Abstract
The tyrosine kinase Syk has been implicated as a key signal transducer from the B cell antigen receptor (BCR). We show here that mutation of the Syk gene completely blocks the maturation of immature B cells into recirculating cells and stops their entry into B cell follicles. Furthermore, using radiation chimeras we demonstrate that this developmental block is due to the absence of Syk in the B cells themselves. Syk-deficient B cells are shown to have the life span of normal immature B cells. If this is extended by over-expression of Bcl-2, they accumulate in the T zone and red pulp of the spleen in increased numbers, but still fail to mature to become recirculating follicular B cells. Despite this defect in maturation, Syk-deficient B cells were seen to give rise to switched as well as nonswitched splenic plasma cells. Normally only a proportion of immature B cells is recruited into the recirculating pool. Our results suggest that Syk transduces a BCR signal that is absolutely required for the positive selection of immature B cells into the recirculating B cell pool.
The initial development of mammalian B cells in the bone marrow is characterized by the sequential rearrangement of heavy and light chain immunoglobulin genes resulting in the production of large numbers of B cells throughout life (1). These newly produced (immature) B cells express antigen receptors (BCRs)1 in the form of membrane-bound IgM; they leave the marrow for the spleen and other secondary lymphoid tissues, where most of them normally die within a week (2–4). A small minority of immature B cells are induced to mature into long-lived recirculating B cells (3–5). These express both IgM and IgD and migrate between the follicles of lymphoid tissues for several weeks (5–8). Comparison of VH gene usage between immature and recirculating B cells shows that recirculating cells use only a subset of the VH genes expressed in immature cells, suggesting that there is selectivity in the recruitment of immature B cells (9–13). It has been proposed that these results may reflect positive selection of immature B cells into the recirculating pool on the basis of binding of the BCR to endogenous selection molecules (14–16). A prediction from this hypothesis is that signaling through the BCR is essential for the selection of immature B cells into the recirculating pool.
Signal transduction through the BCR is dependent on the associated nonpolymorphic receptor subunits CD79α and CD79β (Igα and Igβ; reference 17). In particular, CD79α/β contain peptide motifs termed ITAMs (immune receptor tyrosine-based activation motifs) on their intracellular domains that are crucial for signaling. Cross-linking of the BCR results in the phosphorylation of the tyrosine residues in the ITAMs of CD79α/β and their association with, among others, the protein tyrosine kinase p72Syk (Syk; references 18–21). This association is mediated by an interaction of the SH2 domains of Syk with the ITAM phosphotyrosines and leads to the activation of the Syk kinase, suggesting that Syk may be an important signal transducer of the BCR. This view was strengthened by disruption of the Syk gene in a chicken B cell lymphoma, which led to a severe deficiency in BCR signaling (22).
We and others have previously reported the generation of mice with a targeted disruption of the Syk gene (Syktm1Tyb), which is generally a lethal defect when homozygous (Syk −/−; references 23, 24). By reconstituting irradiated mice with Syk −/− fetal liver cells we observed reduced numbers of cells making the transition from pro-B to pre-B cell, suggestive of a role for Syk in pre-BCR signaling (23). In addition, despite the presence of a small number of immature Syk −/− B cells in the bone marrow, there were no detectable B cells in the spleen and lymph nodes (23). In this report we extend this analysis and demonstrate that although marrow-derived immature Syk −/− B cells migrate to the red pulp and outer T zone of the spleen, they fail to enter B cell follicles. Furthermore, we show that they are completely blocked in their ability to mature into recirculating follicular B cells and that this defect is most likely due to a role for Syk within the B cells themselves. These results strongly suggest that a Syk-mediated BCR signal is required for the transition of immature B cells into the recirculating B cell pool and this may reflect a step at which positive selection of B cells occurs.
Materials and Methods
Generation of Fetal Liver Chimeras.
Fetal liver cells were harvested from embryos at day 16.5 of gestation generated by intercrossing mice that were Syk +/−/3-83μδ/H-2d/Ly9.2/IgHb and Syk +/−/Eμ-bcl-2-36/H-2d/Ly9.2/IgHb. Genotypes of parents and fetuses were determined by Southern blotting for Syk, 3-83, Eμ-bcl-2, H-2, and IgM and by flow cytometry for Ly9. Radiation chimeras were generated by injecting 1.5 × 106 fetal liver cells intravenously into female BALB/c (H-2d/Ly9.1/IgHa) mice that had received two doses of 500 rad from a 60Co source 3 h apart. The recipient mice were given neomycin sulphate (0.16%) in their drinking water for 4 wk after irradiation and were analyzed 2–4 mo after reconstitution.
Immunohistochemistry.
Cryostat sections (5 μm) of frozen splenic tissue were prepared and stained as previously described (25). In the live adult Syk −/− mice, B and T cells were identified using either rat anti-B220 (PharMingen, San Diego, CA) or rat anti-CD3 (Serotec Ltd., Kidlington, Oxford, UK), followed by biotinylated rabbit anti–rat Ig (Dako Ltd., High Wycombe, UK). Donor B cells were identified in tissue sections from radiation chimeras using biotinylated mouse anti-IgMb (PharMingen) or biotinylated anti–3-83 (54.1; gift from D. Nemazee, National Jewish Center for Immunology, Denver, CO). In both cases biotinylated antibodies were revealed using streptavidin-ABComplex-alkaline phosphatase conjugate (Dako Ltd.) and napthol-ASMX-phosphate and Fast Blue BB (Sigma Chemical Co., Poole, Dorset, UK) to give a blue precipitate at the site of antibody binding. Sheep anti–mouse IgD or anti–mouse IgM (The Binding Site, Birmingham, UK) were detected with peroxidase-labeled donkey anti–sheep Ig (The Binding Site) using hydrogen peroxide and diaminobenzidene to give a gold-colored precipitate at the site of antibody binding. Both secondary antibodies were absorbed with 10% normal mouse serum before use to remove cross-reactivity. The thymidine analogue 5′-bromo-2-deoxyuridine (BrdU; Sigma Chemical Co.) in saline was administered as 2 mg intraperitoneally 2 h before death, to label cells in the S phase of the cell cycle. The incorporated BrdU was identified by treating stained slides for 20 min with 1 M HCl at 60°C to expose the incorporated BrdU and destroy the activity of previously bound immunohistological reagents. Anti-BrdU (Dako Ltd.) was then added to the slides after they had been washed. This was detected with biotinylated goat anti–mouse Ig (Dako Ltd.) followed by Streptavidin-ABComplex-alkaline phosphatase. Enzyme activity was demonstrated using naphtol-ASMX-phosphate-free acid (Sigma Chemical Co.) with Fast Red TR salt (Sigma Chemical Co.).
