Abstract
The CD19 cell surface molecule regulates signal transduction events critical for B lymphocyte development and humoral immunity. Increasing the density of CD19 expression renders B lymphocytes hyper-responsive to transmembrane signals, and transgenic mice that overexpress CD19 have increased levels of autoantibodies. The role of CD19 in tolerance regulation and autoantibody generation was therefore examined by crossing mice that overexpress a human CD19 transgene with transgenic mice expressing a model autoantigen (soluble hen egg lysozyme, sHEL) and high-affinity HEL-specific IgMa and IgDa (IgHEL) antigen receptors. In this model of peripheral tolerance, B cells in sHEL/IgHEL double-transgenic mice are functionally anergic and do not produce autoantibodies. However, it was found that overexpression of CD19 in sHEL/IgHEL double-transgenic mice resulted in a breakdown of peripheral tolerance and the production of anti-HEL antibodies at levels similar to those observed in IgHEL mice lacking the sHEL autoantigen. Therefore, altered signaling thresholds due to CD19 overexpression resulted in the breakdown of peripheral tolerance. Thus, CD19 overexpression shifts the balance between tolerance and immunity to autoimmunity by augmenting antigen receptor signaling.
Blymphocyte tolerance to self antigens is achieved by the negative selection and elimination of immature B cells that express high-affinity IgM receptors for autoantigens (1–4). Negative selection is antigen receptor-dependent but also relies on established triggering thresholds for intracellular signals (1, 5). If antigen receptor ligation generates inadequate intracellular signals because of a low affinity for autoantigens, or the valency or concentration of autoantigen is low, autoreactive B cells mature and leave the bone marrow but are rendered functionally anergic (1, 4–7). Intracellular signaling thresholds are likely to also play a major role in the regulation and maintenance of peripheral tolerance.
The CD19 cell surface molecule regulates intracellular signaling thresholds critical for B cell development and humoral immunity (8–13). B lymphocytes from mice that overexpress CD19 are hyper-responsive to antigen receptor crosslinking, which results in serum Ig levels that are increased by ∼40% and humoral responses that are augmented several fold (12, 14, 15). Based on this, it was expected that CD19 overexpression by autoreactive B cells would either lead to their augmented negative selection in the bone marrow or result in a more profound state of peripheral anergy. Unexpectedly however, C57BL/6 mice that overexpress CD19 have two- to fourfold higher levels of anti-DNA autoantibodies and rheumatoid factor (8, 16). Increased autoantibody production in mice overexpressing CD19 correlates with dramatic increases in the number of B1 lineage cells. However, since IgG anti-DNA autoantibodies are preferentially increased in mice that overexpress CD19, the CD19-induced autoantibodies may alternatively result from alterations in conventional B cell tolerance.
Transgenic mouse models for autoreactive B cells (4, 7) provide a mechanism for determining the role of CD19 signaling in regulating peripheral tolerance and autoimmunity. B cells from transgenic mice expressing a model autoantigen (soluble hen egg lysozyme, sHEL1) and high-affinity HEL-specific IgMa and IgDa (IgHEL) antigen receptors enter the peripheral pool but are anergic to antigen receptor ligation and produce little, if any, spontaneous HEL-specific antibody (17). Mice that express a human CD19 (hCD19) transgene provide a model for examining augmented CD19 function in vivo (8, 14–16, 18, 19). Since hCD19 can replace the function of mouse CD19 in vivo, hemizygous hCD19+/– transgenic mice expresses cell surface CD19 at a twofold higher density while hCD19+/+ transgenic mice express threefold higher densities of CD19 (16, 19). Therefore, sHEL/IgHEL double-transgenic mice were crossed with hCD19 transgenic mice to determine whether tolerance would be maintained in sHEL/IgHEL/hCD19 transgenic mice or autoantibodies would be generated. CD19 overexpression in sHEL/IgHEL double-transgenic mice resulted in the production of anti-HEL antibodies at levels similar to those observed in IgHEL mice lacking this model self antigen. Therefore, lowered signaling thresholds due to CD19 overexpression resulted in the breakdown of peripheral tolerance in sHEL/IgHEL double-transgenic mice.
Materials and Methods
Mice.
hCD19 transgenic mice (h19-1 line, C57BL/6) were as described (12, 15). In the h19-1 line of mice, 9–14 copies of the hCD19 transgene are integrated into a single (or closely linked) site(s). These h19-1 mice used in this study were backcrossed onto a wild-type C57BL/6 background for 8 to 10 generations without a diminution of hCD19 expression and all mice express similar levels of cell-surface hCD19. Mice expressing sHEL (ML5 line) and IgHEL (MD4 line) were as described (17, 20). sHEL/IgHEL/hCD19 triple-transgenic mice were generated by appropriate backcrosses of sHEL/IgHEL double-transgenic mice with hCD19+/+ mice. Transgene expression was assessed as described (12, 15, 17, 20). Mice were housed in a specific pathogen-free barrier facility. All studies and procedures were approved by the Duke University Animal Care and Use Committee.
Immunization of Mice.
2-mo-old mice were immunized i.p. with 100 μg of HEL in complete Freund's adjuvant (CFA; Sigma Chemical Co., St. Louis, MO) or PBS in CFA at day 0 and were boosted at day 21. Animals were bled just before the first immunization and 7, 14, and 28 d later.
Mouse Ig Isotype-specific ELISAs.
Serum levels of HEL-specific IgM allotype a (IgMa) antibody were measured by ELISA on HEL-coated plates as described (21). Absolute antibody concentrations were determined relative to a standard curve of HEL-specific IgMa mAb (E1 clone) generated from an IgHEL transgenic mouse immunized with HEL. The ELISA sensitivity limit was ∼20 ng/ml of anti-HEL IgMa antibody.
Immunofluorescence Analysis.
