Abstract
The nature (Th1 versus Th2) and dynamics of the autoimmune response during the development of insulin-dependent diabetes mellitus (IDDM) and after immunotherapy are unclear. Here, we show in nonobese diabetic (NOD) mice that the autoreactive T cell response starts and spreads as a pure Th1 type autoimmunity, suggesting that a spontaneous Th1 cascade underlies disease progression. Surprisingly, induction of antiinflammatory Th2 responses to a single β cell antigen (βCA) resulted in the spreading of Th2 cellular and humoral immunity to unrelated βCAs in an infectious manner and protection from IDDM. The data suggest that both Th1 and Th2 autoimmunity evolve in amplificatory cascades by generating site-specific, but not antigen-specific, positive feedback circuits. Determinant spreading of Th2 responses may be a fundamental mechanism underlying antigen-based immunotherapeutics, explaining observations of infectious tolerance and providing a new theoretical framework for therapeutic intervention.
We have been studying the development of insulin-dependent diabetes mellitus (IDDM) in the nonobese diabetic (NOD) mouse. Concurrent with the onset of insulitis, a spontaneous proliferative T cell response arises to a determinant of glutamic acid decarboxylase (GAD; references 1 and 2). Proliferative T cell responses subsequently spread to other β cell antigen (βCA) determinants. The spontaneously primed GAD-reactive T cells secrete IFN-γ (1), suggesting that a proinflammatory cascade might drive disease progression. However, other studies suggest that Th2 cells could be involved in the disease process, e.g., (a) IFN-γ–deficient mice still develop insulitis and IDDM (3), (b) production of IL-10 in the islets accelerates diabetes in transgenic NOD mice (4), and (c) pathogenic T cell populations express some Th2 cytokines (5). Thus, the characteristics of autoreactive T cell responses during the spontaneous development of murine IDDM remains an open question.
While autoantigen-induced Th2 responses are associated with inhibition of Th1-mediated autoimmune disease (6– 11), the nature of the induced tolerance is a matter of intense debate. Although it is generally accepted that deletion/inactivation of autoreactive lymphocytes is a fundamental tolerance mechanism, infectious tolerance has often been observed (12), which is inconsistent with deletional mechanisms. This regulatory tolerance has been attributed to suppressor cells, and more recently to Th2 cells (6–11). However, Th2 cell lines fail to mitigate Th1-mediated target tissue destruction in adoptive transfer experiments in both NOD mice and in the experimental autoimmune encephalomyelitis model (13, 14), suggesting that a mechanism other than bystander suppression contributes to regulatory tolerance.
The involvement of Th1 and Th2 cells in both the spontaneous autoimmune process and in tolerance states has been difficult to address in nontransgenic mice, primarily because of the very low frequency of autoreactive T cells within the T cell pool. Using an assay capable of characterizing T cells at the single cell level, we examined the natural development of β cell autoimmunity in NOD mice and the immunological impact of neonatal tolerization to a βCA. In the course of these studies, we observed a new phenomenon, Th2 determinant spreading, which may be a fundamental mechanism underlying the efficacy of antigen based immunotherapies and may explain observations of infectious tolerance.
Materials and Methods
Mice.
NOD (Taconic Farms, Germantown, NY), BALB/c, and AKR mice (The Jackson Laboratory, Bar Harbor, ME) were bred under specific pathogen-free conditions. Newborn mice were treated intraperitoneally on days 1 and 3 with 200 μg of the indicated antigen in 50% IFA (GIBCO BRL, Gaithersburg, MD). Only female mice were used in these studies.
Antigens.
Mouse GAD (GAD65), myelin basic protein (MBP), and control Escherichia coli β-galactosidase (β-gal) were purified as previously described (1, 15). The GAD and heat shock protein peptide 277 (HSP) peptides have been reported elsewhere (1, 16, 17). Control hen egg white lysozyme (HEL) peptide HEL11–25, immunogenic in NOD mice, was provided by Eli Sercarz (La Jolla Institute for Allergy and Immunology, La Jolla, CA). Insulin B chain and HEL were purchased from Sigma Chemical Co. (St. Louis, MO).
ELISPOT Analysis.
