Abstract
Itk is a member of the Btk/Tec/Itk family of nonreceptor protein tyrosine kinases (PTKs), and has been implicated in T cell antigen receptor (TCR) signal transduction. Lck and Fyn are the Src-family nonreceptor PTKs that are involved in TCR signaling. To address the question of how these members of different families of PTKs functionally contribute to T cell development and to T cell activation, mice deficient for both Itk and either Lck or Fyn were generated. The Itk/Lck doubly deficient mice exhibited a phenotype similar to that of Lck-deficient mice. The phenotype of the Itk/Fyn doubly deficient mice was similar to that of Itk deficient mice. However the Itk/Fyn doubly deficient mice exhibited a more severe defect in TCR-induced proliferation of thymocytes and peripheral T cells than did mice deficient in either kinase alone. These data support the notion that Itk and Fyn both make independent contributions to TCR-induced T cell activation.
Multiple nonreceptor protein tyrosine kinases (PTKs) are activated after TCR stimulation. These PTKs are responsible for the tyrosine phosphorylation of the TCR-ζ and CD3 chains, the kinases themselves, and a number of cellular substrates (1, 2). Among these cytoplasmic PTKs are the Src-family members such as Fyn and Lck, and the ZAP-70 and Syk kinases. Functionally important interactions as well as physical interactions between these two families of PTKs have been well studied (for review see reference 3).
Recently, a new family of nonreceptor PTKs, expressed specifically in the hematopoietic system (4), has been implicated in antigen receptor–mediated responses. Members of this family include Btk, Itk, and Tec. Itk is highly expressed in T cells and mast cells. Mice deficient in Itk expression have a reduced number of mature T cells, which also are compromised in proliferation after TCR stimulation (5). Thus, this third class of nonreceptor PTKs, in addition to those of the Src and Syk/ZAP-70 families, is involved in T cell development and activation.
The mechanism by which Itk is regulated in T cells is not known. Activation of Btk by Src family PTKs has been observed (6, 7). This observation raises the possibility that an Src family kinase might interact functionally with Itk in T cells. Thymic development and T cell functional responses in mice deficient in Lck or Fyn do not mimic the defects observed in Itk-deficient mice. Lck-deficient mice exhibit a prominent but incomplete developmental arrest at the transition from the CD4−CD8− (double negative, DN) to the CD4+CD8+ (double positive, DP) stage of development. The few peripheral T cells that do develop have markedly impaired TCR functional responses (8). In mice lacking Fyn, thymic development occurs normally, but markedly reduced thymocyte proliferation and some diminution in peripheral T cell proliferation in response to TCR stimulation were observed (9, 10). A complete arrest in the transition of the DN cells to DP cells in mice deficient in both Lck and Fyn has been observed (11, 12). These studies suggest that Lck and Fyn have some redundant roles in TCR signal transduction. To address whether Itk and the Src family members expressed in T cells have overlapping or nonoverlapping functions in T cell development and activation, we generated mice deficient in both Itk and Fyn, as well as mice deficient in both Itk and Lck. This study provides evidence that Fyn and Itk both make important independent contributions to the functional responses of thymocytes and T cells.
Materials and Methods
Generation of Itk/Fyn and Itk/Lck Doubly Deficient Mice.
Itk-deficient mice have been previously reported (5). Fyn-deficient mice were obtained from Paul L. Stein and Philippe Soriano (10) at Fred Hutchinson Cancer Research Center (Seattle, WA). Lck-deficient mice were obtained from Tak W. Mak (Amgen Institute, Toronto, Canada; reference 8). Itk/Fyn and Itk/Lck doubly deficient mice were obtained by breeding Itk-deficient mice with Fyn-deficient mice and Lck-deficient mice, respectively. Genotyping of these mice was carried out using PCR. The oligonucleotides for genotyping the itk locus are as follows: Itk knockout (KO): ASneo+, 5′ ATTGAACAAGATGGATTGCAC 3′; ASneo−, 5′ CGTCCAGATCATCCTGATC 3′; Itk WT: itk420, 5′ TGGGTGCTGACCCTTAAAGAAG 3′; itkintron3a, 5′ GCCGTAAATGAACAGGTGGTGA 3′. The expected size of Itk KO is 475 bp, and that of Itk WT is 218 bp. The ASneo+ and ASneo− primers are located in the neo gene, and thus will also amplify from the neo insertions of the Fyn-deficient mice and Lck-deficient mice. Additional PCR reactions using the itk KO-specific oligoes were performed to distinguish itk +/+ and itk +/− in certain genotype combinations (5).