Antibodies used in this study included: FITC-conjugated and biotin-coupled goat anti–mouse IgM isotype-specific antibodies (Southern Biotechnology Associates, Inc., Birmingham, AL); anti-B220 (CD45RA, RA3-6B2, provided by R. L. Coffman, DNAX Research Inst., Palo Alto, CA), anti-I-A (M5/114.15.2, clone TIB120; American Type Culture Collection [ATCC], Bethesda, MD), anti-HSA (M1/69; PharMingen, San Diego, CA), anti-CD5 (53-7.313, clone TIB104; ATCC), anti-B7-2 (GL-1; PharMingen) and anti-mouse IgMa (DS-1; PharMingen) monoclonal antibodies. Phycoerythrin-conjugated streptavidin (Fisher Scientific, Fair Lawn, NJ) was used to reveal biotin-coupled mAb staining. Phycoerythrin-conjugated goat anti–rat IgG antibodies (Caltag, Burlingame, CA) were used to visualize anti-CD5 mAb staining. Cells reacting with biotin-coupled HEL were stained with phycoerythrin-conjugated streptavidin. Isolated lymphocytes were analyzed on a FACScan® flow cytometer (Becton-Dickinson, San Jose, CA) as described (16).
Measurement of Intracellular Calcium.
Splenocytes were isolated, loaded with indo-1 and stained with FITC-labeled anti-B220 antibodies as described (22). Relative intracellular Ca++ levels ([Ca++]i) were assessed by flow cytometry after gating on the B220+ population of cells. Baseline fluorescence ratios were collected for 1 min before HEL and/or specific mAb were added at final concentrations of: HEL, 100 ng/ml; anti-mouse CD19, 40 μg/ml (MB19-1, IgA) (16); and anti-human CD19, 40 μg/ml (HB12b, IgG1) (23). An increase in the ratio of indo-1 fluorescence indicates an increase in [Ca++]i.
Statistical Analysis.
All data are shown as mean values ± SEM. Analysis of variance (ANOVA) was used to analyze the data, and the Student's t test was used to compare population sample means. The Mann-Whitney test was also used to compare population frequency distributions. The 95% confidence interval for anti-HEL antibody levels observed in sHEL/IgHEL mice was determined using the log normal distribution (mean ± 2 SD) of antibody values with undetectable levels (<20 ng/ml) assigned the value of 10 ng/ml.
Results
Autoantibodies in sHEL/IgHEL/hCD19 Transgenic Mice.
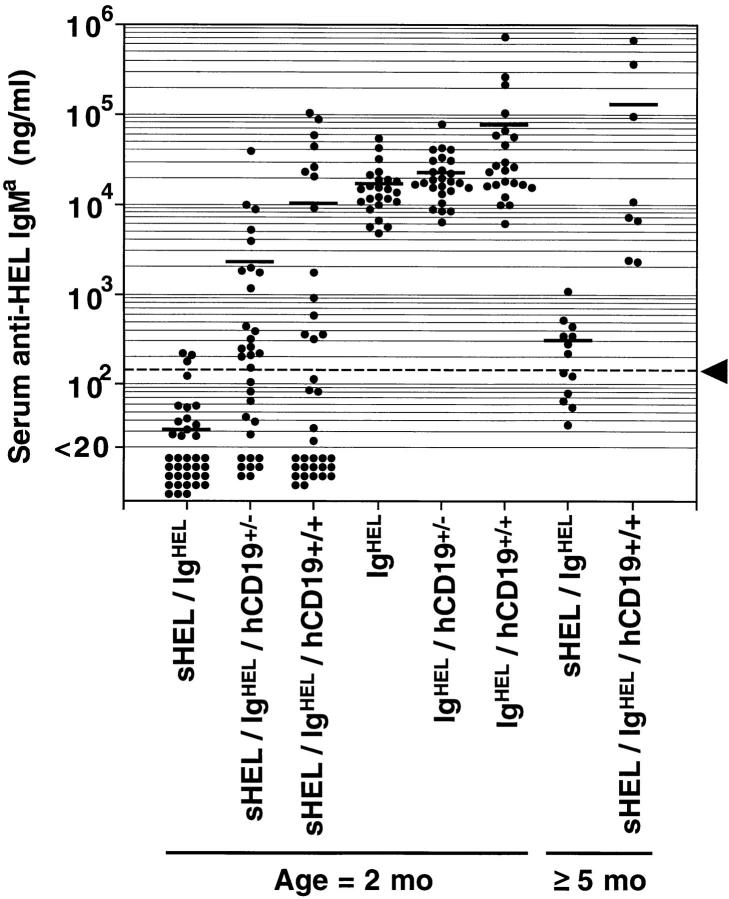
Serum anti-HEL IgMa autoantibody levels in IgHEL transgenic, sHEL/IgHEL double-transgenic, and sHEL/IgHEL/ hCD19 triple-transgenic mice were determined to assess the status of B cell tolerance in each set of mice. Serum antibody levels for each individual mouse are shown in Fig. 1 and mean autoantibody levels for each set of mice are provided to simplify discussion of the results. 2-mo-old sHEL/ IgHEL double-transgenic mice produced very low or undetectable levels of anti-HEL IgMa antibodies (mean levels 31 ng/ml) when compared with IgHEL transgenic mice (mean 16,700 ng/ml, Fig. 1). However, 45% (14 of 33) of sHEL/ IgHEL/hCD19+/– mice had anti-HEL IgMa autoantibody levels (mean 2,430 ng/ml) that were significantly greater than those found in sHEL/IgHEL mice (P ⩽0.001, Fig. 1). Anti-HEL IgMa antibody levels were also elevated in 38% (14 of 36) of sHEL/IgHEL/hCD19+/+ mice (mean 10,500 ng/ml, P ⩽0.01, Fig. 1). Autoantibody levels in some sHEL/IgHEL/hCD19 mice were equivalent to those of IgHEL-transgenic mice not expressing sHEL. In fact, overexpression of CD19 resulted in anti-HEL autoantibody levels in some mice that were 1,000-fold higher than in sHEL/IgHEL mice. By comparison, overexpression of CD19 in IgHEL/CD19+/+ mice resulted in only a fourfold increase in anti-HEL antibody levels (mean 77,300 ng/ml, Fig. 1). Thus, lowered signaling thresholds resulting from the overexpression of CD19 abrogated peripheral anergy in a significant proportion of 2-mo-old sHEL/IgHEL mice.