Splenic T cells were isolated at 4 or 12 wk of age from individual antigen-treated mice as well as unmanipulated mice, and the frequency of antigen-specific T cells secreting IFN-γ, IL-4, and IL-5 was determined using a modified ELISA spot technique (15, 18). In brief, 106 splenic mononuclear cells were added per well (in triplicate) of an ELISPOT plate (Athersys, Cleveland, OH) that had been coated with cytokine capture antibodies and incubated with peptide (20 μM) or whole protein (100 μM) for 24 h for IFN-γ, or for 40 h for IL-4 and IL-5 detection. After washing, biotinylated detection antibodies were added and the plates were incubated at 4°C overnight. Bound secondary antibodies were visualized using horseradish peroxidase (HRP)–streptavidin (DAKO Corp., Carpinteria, CA) and 3-amino-9-ethylcarbazole. Antibodies R4-6A2/XMG 1.2-biotin, 11B11/BVD6-24G2-biotin, and TRFK5/TRFK4-biotin (all from PharMingen, San Diego, CA) were used for capture and detection of IFN-γ, IL-4, and IL-5, respectively.
Autoantibody Characterization.
At the time of sacrifice, sera was collected and the isotype of GAD and insulin autoantibodies were characterized using an ELISA assay as described in the legend to Fig. 1 and in reference 18. In brief, GAD (Synectics Biomedical, Stockholm) or insulin B chain at 10 μg/ml were bound to 96-well plates (Nunc), in 0.1 M NaHCO3, pH 8.5 (GAD) or pH 9.6 (insulin B chain), at 4°C overnight. The wells were rinsed with PBS and then blocked with 3% BSA in PBS for 1 h. Mouse sera was added (0.1 ml of a 1:500 dilution) and incubated for 1 h at 37°C. After washing, bound Ig was characterized using affinity purified HRP-coupled goat anti–mouse IgG+A+M (H+L) (Pierce Chemical Co., Rockford, IL), or HRP-coupled goat anti– mouse isotype-specific antibodies for IgG1 and IgG2a (Southern Biotechnology Associates, Birmingham, AL) and ABTS. Sera from untreated BALB/c and AKR mice were used as negative controls.
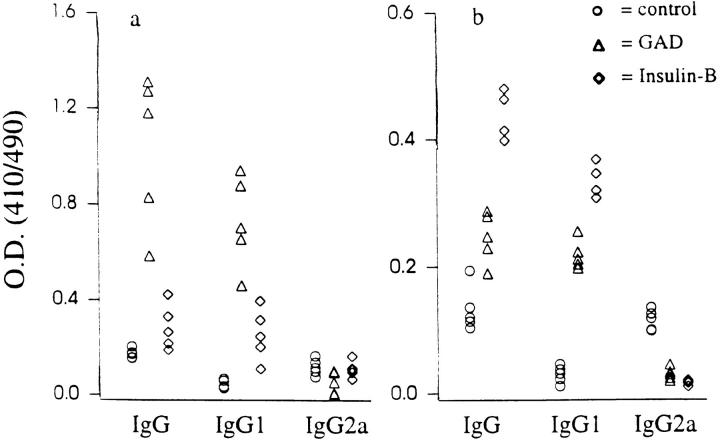
Figure 1.
Propagation of IgG1 responses to βCAs. NOD mice were treated with HEL, GAD, or insulin B chain, as described in Materials and Methods. GAD (a) and insulin (b) antibodies were characterized at 12 wk of age using antigen-specific ELISA assays (18). The background OD was ∼0.05 ± 0.01 for all samples. Serial dilutions of sera showed a linear relationship with resulting OD. The data are represented as the mean absorbance values over background of triplicate samples from individual mice. Experimental and control sera were tested simultaneously in two separate assays (n = 5 for each group). The variance in absorbance values between triplicate samples from the two sets of experiments was <8%. Humoral responses to GAD and insulin in control NOD mice treated with HEL were similar to those of unmanipulated NOD mice. BALB/c mice treated with βCAs developed antibodies only against the injected antigen (data not shown), consistent with the observed lack of Th2 spreading in these mice (Table 1B). Antibodies to GAD and insulin in sera from untreated BALB/c and AKR mice were at background levels (data not shown).
Results and Discussion
When unmanipulated NOD mice were tested at the onset of insulitis (4 wk of age), we detected vigorous IFN-γ, but no IL-4 or IL-5 splenic T cell responses to a single determinant of GAD, GAD peptide 35, (hereafter called GADp35) consistent with a unipolar Th1 response (Table 1A). By 12 wk of age, T cell autoimmunity had spread intramolecularly to additional GAD determinants (GADp6 and GADp15) and intermolecularly to other βCAs (insulin B chain and heat shock protein, supporting earlier observations [refs 1, 2]): all of these second wave reactivities were also purely Th1 in nature (Table 1). Thus, the spontaneously developing autoimmune process is characterized by the spreading of unipolar Th1 type anti-βCA reactivity. Conceivably, the first wave of autoreactive Th1 cells, via secretion of IFN-γ and induction of IL-12, creates an environment that favors Th1 cell differentiation, generating a positive feedback loop of Th1 reactivity and amplifying proinflammatory autoimmune responses to βCAs.
