Abstract
We explored expression and possible function of interferon-γ (IFN-γ) in cultured fetal (E15) rat dorsal root ganglion neurons combining whole cell patch-clamp electrophysiology with single cell reverse transcriptase polymerase chain reaction and confocal laser immunocytochemistry. Morphologically, we located IFN-γ protein in the cytoplasm of the neurons in culture as well as in situ during peri- and postnatal development. Transcripts for classic IFN-γ and for its receptor were determined in probes of cytoplasm sampled from individual cultured neurons, which had been identified by patch clamp electrophysiology. In addition, the cultured neurons expressed both chains of the IFN-γ receptor.
Locally produced IFN-γ acts back on its cellular source. Phosphorylation and nuclear translocation of the IFN-inducible transcriptional factor STAT1 as well as IFN-γ–dependent expression of major histocompatibility complex class I molecules on the neuronal membrane were noted in untreated cultures. However, both processes were substantially blocked in the presence of antibodies neutralizing IFN-γ. Our findings indicate a role of IFN-γ in autocrine regulation of sensory neurons.
Interferon-γ (IFN-γ), or “immune interferon”, is a key mediator required to correctly orchestrate antimicrobial and inflammatory tissue responses. It is remarkably pleiotropic, evoking highly diverse effects in many if not all tissues. The cytokine affects proliferation, differentiation, and the ability to communicate in individual cells. In particular, IFN-γ controls the expression of genes encoding molecules required in immune reactions, such as MHC products, cell adhesion molecules, cytokines, and cytocidal proteins. In contrast to IFN-γ's global activities, the cellular sources of the cytokine are remarkably restricted, with certain sets of activated T lymphocytes and NK cells as the sole known producers (1, 2).
However, IFN-γ is not limited to the immune responses. In addition to its proinflammatory function, some evidence suggests that IFN-γ may also affect differentiation and survival of neuronal cells. For example, in one investigation the cytokine delayed degeneration of sympathetic neurons caused by withdrawal of nerve growth factor (3). Furthermore, in the pheochromocytoma cell line PC12, IFN-γ facilitated nerve growth factor–induced neuronal differentiation (4) and induced long-term excitability by activating transcription of the peripheral nerve type 1 sodium channel (5). Finally, the cytokine promoted cholinergic differentiation of neurons derived from embryonic septal nuclei (6).
Thus far, the cellular source of IFN-γ in healthy nervous tissue has not been determined. Several reports described IFN-γ–like immunoreactivity in dorsal root ganglia (7, 8), and an “IFN-γ–like protein” extracted from sensory trigeminal rat ganglia was shown to share some biological activity with lymphocyte-derived IFN-γ (9). However, the molecular nature of these structures remained elusive. Attempts to identify mRNA for the cytokine were inconclusive (10), and the molecular weight of the “IFN-γ–like activity” differed substantially from that of the classic cytokine.
In this study, we characterized IFN-γ gene transcripts in cultured fetal rat dorsal root ganglion (DRG)1 neurons combining patch-clamp electrophysiology with single cell reverse transcriptase (RT)–PCR amplification. We demonstrate that IFN-γ immunoreactivity in the cytoplasm of cultured DRG neurons is indeed associated with the transcription of mRNA for classic IFN-γ. We also present functional evidence of autocrine/paracrine regulatory activity exerted by neuronal IFN-γ.
Material and Methods
Cell Culture.
DRG were prepared from Wistar rat fetuses (E15) obtained from the breeding facility (Max-Planck-Institute for Psychiatry) as previously described (11). In brief, DRG were removed from the fetuses and were dissociated by 0.1% trypsin (Worthington Biochemical Corporation, Freehold, NJ). Cells were dissociated by trituration and were cultured on poly-l-ornithine (0.1 mg/ml, Sigma Chemical Co., Taufkirchen, Germany) plus laminin-coated (10 μg/ml, gift from Dr. Ries, Max-Planck-Institute for Biochemistry, Martinsried, Germany) dishes in DMEM medium (GIBCO BRL, Eggenstein, Germany) supplemented with 10% fetal calf serum (Pansystems GmbH, Nürnberg, Germany) and 10 ng/ml nerve growth factor (NGF-7S mouse, Sigma Chemical Co.). On the first day cells were treated for 24 h with 10 μM 5-fluoro-2-deoxyuridine (Sigma Chemical Co.) and 10 μM uridine (Sigma Chemical Co.) to inhibit growth of nonneuronal cells. Antibodies neutralizing IFN-γ (30 μg/ml, DB1; Laboserv, Gießen, Germany) were added to the cell culture as described in the text. Hippocampal cell cultures (12) and T cell cultures (13) were performed as previously described.
Patch-clamp RT-PCR.
Whole cell recording and reverse transcription of cytoplasmic RNA from single neurons was performed as previously described (14). Cytoplasmic RNA of individual CD4+ myelin basic protein-specific T lymphoblasts (13) and of satellite cells from the DRG culture was collected with the patch-clamp pipette and was reverse transcribed using the same protocol as previously described (14). Oligonucleotides for PCR amplification were selected with program PRIMER (Whitehead Institute, Cambridge, MA) and were produced by the Max-Planck-Institute for Biochemistry (Martinsried, Germany). Forward primer and reverse primer were always chosen from different exons to detect possible amplification of genomic DNA contamination. The primer sequences were as follows: for rat IFN-γ (These sequence data are available from EMBL/GenBank/DDBJ under accession numbers X02326 and X02327), 5′-AGGATGCATTCATGAGCATCGCC-3′ (385–407) and 5′-CACCGACTCCTTTTCCGCTTCCT-3′ (223–201); and for rat CD4 (W3/25; accession number M15768), 5′-GTGCCGAGGCTTCTCTTTCAGG-3′ (56–77) and 5′-CCCAGAATTGTCTTTTGGTCAGAGG-3′ (247–273). The primer sequences for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), glial fibrillary acidic protein (GFAP), and IFN-γ–receptor have been previously described (14). PCR-amplification was done in a final volume of 50 μl containing 1 μl of transcribed cDNA probe, the four deoxyribonucleotide triphosphates (final 0.2 mM, Pharmacia), 2.5 U AmpliTaq (Perkin-Elmer/Applied Biosystems GmbH, Weiterstadt, Germany) and 1× PCR-buffer (Perkin-Elmer/Applied Biosystems GmbH) covered with two drops of mineral oil (Sigma Chemical Co.). Before PCR amplification on a programmable thermocycler (MultiCycler PTC 200, MJ Research Inc.), denaturated primers (final 100 pmol primers) were added to each tube at 80°C. The cDNA was denaturated at 95°C for 3 min. PCR was performed on cDNA from single cells with 48–50 cycles (93°C for 60 s; ramp with 0.1°C/s from 93°C to 60°C; 60°C for 60 s; 72°C for 60 s) and followed by one final cycle at 72°C for 5 min. 10 μl of the amplified fragments was run along with the molecular weight marker (ΦX 174, HaeIII digested; Pharmacia Biotech, Freiburg, Germany) on a 1.7% agarose gel electrophoresis stained with ethidium bromide. For Southern blot analysis the DNA fragments of the gel were transferred onto a nylon membrane (Hybond N+, Amersham Buchler, Braunschweig, Germany). The resulting blot was hybridized with a rat IFN-γ–specific probe (rat IFN-γ; these sequence data are available from EMBL/ GenBank/DDBJ under accession number X02327: 130–153) using the Digoseigenin Southern blot Hybridization System (Boehringer Mannheim, Mannheim, Germany) according to the manufacturer's instructions. For sequencing, the PCR fragments were gel-purified (QIAGEN, Hilden, Germany) and were sequenced by MediGene (Martinsried, Germany) with an automated sequence analyzer.
