Abstract
Using H-2Kd-restricted photoprobe-specific cytotoxic T lymphocyte (CTL) clones, which permit assessment of T cell receptor (TCR)-ligand interactions by TCR photoaffinity labeling, we observed that the efficiency of antigen recognition by CTL was critically dependent on the half-life of TCR-ligand complexes. We show here that antigen recognition by CTL is essentially determined by the frequency of serial TCR engagement, except for very rapid dissociations, which resulted in aberrant TCR signaling and antagonism. Thus agonists that were efficiently recognized exhibited rapid TCR–ligand complex dissociation, and hence a high frequency of serial TCR engagement, whereas the opposite was true for weak agonists. Surprisingly, these differences were largely accounted for by the coreceptor CD8. While it was known that CD8 substantially decreases TCR–ligand complex dissociation, we observed in this study that this effect varied considerably among ligand variants, indicating that epitope modifications can alter the CD8 contribution to TCR-ligand binding, and hence the efficiency of antigen recognition by CTL.
Modifications of antigenic peptides can affect CTL responses in a diverse manner, such as provoking either antagonism, weak or strong responses or selective activation of Fas (CD95/APO-1) dependent cytotoxicity (1–4). As to the mechanism, it has been shown that TCR antagonists provoke hypophosphorylation of CD3 ξ chains, which fail to recruit and activate the tyrosine kinase ZAP-70, thus impairing the ZAP-70–NFAT pathway of T cell activation (1, 5–7). Two main hypotheses have been proposed to explain these phenomena. First, ligand modification may result in accelerated dissociation of TCR-ligand complexes and thus perturb the cascade of biochemical events that is initiated by TCR-ligation (8–10). Alternatively, ligand modification may confer, via conformational changes in TCR, qualitative changes in signaling by TCR-associated molecules (11, 12).
The observation that blocking of CD8, or in the case of T helper cells CD4, can convert weak peptide agonists into antagonists (7, 13, 14), indicated that the way T cells perceive antigen is determined in part by the coreceptor. Since coreceptors are associated with the cytoplasmic tyrosine kinase p56lck, which plays a key role in T cell activation, it has been proposed that the particular pattern of TCR signaling seen using partial agonists and antagonists is the result of inefficient recruitment of coreceptor to TCR (7). On the other hand, it has been shown that CD8, by coordinate binding of MHC molecules that interact with TCR, significantly strengthens the avidity of TCR-ligand binding by decreasing the dissociation of TCR-ligand complexes (15, 16). The intimate participation of CD8 in TCR-ligand interactions raises the question whether CD8 may play a role in aberrant CTL function by modulating the dynamics of TCR-ligand interactions.
To assess TCR-ligand binding and its dependence on CD8, we used H-2Kd-restricted CTL clones that are specific for a photoreactive peptide derivative and thus permit measurement of TCR-ligand interactions by TCR photoaffinity labeling (4, 15, 17). To this end the Plasmodium berghei circumsporozoite peptide PbCS 252-260 (SYIPSAEKI) was modified by replacing PbCS S-252 with iodo-4-azidosalicylic acid (IASA) and conjugating PbCS K-259 with 4-azidobenzoic acid (ABA). Selective photoactivation of the IASA group permitted covalent attachment of the conjugate to Kd and photoactivation of the ABA group to TCR. By testing 12 peptide derivative variants on 7 CTL clones, we have previously identified 12 cases for which the efficiency of antigen recognition (cytotoxicity) and TCR-ligand binding (TCR photoaffinity labeling) diverged by five-fold or more (4). As these divergences often did not correlate with the avidity of TCR-ligand binding, we examine here whether they are related to the dissociation rates of TCR-ligand complexes and how these depend on the coreceptor CD8.
Materials and Methods
Chromium Release Assay.
