Abstract
Gram-negative bacterial lipopolysaccharide (LPS) stimulates phagocytic leukocytes by interacting with the cell surface protein CD14. Cellular responses to LPS are markedly potentiated by the LPS-binding protein (LBP), a lipid-transfer protein that binds LPS aggregates and transfers LPS monomers to CD14. LBP also transfers LPS to lipoproteins, thereby promoting the neutralization of LPS. LBP present in normal plasma has been shown to enhance the LPS responsiveness of cells in vitro. The role of LBP in promoting LPS responsiveness in vivo was tested in LBP-deficient mice produced by gene targeting in embryonic stem cells. Whole blood from LBP-deficient animals was 1,000-fold less responsive to LPS as assessed by the release of tumor necrosis factor (TNF)-α. Blood from gene-targeted mice was devoid of immunoreactive LBP, essentially incapable of transferring LPS to CD14 in vitro, and failed to support cellular responses to LPS. These activities were restored by the addition of exogenous recombinant murine LBP to the plasma. Despite these striking in vitro findings, no significant differences in TNF-α levels were observed in plasma from wild-type and LBP-deficient mice injected with LPS. These data suggest the presence of an LBP-independent mechanism for responding to LPS. These LBP knockout mice may provide a tool for discovering the nature of the presumed second mechanism for transferring LPS to responsive cells.
Lipopolysaccharide (LPS) is the major lipid component of the outer membrane of Gram-negative bacteria (1) and the major initiator of innate immune responses to bacterial infection. In the sepsis syndrome, exaggerated responses to LPS may have life-threatening side effects such as multiorgan failure and sepsis-related shock.
The earliest cell-mediated events after endotoxin release appear to involve the transfer of LPS to a glycosyl phosphatidyl inositol (GPI)-linked protein known as CD14. Evidence supporting a central role for CD14 in LPS-mediated responses includes the following: (a) LPS binds stoichiometrically to CD14 (2), (b) monoclonal antibodies to CD14 inhibit the ability of LPS to stimulate phagocytes (3), (c) a soluble fragment of CD14 (sCD14) is present in blood (4) and facilitates the activation of cells that do not express membrane CD14 (5), (d) transfection of CD14 into CD14-negative cell lines enhances the responsiveness of these cells to LPS by >1,000-fold (6, 7). The physiological relevance of these observations was underscored by the finding that CD14-gene disrupted mice are 10,000-fold less sensitive to LPS than matched littermates (8).
LPS is a sparingly soluble amphiphile that forms aggregates in aqueous medium. As a consequence, spontaneous diffusion of LPS monomers from aggregates to the binding site on CD14 occurs at a very slow rate (2). A plasma protein known as lipopolysaccharide-binding protein (LBP; reference 9), however, accelerates binding of LPS monomers to CD14 (2) and thereby enhances the sensitivity of cells to LPS (10). LBP acts as a lipid transfer protein, catalytically transferring LPS monomers from aggregates to CD14 (2, 11). This function is in keeping with its sequence homology to the lipid transfer proteins phospholipid transfer protein and cholesterol ester transfer protein. Additional studies have shown that LBP copurifies with HDL particles (12) and also acts to transfer LPS into complexes of high density lipoprotein (HDL) (13) where LPS is functionally neutralized (14). Thus, LBP may serve both to enhance and to neutralize the biological activity of LPS.
We sought to clarify the role of LBP in vivo by deleting the gene from mice. We report that whole blood from the LBP−/− knockout (KO) animal has no LBP activity, and the response to LPS as an activator of cytokine production in vitro was shifted by ∼1,000-fold. Much to our surprise, this remarkable in vitro phenotype was not matched in vivo. Although small deficiencies in response to LPS were observed at the lowest doses of injected LPS, overall, no significant difference in response was observed. Although several potential explanations for this apparent dichotomy exist, we propose that the role of LBP in enhancing LPS signaling is limited to the bloodstream and may contribute little to morbidity and mortality associated with endotoxemia. Furthermore, we propose that a novel protein with LBP-like capabilities may exist in LPS-responsive tissues.
Materials and Methods
Reagents.
Unless otherwise stated, all materials were purchased from Sigma Chemical Co. (St. Louis, MO). Escherichia coli LPS (0111:B4) was purchased from List Biologicals (Campbell, CA).
Construction of the LBP Knockout Vector.
