Abstract
Natural killer (NK) cells are named based on their natural cytotoxic activity against a variety of target cells. However, the mechanisms by which sensitive targets activate killing have been difficult to study due to the lack of a prototypic NK cell triggering receptor. Pharmacologic evidence has implicated protein tyrosine kinases (PTKs) in natural killing; however, Lck-deficient, Fyn-deficient, and ZAP-70–deficient mice do not exhibit defects in natural killing despite demonstrable defects in T cell function. This discrepancy implies the involvement of other tyrosine kinases. Here, using combined biochemical, pharmacologic, and genetic approaches, we demonstrate a central role for the PTK Syk in natural cytotoxicity. Biochemical analyses indicate that Syk is tyrosine phosphorylated after stimulation with a panel of NK-sensitive target cells. Pharmacologic exposure to piceatannol, a known Syk family kinase inhibitor, inhibits natural cytotoxicity. In addition, gene transfer of dominant-negative forms of Syk to NK cells inhibits natural cytotoxicity. Furthermore, sensitive targets that are rendered NK-resistant by major histocompatibility complex (MHC) class I transfection no longer activate Syk. These data suggest that Syk activation is an early and requisite signaling event in the development of natural cytotoxicity directed against a variety of cellular targets.
The NK cell is a type of lymphocyte that is able to mediate natural cytotoxicity against a variety of tumor cells, virus-infected cells, and hematopoietic targets (1). However, little is known regarding the mechanisms by which these targets trigger natural cytotoxicity. In addition to natural cytotoxicity, the NK cell can mediate antibody-dependent cell-mediated cytotoxicity (ADCC)1 through its FcR for IgG, FcγRIII. In contrast to natural cytotoxicity, ADCC is well defined with respect to the receptor–ligand interaction as well as intracellular second messenger involvement (for review see reference 2). In spite of this contrast between the well-defined FcR-dependent and the poorly defined natural cytotoxic mechanisms, both killing mechanisms can be inhibited by MHC-recognizing killer cell inhibitory receptors (KIR). Since KIR inhibition seems to target signaling pathways initiated by immunoreceptor tyrosine-based activation motif (ITAM)–containing receptor complexes (e.g., FcγRIII, TCR, and FcεRI; references 3–9), the ability of both FcR-dependent and natural cytotoxicity to be inhibited by KIR suggests that there may be shared signaling elements in the pathways used during the two alternative routes of initiating NK cell–mediated cytotoxicity.
Evidence using the PTK inhibitors herbimycin A and genistein demonstrates that PTK activation is necessary for both FcR-mediated and natural cytotoxicity (10–14). However, the available genetic evidence from Lck- and Fyn-deficient mice suggests that neither of these specific Src family tyrosine kinases is necessary for NK cell–mediated cytotoxicity (15, 16). This raises the possibility that another Src family PTK (e.g., Lyn) could subserve this role. Alternatively, the receptors initiating NK cell–mediated killing could use a different kind of PTK that might function in a Src family kinase-independent manner. The Syk family member ZAP-70, which is expressed in NK cells, is unlikely to be this PTK because ZAP-70 appears to require activation by a Src family PTK (17–20); and because NK cells from humans and mice lacking ZAP-70 can mediate normal natural cytotoxicity and ADCC (21–23). In contrast, Syk itself could perform this role. Syk is expressed in NK cells (24) and evidence suggests that Syk is able to function in the absence of Src family kinases (18–20, 25). Although chimeric receptors containing the intracellular portion of ZAP-70 require cross-linking with Src family kinases such as Lck or Fyn to mediate cytotoxicity, chimeric receptors containing Syk can initiate killing in the absence of Src family PTK costimulation (18). In addition, Syk, but not ZAP-70, can induce the tyrosine phosphorylation of ITAM-containing receptor subunits in an Src family PTK-independent manner (19, 20, 25).
To test the hypothesis that the Syk tyrosine kinase is functionally involved in natural cytotoxicity, we first stimulated NK cells with a panel of sensitive targets and then characterized their intracellular signaling events. Although stimulation of NK cells with sensitive targets is known to induce increases in intracellular free Ca2+ and inositol phosphate release (26–30), more proximal specific signaling events have not been elucidated. In this study, we describe a rapid and reversible increase in the tyrosine phosphorylation of NK cell–derived Syk after target cell stimulation. In addition, either pharmacologic inhibition of Syk kinase activity or expression of dominant-negative, kinase-inactive Syk in NK cells inhibits natural killing. Finally, tumor cells that are made resistant to NK cell–mediated cytotoxicity by MHC class I transfection no longer activate Syk. Together these data emphasize the central contribution of the Syk tyrosine kinase to generation of natural cytotoxicity.
Materials and Methods
Cells, Chemicals, and Abs.
The P815 cell line (murine mastocytoma) was obtained from American Type Culture Collection (Rockville, MD). The HLA class I–deficient C1R cell line (B-LCL) and its HLA-transfected derivatives were supplied by Peter Cresswell (Yale University, New Haven, CT). The HLA class I–deficient cell line 721.221 (B-LCL) was provided by Peter Parham (Stanford University, Palo Alto, CA). Human PBL were isolated from defibrinated blood by Ficoll-Hypaque density centrifugation. Human CD16+ NK cell lines were isolated and characterized as previously described (29). All chemicals, unless otherwise noted, were obtained from Sigma Chemical Co. (St. Louis, MO). The 3G8 hybridoma (anti-FcγRIII) was provided by Bice Perussia (Thomas Jefferson University, Philadelphia, PA; reference 31) and 3G8 mAb was purified by affinity chromatography over protein A agarose. The generation and characterization of rabbit antisera specific for ZAP-70 have been previously described (32). The 4D10 mAb specific for Syk was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The antiphosphotyrosine mAb 4G10 was purchased from Upstate Biotechnology Inc. (Lake Placid, NY).
Cytotoxicity Assays.
The 51Cr-release assays measuring direct NK cell–mediated cytotoxicity were performed as previously described (29). For experiments evaluating the effects of piceatannol (Boehringer Mannheim, Indianapolis, IN), cloned human NK cells were pretreated with the indicated concentration of the drug for 15 min at 37°C. Lytic units were calculated based on 20% cytotoxicity (33).
Vaccinia Viruses.
ZAP-70K cDNA was excised from pcDNA3. KD.myc.Zap70 (provided by Lawrence E. Samelson, National Institutes of Health, Bethesda, MD; reference 34) with BamHI/ NotI, and blunt-ended with Klenow. The blunt-ended ZAP-70K fragment was inserted into the SmaI cloning site of the vector pSC11 and introduced into WR strain vaccina virus via homologous recombination. Recombinant vaccinia viruses encoding wild-type Syk, wild-type ZAP-70, SykT, and SykK as well as the pSC-65 vector were provided by Jean-Pierre Kinet and Andrew M. Scharenberg (Harvard Medical School, Boston, MA; references 35, 36). For infections, NK cells (2 × 106 cells/ml) were incubated in serum-free RPMI 1640 for 1 h at 37°C at a multiplicity of infection of 20. Cells were then incubated for the remainder of the indicated infection time at 106 cells/ml in RPMI 1640 supplemented with 10% bovine calf serum.
Whole Cell Lysates, Cell Stimulation, Immunoprecipitation, and Immunoblot Analysis.
For whole cell lysates, 1.5 × 106 NK cells were lysed in buffer containing 10 mM Tris (pH 7.4), 50 mM NaCl, 5 mM EDTA, 50 mM NaF, 30 mM Na4P2O7, and 1% Triton X-100 (pH 7.4). Protein levels were determined using the BCA Protein Assay (Pierce, Rockford, IL) and equal amounts of protein were resolved by SDS-PAGE. For cell stimulation, NK cells were resuspended at 108/ml and target cells were resuspended at 5 × 107/ml in RPMI 1640 supplemented with 0.5% BSA. 100 μl of NK cells were mixed with 100 μl of target cells (effector/target ratio of 2:1), pelleted at 5,000 rpm for 5 s, and then incubated at 37°C for the indicated time. For anti-FcR stimulation, NK cells were incubated at 4°C for 3 min with anti-FcγR mAb (3G8; 10 μg/ml). Washed cells were then mixed with goat anti–mouse IgG F(ab′)2 fragments (Organon Teknika-Cappel, Durham, NC), rapidly pelleted, and incubated for 1 min at 37°C. After stimulation, cells were lysed in buffer containing 20 mM Tris-HCl, 40 mM NaCl, 5 mM EDTA, 50 mM NaF, 30 mM Na4P2O7, 0.1% BSA, 1 mM Na3VO4, 1 mM PMSF, 5 μg/ ml aprotinin, 10 μg/ml leupeptin, and 1% Triton X-100 (pH 7.4). Samples were centrifuged at 15,000 g for 5 min to remove insoluble material. Supernatants were subjected to immunoprecipitation with either rabbit antiserum specific for ZAP-70 bound to protein A–Sepharose beads or with anti-Syk mAb 4D10 bound to either protein A–Sepharose or goat anti–mouse IgG agarose beads. Immunoprecipitates were washed and bound proteins were eluted with 50 μl of SDS-sample buffer. Proteins resolved by SDS-PAGE were electrophoretically transferred to Immobilon-P membranes (Millipore, Bedford, MA).