Table 1A.
Intramolecular Spreading of Th2 Responses to GAD Determinants
| Strain | Treatment | Response to antigens | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GADp35 | GADp6 | GADp15 | HEL11–25 | |||||||||||||||||||||||
| IL-4 | IL-5 | IFN-γ | IL-4 | IL-5 | IFN-γ | IL-4 | IL-5 | IFN-γ | IL-4 | IL-5 | IFN-γ | |||||||||||||||
| NOD | none (4 wk) | − | − | 86 | − | − | − | − | − | − | − | − | − | |||||||||||||
| none | − | − | 158 | − | − | 83 | − | − | 73 | − | − | − | ||||||||||||||
| HEL11–25 | − | − | 151 | − | − | 74 | − | − | 76 | 68 | 47 | − | ||||||||||||||
| GADp11 | − | − | 145 | − | − | 88 | − | − | 76 | − | − | − | ||||||||||||||
| GADp35 | 145 | 130 | 63 | 82 | 26 | 56 | 63 | 21 | 55 | − | − | − | ||||||||||||||
| GADp6 | 103 | 34 | 52 | 101 | 82 | 54 | 30 | 36 | 23 | − | − | − | ||||||||||||||
| BALB/c | none | − | − | − | − | − | − | − | − | − | − | − | − | |||||||||||||
| GADp35 | 49 | 42 | − | − | − | − | − | − | − | − | − | − | ||||||||||||||
| GADp6 | − | − | − | 67 | 51 | − | − | − | − | − | − | − | ||||||||||||||
We hypothesized that Th2 autoimmunity, like Th1 autoimmunity, might also spread, since the IL-4 produced by Th2 cells is itself a Th2 differentiation factor (19–22). We tested this hypothesis by inducing Th2 immunity to a single βCA and characterizing the development of T and B cell responses to unrelated βCAs.
NOD mice were neonatally treated with control antigens or βCAs in IFA, a protocol which has recently been shown to induce vigorous Th2 responses (15). NOD mice injected with the control antigens mouse MBP, HEL, or β-gal displayed vigorous IL-4 and IL-5, but no IFN-γ responses to the injected antigen, indicating the induction of unipolar Th2 responses (Table 1). Thus, NOD mice are not generally Th1 biased as has been thought, and can be manipulated to mount Th2 responses. Notably, induction of Th2 immunity to these control antigens did not affect the spontaneous development of Th1-biased anti-βCA responses (Table 1B), nor disease incidence (see below).
Table 1B.
Intermolecular Spreading of Th2 Responses to βCAs
| Response to antigens | ||||||||||||||||||||||||||
| GAD | HSP | Insulin B-chain | β-gal | |||||||||||||||||||||||
| Strain | Treatment | IL-4 | IL-5 | IFN-γ | IL-4 | IL-5 | IFN-γ | IL-4 | IL-5 | IFN-γ | IL-4 | IL-5 | IFN-γ | |||||||||||||
| NOD | none (4 wk) | − | − | 103 | − | − | − | − | − | − | − | − | − | |||||||||||||
| none | − | − | 365 | − | − | 130 | − | − | 70 | − | − | − | ||||||||||||||
| HEL | − | − | 350 | − | − | 105 | − | − | 68 | − | − | − | ||||||||||||||
| MBP | − | − | 359 | − | − | 116 | − | − | 64 | − | − | − | ||||||||||||||
| β-gal | − | − | 303 | − | − | 114 | − | − | 76 | 71 | 43 | − | ||||||||||||||
| GAD | 187 | 113 | 164 | 37 | 26 | 67 | 48 | 48 | 46 | − | − | − | ||||||||||||||
| HSP | 76 | 34 | 245 | 109 | 66 | 54 | 47 | 17 | 60 | − | − | − | ||||||||||||||
| Insulin B | 65 | 26 | 275 | 25 | 16 | 71 | 137 | 80 | 54 | − | − | − | ||||||||||||||
| BALB/c | HEL | − | − | − | − | − | − | − | − | − | − | − | − | |||||||||||||
| GAD | 58 | 40 | − | − | − | − | − | − | − | − | − | − | ||||||||||||||
| Insulin B | − | − | − | − | − | − | 68 | 56 | − | − | − | − | ||||||||||||||
Spreading of Th2 responses to βCAs. Mice were intraperitoneally injected neonatally with control HEL11–25 peptide, GADp35, and GADp6, which constitute determinants (1); GADp11, which does not constitute a determinant; HSPp277; insulin B chain; or whole proteins GAD, MBP, HEL, or β-gal in IFA. T cells from individual spleens were isolated at 12 wk of age (except where indicated) and the frequency of antigen-specific T cells secreting IFN-γ, IL-4, and IL-5 was determined by ELISA spot. The data are represented as the mean number of spot-forming colonies 106 splenic T cells above background. The individual variation within each group was <15%. Most wells without antigen showed no responses, but a background of up to five spots was observed in a few wells. Variation in the spot-forming cells among triplicate samples was <15%. −, no response over background. Experimental and control mice were tested simultaneously (in triplicate) in two separate experiments. n = 5 for each group.
