Abstract
Fas(CD95) and its ligand (FasL) interaction plays a pivotal role in T cell receptor (TCR)-mediated apoptosis. However, the susceptibility of T cells to Fas-mediated apoptosis is tightly regulated during immune responses, a regulation which is thought to maintain the antigen-specificity of T cell apoptosis. Here we show that TCR stimulation enhances the induction of Fas-mediated apoptosis. In addition, using a mutant T cell hybridoma with impaired FasL expression, we show that the synergy provided by TCR stimulation can be mimicked by activators of PKC but not calcium influx. This effect cannot be inhibited by actinomycin D, suggesting that TCR stimulation leads to the alteration in preexisting signaling molecules to enhance Fas-mediated apoptosis. Our results therefore provide a mechanism of how Fas-FasL interactions lead to T cell death in an antigen-specific manner via repetitive antigen stimulation.
The T cell repertoire is determined primarily by positive and negative selection of immature thymocytes in the thymus (1, 2). However, the establishment of antigen-specific T cell tolerance continues in mature T cell compartments to ensure that self-reactive T cells are kept inert (2). Clonal deletion of self-reactive T cells via apoptosis is one of the major mechanisms for establishing such tolerance (2).
Recent experiments have shown that several members of the TNF receptor (TNFR) superfamily play critical roles during antigen-induced T cell apoptosis. For example, the Fas-FasL interaction participate in the induction of antigen-specific apoptosis of both immature thymocytes and mature T cells (3–7), while the TNF-TNFR interaction is primarily involved in mature T cell death (8–10). Thus various members of the TNFR superfamily are thought to play distinct roles during T cell apoptosis depending on the developmental or activation stage of the cells or on the antigens themselves. T cell hybridomas also mimic TCR-mediated apoptosis via Fas-FasL interactions and have been extensively used as an in vitro model system of antigen-specific T cell deletion (11–13).
Some of the biochemical mechanisms that link Fas to the apoptosis machinery has been elucidated by the identification of various signal transducing molecules which interact with the cytoplasmic portion of Fas. Upon binding to FasL, Fas recruits FADD/MORT1 and RIP via the interaction of their death domains (14). Clustering of these signal transducers leads to the further recruitment of FLICE/ MACH (15, 16) and subsequently to the activation of caspases.
Despite the direct interaction of Fas with cell death machinery, Fas-FasL interactions do not always lead to apoptosis. For example, primary T cells have been shown to undergo Fas-mediated apoptosis only after they are repetitively stimulated through TCR (17–19). Although T cells rapidly upregulate both Fas and FasL expression upon initial antigen stimulation, apoptosis does not immediately ensue. Thus a current consensus is that the susceptibility to Fas-mediated apoptosis must be modified or controlled by other factors during immune responses. In this study, we show that TCR signals, independent of de novo gene synthesis, enhance the susceptibility of both normal T cells and T cell hybridomas to Fas-mediated apoptosis.
Materials and Methods
Transfection of Fas cDNA.
Fas cDNA (20) cloned into the retroviral vector pLXSN (21) was transiently transfected into a packaging cell line, BOSC-23, by the calcium phosphate transfection method as described (22). The culture supernatants containing recombinant viruses were collected 2 d later, filtered, and used to infect KIT50 as previously described (22, 23). Transfectants were selected by their G418 (700 μg/ml) resistance (24) and the expression of Fas was confirmed by flow cytometric analysis using the biotinylated anti-Fas Ab, Jo2 (PharMingen, San Diego, CA).
Northern Blot Analysis.
For stimulation, T cell hybridomas were cultured in medium alone or on plates coated with anti-TCR Ab, H57-597 (10 μg/ml) (25). 6 h after stimulation, cells were collected, washed in PBS and poly(A)+ RNA was collected as previously described (23). 2 μg of poly(A)+ RNA was electrophoresed in 1.0% agarose-formaldehyde gel, transferred to GeneScreen membrane (DuPont-NEN, Boston, MA), and hybridized with 32P-labeled cDNA probes as described (23). Probes derived from glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used to control for loading of mRNA.
Isolation and Priming of Lymph Node T cells.