Tyrosine phosphorylated proteins and Syk expression were detected with the 4G10 and 4D10 mAbs, respectively, followed by sheep anti–mouse IgG coupled to horseradish peroxidase (Amersham International, Buckinghamshire, England). ZAP-70 expression was analyzed with ZAP-70–specific rabbit antisera and detected with protein A–horseradish peroxidase and the ECL detection system from Amersham.
32P-labeling and Phosphoamino Acid Analysis.
NK cells were washed with phosphate-free RPMI 1640 medium supplemented with 4% bovine calf serum and 1% l-glutamine. Cells were labeled with 0.5 mCi of [32P]orthophosphate/ml for 3 h at 37°C at 1.5 × 107 cells/ml in phosphate-free medium, washed twice, and then resuspended at 108 cells/ml, and 100 μl of NK cells were used for stimulation. Target cells were also washed with phosphate-free media (not containing [32P]orthophosphate) before use for cell stimulation.
For phosphoamino acid analysis, portions of the membrane corresponding to Syk on the autoradiogram were cut and boiled in 6 N HCl for 1 h at 110°C to elute the protein. Membrane fragments were rinsed twice with water and rinses were combined with eluate. Eluate plus rinse was dried in a speed-vac and resuspended in water/ethanol/methanol (10:5:1) before spotting on thin-layer chromatography (TLC) plates. Two cycles were run in solvent ethanol/ammonium hydroxide/water (105:42:6) with phosphoamino acid standards. TLC plates were sprayed with 0.2% ninhydrin in ethanol before exposure to film.
Results
NK-sensitive Targets Induce Tyrosine Phosphorylation of NK Cell Syk.
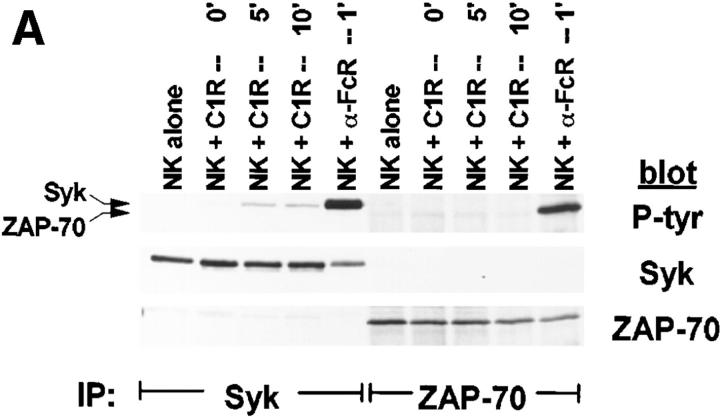
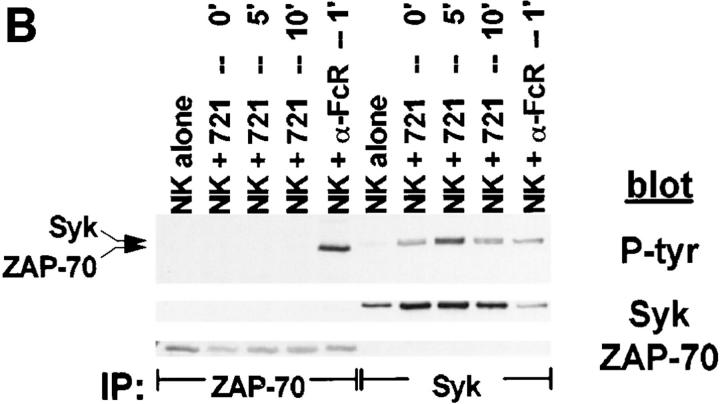
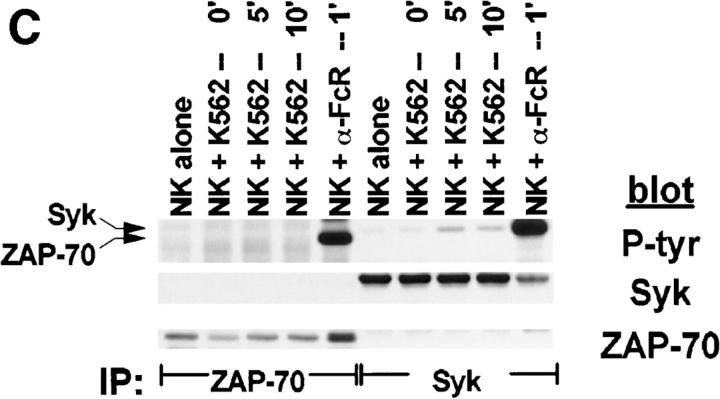
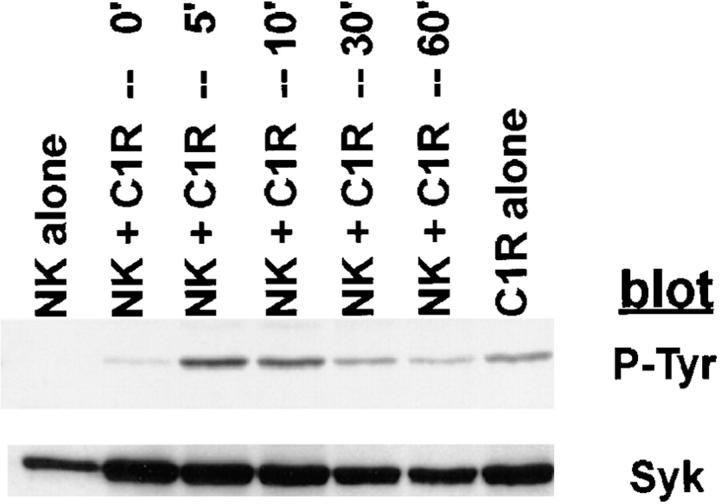
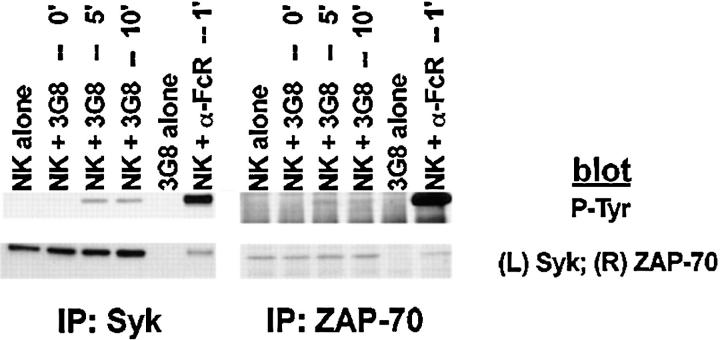
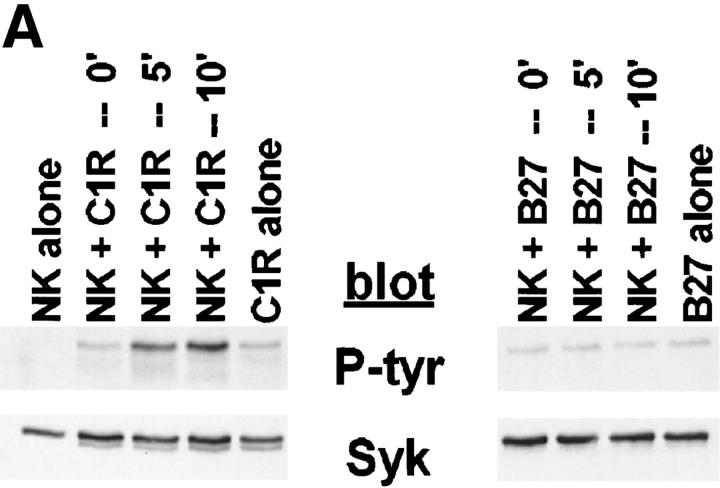
Although PTK activation is an early and requisite event in the generation of natural cytotoxicity (10, 11), genetic evidence suggests that the PTKs Lck, Fyn, and ZAP-70 are not required (15, 16, 21–23). These observations prompted us to evaluate whether the tyrosine kinase Syk is important for natural cytotoxicity. We first determined whether Syk was tyrosine phosphorylated after stimulation of NK cells with sensitive targets since an increase in Syk tyrosine phosphorylation correlates with increased Syk activation. Human NK cell clones were incubated either with the MHC class I–deficient B lymphoblastoid targets C1R or 721.221 or with the prototypic human NK cell target K562. After various times of incubation, cells were lysed and Syk was immunoprecipitated. We observed an increase in Syk tyrosine phosphorylation after NK cells were stimulated with each of the panel of targets (Fig. 1, A–C). This increase in Syk tyrosine phosphorylation was a relatively early event, peaking at 5 min and declining to baseline by 60 min (Fig. 2). Since both Syk family members Syk and ZAP-70 are expressed in NK cells and activated downstream of the FcR (24, 32, 37), we questioned whether NK cell–derived ZAP-70 was also tyrosine phosphorylated after incubation with sensitive targets. ZAP-70 immunoprecipitates from target cell–stimulated lysates revealed no increase in the tyrosine phosphorylation of ZAP-70 after target cell contact (Fig. 1, A–C). This was not a defect in the ability of ZAP-70 to be phosphorylated in these NK cells since cross-linking with FcR-specific antibodies on the same cells results in an increase in ZAP-70 tyrosine phosphorylation (Fig. 1, A–C). Thus, it appears that Syk and not ZAP-70 is activated when this panel of targets is used to stimulate NK cells. Consistent with the observations that both Syk and ZAP-70 are tyrosine phosphorylated after FcR ligation (24, 32, 37), stimulation of NK cells with the 3G8 hybridoma, which expresses membrane-bound antibody to the FcR, resulted in an increase in both Syk and ZAP-70 tyrosine phosphorylation (Fig. 3). These data suggest that while FcR cross-linking can stimulate the tyrosine phosphorylation of Syk and ZAP-70, stimulation of NK cells with this panel of sensitive targets results in the phosphorylation of Syk without detectable ZAP-70 phosphorylation.