Neonatal injection of NOD mice with a single βCA peptide (GADp35, which contains the earliest known target determinant; reference 1), induced clear IL-4 and IL-5 responses not only to the injected peptide, but also to other GAD peptides (GADp6 and GADp15, which contain later target determinants), indicating Th2 type intramolecular spreading (Table 1A). Similarly, neonatal treatment with GADp6 led to the spreading of Th2 immunity to GADp35 and GADp15, indicating that primed Th2 responses can spread to other autoantigen determinants, independent of the order in which spontaneous autoimmune responses arise to these determinants (see Fig. 3 in reference 1). Indeed, after treatment with a GAD peptide, Th2 responses became predominant to the injected peptide, as well as to uninjected GAD peptides through intramolecular spreading. Furthermore, injection of βCAs individually (GAD, HSPp277, or insulin B chain), led to the development of Th2 autoimmunity to noninjected βCAs (intermolecular spreading, Table 1B), creating an amplificatory cascade of this antiinflammatory limb. βCA-treated NOD mice failed to respond to nontarget tissue antigens (MBP, HEL or β-gal), and in other strains of mice, primed Th2 responses to βCAs were restricted to the injected antigen (Table 1 and data not shown). NOD mouse immune responses to control nontarget tissue antigens (MBP, HEL and β-gal) were similar in magnitude to those induced by these antigens in other strains of mice (data not shown) but failed to spread to βCAs (Table 1). Thus, the spreading of Th2 responses was limited to target tissue antigens and was dependent upon a local inflammatory process.
Analysis of humoral responses showed that while unmanipulated and control antigen treated NOD mice had low levels of autoantibodies, GAD-treated animals had elevated autoantibodies to both GAD and insulin (Fig. 1), consistent with the intermolecular spreading of Th2 immunity. Similarly, neonatal treatment with insulin B chain raised the titer of GAD-specific antibodies in addition to the insulin-specific ones. The induced antibodies were of the IgG1 subclass, characteristic of Th2 responses (19). Thus, the spreading of Th2 immunity can lead to the diversification of humoral responses.
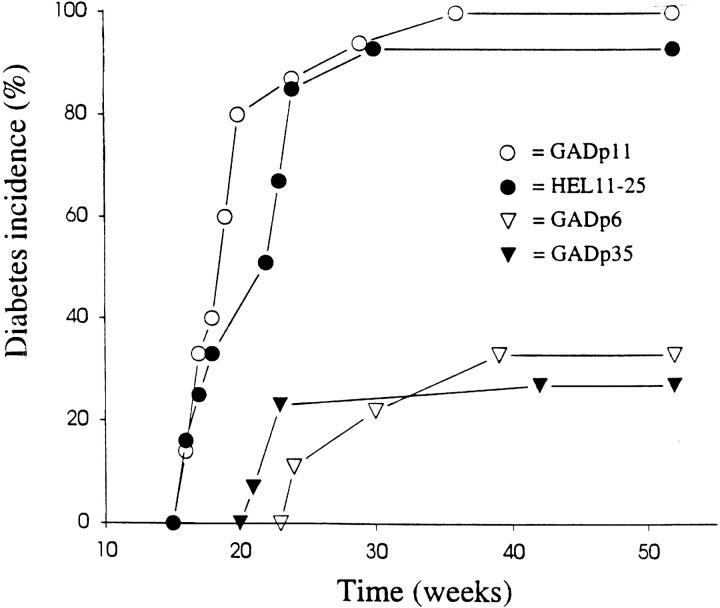
Furthermore, the GAD peptide–treated NOD mice had significantly reduced long-term disease incidence (∼30% of GAD peptide-treated mice versus 96% of controls developed diabetes over 1 yr (P ⩽0.02); Fig. 2). The lack of complete protection by GAD treatment may be due to the diminution of Th2 responses later in life (11, 18). While the induction of Th2 responses to some nontarget tissue antigens has been associated with protection from autoimmune disease (23, 24), the control nontarget tissue antigens used in this study did not induce Th2 spreading or protection from disease.
