining and was calculated as a normalized value as follows: (%EtBr+ (stimulated) − %EtBr+ (unstimulated control))/(100 − %EtBr+ control). Background EtBr staining of cultured DP thymocytes ranged between 18 and 30%. To compare thymocyte apoptosis in response to TCR-CD28 signals among experimental groups with different internal controls (i.e., different mouse strains, different solvents, etc.), individual responses were normalized and expressed as a killing index. The killing index was calculated as follows: (% DP cell death induced by TCR-CD28 under experimental conditions)/(% DP cell death induced by TCR-CD28 under control conditions). Killing index = 1.0 means that the indicated condition did not affect TCR-CD28– induced apoptosis; killing index >1 means that the indicated condition inhibited TCR-CD28–mediated death. All experiments displayed were performed three or more times with similar results.
Results
Surface Molecules Expressed by CD4+CD8+ Thymocytes That Kill Cells by Either TCR-dependent or TCR-independent Mechanisms.
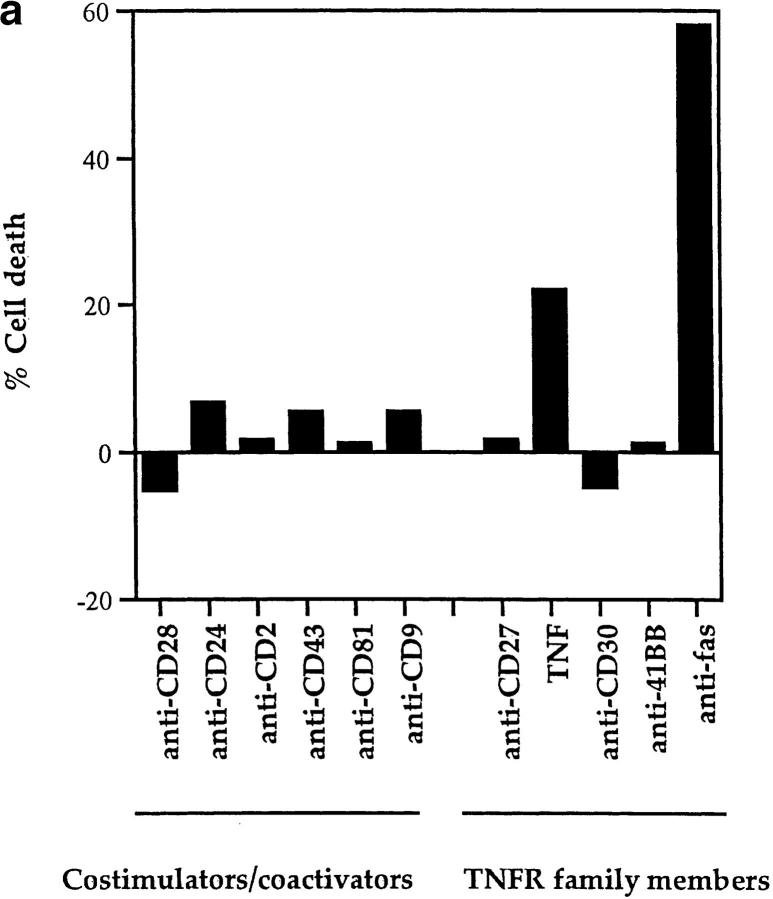
To identify the molecular requirements for TCR-dependent DP thymocyte apoptosis, we stimulated purified DP thymocytes in vitro and assessed them for EtBr uptake, which identifies cells undergoing apoptosis (9, 48). We initially examined surface molecules for their ability to induce apoptosis of DP thymocytes in the absence of TCR engagement (Fig. 1 a). In fact, we identified two stimuli that induced DP thymocyte apoptosis in a TCR-independent manner, namely (a) fas engagement by immobilized platebound antibody and (b) TNFα engagement of TNFR. Antibody engagement of surface fas molecules induced apoptosis of most DP thymocytes, consistent with others' observations and the high expression of fas on DP thymocytes (49); and soluble TNF-α engagement of surface receptors induced apoptosis of 20% of DP thymocytes, which may represent a distinct TNF-α responsive subpopulation (50) (Fig. 1 a). Our present finding that immobilized anti-fas antibody efficiently induces DP thymocyte death differs from that of Ogasawara et al. (51) who found that metabolic inhibitors were necessary to enhance DP thymocyte death induced by soluble anti-fas antibodies. We think that our different observations may be due to differences in the efficiency by which fas is crosslinked by soluble versus immobilized antibody.
Figure 1.
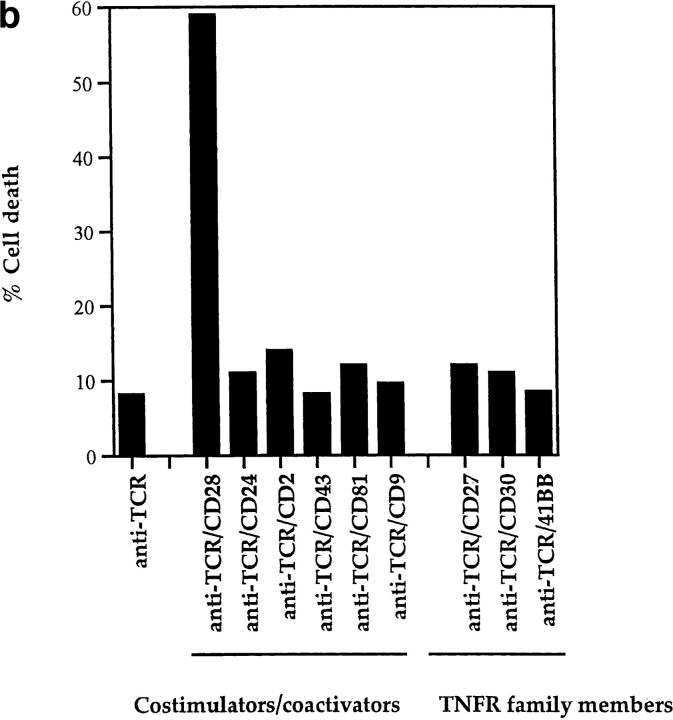
TCR-independent and TCR-dependent mechanisms of DP thymocyte apoptosis. Antibodies against cell surface molecules that possess costimulatory/coactivating activity and antibodies against TNFR family members were assessed for their ability to induce apoptosis of rigorously purified DP thymocytes. Control EtBr percentages ranged between 18 and 30% in all experiments presented in this report. (a) Only TNF-α and fas induce death of DP thymocytes in the absence of TCR stimulation. DP thymocytes isolated from young adult female B6 mice were stimulated by platebound antibodies or by 100 ng/ml recombinant murine TNF-α. Cells were harvested after overnight incubation and percent cell death was quantitated by EtBr staining (see Materials and Methods). (b) Only CD28 cooperates with TCR to induce death of DP thymocytes. Cells were stimulated by platebound antibody combinations as indicated. Anti–TCR-β (H5-597) was plated at a concentration of 10 μg/ml. Even though anti-CD28 antibody was unique in its ability to induce cell death, every experimental antibody enhanced TCR-mediated upregulation of CD5 (data not shown). In a and b, each experimental antibody (other than anti-CD28) was plated at both 10 and 50 μg/ml concentrations. Results from the 10 μg/ml plating preparations are shown and were indistinguishable from results with 50 μg/ml concentrations. Cells were harvested and percent cell death was quantitated by EtBr staining (Materials and Methods).
Even though fas and TNFR are members of the TNFR family of proteins (52), many of which induce cell death, platebound antibody engagement of three other members of the family (CD27, CD30, and 41BB) did not affect DP thymocyte viability (Fig. 1 a, right). In fact, no other molecules examined, including platebound antibodies specific for CD4, CD5, CD8, CD69, and LFA-1 (9 and data not shown), were capable of mediating TCR-independent DP thymocyte apoptosis (Fig. 1 a).
We next assessed molecules that could induce DP thymocyte apoptosis in the presence of TCR engagement (Fig. 1 b). As expected (9), TCR engagement alone failed to induce significant DP thymocyte apoptosis (Fig. 1 b). We examined three specific sets of surface proteins for their ability to cooperate with the TCR to kill DP thymocytes (Fig. 1 b and data not shown), namely (a) molecules thought to be costimulatory (CD9 [20]), CD28 [reviewed in 53], CD43 [19], and CD81 [54], (b) coactivating molecules that enhance TCR signaling (CD2, CD4, CD5, CD8, CD24, CD69, and LFA-1), and (c) selected TNFR family members (CD27, CD30, 41BB), which may also exhibit costimulatory activity (15, 17–18, 21–22, 52). Importantly, antibody engagement of each of these molecules cooperated with TCR signaling to upregulate CD5 expression on DP thymocytes (data not shown). However, only CD28 cooperated with TCR to induce DP thymocyte apoptosis (Fig. 1 b).
Thus, there exist both TCR-independent and TCR-dependent mechanisms of DP thymocyte apoptosis: TCR-independent mechanisms can be mediated by either fas or TNFR engagement, whereas the TCR-dependent mechanisms can be mediated by coengagement of TCR and CD28.
TCR/CD28–induced Apoptosis Is Independent of fas and TNFR Interactions, and Is Strictly Confined to DP Thymocytes That Have Received both TCR and CD28 Signals.