Figure 1.
Tyrosine phosphorylation of Syk after stimulation of NK cells with targets. For each sample, 107 NK cells were mixed with 5 × 106 cells of either (A) C1R, (B) 721.221, or (C) K562, pelleted, and incubated at 37°C for the indicated times. For anti-FcR stimulation, NK cells were incubated for 1 min with cross-linked anti-FcR mAb (3G8). Syk or ZAP-70 immunoprecipitates were resolved by SDS-PAGE, transferred to membrane, and probed sequentially with antiphosphotyrosine mAb (P-tyr), anti-Syk mAb (Syk), and anti-ZAP-70 antiserum (ZAP-70).
Figure 2.
Kinetics of Syk tyrosine phosphorylation after stimulation of NK cells with targets. For each sample, 107 NK cells were mixed with 5 × 106 C1R target cells, pelleted, and incubated at 37°C for the indicated times. Syk immunoprecipitates were resolved by SDS-PAGE, transferred to membrane, and probed sequentially with antiphosphotyrosine mAb (P-Tyr) and anti-Syk mAb (Syk).
Figure 3.
Anti-FcR–bearing hybridomas induce tyrosine phosphorylation of Syk and ZAP-70. NK cells were stimulated with the 3G8 hybridoma or cross-linked 3G8 mAb as in Fig. 2. Syk or ZAP-70 immunoprecipitates were resolved by SDS-PAGE, transferred to membrane, and probed with antiphosphotyrosine mAb (P-tyr) and either anti-Syk mAb or anti-ZAP-70 antiserum.
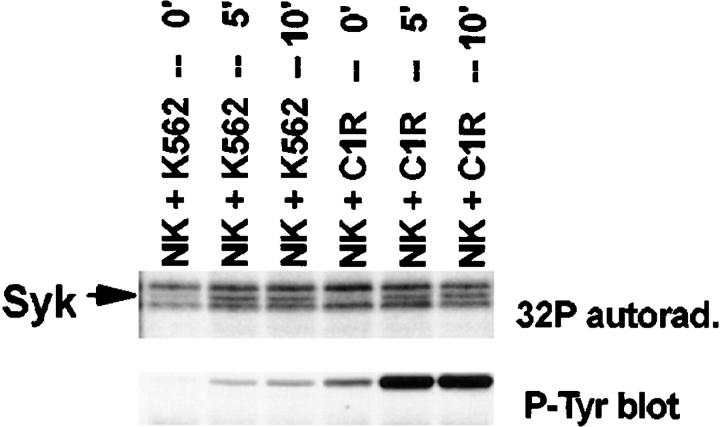
Although Syk activation has not been described in tumor cells undergoing cell death, we could not formally exclude the possibility that the endogenous Syk in the above panel of targets was undergoing tyrosine phosphorylation. To ensure that the observed increase in tyrosine phosphorylation was NK cell–derived Syk, NK cell clones were labeled with [32P]orthophosphate before cellular target stimulation. Indeed, Syk immunoprecipitates showed an increase in 32P incorporation of a band at 72 kD after stimulation (Fig. 4). A phosphotyrosine blot overlays a 72-kD radiolabeled band on the autoradiogram. Phosphoaminoacid analysis of the 72-kD band illustrates that the increase in 32P incorporation is due to an increase in phosphotyrosine and phosphoserine (data not shown). These data support the conclusion that NK cell–derived Syk is tyrosine phosphorylated after stimulation with NK-sensitive tumor targets.
Figure 4.
32P incorporation into NK cell–derived Syk. 107 32P-labeled NK cells were mixed with 5 × 106 cells of the indicated target (K562 or C1R), pelleted, and incubated at 37°C for the indicated times. Syk immunoprecipitates were resolved by SDS-PAGE, transferred to membrane, and exposed to x-ray film (Syk) followed by probing with antiphosphotyrosine mAb (P-tyr blot). cpm from phosphoamino acid analysis were: NK + K562 — 5′: 2.3-fold increase in [32P]Tyr, 2-fold increase in [32P]Ser; NK + K562 — 10′: 1.5-fold increase in [32P]Tyr, 1.5-fold increase in [32P]Ser; NK + C1R — 5′: 2.4-fold increase in [32P]Tyr, 1.6-fold increase in [32P]Ser; NK + C1R — 10′: 5.4-fold increase in [32P]Tyr, 2.5-fold increase in [32P]Ser.
Piceatannol Pretreatment Inhibits Natural Cytotoxicity.
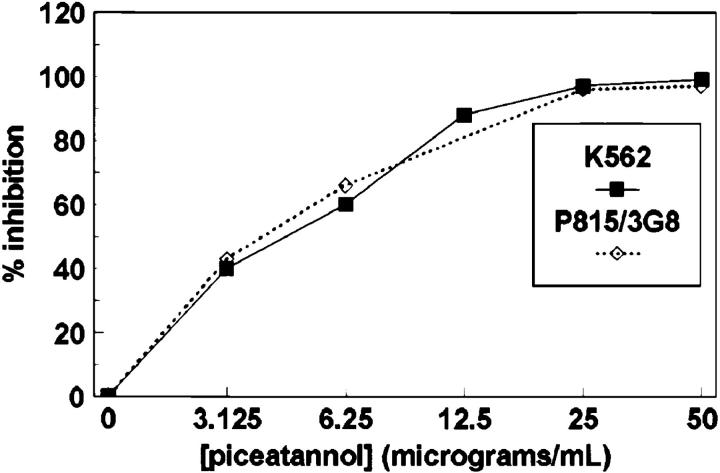
Although the above data demonstrate biochemically that Syk is modified in NK cells after target cell stimulation, a functional requirement of Syk for cytotoxicity remained to be demonstrated. To assess whether Syk family tyrosine kinase activity is required for natural cytotoxicity, we used the PTK inhibitor piceatannol (38). Syk has previously been shown to be 10-fold more sensitive to piceatannol treatment than Lyn, an Src family member, in in vitro kinase assays (38). Pretreatment of NK cell clones with piceatannol inhibited natural cytotoxicity and FcR-initiated killing in a concentration-dependent fashion with half-maximal inhibition at a concentration of ∼6 μg/ml (Fig. 5). This 50% inhibitory concentration is consistent with the concentration that exhibits specific inhibition of Syk in vitro kinase activity. These results suggest that the tyrosine kinase activity of the Syk family is functionally required for natural cytotoxicity.