Screening of a murine 129/SVJ genomic library in the P1 cloning vector was performed by Genome Systems, Inc. (St. Louis, MO). A genomic clone was identified using primer pairs to generate PCR products corresponding to portions of the murine LBP 5′ untranslated region (UTR) (5′-CGGGGCCTCTCTTTCCCGC-3′, 5′-CCTGGATGCTCCGTGGGGG-3′) and 3′ UTR (5′-GGGTCTCAGTGGCCACAGC-3′, 5′-CAGGTCTCCCACCCAGTGTTG-3′). Exons 1–3 were sequenced and found to be identical to the murine LBP cDNA sequence (These sequence data are available from EMBL/GenBank/DDBJ under accession number X99347). A 8.4-kb NheI–NdeI subclone was used to construct the targeting vector. A 1.4-kb SpeI fragment was replaced with the neomycin phosphotransferase gene (neo) in the opposite transcriptional orientation from the LBP gene. Replacement of LBP sequences with the neo cassette deleted a portion of the 5′ UTR, the start codon and subsequent 40 amino acids, and introduced a new EcoRV restriction site. A map of the 5′ portion of the LBP gene and the targeting vector is shown in Fig. 1.
Figure 1.
Targeting vector. The genomic organization of murine LBP is shown and compared with the targeting vector. The mutant construct removes a portion of the 5′ UTR, the translational start site, the next 40 amino acids, and the first splice donor site.
Generation of Mutant Embryonic Stem Cell Line.
The vector (15 μg) was linearized with XhoI and transfected into the R1 embryonic stem (ES) cells (15). ES cells were transferred onto irradiated neoresistant primary embryonic fibroblasts and stable integrants were selected using G418 (350 μg/ml, GIBCO BRL, Gaithersburg, MD). To identify clones that underwent homologous recombination, ES cell DNA was digested with EcoRV, and analyzed by Southern blot hybridization using a PCR-generated probe derived from sequence external to the targeting vector (Fig. 1; 5′-CGGTTCTCAAATATTTGCGTCACCCC-3′, 5′-CTTTGGGCTTCCATGTCCCAGGAACC-3′).
Generation of Germline Chimeras and Homozygous Mutant Mice.
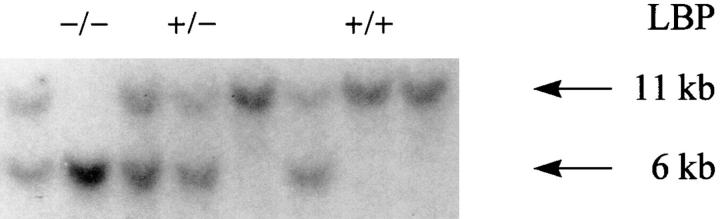
An ES cell clone that underwent homologous recombination was expanded and injected into C57BL/6J blastocysts. The resulting chimeric animals were backcrossed to C57BL/6J (Jackson Laboratories, Bar Harbor, ME) mice. Germline transmission was identified by Southern blot analysis of tail DNA. Hemizygous animals were crossed and homozygous offspring were identified by Southern blot as shown in Fig. 2.
Figure 2.
Southern blotting analysis of DNA from mutant LBP−/−, hemizygous LBP+/−, and homozygous LBP+/+ F1 littermates. Southern blots of tail-tip DNA of mice from the first heterozygote/heterozygote crossing are shown. The mutant allele is ∼6 kb and the wild-type allele is 11 kb.
Reverse Transcriptase PCR of LBP Messenger RNA from Mouse Liver.
To design primer pairs, a genomic fragment containing the second and third exons of LBP was sequenced. Similarly, a downstream genomic fragment containing a portion of the 3′-UTR was sequenced at the 3′ end. The presence of LBP messenger RNA (mRNA) was detected using a 5′ primer within the sequence in exon 3 (5′-GGCTCTGCAGAGAGAGCTGTACAA-3′) and a 3′ primer from the penultimate exon (5′-TAGTTAAGGAATGCCTGGAACAGG-3′). Using these primers, the predicted product from mRNA is 1.1 kb; the predicted product from the amplification of genomic DNA is ⩾10 kb. glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified as a control (5′ primer: GTCATCATCTCCGCCCCTTCTGC; 3′ primer: GATGCCTGCTTCACCACCTTCTTG).