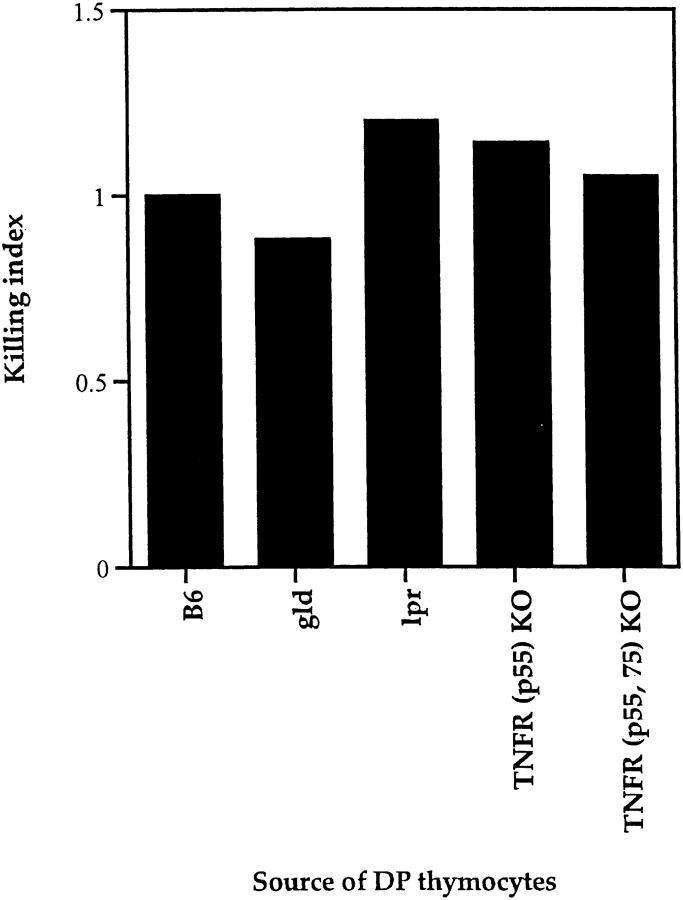
The ability of fas and TNF-α to mediate DP thymocyte apoptosis raised the possibility that both mechanisms of DP thymocyte apoptosis (TCR-independent and TCR-dependent) may ultimately result from engagement of fas or TNFR. To address this possibility, we examined the ability of TCR-CD28 coengagement to kill purified DP thymocytes from mice deficient in (a) fas (lpr, reviewed in reference 55), (b) fas ligand (gld), (c) the p55 murine TNFR, or (d) both the p55 and p75 murine TNFRs (38, 38a) (Fig. 2). None of the mutations significantly affected the ability of TCR-CD28 coengagement to induce apoptosis of DP thymocytes, indicating that neither fas nor the TNF receptors p55 or p75 were required (Fig. 2). In addition, neutralizing anti–TNF-α antibodies had no effect on TCR-CD28– induced apoptosis of wild-type DP thymocytes (data not shown). We also found that TCR-CD28–induced death was not blocked by CD30 Ig, a fusion protein that blocks CD30-CD30L interactions (data not shown). We conclude that TCR-CD28 coengagement induces DP thymocyte apoptosis by a mechanism that is independent of fas/fasL, TNF-TNFR, and CD30-CD30L interactions.
Figure 2.
TCR-CD28 killing of DP thymocytes is not mediated by fas-fasL or TNF-TNFR interactions. DP thymocytes were isolated from the mice indicated (wild-type B6, gld/gld (fasL deficient), lpr/lpr (fas deficient), TNFR (p55)–deficient, and TNFR (p55 and p75)–deficient mice strains. Single-cell suspensions were stimulated overnight by platebound anti–TCR-β and anti-CD28 antibodies, and then were harvested and stained with EtBr. To compare thymocyte apoptosis from different mouse strains with different internal controls, we have normalized individual responses to their respective controls. The normalized value is referred to as a killing index.
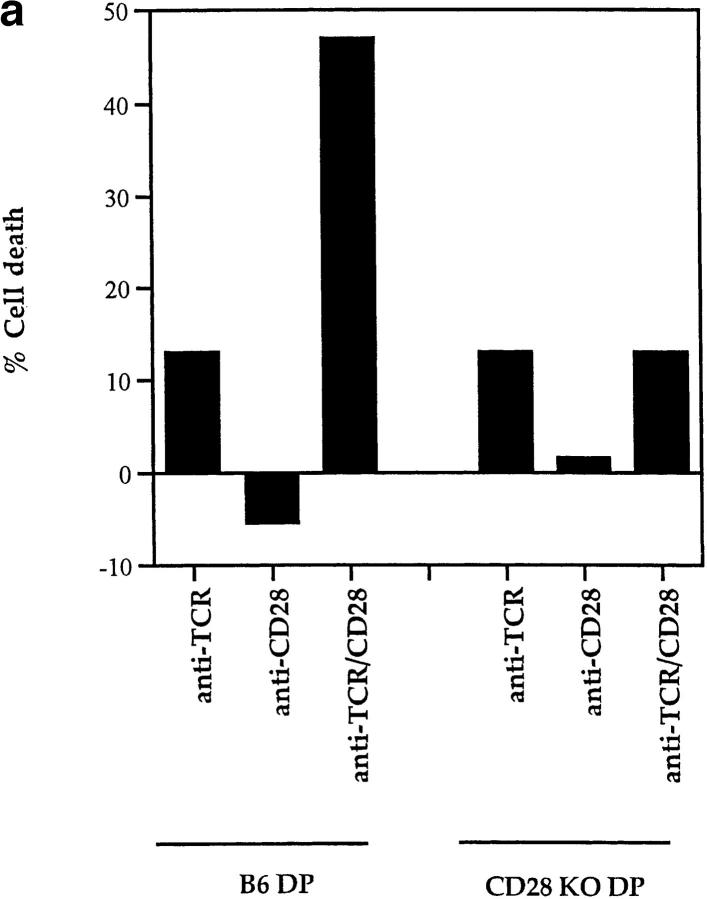
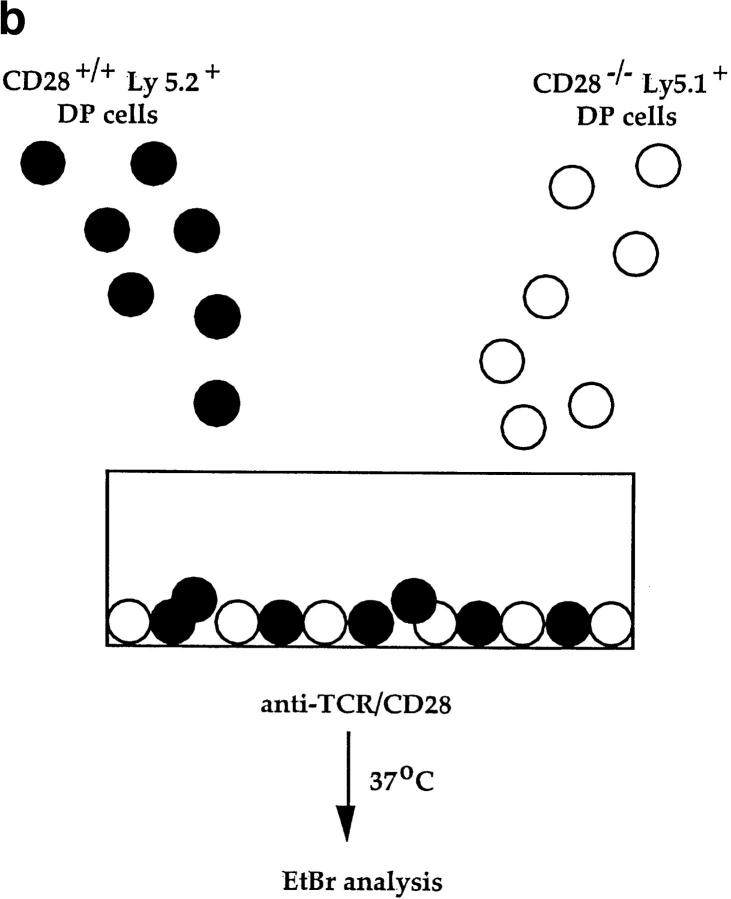
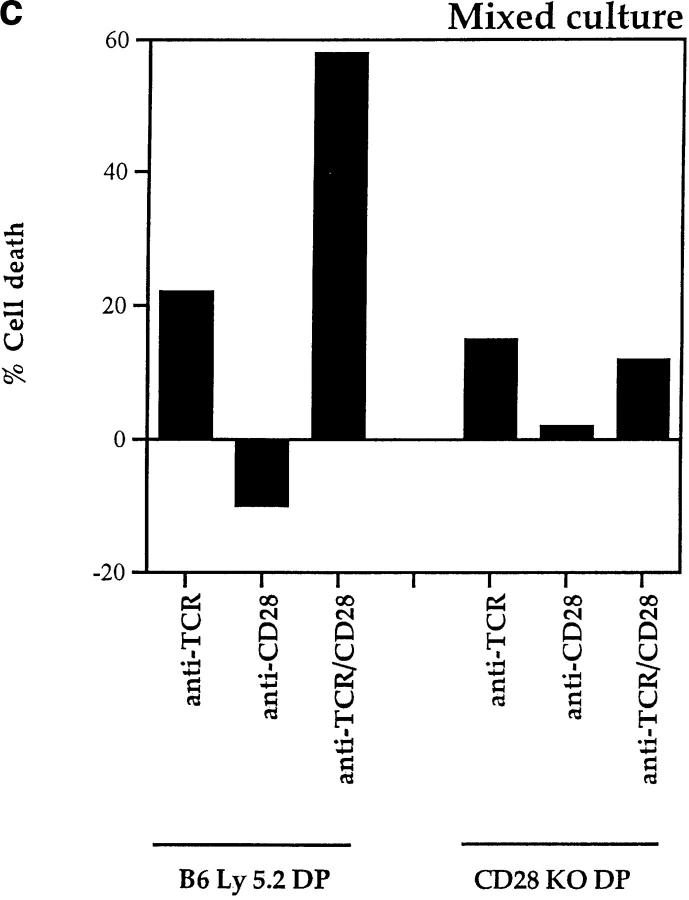
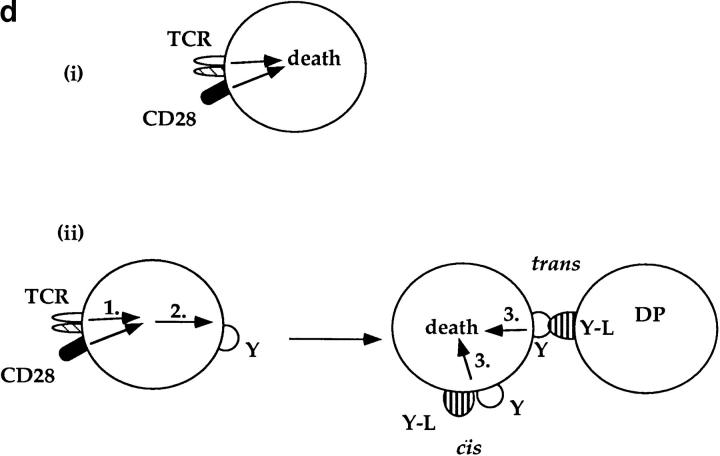
While fas and TNFR are not involved in TCR-CD28– induced apoptosis, additional members of the TNFR family expressing the “death domain” associated with apoptotic signaling continue to be identified (56–58). To determine if TCR-CD28 coengagement induced apoptosis in trans of bystander thymocytes via ligands (known and unknown), we used thymocytes from CD28 KO mice as bystander cells for they do not die in response to TCR-CD28 stimulation (Fig. 3 a). In this mixed culture experiment, we stimulated cocultures of DP thymocytes from wild-type (B6-Ly5.2) and CD28 KO mice with both anti-TCR and anti-CD28 platebound antibodies (Fig. 3 b). We found that the majority of wild-type DP thymocytes died in cocultures in response to TCR-CD28 coengagement. In contrast, bystander CD28 KO DP thymocytes did not die even though they were present in the same cocultures and even though they were stimulated by the immobilized anti-TCR antibodies (Fig. 3 c). These results demonstrate that TCR-CD28 coengagement only induces death of DP thymocytes that had been stimulated by both TCR and CD28 signals, and suggests one of two scenarios as illustrated in Fig. 3 d. Either TCR-CD28 transduces intracellular signals that directly activate an intrinsic death program in DP thymocytes, or TCR-CD28 coengagement stimulates surface expression of an unknown death domain bearing molecule that signals cell death upon interaction with its ligand which must also be expressed on DP thymocytes. In the latter case, the death receptor-ligand interaction could conceivably operate either on an individual DP thymocyte (i.e., cis) or between interacting DP thymocytes (trans).