Figure 5.
Piceatannol pretreatment inhibits NK cell– mediated cytotoxicity. NK cells were treated for 15 min at 37°C with the indicated concentration of piceatannol, washed, and then incubated for 4 h with either 51Cr-labeled K562 cells or 51Cr-labeled P815 cells coated with 0.15 μg/ml of the anti-FcR mAb 3G8. Data are expressed as percentage of inhibition of lytic U/106 cells calculated from NK cells incubated in vehicle alone.
Syk Overexpression Enhances Natural Cytotoxicity Whereas Kinase-inactive Syk Expression Inhibits Killing.
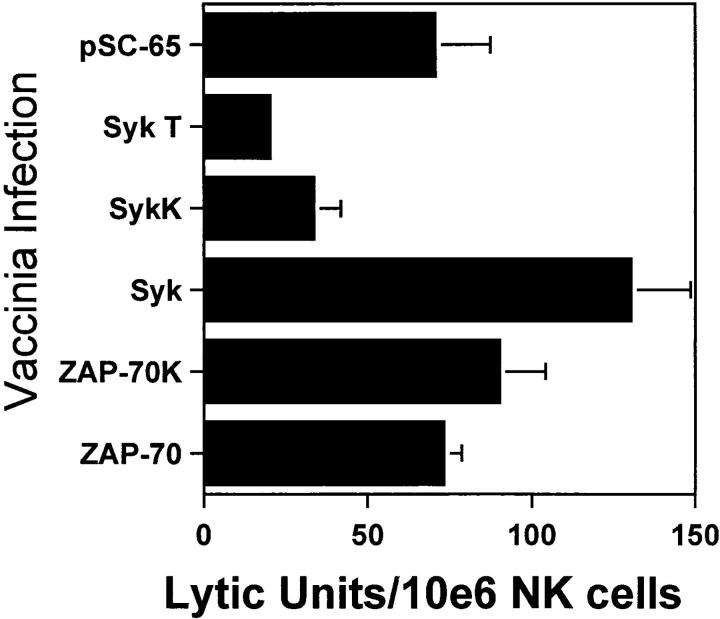
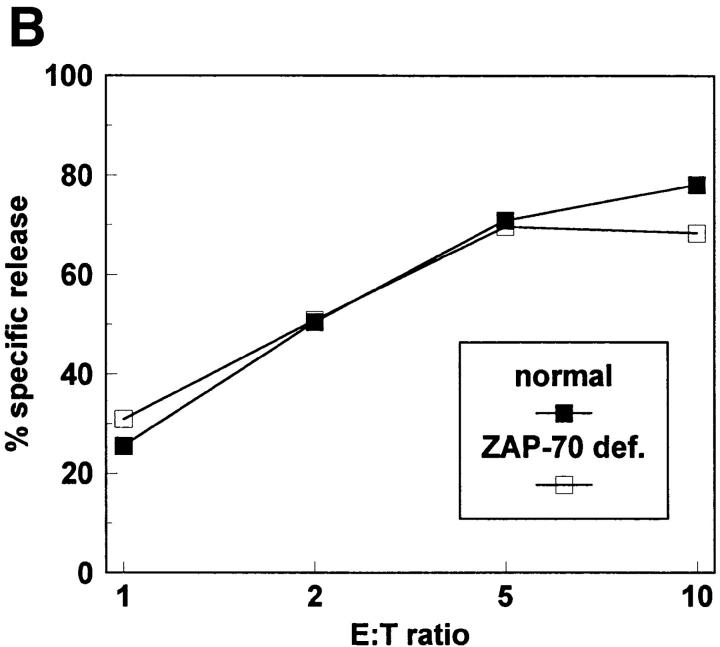
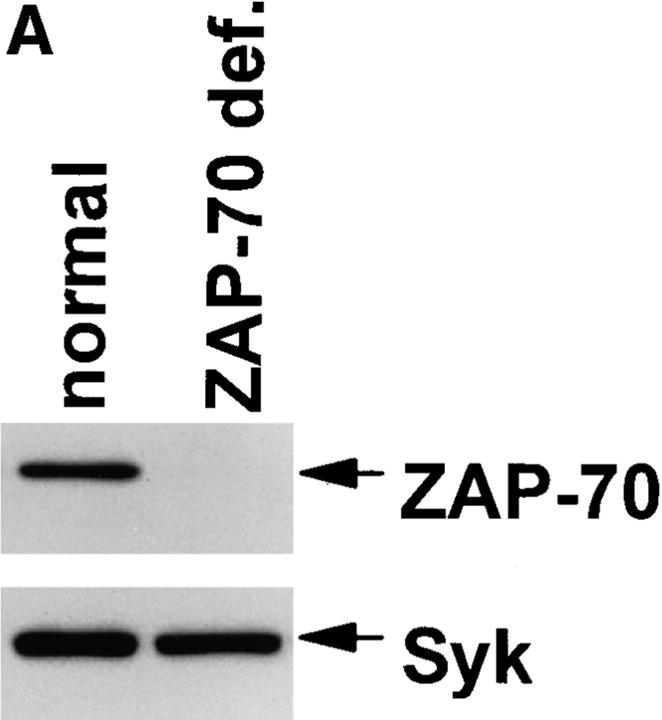
Although the data using piceatannol are consistent with a required role for Syk family PTK in the development of natural cytotoxicity, conclusions from pharmacologic approaches must be tempered by concerns regarding potential drug effects on other second messenger molecules. To further define a role for Syk in natural cytotoxicity, we took a genetic approach to express wild-type or kinase-inactive forms of Syk family members and to evaluate the effects on natural cytotoxicity. We used the vaccinia virus expression system to overexpress wild-type or kinase-inactive forms of the two Syk family members, Syk and ZAP-70, in NK cell clones. Two different Syk mutations were used. The SykT mutant, which has recently been shown to act as a dominant-negative in mast cells (35), is truncated at residue 395 so as to lack the kinase domain but contain the SH2 domains. The kinase-inactive SykK mutant has a point mutation at residue 395 (K to R) that is within the ATP-binding region of the kinase domain. Kinase inactive ZAP-70 (ZAP-70K) also has a K to R point mutation at residue 369 within the kinase domain. After a 4 h infection with virus containing the coding sequences either for wild-type Syk family PTK, mutant Syk family PTK, or the control vector, NK cell function was assessed in a 51Cr-release assay. Overexpression of wild-type Syk with vaccinia enhanced natural killing whereas wild-type ZAP-70 overexpression did not (Fig. 6). The kinase-inactive mutants of Syk (SykT, SykK) but not ZAP-70 (ZAP-70K) acted as dominant-negatives to inhibit natural killing (Fig. 6). Although the latter observation is consistent with the notion that ZAP-70 is not required for natural cytotoxicity, one cannot exclude the possibility that the inability of ZAP-70K to inhibit killing is due to insufficient expression of the transfected gene product. Therefore, we took a genetic approach to directly analyze this issue. Specifically, we derived NK cell clones from a patient with ZAP-70 deficiency (Fig. 7 A) and found that these clones mediated wild-type levels of natural killing (Fig. 7 B) as previously reported (21, 22). Taken together, the above results suggest a required role for Syk, but not ZAP-70, in natural killing. It should be emphasized that although our data indicate that ZAP-70 activity is not necessary for natural cytotoxicity, they do not exclude the possibility that under normal circumstances ZAP-70 may subserve some signaling role.
Figure 6.
Kinase-inactive Syk inhibits the generation of natural cytotoxicity. NK cells were infected for 4 h either with vector control vaccinia (pSC-65) or recombinant vaccinia encoding wild-type Syk, wild-type ZAP-70, truncated Syk (SykT), kinase-inactive Syk (SykK), or kinase-inactive ZAP-70 (ZAP-70K). Infected cells were incubated for 4 h with 51Cr-labeled 721.221 cells. Data are expressed as lytic U/106 cells ± one standard deviation.