Figure 1.
Anti-HEL IgMa antibody levels in IgHEL and sHEL/IgHEL mice that overexpress CD19. Each value indicates serum levels of HEL-specific IgMa from individual 2-mo-old (2 mo) or 5– 10-mo-old (⩾5 mo) mice measured by ELISA. Horizontal bars indicating mean anti-HEL antibody concentrations for each group are provided for reference. The dashed horizontal line (arrowhead) delimits the 95% confidence interval for the log normal distribution of anti-HEL antibody levels observed in unimmunized 2-mo-old sHEL/IgHEL mice as described in Materials and Methods.
The breakdown in peripheral tolerance and the development of autoantibodies in sHEL/IgHEL mice that overexpressed CD19 correlated with mouse age. By 5–10 mo of age, all sHEL/IgHEL/hCD19+/+ mice produced significantly higher levels of autoantibodies (mean 144,222 ng/ ml) than sHEL/IgHEL mice (303 ng/ml, P <0.01, Fig. 1). The lowest autoantibody level found in a 5-mo-old sHEL/ IgHEL/hCD19+/+ mouse was 2,255 ng/ml. Therefore, the breakdown of tolerance in sHEL/IgHEL/hCD19+/+ mice had 100% penetrance by 5 mo of age.
Abrogation of Peripheral Tolerance in sHEL/IgHEL/hCD19 Mice.
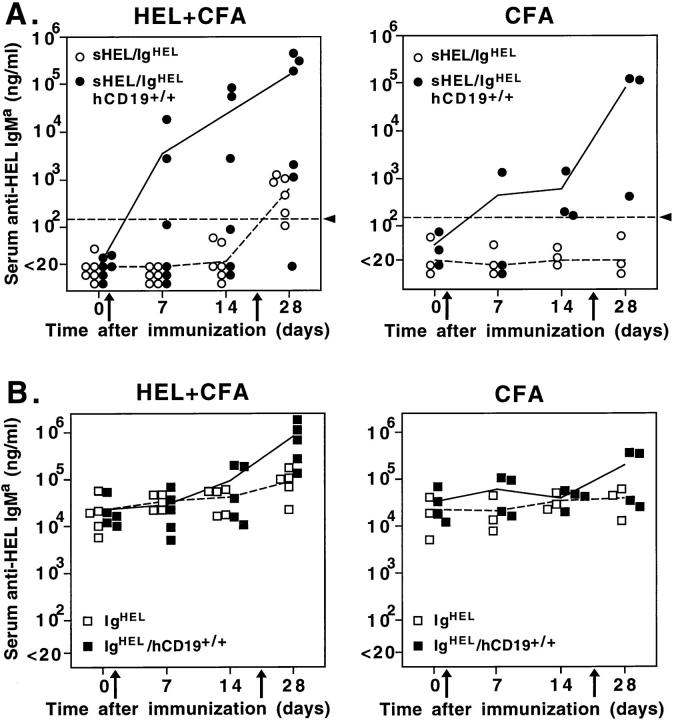
Whether B cell anergy could be surmounted in young mice that overexpress CD19 was assessed by immunizing 2-mo-old mice with HEL in CFA. Mice without detectable levels of spontaneous anti-HEL antibodies were also injected with CFA alone to mimic a nonspecific inflammatory stimulus. Immunization of sHEL/IgHEL/hCD19+/+ mice with HEL generated primary anti-HEL antibody responses in some mice, and a mean secondary antibody response that was 200-fold higher (P <0.05) than that of sHEL/IgHEL mice (Fig. 2 A). A measurable antibody response was only detected in sHEL/IgHEL mice after secondary immunization (Fig. 2 A). A striking result was that the inflammation induced by CFA alone induced sHEL/IgHEL/ hCD19+/+ mice to produce anti-HEL antibodies in response to endogenous sHEL autoantigen (Fig. 2 A). In this case, the mean secondary antibody response was 4,000-fold higher than in sHEL/IgHEL mice (P <0.05). In fact, the anti-HEL antibody levels induced in some sHEL/IgHEL/hCD19+/+ mice were equivalent to those of IgHEL mice (Fig. 2 B). Similar results were obtained with sHEL/IgHEL/hCD19+/– mice although anti-HEL autoantibody levels were intermediate (data not shown). In sHEL/IgHEL/hCD19+/+ mice that already expressed detectable anti-HEL antibodies, autoantibody levels were also dramatically augmented after CFA administration (data not shown). Therefore, inflammatory responses induced by the administration of CFA revealed a breakdown in tolerance and resulted in autoantibody production in anergic mice that overexpressed CD19.
Figure 2.
Humoral immune responses of sHEL/IgHEL (A) and IgHEL(B) mice that overexpress CD19 in response to immunization with HEL. 2-mo-old mice were injected i.p. with HEL or PBS mixed with CFA on days 0 and 21 (arrows), and were bled at the indicated times. Levels of serum anti-HEL IgMa antibodies for individual mice (dots or squares) were determined by ELISA. Mean antibody levels are shown as solid (hCD19+/+) or dashed (hCD19–/–) lines. The dashed horizontal lines (arrowhead) delimit the 95% confidence interval for the log normal distribution of anti-HEL antibody levels observed in unimmunized sHEL/IgHEL mice.
Effects of CD19 Overexpression on B Cell Development.