Staining of Cells with Fluorescent Antibodies.
Bone marrow and spleen cells were stained with monoclonal antibodies as previously described (23). Fluorescence was analyzed on a FACStar® or FACSVantage® flow cytometer with Cellquest software (Becton Dickinson, Mountain View, CA). Dead cells were excluded on the basis of low forward light scatter and only live cells falling within the lymphocyte scatter gate are shown. Anti-B220-allophycocyanin, anti-IgMa-PE, anti-IgDa-FITC, and anti-CD43-biotin were purchased from PharMingen, anti-3-83 idiotype (54.1; gift of D. Nemazee) and anti-IgD (1.19) were purified from tissue culture supernatants and conjugated to FITC or biotin by standard methods. Biotinylated antibodies were revealed with streptavidin-PE (Biogenesis, Poole, UK) or streptavidin-RED613 (GIBCO-BRL, Gaithersburg, MD).
Results
Syk−/− Immature B Cells Accumulate in the Outer T Zone and Do Not Mature into Recirculating Follicular B Cells.
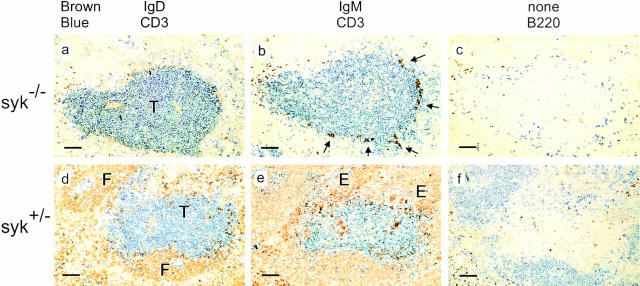
We had earlier shown, using irradiated mice reconstituted with Syk −/− fetal liver, that there was a partial block at the pro-B to pre-B cell transition, suggestive of a role for Syk in pre-BCR signaling (23). Furthermore, we noted that despite the presence of a small number of immature Syk −/− B cells in the marrow, no detectable recirculating B cells accumulated in the peripheral lymphoid organs, at least as judged by flow cytometric analysis (23). To investigate further the fate of Syk −/− immature B cells, we turned to the more sensitive technique of immunohistology to look for the presence of Syk −/− B lineage cells in the spleen, since this is at least one of the sites to which immature B cells migrate upon leaving the marrow (3, 4). Initially, analysis was performed on spleens of two Syk −/− mice that, exceptionally, had survived to adulthood. Strikingly, this showed small numbers of immature (IgM+IgD−) B cells lined up along the edge of the T zone, but a total absence of both follicles and recirculating (IgM+IgD+) B cells (Fig. 1). Thus Syk −/− immature B cells migrate from the marrow to the spleen but do not mature into recirculating follicular B cells.
Figure 1.
Immunohistochemistry of splenic B cells in an adult Syk −/− mouse. Sections were stained with antibodies to IgD or IgM; these were revealed with peroxidase (brown) or with antibodies to CD3 or B220 revealed with alkaline phosphatase (blue) as indicated. Sections labeled “none” were stained only with the peroxidase-coupled secondary antibody to reveal endogenous peroxidase activity. (a–c) Serial sections from the spleen of an exceptional Syk −/− mouse that survived to adulthood; small numbers of B220+ cells are found in the outer T zone (T) and a proportion of these are IgM+ (arrows in b), but none are IgD+ nor have they entered the follicles. The B220+IgM− cells in this spleen were shown to be CD3− (data not shown). Note in c the brown staining of eosinophils which contain endogenous peroxidase activity; the same eosinophils are seen staining brown in a and b. (d–f ) Serial sections from a heterozygous littermate showing the development of normal follicles (F), which contain IgM+IgD+ recirculating B cells; IgM+IgD− plasma cells are present in extra-follicular foci (E) in this spleen. Scale: black bar represents 100 μm. Analysis of a second Syk −/− mouse gave similar results.
Failure of Follicular Entry by Syk−/− B Cells Is a Cell-autonomous Defect.
It was unclear from our preliminary study if this developmental block is due to a malfunction within B cells, or some other cell type required for the formation of follicles, or both. To address this issue, chimeras were constructed by transferring Syk −/− fetal liver into irradiated wild-type mice which differed in their immunoglobulin heavy chain allotype; this allotype difference allowed B cells of host and donor origin to be distinguished. Flow cytometric analysis of these mice confirmed that they contained Syk −/− immature B cells in the marrow, but none were detectable in peripheral lymphoid tissue (data not shown). The spleens from 20 of these chimeras were examined by immunohistology, and all were found to have small numbers of donor Syk −/− immature B cells (IgMb+IgD−) in the T zones and red pulp, but no Syk-deficient B cells in the follicles (Fig. 2 a); the B cells in these follicles were exclusively host (IgMa+) cells (Fig. 2 a and data not shown). Furthermore, analysis of lymph nodes from the same chimeras failed to find any donor Syk-deficient B cells, although again host IgMa+IgD+ cells were found in the follicles (Fig. 2 b). In contrast, chimeras made with Syk +/+ or Syk +/− fetal liver had normal follicles filled with donor IgMb+IgD+ B cells (data not shown). Effective reconstitution of the irradiated mice with Syk −/− donor hematopoietic cells could be clearly documented in the T lineage (data not shown); however, there was always a small, although variable, level of residual host lymphopoiesis. The presence of significant numbers of host (Syk +/+) follicular IgMa+IgD+ B cells, particularly in lymph nodes, demonstrates that wild-type B cells can mature in these Syk −/− chimeras; consequently the maturational arrest of the Syk −/− immature B cells appears to be due to the absence of Syk within the B cells themselves.