Figure 7.
ZAP-70 is not required for natural cytotoxicity. (A) Whole cell lysates from normal and ZAP-70–deficient NK cells were resolved by SDS-PAGE, transferred to membrane, and probed with anti–ZAP-70 antisera (ZAP-70) or anti-Syk mAb (Syk). (B) Normal and ZAP-70–deficient NK cells were incubated for 4 h with 51Cr-labeled 721.221 cells.
NK-resistant Targets Fail to Induce Syk Tyrosine Phosphorylation.
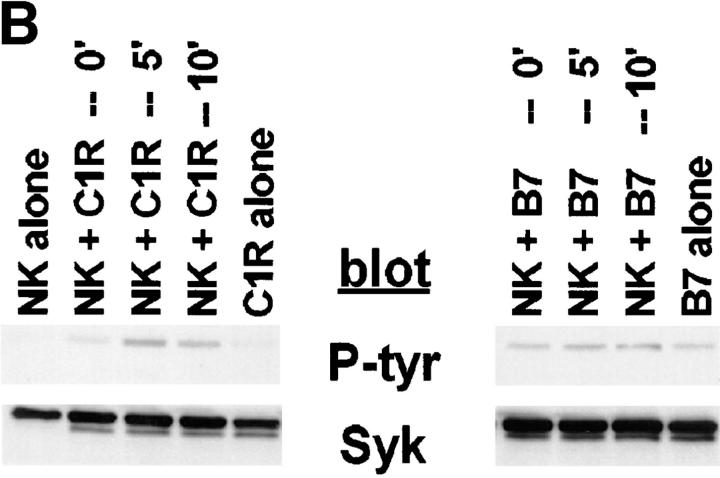
Since our data suggest that Syk is functionally involved in the development of natural killing, it would follow that an NK-resistant target might not induce Syk tyrosine phosphorylation. Targets sensitive to NK lysis can be made resistant by transfection with an HLA class I molecule, provided the NK cell expresses a KIR specific for that class I (for review see reference 39). Since the parental target cell has only been altered by the addition of class I, we can use these cells to compare sensitive and resistant targets with the same triggering ligands. We compared NK-resistant, MHC class I–transfected C1R cells to the parental class I–deficient NK-sensitive C1R in their ability to induce Syk tyrosine phosphorylation. A DX9+ NK cell clone expressing the p70 immunoglobulin superfamily KIR specific for HLA-B27 (and related HLA-A and -B alleles) was stimulated with either C1R or C1R-B27 targets before lysing the cells and immunoprecipitating Syk. The sensitive C1R target stimulated an increase in Syk tyrosine phosphorylation, whereas stimulation with the resistant C1R-B27 did not result in a detectable change in Syk tyrosine phosphorylation (Fig. 8 A). This result was extended to include the CD94/ NKG2A/B inhibitory receptors of the C-type lectin superfamily. An NK cell clone expressing the CD94/NKG2A/B inhibitory receptor that can recognize HLA-B7 was stimulated with C1R or C1R-B7 targets before lysing and immunoprecipitating Syk. Again, the sensitive C1R target stimulated an increase in Syk tyrosine phosphorylation, whereas the resistant C1R-B7 target did not (Fig. 8 B). C1R-B27 and C1R-B7 have somewhat higher basal levels of tyrosine phosphorylated Syk than C1R (see B27 alone versus C1R alone, Fig. 8 A, B7 alone versus C1R alone, Fig. 8 B). However, increases in the tyrosine phosphorylation of NK cell–derived Syk were only seen after incubation with the NK-sensitive targets. These data suggest that the ability of a target to generate NK cell–mediated killing is associated with its capacity to stimulate Syk activation in NK cells.
Figure 8.
MHC class I–bearing, NK-resistant targets do not stimulate an increase in Syk tyrosine phosphorylation. 107 NK cells were mixed with 5 × 106 cells of (A) C1R or C1R-B27, or (B) C1R or C1R-B7. The reaction mixtures were pelleted and incubated at 37°C for the indicated times. Syk immunoprecipitates were resolved by SDS-PAGE, transferred to membrane, and probed with antiphosphotyrosine mAb (P-tyr) or anti-Syk mAb (Syk).
Discussion
NK cells exhibit unprimed, natural cytotoxicity against many different targets (1). Here we examine the mechanisms by which NK cells generate cytotoxicity against a panel of tumor targets. We demonstrate that the PTK Syk plays a central role in NK cell–mediated cytotoxicity.
Mechanisms of natural cytotoxicity have been difficult to study due to the lack of defined triggering receptors. A number of candidate receptors have been described which can induce certain aspects of NK cell activation and/or cytotoxicity. Among these receptors are adhesion molecules such as the β1- and β2-integrins (40–44), and other receptors such as CD2 (45), CD69 (46), Lag-3 (47), DNAM-1 (48), KAR (4, 49–57), NK-TR (58), and NKR-P1 (59– 61). Antibodies to some of these receptors can enhance the killing of FcR-bearing resistant targets (“reverse ADCC”) as well as stimulating the release of inositol phosphates and intracellular free Ca2+. However, proving the direct involvement of such receptors in the natural killing of specific tumor targets has often been problematic. Given the number of NK cell receptors that are candidate triggering receptors and the broad range of sensitive target cells, the receptor–ligand interactions involved may be relatively specific for each effector–target combination. In addition to the mere presence or absence of triggering receptors on NK cells or triggering ligands on target cells, the distribution of these proteins in the cell membrane may be critical. Helander et al. (44) propose the β2-integrin LFA-1 as a receptor able to trigger natural cytotoxicity by recognizing its redistributed ligand ICAM (intracellular adhesion molecule)-2 on diseased versus normal cells. Their results suggest that when ICAM-2 is concentrated on the target cell membrane, LFA-1 can participate in triggering cytotoxicity. Given the potential heterogeneity in triggering receptors required for natural cytotoxicity and the possibility that ligand distribution on the target cell may be involved, we used a panel of NK-sensitive tumor targets to stimulate NK cell clones rather than isolating receptor–ligand interactions to examine the molecular mechanisms involved in natural killing. Indeed, our data suggest that for this panel of targets, the Syk tyrosine kinase plays a common regulatory role in the generation of natural cytotoxicity.
The molecular mechanisms by which Syk is activated during natural cytotoxicity are unknown. In contrast to the poorly defined receptor–ligand interactions involved in natural cytotoxicity, FcR-initiated killing has a well-defined receptor and signaling pathway. FcγRIII consists of a ligand-binding α chain noncovalently associated with homo- or heterodimers of the CD3-ζ and FcεRIγ chains (62–65). The ζ and γ chains contain ITAMs that are thought to serve as substrates for Src family PTK (for reviews see references 66 and 67). Once these ITAMs are phosphorylated by Src family PTK, Syk family PTKs can bind to the ITAM via their SH2 domains and activate downstream pathways. FcγRIII can induce tyrosine phosphorylation and activation of both Syk and ZAP-70 (24, 32, 37) but, similar to natural cytotoxicity, expression of dominant-negative, kinase-inactive forms of Syk but not ZAP-70 inhibit FcR-initiated killing (data not shown). This suggests that although Syk and ZAP-70 are activated after FcR cross-linking, ZAP-70 is not required for FcR-mediated cytotoxicity. These results are consistent with results from ZAP-70–deficient humans and mice showing no defects in natural cytotoxicity or ADCC (21–23).
Since our data suggest that Syk is a common signaling element used by FcR-initiated killing and natural cytotoxicity and since Syk family kinases are thought to dock to ITAM-containing molecules, this raises the possibility that ITAM-containing receptor complexes might be involved in natural cytotoxicity. The known ITAM-containing subunits expressed in NK cells, ζ and γ, do not appear to subserve this role in initiating natural cytotoxicity since NK cells from animals lacking these genes mediate normal natural killing (68–70). Killer cell activating receptors (KARs), variants of the KIRs that lack the cytoplasmic inhibitory motif required to bind the phosphatase SHP-1, have the capability to initiate NK cell–mediated killing (4, 49–57). Recent data suggest that KARs coprecipitate proteins with molecular weights of ∼12–16 kD (KAR-associated proteins or KARAPs) that are not ζ or γ (71). Although the molecular identities of these molecules are unknown, two-dimensional gel analysis suggests that, similar to ζ and γ, they may be expressed as disulfide-linked dimers. Expression of KARAPs appears to correlate with the ability of KARs to activate granule release, since cross-linking KAR on NK cells, which express KARAP, can trigger cytotoxicity while cross-linking KAR-transfected rat basophilic leukemia cells, which do not express detectable KARAP, does not induce granule release (71). Molecular cloning of the KARAP protein will be needed to determine if these are indeed additional ITAM-containing subunits that are shared by other triggering–receptor complexes.