Figure 3.
Only TCR-CD28–stimulated DP thymocytes die in response to TCR-CD28 coengagement. (a) TCR-CD28 stimulation will not kill bystander CD28 KO DP thymocytes. Individual populations of DP thymocytes from wild-type B6 mice and CD28 KO mice were cultured and stimulated independently with platebound antibodies. % Cell death of each population of DP thymocytes was quantitated and normalized as described in Materials and Methods. (b) Experimental design. DP thymocytes from wild-type mice (CD28+Ly5.2+) were mixed in a 1:1 ratio with DP thymocytes from CD28-deficient mice (CD28−/−Ly 5.1+) and stimulated by platebound anti–TCR-β and anti-CD28. Thymocytes were harvested after overnight culture and percent cell death in each population was determined. CD28+/+ and CD28−/− DP thymocytes were distinguished by the presence or absence of Ly5.1 staining. (c) Bystander CD28 KO DP thymocytes are not killed by TCR-CD28 signals. DP thymocytes were isolated from wild-type and CD28-deficient (CD28 KO) mice which differed in Ly5 expression such that wild-type DP thymocytes were Ly5.2+ and CD28 KO thymocytes were Ly5.1+. Harvested cells were stained with both anti-Ly5.1 antibody and EtBr to determine cell death in each population of cocultured DP thymocytes. Percent cell death was quantitated and normalized as described in Materials and Methods. (d) Schematic of the mechanism by which TCR-CD28 coengagement kills DP thymocytes. This figure illustrates two possible CD28-dependent mechanisms of TCR-mediated apoptosis of DP thymocytes, both of which result in death exclusively of TCR-CD28–stimulated DP thymocytes. The upper figure (i) illustrates one scenario in which simultaneous coengagement of TCR and CD28 molecules directly and cell-autonomously induces an apoptotic program. The lower figure (ii) illustrates an alternative scenario in which simultaneous coengagement of TCR and CD28 induces expression of a death domain containing receptor (Y) that signals apoptosis upon interaction with its ligand (Y-L, Y ligand) that is also expressed on DP thymocytes. In this latter case, the ligand could conceivably engage the death receptor in either cis or trans.
Identification of Apoptotic Signals Generated by TCR/CD28 Stimulation.
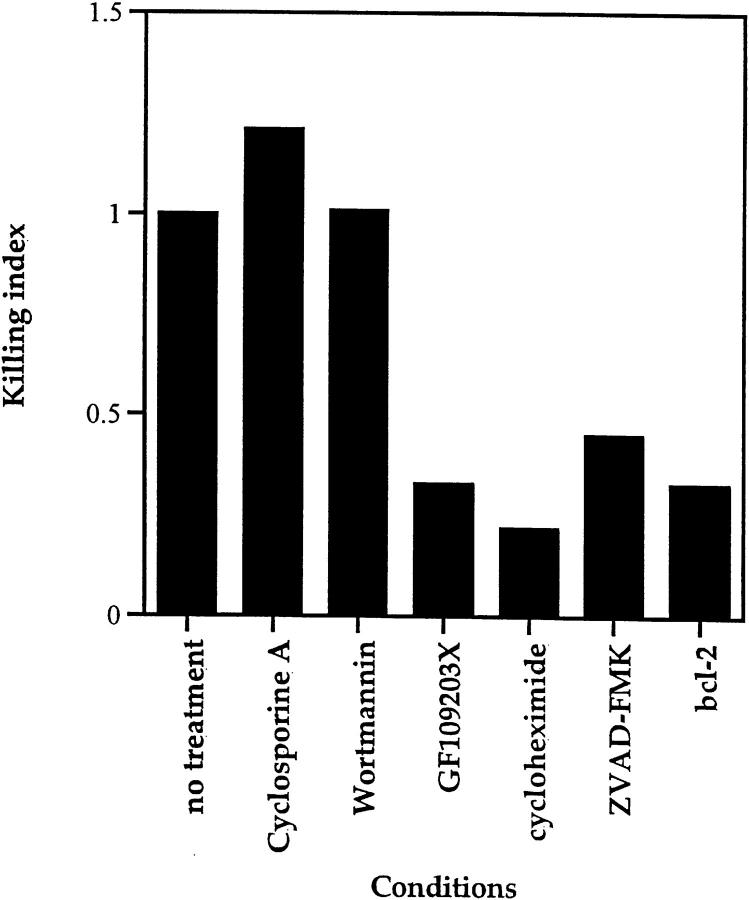
To characterize the molecular basis for TCR-CD28–induced apoptosis of DP thymocytes, we assessed specific inhibitors for their ability to abrograte DP thymocyte death (Fig. 4). We found that neither cyclosporine A (reviewed in reference 59) nor wortmannin (60, 61) inhibited TCR-CD28–mediated death (Fig. 4), indicating that calcineurin and PI 3-kinase activity are not required. In contrast, DP thymocyte apoptosis induced by TCR-CD28 coengagement was inhibited by (a) GF109203X, a specific inhibitor of protein kinase Cγ (PKCγ (61, 62), (b) the protein synthesis inhibitor, cycloheximide, and (c) the caspase (ICE-family protease) inhibitor, ZVAD-FMK (63, 64) (Fig. 4). Consistent with the participation of caspases, TCR-CD28 coengagement failed to induce significant apoptosis in DP thymocytes from bcl-2 transgenic mice which constitutively overexpress the anti-apoptotic bcl-2 protein (Fig. 4).
Figure 4.
Inhibitors of TCR-CD28–mediated death of DP thymocytes. DP thymocytes from wild-type (B6) mice were stimulated by platebound anti–TCR-β and anti-CD28 in the presence or absence of the following pharmacological agents: the calcineurin inhibitor, cyclosporine A (1 μg/ml); the PI-3-kinase inhibitor, wortmannin (800 ng/ml); the PKCγ inhibitor, GF109203x (800 ng/ml); the protein synthesis inhibitor, cycloheximide (10 μg/ml); and the caspase inhibitor, ZVAD-FMK (100 μM). To compare the effects of various reagents on TCR-CD28–mediated DP thymocyte apoptosis in experiments performed with different solvent controls, individual responses were normalized to their respective controls (killing index). As positive controls for the pharmacologic agents used: cyclosporine A and GF109203x used in this experiment inhibited TCR-mediated CD5 upregulation, and wortmannin used in this experiment blocked NK-mediated target cell lysis (data not shown). Also displayed in the same format are the results of anti–TCR-CD28 stimulation of DP thymocytes isolated from bcl-2 transgenic mice.
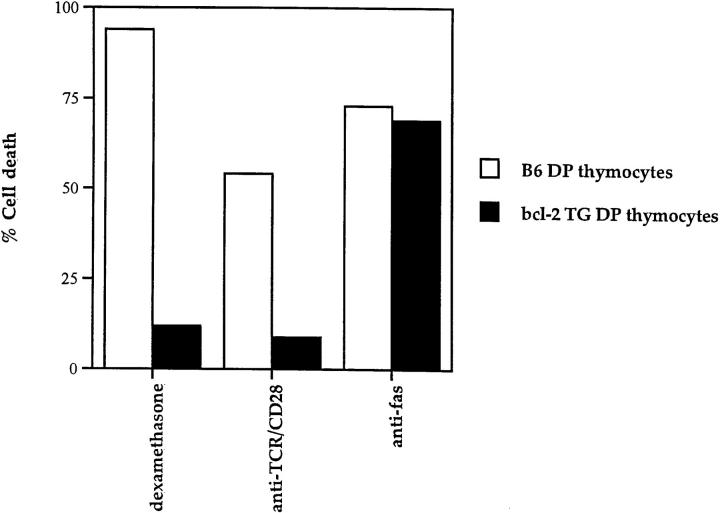
These data further suggest that the death mechanism initiated by TCR-CD28 coengagement is distinct from that initiated by fas engagement, for unlike TCR-CD28–mediated death, fas mediated death of thymocytes is not inhibitible by cycloheximide (65, 66) and may not be inhibitable by bcl-2 (67). To demonstrate this directly, we compared the effect of bcl-2 on DP thymocyte apoptosis induced by fas and TCR-CD28 engagement (Fig. 5). In this experiment, we cultured DP thymocytes from wild-type and bcl-2 transgenic mice with three different stimulators of apoptosis: dexamethasone, platebound antibodies against fas, and platebound antibodies against TCR-CD28. As expected, dexamethasone killed wild-type DP thymocytes but not DP thymocytes from bcl-2 transgenic mice (Fig. 5). More importantly, DP thymocytes that expressed bcl-2 were susceptible to fas mediated death but resistant TCR/CD28-induced death (Fig. 5). Thus, the death program initiated by TCR-CD28 coengagement is distinct from that initiated by fas engagement.