Livers and kidneys from hemizygous or homozygous age- and gender-matched mice were removed under anesthesia (Metofane; Henry Schein, Port Washington, NY). RNA was extracted using RNazol (Tel-test, Friendswood, TX). Poly-A mRNA was purified using the PolyATtract mRNA Isolation System III kit (Promega, Madison, WI), and 10 ng of mRNA was reverse transcribed with Superscript II (GIBCO BRL). One tenth of the total reverse-transcribed product was used as template per PCR reaction. Each reaction contained PCR primers (500 nM), MgCl2 (2 mM), deoxy nucleotides triphosphates (dNTPs) (200 μM), and 1 unit Taq polymerase in a total volume of 25 μl. PCR conditions were 94°C for 1 min, 60°C for 45 s, and 1.5 min at 72°C for 35 cycles. Amplified fragments were resolved on a 3% agarose gel.
LBP/LPS Binding Assay (16).
ELISA plates (MaxiSorp; Nunc, Wiesbaden, Germany) were coated with LPS, washed, and dried. Plates were blocked with incubation buffer (50 mM Hepes, 150 mM NaCl, pH 7.4) containing 10 mg/ml BSA. Mouse plasma was serially diluted in incubation buffer plus BSA, added to each well, and incubated for 2 h at 37°C. After washing, 100 μl/well of a rabbit polyclonal antirecombinant murine LBP antibody (5 μg/ml) was added and incubated for 1 h at 37°C. Wells were developed using an anti–rabbit Ig horseradish peroxidase conjugate (BioGenes, Berlin, Germany) with o-phenylenediamine dihydrochloride as substrate.
Measurement of LPS Transfer Activity.
LPS transfer assays with boron dipyrromethane (BODIPY)-labeled LPS were as described (17) except that the assay was adapted to a 96-well format, and fluorescence was recorded with a Cytofluor II fluorescence plate reader (PerSeptive Biosystems, Framingham, MA). All reagents were diluted in Dulbecco's PBS to a total volume of 200 μl/well using Immulon-1 plates (Dynatech Laboratories, Inc., Chantilly, VA). All readings were blanked against Dulbecco's PBS.
LPS Stimulation of TNF-α Release.
KO mice and hemizygous littermates were matched for gender and weight. Blood was drawn in heparinized capillary tubes via the retroorbital plexus under anesthesia. Whole blood (40 μl/tube) was mixed with an equal volume of LPS suspended as a dispersion in PBS and 4 U/ ml heparin and incubated for 4 h at 37°C. TNF-α concentrations in the supernatants were measured by ELISA (Genzyme, Cambridge, MA).
In some experiments, washed peripheral blood cells containing a mixed population of leukocytes were also used as a source of LPS-inducible cytokines. Heparinized whole blood was subjected to centrifugation at 400 g, and the cellular pellet was washed twice and resuspended in the original volume of PBS. A 40-μl portion of these cells was then mixed with pooled plasma from LBP+/− or LBP−/− mice and incubated with LPS (1 ng/ml) for 4 h at 37°C. TNF-α concentrations in the supernatants were then measured by ELISA.
In Vivo Stimulation of TNF-α Production after Intravenous Injection of LPS.
LPS was suspended in 0.2 ml of sterile nonpyrogenic saline (Abbott Laboratories, Chicago, IL) at the stated concentration. Hemizygous (LBP+/−) and homozygous (LBP−/−) mice (3/ group) were anesthetized and injected intravenously via the retroorbital plexus. Plasma TNF-α levels were measured by ELISA from blood collected 90 min after injection.
Results
A Mutant LBP Transcript Is Expressed at Low Levels in LBP−/− Mice.
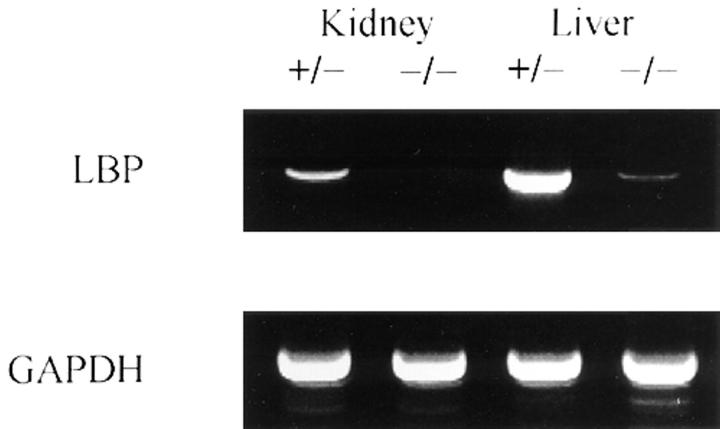
Initial restriction mapping of the murine LBP gene demonstrated that it is at least 14 kb in length. Since the gene was too large to delete in its entirety, we constructed a vector to delete the first exon, including the translational start site, and part of the first intron. PCR primers derived from the deleted region of the 5′ UTR to the 3′ UTR amplified the predicted products from RNA derived from wild-type mice but not KO mice, thus confirming the deletion (data not shown). To determine if the remaining portion of the gene was transcribed in KO animals, RNA was prepared and subjected to reverse transcriptase PCR analysis using primers that were not in the region of the gene deletion. Liver mRNA from LBP−/− mice expressed only a small fraction of LBP transcript in comparison to wild-type mice and no LBP mRNA was detected in kidneys from −/− mice (Fig. 3). These data confirmed that the KO mouse expresses very low levels of mutant LBP transcript. Even if the mutant mRNA were translated successfully, the resultant protein would be truncated and probably expressed at very low levels.