Immunofluorescence Labeling and Confocal Laser Scanning Microscopy.
Cultured rat DRG neurons or frozen sections of rat lumbar DRG were fixed with 4% paraformaldehyde in PBS. For labeling of MHC class I molecules on the cell membrane, mouse monoclonal antibodies directed against MHC class I (Ox 18, 10 μg/ml; Serotec Ltd., Oxford, England) were added to the cell culture for 60 min before fixation. Nonspecific binding sites were blocked with PBS containing 2% goat serum and 2% bovine albumin. For intracellular labeling, cultured cells were treated with PBS containing 0.1% Triton X-100 for 30 min. In the first step, cells or tissue sections were labeled with mouse monoclonal antibodies recognizing rat IFN-γ (DB1; 10 μg/ml), mouse monoclonal antibodies directed against STAT1 (5 μg/ml; PharMingen, Hamburg, Germany), rabbit polyclonal antibodies recognizing an epitope of IFN-γ–receptor α chain corresponding to amino acids 461–477 (2 μg/ml, K-17; Santa Cruz Biotechnology, Heidelberg, Germany), or rabbit polyclonal antibodies recognizing an epitope of IFN-γ–receptor β chain corresponding to amino acids 312–331 (2 μg/ml, M-20; Santa Cruz Biotechnology) followed by secondary fluorochrome Cy3–conjugated goat antibodies directed against mouse or rabbit immunoglobulin (10 μg/ml; Dianova, Hamburg, Germany). In a second step, cells were double-labeled with mouse monoclonal antibodies recognizing neurofilament (2 μg/ml, NN18; Boehringer Mannheim) followed by fluorochrome (dichlorotriazinyl) aminofluorescein–conjugated antibodies to mouse immunoglobulin (5 μg/ml; Dianova, Hamburg, Germany). Conventional photographs were taken with an immunofluorescence microscope (Carl Zeiss, Oberkochen, Germany). Confocal images were acquired with a confocal laser scanning microscope (Leica, Bensheim, Germany) equipped with ×63 oil objectives. Baseline labeling for the IFN-γ, STAT1, and MHC class I staining procedures was revealed with irrelevant mouse monoclonal antibodies (10 μg/ml; Dianova) and secondary fluorochrome Cy3–conjugated goat antibodies to mouse immunoglobulin (10 μg/ml; Dianova). Baseline labeling for the IFN-γ–receptor α and β chains was revealed by adding the corresponding (blocking) peptides (2 μg/ml; Santa Cruz Biotechnology) to the polyclonal rabbit antibodies, followed by secondary fluorochrome Cy3–conjugated goat antibodies to rabbit immunoglobulin (10 μg/ml; Dianova).
Statistical Analyses.
Data were determined by three independent experiments and are indicated as mean ± SEM. Analyses of STAT1 as well as of MHC class I by confocal laser scanning microscopy revealed significant (P <0.01) difference between the untreated and IFN-γ neutralizing antibody–treated group in the unpaired two-tail Student's t test.
Results
IFN-γ Expression in Sensory Neurons.
Histological sections were performed from rat lumbar DRG tissue. IFN-γ immunolabeling was detectable in a subpopulation of DRG cells during peri- and postnatal development (Fig. 1). Histological sections showed strong IFN-γ expression at embryonic day 18 and during the first 2 wk after birth. The in situ IFN-γ labeling was very prominent in the neuronal perikarya, but was also demonstrable in the neuronal processes (Fig. 1).
Figure 1.
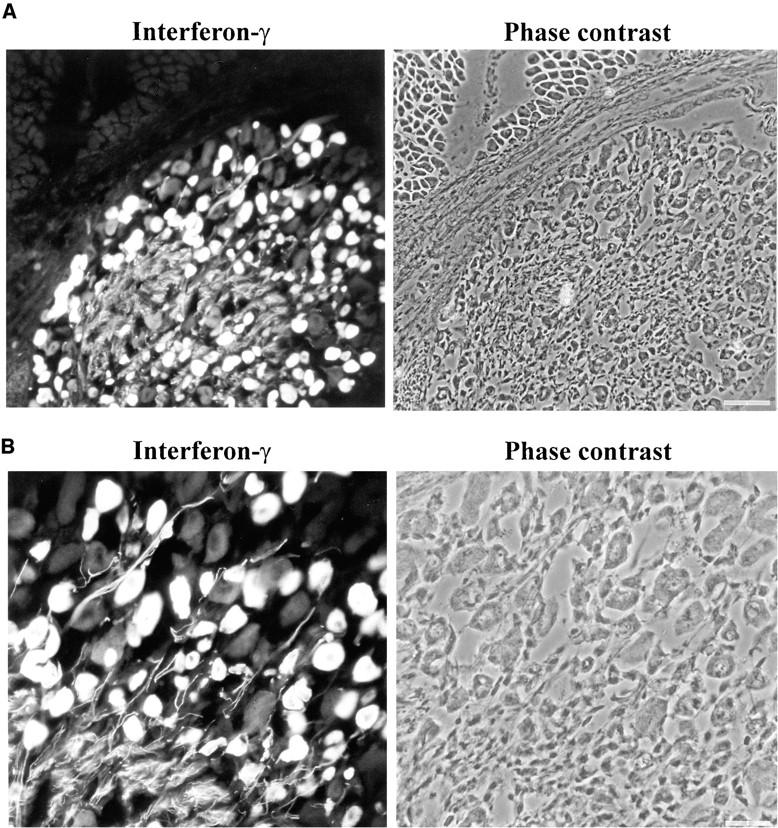
IFN-γ immunoreactivity in the DRG during postnatal development. (A) Frozen section from rat postnatal day 9 DRG was immunolabeled with antibodies directed against IFN-γ. The IFN-γ immunoreactivity was detectable in the DRG, but not in the adjacent muscle tissue. Scale bar: 50 μm. (B) The perikarya and processes of a subpopulation of neurons were immunolabeled with IFN-γ–specific antibodies in a frozen section of postnatal day 9 DRG. Scale bar: 20 μm.