The oligonucleotides for genotyping the fyn locus are as follows: Fyn KO: neo3′, 5′ GCATCGCCTTCTATCGCCTTCTTGACG 3′; fynR, 5′ GCAAAACAACCCACACAGAG 3′; Fyn WT: fynF, 5′ TTACCCTCTGAGCATCTGAC 3′; fynR, see above. The expected size of Fyn KO is ∼400 bp, and that of Fyn WT is ∼550 bp. The oligonucleotides for genotyping the lck locus are as follows: Lck KO: neo.lck, 5′ TATCAGGACATAGCGTTGGCTACCC 3′; lckR, 5′ CTTAGACTCACGTGCTCCACAGGTA 3′; Lck WT: lckF, 5′ TGGTGAGACCTGACAACTGTCCGGA 3′; lckR, see above. The expected size of Lck KO is 560 bp, and that of Lck WT is 375 bp. Each PCR reaction was carried out individually under similar conditions. The typical conditions are 93°C for 30 min for denaturation, 55°C for 45 min for annealing, and 74°C for 45 min for amplification, with a total of 35 cycles of the above three steps, followed by a 74°C for 3-h step to finish up the reaction.
Flow Cytometric Analysis.
Flow cytometry was performed by staining 106 freshly isolated thymocytes and lymph node cells in 100 μl of PBS supplemented with 0.3% BSA and 0.01% sodium azide on ice for 30 min. For three-color analysis, cells were first stained with various biotin-conjugated antibodies against different lymphocyte surface proteins, followed by staining with a cocktail of streptavidin-TRICOLOR (Caltag Labs, San Francisco, CA), CD4-PE (YTS191.1; Caltag), and CD8-FITC (CT-CD8α, Caltag). Cells were analyzed on a FACScan® or FACSCalibur using the Lysis II or the CellQuest softwares. Viable cells were gated on the basis of forward- and side-light scattering. Additional antibodies used included anti-CD69 (H1.2F3), anti-heat stable antigen (M1/69), anti-TCRαβ (H57-597), and anti-IgM (R6-60.2), all from PharMingen (San Diego, CA).
Cell Proliferation Assays.
Complete medium (RPMI 1640 supplemented with 10% FCS, 2 mM l-glutamine, 50 μM β-mercaptoethanol, 100 μM nonessential amino acids, 50 U/ml penicillin, and 50 μg/ml streptomycin) was used for cell culture and preparation. Responder cells were isolated from thymi or spleens of age-matched wild-type, itk +/− fyn +/+, itk +/− fyn −/−,itk −/− fyn +/+, and itk −/− fyn −/− mice (8–10-wk old, H-2b/b). Splenocytes from BALB/c (H-2d/d) and C57BL/6 (H-2b/b) were inactivated by irradiation (∼2,000 rad). Proliferation assays were set up in a volume of 200 μl in flat-bottomed wells of microtiter plates. For the MLR, serial dilutions of 4 × 105, 2 × 105, 1 × 105, and 0.5 × 105 splenocytes were mixed with 5 × 105 stimulator cells of various types in each well, and were incubated for 96 h before pulsing for 16 h with 1 μCi of [3H]thymidine per well. For other cell proliferation assays, 5 × 104 cells were mixed with 5 × 105 irradiated C57BL/6 splenocytes plus anti-CD3 (purified 145-2C11 at 1.5, 0.5, 0.167, and 0.056 μg/ml final concentration) in the presence of PMA (1 ng/ml). Cells were incubated for 48 h before pulsing for 16 h with 1 μCi of [3H]thymidine per well. Cellular radioactivity was harvested using a microtiter plate cell harvester (Tomtec, Orange, CT). All assays were done in triplicates, and the mean and standard deviation are presented.