Figure 3.
Reverse transcriptase PCR of LBP mRNA from hemizygous mice and homozygous LBP−/− littermates. RNA was extracted from F4 mice, and analyzed by reverse transcriptase PCR. Intron spanning primers were used; the DNA product resulting from the reverse transcription and amplification of LBP mRNA was 1.1 kb, whereas contaminating genomic DNA had a predicted size of >10 kb. Shown is one of four representative experiments.
Plasma from LBP−/− Mice Appears to Lack Functional LBP.
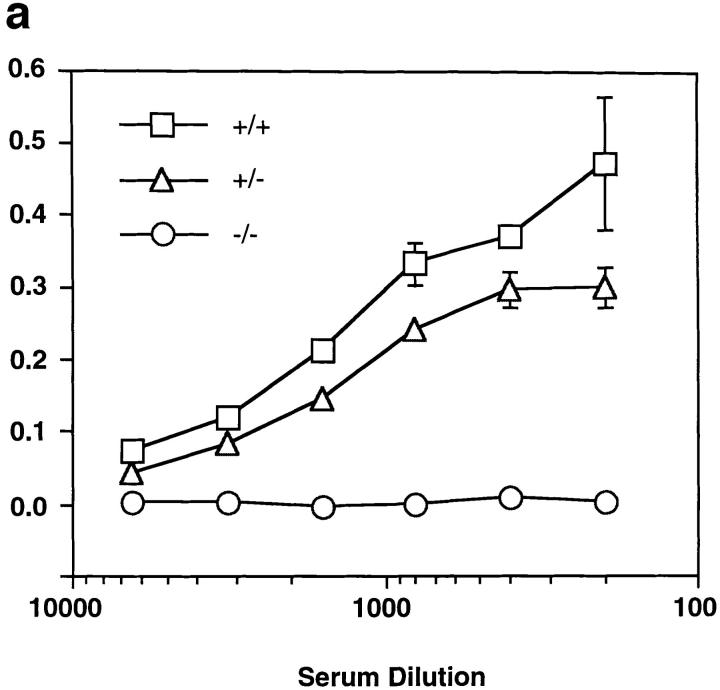
To determine if blood contained a functional mutant LBP molecule, we analyzed plasma from KO mice for LBP-like activity. The ability of LBP to bind LPS and to form a stable complex has been well described (9, 16). We incubated plasma from the KO mouse with LPS-coated plastic, washed the wells with buffer, and developed the plate with a polyclonal antiserum against murine LBP. Although hemizygous mice expressed wild-type levels of LBP, their homozygous gene-targeted littermates had no detectable LBP (Fig. 4 a).
Figure 4.