Figure 2.
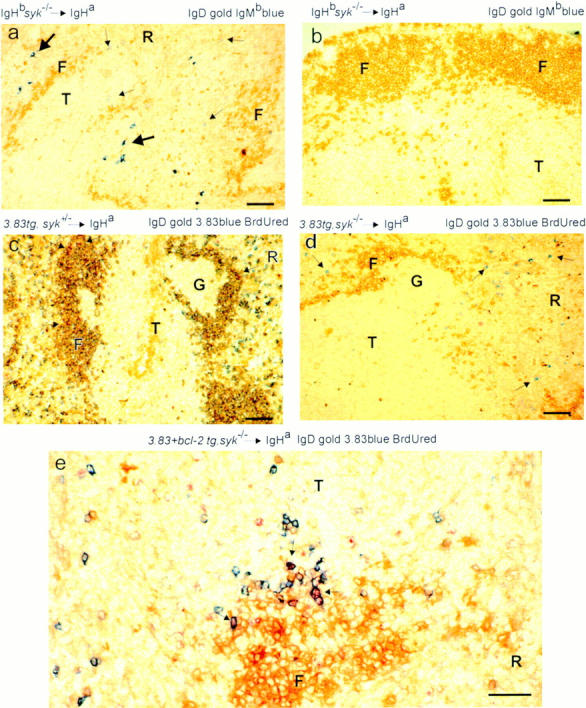
Immature Syk −/− B cells migrate to the spleen but do not mature into follicular B cells. Immunohistochemistry of peripheral B cells in radiation chimeras whose nature is shown to the left above each micrograph; i.e., whether the reconstituting livers were Syk +/− or Syk −/− and if they contained the 3-83 or Bcl-2 transgenes (29, 33). All fetal liver donors were of the IgHb immunoglobulin allotype; all hosts were IgHa. 20 Syk −/− nontransgenic chimeras and at least three chimeras from each group of transgenic donors were analyzed. The specificity of the antibodies used for staining the sections is shown to the right above each micrograph; the molecule stained gold by immunoperoxidase is shown first, the one stained blue by immunoalkaline phosphatase is shown second and, where used, BrdU staining is in red. Cells double positive for gold and blue appear dark brown. Scale: black bar represents 100 μm. (a) Donor IgMb+ Syk −/− immature B cells (small arrows) are present in the T zone (T) and red pulp (R) of the spleen, but not in the partially filled follicles (F), which contain mature IgD+ (gold) cells of host origin. Donor IgMb+ Syk −/− plasma cells in the red pulp are indicated by large arrows. (b) Section through a lymph node from a similar chimera to that in a. Well-developed primary follicles are present but no donor IgMb+ Syk −/− B cells were detected in this or in any lymph node from similar chimeras. (c) Spleen section from a Syk +/−/3-83 chimera that, like the ones shown in d and e, had been given BrdU for 12 h, 8 d before the spleen was taken. The follicular mantles are filled with mature 3-83+IgD+ (dark brown) donor B cells; occasional cells (arrowed) have a red-stained nucleus, indicating that they had taken up BrdU during the 12-h pulse 8 d previously. Some 3-83+IgD− (blue) donor B cells can be seen in the outer T zone and red pulp; some of these may be immature B cells, but none are BrdU+. The germinal center (G) is filled with donor-derived 3-83−IgHb+ B cells (data not shown) that arise from occasional rearrangement of endogenous Ig genes in the 3-83 transgenics. (d) Spleen section from a Syk −/−/3-83 chimera. Small numbers of 3-83+IgD− donor B cells (blue and arrowed) can be seen in the outer T zone and red pulp; none of these are BrdU+. As in a the follicles exclusively contain host IgD+ cells (gold) but no donor B cells. (e) Spleen section from a Syk −/−/3-83/Bcl-2Tg chimera. More donor B cells are present in the T zone and red pulp than in the chimera in d not carrying Bcl-2Tg. Nevertheless, there were no donor B cells in follicles. Some of the donor 3-83+ B cells had BrdU in their nuclei (arrowed and red). These must have been in cell cycle as pre-B cells 8 d previously, indicating an extended life span for these immature B cells.
In wild-type rodents, when the recirculating pool of B cells is selectively depleted so that B lymphopoiesis continues unimpaired, the proportion of immature B cells recruited to become recirculating cells increases dramatically until the pool is filled. This replenishment takes 4–5 d. This study shows that most immature B cells have the potential to become recirculating cells and are not deleted in some central selection process within the marrow. This indicates that under normal circumstances there is competition between immature B cells for limited numbers of slots in the recirculating pool (2). Furthermore, the slots in the recirculating pool become available because of competition between recirculating and immature B cells. This is shown in experiments in which the recirculating pool of SCID mice is partially reconstituted with recirculating cells (26). In these chimeras, which produce no immature B cells, the number of recirculating cells remains constant; their life-span is indefinite, rather than the few weeks for which they persist in normal mice (5, 8), and they are not able to replicate to fully fill the partially reconstituted recirculating pool (26). These data indicate that it is the presence of immature cells that results in the physiological loss of recirculating cells. It is notable that Syk −/− immature B cells fail to mature in chimeras where host B cell production was insufficient to fill the follicles completely. In these mice the mutant cells are not being competitively excluded from follicles; rather they have an intrinsic inability to mature.
Although Syk −/− B cells do not enter the recirculating B cell pool, plasma cells of donor origin were present in the red pulp of many chimeras (Fig. 2 a); some of these had switched to IgG production (data not shown). This indicates that Syk −/− immature B cells can be induced to become plasma cells, apparently without first becoming recirculating cells, and suggests that some signals can be delivered through the BCR in the absence of Syk. Isolated cells with an immature B cell phenotype have been induced to become plasma cells in free cultures or splenic fragment cultures, but the possibility that the responding cells matured to recirculating cells in culture was not excluded (27, 28).