DRG cells from embryonic rats (E15) were cultured in the presence of nerve growth factor. Most of the DRG neurons that differentiated in vitro bound monoclonal antibodies recognizing IFN-γ. Specific immunofluorescence was detected after 7 d in culture in 83 ± 8% of DRG neurons identified by neurofilament labeling, but in none of the nonneuronal cells (Fig. 2). Confocal laser scanning microscopy localized the IFN-γ proteins in the cytoplasm, sparing the nucleus and the cell membrane (Fig. 2), whereas processes and dendrites showed no or only very weak reactivity.
Figure 2.
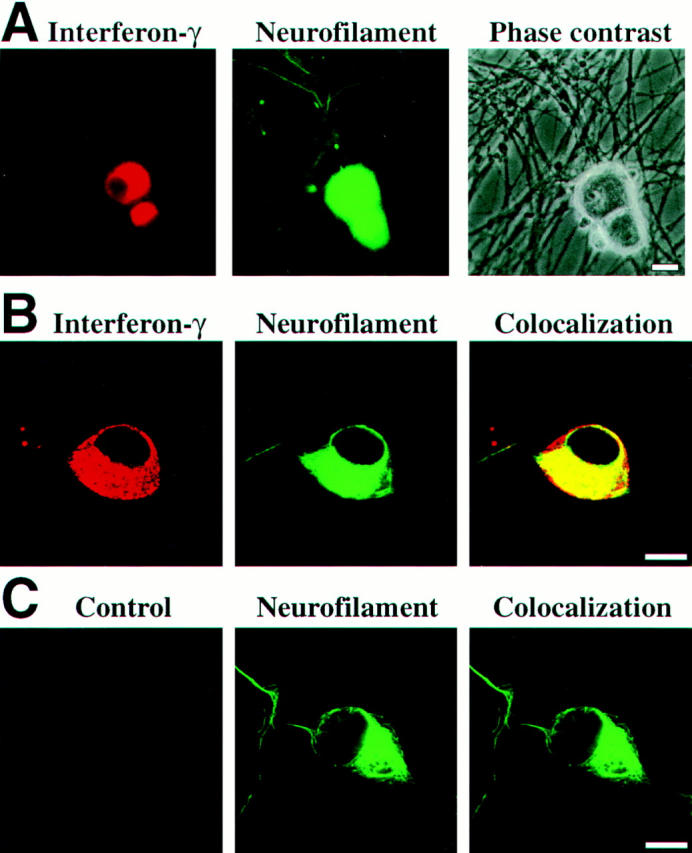
IFN-γ immunoreactivity in cultured sensory neurons. (A) IFN-γ immunofluorescence labeling in neurons. Embryonic sensory neurons cultured for 7 d were identified by antibodies recognizing neurofilament and were double-labeled with antibodies directed against IFN-γ. Scale bar: 10 μm. (B) Confocal laser scanning microscopy of sensory neurons. Neurofilament labeling is located in the cytoplasm and the neuronal processes, whereas IFN-γ labeling is only localized in the perinuclear cytoplasm. Scale bar: 10 μm. (C) Incubation with primary control antibodies instead of IFN-γ–specific antibodies showed baseline labeling intensity. Scale bar: 10 μm.
IFN-γ Gene Transcription in Sensory Neurons.
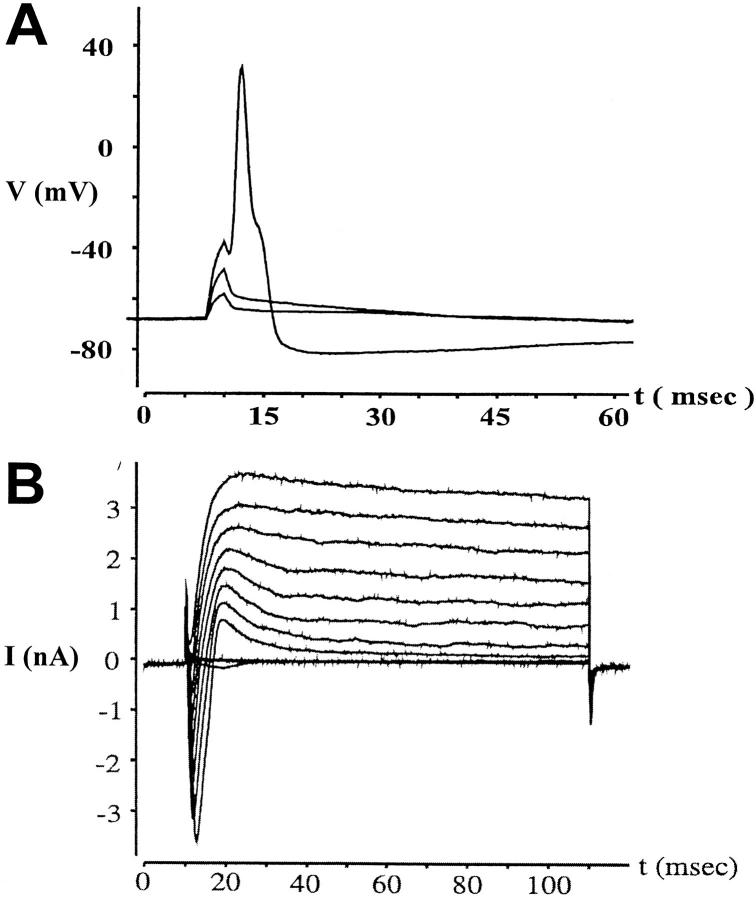
After 7 d in culture, neurons could be distinguished morphologically from nonneuronal cells. Their neuronal identity was ascertained by whole cell patch-clamp electrophysiology (15). As expected, the DRG-derived neurons showed sodium currents activated at different membrane potentials, and responded to current pulses of increasing amplitude with sodium action potentials (Fig. 3).