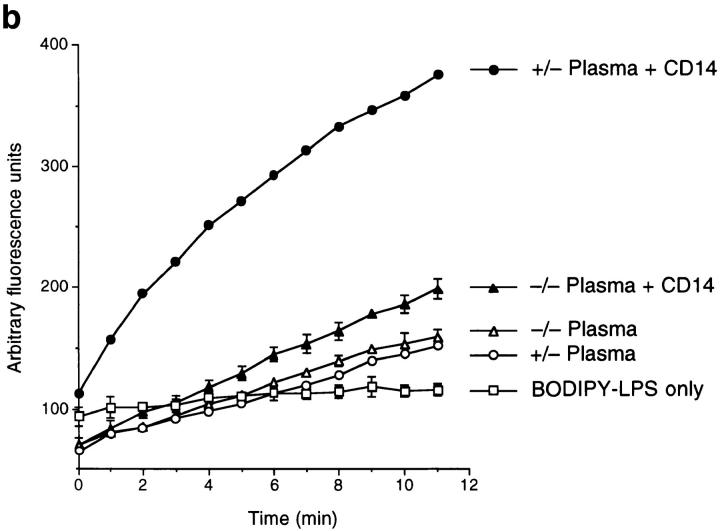
Plasma from the knock-out mice does not contain functional LBP. (a) LPS-coated plastic was incubated with plasma from wild-type LBP+/+, hemizygous LBP+/−, and homozygous LBP−/− mice. LPS-bound LBP was detected by incubating washed wells with polyclonal anti-LBP serum as described in Materials and Methods. No detectable LBP remained bound to the plastic in plasma from the KO animals. (b) Plasma from LBP+/− animals was compared to plasma from homozygous LBP−/− animals for its ability to mediate transfer of LPS to sCD14. B-LPS plus LBP+/− plasma plus sCD14 (•), B-LPS plus LBP−/− plasma plus sCD14 (▴), B-LPS plus LBP+/− plasma (○), B-LPS plus LBP−/− plasma (▵), B-LPS alone (□). The KO plasma had virtually no ability to transfer LPS to sCD14. Addition of recombinant LBP to LBP−/− plasma restored activity (not shown). Results are representative of four separate experiments.
LBP not only binds LPS aggregates but also transfers LPS monomers from LPS aggregates to CD14 (2). This property of LBP can be studied in a fluorescent assay, using BODIPY-labeled LPS, in which fluorescence is quenched in its aggregate state (17). As labeled LPS monomers exit from LPS aggregates and bind to CD14, an increased fluorescent signal can be observed. This process is greatly accelerated by the presence of substoichiometric quantities of LBP (2, 17). Thus, it was possible to ask if the KO plasma contained functional LBP. Although the plasma from the hemizygous mice rapidly transferred LPS to soluble CD14, plasma from the KO mice was virtually inactive (Fig. 4 b). Taken together, the experiments demonstrated in Fig. 4 a and b suggest that mutant mice either fail to translate their mutant transcript into secreted protein, or that the resulting protein produced by the KO mice fails to properly bind or transfer LPS to CD14.
Whole Blood from the LBP KO Mice Failed to Produce TNF-α in Response to Low Concentrations of LPS.
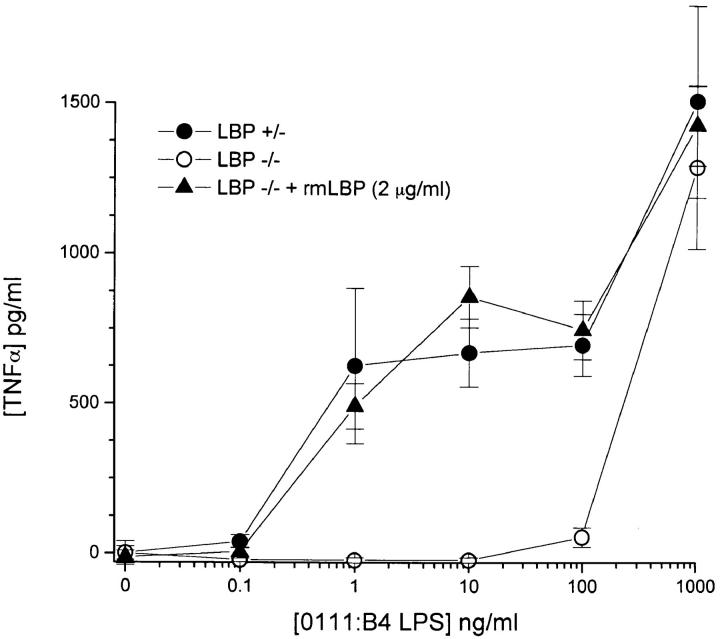
We measured the ability of LPS to induce TNF-α secretion in whole blood ex vivo from the LBP−/− animals and their hemizygous littermates. Careful dose response studies of the KO animal revealed that the absence of functional LBP shifted the dose-response relationship of LPS by at least 1,000-fold (Fig. 5). The addition of recombinant LBP to whole blood from the LBP−/− mouse shifted the curve to closely resemble that of the hemizygous mouse (Fig. 5). Thus, blood from mice with the targeted deletion of the LBP gene was markedly hyporesponsive to LPS, presumably as a result of the inability of LPS to rapidly associate with CD14.