Lymph node T cells were prepared using T cell enrichment column (Biotex Laboratories Inc., Alberta, Canada) from 6–8-wk-old BALB/c mice (Jackson ImmunoResearch Labs., Inc., West Grove, PA). Cells were primed for apoptosis as previously described (18). In brief, purified T cells were stimulated with concanavalin A (5 μg/ml) and anti-CD28 Ab (1 μg/ml) for 48 h, washed three times with 10 mg/ml α-methylmannoside in balanced salt solution and then incubated in media in the presence of 50 U/ml IL-2. After 48 h, activated T cells (50,000 cells/well) were cultured in 96-well plates coated with anti-CD3 Ab, 2C11 (10 μg/ml), anti-Fas Ab (Jo2, 10 μg/ml) or anti-CD3 plus anti-Fas Abs.
Stimulation of TCR or Fas by Antibody Cross-linking in the Presence of Various Biological Inhibitors and Activators.
96-well plates were coated with various amounts of anti-TCR Ab (H57-597) (25), anti-CD3 Ab (2C11), or anti-Fas Ab (Jo2) as indicated in the figure legends to cross-link the TCR and/or the Fas antigen, respectively. Cells were added to the plates at 2 × 104 cells/well (T cell hybridomas) or 5 × 104 cells/well (primed LNTC). Various inhibitors or activators were added to the wells at the final concentrations: PMA (Calbiochem-Novabiochem, La Jolla, CA) at 20 ng/ml, ionomycin (Calbiochem-Novabiochem) at 500 ng/ml, dexamethasone (Sigma Chem. Co., St. Louis, MO) at 10 μM, cyclosporin A (Sandoz Pharmaceuticals, East Hanover, NJ) at 50 ng/ ml, EGTA (Sigma) at 5 mM to chelate extracellular Ca2+, actinomycin D (Sigma) at 31.25 ng/ml.
Cell Death Analysis.
Percentage of apoptotic cells were measured by propidium iodide (PI; Sigma) uptake on a FACSCalibur® flow cytometer (Becton Dickinson, Mountain View, California) as previously described (8). Specific cell death was determined as follows: % specific death = [(% PI+ stimulated − % PI+ control)/(100% − % PI+ control)] × 100%.
Results and Discussion
Isolation and Characterization of T Cell Hybridomas Which Have Defective Fas and FasL Expression.
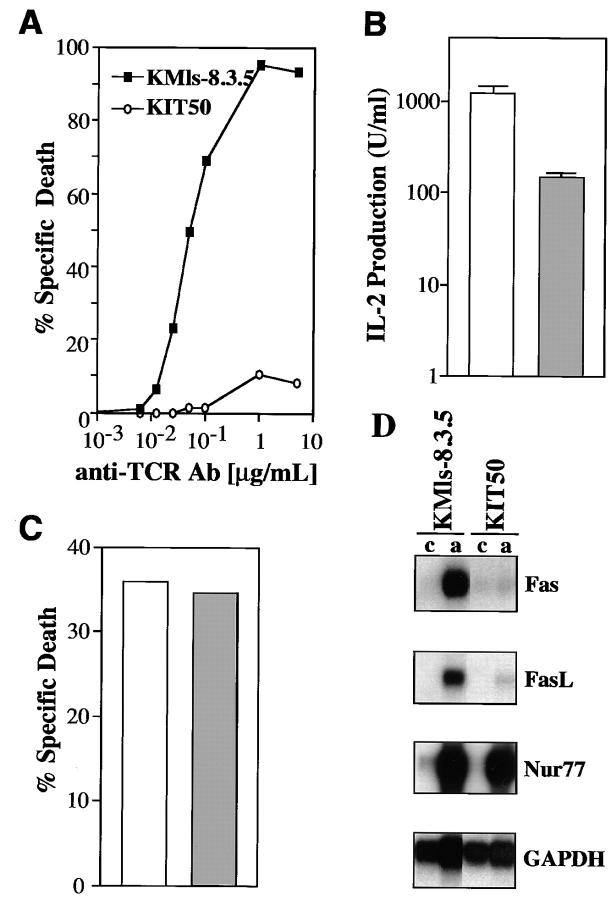
To study the signaling pathway specific for TCR-mediated apoptosis, we have previously generated several mutant T cell hybridoma clones which are resistant to TCR-mediated apoptosis (23, 26). One of those mutant clones, KIT50, expressed surface TCR similar to the parental cell line, KMls-8.3.5 (data not shown), but was resistant to the apoptosis induced by anti-TCR Ab treatment (Fig. 1 A) or by PMA plus ionomycin (data not shown). In addition, TCR-mediated IL-2 production in KIT50 was significantly lower than that in the parental cell line (Fig. 1 B). These results suggest that some of the signals common to both TCR-mediated IL-2 production and apoptosis are likely to be affected in KIT50. That these defects in apoptosis are specific for signals emanating from the TCR is suggested by the finding that dexamethasone treatment induced apoptosis in KIT50 as much as in the parental cell line (Fig. 1 C). KIT50 thus contains the necessary molecular machinery to carry out the apoptosis program, with the biochemical lesions likely to reside between the TCR-proximal signaling components and the apoptosis effector molecules (i.e., caspases) common to both TCR- and dexamethasone-mediated apoptosis.