Our data clearly suggest that Syk and ZAP-70 are not functionally redundant in the generation of natural cytotoxicity. This observation is consistent with reports demonstrating different activation requirements for each of the Syk family members. In vitro studies using peptides containing phosphorylated ITAMs from the FcεRI γ chain suggest that these peptides can activate Syk kinase activity (72, 73), whereas ZAP-70 kinase activity is only activated upon binding a dimeric form of phosphorylated ζ (74) rather than peptide fragments (75, 76). In vivo studies in COS cells suggest that ZAP-70 is only phosphorylated upon the addition of Lck or Fyn (17), whereas Syk is phosphorylated when transfected alone (77, 78). The intrinsic enzymatic activities of Syk and ZAP-70 also differ when expressed in COS cells (79). Syk autophosphorylation and catalytic activity toward the exogenous substrate erythrocyte band 3 was ∼100-fold greater than that of ZAP-70. Further studies in COS cells expressing chimeric receptors which cytoplasmically express FcεRIγ chain coexpressed with Syk or ZAP-70 showed that the chimeric γ plus Syk led to γ phosphorylation and Syk kinase activity while γ plus ZAP-70 induced neither γ phosphorylation nor ZAP-70 activation (20).
The final outcome of an NK cell interacting with a target cell is determined by the balance of positive and negative signals transmitted. Positive signals generated during natural cytotoxicity include activation of PTKs and increases in inositol phosphate and intracellular free Ca2+ release. Negative signals are derived, at least in part, from the protein-tyrosine phosphatase (PTPase) SHP-1 that is associated with MHC–recognizing KIR (5, 8, 80–82). Although it appears the overall balance of positive and negative signals generated determines the outcome of an NK cell interacting with a target cell, our data suggest that the two pathways may directly interact. KIR may preempt the initiation of a positive signal by acting on the activating receptor complex or its most proximal signaling molecules. Since Syk activation is central to the generation of both natural cytotoxicity and ADCC, SHP-1–mediated dephosphorylation of Syk could inhibit cellular activation. Alternatively, SHP-1 may act by inactivating a kinase that may phosphorylate and activate Syk. Recent data suggest that Syk may be activated by an Src kinase–initiated activation loop in which, once a small amount of Syk is phosphorylated, it can activate other Syk molecules to allow rapid Syk activation (36). Another potential SHP-1 target is the ITAM-containing receptor protein that upon phosphorylation docks Syk family molecules to the receptor complex. SHP-1 targeting any of these activation steps could inhibit the Syk tyrosine kinase by either preventing the association of Syk with the receptor complex or by preventing its full activation. Valiante et al. (83) have suggested that KIR-associated SHP-1 inhibits NK cell–mediated natural killing by dephosphorylating such downstream targets as the phospholipase C (PLC)-γ-adaptor protein pp36, thereby preventing PLC-γ association and activation. Our results here, together with previous data from our lab (4,5), suggest that inhibition may occur at a more proximal step in activation. If inhibition of Syk activation, a proximal step in the generation of natural cytotoxicity and ADCC, is the target for KIR inhibition, then this supports a role for Syk as a common second messenger in use during many forms of cytotoxicity.
Acknowledgments
The authors wish to thank Jean-Pierre Kinet and Andrew M. Scharenberg for generously providing the pSC-65, Syk, ZAP-70, SykT, and SykK recombinant vaccinia viruses, and Theresa Lee for her skillful assistance with the preparation of the manuscript.
Footnotes
This research was supported by the Mayo Foundation and by grant CA-47752 from the National Institutes of Health.
Abbreviations used in this paper: ADCC, antibody-dependent cell-mediated cytotoxicity; ITAM, immunoreceptor tyrosine-based activation motif; KAR, killer cell activating receptor; KARAP, KAR-associated protein; KIR, killer cell inhibitory receptor; PTK, protein tyrosine kinase.
Kathryn M. Brumbaugh and Bryce A. Binstadt contributed equally to this work.
References
- 1.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leibson PJ. Signal transduction during natural killer cell activation: inside the mind of a killer. Immunity. 1997;6:655–661. doi: 10.1016/s1074-7613(00)80441-0. [DOI] [PubMed] [Google Scholar]
- 3.Vitale M, Sivori S, Pende D, Moretta L, Moretta A. Coexpression of two functionally independent p58 inhibitory receptors in human natural killer cell clones results in the inability to kill all normal allogeneic target cells. Proc Natl Acad Sci USA. 1995;92:3536–3540. doi: 10.1073/pnas.92.8.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brumbaugh KM, Pérez-Villar JJ, Dick CJ, Schoon RA, López-Botet M, Leibson PJ. Clonotypic differences in signaling from CD94 (kp43) on NK cells lead to divergent cellular responses. J Immunol. 1996;157:2804–2812. [PubMed] [Google Scholar]
- 5.Binstadt BA, Brumbaugh KM, Dick CJ, Scharenberg AM, Williams BL, Colonna M, Lanier LL, Kinet J-P, Abraham RT, Leibson PJ. Sequential involvement of Lck and SHP-1 with MHC-recognizing receptors on NK cells inhibits FcR-initiated tyrosine kinase activation. Immunity. 1996;5:629–638. doi: 10.1016/s1074-7613(00)80276-9. [DOI] [PubMed] [Google Scholar]
- 6.Ferrini S, Cambiaggi A, Meazza R, Sforzini S, Marciano S, Mingari MC, Moretta L. T cell clones expressing the natural killer cell–related p58 receptor molecule display heterogeneity in phenotypic properties and p58 function. Eur J Immunol. 1994;24:2294–2298. doi: 10.1002/eji.1830241005. [DOI] [PubMed] [Google Scholar]
- 7.Phillips JH, Gumperz JE, Parham P, Lanier LL. Superantigen-dependent, cell-mediated cytotoxicity inhibited by MHC class I receptors on T lymphocytes. Science. 1995;268:403–405. doi: 10.1126/science.7716542. [DOI] [PubMed] [Google Scholar]
- 8.Fry AM, Lanier LL, Weiss A. Phosphotyrosines in the killer cell inhibitory receptor motif of NKB1 are required for negative signaling and for association with protein tyrosine phosphatase 1C. J Exp Med. 1996;184:295–300. doi: 10.1084/jem.184.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bléry, M., J. Delon, A. Trautmann, A. Cambiaggi, L. Olcese, R. Biassoni, L. Moretta, P. Chavrier, A. Moretta, M. Daëron, and E. Vivier. Reconstituted killer cell inhibitory receptors for major histocompatibility complex class I molecules control mast cell activation induced via immunoreceptor tyrosine-based activation motifs. J. Biol. Chem. 272:8989– 8996. [DOI] [PubMed]
- 10.Einspahr KJ, Abraham RT, Binstadt BA, Uehara Y, Leibson PJ. Tyrosine phosphorylation provides an early and requisite signal for the activation of natural killer cell cytotoxic function. Proc Natl Acad Sci USA. 1991;88:62–69. doi: 10.1073/pnas.88.14.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Shea JJ, McVicar DW, Kurhns DB, Ortaldo JR. A role for protein tyrosine kinase activity in natural cytotoxicity as well as antibody-dependent cellular cytotoxicity. Effects of herbimycin A. J Immunol. 1992;148:2497–2502. [PubMed] [Google Scholar]