Results
T Cell Development in the Absence of Both Itk and Fyn.
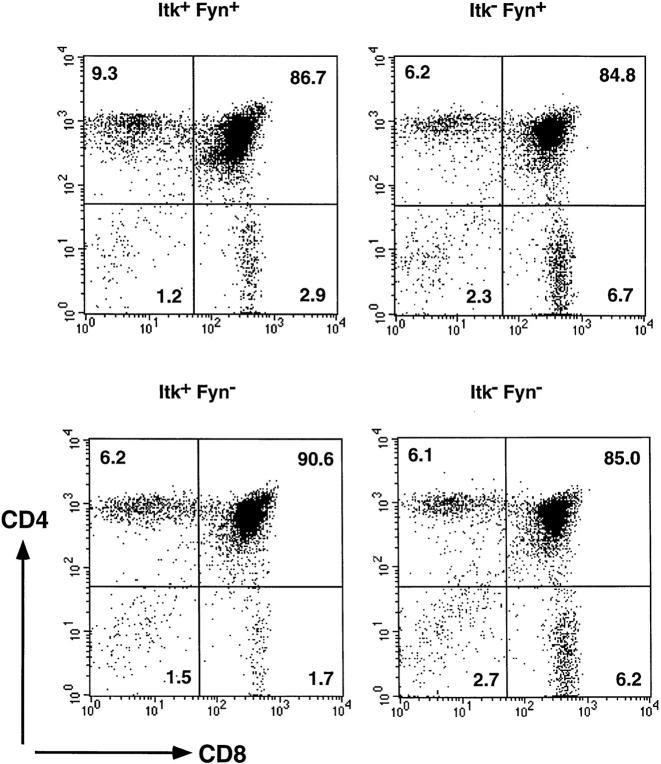
Maturation of thymocytes takes place in an ordered sequence of developmental events (13). Early immature thymocytes do not express either the CD4 or the CD8 coreceptors (DN). As a result of pre-TCR signaling events, the DN cells acquire expression of both CD4 and CD8 to become DP cells. Subsequently, expression of one of the coreceptors is extinguished, and the thymocytes become mature CD4+ and CD8+ single positive T cells. Mice lacking Fyn, an Src family nonreceptor PTK have normal numbers of thymocyte and T cell subpopulations as well as normal levels of surface expression of the TCR chains (Table 1 and Fig. 1), consistent with previous reports (9, 10). On the other hand, mice deficient in Itk have a partial block in T cell development (Table 1 and Fig. 1) in agreement with our previous studies (5). Itk-deficient mice have reduced numbers of mature lymph node T cells as well as a reduced ratio of CD4 to CD8 single positive cells in the thymus and the periphery.
Table 1.
T Cell Populations in the Thymus and Lymph Nodes of +/+, fyn−/−, itk−/−, and fyn−/−itk−/− Mice
| Genotype | Source | Total cells | Percentage T cells§ | Total T cells§ | Percentage of total cells | CD4/CD8 ratio | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4−CD8− | CD4+CD8+ | CD4+CD8− | CD4−CD8+ | |||||||||||||||
| 10−7 | 10−7 | |||||||||||||||||
| +/+ (n = 12)* | Thymus | 10.2 ± 4.3 | 2.1 ± 0.8 | 85.7 ± 4.5 | 9.0 ± 3.5 | 3.2 ± 1.3 | 3.0 ± 1.0 | |||||||||||
| fyn −/− (n = 3)* | Thymus | 17.2 ± 3.3 | 2.0 ± 1.2 | 88.6 ± 1.9 | 6.9 ± 2.6 | 2.6 ± 0.3 | 2.6 ± 0.7 | |||||||||||
| itk −/− (n = 16)* | Thymus | 8.7 ± 1.8 | 3.2 ± 1.2 | 80.7 ± 8.0 | 7.1 ± 2.6 | 8.9 ± 6.2 | 1.0 ± 0.5 | |||||||||||
| fyn −/− itk −/− (n = 7)* | Thymus | 12.3 ± 4.2 | 3.7 ± 1.5 | 84.7 ± 4.5 | 6.7 ± 2.8 | 5.0 ± 1.5 | 1.4 ± 0.5 | |||||||||||
| +/+ (n = 12)* | Lymph nodes‡ | 6.1 ± 1.4 | 77.8 ± 8.0 | 4.7 ± 1.1 | 3.3 ± 1.5 | 1.2 ± 0.9 | 47.4 ± 9.2 | 26.0 ± 2.4 | 1.8 ± 0.4 | |||||||||
| fyn −/− (n = 3)* | Lymph nodes‡ | 6.6 ± 1.6 | 74.8 ± 6.9 | 5.0 ± 1.6 | 1.9 ± 1.0 | 1.4 ± 1.9 | 47.0 ± 4.7 | 24.4 ± 3.0 | 2.0 ± 0.3 | |||||||||
| itk −/− (n = 16)* | Lymph nodes‡ | 6.1 ± 2.3 | 60.7 ± 9.2 | 3.6 ± 1.4 | 5.2 ± 2.3 | 1.1 ± 1.9 | 27.2 ± 5.5 | 27.2 ± 7.9 | 1.2 ± 0.7 | |||||||||
| fyn −/− itk −/− (n = 7)* | Lymph nodes‡ | 5.9 ± 2.0 | 54.8 ± 7.6 | 3.2 ± 1.0 | 5.8 ± 3.7 | 0.7 ± 0.4 | 25.7 ± 3.2 | 22.6 ± 5.1 | 1.2 ± 0.4 | |||||||||
Data are expressed as mean ± standard deviation.
Mice, 7–12 wk old; numbers of animals studied are shown in parentheses.