Figure 1.
Isolation and characterization of a T cell hybridoma mutant with impaired Fas and FasL expression. (A) The T cell hybridoma mutant KIT50 is resistant to anti-TCR Ab (H57-597). Cells were incubated for 24 h on 96-well plates coated with increasing amounts of H57-597 and then assayed for apoptosis by PI uptake and FACS® analysis. Specific cell death was determined as described in Materials and Methods. (Squares) The parental cell line, KMls-8.3.5; (circles) the mutant cell line, KIT50. The representative results of at least 10 independent experiments are shown. (B) IL-2 production upon TCR stimulation. Cells were stimulated for 18 h on plates coated with anti-TCR Ab (10 μg/ml). Supernatants were collected and their activity was assayed using HT-2 cells as previously described (23). (White bar) The parental cell line, KMls-8.3.5; (hatched bar) the mutant cell line, KIT50. The representative results of three to seven independent experiments are shown. (C) Apoptotic cell death induced by dexamethasone. KMls-8.3.5 and KIT50 cells were incubated in the presence of 10 μM dexamethasone or in the media alone for 24 h and cell viability was tested by PI uptake. Spontaneous cell death in the media alone was <2–3%. (White bar) the parental cell line, KMls-8.3.5; (hatched bar) the mutant cell line, KIT50. The representative results of at least six independent experiments are shown. (D) Fas and FasL mRNA expression was impaired in KIT50. RNA was prepared from control or TCR-stimulated cells and then the expression of various genes was tested by Northern blot hybridization. (c) Control, unstimulated, (a) stimulated with anti-TCR Ab (10 μg/ml).
To further study the defect(s) in KIT50, we analyzed the expression of Nur77, Fas and FasL which were shown to play a critical role in TCR-mediated apoptosis of T cell hybridomas (11–13, 27, 28). Upon TCR-stimulation, the induction of Nur77 was unaffected in KIT50 (Fig. 1 D). However, both Fas and FasL mRNA induction were markedly affected in the mutant cell line KIT50 (Fig. 1 D). The induction of IL-2 mRNA was also impaired in KIT50 (data not shown), reflecting the reduced level of IL-2 production shown in Fig. 1 B. These results suggest that some (e.g., IL-2, FasL, and Fas expression) but not all (e.g., Nur77) of the signals triggered by the TCR was affected in the mutant cell line, KIT50. In addition, the impairment in both Fas and FasL expression appear to result in the resistance of KIT50 to TCR-mediated apoptosis.
TCR-mediated Signals Enhance Fas-mediated Apoptosis.
The susceptibility to Fas-mediated apoptosis varies among different cell types. It has been proposed that T cell proliferation is one of the requirements for susceptibility to Fas-mediated apoptosis (17–19). However, it is not clear whether TCR-mediated proliferation (or cell cycle progression) is the only requirement for T cells to become susceptible to Fas-mediated apoptosis.