- 12.Vivier E, Morin P, O'Brien C, Druker B, Schlossman SF, Anderson P. Tyrosine phosphorylation of the FcγRIII(CD16): ζ complex in human natural killer cells. Induction by antibody-dependent cytotoxicity but not by natural killing. J Immunol. 1991;146:206–210. [PubMed] [Google Scholar]
- 13.O'Shea JJ, Weissman AM, Kennedy ICS, Ortaldo JR. Engagement of the natural killer cell IgG Fc receptor results in tyrosine phosphorylation of the ζ chain. Proc Natl Acad Sci USA. 1991;88:350–354. doi: 10.1073/pnas.88.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ting AT, Einspahr KJ, Abraham RT, Leibson PJ. Fcγ receptor signal transduction in natural killer cells. Coupling to phospholipase C via a G protein–independent, but tyrosine kinase–dependent pathway. J Immunol. 1991;147:3122–3127. [PubMed] [Google Scholar]
- 15.Wen T, Zhang L, Kung SKP, Molina TJ, Miller RG, Mak TW. Allo-skin graft rejection, tumor rejection and natural killer activity in mice lacking p56lck . Eur J Immunol. 1995;25:3155–3159. doi: 10.1002/eji.1830251125. [DOI] [PubMed] [Google Scholar]
- 16.van Oers N S C, Lowin-Kropf B, Finlay D, Connolly K, Weiss A. αβ T cell development is abolished in mice lacking both Lck and Fyn protein tyrosine kinases. Immunity. 1996;5:429–436. doi: 10.1016/s1074-7613(00)80499-9. [DOI] [PubMed] [Google Scholar]
- 17.Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR ζ chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 18.Kolanus W, Romeo C, Seed B. T cell activation by clustered tyrosine kinases. Cell. 1993;74:171–183. doi: 10.1016/0092-8674(93)90304-9. [DOI] [PubMed] [Google Scholar]
- 19.Chu DH, Spits H, Peyron J-F, Rowley RB, Bolen JB, Weiss A. The Syk protein tyrosine kinase can function independently of CD45 or Lck in T cell antigen receptor signaling. EMBO (Eur Mol Biol Organ) J. 1996;15:6251–6261. [PMC free article] [PubMed] [Google Scholar]
- 20.Zoller KE, MacNeil EA, Brugge JS. Protein tyrosine kinases Syk and ZAP-70 display distinct requirements for Src family kinases in immune response receptor signal transduction. J Immunol. 1997;158:1650–1659. [PubMed] [Google Scholar]
- 21.Elder ME, Lin D, Clever J, Chan AC, Hope TJ, Weiss A, Parslow TG. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science. 1994;264:1596–1599. doi: 10.1126/science.8202712. [DOI] [PubMed] [Google Scholar]
- 22.Chan AC, Kadlecek TA, Elder ME, Filipovich AH, Kuo W-L, Iwashima M, Parslow TG, Weiss A. ZAP-70 deficiency in an autosomal recessive form of severe combined immunodeficiency. Science. 1994;264:1599–1601. doi: 10.1126/science.8202713. [DOI] [PubMed] [Google Scholar]
- 23.Negishi I, Motoyama N, Nakayama K-I, Nakayama K, Senju S, Hatakeyama S, Zhang Q, Chan AC, Loh DY. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376:435–438. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- 24.Stahls A, Liwszyc GE, Couture C, Mustelin T, Andersson LC. Triggering of human natural killer cells through CD16 induces tyrosine phosphorylation of the p72sykkinase. Eur J Immunol. 1994;24:2491–2496. doi: 10.1002/eji.1830241035. [DOI] [PubMed] [Google Scholar]
- 25.Pao LI, Cambier JC. Syk, but not Lyn, recruitment to B cell antigen receptor and activation following stimulation of CD45−B cells. J Immunol. 1997;158:2663–2669. [PubMed] [Google Scholar]
- 26.Gerrard JM, Hildes E, Atkinson EA, Greenberg AH. Activation of inositol cycle in large granular lymphocyte leukemia RNK following contact with an NK-sensitive tumor. Adv Prostaglandin Thromboxane Leukotriene Res. 1987;17:573–576. [PubMed] [Google Scholar]
- 27.Steele TA, Brahmi Z. Phosphatidylinositol metabolism accompanies early activation events in tumor target cell–stimulated human natural killer cells. Cell Immunol. 1988;112:402–413. doi: 10.1016/0008-8749(88)90309-7. [DOI] [PubMed] [Google Scholar]
- 28.Chow SC, Ng J, Nordstedt C, Fredholm BB, Jondal M. Phosphoinositide breakdown and evidence for protein kinase C involvement during human NK killing. Cell Immunol. 1988;114:96–103. doi: 10.1016/0008-8749(88)90257-2. [DOI] [PubMed] [Google Scholar]
- 29.Windebank KP, Abraham RT, Powis G, Olsen RA, Barna TJ, Leibson PJ. Signal transduction during human natural killer cell activation: Inositol phosphate generation and regulation by cyclic AMP. J Immunol. 1988;141:3951–3957. [PubMed] [Google Scholar]
- 30.Stahls AK, Carpen O. Generation of inositol phosphates during triggering of cytotoxicity in human natural killer and lymphokine-activated killer cells. Scand J Immunol. 1989;29:211–216. doi: 10.1111/j.1365-3083.1989.tb01118.x. [DOI] [PubMed] [Google Scholar]
- 31.Perussia B, Trinchieri G. Antibody 3G8, specific for the human neutrophil Fc receptor, reacts with natural killer cells. J Immunol. 1984;132:1410–1415. [PubMed] [Google Scholar]
- 32.Ting AT, Dick CJ, Schoon RA, Karnitz LM, Abraham RT, Leibson PJ. Interaction between lck and syk family tyrosine kinases in Fcγ receptor–initiated activation of natural killer cells. J Biol Chem. 1995;270:16415–16421. doi: 10.1074/jbc.270.27.16415. [DOI] [PubMed] [Google Scholar]
- 33.Pross, H.F., D. Callewaert, and P. Rubin. 1986. Assays for NK cell cytotoxicity—their values and pitfalls. In Immunobiology of Natural Killer Cells, Volume I. E. Lotzová and R.B. Herberman, editors. CRC Press, Inc., Boca Raton, FL. pp. 2–20.
- 34.Wange RL, Guitian R, Isakov N, Watts JD, Aebersold R, Samelson LE. Activating and inhibitory mutations in adjacent tyrosines in the kinase domain of ZAP-70. J Biol Chem. 1995;270:18730–18733. doi: 10.1074/jbc.270.32.18730. [DOI] [PubMed] [Google Scholar]
- 35.Scharenberg AM, Lin S, Cuenod B, Yamamura H, Kinet JP. Reconstitution of interactions between tyrosine kinases and the high affinity IgE receptor which are controlled by receptor clustering. EMBO (Eur Mol Biol Organ) J. 1995;14:3385–3394. doi: 10.1002/j.1460-2075.1995.tb07344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Hillal O, Kurosaki T, Yamamura H, Kinet J-P, Scharenberg AM. Syk kinase activation by a src kinase-initiated activation loop phosphorylation chain reaction. Proc Natl Acad Sci USA. 1997;94:1919–1924. doi: 10.1073/pnas.94.5.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vivier E, da Silva AJ, Ackerly M, Levine H, Rudd CE, Anderson P. Association of a 70-kDa tyrosine phosphoprotein with the CD16:ζ:γ complex expressed in human natural killer cells. Eur J Immunol. 1993;23:1872–1876. doi: 10.1002/eji.1830230821. [DOI] [PubMed] [Google Scholar]
- 38.Oliver JM, Burg DL, Wilson BS, McLaughlin JL, Geahlen RL. Inhibition of mast cell FcεR1-mediated signaling and effector function by the Syk-selective inhibitor, Piceatannol. J Biol Chem. 1994;269:29697–29703. [PubMed] [Google Scholar]
- 39.Parham, P., editor. 1997. NK Cells, MHC Class I Antigens and Missing Self. Immunol. Rev. 155:5–221.