The effects of CD19 overexpression on B cell development was assessed to elucidate the cellular basis for the breakdown of peripheral tolerance in sHEL/IgHEL mice. The breakdown in tolerance did not result from relaxed negative selection, since the number of mature IgM+ B220hi or HSAlo B220hi B cells in the bone marrow, blood, and spleen of IgHEL/hCD19+/+ mice was significantly reduced in the absence or presence of sHEL (Fig. 3, Table I). A similar decrease in the generation of mature B cells occurs, presumably as a consequence of increased clonal elimination, in wild-type mice that overexpress CD19 (15). However, since all B cells bear the same receptor with the same affinity for antigen, the partial decrease in generation of mature B cells in the bone marrow of IgHEL/hCD19+/+ mice and sHEL/IgHEL/hCD19+/+ mice suggests that this developmental bottleneck occurs independent of antigen receptor ligation. Further, it is difficult to imagine a deleting antigen that binds to the transgenic receptor better than HEL, and deletion also occurs in mice lacking sHEL. Therefore, overexpression of CD19 may alter the generation of mature B cells through mechanisms in addition to increased negative selection. Nonetheless, the breakdown in tolerance did not result from relaxed negative selection.
Figure 3.

B cell development in IgHEL and sHEL/IgHEL mice that overexpress CD19. Representative two-color immunofluorescence staining of B cells from bone marrow (A), blood (B), spleens (C), and peritoneum of littermate pairs (D). B lymphocytes were revealed by B220 or IgM expression. (A) Quadrants delineated by squares indicate the pre–B cell (B220lo IgM−), immature B cell (B220lo IgM+) and mature B cell (B220hi IgM+) compartments, with numbers representing the percentage of cells within quadrants. The gates that define mature B lymphocytes for sHEL/ IgHEL mice were different from the gate used for IgHEL mice since surface IgM levels are downregulated in sHEL/IgHEL mice. (B and C) Quadrants delineated by squares indicate the B cell (B220+ IgM+) compartments. Spleen cells were also stained for B220 or IgM and counterstained for sHEL binding or I-A expression. (D) The gates used to determine the frequency of the CD5+ B220+ population and CD5− B220+ population of cells for Table I are shown as polygons and squares. Populations of cells lacking surface antigen expression were determined using unreactive monoclonal antibodies as controls. All samples were stained in parallel and analyzed sequentially by flow cytometry with identical instrument settings. Relative fluorescence intensity is shown on a four decade log scale, with 50% log density contour levels. Horizontal dashed lines in some histograms are provided for reference. These results are representative of those obtained with at least five sets of mice. Equivalent results were obtained by using anti-IgMa antibody instead of anti-IgM antibody.
Table I.
Phenotype and Frequency of B lymphocytes in Lymphoid Tissues
| Tissue | Phenotype | IgHEL | IgHEL hCD19+/+ | sHEL IgHEL | sHEL IgHEL hCD19+/+ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency (%) and number (No. × 10−6) of B cells* | ||||||||||||
| bone | % IgM−B220lo | 14 ± 2 | 14 ± 2 | 13 ± 2 | 13 ± 2 | |||||||
| marrow | % IgM+B220lo | 32 ± 4 | 49 ± 10 | 46 ± 2 | 53 ± 3 | |||||||
| % IgM+B220hi | 16 ± 3 | 8 ± 3§ | 12 ± 2 | 8 ± 1 | ||||||||
| % HSAlo B220hi | 25 ± 3 | 9 ± 3‖ | 17 ± 5 | 5 ± 1§ | ||||||||
| blood | % B220+ | 43 ± 3 | 7 ± 2‖ | 22 ± 3 | 6 ± 2‖ | |||||||
| # B220+ | 2.4 ± 0.5 | 0.3 ± 0.1‖ | 0.9 ± 0.2 | 0.2 ± 0.1‖ | ||||||||
| spleen | % B220+ | 36 ± 6 | 28 ± 5 | 44 ± 4 | 17 ± 3‖ | |||||||
| # B220+ | 24 ± 5 | 14 ± 3§ | 29 ± 5 | 15 ± 6§ | ||||||||
| peritoneum | % CD5+ B220lo | 2.2 ± 0.3 | 1.3 ± 0.4 | 4.5 ± 1.1 | 1.9 ± 0.7 | |||||||
| # CD5+ B220lo | 0.05 ± 0.01 | 0.05 ± 0.02 | 0.10 ± 0.02 | 0.04 ± 0.02 | ||||||||
| % CD5− B220hi | 38 ± 6 | 2.7 ± 0.7‖ | 14 ± 2 | 1.2 ± 0.4‖ | ||||||||
| # CD5− B220hi | 1.0 ± 0.3 | 0.07 ± 0.03§ | 0.33 ± 0.03 | 0.02 ± 0.01‖ | ||||||||
| Expression | Source of B cells | Levels relative to IgHEL mice (% ± SEM)‡ | ||||||||||
| IgM levels: | bone | B220lo | 100 | 69 ± 12‖ | 41 ± 9 | 22 ± 8 | ||||||
| marrow: | B220hi | 100 | 63 ± 4‖ | 4.1 ± 0.5 | 4.3 ± 0.7 | |||||||
| blood: | B220+ | 100 | 68 ± 15‖ | 19 ± 3 | 25 ± 4 | |||||||
| spleen: | B220+ | 100 | 62 ± 4‖ | 16 ± 2 | 7 ± 1‖ | |||||||
| I-A levels: | blood: | IgM+ | 100 | 126 ± 5‖ | 169 ± 7 | 254 ± 33‖ | ||||||
| spleen: | IgM+ | 100 | 259 ± 55§ | 184 ± 13 | 283 ± 30§ | |||||||
Cumulative mean (± SEM) frequencies of different cell populations from at least five 2-mo-old mice of each genotype. Flow cytometry gates similar to those shown in Fig. 3 were used to determine the frequency of each cell type within the lymphocyte population. B cell numbers for blood indicate the number of cells/ml. B cell numbers from spleen and peritoneum were determined based on the total number of lymphocytes recovered.
Relative cell surface antigen densities were determined by comparing the channel numbers of mean linear fluorescence intensity between IgHEL B cells and B cells fom other mice. Values represent the mean expression levels obtained from at least three sets of mice of each genotype. All samples in each set of mice were stained in parallel and analyzed sequentially by flow cytometry with identical instrument settings.