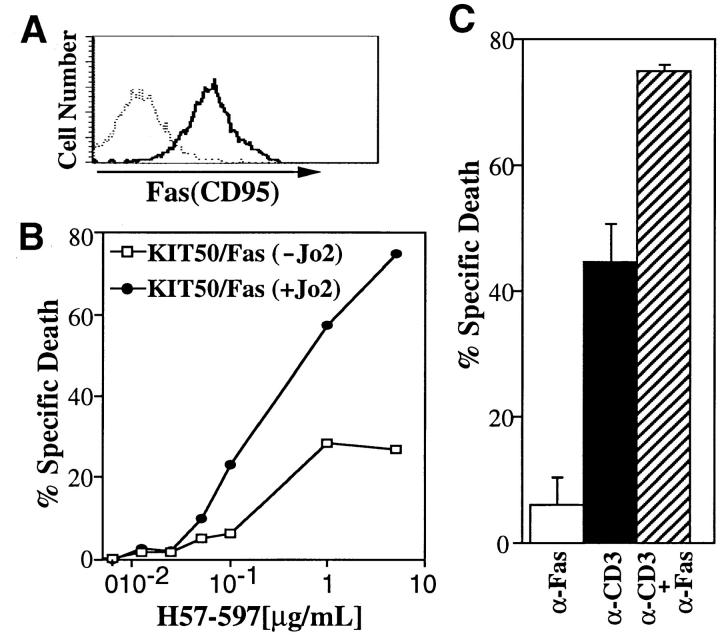
Since T cell hybridomas are continuously progressing through the cell cycle, we tested whether additional signals from the TCR are required for Fas-mediated apoptosis using KIT50-Fas which are derived from KIT50 by transfection of Fas (Fig. 2 A). KIT50-Fas is suitable for this study because TCR-stimulation itself does not induce significant apoptosis (<20% specific cell death) due to its impaired FasL induction (Fig. 2 B). When KIT50/Fas was treated with anti-Fas Ab alone, only a low level of apoptosis (<5% specific cell death) was seen (Fig. 2 B). Thus cell cycle progression or continuous proliferation itself appears insufficient to render T cells susceptible to Fas-mediated cell death. When KIT50-Fas cells were co-stimulated with anti-TCR Ab and anti-Fas Ab, there was a synergistic effect in the induction of apoptosis (Fig. 2 B), suggesting that TCR-mediated signals enhance Fas-mediated apoptosis in T cell hybridomas. This enhancement is likely due to the intracellular biochemical changes upon TCR stimulation, but not due to the residual upregulation of FasL expression (see below).
Figure 2.
TCR-mediated signals enhance Fas-mediated apoptosis. (A) KIT50/Fas expresses relatively high levels of Fas on the cell surface. Surface Fas expression on KIT50-Fas cells were determined by FACS® analysis using biotinylated Jo2 Ab (PharMingen) and phycoerythrin (PE)- labeled streptavidin (Becton Dickinson). (Dotted line) PE-streptavidin alone; (solid line) biotinylated Jo2 plus PE-streptavidin. (B) TCR stimulation enhances Fas-mediated cell death. KIT50-Fas cells were cultured in 96-well plates coated with increasing amounts of H57-597 ± anti-Fas Ab (Jo2, 10 μg/ml). Cells were collected after 24 h and cell death was detected by PI uptake by FACS® analysis. The representative results of at least three independent experiments are shown. (Filled circles) KIT50/Fas stimulated with anti-Fas and anti-TCR Abs; (white squares) KIT50/Fas stimulated with anti-TCR Ab alone. (C) Synergistic effect of simultaneous Fas and TCR stimulation on activated lymph node T cells. ConA-IL-2–activated LNTC were incubated on plates coated with anti-CD3 (10 μg/ml) and/or anti-Fas Abs (10 μg/ml). Cell death was determined 48 h later by PI uptake. The representative results of three independent experiments are shown. Spontaneous cell death in the media alone was <5%. These experiments were done in the absence of cycloheximide. (White bar) anti-Fas Ab alone; (black bar) anti-CD3 Ab alone; (hatched bar) anti-Fas Ab plus anti-CD3 Ab.