Figure 5.
Distinct signaling mechanisms of DP thymocyte apoptosis as revealed by differential sensitivity to bcl-2. DP thymocytes isolated from wild-type (B6) and bcl-2 transgenic (bcl-2 TG) mice were cultured with three distinct apoptotic stimuli: dexamethasone (10−6 M), platebound anti-fas antibodies, and platebound anti-TCR and anti-CD28. It can be seen that transgenic bcl-2 expression abrograted TCR-CD28–mediated apoptosis but not fas-mediated apoptosis of DP thymocytes. It might be noted that thymocyte death by TCR-CD28 engagement does not involve glucocorticoids as the steroid inhibitor RU486 only blocked death induced by dexamethasone but not by TCR-CD28 (data not shown).
We conclude that apoptotic signals generated by TCR-CD28 coengagement are mediated by PKCγ, require protein synthesis, and result in the activation of intracellular caspases that are regulated by bcl-2.
Identification of a TCR-dependent but CD28-independent Mechanism of DP Thymocyte Apoptosis.
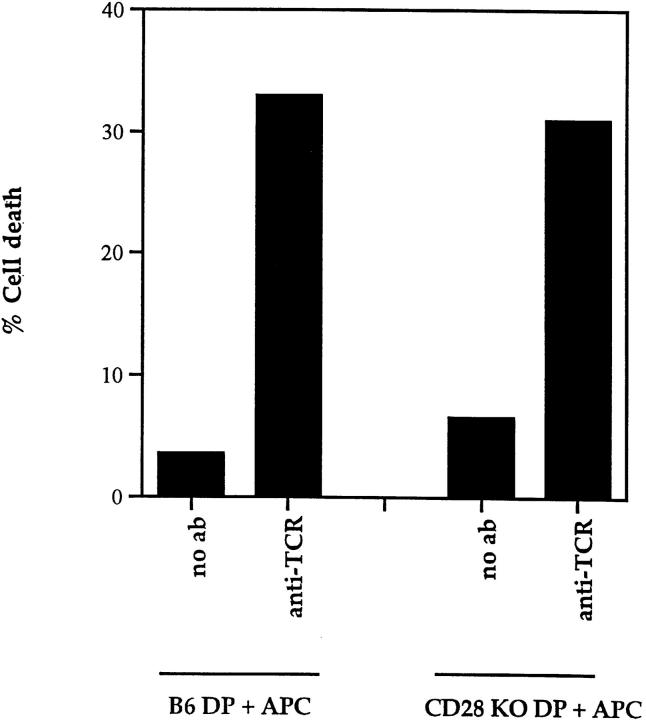
Our studies using an array of antibodies to known molecules on the surface of DP thymocytes did not identify any proteins other than CD28 that could cooperate with TCR to induce DP thymocyte apoptosis. To determine if any such molecule existed, we asked whether APC expressed ligands, known or unknown, for surface molecules that would cooperate with TCR to induce DP thymocyte apoptosis. In this coculture experiment, we stimulated DP thymocytes from wild-type and CD28 KO mice with platebound anti-TCR in the presence of APC. We confined our assessment of EtBr staining to DP thymocytes by excluding APCs from the analysis using an allelic marker, Ly5. DP thymocytes from wild-type mice died in response to TCR stimulation in the presence of APC. More importantly, DP thymocytes from CD28 KO mice also died in response to TCR stimulation in the presence of APC, indicating that APC possess ligands that engage molecules other than CD28 that can induce death of TCR-stimulated thymocytes. It is important to note that this CD28-independent mechanism of DP thymocyte death strictly requires engagement of TCR on DP thymocytes, as APCs did not kill unstimulated CD28 KO DP thymocytes (Fig. 6). From these data we can conclude that APC express or secrete ligands for surface molecules on DP thymocytes that are capable of cooperating with the TCR to induce TCR-dependent but CD28-independent apoptosis.
Figure 6.
A CD28-independent mechanism of TCR-mediated death of DP thymocytes. DP thymocytes from either B6 (Ly 5.1+) or CD28 KO (Ly5.1+) mice were co-cultured with Ly 5.2+ APC in the presence or absence of platebound anti-TCR. Harvested cells were stained with both anti-Ly5.1 antibody and EtBr. DP thymocytes were distinguished from APCs by expression of Ly5.1.
CD28-dependent and CD28-independent Mechanisms of TCR Induced DP Thymocyte Apoptosis Are Distinct.
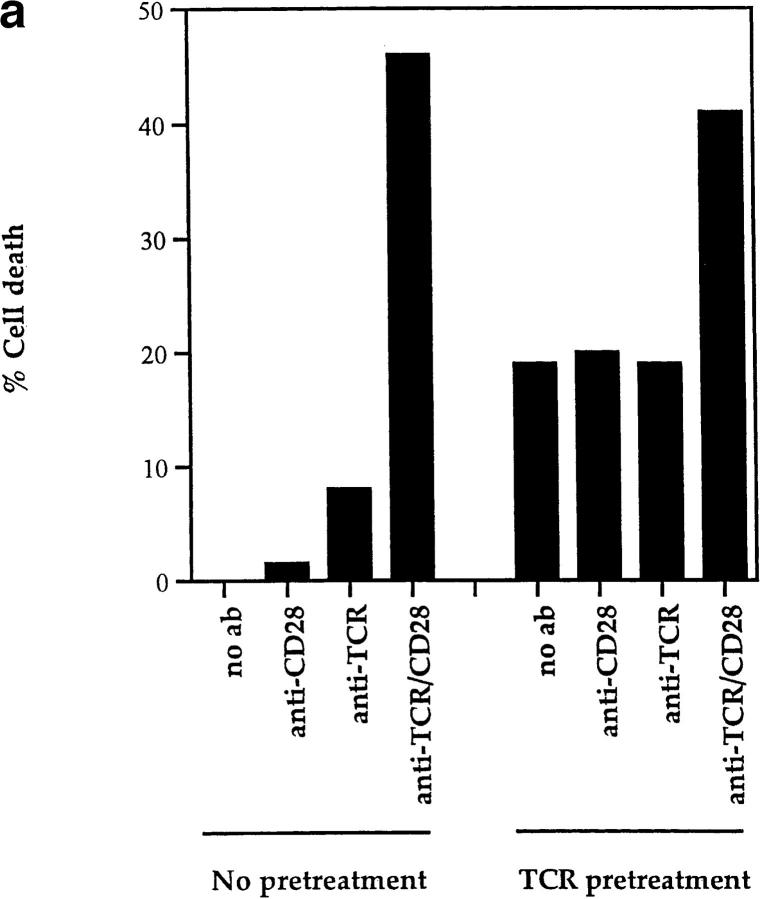
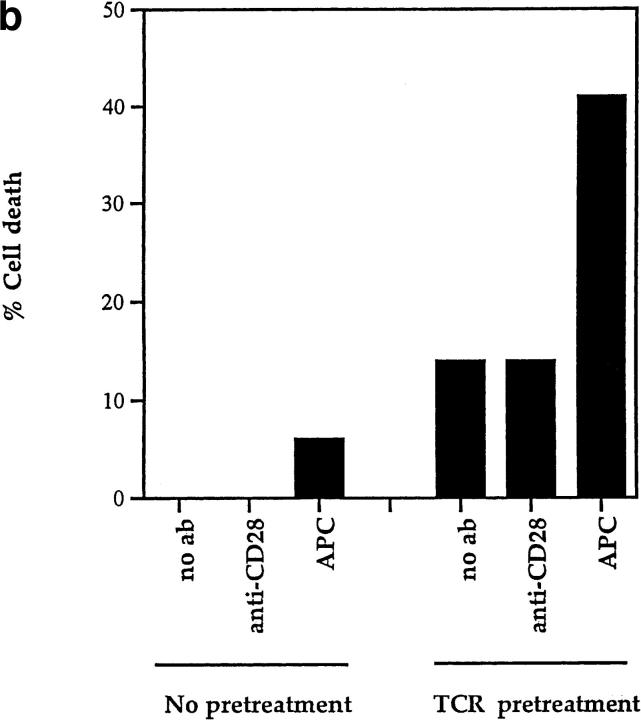
The present results clearly indicate that CD28 is not the only surface molecule that the TCR can use to kill DP thymocytes. However, it is possible that even though CD28-dependent and CD28-independent mechanisms of TCR-induced DP thymocyte apoptosis are mediated by different surface receptor molecules on DP thymocytes, the second signals induced by these different proteins might be identical. That this is not the case was revealed by experiments in which TCR and second signals were induced sequentially rather than simultaneously. In this experiment, we prestimulated DP thymocytes with immobilized anti-TCR for 6 h and subsequently transferred them to cultures with platebound anti-CD28. We found that death of prestimulated DP thymocytes was not affected by subsequent stimulation through CD28 (Fig. 7 a). In fact, only subsequent coengagement of both TCR and CD28 together increased apoptosis of prestimulated DP thymocytes above background (Fig. 7 a). In contrast, we found in the same experiment that DP thymocytes from CD28 KO mice similarly prestimulated with immobilized anti-TCR and subsequently transferred to APCs were killed efficiently (Fig. 7 b). Thus, to stimulate DP thymocyte apoptosis, TCR and second signals induced by CD28 must be generated simultaneously, whereas TCR and second signals induced by APC can be generated sequentially, as illustrated in Fig. 7 c. Consequently, CD28-dependent and CD28-independent pathways of cell death represent two distinct mechanisms by which TCR can induce DP thymocyte apoptosis.