Figure 2.
βCA treatment inhibits insulin-dependent diabetes. Neonatal female NOD mice were treated at days 1 and 3 with 200 μg HEL11–25, GADp11, GADp35, or GADp6 in IFA and were followed up until they reached 1 y of age in order to determine the effect of treatment on long-term disease incidence. Two consecutive blood glucose levels of >300 mg/dl was considered disease onset. n = 15 for each group.
Although we induced Th2 immunity before the onset of insulitis, βCA-treated NOD mice still developed βCA-reactive Th1 cells, suggesting that there is an inherent islet perturbation which promotes Th1 autoimmunity to βCAs. However, the Th1 responses to βCAs were markedly reduced in βCA-treated animals relative to control groups (Table 1). Despite the presence of significant Th1 responses, βCA (but not control) -treated mice displayed almost no splenic T cell proliferative responses to the injected antigen and had greatly reduced proliferative responses to other βCAs (data not shown). As the autoantibody responses in βCA-treated mice were predominantly of the Th2 type and the mice were protected from disease, it appears that Th2 immunity can be functionally dominant and a potent regulator of pathogenic Th1 activity.
In summary, we have shown in unmanipulated NOD mice that the anti-β cell response starts and spreads as a pure Th1 type autoimmunity, suggesting that a Th1 cascade underlies disease progression. Induction of Th2 autoimmunity to a single βCA resulted in the spreading of Th2 type T cell and antibody responses to other βCAs in an infectious manner. These data suggest that both Th1 and Th2 autoimmunity evolve in amplificatory cascades defined by site-specific, but not antigen-specific, positive feedback circuits, which may be generated by the cytokine milieu (24) or induced changes in accessory molecule expression (25–27). Th2 type determinant spreading introduces a novel mechanism for Th2-mediated protection, providing an explanation for the infectious nature of tolerance and why different autoantigens can be successfully used for immune therapy. Conversely, in Th2-mediated diseases, Th2 determinant spreading may contribute to the disease process, perhaps accounting for observations that in allergic conditions, individuals gradually become sensitized to an increasing number of antigens. Thus, these findings may provide a new theoretical framework for understanding disease progression in autoimmune and allergic conditions, as well as for therapeutic intervention.
Acknowledgments
This work was supported by grants from the National Institutes of Health (Bethesda, MD), the Juvenile Diabetes Foundation International, and the Riva Foundation.
References
- 1.Kaufman DL, Clare-Salzler M, Tian J, Forsthuber T, Ting GSP, Robinson P, Atkinson MA, Sercarz EE, Tobin AJ, Lehmann PV. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature. 1993;366:69–72. doi: 10.1038/366069a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tisch R, Yang XD, Singer SM, Liblau RS, Fugger L, McDevitt HO. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993;366:72–75. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- 3.Hultgren B, Huang X, Dybdal N, Stewart TA. Genetic absence of gamma-interferon delays but does not prevent diabetes in NOD mice. Diabetes. 1996;45:812–817. doi: 10.2337/diab.45.6.812. [DOI] [PubMed] [Google Scholar]
- 4.Lee MS, Mueller R, Wicker LS, Peterson LB, Sarvetnick N. IL-10 is necessary and sufficient for autoimmune diabetes in conjunction with NOD MHC homozygosity. J Exp Med. 1996;183:2663–2668. doi: 10.1084/jem.183.6.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson JT, Cornelius JG, Jarpe AJ, Winter WE, Peck AB. Insulin-dependent diabetes in the NOD mouse model. II. Beta cell destruction in autoimmune diabetes is a TH2 and not a TH1 mediated event. Autoimmunity. 1993;15:113–122. doi: 10.3109/08916939309043886. [DOI] [PubMed] [Google Scholar]