Figure 5.
Ex vivo cytokine responses by blood from LBP−/− mice was markedly hyporesponsive to exogenously added LPS. Whole blood was obtained from +/− and −/− mice, pooled, and assessed for LPS responsiveness as described in Materials and Methods. Recombinant LBP was added to achieve a final concentration of 2 μg/ml. Each data point is expressed as the mean +/− range of two separate experimental wells (each of which was measured in duplicate). Shown is one of four similar experiments.
Additional studies showed that responses of LBP−/− cells could be restored with plasma from LBP+/− animals. Blood cells were washed out of whole blood from LBP−/− animals and their hemizygous littermates and were resuspended in PBS containing pooled sera from the LBP+/− or LBP−/− littermates. When cells from either mouse were stimulated with LPS (1 ng/ml) in plasma from hemizygotes, they responded to LPS by releasing TNF-α. In contrast, cells were unresponsive to LPS when incubated with LPS in the presence of LBP−/− plasma, regardless of whether they were derived from hemizygous LBP+/− or homozygous KO animals (data not shown).
Injection of LPS into LBP KO Animals Results in Normal Expression of TNF-α.
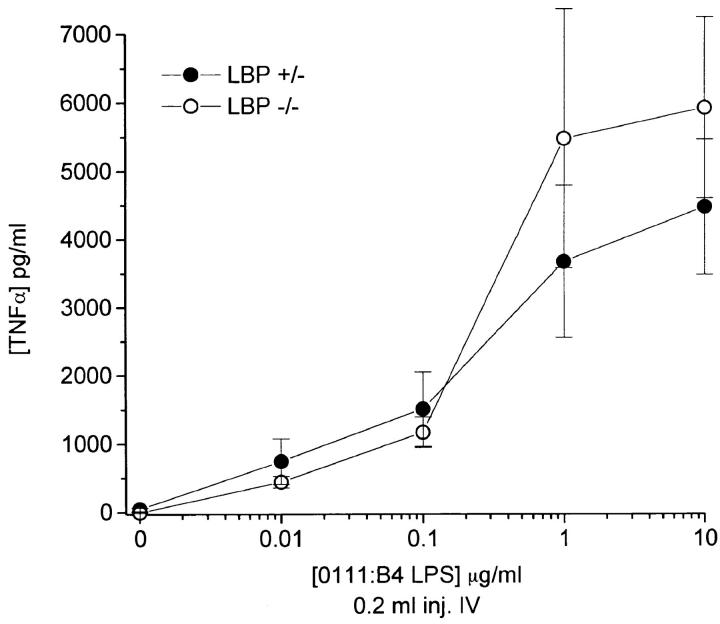
We predicted that the LBP KO mouse would be markedly hyporesponsive to the systemic administration of LPS. KO mice, representing F2 through F4 generations, were matched for age and sex and injected intravenously with graded concentrations of LPS. Surprisingly, the KO mice responded nearly identically to the hemizygous littermates over a wide range of doses (Fig. 6). Although at the lowest dose of LPS, a trend towards less response may have been seen in the KO animals, these doses were far lower than the dose of LPS needed to kill an animal (or even produce visible signs of morbidity). This was true regardless of the route of administration, as intraperitoneal injections of endotoxin produced similar results (data not shown). These results contrast dramatically with the differences observed ex vivo in which blood from the mutant LBP−/− mice was ∼1,000-fold less responsive than blood from hemizygous LBP+/− littermates.
Figure 6.
In vivo cytokine response to LPS injection in homozygous −/− KO and hemizygous +/− mice are nearly identical. Age- and sex-matched mice were injected intravenously under anesthesia with 200 μl of LPS at the indicated concentrations via the retroorbital plexus. After 90 min, mice were reanesthetized and phlebotomized using heparinized capillary tubes. Each of three mice was assessed separately per condition. Plasma TNF-α was determined by ELISA. Shown is a representative experiment from six similar experiments (three intravenous and three intraperitoneal injections).