Figure 7.
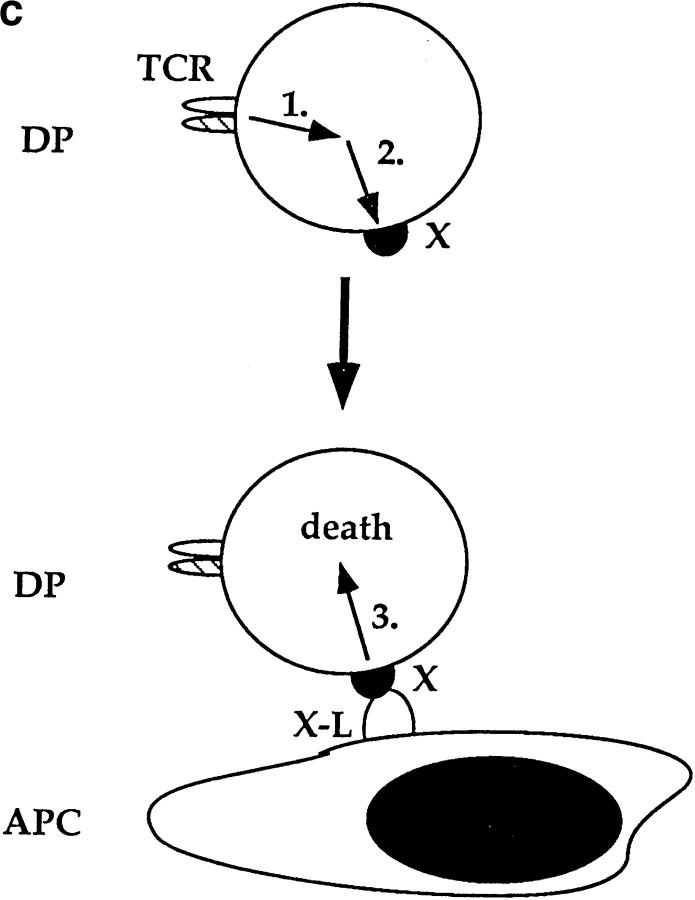
CD28-dependent and CD28-independent mechanisms of DP thymocyte apoptosis are distinct. (a) TCR and CD28 signals must be received simultaneously to mediate death of DP thymocytes. DP thymocytes from B6 mice were prestimulated by platebound anti-TCR-β for 6 h. They were then removed from this stimulus and transferred to wells that had been precoated with the antibodies indicated on the x-axis. Cells were harvested and stained with EtBr. The background cell death observed in TCR pretreated groups is likely due to cell damage inflicted by their physical removal from platebound anti-TCR antibody. (b) TCR and second signals derived from APCs do not have to be simultaneous to induce CD28-independent DP thymocyte death. DP thymocytes from CD28 KO (Ly 5.11) mice were prestimulated by platebound anti–TCR-β for 6 h. They were then removed from this stimulus and transferred either into wells that had been precoated with anti-CD28 or into wells containing APCs from B6 Ly5.2 mice (in a 2:1 ratio with the DP cells). Cells were harvested and stained with both Ly5.1 and EtBr. DP thymocytes were distinguished from APC by expression of Ly5.1. As can be seen, preengagement of TCR on DP thymocytes made them susceptible to APC-induced cell death. (c) Schematic of the CD28-independent mechanism of DP thymocyte apoptosis. This figure illustrates the proposed mechanism by which TCR and subsequent APC signals induce apoptosis of DP thymocytes in a CD28-independent manner. TCR prestimulation of DP thymocytes (1) induces upregulation of a molecule X which might express death domains (2). Subsequent engagement of molecule X with a ligand expressed by APCs (X ligand or X-L) induces apoptosis of only prestimulated DP thymocytes (3).
Characterization of TCR-dependent but CD28-independent Mechanisms of DP Thymocyte Apoptosis.
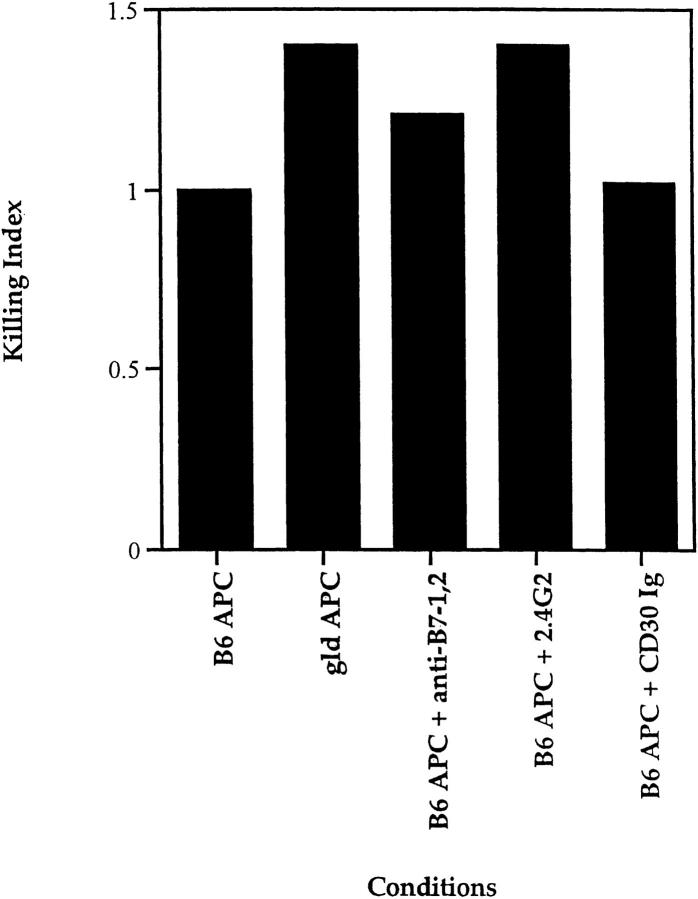
To determine if fas/ fasL interactions were involved in APC-induced cell death, we prestimulated CD28 KO DP thymocytes with platebound anti-TCR and then transferred them to cultures containing APCs isolated from fasL-deficient (gld) mice (Fig. 8). FasL-deficient APCs from gld mice mediated the death of TCR-stimulated DP thymocytes as efficiently, if not more efficiently, than wild-type APCs (Fig. 8). Thus, the CD28-independent mechanism of TCR-mediated DP cell death is also independent of fas/fasL interactions.
Figure 8.
Characterization of the CD28-independent mechanism of DP thymocyte apoptosis. DP thymocytes from CD28 KO mice were presetimulated with platebound anti-TCR antibody for 6 h, then cocultured with APC from either gld mice or B6 Ly5.2 mice in the presence or absence of the following reagents: anti-FcR antibody (2.4G2, 10 μg/ml); the fusion protein CD30 Ig (10 μg/ml) which blocks CD30-CD30L interactions; or a combination of anti–B7-1 and anti–B7-2 antibodies (10 μg/ml each) which blocks B7 ligand engagement by both CD28 and CTLA-4. To compare the effects of various reagents on TCR-CD28– mediated DPthymocyte apoptosis in experiments performed over time, individual responses were normalized to their respective controls and the normalized value is referred to as a killing index.
It was conceivable that the CD28-independent mechanism of cell death involved a CD28-like molecule such as CTLA-4 that could be engaged by B7 ligands on APC (68). In fact, this is not the case, as blocking antibodies to B7-1 and B7-2 failed to inhibit the ability of APCs to kill TCR-prestimulated DP thymocytes from CD28 KO mice (Fig. 8). Similar results were obtained with soluble CTLA-4 Ig (data not shown). Furthermore, the apoptosis observed was not a consequence of the transfer of small amounts of anti-TCR which could bind to Fc receptors (FcR) on APC, for the anti-FcR antibody 2.4G2 did not inhibit the apoptotic effects of sequential TCR and APC engagement (Fig. 8).
Finally, these data suggest that TCR prestimulation (in the absence of CD28 coengagement) “prepares” DP thymocytes to undergo apoptosis upon subsequent interaction with APC, possibly by inducing expression of surface molecules containing death domains that bind APC-derived ligands. Consistent with such a possibility, cycloheximide reduced by over 70% the number of TCR prestimulated DP thymocytes that died upon subsequent exposure to APC (data not shown), indicating that new protein synthesis is required to make TCR prestimulated DP thymocytes vulnerable to death. TNFR family members are attractive candidate molecules whose synthesis could be induced on DP thymocytes by TCR prestimulation. Indeed, CD30 has been reported to play a role in thymocyte negative selection (21). Nevertheless, CD30 does not appear to be involved as neither soluble CD30-Ig fusion protein (45) (Fig. 8) nor antibodies specific for the CD30 ligand (data not shown) blocked the ability of APCs to mediate CD28-independent apoptosis of prestimulated DP thymocytes.
Discussion
The present report identifies two distinct mechanisms by which DP thymocytes can be induced to undergo apoptosis in response to TCR signals. In the CD28-dependent mechanism, TCR signals are delivered simultaneously with CD28 costimulatory signals, resulting in DP thymocyte death. In the CD28-independent mechanism, TCR signals are not accompanied by costimulatory signals and death signals are generated upon subsequent interaction with APCs. In addition the present study demonstrates that purified DP thymocytes can also be induced to undergo apoptosis in the absence of TCR signals by fas-fasL and TNF-TNFR interactions, but such TCR-independent mechanisms of apoptosis cannot be the basis for TCR-specific clonal deletion of DP thymocytes in vivo.