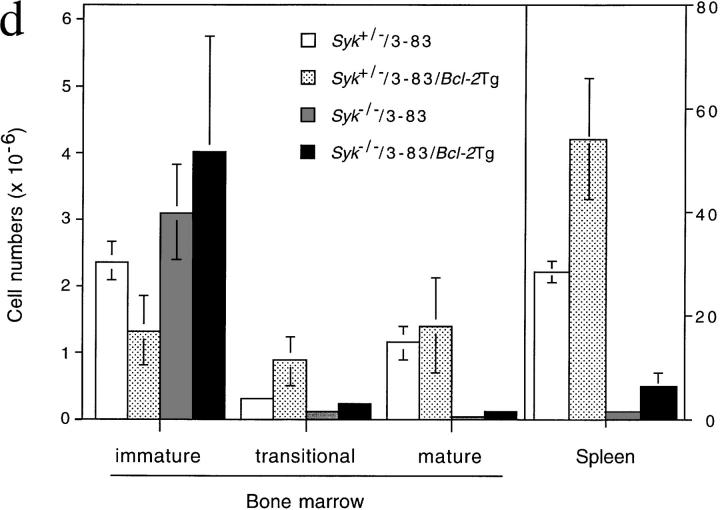
Expression of a Rearranged Transgenic BCR Increases the Number of Immature Syk− /− B Cells, but Does Not Overcome the Maturational Block.
It is possible that the block in maturation of Syk −/− immature B cells was not complete, but simply inefficient, and the failure to observe any Syk −/− recirculating cells was due to the reduced number of immature B cells produced in the marrow of the Syk −/− chimeras (23). To attempt to overcome this, we made further chimeras with Syk −/− and control fetal livers containing the 3-83μδ (3-83) BCR transgene; this encodes IgM and IgD specific for H-2Kk and H-2Kb, but not H-2Kd; the background of both the donor and host in all these chimeras was H-2d (29). Although 3-83–expressing B cells were abundant in the bone marrow of the Syk-deficient chimeras, all these cells expressed IgM with little or no IgD and thus do not represent recirculating cells, which are typically IgM+IgDhi (Fig. 3, a and d). In Syk −/− chimeras made without a BCR transgene, most B lineage cells in the marrow are pro-B cells, and thus it was possible that the 3-83–expressing cells in the Syk −/−/3-83 chimeras would still be phenotypically pro-B cells, despite the expression of a rearranged BCR (23). However, flow cytometric analysis demonstrated that most of the 3-83–expressing cells in these chimeras do not express CD43, a marker of pro-B cells that is turned off as the cells mature into pre-B/immature B cells (Fig. 3 b; reference 30). Thus, the Syk −/−/3-83 chimeras contained large numbers of Syk −/− cells with an immature B cell phenotype. The mechanism whereby the expression of the 3-83 BCR partially rescues the pro-B→ pre-B cell developmental block is unclear. Despite the abundance of these immature cells in the bone marrow, very few Syk-deficient cells could be detected in the spleen and lymph nodes of the chimeras and again these were immature in phenotype (IgM+IgD−/low; Fig. 3, c and d, and data not shown). Furthermore, immunohistology demonstrated that in contrast to wild-type 3-83+ B cells, the few Syk-deficient cells were again confined to the outer T zone and red pulp of the spleen (Fig. 2, c and d).
Figure 3.
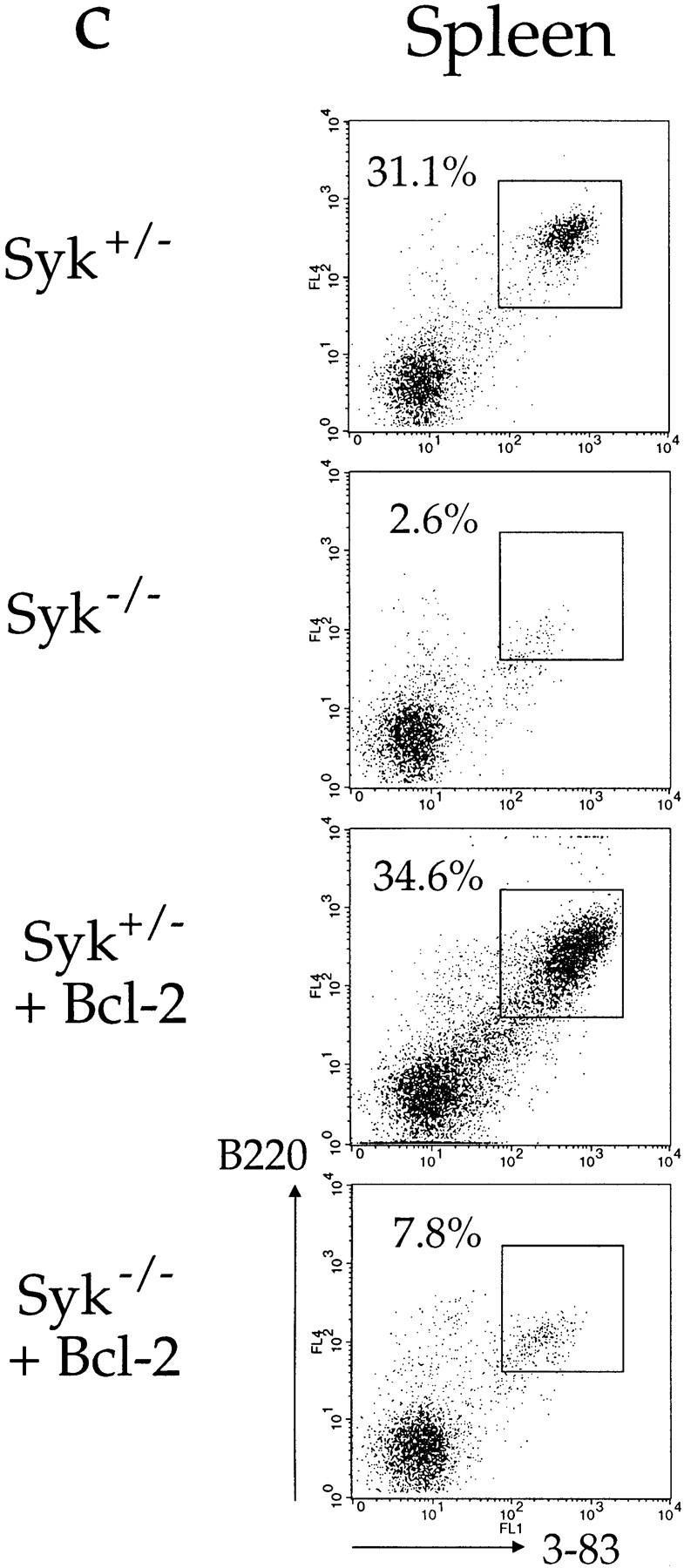
Positive selection of immature B cells. Flow cytometric analysis of cells from radiation chimeras reconstituted with liver cells from Syk +/−/3-83 (Syk+/−), Syk −/−/3-83 (Syk−/−), and Syk −/−/3-83/Bcl-2Tg (Syk−/− + Bcl-2) fetuses or from an adult Syk +/−/3-83/Bcl-2Tg (Syk+/− + Bcl-2) mouse. In separate experiments chimeras made with Syk +/−/3-83/Bcl-2Tg fetal liver gave essentially the same results as presented in this figure (data not shown). Chimeras made with Syk +/+/3-83 fetal liver gave results indistinguishable from Syk +/−/3-83 chimeras (data not shown). (a) The first column from the left shows the expression of B220 and the transgenic BCR (3-83) on bone marrow cells; B220+3-83+ cells are boxed and their fraction as a percentage of all