To confirm that Fas-mediated apoptosis is enhanced by anti-TCR Ab in primary T cells, purified T cells were primed for anti-TCR induced apoptosis by culturing them with ConA and a high dose of IL-2 as previously described (18). Proliferating T cells were then restimulated with anti-CD3 Ab to induce apoptosis (18). When restimulated with anti-CD3 Ab, a significant portion of T cells died, whereas treatment with anti-Fas Ab alone induced only few cells (<5%) to undergo apoptosis (Fig. 2 C). When T cells were stimulated with both anti-CD3 and anti-Fas Ab, however, there was a significant increase (P <0.001) in the induction of apoptosis compared to treatment with anti-CD3 or anti-Fas Ab alone. These results suggest that TCR- and Fas-mediated signals synergize to induce apoptosis in primary T cells (Fig. 2 C). Thus concomitant signals from TCR and Fas appears to be a prerequisite for efficient induction of apoptosis via Fas.
Calcium-independent Signals Are Required for Synergistic Effect by TCR-stimulation.
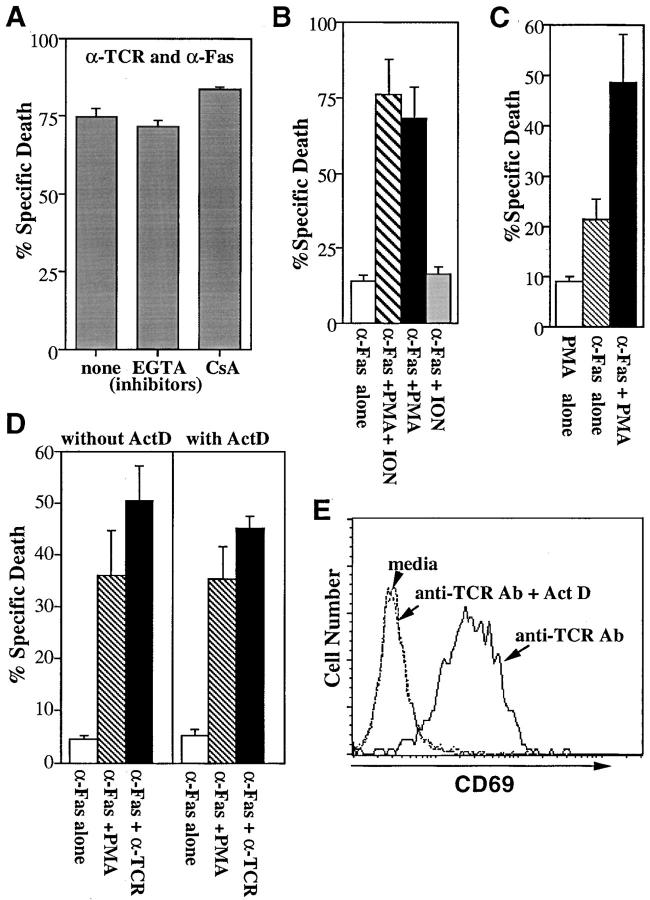
To study how TCR-mediated signals enhance Fas-mediated apoptosis, we have tested whether cyclosporin A (CsA) can interfere with this synergy of TCR and Fas signals. CsA treatment, which abrogates the TCR-mediated apoptosis of T cell hybridomas by inhibiting FasL expression (data not shown) (13), did not affect the enhancement of Fas-mediated cell death by anti-TCR Ab (Fig. 3 A). These results suggest that calcium/calcineurin-dependent signals or FasL expression activated by the TCR, the latter of which is also inhibited by CsA, are not involved in the enhancement of Fas-mediated apoptosis. In support of this, the enhancement of Fas-mediated apoptosis by TCR-stimulation was not inhibited in the presence of a large excess EGTA (Fig. 3 A). These results are consistent with previous reports that Fas-mediated apoptosis does not involve calcium-regulated signals (29).
Figure 3.