Cervical, brachial, axillary, superficial inguinal, and mesenteric.
The number of T cells in the lymph nodes was calculated based on the percentages of cells staining positive with anti-CD3ε.
Figure 1.
Itk/Fyn doubly deficient mice showed similar phenotypes as Itk− mice in T cell development. Thymocytes from +, itk −/−, fyn −/−, and itk −/− fyn −/− mice were stained with antibodies against CD4, CD8, and various other cell surface molecules, and were analyzed with flow cytometry. Shown are profiles of the thymocyte subpopulations with the percentage of total cells in each quadrant as indicated.
To address whether Itk and Fyn might functionally interact, we examined the developmental consequences of a defect in both of these kinases. Mice deficient for both Fyn and Itk expression were derived by breeding. Analysis of the Itk/Fyn doubly deficient mice demonstrated similar thymic and peripheral T cell phenotypes as the Itk-deficient mice (Table 1 and Fig. 1). Hence, the absence of Fyn did not further enhance the developmental consequences of Itk-deficiency.
A few mice deficient in both Lck and Itk were also obtained. The developmental consequences of this breeding revealed that mice deficient in both Itk and Lck had thymic phenotypes indistinguishable from Lck-deficient mice (data not shown). A partial developmental arrest at the DN to DP transition and a 10-fold reduction in thymocyte number were observed, consistent with studies of the Lck-deficient mice previously reported (8). As observed in the Lck-deficient mice, only a few peripheral T cells were detectable in the spleens and lymph nodes of the Itk/Lck doubly deficient animals. Thus, the absence of Itk does not further augment the already severe phenotype observed in the Lck deficient mice.
Thymocyte Proliferation in the Absence of Both Itk and Fyn.
To address whether Itk and Fyn make independent contributions to the processes that lead to T cell activation, thymocytes and splenocytes were prepared from Itk/Fyn doubly deficient mice and compared with those from mice lacking either kinase alone in TCR-mediated proliferative responses. Thymocytes lacking either Fyn or Itk consistently showed a reduction in proliferation to anti-CD3 mAb over a broad dose range when compared to control Itk+ Fyn+ thymocytes (Fig. 2 A), consistent with previous studies (9, 10). Interestingly, thymocytes lacking both Itk and Fyn exhibited a much more severe proliferative defect, detectable over all doses of anti-CD3 mAb used. These results suggest that both Fyn and Itk contribute to the proliferative response induced by TCR stimulation in thymocytes.
Figure 2.