Differences between mice not expressing hCD19 and those expressing hCD19 were significant, P <0.05.
P <0.01.
Peripheral B cell numbers were significantly reduced in both IgHEL mice and sHEL/IgHEL mice overexpressing CD19 (Fig. 3, Table I). Overexpression of CD19 reduced circulating B cell numbers by 87% in IgHEL mice and 78% in sHEL/IgHEL mice. CD19 overexpression reduced spleen B cell numbers by 42% in IgHEL mice and 48% in sHEL/IgHEL mice. Conventional B cells within the peritoneum were also reduced by >90% in IgHEL/hCD19+/+ and sHEL/IgHEL/hCD19+/+ mice. Overexpression of CD19 did not induce the generation of B cells with the phenotypic characteristics of either B1a or B1b cells and only small numbers of CD5+ B220lo B cells were observed in any of the 2-mo-old transgenic mouse lines (Fig. 3, Table I). In addition, all of the HEL-binding B cells in each line of mice were conventional B cells since they were CD5−, CD23+, IgDhi, and B220hi (data not shown). Thus, the dramatic increase in the levels of autoantibodies generated in sHEL/IgHEL/hCD19+/+-transgenic mice were even more significant given the >50% reduction in numbers of peripheral B cells in these mice.
Chronic stimulation through the B cell antigen receptor in sHEL/IgHEL mice results in a unique IgMlo IgDhi phenotype with increased expression of class II (I-A) antigens (17, 21, 24). In comparison, the overexpression of hCD19+/+ in these mice resulted in even lower IgM expression and higher I-A expression (Fig. 3, Table I). Despite the decrease in surface IgM, all B cells from sHEL/IgHEL/ hCD19+/+ mice still bound sHEL in vitro in proportion to their IgMa density (Fig. 3 C). B cells from IgHEL/hCD19+/+ transgenic mice had an intermediate IgMlo I-Ahi phenotype even in the absence of sHEL (Fig. 3, Table I). B cells from mice that overexpressed CD19 also expressed significantly elevated levels of cell surface CD86 (B7-2) (data not shown). Therefore, CD19 overexpression appeared to augment the phenotypic outcome of signaling through the B cell antigen receptor in the absence or presence of autoantigen. However, B cells from sHEL/IgHEL/hCD19+/+ mice still exhibited a phenotype that is characteristic of anergic B cells.
[Ca++]i Responses in B Cells from sHEL/IgHEL/hCD19 Mice.
Peripheral tolerance in sHEL/IgHEL mice results in the failure of anergic B cells to mobilize intracellular Ca++ in response to HEL-mediated antigen receptor crosslinking in vitro (25). B cells from sHEL/IgHEL/hCD19+/+ mice were equivalent to anergic B cells from sHEL/IgHEL mice in their failure to mobilize Ca++ in response to HEL (Fig. 4 A). B cells from sHEL/IgHEL/hCD19+/+ mice that generated high levels of autoantibodies also failed to mobilize Ca++ in response to HEL (data not shown). B cells from IgHEL/hCD19+/+ mice generated normal Ca++ responses (Fig. 4 B). Therefore, the development of autoimmunity in sHEL/IgHEL/hCD19+/+ mice does not result from a CD19-induced recovery of early signaling responses in the bulk of anergic B cells.
Figure 4.
Signal transduction through surface IgM and CD19 in B cells from sHEL/IgHEL/hCD19+/+ (A) or IgHEL/hCD19+/+ mice (B). Relative [Ca++]i levels were assessed by flow cytometry after gating on the B220+ population of indo-1 loaded splenocytes. Baseline fluorescence ratios were collected for 1 min before HEL and/or specific monoclonal antibodies were added (arrows) at final concentrations of: HEL, 100 ng/ml; anti-mouse CD19, 40 μg/ml; anti-human CD19, 40 μg/ml. An increase in [Ca++]i over time is shown as an increase in the ratio of indo-1 fluorescence. Values represent the ratios of fluorescence intensity of cell populations after treatment relative to the fluorescence intensity of untreated cells. These results are representative of those obtained from three littermate pairs of mice.
Although antigen receptor ligation did not induce Ca++ responses in anergic B cells, crosslinking human and mouse CD19 induced a normal Ca++ response in anergic B cells from sHEL/IgHEL/hCD19+/+ mice (Fig. 4 A). Crosslinking mouse CD19 in sHEL/IgHEL mice also induced a normal Ca++ response (data not shown). The presence of HEL during CD19 crosslinking resulted in a Ca++ response that was significantly greater than that observed with CD19 crosslinking alone in sHEL/IgHEL/hCD19+/+ mice (P <0.01, Fig. 4 A). However, the magnitude of the CD19/HEL-induced Ca++ response in sHEL/IgHEL/hCD19+/+ mice (Fig. 4 A) was less than that observed in IgHEL/hCD19+/+ mice (Fig. 4 B). Of interest was the observation that the magnitude of the CD19-induced Ca++ response in sHEL/IgHEL/hCD19+/+ mice was always significantly higher than the Ca++ response in IgHEL/hCD19+/+ mice (n = 3, P <0.05). The increased Ca++ responses of B cells from sHEL/IgHEL/ hCD19+/+ mice presumably results from the endogenous ligation of antigen receptors by sHEL encountered in vivo. These results indicate that CD19 ligation can induce a relatively normal Ca++ response in anergic B cells. Moreover, CD19 ligation can also augment transmembrane signals generated through the B cell antigen receptor despite clonal anergy.