lymphoid cells is shown. The second column shows the expression of IgMa and IgDa by B220+3-83+ cells with gating to show immature (IgM+IgD−), transitional (IgM+IgDlow), and mature (IgM+IgDhigh) B cells; the percentage for each gated population is expressed as a fraction of all lymphocytes. (b) The first column shows the gating on immature (B220low3-83low) cells that was used to generate the histograms of CD43 expression on B220+3-83+ bone marrow cells shown in the second column; percentages represent the fraction of gated immature B220low3-83low cells that are CD43−. (c) Expression of B220 and 3-83 in the spleen; B220+3-83+ cells are boxed and their fraction as a percentage of all lymphoid cells is shown. (d) Mean numbers ± SEM of B220+3-83+ cells in the bone marrow (per femur) and spleen are shown. In the marrow, these have been subdivided into immature (IgM+IgD−), transitional (IgM+IgDlow) and mature (IgM+IgDhigh) B cells using the gating shown in a. Data in (a–c) are representative of at least six mice of each genotype. Absolute cell numbers in the marrow were determined using eight Syk +/−/3-83 mice, six Syk −/−/3-83 mice, three Syk +/−/3-83/Bcl-2Tg mice, and four Syk −/−/3-83/Bcl-2Tg mice; data for the spleen cell numbers was determined using four Syk +/−/3-83 mice, four Syk −/−/3-83 mice, three Syk +/−/3-83/Bcl-2Tg mice, and two Syk −/−/3-83/Bcl-2Tg mice.
Constitutive Expression of Bcl-2 Is Not Sufficient to Overcome the Maturational Block in Syk− /− Immature B Cells.
The transition of immature to recirculating B cells is accompanied by an increase in expression of the anti-apoptotic gene Bcl-2 (31, 32). Thus it was possible that Syk-deficient immature B cells failed to differentiate into recirculating B cells because, unlike control cells, they had not expressed Bcl-2 and hence died prematurely. To address this, we generated radiation chimeras using Syk-deficient, 3-83–expressing fetal livers containing the Eμ-Bcl-2-36 (Bcl-2Tg) transgene, which constitutively expresses Bcl-2 in the B lineage (33). Expression of Bcl-2 resulted in a greater accumulation of immature Syk-deficient B cells both in the marrow and spleen, and in the generation of some IgM+ IgDlow cells which probably correspond to transitional B cells as described by Carsetti et al. (34; Fig. 3, a, c, and d). However, we were unable to detect any Syk −/− recirculating IgM+IgDhigh B cells (Fig. 3, a and d). Immunohistology confirmed that the Bcl-2 transgene had caused the accumulation of many more Syk-deficient B cells in the spleens of Syk −/−/3-83/Bcl-2Tg chimeras, and once again they were exclusively located in the outer T zone and red pulp, but not in follicles (Fig. 2 e). Thus, the expression of Bcl-2 allows Syk-deficient immature B cells to proceed a little further in their differentiation into transitional cells and to accumulate in larger numbers in the spleen, but is not sufficient to rescue the developmental block in their maturation into recirculating follicular B cells.