A PKC dependent, calcium-independent pathway mediates synergy between TCR and Fas signaling. (A) EGTA or CsA does not block the synergy between TCR and Fas. KIT50-Fas cells were stimulated with plate-bound H57-597 (10 μg/ml) plus Jo2 (10 μg/ml) in the presence of media alone, EGTA or CsA. Cell death was measured 24 h later by PI uptake. The representative results of three independent experiments are shown. The similar treatment of EGTA or CsA completely blocked cell death induced by anti-TCR Ab alone (data not shown). The low level of apoptosis induced by anti-Fas Ab alone (2–10% specific cell death) was not affected by EGTA or CsA (data not shown). Spontaneous cell death in the media alone ± EGTA or CSA was <5%. (B) PMA can substitute for TCR signals to enhance Fas-mediated apoptosis. KIT50-Fas cells were cultured in 96-well plates coated with anti-Fas Ab (Jo2, 10 μg/ ml) in the presence of various stimuli. Cell death was assayed 24 h later by PI uptake. The representative results of at least three independent experiments are shown. Cells were stimulated with Jo2 in the presence of media alone (white bar), PMA plus ionomycin (hatched bar), PMA alone (black bar), or ionomycin alone (gray bar). In the absence of Jo2, PMA or ionomycin alone did not induce cell death and PMA plus ionomycin induced a low level (<15%) of apoptosis in these cells (data not shown). (C) PMA alone can enhance Fas-mediated cell death of another Fas-transfected T cell hybridoma, KCIT1-8.5/Fas. KCIT1-8.5/Fas cells (23) were stimulated with PMA alone (white bar), plate-bound 10 μg/ml anti-Fas Ab alone (hatched bar), or PMA plus plate-bound anti-Fas Ab (black bar). Cell death was assayed 24 h later by PI uptake. The representative results of three independent experiments are shown. (D) Actinomycin D does not inhibit the synergy mediated by PMA or anti-TCR Ab. KIT50/Fas cells were stimulated with various reagents in the presence or absence of actinomycin D. Cell death was determined 24 h later by PI uptake. The representative results of three independent experiments are shown. (White bars) plate-bound anti-Fas Ab alone, 10 μg/ml; (hatched bars) PMA plus plate-bound anti-Fas Ab; (black bars) plate-bound anti-TCR Ab plus anti-Fas Ab at 10 μg/ml each. In the absence of anti-Fas Ab, PMA alone did not induce significant cell death (<5%). Anti-TCR Ab without anti-Fas Ab induced ∼10% specific cell death (data not shown). (E) Actinomycin D inhibits CD69 induction by TCR stimulation. KIT50/Fas cells from D were stained by FITC-labeled anti-CD69 antibody and analyzed by FACS®.
Since most of the cellular responses (e.g., IL-2 production or apoptosis) induced by TCR-stimulation can be mimicked by treatment of a phorbol ester (PMA) and a calcium ionophore (ionomycin) (30), we tested whether these reagents could also enhance Fas-mediated apoptosis. Similar to anti-TCR Ab, PMA plus ionomycin treatment greatly enhanced Fas-mediated apoptosis (Fig. 3 B). When PMA or ionomycin was treated separately, PMA but not ionomycin alone enhanced Fas-mediated apoptosis (Fig. 3 B) although it did not induce FasL expression (data not shown) (13). Thus the enhanced Fas-mediated apoptosis observed in these experiments is not due to increased FasL expression. Similar synergy with PMA and anti-Fas Ab in the induction of cell death was demonstrated in another Fas-transfected T cell hybridoma, KCIT1-8.5/Fas (23) (Fig. 3 C). These results suggest that cellular components activated by PMA are responsible for the enhancement of Fas-mediated apoptosis in T cells.