Figure 3.
Identification of neurons by whole cell patch-clamp electrophysiology. (A) Action potentials were evoked in sensory neurons cultured for 7 d by current pulses of increasing amplitude (200, 300, and 400 pA) and were recorded in the current clamp mode. (B) Whole cell membrane currents were evoked by successive depolarization steps of 10 mV (from a holding potential of −80 mV to voltages ranging from −50 to +50 mV) and were recorded in the voltage clamp mode.
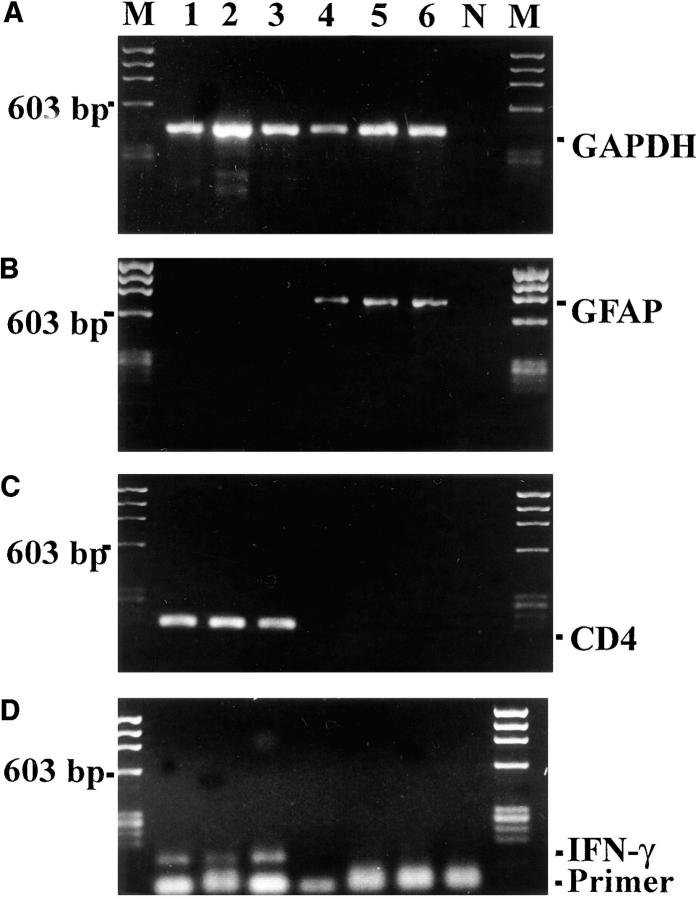
We then used single-cell RT-PCR analysis to identify the intraneuronal IFN-γ–like material. After electrophysiological characterization, we sampled cytoplasmic specimens from individual neurons through the patch-clamp micropipette, and assessed current gene transcription by RT-PCR. Oligonucleotides specific for IFN-γ were used to amplify gene transcripts for the cytokine. In addition, as in our previous studies, coamplification of mRNA for the house-keeping enzyme GAPDH and for cell lineage markers served as internal quality standards (12, 14). The validity of our method was corroborated by parallel analyses of activated CD4+ T lymphocytes with a Th1-like phenotype (13) and DRG-derived glia cells (Fig. 4). Each single T lymphoblast analyzed contained mRNA for the lineage marker gene CD4 along with IFN-γ. In contrast, satellite cells from DRG cultures, which expressed glia specific GFAP gene transcripts, and were negative for IFN-γ and CD4 gene transcripts (Fig. 4). In a subsequent cytokine study of 19 individual DRG neurons, mRNA for IFN-γ was identified in 13 cells cultured for 7 d (Fig. 5). Southern blot analysis and sequencing confirmed the identity of the amplified fragments with classic IFN-γ. IFN-γ gene expression in cultured DRG neurons developed steadily over time. Among neurons cultured for 2 h, only a minority (1/11) of the (electrophysiologically immature) cells expressed IFN-γ gene transcripts (Fig. 5). However, the percentage of IFN-γ–expressing neurons continuously increased through the first 24 h in culture. After 6 h, 3/13 neurons, and after 24 h, 7/11 DRG neurons, expressed IFN-γ gene transcripts. In contrast, differentiated neurons derived from hippocampus tissue transcribed IFN-γ at no time.
Figure 4.
Single cell RT-PCR of CD4+ T lymphoblasts and DRG satellite cells. Gene transcripts for GAPDH (A), GFAP (B), CD4 (C), and IFN-γ (D) were analyzed by single-cell RT-PCR of activated T cells (lanes 1–3) and of DRG satellite cells (lanes 4–6). N and M show negative PCR control and molecular weight marker, respectively.
Figure 5.