Discussion
Gene targeting and homologous recombination were used to delete the LBP gene from mice. Plasma from the mice appeared devoid of LBP since LPS-coated plastic treated with this plasma did not accumulate immunoreactive LBP and plasma derived from the KO animal demonstrated a strikingly reduced ability to transfer LPS to sCD14. LBP is thought to promote responses to LPS by catalyzing the transfer of LPS to CD14. Consistent with this notion, we observed that whole blood from LBP−/− animals showed dramatically reduced ability to produce TNF-α, requiring nearly 1,000-fold more LPS for a half-maximal response than blood from LBP+/− animals. The LBP necessary for cellular responses in these studies was clearly due to differences between the two sources of plasma since adding LBP+/− plasma to LBP−/− cells fully restored sensitivity to LPS, and hyporesponsiveness was reversed by the addition of recombinant murine LBP. These experimental findings confirm the important role of LBP in mediating sensitive responses of monocytes and macrophages to LPS.
Studies on the responses of living mice yielded a surprisingly different result. The production of TNF-α in response to LPS was nearly indistinguishable for LBP−/− and LBP+/− mice, especially at doses sufficient to result in shock and death. This result was surprising in light of the report that a CD14−/− mouse showed strongly depressed responses to a similar LPS challenge (8). It thus appears that the LBP−/− mouse reported here may respond to LPS in a CD14-dependent but LBP-independent fashion. One tentative conclusion that might be drawn from these data is that blood is not the compartment that is primarily responsible for cytokinemia resulting from endotoxemia. Even so, an additional mechanism is needed to explain how tissue phagocytes and other TNF-α–producing cells can respond to LPS independently of LBP. We envision two potential explanations for these results.
Low levels of a functional, truncated LBP might be produced in the liver but might fail to be expressed in blood due to the introduced mutation. We do not favor this hypothesis. Previous studies have shown that the NH2-terminal half of LBP is responsible for its LPS binding activity and the COOH-terminal half contains the activity responsible for transferring LPS to CD14 (18, 19). Although it is possible that a truncated LBP gene product could be generated by translation of an LBP transcript at an internal methionine codon, the first potential initiation codon within the LBP locus (methionine 146) is beyond the putative LPS-binding domain, and the resulting protein would not possess LPS-binding activity. Moreover, the mutant LBP partial transcript was expressed at very low levels in the livers of the KO mice (Fig. 3), and any resulting protein would be expressed at vanishingly low levels.
A more attractive explanation for the LPS responsiveness of the LBP KO mice is that a protein distinct from LBP may transfer LPS to CD14 within tissues. In this regard, Acton et al. (20) have recently described a membrane protein (the class B scavenger receptor, SR-BI) that transfers lipids from high density lipoproteins to the plasma membranes of hepatocytes, and Vassellon et al. have described a protease-sensitive activity on the surface of leukocytes that appears to transfer LPS from CD14 to the membranes (21). These studies offer precedence for cell-associated lipid transfer proteins that might exist on endothelial cells or other elements in tissues.
We wish to stress that our results do not rule out a contribution of LBP to LPS responsiveness under all circumstances in vivo. The proteins contributing to a response might vary with the type and physical state of the LPS, with the route of administration, and with the physiological state of the animals (resting or acute phase). In fact, preliminary studies suggest that LBP−/− animals may show depressed responses to LPS under some dosing regimens (not shown). This observation is in keeping with the ability of LBP to enable responses in whole blood and the ability of anti-LBP to block responses of animals to LPS (22). Redundant or overlapping mechanisms are commonly observed in vital physiological functions and should be expected in host response to Gram-negative infection. The most important conclusion from our work is that animals do possess machinery that acts in parallel or in addition to LBP to initiate cellular responses to LPS. The availability of the LBP−/− animal makes it possible to seek the nature of this additional machinery.
Acknowledgments
The authors would like to thank Drs. Mason Freeman and Amin Arnaout for their insightful comments.
This work was supported in part by National Institutes of Health grants DK-50305, AI-38515, and GM-54060 (D.T. Golenbock), AI-30556 (S.D. Wright), and K08 AI-01476 (R.R. Ingalls).