Phorbol ester treatment of T cells mimics activation of PKC- and Ras-dependent cellular responses initiated by the TCR (30). Since Ras/MAP kinase activation can lead to the induction of gene transcription, we tested whether the enhancement of Fas-mediated apoptosis by PMA requires de novo macromolecular synthesis. When cells were treated with PMA and anti-Fas Ab in the presence of actinomycin D (ActD), the level of apoptosis was comparable to that in the absence of ActD (Fig. 3 D). Similar to PMA, TCR signals enhanced Fas-mediated apoptosis even in the presence of ActD (Fig. 3 D). At the same concentration of ActD, upregulation of CD69 by TCR was efficiently blocked (Fig. 3 E). These results suggest that TCR (or PMA)-induced enhancement of Fas-mediated apoptosis does not depend on new gene products. Therefore, TCR-mediated activation of PKC (or other cellular signals) is likely to modify pre-existing signaling components downstream of Fas. It is not clear at this point which cellular proteins activated by TCR are responsible to modulate the susceptibility to Fas-mediated apoptosis. It was previously shown that Ras, which is also activated by TCR, is activated during Fas-mediated apoptosis and that the interference of Ras activation impairs Fas-mediated apoptosis (31). Therefore, it is possible that TCR-mediated Ras activation may increase the susceptibility of cells to Fas-mediated apoptosis. It is also possible that Fas-associated signaling molecules can be regulated by phosphorylation events because some of these molecules were found to be differentially phosphorylated (32). In addition, TCR-mediated signals may down-modulate anti-apoptotic signals which are associated with the protein phosphatase, FAP1 (33).
Antigen-specific deletion of T cells is necessary for the control of T cell immune responses and for the maintenance of T cell homeostasis, for which Fas and FasL play critical roles. It was previously shown that antigen (anti-TCR)-induced proliferation and cell cycle induction of mature T cells is a prerequisite for Fas-mediated apoptosis, thus proposing a model of how antigen specificity is attained during T cell deletion by repetitive antigen stimulation (17, 19). In this study, we provide evidence that additional TCR-mediated signals independent of cell cycle progression and de novo gene synthesis can synergize with Fas to trigger apoptosis. Therefore, it is likely that antigen-specificity of T cell deletion may be accomplished by coupling the TCR to Fas-mediated apoptosis via several check-points: those which are proliferation-dependent as well as others involving PKC activation.
Acknowledgments
We would like to thank Dr. John MacMicking for his critical comments and Angela Santana and Elizabeth Robinson for their excellent technical help.
This work was supported in part by National Institutes of Health grant R01 AI/CA41082 (Y. Choi). Y. Choi is an assistant investigator of the Howard Hughes Medical Institute.
Footnotes
Note added in proof. After this paper was accepted for publication, we found that Hornung et al. also reported that TCR signals can increase Fas-mediated apoptosis (Hornung, S., L. Zheng, and M.J. Lenardo. 1997. J. Immunol. 159:3816–3822).
References
- 1.von Boehmer H. Positive selection of lymphocytes. Cell. 1994;76:219–228. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 2.Nossal GJV. Negative selection of lymphocytes. Cell. 1994;76:229–239. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 3.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 4.Lynch DH, Ramsdell F, Alderson MR. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569–574. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 5.van Parijs L, Abbas AK. Role of Fas-mediated cell death in the regulation of immune responses. Curr Opin Immunol. 1996;8:355–361. doi: 10.1016/s0952-7915(96)80125-7. [DOI] [PubMed] [Google Scholar]
- 6.Castro JE, Listman JA, Jacobson BA, Wang Y, Lopez PA, Ju S, Finn PW, Perkins DL. Fas modulation of apoptosis during negative selection of thymocytes. Immunity. 1996;5:617–627. doi: 10.1016/s1074-7613(00)80275-7. [DOI] [PubMed] [Google Scholar]
- 7.Kishimoto H, Sprent J. Negative selection in the thymus includes semimature T cells. J Exp Med. 1997;185:263–271. doi: 10.1084/jem.185.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumor necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 9.Sytwu H-K, Libau RS, McDevitt HO. The roles of Fas/APO-1(CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity. 1996;5:17–30. doi: 10.1016/s1074-7613(00)80306-4. [DOI] [PubMed] [Google Scholar]