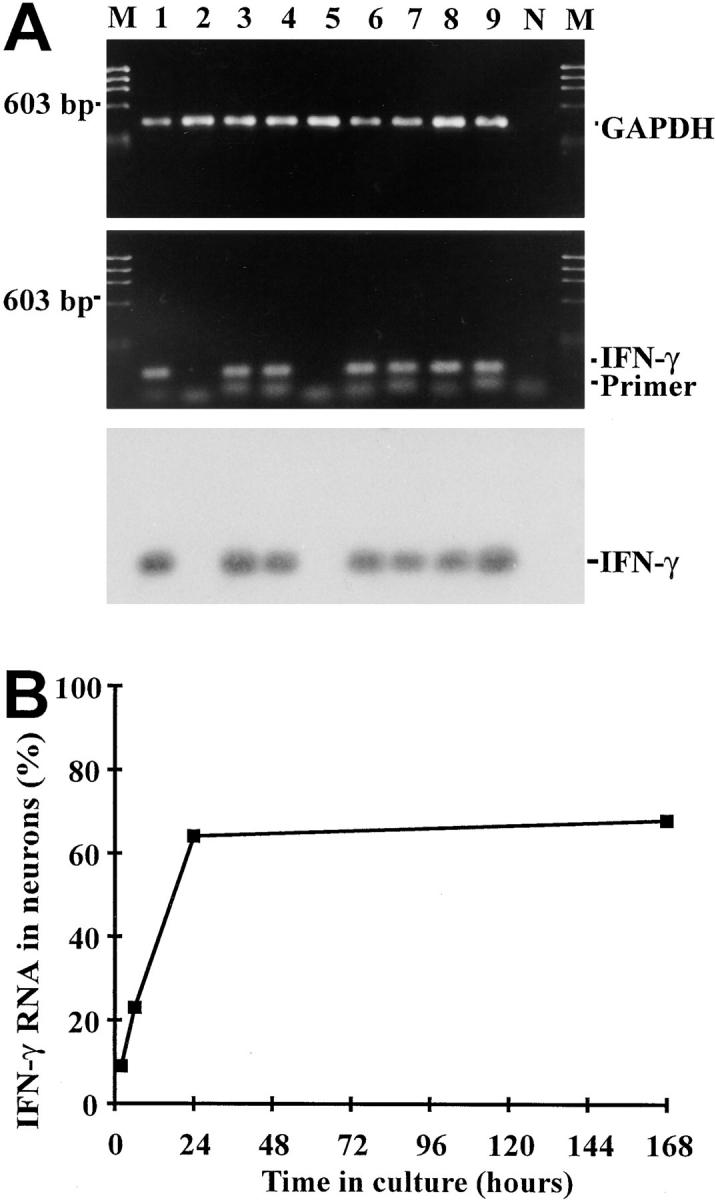
Gene transcripts for IFN-γ detected in sensory neurons by single-cell RT-PCR. (A) IFN-γ gene transcripts amplified from individual neurons. Embryonic sensory neurons were cultured for 7 d and were identified by electrophysiology. Gene transcripts for GAPDH (lanes 1–9) and IFN-γ (lanes 1, 3, 4, 6–9) were detected by single-cell RT-PCR in individual neurons. N and M show negative PCR control and molecular weight marker, respectively. (B) Frequency of DRG neurons transcribing IFN-γ in relation to time in culture.
IFN-γ–receptor Expression of Sensory Neurons.
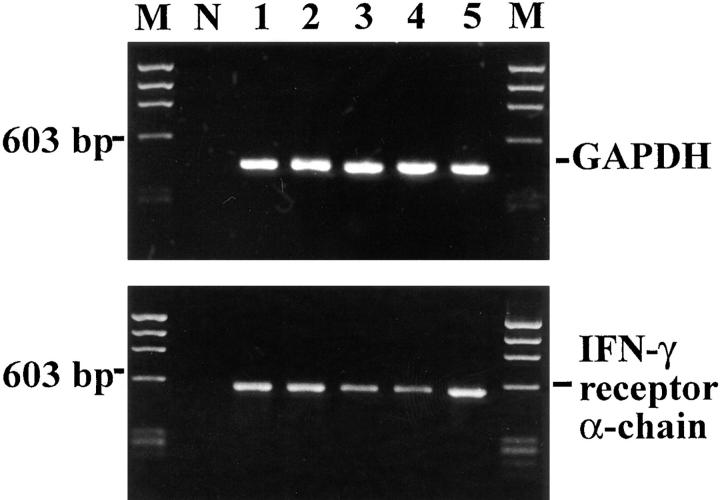
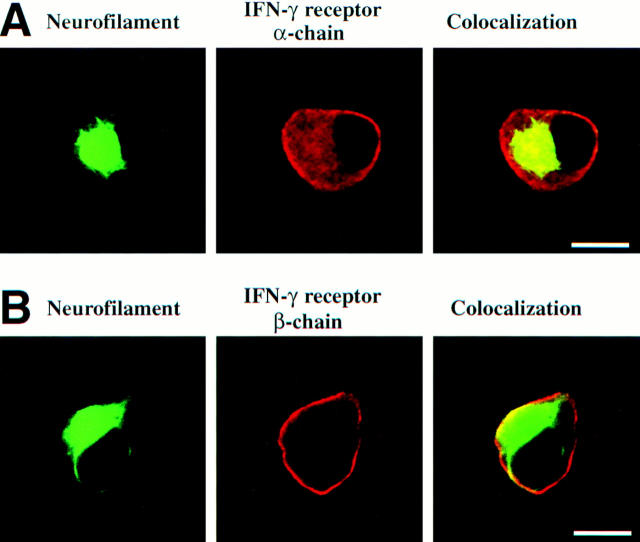
IFN-γ acts on its target cells by binding to and activating membrane-bound IFN-γ receptors, heterodimeric proteins composed by a cytokine-binding α chain and signal-transducing β chain (16). Gene transcripts for the IFN-γ receptor α chains were detected in all DRG neurons analyzed (Fig. 6) Membrane expression of the receptor complex was confirmed by immunofluorescence and confocal laser scanning microscopy. IFN-γ–receptor α chain–specific antibodies labeled 98 ± 4% of neurofilament-positive DRG neurons, predominantly on the cell membrane (Fig. 7). The signal-transducing β chain was detected on 97 ± 5% sensory neurons (Fig. 7). Simultaneous expression of a cytokine along with its specific receptor in the same cell provides a necessary, although insufficient, structural basis for autocrine regulation. This is the proven case in IFN-γ production by activated T lymphocytes (17), and could also hold true for our cultured DRG neurons. Since the binding of IFN-γ to its receptors results in phosphorylation and translocation of the transcriptional factor STAT1 from the cytoplasm to the nucleus (16), both features of STAT1 activation should be demonstrable in the cells of our DRG cultures, and should be reversed by the addition of neutralizing antibodies against IFN-γ.
Figure 6.
IFN-γ receptor gene transcripts in sensory neurons. Gene transcripts for GAPDH (lanes 1–5) and IFN-γ–receptor α chain (lanes 1–5) were amplified from single neurons by RT-PCR. N and M show negative PCR control and molecular weight marker, respectively.
Figure 7.
IFN-γ receptor expression detected on sensory neurons by confocal laser scanning microscopy. (A) Confocal localization of IFN-γ receptor (α chain) of sensory neurons. Embryonic sensory neurons (cultured for 2 d) were identified by antibodies recognizing neurofilament and were double labeled with antibodies directed against IFN-γ–receptor α chain. (B) Immunolabeling of IFN-γ receptor (β chain) on the neuronal cell membrane. Sensory neurons (cultured for 2 d) were identified by double-labeling with neurofilament. Scale bar A and B: 10 μm.