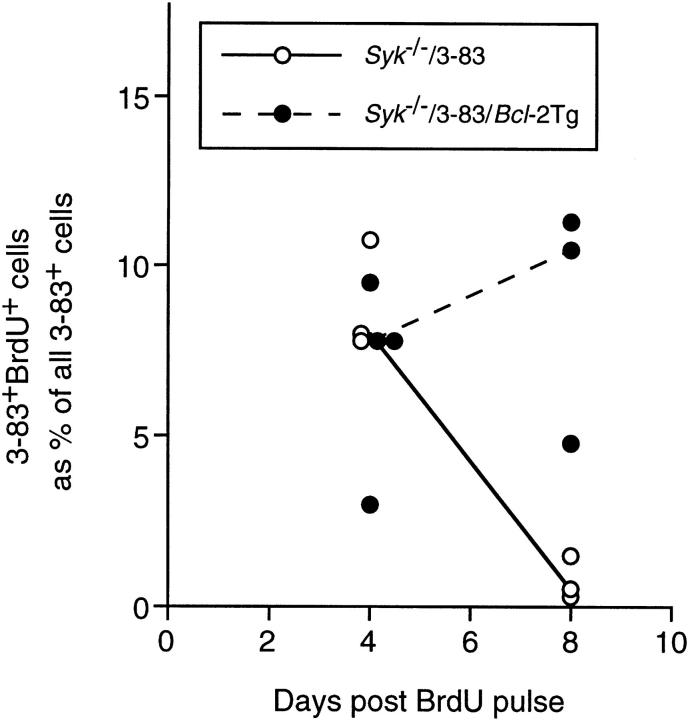
To confirm that the Bcl-2Tg had indeed increased the life-span of Syk-deficient immature B cells, Syk −/−/3-83 chimeras with and without Bcl-2Tg were given a 12-h pulse with the thymidine analogue BrdU to identify cells proliferating during the pulse that had subsequently gone out of cell cycle. Pre-B cells exit the cell cycle ∼24 h before first expressing surface immunoglobulin (35) and immature and recirculating B cells do not subsequently proliferate unless they are recruited into an antibody response (4). In wild-type rodents, pulse-labeled immature B cells enter the spleen on the second and third day after a 12-h BrdU pulse and are lost within 7 d (4, 36). In this experiment, spleens of the chimeric mice were examined 4 and 8 d after the BrdU pulse. At 4 d, BrdU-labeled 3-83+ B cells were present in roughly equal proportions in mice with and without Bcl-2Tg (Fig. 4). By 8 d, as expected, BrdU-labeled B cells were largely absent from the chimeras without the Bcl-2Tg (Fig. 2 d) but were present in an undiminished proportion of the B cells carrying the Bcl-2Tg (Fig. 2 e). Thus, in the absence of Bcl-2Tg, Syk −/− immature cells turn over with normal kinetics. However, in chimeras with Bcl-2Tg, the life span of Syk-deficient immature B cells was significantly extended.
Figure 4.
Life span of Syk −/− immature B cells is increased by expression of Bcl-2. Chimeric mice reconstituted with Syk −/−/3-83 fetal livers with and without Bcl-2Tg were given a 12-h pulse of BrdU. Graph shows the percentage of splenic 3-83+ B cells that were also labeled with BrdU either 4 or 8 d after the BrdU pulse. At each time point, spleens from three or four mice were analyzed by quantitative immunohistology as previously described (4). The lines are drawn between median values. The number of cells enumerated was 168–263 cells/spleen in the Syk −/−/ 3-83 chimeras and 673–994 cells/spleen in the Syk −/−/3-83/Bcl-2Tg chimeras.
Discussion
The finding that Syk deficiency causes a total block in recruitment of immature B cells into the recirculating B cell pool provides insight into the signals involved in this maturation step. A similar, although incomplete, block in maturation was described in mice carrying a CD79α (Igα) gene with a mutated intracellular signalling domain (37). Since CD79α is a signaling component of the BCR that may act by directly binding and activating Syk (18–21), we propose that the maturation of immature B cells into recirculating follicular B cells requires a BCR signal, and that Syk is an essential transducer of at least part of this signal.
What is the purpose of such a signal? The restricted VH usage of recirculating B cells in contrast to immature B cells has been used to suggest that a subset of immature cells is positively selected into the recirculating pool on the basis of BCR binding to endogenous selection molecules, although it cannot be excluded that this effect is due to selective deletion (9–16). In any case, since cells of the recirculating B cell pool typically express surface immunoglobulin, it may be that selection ensures that only cells with a functional BCR are recruited into the recirculating B cell compartment; such a process would necessarily require a BCR signal.
Mutations in a number of other signaling molecules have been reported to affect the transition of immature B cells into recirculating cells. Mice with a mutation in Btk, a tyrosine kinase involved in BCR signaling, contain some recirculating B cells, but have an accumulation of transitional cells (38, 39). Furthermore, in the presence of normal competitor B cells, Btk-mutated B cells are completely blocked in their development at the immature B cell stage (40). Studies of mice deficient in the tyrosine phosphatase CD45 demonstrated that this too is involved in BCR signaling (41–43). These mice have plenty of recirculating B cells, but these cells are short-lived and have abnormally high IgM and IgD expression; engagement of surface immunoglobulin in these mice partially rescues this phenotype (44). These results are consistent with a requirement for BCR-mediated signals in the development of immature B cells into long-lived recirculating B cells.
The failure of Syk −/− B cells to be recruited into the recirculating B cell pool is superficially reminiscent of the fate of autoreactive B cells that develop in the presence of soluble autoantigen (45). These B cells accumulate in the outer T zone of the spleen and do not compete with nonautoreactive immature B cells for signals to enter the recirculating B cell pool. They differ from the Syk −/− B cells described here, and indeed from normal immature B cells (3, 4), in that they do not migrate to the red pulp as well as the T zone. It seems that these antigen-engaged B cells in the outer T zone are programmed to interact with cells that can deliver accessory signals that induce B cells to migrate to and proliferate in follicles or extrafollicular foci (46, 47). Such cells concomitantly become inefficient in competing for signals that induce maturation into recirculating B cells (45). In contrast, the Syk −/− immature cells described here are not antigen-engaged, migrate to both the red pulp and T zones of the spleen, and fail to enter follicles even in the absence of significant competition. It is more likely that the follicular exclusion reflects the lack of a Syk-mediated signal required for maturation into a recirculating B cell. In this regard, it is interesting to note that B cells lacking the putative chemokine receptor Burkitt's lymphoma receptor 1 (BLR1) are also unable to enter splenic follicles, but remain trapped in the outer T zone (48). Since the expression of BLR1 is increased upon maturation from immature to recirculating B cells, it is possible that Syk −/− B cells fail to enter splenic follicles because they have not induced the expression of BLR1. However this cannot explain the failure of Syk-deficient B cells to enter lymph node follicles, since apparently this occurs normally in the BLR1 mutants.
In conclusion, we have shown that the tyrosine kinase Syk is absolutely required for the maturation of immature B cells into the recirculating follicular B cell pool. Furthermore, although Syk-deficient immature B cells reach the red pulp and outer T zone of the spleen, they fail to enter follicles. The T zone or red pulp may be the site where immature B cells normally receive a BCR signal that drives their positive selection into the recirculating pool.