The generation of the IASA-YIPSAEK(ABA)I-specific CTL clones S4, S14, S17, and T1 and their culture conditions have been described previously (4, 17). Antigenic activities of peptide derivatives were determined in a 51Cr-release assay as described (4, 17). In brief, 51Cr-labeled P815 cells (2 × 103 per well) were incubated in DMEM supplemented with FCS (5%) and Hepes (10 mM) with 10-fold dilutions of peptide derivative ranging from 10−6–10−13 M. Cloned CTL (6 × 103 per well) were added and after 4 h of incubation at 37°C the 51Cr content of supernatants was determined. The specific lysis was calculated as 100 × (experimental−spontaneous release)/(total− spontaneous release). The relative antigenic activities were calculated by dividing the concentrations of IASA-YIPSAEK(ABA)I required for half-maximal lysis by that required for the variant peptide derivatives. For sake of comparison the relative antigenic activities of the compounds were normalized by dividing by the relative Kd competitor activity, which expresses the ability of a peptide derivative to bind to Kd (4, 17). In experiments where Fab′ fragments of the anti-Kd α3 mAb SF1-1.1.1 were used, and in the antagonist assays, the peptide derivatives were covalently attached to P815 cell-associated Kd (see below).
Interferon γ Assay.
The CTL were incubated with sensitized target cells under the same conditions as for the cytotoxic assay, except that peptide derivative concentrations ranged from 10−6– 10−11 M. After 24 h incubation the IFN-γ content in supernatants was assessed by ELISA as previously described (18).
Kd Photoaffinity Labeling.
Synthesis and photoaffinity-labeling procedures were performed essentially as described (4, 17, 19). In brief, for photoaffinity labeling of cell-associate Kd molecules, P815 mastocytoma cells were incubated with the respective peptide derivatives for 60 min at 37°C, followed by UV irradiation at ⩾350 nm. Preparation of 125I-labeled soluble covalent Kd-peptide derivative complexes was performed as described (4). The stock solutions had concentrations of 1–5 × 108 cpm/ml and specific radioactivities of ∼2,000 Ci/mMol.
TCR Photoaffinity Labeling.
TCR photoaffinity labeling was performed as described (4, 15, 17, 19), except that the samples were incubated at 26°C for 30–60 min before UV irradiation at 312 ± 40 nm. For TCR-ligand complex dissociation experiments CTL (6 × 106–2 × 107 cells/ml) were preincubated in medium with 125I-labeled Kd-peptide derivatives complexes (1–6 × 107 cpm/ml for 90–150 min at 0–4°C or for 30–60 min in medium containing SF1-1.1.1 Fab′ (20 μg/ml). Aliquots (100–700 μl) were added to 10-ml volumes of 26°C medium containing 0.7% FCS and anti-Kd α1 mAb 20-8-4S (10 μg/ml), which prevents TCR-ligand binding (15), and incubated at 26°C for the indicated periods. After UV irradiation for 20 s with a 500 W UV B lamp (Dr. Hönle Inc., Munich, Germany, equipped with an infrared and an UV 296-nm filter), TCR were immunoprecipitated and analyzed by SDS-PAGE. TCR photoaffinity labeling was quantified by phosphor-imaging and normalized relative to the wild-type as described (4). Mean values and standard deviations were calculated from three to six independent experiments.
TCR-ligand Binding.
TCR-ligand binding was determined by a direct binding assay as previously described (4), except that the incubations of CTL (1.2 × 106 cells/200 μl) with 125I-labeled Kd-peptide derivative complexes (3–10 × 106 cpm/incubation) were performed for 30–60 min at 26°C. Specific TCR-ligand binding was calculated by subtracting from the total cell-associated cpm, the cpm measured in presence of anti-Kd mAb 20-8-4S (10 μg/ml). The specific binding (cpm) for the wild-type (wt) ligand for a given clone was referred to as 1 and the binding of the variant ligands expressed relative to this value. Due to high background the difference between TCR-ligand binding in the presence and absence of SF1-1.1.1 Fab′ was assessed by TCR photoaffinity labeling. Each experiment was performed in triplicates and repeated at least twice.