Impaired proliferative responses upon TCR stimulation of Itk−Fyn− thymocytes and splenocytes. (A) Thymocytes from +, itk −/−, fyn −/−, and itk −/− fyn −/− mice were stimulated with anti-CD3 at indicated concentrations in the presence of PMA (1 ng/ml) for 48 h. Cell proliferation was measured by [3H]thymidine incorporation after an additional 16 h. The genotypes of mice are as indicated: I, itk; F, fyn. Standard deviations for triplicate samples are shown as error bars along the y axis. (B) Splenocytes from +, itk −/−, fyn −/−, and itk −/− fyn −/− mice were stimulated with anti-CD3 at indicated concentrations in the presence of PMA (1 ng/ml) for 48 h. Cell proliferation was measured by [3H]thymidine incorporation after an additional 16 h. The genotypes of mice are as indicated in A. Standard deviations for triplicate samples are shown as error bars along the y axis. Data are representative of two independent experiments.
Proliferation of Mature T cells in Response to anti-TCR and Allogeneic MHC Stimulations.
To examine the response of mature peripheral T cells, splenocytes from Itk/Fyn doubly deficient mice as well as from mice deficient in either kinase alone or control mice were stimulated with anti-CD3 mAb in the presence or absence of PMA. As has been previously reported (9, 10), the proliferation of Fyn− splenocytes was similar to that of wild-type (Itk+Fyn+) splenocytes. On the other hand, splenocytes lacking Itk showed a proliferative response of 15–30% of that of the Itk+Fyn+ cells (Fig. 2 B and data not shown). Consistent with the observation using thymocytes, Itk−Fyn− splenocytes had a much more profound reduction in their proliferative response. Taken together, these results suggest that Itk and Fyn both make at least some independent contributions to TCR-regulated pathways that lead to T cell proliferative responses.
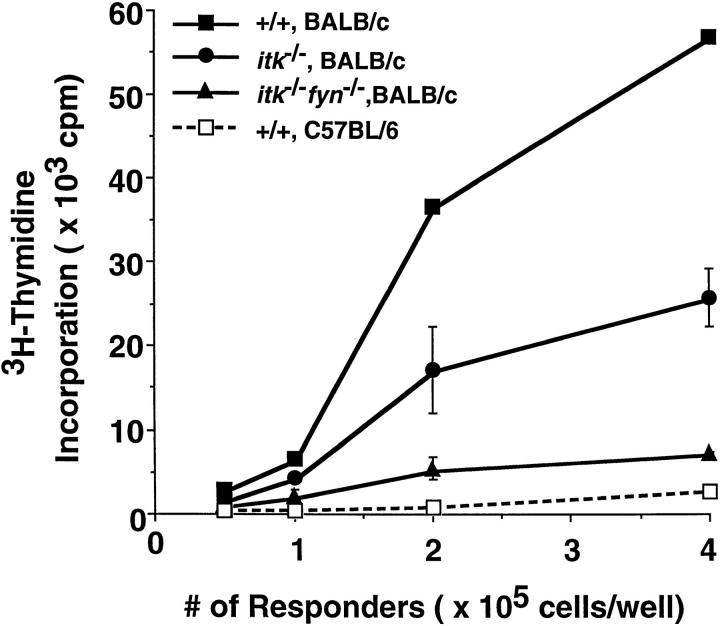
To examine the impact of these kinases on antigen-dependent responses, mature T cells from mutant mice were also examined in MLR. Consistent with the results with anti-CD3 induced proliferation, Itk−Fyn− splenocytes responded very poorly to allogeneic MHC stimulation, whereas Itk-deficient splenocytes had an intermediate level of proliferation (Fig. 3). On the other hand, splenocytes lacking Fyn exhibited near normal alloantigen-induced proliferation (data not shown), consistent with previous studies (9, 10). These results support the notion that both Itk and Fyn functionally contribute to T cell responses to alloantigens.
Figure 3.
Lack of a proliferative response with Itk−Fyn− splenocytes in a MLR. Splenocytes from mice of the indicated genotypes were stimulated in vitro with irradiated splenocytes from C57BL/6 mice (broken line), or from BALB/c mice (solid lines) for 96 h. Cell proliferation was measured by [3H]thymidine incorporation after an additional 16 h. The genotypes of mice are as indicated. Standard deviations for triplicate samples are shown as error bars along the y axis.