- 6.Powrie F, Coffman RL. Cytokine regulation of T cell function: potential for therapeutic intervention. Immunol Today. 1993;14:270–274. doi: 10.1016/0167-5699(93)90044-L. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Kuchroo VJ, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 8.Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 9.Röcken M, Racke MK, Shevach EM, IL -4 induced immune deviation as antigen-specific therapy for inflammatory autoimmune disease Immunol Today. 1996;17:225–231. doi: 10.1016/0167-5699(96)80556-1. [DOI] [PubMed] [Google Scholar]
- 10.Brocke S, Gijbels K, Allegretta M, Ferber I, Piercy C, Blankenstein T, Martin R, Utz U, Karin N, Mitchell D, et al. Treatment of experimental encephalomyelitis with a peptide analog of myelin basic protein. Nature. 1996;379:343–346. doi: 10.1038/379343a0. [DOI] [PubMed] [Google Scholar]
- 11.Tian J, Atkinson MA, Clare-Salzler M, Herschenfeld A, Forsthuber T, Lehmann PV, Kaufman DL. Nasal administration of glutamate decarboxylase (GAD65) peptides induces Th2 responses and prevents murine insulin–dependent diabetes. J Exp Med. 1996;183:1561–1567. doi: 10.1084/jem.183.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, Waldmann H. “Infectious” transplantation tolerance. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 13.Katz JD, Benoist C, Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995;268:1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 14.Khoruts A, Miller SD, Jenkins MK. Neuroantigen-specific Th2 cells are inefficient suppressors of experimental autoimmune encephalomyelitis induced by effector Th1 cells. J Immunol. 1995;155:5011–5017. [PubMed] [Google Scholar]
- 15.Forsthuber T, Yip HC, Lehmann PV. Induction of Th1 and Th2 immunity in neonatal mice. Science. 1996;271:1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 16.Atkinson MA, Bowman MA, Campbell L, Kaufman DL, Maclaren NK. Cellular immunity to an epitope common to glutamate decarboxylase and Coxsackie virus in insulin-dependent diabetes. J Clin Invest. 1994;94:2125–2129. doi: 10.1172/JCI117567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elias D, Cohen IR. Peptide therapy for diabetes in NOD mice. Lancet. 1994;343:704–706. doi: 10.1016/s0140-6736(94)91582-2. [DOI] [PubMed] [Google Scholar]
- 18.Tian J, Clare-Salzler M, Herschenfeld A, Middleton B, Newman BD, Evans C, Atkinson M, Mueller R, Mullen Y, Sarvetnick N, et al. Modulating autoimmune responses to GAD inhibits disease progression and prolongs islet graft survival in diabetes-prone mice. Nat Med. 1996;2:1348–1353. doi: 10.1038/nm1296-1348. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 20.O'Garra A, Murphy K. Role of cytokines in determining T-lymphocyte function. Curr Opin Immunol. 1994;6:458–466. doi: 10.1016/0952-7915(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 21.Janeway CA, Jr, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275–285. doi: 10.1016/0092-8674(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 22.Reiner SL, Seder RA. T helper cell differentiation in the immune response. Curr Opin Immunol. 1995;7:360–366. doi: 10.1016/0952-7915(95)80111-1. [DOI] [PubMed] [Google Scholar]
- 23.Vaysburd M, Lock C, McDevitt H. Prevention of insulin-dependent diabetes mellitus in nonobese diabetic mice by immunogenic but not by tolerated peptides. J Exp Med. 1995;182:897–902. doi: 10.1084/jem.182.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falcone M, Bloom BR. A T helper cell 2 (Th2) immune response against non-self antigens modifies the cytokine profile of autoimmune T cells and protects against experimental allergic encephalomyelitis. J Exp Med. 1997;185:901–907. doi: 10.1084/jem.185.5.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Glimcher LH. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 26.Lenschow DJ, Herold KC, Rhee L, Patel B, Koons A, Qin HY, Fuchs E, Singh B, Thompson CB, Bluestone JA. CD28/B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity. 1996;5:285–293. doi: 10.1016/s1074-7613(00)80323-4. [DOI] [PubMed] [Google Scholar]
- 27.Miller SD, Vanderlugt CL, Lenschow DJ, Pope JG, Karandikar NJ, Dal MC, Canto, Bluestone JA. Blockade of CD28/B7-1 interaction prevents epitope spreading and clinical relapses of murine EAE. Immunity. 1995;3:739–745. doi: 10.1016/1074-7613(95)90063-2. [DOI] [PubMed] [Google Scholar]