Results and Discussion
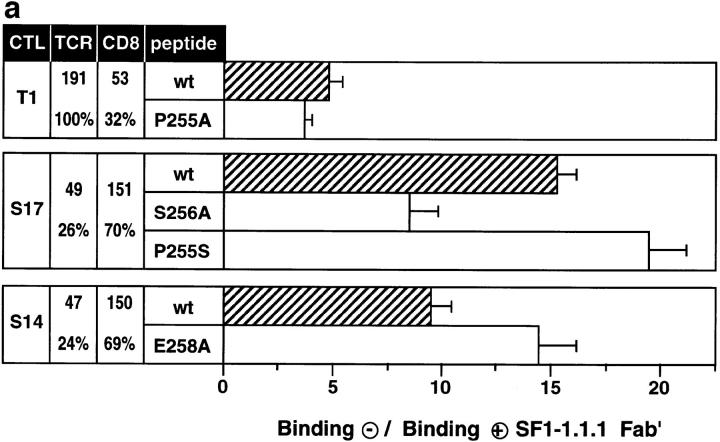
To assess the significance of TCR-ligand complex dissociation for CTL function, we selected two cases, in which antigen recognition was more efficient than TCR-ligand binding (strong agonists) (peptide derivative variant P255A on T1 CTL and S256A on S17 CTL) and two examples where recognition was less efficient than binding (weak agonists) (P255S on S17 CTL and E258A on S14 CTL) (Fig. 1 a). Since perforin dependent cytotoxicity, as mainly assessed in the used chromium release assay, is a rapid CTL response and takes place at very low peptide concentrations (20, 21), we also assessed the interferon-γ (IFN-γ) response, which requires higher peptide concentrations and sustained TCR signaling for extended periods of time (21). In spite of these differences both CTL responses were remarkably similar, except that on S14 CTL for E258A the IFN-γ production was more efficient than cytotoxicity (Fig. 1 b). Occasional divergences between cytolytic and IFN-γ CTL responses among peptide variants have also been observed in other systems (24).
Figure 1.

Antigen recognition, IFN-γ production and TCR-ligand binding for IASA-YIPSAEK(ABA)I (wt) and variants on cloned CTL. The normalized relative antigenic activities (cytotoxicity) and TCR-ligand binding at 26°C are shown as open and full bars, respectively (a), and the IFN-γ production as open bars (b). All values were normalized relative to IASA-YIPSAEK(ABA)I. The cases for which the CTL response was ⩾5-fold lower than TCR-ligand binding are shown in red and those for which it was ⩾5-fold higher in green. TCR antagonists are shown in purple. The variants tested include IASA-YIASAEK(ABA)I (P255A), IASA-YIPSAAK(ABA)I (E258A), IASA-YISSAEK(ABA)I (P255S), IASA-YIPAAEK(ABA)I (S256A), IASA-YILSAEK(ABA)I (P255L), and the CTL clones T1, S14, and S17. Some of the experiments were performed in the presence of SF1-1.1.1 Fab′. Each experiment was performed in triplicates and the mean values and standard deviations were calculated from at least two experiments.
Assessment of the kinetics of TCR-ligand complex dissociation showed that for both strong agonists the dissociation was significantly faster than for the wild-type ligand (Fig. 2, a and c). In contrast, for the two weak agonists dissociation was markedly slower, even though in one case (E258A on S14 CTL) TCR-ligand binding was weak (Fig. 2, b and c). For sake of increased accuracy, these kinetics were assessed at 26°C. With S17 CTL kinetics were also measured at 37°C (Fig. 2 d), showing that on living CTL TCR-ligand complex dissociation increases considerably with temperature, as has been reported for T1 CTL (15). These findings are consistent with the concept of serial TCR engagement (21, 23, 24), namely that CTL activation depends on the frequency of serial TCR engagement, which is determined by the rate of TCR-ligand complex dissociation.
Figure 2.