Autocrine Activation of STAT1 and MHC class I in Sensory Neurons.
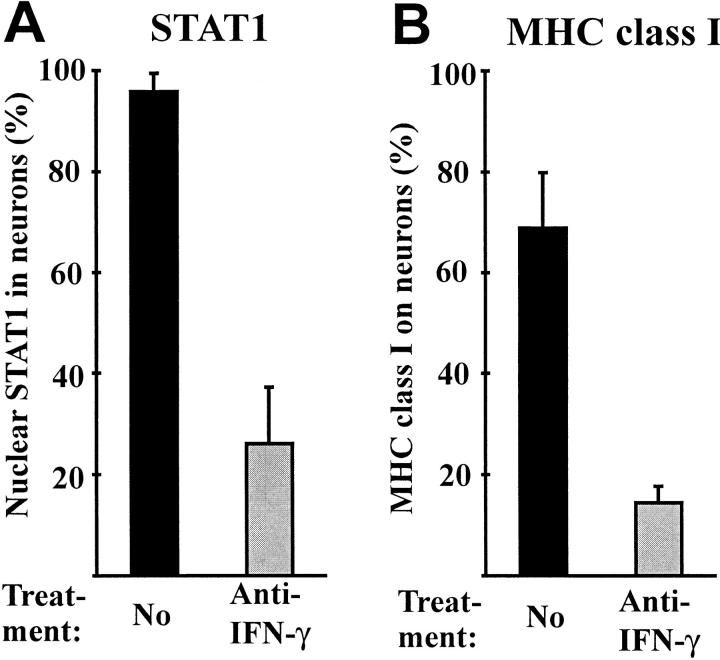
Western blot analysis of total cell lysate of DRG cultures demonstrated phosphorylated STAT1 (data not shown), but this method does not identify the cell type in the mixed DRG culture that has the active form of STAT1. However, confocal laser scanning microscopy clearly located STAT1 in the nuclei of almost all cultured neurofilament-positive DRG neurons (96 ± 4%, Figs. 8 and 9), and also in the glia cells. In contrast, in differentiated hippocampal neuronal cultures, where no IFN-γ synthesis is demonstrable, all STAT1 immunoreactivity was confined to the cytoplasm (Fig. 8). IFN-γ–neutralizing antibodies to DRG cultures profoundly interfered with nuclear translocation of STAT1. After neutralization of IFN-γ, most neurons displayed STAT1 within their cytoplasm, with nuclear location seen only in a minority of all cells (26 ± 11%, Figs. 8 and 9).
Figure 8.
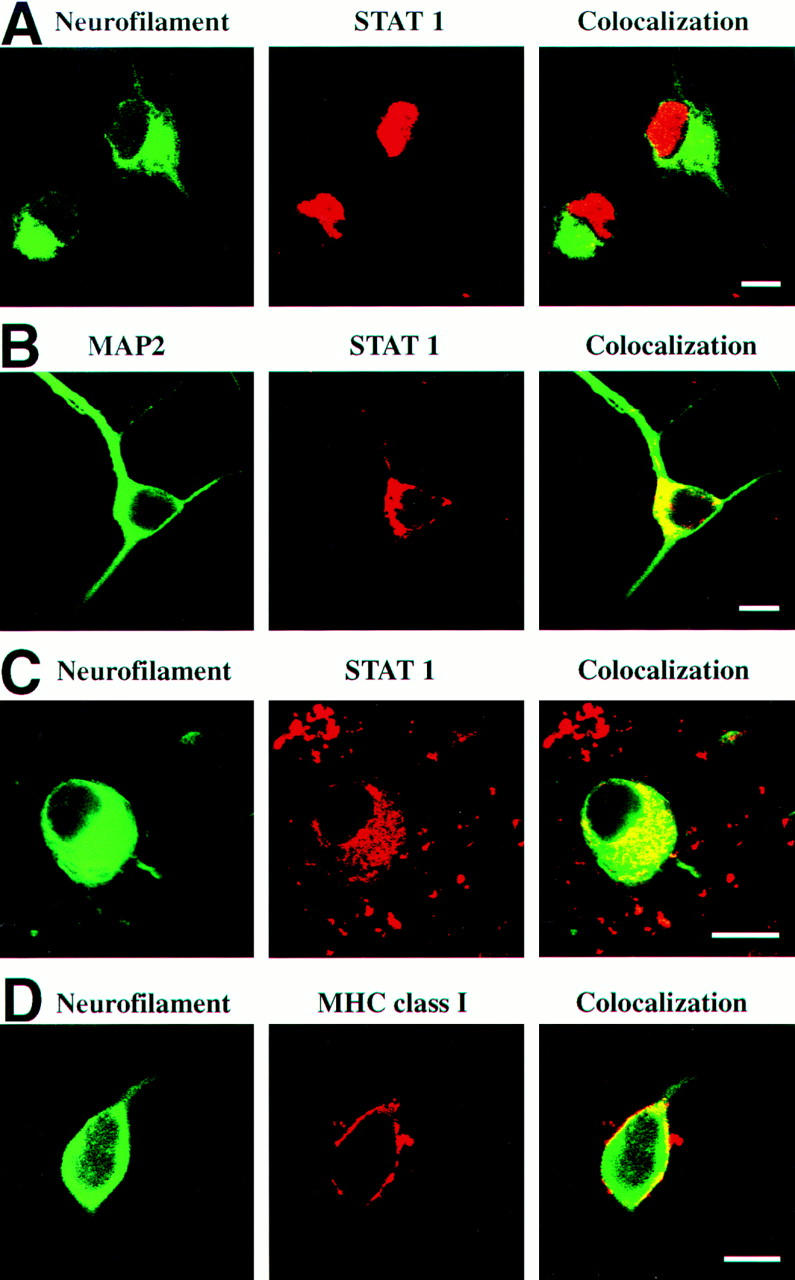
Nuclear localization of STAT1 and cell membrane expression of MHC class I molecules. (A) Nuclear localization of STAT1 in sensory neurons. Embryonic sensory neurons were cultured for 2 d and analyzed by confocal laser scanning microscopy for neurofilament and STAT1. (B) Cytoplasmic localization of STAT1 in neurons of hippocampal cultures. (C) Cytoplasmic localization of STAT1 in sensory neurons (cultured for 2 d) in the presence of neutralizing antibodies directed against IFN-γ. (D) MHC class I molecules detected on the cell membrane of untreated sensory neurons. Sensory neurons (cultured for 5 d) were identified by antibodies recognizing neurofilament and were immunolabeled with antibodies directed against MHC class I on the cell surface. Scale bar A, B, C, and D: 10 μm.