Discussion
The striking induction of autoantibody production in sHEL/IgHEL mice that are normally functionally anergic directly implicates CD19 signaling thresholds as a regulator of peripheral tolerance in B cells. CD19 overexpression by only twofold to threefold caused a breakdown of peripheral B cell tolerance in a clear and dramatic fashion, with autoantibody levels increased several thousandfold in some sHEL/IgHEL/CD19+/+ mice (Figs. 1 and 2). These dramatic increases in autoantibody levels in sHEL/IgHEL/ hCD19+/+ mice are even more significant given the >50% reduction in numbers of peripheral B cells in mice that overexpress CD19 (Fig. 3, Table I). Overexpression of CD19 alone did not induce anergic B cells to produce autoantibodies, as evidenced by the fact that some sHEL/IgHEL/ hCD19 mice were anergic and did not produce spontaneous autoantibodies until 5 mo of age (Fig. 1). However, significant autoantibody production was induced in 2-mo-old anergic sHEL/IgHEL/hCD19 mice by inducing inflammation with CFA (Fig. 2). Autoantibodies in sHEL/IgHEL mice that overexpressed CD19 are likely to have originated from a breakdown in tolerance in conventional B cells, since HEL-specific B1a or B1b cells were not detected in IgHEL (26), sHEL/IgHEL or sHEL/IgHEL/hCD19 mice (Fig. 3, Table I). These findings suggest that alterations in CD19-related signaling thresholds breaks peripheral tolerance, which predisposes B cells to the induction of autoantibodies.
The levels of autoantibody production observed in sHEL/ IgHEL mice that overexpress CD19 (Figs. 1 and 2) clearly demonstrates that tolerance was abrogated in a significant portion of B cells. Since IgHEL B cells are constantly exposed to antigen in transgenic sHEL mice, autoantibody production in sHEL/IgHEL/CD19+/+ mice is most likely induced through an antigen receptor-dependent process. Autoantibody production in sHEL/IgHEL/CD19+/+ mice may relate to the observation that CD19 ligation can augment transmembrane signals generated through the antigen receptor despite clonal anergy (Fig. 4). We have recently demonstrated that genetic alterations in CD19 expression have significant effects on the signal transduction pathways activated after B cell antigen receptor engagement (27). Therefore, one pathway to autoantibody production in sHEL/IgHEL mice may be via concomitant CD19 overexpression, chronic antigen receptor ligation, and the influence of inflammatory mediators triggering the simultaneous breakdown of tolerance and autoantibody production in anergic IgHEL B cells. Alternatively, inflammatory mediators such as those generated by CFA administration may induce the expansion or differentiation of antigen-stimulated IgHEL B cell clones subsequent to a CD19-induced breakdown in tolerance. The latter possibility is supported by the finding that B cells from mice that overexpressed CD19 maintained a phenotype characteristic of anergic B cells (Fig. 3) and failed to generate Ca++ responses after antigen receptor ligation (Fig. 4). The spontaneous development of autoantibodies in sHEL/IgHEL/hCD19 mice may also require a breakdown in T cell tolerance, since HEL-specific helper T cells are anergic due to chronic sHEL exposure (6). Thereby, soluble factors induced by CFA administration may replace the requirement for T cell help during autoantibody production in sHEL/IgHEL mice. Although the exact pathway to autoantibody production has yet to be determined, the current results suggest strongly that inappropriate CD19 expression or function contributes to autoimmunity by disrupting tolerance.
Variability in the timing and magnitude of autoantibody production in individual sHEL/IgHEL/CD19+/+ mice is similar to what has been observed in many mouse models of autoimmunity (28). Overexpression of CD19 in sHEL/ IgHEL mice resulted in significant autoantibody production in a large portion of 2-mo-old mice, while all triple-transgenic mice produced significant autoantibodies by 5 mo of age (Fig. 1). The expansion and/or accumulation of B cell clones that have escaped tolerance may explain why all sHEL/IgHEL/hCD19+/+ mice produced high levels of spontaneous autoantibodies by 5 mo of age. This contrasts markedly with old sHEL/IgHEL mice which did not produce significant levels of anti-HEL autoantibodies (Fig. 1). Previous studies of sHEL/IgHEL mice have demonstrated that functional inactivation of autoreactive B cells is maintained throughout life (29). The variability and delayed onset of autoantibody production in sHEL/IgHEL/hCD19+/+ mice may also result from their confinement to a specific-pathogen-free barrier facility. Autoantibodies first appear in some mouse models of lupus (NZB and MRL strains) around 2 mo of age, but are dramatically increased by 4–5 mo of age in all mice. Variability in onset and magnitude of autoantibody production also occurs in individual mice of these inbred mouse strains. Therefore, variability between the triple transgenic mice examined in this study is not surprising despite their identical genetic background and the fact that the hCD19 transgene is expressed to the same extent in all animals. Consistent with the current studies, an association between CD19 overexpression and autoimmunity in humans has been suggested (30). The etiology of autoimmunity in humans has also been historically linked with the accumulation of inflammatory episodes or infectious agents and often varies in degree and time of onset. Therefore, many of the current findings in sHEL/IgHEL mice mimic the evolution of autoreactive B cells and autoantibodies in humans.
The role of antigen receptor signaling strength in the development of autoreactive B cells has recently been examined in sHEL/IgHEL mice (26, 31). Mutations in the SHP1 protein tyrosine phosphatase of motheaten viable (mev) mice abrogates the negative regulatory role of SHP1 in antigen receptor signaling, resulting in the generation of autoantibodies in nontransgenic mice. In IgHEL mice, the mev mutation lowers signaling thresholds, which incites the negative selection of IgHEL B cells in the bone marrow of sHEL mice (26). The SHP1 deficiency thereby prevents autoantibody generation but facilitates the development of peritoneal B1 cells reactive with HEL (26). These results contrast markedly with the results of the current study, in which lowering B cell signaling thresholds by increased CD19 expression resulted in a breakdown in tolerance and autoantibody production rather than the total negative selection of IgHEL B cells in the bone marrow of sHEL mice (Figs. 1 and 3). Thus, SHP1 may play a key role in setting thresholds for negative selection in the bone marrow, while CD19 may preferentially regulate peripheral tolerance. Alternatively, tolerance may be finely tuned, with CD19 and SHP1 altering signaling strengths to differing extents. In contrast with CD19 overexpressing B cells, signaling in response to antigen receptor ligation is diminished in CD45-deficient IgHEL B cells (31). Diminished signaling in CD45-deficient B cells leads to reduced negative selection in the bone marrow and prolonged retention in peripheral lymphoid tissues of mice expressing sHEL. Since the in vivo functional capacity of peripheral CD45-deficient IgHEL B cells or their production of autoantibodies has not been examined, it is difficult to assess how diminished signaling in those studies relates to the current studies. Nonetheless, all of these studies demonstrate that antigen receptor signaling strength influences positive or negative selection, and the current studies demonstrate a direct and active role for CD19 in regulating peripheral tolerance and autoantibody generation.