References
- 1.Raetz CRH. Biochemistry of Endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- 2.Hailman E, Lichenstein HS, Wurfel MM, Miller DS, Johnson DA, Kelley M, Busse LA, Zukowski MM, Wright SD. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS-binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 4.Bazil V, Baudys M, Hilgert I, Stefanova I, Low MG, Zbrozek J, Horejsi V. Structural relationship between the soluble and membrane-bound forms of human monocyte surface glycoprotein CD14. Mol Immunol. 1989;26:657–662. doi: 10.1016/0161-5890(89)90048-5. [DOI] [PubMed] [Google Scholar]
- 5.Frey EA, Miller DS, Jahr TG, Sundan A, Bazil V, Espevik T, Finlay BB, Wright SD. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golenbock D, Liu Y, Millham F, Freeman M, Zoeller R. Surface expression of human CD14 in Chinese hamster ovary fibroblasts imparts macrophage-like responsiveness to bacterial endotoxin. J Biol Chem. 1993;268:22055–22059. [PubMed] [Google Scholar]
- 7.Lee J-D, Kato K, Tobias PS, Kirkland TN, Ulevitch RJ. Transfection of CD14 into 70Z/3 cells dramatically enhances the sensitivity to complexes of lipopolysaccharide (LPS) and LPS binding protein. J Exp Med. 1992;175:1697–1705. doi: 10.1084/jem.175.6.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haziot A, Ferrero E, Kontgen F, Hijiya N, Yamamoto S, Silver J, Stewart CL, Goyert SM. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407–414. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 9.Tobias PS, Soldau K, Ulevitch RJ. Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. J Exp Med. 1986;164:777–793. doi: 10.1084/jem.164.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathison JC, Tobias PS, Wolfson E, Ulevitch RJ. Plasma lipopolysaccharide (LPS)-binding protein. A key component in macrophage recognition of gram-negative LPS. J Immunol. 1992;149:200–206. [PubMed] [Google Scholar]
- 11.Tobias P, Soldau K, Gegner J, Mintz D, Ulevitch R. Lipopolysaccharide binding protein-mediated complexation of lipopolysaccharide with soluble CD14. J Biol Chem. 1995;270:10482–10488. doi: 10.1074/jbc.270.18.10482. [DOI] [PubMed] [Google Scholar]
- 12.Wurfel MM, Kunitake ST, Lichenstein H, Kane JP, Wright SD. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J Exp Med. 1994;180:1025–1035. doi: 10.1084/jem.180.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wurfel MM, Hailman E, Wright SD. Soluble CD14 acts as a shuttle in the neutralization of lipopolysaccharide (LPS) by LPS-binding protein and reconstituted high density lipoprotein. J Exp Med. 1995;181:1743–1754. doi: 10.1084/jem.181.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skarnes RC. The inactivation of endotoxin after interaction with certain proteins of normal serum. Ann NY Acad Sci. 1966;133:644–662. doi: 10.1111/j.1749-6632.1966.tb52395.x. [DOI] [PubMed] [Google Scholar]
- 15.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tobias PS, Soldau K, Ulevitch RJ. Identification of a lipid A binding site in the acute phase reactant lipopolysaccharide binding protein. J Biol Chem. 1989;264:10867–10871. [PubMed] [Google Scholar]
- 17.Yu B, Wright SD. Catalytic properties of lipopolysaccharide (LPS) binding protein. Transfer of LPS to soluble CD14. J Biol Chem. 1996;271:4100–4105. doi: 10.1074/jbc.271.8.4100. [DOI] [PubMed] [Google Scholar]
- 18.Abrahamson SL, Wu HM, Williams RE, Der K, Ottah N, Little R, Gazzano-Santoro H, Theofan G, Bauer R, Leigh S, et al. Biochemical characterization of recombinant fusions of lipopolysaccharide binding protein and bactericidal/permeability-increasing protein. Implications in biological activity. J Biol Chem. 1997;272:2149–2155. doi: 10.1074/jbc.272.4.2149. [DOI] [PubMed] [Google Scholar]
- 19.Lamping N, Hoess A, Yu B, Park TC, Kirschning CJ, Pfeil D, Reuter D, Wright SD, Herrmann F, Schumann RR. Effects of site-directed mutagenesis of basic residues (Arg 94, Lys 95, Lys 99) of lipopolysaccharide (LPS)- binding protein on binding and transfer of LPS and subsequent immune cell activation. J Immunol. 1996;157:4648–4656. [PubMed] [Google Scholar]
- 20.Acton SL, Scherer PE, Lodish HF, Krieger M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J Biol Chem. 1994;269:21003–21009. [PubMed] [Google Scholar]
- 21.Vasselon T, Pironkova R, Detmers PA. Sensitive responses of leukocytes to LPS requires a protein distinct from CD14 at the cell surface. J Immunol. 1997;159:4498–4505. [PubMed] [Google Scholar]
- 22.Gallay P, Heumann D, Le RD, Barras C, Glauser MP. Lipopolysaccharide-binding protein as a major plasma protein responsible for endotoxemic shock. Proc Natl Acad Sci USA. 1993;90:9935–9938. doi: 10.1073/pnas.90.21.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]