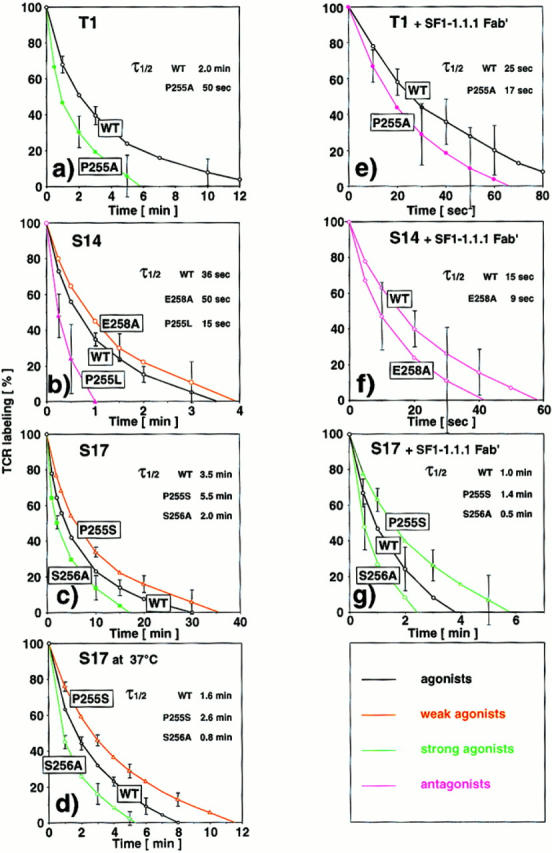
TCR-ligand complex dissociation kinetics on cloned T1, S17 and S14 CTL. Aliquots of CTL, preincubated in the absence or presence of SF1.-1.1.1 Fab′ with soluble covalent complexes of Kd and IASA-YIPSAEK(ABA)I or variants, were diluted into aliquots of DMEM containing anti-Kd α1 mAb 20-8-4S and UV irradiated after the indicated periods of incubation. All kinetic experiments were performed at 26°C, except the one shown in d, which was assessed at 37°C. The TCR photoaffinity labeling observed at time 0 was defined as 100%. τ1/2 designates the time required for 50% dissociation.
To test this hypothesis, we repeated these experiments in the presence of Fab′ fragments of the anti-Kd α3 mAb SF1-1.1.1, which prevents participation of CD8 in TCR-ligand binding, but not CD8 dependent adhesion (15). Remarkably, in the presence of this reagent the efficiency of antigen recognition (cytotoxicity) was often unchanged or even increased (Fig. 1 a). This was also true for IFN-γ production; indeed, in two instances blocking of CD8 substantially increased this response (wt and P255S on S17 CTL) (Fig. 1 b). However in other cases SF1-1.1.1 Fab′ dramatically reduced the cytotoxic or IFN-γ response (P255A on T1, wt and E258A on S14 CTL) (Fig. 1). With the exception of the latter cases, blocking of CD8 reduced TCR-ligand binding more than it decreased the efficiency of antigen recognition, as a result the efficiency of antigen recognition was increased relative to TCR-ligand binding; i.e., agonists or weak agonists were converted into strong agonists (Fig. 1). TCR-ligand complex dissociation was always considerably faster in the presence of SF1-1.1.1 Fab′ than in its absence (Fig. 2). This is consistent with reports showing that CD8 stabilizes TCR-ligand binding by decreasing dissociation of TCR-ligand complexes (15, 16). Our finding that acceleration of TCR-ligand complex dissociation increased the relative efficiency of antigen recognition supports the concept that CTL activation depends on the frequency of serial TCR engagement. It has been reported that CD8 expression on CTL can be downmodulated by antigen (25, 26), which is likely to have similar effects as the CD8 blocking experiments described here.
Exceptions to this rule were the cases where blocking of CD8 participation in TCR-ligand binding dramatically impaired antigen recognition. As it has been shown that blocking of CD4 can convert agonists into antagonists (7, 13), we examined whether, in the presence of SF1-1.1.1 Fab′, the variants P255A and E258A antagonized the cytotoxic responses of T1 and S14 CTL, respectively. As shown in Fig. 3, this was indeed the case. In the presence of this reagent the recognition of IASA-YIPSAEK(ABA)I by T1 (Fig. 3 a) and S14 CTL (Fig. 3 b) was significantly reduced on target cells that expressed covalent Kd-P255A or Kd-S256A complexes, compared to target cells that expressed irrelevant Kd-P255H complexes (4). Consistent with these findings we observed in both cases predominant pp21 ξ chain phosphorylation in the presence, but not in the absence of SF1-1.1.1 Fab′ (data not shown). This form of ξ chain phosphorylation has been shown to be preferentially or exclusively induced by TCR antagonists (1, 5–7). Remarkably, in both cases exceedingly fast TCR-ligand complex dissociations were observed (Fig. 2, e and f). The only other comparably fast dissociation was recorded for variant P255L on S14 CTL, which is an antagonist for this clone (Fig. 2 b and reference 4). These observations are in agreement with the kinetic proofreading concept (8, 9) and a study by Lyons et al. (10) demonstrating that TCR-ligand complex dissociation is more rapid for antagonists than for agonists. Taken collectively our findings indicate that acceleration of TCR-ligand complex dissociation increases the efficiency of antigen recognition by CTL up to a critical threshold, beyond which TCR engagement results in aberrant TCR signaling and TCR antagonism.