Discussion
We describe here the characterization of Itk/Fyn doubly deficient mice in T cell development and activation. Our data failed to reveal an additive effect upon thymocyte and T cell development in mice that lack both Fyn and Itk. On the other hand, we observed a synergistic loss of proliferative responses of these cell populations in the absence of both Itk and Fyn. The severe defect in proliferative responses induced with TCR stimulation suggests that these kinases provide at least some independent functions in the events leading to T cell and thymocyte proliferation.
Itk-deficient mice and Fyn-deficient mice have distinctive phenotypes. Mice lacking Itk have a reduced ratio of CD4 to CD8 single positive thymocytes (5). The absolute numbers of mature CD4+ T cells are also reduced by about twofold compared to wild-type mice expressing Itk (Table 1). Fyn-deficient mice, on the other hand, do not exhibit an obvious developmental phenotype (9, 10). The main immune system defect of Fyn-deficient mice is in mature thymocytes that have diminished proliferative responses as well as reduced tyrosine phosphorylation of cellular proteins upon TCR stimulation (9, 10). Overall, the developmental consequences resulting from a lack of either Itk or Fyn is quite modest.
Itk/Fyn doubly deficient mice resembled Itk-deficient mice in their T cell developmental phenotypes, suggesting that even in the absence of Itk, Fyn is not critically required for T cell development, nor does it play a function that is redundant with Itk. In contrast, thymocytes and mature T cells in Itk/Fyn doubly deficient mice exhibited a severe defect in proliferative responses upon TCR stimulation. This defect was much more pronounced than would be predicted by an additive effect of lacking either kinase alone. These data suggest that both Itk and Fyn are important in T cells activation and are likely to play partially distinct functions.
To address whether Fyn is the only specific and functional partner of Itk in T cell activation, we also generated mice deficient for both Itk and Lck, another Src family member expressed in T cells. The developmental phenotypes of Itk/Lck double-deficient mice resembled those of Lck-deficient mice (data not shown; reference 8). There was an early block of thymocyte maturation resulting in reduced numbers of CD4+CD8+ double-positive thymocytes, and very few mature T cells. Because of this early block in development, it is difficult to assess whether Lck deficiency exacerbates the later defect due to the absence of Itk. The phenotype of Itk/Lck-deficient mice is also distinct from Fyn/Lck doubly deficient mice (11, 12), which have a complete lack of double-positive thymocytes. The results of Fyn/ Lck doubly deficient mice indicate that there is a certain degree of functional redundancy between Fyn and Lck in promoting transition of thymocytes from double-negative to double-positive stages. The results also suggest that Lck-deficiency provides a good platform to reveal Fyn's contribution to thymocyte development. The lack of similarity between Itk/Lck double-deficient mice and Fyn/Lck doubly deficient mice, however, suggests that Itk is not the sole molecule mediating signals from Fyn at these specific stages of thymocyte maturation. This interpretation is consistent with our studies of Itk/Fyn doubly deficient mice demonstrating that both Itk and Fyn contribute to T cell activation; lack of either kinase alone is not sufficient to abolish T cell activation.
In B lymphocytes, Btk is thought to have functions similar to those of Itk in T cells. Mice deficient in Btk exhibit defects in B cell development and in BCR-mediated proliferative responses. The Src-family PTK Lyn has been shown to regulate the activity of Btk, and mice lacking Lyn have decreased proliferative responses. In T cells, Lck and Fyn may have functions similar to Lyn in B cells. Thus, these PTKs may be involved in activating Itk. The finding that the absence of Fyn exacerbates the proliferative defect of Itk-null T cells suggests that Fyn is additionally involved in pathways distinct from those involving Itk. At least one of these Fyn-specific pathways may involve yet another cytoplasmic PTK. Recent studies have shown that in T cells, Fyn specifically regulates TCR-mediated activation of Pyk2, a PTK homologous to the focal adhesion kinase (14). Thus, Fyn and Itk are likely to regulate at least some distinct events involved in the proliferative response resulting from TCR stimulation. Further work will be required to define these distinct events.