Acknowledgments
We thank C. Atkins for expert assistance in flow cytometric analysis; D. Nemazee for 3-83μδ transgenic mice and the hybridoma 54.1; S. Cory and M. Merkenschlager for permission to use and provision of the Eμ-bcl-2-36 transgenic mice, respectively. We thank E. Schweighoffer for critical reading of the manuscript.
Footnotes
The work in London was supported by the Medical Research Council and the work in Birmingham was supported by a Medical Research Council program grant.
Abbreviations used in this paper: BCR, B cell antigen receptor; BLR1, Burkitt's lymphoma receptor 1; BrdU, 5′-bromo-2-deoxyuridine; ITAM, Immune receptor tyrosine-based activation motif.
References
- 1.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 2.Bazin H, Platteau B, MacLennan ICM, Johnson GD. B-cell production and differentiation in adult rats. Immunology. 1985;54:79–88. [PMC free article] [PubMed] [Google Scholar]
- 3.Lortan JE, Roobottom CA, Oldfield S, MacLennan ICM. Newly produced virgin B-cells migrate to secondary lymphoid organs but their capacity to enter follicles is restricted. Eur J Immunol. 1987;17:1311–1316. doi: 10.1002/eji.1830170914. [DOI] [PubMed] [Google Scholar]
- 4.Chan EY-T, MacLennan ICM. Only a small proportion of splenic B cells in adults are short-lived virgin cells. Eur J Immunol. 1993;23:357–363. doi: 10.1002/eji.1830230209. [DOI] [PubMed] [Google Scholar]
- 5.Gray D. Population kinetics of rat peripheral B cells. J Exp Med. 1988;167:805–816. doi: 10.1084/jem.167.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard JC, Hunt SV, Gowans JL. Identification of marrow-derived and thymus-derived small lymphocytes in the lymphoid tissue and thoracic duct lymph of normal rats. J Exp Med. 1972;135:200–219. doi: 10.1084/jem.135.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieuwenhuis P, Ford WL. Comparative migration of B- and T-lymphocytes in the rat spleen and lymph nodes. Cell Immunol. 1976;23:254–267. doi: 10.1016/0008-8749(76)90191-x. [DOI] [PubMed] [Google Scholar]
- 8.Förster I, Rajewsky K. The bulk of the peripheral B-cell pool in mice is stable and not rapidly renewed from the bone marrow. Proc Natl Acad Sci USA. 1990;87:4781–4784. doi: 10.1073/pnas.87.12.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malynn BA, Yancopoulos GD, Barth JE, Bona CA, Alt FW. Biased expression of JH-proximal VHgenes occurs in the newly generated repertoire of neonatal and adult mice. J Exp Med. 1990;171:843–859. doi: 10.1084/jem.171.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freitas AA, Andrade L, Lembezat MP, Coutinho A. Selection of VHgene repertoires: differentiating B cells of adult bone marrow mimic fetal development. Int Immunol. 1990;2:15–23. doi: 10.1093/intimm/2.1.15. [DOI] [PubMed] [Google Scholar]
- 11.Gu H, Tarlinton D, Muller W, Rajewsky K, Förster I. Most peripheral B cells in mice are ligand selected. J Exp Med. 1991;173:1357–1371. doi: 10.1084/jem.173.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandey A, Tjoelker LW, Thompson CB. Restricted immunoglobulin junctional diversity in neonatal B cells results from developmental selection rather than homology-based V(D)J joining. J Exp Med. 1993;177:329–337. doi: 10.1084/jem.177.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huetz F, Carlsson L, Tornberg UC, Holmberg D. V-region directed selection in differentiating B lymphocytes. EMBO (Eur Mol Biol Organ) J. 1993;12:1819–1826. doi: 10.1002/j.1460-2075.1993.tb05830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cyster JG. Signaling thresholds and interclonal competition in preimmune B-cell selection. Immunol Rev. 1997;156:87–101. doi: 10.1111/j.1600-065x.1997.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Martin F, Forbush KA, Perlmutter RM, Kearney JF. Evidence for selection of a population of multi-reactive B cells into the splenic marginal zone. Int Immunol. 1997;9:27–41. doi: 10.1093/intimm/9.1.27. [DOI] [PubMed] [Google Scholar]
- 16.Hachemi-Rachedi S, Cumano A, Drapier A-M, Cazenave P-A, Sanchez P. Does positive selection determine the B cell repertoire? . Eur J Immunol. 1997;27:1069–1074. doi: 10.1002/eji.1830270505. [DOI] [PubMed] [Google Scholar]
- 17.Kurosaki T. Molecular mechanisms in B cell antigen receptor signalling. Curr Opin Immunol. 1997;9:309–318. doi: 10.1016/s0952-7915(97)80075-1. [DOI] [PubMed] [Google Scholar]
- 18.Law DA, Chan VWF, Datta SK, DeFranco AL. B-cell antigen receptor motifs have redundant signalling capabilities and bind the tyrosine kinases PTK72, Lyn and Fyn. Curr Biol. 1993;3:645–657. doi: 10.1016/0960-9822(93)90062-s. [DOI] [PubMed] [Google Scholar]
- 19.Yamada T, Taniguchi T, Yang C, Yasue S, Saito H, Yamamura H. Association with B-cell-antigen receptor with protein-tyrosine kinase p72syk and activation by engagement of membrane IgM. Eur J Biochem. 1993;213:455–459. doi: 10.1111/j.1432-1033.1993.tb17781.x. [DOI] [PubMed] [Google Scholar]
- 20.Saouaf SJ, Mahajan S, Rowley RB, Kut SA, Fargnoli J, Burkhardt AL, Tsukada S, Witte ON, Bolen JB. Temporal differences in the activation of three classes of non-transmembrane protein tyrosine kinases following B-cell antigen receptor surface engagement. Proc Natl Acad Sci USA. 1994;91:9524–9528. doi: 10.1073/pnas.91.20.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burg DL, Furlong MT, Harrison ML, Geahlen RL. Interactions of Lyn with the antigen receptor during B cell activation. J Biol Chem. 1994;269:28136–28142. [PubMed] [Google Scholar]