Figure 3.
Blocking of CD8 converts agonists P255A and S256A into antagonists for T1 and S14 CTL, respectively. T1 (a) or S14 CTL (b) were incubated in the presence of IASA-YIPSAEK(ABA)I 10−9 M in (a), 10−7 M in (b) and SF1-1.1.1 Fab′ (20 μg/ml) with 51Cr-labeled P815 cells, expressing Kd molecules crosslinked with variant P255A, 10−9 M (a) or E258A, 10−8 M (b) or P255H, 10−9 M in a and 10−8 M in b, a derivative not recognized by either TCR (4). The mean values and standard deviations were calculated from triplicate values of a representative experiment.
It is interesting to note that the half-life of TCR-ligand complexes varied considerably among different CTL clones (Fig. 2, a–c), which may explain why certain CTL clones are more prone to TCR antagonism than others (4). For example with S17 CTL, which exhibited remarkably slow dissociation kinetics, TCR antagonism was never observed, even when testing large panels of epitope variants, whereas the opposite was true for S14 CTL and, to a lesser extent, T1 CTL, especially in the presence of SF1-1.1.1 Fab′(Figs. 1 and 2, reference 4 and unpublished data).
A striking finding of our study is that the contribution of CD8 to TCR-ligand binding varied not only among CTL clones, but also among epitope variants on given clones (Fig. 4 a). While the former differences can be explained in part by variations in CD8 and TCR expression (Fig. 4 a), the latter indicate that epitope modifications can alter the avidity of CD8 participation in TCR-ligand binding. This explains, at least in part, why blocking of CD8 accelerated TCR-ligand complex dissociation in a diverse manner, and why one cannot reliably predict from the avidity of TCR-ligand binding the dissociation rates and hence the functional phenotype of epitope variants (Figs. 1 and 2 and reference 4). Moreover, several ligand variants displayed different ratios of TCR α versus β chain photoaffinity labeling (Fig. 4 b). Similar findings were obtained at 0–4°C (unpublished data), i.e., in the absence of metabolically active cellular processes, suggesting that epitope modification can induce either conformational changes in TCR or slightly alter the orientation of TCR-ligand binding, as a result of which CD8 may participate more or less avidly in TCR-ligand binding. This view is consistent with the observation that anti-TCR mAb and their Fab′ can substantially affect T cell responses (11, 12), and suggests that the conformational changes induced by such reagents, can affect T cell function by interfering with TCR-coreceptor cooperation. It is also interesting to note that TCR-ligand binding apparently can induce structural changes in the α3 domain of MHC class I molecules, which may alter their interaction with CD8 (27, 28).
Figure 4.
Epitope modifications alter the contribution of CD8 to TCR-ligand binding. The degree of CD8 participation in TCR-ligand binding is expressed as the ratio of TCR-ligand binding in the absence, divided by the binding in the presence of SF1-1.1.1 Fab′ (a). The surface expression of TCR and CD8 was assessed by flow cytometry after staining with antibodies H57-597 and H35-17, respectively. The values indicate mean fluorescence intensities for a representative experiment. (b) Different ligand variants exhibit different ratios of TCR-α versus -β chain TCR photoaffinity labeling on T1 and S17 CTL. An autoradiogram of SDS-PAGE (10%, reducing conditions) of a representative experiment is shown.