Figure 9.
Autocrine activation of STAT1 and MHC class I in sensory neurons. (A) Frequency of sensory neurons (cultured for 2 d with or without IFN-γ neutralizing antibodies) demonstrating the nuclear localization of STAT1. (B) MHC class I molecules on the cell membrane of sensory neurons (cultured for 5 d) untreated or treated with IFN-γ neutralizing antibodies.
The possible auto/paracrine action of neuronal IFN-γ was supported by an investigation of MHC class I expression on cultured DRG neurons. As previously reported, IFN-γ readily induces MHC class I genes in electrically silent neurons, but much less so in firing neurons (12, 14). Adult sensory DRG neurons are silent in culture, and are susceptible to MHC class I induction by IFN-γ (18). Thus, in the case of autocrine activity of neuronal IFN-γ, cultured IFN-γ–secreting neurons should constitutively express MHC class I on their membranes. That this is the case was documented by confocal microscopy with MHC class I expression on 67 ± 11% of all sensory neurons (cultured for 5 d and identified by a neurofilament marker, Figs. 8 and 9). Treatment of the DRG culture with neutralizing anti–IFN-γ monoclonal antibodies reduced the proportion of MHC class I–positive neurons to 14 ± 3% (Fig. 9).
Discussion
Over the past few years it has become clear that numerous humoral mediators that originally had been thought to act exclusively either in the immune system or in the nervous system in fact interconnect both organ systems. For example, neurotrophic factors like nerve growth factor are not restricted to neural cells, but are produced by and act on immune cells as well (19). Conversely, a number of cytokines with classical immune functions are also effective in nervous tissues. Proinflammatory cytokines like TNF-α or IL-1 influence neuronal function and behavior (20). A proportion of these mediators may be imported through the blood brain barrier or discharged in the central nervous system by immigrant inflammatory cells. But it has also become clear that many of these cytokines are produced and released within the nervous system by autochthonous cells (21, 22). In this study, we have shown that classical IFN-γ is produced and used by neurons in the peripheral nervous system.
Autocrine gene regulation is a common feature of the cytokine network. For example, IFN-γ produced by subsets of activated T cells activates STAT1 factor and thereby upregulates its own gene expression (17, 23). Likewise, in the brain, neurotrophils such as brain-derived neurotrophic factor prevent death of adult sensory neurons acting in autocrine loops (24). Our results now suggest that STAT1 activation in neurons is at least partially regulated by IFN-γ produced by the same cells. Although neutralization of IFN-γ by monoclonal antibodies suppressed MHC class I membrane expression on neurons almost completely, this procedure inhibited phosphorylation and translocation of STAT1 only partially. This can be explained by the activity of neural mediators ligating the gp130 receptor of the IL-6/CNTF family of cytokines, which uses besides STAT3 also the STAT1 activation pathway (25).
Most reports on IFN-γ activity within the nervous system are related to inflammatory responses, as represented by experimental autoimmune encephalomyelitis, multiple sclerosis, or microbial brain infections. In these situations, the cytokine is mainly produced and released by infiltrating immune cells (26). This is in contrast to neuronal IFN-γ, whose particular temporal and spatial expression patterns suggest a developmental function, possibly related to the differentiation of peripheral nerves.
Autocrine IFN-γ secretion by DRG neurons may contribute signals controlling neuronal differentiation (4), like activation of neuron-specific genes encoding the peripheral nerve type 1 sodium channel (5). Other possible targets of IFN-γ–dependent regulation are the genes controlling neuron transmitter production (6). Alternatively, DRG neuronal IFN-γ could regulate glia cells surrounding the neurons in situ. In vitro, IFN-γ interferes with myelination of central nervous system (27) and peripheral nerve myelination (28). In agreement with this, transgenic mice constitutively producing IFN-γ in their central nervous system display a “tremoring” phenotype coinciding with a “dramatic overall decrease in central nervous system myelin” (29).
Demonstration of local production of IFN-γ by small and medium sized DRG neurons (which are enriched in our nerve growth factor–dependent culture model) and its auto/paracrine action on local cells predicts a new and unexpected function of this unusually pleiotropic proinflammatory cytokine.
Acknowledgments
We thank Ms. L. Penner for expert technical assistance; Drs. R. Böhm-Matthaei, E. Hoppe, R. Kiefer, and H.-P. Hartung for technical advice; Dr. A. Ries for supplying us with laminin; Dr. G. Kääb for culturing T lymphoblasts; and Dr. I. Medana for helpful discussion.
Footnotes
The project was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 391) and the European Community (CHRX-CT94-0670).
Abbreviations used in this paper: DRG, dorsal root ganglion; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFAP, glial fibrillary acidic protein; RT, reverse transcriptase.