- 22.Takata M, Sabe H, Hata A, Inazu T, Homma Y, Nukada T, Yamamura H, Kurosaki T. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+mobilization through distinct pathways. EMBO (Eur Mol Biol Organ) J. 1994;13:1341–1349. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz VLJ. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 24.Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378:303–306. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 25.Gulbranson-Judge A, MacLennan I. Sequential antigen-specific growth of T cells in the T zones and follicles in response to pigeon cytochrome c. Eur J Immunol. 1996;26:1830–1837. doi: 10.1002/eji.1830260825. [DOI] [PubMed] [Google Scholar]
- 26.Sprent J, Schaefer M, Hurd M, Surh CD, Ron Y. Mature murine B and T cells transferred into SCID mice can survive indefinitely and many maintain a virgin phenotype. J Exp Med. 1991;174:717–728. doi: 10.1084/jem.174.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allman DM, Ferguson SE, Cancro MP. Peripheral B cell maturation. I. Immature peripheral B cells in adults are heat-stable antigenhiand exhibit unique signaling characteristics. J Immunol. 1992;149:2533–2540. [PubMed] [Google Scholar]
- 28.Linton P-JL, Decker DJ, Klinman NR. Primary antibody-forming cells and secondary B cells are generated from separate precursor cell subpopulations. Cell. 1989;59:1049–1059. doi: 10.1016/0092-8674(89)90761-7. [DOI] [PubMed] [Google Scholar]
- 29.Russell DM, Dembic Z, Morahan G, Miller JFAP, Burki K, Nemazee D. Peripheral deletion of self-reactive B cells. Nature. 1991;354:308–311. doi: 10.1038/354308a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre–pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y-S, Hayakama K, Hardy RR. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med. 1993;178:951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merino R, Ding L, Veis DJ, Korsmeyer SJ, Nunez G. Developmental regulation of the Bcl-2 protein and susceptibility to cell death in B lymphocytes. EMBO (Eur Mol Biol Organ) J. 1994;13:683–691. doi: 10.1002/j.1460-2075.1994.tb06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strasser A, Harris AW, Vaux DL, Webb E, Bath ML, Adams JM, Cory S. Abnormalities of the immune system induced by dysregulated bcl-2expression in transgenic mice. Curr Top Microbiol Immunol. 1990;166:175–181. doi: 10.1007/978-3-642-75889-8_22. [DOI] [PubMed] [Google Scholar]
- 34.Carsetti R, Köhler G, Lamers MC. Transitional B cells are the target of negative selection in the B cell compartment. J Exp Med. 1995;181:2129–2140. doi: 10.1084/jem.181.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osmond DG, Nossal GJV. Differentiation of lymphocytes in mouse bone marrow. II. Kinetics of maturation and renewal of antiglobulin-binding cells studied by double labeling. Cell Immunol. 1974;13:132–145. doi: 10.1016/0008-8749(74)90233-0. [DOI] [PubMed] [Google Scholar]
- 36.Allman DM, Ferguson SE, Lentz VM, Cancro MP. Peripheral B cell maturation. II. Heat-stable antigenhisplenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells. J Immunol. 1993;151:4431–4444. [PubMed] [Google Scholar]
- 37.Torres RM, Flaswinkel H, Reth M, Rajewsky K. Aberrant B cell development and immune response in mice with a compromised BCR complex. Science. 1996;272:1804–1808. doi: 10.1126/science.272.5269.1804. [DOI] [PubMed] [Google Scholar]
- 38.Hardy RR, Hayakawa DR, Parks DR, Herzenberg LA. Demonstration of B-cell maturation in X-linked immunodeficient mice by simultaneous three-colour immunofluorescence. Nature. 1983;306:270–272. doi: 10.1038/306270a0. [DOI] [PubMed] [Google Scholar]
- 39.Klaus, G.G.B., M. Holman, C. Johnson-Leger, C. Elgueta-Karstegl, and C. Atkins. 1997. A re-evaluation of the effects of X-linked immunodeficiency (xid) mutation on B cells differentiation and function in the mouse. Eur. J. Immunol. In press. [DOI] [PubMed]
- 40.Forrester LM, Ansell JD, Micklem HS. Development of B lymphocytes in mice heterozygous for the X-linked immunodeficiency (xid) mutation. J Exp Med. 1987;165:949–958. doi: 10.1084/jem.165.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kishihara K, Penninger J, Wallace VA, Kundig TM, Kawai K, Wakeham A, Timms E, Pfeffer K, Ohashi PS, Thomas ML, et al. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase–deficient mice. Cell. 1993;74:143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- 42.Benatar T, Carsetti R, Furlonger C, Kamalia N, Mak T, Paige CJ. Immunoglobulin-mediated signal transduction in B cells from CD45-deficient mice. J Exp Med. 1996;183:329–334. doi: 10.1084/jem.183.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Byth KF, Conroy LA, Howlett S, Smith AJH, May J, Alexander DR, Holmes N. CD45-null transgenic mice reveal a positive regulatory role for CD45 in early thymocyte development, in the selection of CD4+CD8+thymocytes, and in B cell maturation. J Exp Med. 1996;183:1707–1718. doi: 10.1084/jem.183.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cyster JG, Healy JI, Kishihara K, Mak TW, Thomas ML, Goodnow CC. Regulation of B-lymphocyte negative and positive selection by tyrosine phosphatase CD45. Nature. 1996;381:325–328. doi: 10.1038/381325a0. [DOI] [PubMed] [Google Scholar]
- 45.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 46.Toellner KM, Gulbranson-Judge A, Taylor DR, Sze DM, MacLennan ICM. Immunoglobulin switch transcript production in vivo related to the site and time of antigen-specific B cell activation. J Exp Med. 1996;183:2303–2312. doi: 10.1084/jem.183.5.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cook MC, Basten A, Fazekas de St B, Groth Outer periarteriolar lymphoid sheath arrest and subsequent differentiation of both naive and tolerant immunoglobulin transgenic B cells is determined by B cell receptor occupancy. J Exp Med. 1997;186:631–643. doi: 10.1084/jem.186.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Förster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]