The present study shows that the half-life of TCR-ligand complexes is of critical importance for CTL activation and is determined to a considerable degree by the coreceptor CD8. The finding that epitope modifications can alter the participation of CD8 in TCR-ligand binding, and hence TCR-ligand complex dissociation, reveals a new principle by which CD8 can affect CTL function. However CD8 plays other roles in CTL function as well, such as mediating CTL-target cell adhesion and participation in CTL activation by CD8-dependent signaling (29, 30), which must also be taken into account for a comprehensive understanding of the role of CD8 in normal and aberrant CTL function.
Acknowledgments
We thank C. Horvath and P. Bassanini for excellent technical assistance, Dr. C. Servis for peptide and conjugate synthesis, Dr. P. Romero for helpful discussions and Anna Zoppi for preparing the manuscript.
Footnotes
Dr. Denis Hudrisier was supported by an “Association Française pour la Recherche Thérapeutique” fellowship.
References
- 1.Kersh GJ, Allen PM. Essential flexibility in the T-cell recognition of antigen. Nature. 1996;380:495–498. doi: 10.1038/380495a0. [DOI] [PubMed] [Google Scholar]
- 2.Jameson SC, Carbone FR, Bevan MJ. Clone-specific T cell receptor antagonists of major histocompatibility complex class I-restricted cytotoxic T cells. J Exp Med. 1993;177:1541–1550. doi: 10.1084/jem.177.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao W, Tykodi SS, Esser MT, Braciale VL, Braciale TJ. Partial activation of CD8+T cells by a self-derived peptide. Nature. 1995;378:295–298. doi: 10.1038/378295a0. [DOI] [PubMed] [Google Scholar]
- 4.Kessler BM, Bassanini P, Cerottini J-C, Luescher IF. Effects of epitope modification on T cell receptor-ligand-binding and antigen recognition by seven H-2Kd-restricted cytotoxic T lymphocyte clones specific for a photoreactive peptide derivative. J Exp Med. 1997;185:653–662. doi: 10.1084/jem.185.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reis e Sousa C, Levine EH, Germain RN. Partial signalling by CD8+T cells in response to antagonist ligands. J Exp Med. 1996;184:149–157. doi: 10.1084/jem.184.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signalling: altered phospho-ξ and lack of ZAP70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 7.Madrenas J, Chau LA, Smith J, Bluestone JA, Germain RN. The efficiency of CD4 recruitment to ligand-engaged TCR controls the agonist/partial agonist properties of peptide-MHC molecule ligands. J Exp Med. 1997;185:219–230. doi: 10.1084/jem.185.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKeithan TW. Kinetic proofreading in T-cell receptor signal transduction. Proc Natl Acad Sci USA. 1995;92:5042–5046. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabinowitz JD, Beeson C, Lyons DS, Davis MM, McConnell HM. Kinetic discrimination in T-cell activation. Proc Natl Acad Sci USA. 1996;93:1401–1405. doi: 10.1073/pnas.93.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyons DS, Lieberman SA, Hampl J, Boniface JJ, Chien Y-H, Berg LJ, Davis MM. A TCR binds to antagonist ligands with lower affinities and faster dissociation rates than to agonists. Immunity. 1996;5:53–61. doi: 10.1016/s1074-7613(00)80309-x. [DOI] [PubMed] [Google Scholar]
- 11.Yoon ST, Dianzani U, Bottomly K, Janeway CJ. Both high and low avidity antibodies to the T cell receptor can have agonist or antagonist activity. Immunity. 1994;1:563–569. doi: 10.1016/1074-7613(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 12.Janeway CJ. Ligands for the T-cell receptor: hard times for avidity models. Immunol Today. 1995;16:223–225. doi: 10.1016/0167-5699(95)80163-4. [DOI] [PubMed] [Google Scholar]