It could be argued that the breakdown in tolerance observed in this study results from the inadvertent insertion of the CD19 transgene into a locus that controls B cell tolerance. However, this is unlikely for several reasons. First, the human CD19 transgenic mice used in these studies reconstitute normal B cell function when crossed with CD19-deficient mice (19). The h19-1 line of mice has also been backcrossed extensively onto a wild-type C57BL/6 background without a diminution of human CD19 expression. This suggests that only one transgene integration site exists and that the heterogeneity in autoantibody production observed between triple transgenic mice does not reflect the segregated inheritance of transgenes that have integrated into multiple sites. We have also generated and analyzed seven independent lines of hCD19 transgenic mice (15, 18). In all cases, B cells from each mouse line demonstrate identical functional abnormalities; hyper-responsive B cells and enhanced autoantibody production. The magnitude of these abnormalities correlates directly and linearly with the level of hCD19 overexpression (19). In addition, the abnormalities observed in hCD19 transgenic mice are reciprocal of what we have observed in CD19-deficient mice (12, 14, 16). Therefore, the effects observed in the current study most likely relate directly to augmented CD19 function rather than an interruption of other genes involved in tolerance regulation.
CD19 is a signaling component of a multimeric complex that includes CD21, the receptor for the C3d fragment of complement that covalently associates with antigens during complement activation (23, 32). C3d binding to CD21 can thereby act as a ligand for the CD19 complex that links complement activation with B cell function (33–35). Since CD21-deficient mice manifest developmental and functional defects similar to those of CD19-deficient mice (12, 13, 36, 37), overexpression of CD19 in vivo may mimic C3d ligation of CD21 by augmented signaling through the CD19 complex (8). Because the roadblock to B cell Ca++ responses in anergic B cells was transcended in vitro by simultaneous CD19 ligation and antigen receptor signaling (Fig. 4), C3 cleavage products binding to the CD19 complex may provide a molecular mechanism for bypassing peripheral B cell anergy in vivo. The inappropriate or prolonged generation of C3d during inflammatory or infectious episodes in vivo may increase the responsiveness of autoreactive B cells to weak self antigens through augmented CD19 function, resulting in a breakdown of tolerance and the clonal amplification of autoantibody-producing B cells. Because altering CD19 complex function provides a mechanism for breaking self-tolerance in vivo, CD19 function may be a molecular mechanism linking inflammation with the development of autoimmune disease.
Acknowledgments
This work was supported by National Institutes of Health grants AI-26872, HL-50985 and CA-54464 (T.F. Tedder), the Howard Hughes Medical Institute (C.C. Goodnow), and DRG-1409 from the Damon Runyon-Walter Winchell Foundation (B.C. Weintraub).
Footnotes
1. Abbreviations used in this paper: hCD19, human CD19; IgHEL, high-affinity HEL-specific IgMa and IgDa; sHEL, soluble hen egg lysozyme.
References
- 1.Goodnow CC. Balancing immunity and tolerance: deleting and tuning lymphocyte repertoires. Proc Natl Acad Sci USA. 1996;93:2264–2271. doi: 10.1073/pnas.93.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartley SB, Cooke MP, Fulcher DA, Harris AW, Cory S, Basten A, Goodnow CC. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell. 1993;72:325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 3.Nemazee D, Bürki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature (Lond) 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 4.Goodnow CC. Transgenic mice and analysis of B-cell tolerance. Annu Rev Immunol. 1992;10:489–518. doi: 10.1146/annurev.iy.10.040192.002421. [DOI] [PubMed] [Google Scholar]
- 5.Klinman NR. The “clonal selection hypothesis” and current concepts of B cell tolerance. Immunity. 1996;5:189–195. doi: 10.1016/s1074-7613(00)80314-3. [DOI] [PubMed] [Google Scholar]
- 6.Adelstein S, Pritchard-Briscoe H, Anderson TA, Crosbie J, Gammon G, Loblay RH, Basten A, Goodnow CC. Induction of self-tolerance in T cells but not B cells of transgenic mice expressing little self antigen. Science (Wash DC) 1991;251:1223–1225. doi: 10.1126/science.1900950. [DOI] [PubMed] [Google Scholar]
- 7.Nemazee D, Russell D, Arnold B, Haemmerling G, Allison J, Miller JF, Morahan G, Buerki K. Clonal deletion of autospecific B lymphocytes. Immunol Rev. 1991;122:117–132. doi: 10.1111/j.1600-065x.1991.tb00600.x. [DOI] [PubMed] [Google Scholar]
- 8.Tedder TF, Inaoki M, Sato S. The CD19/21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity. Immunity. 1997;6:107–118. doi: 10.1016/s1074-7613(00)80418-5. [DOI] [PubMed] [Google Scholar]
- 9.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science (Wash DC) 1996;272:50–54. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 10.Carter RH, Fearon DT. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science (Wash DC) 1992;256:105–107. doi: 10.1126/science.1373518. [DOI] [PubMed] [Google Scholar]
- 11.Dempsey PW, Allison MED, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science (Wash DC) 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 12.Engel P, Zhou L-J, Ord DC, Sato S, Koller B, Tedder TF. Abnormal B lymphocyte development, activation and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 1995;3:39–50. doi: 10.1016/1074-7613(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 13.Rickert RC, Rajewsky K, Roes J. Impairment of T-cell–dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature (Lond) 1995;376:352–355. doi: 10.1038/376352a0. [DOI] [PubMed] [Google Scholar]