The CD28-dependent mechanism of apoptosis is a consequence of simultaneous TCR and CD28 coengagement on DP thymocytes and results exclusively in death of DP thymocytes that have simulataneously received both TCR and CD28 signals. Such apoptotic signals do not require the activity of PI 3-kinase, a signaling molecule associated with CD28 (69), but are resistant to the effects of cyclosporine A, a hallmark of CD28 involvement in mature T cells (70). Apoptosis induced by TCR-CD28 coengagement requires PKCγ and employs a common effector pathway involving caspases that is inhibitible by bcl-2. The present results are consistent with either of two possibilities: simultaneous TCR-CD28 signals induce DP thymocyte death either by directly initiating a novel intracellular apoptotic program or by inducing surface expression of an uncharacterized death receptor (e.g., a TNFR family member) that is engaged by surface ligands. In either case, the CD28-dependent mechanism of death requires that both TCR and CD28 signals be delivered simultaneously and requires that the death ligand, if it exists, be expressed on DP thymocytes for these were the only cells present in our cultures. We favor the possibility that TCR-CD28 directly signals an apoptotic program because we have found that TCR-CD28–induced apoptosis of DP thymocytes occurs efficiently at very low cell densities at which cell-cell interactions are unlikely to occur (data not shown). However, we cannot exclude the possibility that TCR/CD28 stimulation results in lethal death receptor/ligand interactions occurring in cis on the surface of individual DP thymocytes (Fig. 3 d).
The mechanism responsible for CD28-independent DP apoptosis is distinct from that of TCR-CD28–induced apoptosis. CD28-independent DP thymocyte apoptosis does not require costimulatory signals. Rather, TCR engagement alone on DP thymocytes appears sufficient to induce surface expression of a molecule which triggers death upon subsequent engagement by ligands expressed by APCs. This proposed surface molecule has features of death domain containing receptors, such as some members of the TNFR family. Thus, TCR signals may stimulate expression of a TNFR family member which is neither fas, nor CD30, nor 41BB, but which generates apoptotic signals when engaged by an appropriate ligand expressed or secreted by APC.
It is conceivable that all programmed cell death is initiated by signals transduced by death domains on specialized surface receptors. From this perspective, surface death receptors would be responsible for death of DP thymocytes stimulated either by TCR-CD28 costimulatory signals or by CD28-independent TCR signals alone. However, it is important to appreciate that the identity and specificity of the death receptors induced by TCR-CD28 signals must be different than those induced by TCR signals alone. This conclusion is based on our data showing that TCR-CD28– stimulated DP thymocytes kill themselves or each other, but do not kill TCR-stimulated DP bystander cells (Fig. 3 c). Rather, TCR-stimulated DP thymocytes are induced to die upon subsequent interaction with APCs (Fig. 7 b). Therefore, from the perspective that all cell death is mediated by death domain containing receptors, the ligand for TCR-CD28–induced death receptors must be expressed on DP thymocytes themselves, whereas the ligand for TCR induced death receptors is not expressed on DP thymocytes but is expressed on APCs.
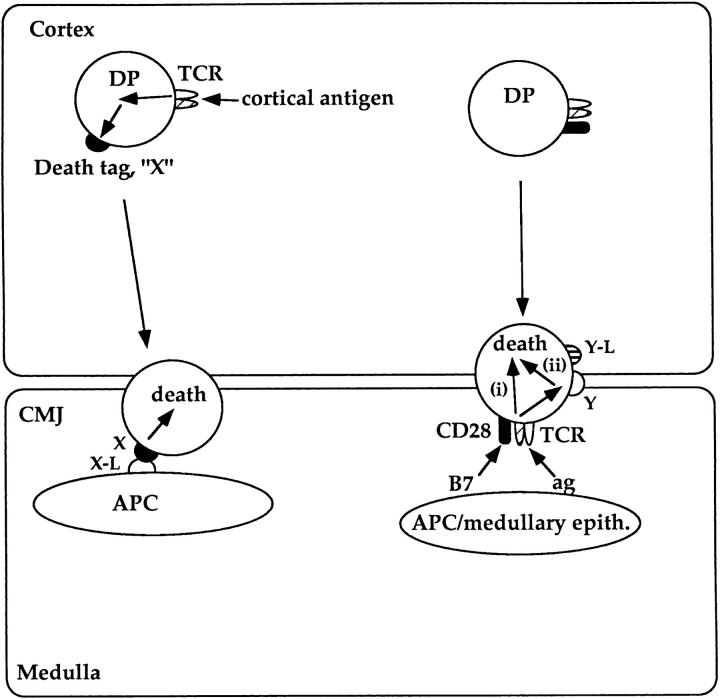
The importance of APCs in inducing clonal deletion in the thymus has long been appreciated (8, 71–73). However, the mechanism by which APCs induce DP thymocyte apoptosis has not been fully understood. Here we show for the first time that APCs can induce death of TCR stimulated DP thymocytes in two distinct ways via two distinct sets of ligands. Our observation that TCR signals can induce DP thymocyte apoptosis by two different mechanisms is relevant to an understanding of clonal deletion of DP thymocytes during normal in vivo development. In Fig. 9 we illustrate a model of in vivo clonal deletion that is based on our present data. We propose that the two mechanisms of TCR-mediated DP apoptosis identified in this report represent two distinct strategies to rid the developing DP thymocyte repertoire of autoreactive specificities during thymocyte development (Fig. 9). Only DP thymocytes whose TCRs bind MHC–peptide complexes on cells expressing the CD28 ligands B7-1 or B7-2 will be killed as a consequence of TCR and CD28 coengagement. Because B7-1 and B7-2 are not expressed in the thymic cortex but are only expressed on APCs and medullary epithelial cells, this form of clonal deletion would be confined to DP thymocytes autoreactive to self-antigens encountered either at the corticomedullary junction, where most APCs are located, or on medullary epithelium. In contrast, high-affinity interactions of TCR on DP thymocytes with self-antigens in the cortex would induce expression of a surface molecule that “tags” DP thymocytes for future disposal (i.e., a “death tag”). Such DP thymocytes would not die in response to TCR engagement per se, but would travel through the cortex and enter the corticomedullary junction where they would encounter a “screen” of APCs constitutively expressing or secreting a ligand that bind the putative death tag and induce apoptosis.
Figure 9.
Proposed model of two distinct mechanisms of intrathymic DP clonal deletion. Applying the present data to in vivo thymocyte development, we propose that there are two distinct mechanisms by which autoreactive DP thymocytes are deleted in the thymus. CD28-dependent mechanism (right): DP thymocytes recognizing self-antigens on B7+ cells (i.e., medullary epithelial cells or APCs) are killed by signals generated by simultaneous engagement of TCR and CD28. TCR-CD28 coengagement may directly initiate an apoptotic program (i) or may upregulate a death domain containing receptor that signals death upon interaction with its ligand on DP thymocytes (ii). CD28-independent mechanism (left): DP thymocyte expressing TCR's recognizing self-antigens on cortical epithelium do not die as a consequence of TCR engagement. Instead, they are induced to express a surface molecule (i.e., a death tag, X) that signals them to undergo apoptosis upon subsequent interaction with APC's present in the CMJ. It should be noted that in this model, both mechanisms of clonal deletion of autoreactive DP thymocytes are proposed to occur in the CMJ or medulla even if they encounter self-antigen in the cortex.
This model provides thre main insights. First, apoptosis of DP thymocytes targeted for deletion occurs either at the corticomedullary junction (CMJ) or in the thymic medulla, regardless of whether DP cells encountered antigen in the cortex. As a result, DP thymocyte deletion requires that DP cells migrate out of the thymic cortex to the CMJ or medulla which is considered to be a consequence of positive selection. Thus, our model predicts that DP thymocytes can only be clonally deleted in vivo if they are first positively selected. Observations that TCR-mediated apoptosis is confined to the thymic medulla are consistent with this perspective (74–75). Indeed, only DP thymocytes dying of neglect appear to undergo apoptosis in the cortex (75).
Second, the model proposes that CD28-dependent and CD28-independent mechanisms of TCR-mediated DP apoptosis eliminate clones bearing TCR specific for distinct sets of antigens: the CD28-dependent mechanism disposes of TCR reactivities to APC and medullary antigens, whereas the indirect, CD28-independent mechanism of TCR-mediated DP apoptosis disposes of TCR reactivities to cortical antigens. Recently, experimental mice were generated in which expression of MHC class II was confined to cortical epithelium (76). Our model predicts that these experimental mice would lack the CD28-dependent mechanism of death because medullary cells expressing B7 (thymic APC or medullary epithelium) would lack MHC class II expression and fail to engage TCR on DP thymocytes. Indeed, T cells in these animals were not tolerant to self-antigens on Class II+ APCs. Importantly, while CD28-dependent clonal deletion is absent in these mice, our model predicts that the CD28-independent mechanism of clonal deletion should be intact, so that their CD4+ T cells would be tolerant to self-antigens expressed on class II+ cortical epithelium.
Third, the model predicts that there can be a delay between the receipt of a negative selecting TCR signal and the death of the cell by the CD28-independent mechanism. Thus, the presence of autoreactive TCR specificities among DP thymocytes is not necessarily indicative of a failure of negative selection.
Our efforts to define the molecular basis for CD28-dependent mechanisms of TCR-mediated DP apoptosis revealed that CD28 is surprisingly unique in its capacity to cooperate with TCR to directly produce apoptotic signals. Even molecules that have been described as augmenting proliferation of mature T cells, such as CD9, CD43, CD81, CD27, 41BB, and CD30, were not able to stimulate TCR-mediated death of DP thymocytes, so DP thymocyte apoptosis is not the result of simultaneous engagement of TCR with any costimulatory molecule. Rather, CD28 appears to generate unique second signals whose identities have not yet been elucidated. Interestingly, TCR-CD28–induced thymocyte apoptosis does not require the activity of PI 3-kinase, one molecule known to associate with CD28.
The identity of the APC-derived signals responsible for CD28-independent thymocyte apoptosis is not known. While such signals may well involve molecules containing death domains, this study has ruled out two attractive candidates, CD30 and 41BB (Fig. 1 b). However, proteins expressing death domains continue to be identified. In fact, three newly identified molecules containing death domains (DR-3, DR-4, and TRAMP) are expressed in lymphopoietic tissues (56–58).