- 10.Speiser DE, Sebzda E, Ohteki T, Bachmann MF, Pfeffer K, Mak TW, Ohashi PS. TNF receptor p55 mediates in vivo deletion of peripheral cytotoxic T lymphocytes. Eur J Immunol. 1996;26:3055–3060. doi: 10.1002/eji.1830261235. [DOI] [PubMed] [Google Scholar]
- 11.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, Green DR. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 12.Ju S-T, Panka DJ, Cui H, Ettinger R, El-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Mercep M, Ware CF, Ashwell JD. Fas and activation-induced Fas ligand mediate apoptosis of T cell hybridomas: Inhibition of Fas ligand expression by retinoic acid and glucocorticoids. J Exp Med. 1995;181:1673–1682. doi: 10.1084/jem.181.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chinnaiyan AM, Dixit VM. Portrait of an executioner: the molecular mechanism of Fas/APO-1-induced apoptosis. Semin Immunol. 1997;9:69–76. doi: 10.1006/smim.1996.0055. [DOI] [PubMed] [Google Scholar]
- 15.Muzio M, Cinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Brentz JD, Zhang M, Gentz R, et al. FLICE, a novel FADD-homologous ICE/ CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 16.Boldin MP, Goncharov TM, Golstev YV, Wallach D. Involvement of MACH, a novel MORT1/ FADD-interacting protease in Fas/APO-1 and TNF receptor-induced cell death. Cell. 1996;81:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 17.Boehme SA, Lenardo M. Propriocidal apoptosis of mature T lymphocytes occurs at S phase in the cell cycle. Eur J Immunol. 1993;23:1552–1560. doi: 10.1002/eji.1830230724. [DOI] [PubMed] [Google Scholar]
- 18.Lenardo MJ. Interleukin-2 programs mouse αβ T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 19.Lenardo MJ, Boehme S, Chen L, Combadiere B, Fisher G, Freedman M, McFarland H, Pelfrey C, Zheng L. Autocrine feedback death and the regulation of mature T lymphocyte antigen receptors. Intl Rev Immunol. 1995;13:115–134. doi: 10.3109/08830189509061742. [DOI] [PubMed] [Google Scholar]
- 20.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 21.Miller AD, Rosman GJ. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989;7:980–982. [PMC free article] [PubMed] [Google Scholar]
- 22.Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park CG, Lee SY, Kandala G, Lee SY, Choi Y. A novel gene product that couples TCR signaling to Fas(CD95) expression in activation-induced cell death. Immunity. 1996;4:583–591. doi: 10.1016/s1074-7613(00)80484-7. [DOI] [PubMed] [Google Scholar]
- 24.Yazdanbakhsh K, Choi J-W, Li Y, Lau LF, Choi Y. Cyclosporin A blocks apoptosis by inhibiting the DNA biding activity of the transcription factor Nur77. Proc Natl Acad Sci USA. 1995;92:437–441. doi: 10.1073/pnas.92.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubo RT, Born W, Kappler JW, Marrack P, Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989;142:2736–2742. [PubMed] [Google Scholar]
- 26.Wong B, Park CG, Choi Y. Identifying the molecular control of T cell death. Semin Immunol. 1997;9:7–16. doi: 10.1006/smim.1996.0051. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Smith SW, McLaughlin KA, Schwartz LM, Osborne BA. Apoptotic signals delivered through the T-cell receptor of a T-cell hybrid require the immediate-early gene nur77. Nature. 1994;367:281–284. doi: 10.1038/367281a0. [DOI] [PubMed] [Google Scholar]
- 28.Woronicz J, Calnan B, Ngo V, Winoto A. Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature. 1994;367:277–281. doi: 10.1038/367277a0. [DOI] [PubMed] [Google Scholar]
- 29.Rouvier E, Luciani M-F, Golstein P. Fas involvement in Ca2+-independent T cell mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 31.Gulbins E, Bissonnette R, Mahboubi A, Martin S, Nishioka W, Brunner T, Baier G, Baier-Bitterlich G, Byrd C, Lang F, et al. FAS-induced apoptosis is mediated via a ceramide-initiated RAS signaling pathway. Immunity. 1995;2:341–351. doi: 10.1016/1074-7613(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 32.Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxic-dependent APO-1(Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO (Eur Mol Biol Organ) J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato T, Irie S, Kitada S, Reed JC. FAP-1: a protein tyrosine phosphatase that associates with Fas. Science. 1995;268:411–415. doi: 10.1126/science.7536343. [DOI] [PubMed] [Google Scholar]