- 14.Sato S, Steeber DA, Tedder TF. The CD19 signal transduction molecule is a response regulator of B-lymphocyte differentiation. Proc Natl Acad Sci USA. 1995;92:11558–11562. doi: 10.1073/pnas.92.25.11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou L-J, Smith HM, Waldschmidt TJ, Schwarting R, Daley J, Tedder TF. Tissue-specific expression of the human CD19 gene in transgenic mice inhibits antigen-independent B lymphocyte development. Mol Cell Biol. 1994;14:3884–3894. doi: 10.1128/mcb.14.6.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato S, Ono N, Steeber DA, Pisetsky DS, Tedder TF. CD19 regulates B lymphocyte signaling thresholds critical for the development of B-1 lineage cells and autoimmunity. J Immunol. 1996;156:4371–4378. [PubMed] [Google Scholar]
- 17.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature (Lond) 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 18.Sato S, Miller AS, Howard MC, Tedder TF. Regulation of B lymphocyte development and activation by the CD19/CD21/CD81/Leu 13 complex requires the cytoplasmic domain of CD19. J Immunol. 1997;159:3278–3287. [PubMed] [Google Scholar]
- 19.Sato S, Steeber DA, Jansen PJ, Tedder TF. CD19 expression levels regulate B lymphocyte development: human CD19 restores normal function in mice lacking endogenous CD19. J Immunol. 1997;158:4662–4669. [PubMed] [Google Scholar]
- 20.Hartley SB, Crosbie J, Brink R, Kantor AA, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature (Lond) 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 21.Goodnow CC, Crosbie J, Jorgensen H, Brink RA, Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature (Lond) 1989;342:385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- 22.Sato S, Miller AS, Inaoki M, Bock CB, Jansen PJ, Tang MLK, Tedder TF. CD22 is both a positive and negative regulator of B lymphocyte antigen receptor signal transduction: altered signaling in CD22-deficient mice. Immunity. 1996;5:551–562. doi: 10.1016/s1074-7613(00)80270-8. [DOI] [PubMed] [Google Scholar]
- 23.Bradbury LE, Kansas GS, Levy S, Evans RL, Tedder TF. The CD19/CD21 signal transducing complex of human B lymphocytes includes the target of antiproliferative antibody-1 and Leu-13 molecules. J Immunol. 1992;149:2841–2850. [PubMed] [Google Scholar]
- 24.Mason DY, Jones M, Goodnow CC. Development and follicular localization of tolerant B lymphocytes in lysozyme/anti-lysozyme IgM/IgD transgenic mice. Intl Immunol. 1992;4:163–175. doi: 10.1093/intimm/4.2.163. [DOI] [PubMed] [Google Scholar]
- 25.Cooke MP, Heath AW, Shokat KM, Zeng Y, Finkelman FD, Linsley PS, Howard M, Goodnow CC. Immunoglobulin signal transduction guides the specificity of B cell-T cell interactions and is blocked in tolerant self-reactive B cells. J Exp Med. 1994;179:425–438. doi: 10.1084/jem.179.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cyster JG, Goodnow CC. Protein tyrosine phosphatase 1C negatively regulates antigen receptor signaling in B lymphocytes and determines thresholds for negative selection. Immunity. 1995;2:13–24. doi: 10.1016/1074-7613(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 27.Sato, S., P.J. Jansen, and T.F. Tedder. 1997. CD19 and CD22 reciprocally regulate Vav tyrosine phosphorylation during B lymphocyte signaling. Proc. Natl. Acad. Sci. USA. In press. [DOI] [PMC free article] [PubMed]
- 28.Theofilopoulos, A.N. 1992. Murine models of lupus. In Systemic Lupus Erythematosus. R.G. Lahita, editor. Churchill Livingston, Edinburgh. 121–194.
- 29.Rathmell JC, Goodnow CC. Effects of the lprmutation on elimination and inactivation of self-reactive B cells. J Immunol. 1994;153:2831–2842. [PubMed] [Google Scholar]
- 30.Oooto Y, Ikemoto T, Nakagawa T, Shimizu A. Quantitative flocytometric analysis of B cell surface antigens in patients with autoimmune diseases. Jpn J Clin Pathol. 1995;43:381–384. [PubMed] [Google Scholar]
- 31.Cyster JG, Healy JI, Kishihara K, Mak TW, Thomas ML, Goodnow CC. Regulation of B-lymphocyte negative and positive selection by tyrosine phosphatase CD45. Nature (Lond) 1996;381:325–328. doi: 10.1038/381325a0. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto AK, Kopicky-Burd J, Carter RH, Tuveson DA, Tedder TF, Fearon DT. Intersection of the complement and immune systems: a signal transduction complex of the B lymphocyte containing complement receptor type 2 and CD19. J Exp Med. 1991;173:55–64. doi: 10.1084/jem.173.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pepys MB. Role of complement in the induction of immunological responses. Transplant Rev. 1976;32:93–120. doi: 10.1111/j.1600-065x.1976.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 34.Melchers F, Erdei A, Schulz T, Dierich MP. Growth control of activated, synchronized murine B cells by the C3d fragment of human complement. Nature (Lond) 1985;317:264–267. doi: 10.1038/317264a0. [DOI] [PubMed] [Google Scholar]
- 35.Fearon DT, Carter RH. The CD19/CR2/ TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu Rev Immunol. 1995;13:127–149. doi: 10.1146/annurev.iy.13.040195.001015. [DOI] [PubMed] [Google Scholar]
- 36.Ahearn JM, Fischer MB, Croix D, Goerg S, Ma M, Xia J, Zhou X, Howard RG, Rothstein TL, Carroll MC. Disruption of the Cr2locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity. 1996;4:251–262. doi: 10.1016/s1074-7613(00)80433-1. [DOI] [PubMed] [Google Scholar]
- 37.Molina H, Holers VM, Li B, Fang Y-F, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr RW, Chaplin DD. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci USA. 1996;93:3357–3361. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]