Acknowledgments
We thank Paul Stein (Wistar Institute, Philadelphia, PA) and Philippe Soriano (Fred Hutchinson Cancer Research Institute, Seattle, WA) for providing the Fyn-deficient mice and Clifford Lowell (University of California San Francisco) for advice on genotyping them using the PCR reactions; Tak Mak (Amgen Institute, Toronto) for providing the Lck-deficient mice; N. Killeen, D. Qian, and N. van Oers for valuable discussions.
Footnotes
X.C. Liao is supported by the Cancer Research Fund of the Damon Runyon–Walter Winchell Foundation (1992–1995) and by a Special Fellowship from the Leukemia Society of America, Inc. (1995–1996). D.R. Littman and A. Weiss are investigators of the Howard Hughes Medical Institute.
References
- 1.Wange RL, Samelson LE. Complex complexes: signaling at the TCR. Immunity. 1996;5:197–205. doi: 10.1016/s1074-7613(00)80315-5. [DOI] [PubMed] [Google Scholar]
- 2.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 3.Qian D, Weiss A. T cell antigen receptor signal transduction. Curr Opin Cell Biol. 1997;9:205–212. doi: 10.1016/s0955-0674(97)80064-6. [DOI] [PubMed] [Google Scholar]
- 4.Desiderio S, Silicano JD. The Itk/Btk/Tec family of protein-tyrosine kinases. Chem Immunol. 1994;59:191–210. [PubMed] [Google Scholar]
- 5.Liao XC, Littman DR. Altered T cell receptor signaling and disrupted T cell development in mice lacking Itk. Immunity. 1995;3:757–769. doi: 10.1016/1074-7613(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 6.Rawlings DJ, Scharenberg AM, Park H, Wahl MI, Lin S, Kato RM, Fluckiger A-C, Witte ON, Kinet J-P. Activation of BTK by a phosphorylation mechanism initiated by SRC family kinases. Science. 1996;271:822–825. doi: 10.1126/science.271.5250.822. [DOI] [PubMed] [Google Scholar]
- 7.Alexandropoulos K, Cheng G, Baltimore D. Proline-rich sequences that bind to Src homology 3 domains with individual specificities. Proc Natl Acad Sci USA. 1995;92:3110–3114. doi: 10.1073/pnas.92.8.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molina TJ, Kishihara K, Siderovski DP, van Ewijk W, Narendran A, Timms E, Wakeham A, Paige CJ, Hartmann K-U, Veillette A, et al. Profound block in thymocyte development in mice lacking p56lck . Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 9.Appleby, M.W., J.A. Gross, M.P. Cooke, S.D. Levin, X. Qian, and R.M. Perlmutter. 1992. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell. 70:751–763. [DOI] [PubMed]
- 10.Stein PL, Lee H-M, Rich S, Soriano P. pp59fynmutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1992;70:741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- 11.van Oers NSC, Lowin-Kropf B, Finlay D, Connolly K, Weiss A. αβ T cell development is abolished in mice lacking both Lck and Fyn protein tyrosine kinases. Immunity. 1996;5:429–436. doi: 10.1016/s1074-7613(00)80499-9. [DOI] [PubMed] [Google Scholar]
- 12.Groves T, Smiley P, Cooke MP, Forbush K, Perlmutter RM, Guidos CJ. Fyn can partially substitute for Lck in T lymphocyte development. Immunity. 1996;5:417–428. doi: 10.1016/s1074-7613(00)80498-7. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka Y, Arbouin L, Gillet A, Lin S-Y, Magnan A, Malissen B, Malissen M. Early T-cell development in CD3-deficient mice. Immunol Rev. 1995;148:172–199. doi: 10.1111/j.1600-065x.1995.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 14.Qian D, Lev S, van Oers NSC, Dikic I, Schlessinger J, Weiss A. Tyrosine phosphorylation of Pyk2 is selectively regulated by Fyn during TCR signaling. J Exp Med. 1997;185:1253–1259. doi: 10.1084/jem.185.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]