Finally, it is important to draw a distinction between TCR-mediated apoptosis of DP thymocytes and the phenomenon of negative selection. Negative selection refers to any process that rids a developing T cell repertoire of an autospecificity. Although TCR-mediated clonal deletion of DP thymocytes is thought to be a major component of negative selection, it is clearly not the only mechanism responsible for negative selection. Indeed, we have previously shown that thymocytes can be developmentally arrested before the DP stage in response to TCR signals (3) and others have shown that negative selection can occur after the DP stage of development (77–79). In all likelihood the molecular mechanisms that operate at these other developmental stages (both pre- and post-DP) are distinct from those responsible for TCR-dependent apoptosis of DP thymocytes. In support of this possibility, it is interesting to note that (a) transgenic bcl-2 expression does not always affect negative selection (36) even though it abrogates clonal deletion of DP thymocytes and (b) fas appears to play a role in the TCR-mediated death of semimature T cells in the thymus (79) but clearly does not play a role in the TCR-mediated death of rigorously purified DP thymocytes.
In conclusion, this study identified both TCR-independent and TCR-dependent mechanisms of DP thymocyte apoptosis and reveals that TCR-dependent mechanisms of DP thymocyte death occur by two mechanisms: (a) a CD28-dependent mechanism in which TCR and CD28 costimulatory signals must be received simultaneously to generate apoptotic signals and (b) a CD28-independent mechanism in which TCR signals are indirectly responsible for apoptosis by upregulating molecules which, when subsequently engaged by APCs, will induce cell death. We propose that these two mechanisms represent two distinct strategies used by the thymus to dispose of autoreactive DP thymocytes and that the strategy used depends on where the antigen is encountered. Hence, DP thymocytes autoreactive to cortical antigens will be removed from the T cell repertoire by the indirect CD28-independent mechanism, whereas DP thymocytes autoreactive to APC or medullary cell antigens will be removed from the repertoire by CD28-dependent mechanisms (Fig. 9). Thus, the present study reveals an unexpected diversity of molecular mechanisms responsible for TCR-specific clonal deletion.
Acknowledgments
We are grateful to Drs. Eckhardt Podack, Philip J. Morrissey, Charles Janeway, and Karen Hathcock for generously providing reagents and mice; to Shabnam Shahabadi for excellent technical assistance; to Drs. Barbara Osborne, E. Wendy Shores, Susan Sharrow, and Pierre Henkart for critically reviewing the manuscript.
Footnotes
This report was supported in part by National Medical Research and Development Command EQ.0095.003.1412. The views expressed in the article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, or the US Government.
Abbreviations used in this paper: CMJ, cortico medullary junction; DP, double positive; EtBr, ethidium bromide; fasL, fas ligand; FcR, Fc receptor; SP, single positive; TNFR, TNF receptor.
References
- 1.Burnet FM. A modification of Jerne's theory of antibody production using the concept of clonal selection. Aust J Sci. 1957;20:67–69. doi: 10.3322/canjclin.26.2.119. [DOI] [PubMed] [Google Scholar]
- 2.Lederberg J. Genes and antibodies: do antigens bear instructions for antibody specificity or do they select cell lines that arise by mutation? . Science. 1959;129:1649–1653. doi: 10.1126/science.129.3364.1649. [DOI] [PubMed] [Google Scholar]
- 3.Takahama Y, Shores EW, Singer A. Negative selection of precursor thymocytes before their differentiation into CD4+CD8+cells. Science. 1992;258:653–656. doi: 10.1126/science.1357752. [DOI] [PubMed] [Google Scholar]
- 4.Fowlkes BJ, Ramsdell F. T Cell tolerance. Curr Opin Immunol. 1993;5:873–879. doi: 10.1016/0952-7915(93)90099-e. [DOI] [PubMed] [Google Scholar]
- 5.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 6.Fowlkes BJ, Schwartz R, Pardoll DM. Deletion of self-reactive thymocytes occurs at a CD46+CD8+precursor stage. Nature. 1988;334:620–623. doi: 10.1038/334620a0. [DOI] [PubMed] [Google Scholar]
- 7.Robey E, Fowlkes BJ. Selective events in T cell development. Annu Rev Immunol. 1994;12:675–705. doi: 10.1146/annurev.iy.12.040194.003331. [DOI] [PubMed] [Google Scholar]
- 8.Page DM, Kane LP, Allison JP, Hedrick SM. Two signals are required for negative selection of CD4+CD8+thymocytes. J Immunol. 1993;151:1868–1880. [PubMed] [Google Scholar]
- 9.Punt JA, Osborne BA, Takahama Y, Sharrow SO, Singer A. Negative selection of CD4+CD8+thymocytes by T cell receptor-induced apoptosis requires a costimulatory signal that can be provided by CD28. J Exp Med. 1994;179:709–713. doi: 10.1084/jem.179.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amsen D, Kruisbeek AM. CD28-B7 interactions function to co-stimulate clonal deletion of double-positive thymocytes. Int Immunol. 1996;8:1927–1936. doi: 10.1093/intimm/8.12.1927. [DOI] [PubMed] [Google Scholar]
- 11.Kishimoto H, Cai Z, Brunmark A, Jackson MR, Peterson PA, Sprent J. Differing roles for B7 and intercellular adhesion molecule-1 in negative selection of thymocytes. J Exp Med. 1996;184:531–537. doi: 10.1084/jem.184.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groves T, Parsons M, Miyamoto NG, Guidos CJ. TCR engagement of CD4+CD8+thymocytes in vitro induces early aspects of positive selection, but not apoptosis. J Immunol. 1997;158:65–75. [PubMed] [Google Scholar]
- 13.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 14.Shahinian A, Pfeffer K, Lee KP, Kundig TM, Kishihara K, Wakeman A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 15.Hubbe M, Altevogt P. Heat-stable antigen/CD24 on mouse T lymphocytes: evidence for a costimulatory function. Eur J Immunol. 1994;24:731–737. doi: 10.1002/eji.1830240336. [DOI] [PubMed] [Google Scholar]
- 16.Cocks BG, Chang CC, Carballido JM, Yseel H, de Vries JE, Aversa G. A novel receptor involved in T-cell activation. Nature. 1995;376:260–263. doi: 10.1038/376260a0. [DOI] [PubMed] [Google Scholar]
- 17.Del Prete G, De Carli M, D'Elios MM, Daniel KC, Almerigogna F, Alderson M, Smith CA, Thomas E, Romagnani S. CD30-mediated signaling promote the development of human T helper type 2-like T cells. J Exp Med. 1995;182:1655–1661. doi: 10.1084/jem.182.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gravestein LA, Nieland JD, Kruisbeek AM, Borst J. Novel mAbs reveal potent co-stimulatory activity of murine CD27. Int Immunol. 1995;7:551–557. doi: 10.1093/intimm/7.4.551. [DOI] [PubMed] [Google Scholar]
- 19.Sperling AI, Green JM, Mosley RL, Smith PL, DiPaolo RJ, Klein JR, Bluestone JA, Thompson CB. CD43is a murine T cell costimulatory receptor that functions independently of CD28. J Exp Med. 1995;182:139–146. doi: 10.1084/jem.182.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tai X-G, Yashiro Y, Abe R, Kazuhito T, Wood CR, Morris J, Long A, Ono S, Kobayashi M, Hamaoka T, et al. A role for CD9 molecules in T cell activation. J Exp Med. 1996;184:753–758. doi: 10.1084/jem.184.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amakawa R, Hakem A, Kundig TM, Matsuyama T, Simard JJ, Timms E, Wakeham A, Mittruecker HW, Griesser H, Takimot H, et al. Impaired negative selection of T cells in Hodgkin's disease antigen in CD30-deficient mice. Cell. 1996;84:551–562. doi: 10.1016/s0092-8674(00)81031-4. [DOI] [PubMed] [Google Scholar]
- 22.DeBenedette MA, Shahinian A, Mak TW, Watts TH. Costimulation of CD28− T lymphocytes by 4-1BB ligand. J Immunol. 1997;158:551–559. [PubMed] [Google Scholar]
- 23.Jones LA, Izon DJ, Nieland JD, Linsley PS, Kruisbeek AM. CD28-B7 interactions are not required for intrathymic clonal deletion. Int Immunol. 1993;5:503–512. doi: 10.1093/intimm/5.5.503. [DOI] [PubMed] [Google Scholar]
- 24.Walunas TL, Sperling AI, Khattri R, Thompson CB, Bluestone JA. CD28 expression is not essential for positive or negative selection of thymocytes or peripheral T cell tolerance. J Immunol. 1996;153:1006–1013. [PubMed] [Google Scholar]
- 25.Castro JE, Listman JA, Jacobson BA, Wang Y, Lopez PA, Ju S, Finn PW, Perkins DL. Fas modulation of apoptosis during negative selection of thymocytes. Immunity. 1996;5:617–627. doi: 10.1016/s1074-7613(00)80275-7. [DOI] [PubMed] [Google Scholar]
- 26.Herron LR, Eisenberg RA, Roper RE, Kakkanaiah VN, Cohen PL, Kotzin BL. Selection of the T cell receptor repertoire in lpr mice. J Immunol. 1993;151:3450–3459. [PubMed] [Google Scholar]
- 27.Singer GG, Abbas A. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994;1:365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 28.Suda T, Nagata S. Purification and characterization of the Fas-ligand that induced apoptosis. J Exp Med. 1994;179:873–879. doi: 10.1084/jem.179.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sytwu HK, Liblau RS, McDevitt HO. The roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity. 1996;5:17–30. doi: 10.1016/s1074-7613(00)80306-4. [DOI] [PubMed] [Google Scholar]
- 30.Lenardo MJ. Fas and the art of lymphocyte maintenance. J Exp Med. 1996;183:721–724. doi: 10.1084/jem.183.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell JH. Activation-induced death of mature T cells in the regulation of immune responses. Curr Opin Immunol. 1995;7:382–388. doi: 10.1016/0952-7915(95)80114-6. [DOI] [PubMed] [Google Scholar]
- 32.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, Green DR. Cell-autonomous Fas (CD95) Fas-ligand interaction mediates activation induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 33.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1 (Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 34.Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, Stanger BZ, Marshak RA. Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 35.Golstein P, Marguet D, Depraetere V. Fas bridging cell death and cytotoxicity: the reaper connection. Immunol Rev. 1995;146:45–56. doi: 10.1111/j.1600-065x.1995.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 36.Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 37.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 38.Leon LR, Kozak W, Peschon J, Kluger MJ. Exacerbated febrile responses to LPS, but not turpentine, in TNF double receptor-knockout mice. Am J Physiol. 1997;272:R563–R569. doi: 10.1152/ajpregu.1997.272.2.R563. [DOI] [PubMed] [Google Scholar]
- 38a.Peschon, J.J., D.S. Torrance, K. Stocking, C.O. Evans, C. Willis, K. Charrier, O.P.J. Morrissey, C.B. Ware, R.G. Goodwin, C.A. Smith, et al. 1998. Pulmonary and systemic inflammatory responses in mice genetically deficient in p55 and p75 tumor necrosis factor receptors. J. Immunol. In press.