- 13.Vidal K, Hsu BL, Williams CB, Allen PM. Endogenous altered peptide ligands can affect peripheral T cell responses. J Exp Med. 1996;183:1311–1321. doi: 10.1084/jem.183.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jameson SC, Hogquist KA, Bevan MJ. Specificity and flexibility in thymic selection. Nature. 1994;369:750–752. doi: 10.1038/369750a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luescher IF, Vivier E, Layer A, Mahiou J, Godeau F, Malissen B, Romero P. CD8 modulation of T-cell antigen receptor-ligand interactions on living cytotoxic T lymphocytes. Nature. 1995;373:353–356. doi: 10.1038/373353a0. [DOI] [PubMed] [Google Scholar]
- 16.Garcia KC, Scott CA, Brunmark A, Carbone FR, Peterson PA, Wilson IA, Teyton L. CD8 enhances formation of stable T-cell receptor MHC class I molecule complexes. Nature. 1996;384:577–581. doi: 10.1038/384577a0. [DOI] [PubMed] [Google Scholar]
- 17.Luescher IF, Anjuere F, Peitsch MC, Jongeneel CV, Cerottini J-C, Romero P. Structural analysis of TCR-ligand interactions studied on H-2Kd-restricted cloned CTL specific for a photoreactive peptide derivative. Immunity. 1995;3:51–63. doi: 10.1016/1074-7613(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 18.Mattner F, Magram J, Ferrante J, Launois P, Di Padova K, Behin R, Gately MK, Louis JA, Alber G. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania majorand mount a polarized Th2 cell response. Eur J Immunol. 1996;26:1553–1559. doi: 10.1002/eji.1830260722. [DOI] [PubMed] [Google Scholar]
- 19.Luescher IF, Cerottini J-C, Romero P. Photoaffinity labeling of the T cell receptor on cloned cytotoxic T lymphocytes by covalent photoreactive ligand. J Biol Chem. 1994;269:5574–5582. [PubMed] [Google Scholar]
- 20.MacDonald HR. Early detection of potentially lethal events in T cell-mediated cytolysis. Eur J Immunol. 1975;5:251–254. doi: 10.1002/eji.1830050406. [DOI] [PubMed] [Google Scholar]
- 21.Valitutti S, Müller S, Dessing M, Lanzavecchia A. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J Exp Med. 1996;183:1917–1921. doi: 10.1084/jem.183.4.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin S, Kohler H, Weltzien HU, Leipner C. Selective activation of CD8 T cell effector functions by epitope variants of lymphocytic choriomeningitis virus glycoprotein. J Immunol. 1996;157:2358–2365. [PubMed] [Google Scholar]
- 23.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering on many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 24.Valitutti S, Lanzavecchia A. Serial triggering of TCRs: a basis for the sensitivity and specificity of antigen recognition. Immunol Today. 1997;18:299–304. [PubMed] [Google Scholar]
- 25.Hämmerling GJ, Schönrich G, Momburg F, Auphan N, Malissen M, Malissen B, Schmitt-Verhulst A-M, Arnold B. Non-deletional mechanisms of peripheral and central tolerance: studies with transgenic mice with tissue-specific expression of a foreign MHC class I antigen. Immun Rev. 1991;122:47–67. doi: 10.1111/j.1600-065x.1991.tb00596.x. [DOI] [PubMed] [Google Scholar]
- 26.Robbins PA, McMichel HJ. Immune recognition of HLA molecules downmodulates CD8 expression on cytotoxic T lymphocytes. J Exp Med. 1991;173:221–230. doi: 10.1084/jem.173.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia KC, Degano M, Stanfield RL, Brunmark A, Jackson MR, Peterson PA, Teyton L, Wilson IA. An αβ T cell receptor structure at 2.5 A and its orientation in the TCR-MHC complex. Science. 1996;274:209–219. doi: 10.1126/science.274.5285.209. [DOI] [PubMed] [Google Scholar]
- 28.Garboczi DN, Gosh P, Utz U, Fan QR, Biddison WE, Wiley DC. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- 29.Kane KP, Mescher MF. Activation of CD8-dependent cytotoxic T lymphocyte adhesion and degranulation by peptide class I antigen complexes. J Immunol. 1993;150:4788–4797. [PubMed] [Google Scholar]
- 30.Anel A, O'Rourke AM, Kleinfeld AM, Mescher MF. T cell receptor and CD8-dependent tyrosine phosphorylation events in cytotoxic T lymphocytes: activation of p56lckby CD8 binding to class I protein. Eur J Immunol. 1996;26:2310–2319. doi: 10.1002/eji.1830261007. [DOI] [PubMed] [Google Scholar]