References
- 1.Farrar MA, Schreiber RD. The molecular cell biology of interferon-γ and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 2.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 3.Chang JY, Martin DP, Johnson EM. Interferon suppresses sympathetic neuronal cell death caused by nerve growth factor deprivation. J Neurochem. 1990;55:436–445. doi: 10.1111/j.1471-4159.1990.tb04155.x. [DOI] [PubMed] [Google Scholar]
- 4.Improta T, Salvatore AM, DiLuzio A, Romeo G, Coccia EM, Calissano P. IFN-γ facilitates NGF-induced neuronal differentiation in PC-12 cells. Exp Cell Res. 1988;179:1–9. doi: 10.1016/0014-4827(88)90342-4. [DOI] [PubMed] [Google Scholar]
- 5.Toledo-Aral JJ, Brehm P, Halegoua S, Mandel G. A single pulse of nerve growth factor triggers long-term neuronal excitability through sodium channel gene induction. Neuron. 1995;14:607–611. doi: 10.1016/0896-6273(95)90317-8. [DOI] [PubMed] [Google Scholar]
- 6.Jonakait GM, Wei R, Sheng Z-L, Hart RP, Ni L. Interferon-γ promotes cholinergic differentiation of embryonic septal nuclei and adjacent forebrain. Neuron. 1994;12:1149–1159. doi: 10.1016/0896-6273(94)90322-0. [DOI] [PubMed] [Google Scholar]
- 7.Eneroth A, Kristensson K, Ljungdahl Å, Olsson T. Interferon-γ–like immunoreactivity in developing rat spinal ganglia neurons in vivo and in vitro. J Neurocytol. 1991;20:225–231. doi: 10.1007/BF01186995. [DOI] [PubMed] [Google Scholar]
- 8.Kiefer R, Kreutzberg GW. Gamma interferon-like immunoreactivity in the rat nervous system. Neuroscience. 1990;37:725–734. doi: 10.1016/0306-4522(90)90103-b. [DOI] [PubMed] [Google Scholar]
- 9.Olsson T, Kelic S, Edlund C, Bakhiet M, Höjeberg B, Van der Meide P, Ljungdahl Å, Kristensson K. Neuronal interferon-γ immunoreactive molecule: bioactivities and purification. Eur J Immunol. 1994;24:308–314. doi: 10.1002/eji.1830240205. [DOI] [PubMed] [Google Scholar]
- 10.Kiefer R, Haas CA, Kreutzberg GW. Gamma interferon–like immunoreactive material in rat neurons: evidence against a close relationship to gamma interferon. Neuroscience. 1991;45:551–560. doi: 10.1016/0306-4522(91)90270-x. [DOI] [PubMed] [Google Scholar]
- 11.Wood PM. Separation of functional Schwann cells and neurons from normal peripheral nerve tissue. Brain Res. 1976;115:361–375. doi: 10.1016/0006-8993(76)90355-3. [DOI] [PubMed] [Google Scholar]
- 12.Neumann H, Cavalié A, Jenne DE, Wekerle H. Induction of MHC class I genes in neurons. Science. 1995;269:549–552. doi: 10.1126/science.7624779. [DOI] [PubMed] [Google Scholar]
- 13.Kääb G, Brandl G, Marx A, Wekerle H, Bradl M. The myelin basic protein specific T cell repertoire in (transgenic) Lewis rat/SCID mouse chimeras: preferential Vb8.2 T cell receptor usage depends on an intact Lewis thymic microenvironment. Eur J Immunol. 1996;26:981–988. doi: 10.1002/eji.1830260504. [DOI] [PubMed] [Google Scholar]
- 14.Neumann H, Schmidt H, Cavalié A, Jenne D, Wekerle H. MHC class I gene expression in single neurons of the central nervous system: differential regulation by interferon-γ and tumor necrosis factor–α. J Exp Med. 1997;185:305–316. doi: 10.1084/jem.185.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohrer H, Henke-Fahle S, El-Sharkawy T, Lux HD, Thoenen H. Progenitor cells from embryonic chick dorsal root ganglia differentiate in vitro to neurons: biochemical and neurophysiological evidence. EMBO (Eur Mol Biol Organ) J. 1985;4:1709–1714. doi: 10.1002/j.1460-2075.1985.tb03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bach EA, Aguet M, Schreiber RD. The IFN-γ receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 17.Hardy KJ, Sawada T. Human γ interferon strongly upregulates its own gene expression in peripheral blood lymphocytes. J Exp Med. 1989;170:1021–1026. doi: 10.1084/jem.170.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujimaki H, Hikawa N, Nagoya T, Minami M. IFN-γ induces expression of MHC class I molecules in adult mouse dorsal root ganglion neurons. Neuroreport. 1997;7:2951–2955. doi: 10.1097/00001756-199611250-00030. [DOI] [PubMed] [Google Scholar]
- 19.Torcia M, Bracci-Laudiero L, Lucibello M, Nencioni L, Labardi D, Rubatelli A, Cozzolino F, Aloe L, Garaci E. Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell. 1996;85:345–356. doi: 10.1016/s0092-8674(00)81113-7. [DOI] [PubMed] [Google Scholar]
- 20.Rothwell NJ, Hopkins SJ. Cytokines and the nervous system. II. Actions and mechanisms of action. Trends Neurosci. 1995;18:130–136. doi: 10.1016/0166-2236(95)93890-a. [DOI] [PubMed] [Google Scholar]
- 21.Murphy PG, Grondin J, Altares M, Richardson PM. Induction of interleukin-6 in axotomized sensory neurons. J Neurosci. 1995;15:5130–5138. doi: 10.1523/JNEUROSCI.15-07-05130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tchelingerian J-L, Quinonero J, Booss J, Jacque C. Localization of TNF-α and IL-1α immunoreactivities in striatal neurons after surgical injury to the hippocampus. Neuron. 1993;10:213–224. doi: 10.1016/0896-6273(93)90312-f. [DOI] [PubMed] [Google Scholar]
- 23.Girdlestone J, Wing M. Autocrine activation by interferon-γ of STAT factors following T cell activation. Eur J Immunol. 1996;26:704–709. doi: 10.1002/eji.1830260329. [DOI] [PubMed] [Google Scholar]
- 24.Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD, Lindsay RM. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- 25.Zhong Z, Wen Z, Darnell JE. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 26.Benveniste EN, Benos DJ. TNF-α and interferon-γ–mediated signal transduction pathways: effects on glial cell gene expression and function. FASEB (Fed Am Soc Exp Biol) J. 1995;9:1577–1584. doi: 10.1096/fasebj.9.15.8529837. [DOI] [PubMed] [Google Scholar]
- 27.Agresti C, D'Urso D, Levi G. Reversible inhibitory effects of interferon-γ and tumor necrosis factor-α on oligodendroglial lineage cell proliferation and differentiation in vitro. Eur J Neurosci. 1996;8:1106–1116. doi: 10.1111/j.1460-9568.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 28.Schneider-Schaulies J, Kirchhoff F, Archelos J, Schachner M. Down-regulation of myelin-associated glycoprotein on Schwann cells by interferon-γ and tumor necrosis factor–α affects neurite outgrowth. Neuron. 1991;7:995–1005. doi: 10.1016/0896-6273(91)90344-y. [DOI] [PubMed] [Google Scholar]
- 29.Corbin JG, Kelly D, Rath EM, Baerwald KD, Suzuki K, Popko B. Targeted CNS expression of interferon-γ in transgenic mice leads to hypomyelination, reactive gliosis, and abnormal cerebellar development. Mol Cell Neurosci. 1996;7:354–370. doi: 10.1006/mcne.1996.0026. [DOI] [PubMed] [Google Scholar]