- 39.Nakayama T, June CH, Munitz TI, Sheard M, McCarthy SA, Sharrow SO, Samelson LE, Singer A. Inhibition of T cell receptor expression and function in immature CD4+CD8+cells by CD4. Science. 1990;249:1558–1561. doi: 10.1126/science.2120773. [DOI] [PubMed] [Google Scholar]
- 40.Ernst B, Surh CD, Sprent J. Thymic selection and cell division. J Exp Med. 1995;182:961–971. doi: 10.1084/jem.182.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gross JA, Callas E, Allison JP. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992;149:380–388. [PubMed] [Google Scholar]
- 42.Kubo RT, Born W, Kappler JW, Marrack P, Pigeon M. Characterisation of a monoclonal antibody which detects all mature αβ T cell receptors. J Immunol. 1989;142:2736–2742. [Google Scholar]
- 43.Boismenu R, Rhein M, Fisher WH, Havran WL. A role for CD81 in early T cell development. Science. 1996;271:198–200. doi: 10.1126/science.271.5246.198. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Jones B, Brady W, Janeway CA, Jr, Linsley PS. Co-stimulation of murine CD4 T cell growth: cooperation between B7 and heat-stable antigen. Eur J Immunol. 1992;22:2855–2859. doi: 10.1002/eji.1830221115. [DOI] [PubMed] [Google Scholar]
- 45.Bowen MA, Lee RK, Miragliotta G, Nam SY, Podack ER. Structure and expression of murine CD30 and its role in cytokine production. J Immunol. 1996;156:442–449. [PubMed] [Google Scholar]
- 46.Wiley SR, Goodwin RG, Smith CA. Reverse signaling via CD30 ligand. J Immunol. 1996;157:3635–3639. [PubMed] [Google Scholar]
- 47.Hathcock KS, Laszlo G, Pucillo C, Linsley P, Hodes RJ. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994;180:631–640. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lyons AB, Samuel K, Sanderson A, Maddy AH. Simultaneous analysis of immunophenotype and apoptosis of murine thymocytes by single laser flow cytometry. Cytometry. 1992;13:1809–1814. doi: 10.1002/cyto.990130803. [DOI] [PubMed] [Google Scholar]
- 49.Nishimura Y, Ishii A, Kobayashi Y, Yamaski Y, Yonehara S. Expression and function of mouse Fas antigen on immature and mature T cells. J Immunol. 1995;154:4395–4403. [PubMed] [Google Scholar]
- 50.Hernandez-Caselles T, Stutman O. Immune functions of tumor necrosis factor. I. Tumor necrosis factor induces apoptosis of mouse thymocytes and can also stimulate or inhibit IL-6-induced proliferation depending on the concentration of mitogenic constimulation. J Immunol. 1993;151:3999–4012. [PubMed] [Google Scholar]
- 51.Ogasawara J, Suda T, Nagata S. Selective apoptosis of CD4+CD8+thymocytes by the anti-fas antibody. J Exp Med. 1995;181:485–491. doi: 10.1084/jem.181.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith CA, Rarrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 53.Rudd CE. Upstream-downstream: CD28 cosignaling pathways and T cell function. Immunity. 1996;4:527–534. doi: 10.1016/s1074-7613(00)80479-3. [DOI] [PubMed] [Google Scholar]
- 54.Todd SC, Lipps SG, Crisa L, Salomon DR, Tsoukas CD. CD81 expressed on human thymocytes mediates integrin activation and interleukin 2-dependent proliferation. J Exp Med. 1996;184:2055–2060. doi: 10.1084/jem.184.5.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 56.Bodmer J-L, Burns K, Schneider P, Hofmann K, Steiner V, Thorne M, Bornand T, Hahne M, Schroter M, Becker K, et al. TRAMP, a novel apoptosis-mediating receptor with sequence homology to tumor necrosis factor receptor 1 and Fas (Apo-1/CD95) Immunity. 1997;6:79–88. doi: 10.1016/s1074-7613(00)80244-7. [DOI] [PubMed] [Google Scholar]
- 57.Chinnaiyan AM, O'Rourke K, Yu G-L, Lyons RH, Garg M, Duan DR, Xing L, Gentz R, Ni J, Dixit VM. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science. 1996;274:990–992. doi: 10.1126/science.274.5289.990. [DOI] [PubMed] [Google Scholar]
- 58.Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 59.Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 60.Yano H, Nakanishi S, Kimura K, Hanai N, Saitoh Y, Fukui Y, Nonomura Y, Matsuda Y. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol-3 kinase in RBL-2H3 cells. J Biol Chem. 1993;268:25846–25856. [PubMed] [Google Scholar]
- 61.Bonnema JD, Karnitz LM, Schoon RA, Abraham RT, Leibson PJ. Fc receptor stimulation of phosphatidylinositol 3-kinase in natural killer cells is associated with protein kinase C-independent granule release and cell-mediated cytotoxicity. J Exp Med. 1994;180:142–1435. doi: 10.1084/jem.180.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paya CV, Virelizier JL, Michelson S. Modulation of T-cell activation through protein kinase C- or A-dependent signalling pathways synergistically increases human immunodeficiency virus long terminal repeat induction by cytomegalovirus immediate-early proteins. J Virol. 1991;65:5477–5484. doi: 10.1128/jvi.65.10.5477-5484.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shaw E. Cysteinyl proteinases and their selective inactivation. Adv Enzymol Relat Areas Mol Biol. 1990;63:271–346. doi: 10.1002/9780470123096.ch5. [DOI] [PubMed] [Google Scholar]
- 64.Sarin A, Wu M-L, Henkart P. Different interleukin-1β converting enzyme (ICE) family protease requirements for the apoptotic death of T lymphocytes triggered by diverse stimuli. J Exp Med. 1996;184:2445–2450. doi: 10.1084/jem.184.6.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogasawara J, Suda T, Nagata S. Selective apoptosis in CD4+CD8+thymocytes by the anti-fas antibody. J Exp Med. 1995;181:485–491. doi: 10.1084/jem.181.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson KL, Anderson G, Michell RH, Jenkinson EJ, Owen JJT. Intracellular signaling pathways involved in the induction of apoptosis in immature thymic T lymphocytes. J Immunol. 1996;156:4083–4091. [PubMed] [Google Scholar]
- 67.Strasser A, Harris AW, Huang DC, Krammer PH, Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO (Eur Mol Biol Organ) J. 1995;14:6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Truitt KE, Hicks CM, Imboden JB. Stimulation of CD28 triggers an association between CD28 and phosphatidylinositol 3-kinase in Jurkat T cells. J Exp Med. 1994;179:1071–1076. doi: 10.1084/jem.179.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.June CH, Ledbetter JA, Gillespie MM, Lindsten T, Thompson CB. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol Cell Biol. 1987;7:4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lo D, Sprent J. Identity of cells that imprint H-2-restricted T-cell specificity in the thymus. Nature. 1986;319:672–675. doi: 10.1038/319672a0. [DOI] [PubMed] [Google Scholar]
- 72.Swat W, Ignatowicz L, von Boehmer H, Kisielow P. Clonal deletion of immature CD4+8+ thymocytes in suspension culture by extrathymic antigen-presenting cells. Nature. 1991;351:150–153. doi: 10.1038/351150a0. [DOI] [PubMed] [Google Scholar]
- 73.Matzinger P, Guerder S. Does T-cell tolerance require a dedicated antigen-presenting cell? . Nature. 1989;338:74–76. doi: 10.1038/338074a0. [DOI] [PubMed] [Google Scholar]
- 74.Degermann S, Surh CD, Glimcher LH, Sprent J, Lo D. B7 expression on thymic medullary epithelium correlates with epithelium-mediated deletion of V beta 5+ thymocytes. J Immunol. 1994;152:3254–3263. [PubMed] [Google Scholar]
- 75.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. [PubMed] [Google Scholar]
- 76.Laufer TM, DeKoning J, Markowitz JS, Lo D, Glimcher LH. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex. Nature. 1996;383:81–85. doi: 10.1038/383081a0. [DOI] [PubMed] [Google Scholar]
- 77.MacDonald HR, Lees RK. Programmed death of autoreactive thymocytes. Nature. 1990;343:642–644. doi: 10.1038/343642a0. [DOI] [PubMed] [Google Scholar]
- 78.Guidos CJ, Danska JS, Fathman CG, Weissman IL. T cell receptor-mediated negative selection of autoreactive T lymphocyte precursors occurs after commitment to the CD4 or CD8 lineages. J Exp Med. 1990;12:835–845. doi: 10.1084/jem.172.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kishimoto H, Sprent J. Negative selection in the thymus includes semimature T cells. J Exp Med. 1997;185:263–271. doi: 10.1084/jem.185.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]