- 40.Gismondi A, Milella M, Palmieri G, Piccoli M, Frati F, Santoni A. Stimulation of protein tyrosine phosphorylation by interaction of NK cells with fibronectin via α4β1 and α5β1. J Immunol. 1995;154:3128–3137. [PubMed] [Google Scholar]
- 41.Rabinowich H, Lin W-C, Manciulea M, Herberman RB, Whiteside TL. Induction of protein tyrosine phosphorylation in human natural killer cells by triggering via α4β1 or α5β1 integrins. Blood. 1995;85:1858–1864. [PubMed] [Google Scholar]
- 42.Schmidt RE, Bartley G, Levine H, Schlossman SF, Ritz J. Functional characterization of LFA-1 antigens in the interaction of human NK clones and target cells. J Immunol. 1985;135:1020–1025. [PubMed] [Google Scholar]
- 43.Timonen T, Patarroyo M, Gahmberg CG. Cd11a-c/CD18 and GP84 (LB-2) adhesion molecules on human large granular lymphocytes and their participation in natural killing. J Immunol. 1988;141:1041–1046. [PubMed] [Google Scholar]
- 44.Helander TS, Carpen O, Turunen O, Kovanen PE, Vaheri A, Timonen T. ICAM-2 redistributed by ezrin as a target for killer cells. Nature. 1996;382:265–268. doi: 10.1038/382265a0. [DOI] [PubMed] [Google Scholar]
- 45.Siliciano RF, Pratt JC, Schmidt RE, Ritz J, Reinherz EL. Activation of cytolytic T lymphocyte and natural killer cell function through the T11 sheep erythrocyte binding protein. Nature. 1985;317:428–430. doi: 10.1038/317428a0. [DOI] [PubMed] [Google Scholar]
- 46.Moretta A, Poggi A, Pende D, Tripodi G, Orengo AM, Pella N, Augagliaro R, Bottino C, Ciccone E, Moretta L. CD69 mediated pathway of lymphocyte activation: anti-CD69 monoclonal antibodies trigger the cytolytic activity of different lymphoid effector cells with the exception of cytolytic T lymphocytes expressing T cell receptor α/β. J Exp Med. 1991;174:1393–1398. doi: 10.1084/jem.174.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyazaki T, Dierich A, Benoist C, Mathis D. Independent modes of natural killing distinguished in mice lacking Lag3. Science. 1996;272:405–408. doi: 10.1126/science.272.5260.405. [DOI] [PubMed] [Google Scholar]
- 48.Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, McClanahan T, Kitamura T, Nicholl J, Sutherland GR, Lanier LL, Phillips JH. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4:573–581. doi: 10.1016/s1074-7613(00)70060-4. [DOI] [PubMed] [Google Scholar]
- 49.Mason LH, Yagita H, Ortaldo JR. LGL-1: a potential triggering molecule on murine NK cells. J Leukocyte Biol. 1994;55:362–370. doi: 10.1002/jlb.55.3.362. [DOI] [PubMed] [Google Scholar]
- 50.Moretta A, Sivori S, Vitale M, Pende D, Morelli L, Augugliaro R, Bottino C, Moretta L. Existence of both inhibitory (p58) and activatory (p50) receptors for HLA-C molecules in human natural killer cells. J Exp Med. 1995;182:875–884. doi: 10.1084/jem.182.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pérez-Villar JJ, Melero I, Rodriguez A, Carretero M, Aramburu J, Sivori S, Orengo AM, Moretta A, López-Botet M. Functional ambivalence of the Kp43 (CD94) NK cell–associated surface antigen. J Immunol. 1995;154:5779–5788. [PubMed] [Google Scholar]
- 52.Biassoni R, Cantoni C, Falco M, Verdiani S, Bottino C, Vitale M, Conte R, Poggi A, Moretta A, Moretta L. The human leukocyte antigen (HLA)-C–specific “activatory” or “inhibitory” natural killer cell receptors display highly homologous extracellular domains but differ in their transmembrane and intracytoplasmic portions. J Exp Med. 1996;183:645–650. doi: 10.1084/jem.183.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bottino C, Sivori S, Vitale M, Cantoni C, Falco M, Pende D, Morelli L, Augugliaro R, Semenzato G, Biassoni R, et al. A novel surface molecule homologous to the p58/p50 family of receptors is selectively expressed on a subset of human natural killer cells and induces both triggering of cell functions and proliferation. Eur J Immunol. 1996;26:1816–1824. doi: 10.1002/eji.1830260823. [DOI] [PubMed] [Google Scholar]
- 54.Mandelboim O, Davis DM, Reyburn HT, Vales-Gomez M, Sheu EG, Pazmany L, Strominger JL. Enhancement of class II–restricted T cell responses by costimulatory NK receptors for class I MHC proteins. Science. 1996;274:2097–2100. doi: 10.1126/science.274.5295.2097. [DOI] [PubMed] [Google Scholar]
- 55.Mason LH, Anderson SK, Yokoyama WM, Smith HRC, Winkler-Pickett R, Ortaldo JR. The Ly-49D receptor activates murine natural killer cells. J Exp Med. 1996;184:2119–2128. doi: 10.1084/jem.184.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carretero, M., C. Cantoni, T. Bellon, C. Bottino, R. Biassoni, A. Rodriguez, J.J. Perez-Villar, L. Moretta, A. Moretta, and M. Lopez-Botet. The CD94 and NKG2-A C-type lectins covalently assemble to form a natural killer cell inhibitory receptor for HLA class I molecules. Eur. J. Immunol. 27:563– 567. [DOI] [PubMed]
- 57.Houchins JP, Lanier LL, Niemi EC, Phillips JH, Ryan JC. Natural killer cell cytolytic activity is inhibited by NKG2-A and activated by NKG2-C. J Immunol. 1997;158:3603–3609. [PubMed] [Google Scholar]
- 58.Frey JL, Bino T, Kantor RRS, Segal DM, Giardina SL, Roder J, Anderson S, Ortaldo JR. Mechanism of target cell recognition by natural killer cells: characterization of a novel triggering molecule restricted to CD3−large granular lymphocytes. J Exp Med. 1991;174:1527–1536. doi: 10.1084/jem.174.6.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chambers WH, Vujanovic NL, DeLeo AB, Olszowy MW, Herberman RB, Hiserodt JC. Monoclonal antibody to a triggering structure expressed on rat natural killer cells and adherent lymphokine-activated killer cells. J Exp Med. 1989;169:1373–1389. doi: 10.1084/jem.169.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giorda R, Rudert WA, Vavassori C, Chambers WH, Hiserodt JC, Trucco M. NKR-P1, a signal transduction molecule on natural killer cells. Science. 1990;249:1298–1300. doi: 10.1126/science.2399464. [DOI] [PubMed] [Google Scholar]
- 61.Ryan JC, Niemi EC, Nakamura MC, Seaman WE. NKR-P1A is a target-specific receptor that activates natural killer cell cytotoxicity. J Exp Med. 1995;181:1911–1915. doi: 10.1084/jem.181.5.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anderson P, Caligiuri M, Ritz J, Schlossman SF. CD3-negative natural killer cells express ζ TCR as part of a novel molecular complex. Science. 1989;341:159–162. doi: 10.1038/341159a0. [DOI] [PubMed] [Google Scholar]
- 63.Lanier LL, Yu G, Phillips JH. Co-association of CD3ζ with a receptor (CD16) for IgG Fc on human natural killer cells. Nature. 1989;342:803–805. doi: 10.1038/342803a0. [DOI] [PubMed] [Google Scholar]
- 64.Orloff DG, Ra C, Frank SJ, Klausner RD, Kinet J-P. Family of disulphide-linked dimers containing the ζ and η chains of the T-cell receptor and the γ chain of Fc receptors. Nature. 1990;347:189–191. doi: 10.1038/347189a0. [DOI] [PubMed] [Google Scholar]
- 65.Kurosaki T, Gander I, Ravetch JV. A subunit common to an IgG Fc receptor and the T-cell receptor mediates assembly through different interactions. Proc Natl Acad Sci USA. 1991;89:3837–3841. doi: 10.1073/pnas.88.9.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 67.Jouvin M-H, Numerof RP, Kinet J-P. Signal transduction through the conserved motifs of the high affinity IgE receptor FcεRI. Sem Immunol. 1995;7:29–35. doi: 10.1016/1044-5323(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 68.Love PE, Shores EW, Johnson MD, Tremblay ML, Lee EJ, Grinberg A, Huang SP, Singer A, Westphal H. T cell development in mice that lack the zeta chain of the T cell antigen receptor complex. Science. 1993;261:918–921. doi: 10.1126/science.7688481. [DOI] [PubMed] [Google Scholar]
- 69.Ohno H, Aoe T, Taki S, Kitamura D, Ishida Y, Rajewsky K, Saito T. Developmental and functional impairment of T cells in mice lacking CD3 zeta chains. EMBO (Eur Mol Biol Organ) J. 1993;12:4357–4366. doi: 10.1002/j.1460-2075.1993.tb06120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 71.Olcese L, Cambiaggi A, Semenzato G, Bottino C, Moretta A, Vivier E. Human killer cell activatory receptors for MHC class I molecules are included in a multimeric complex expressed by natural killer cells. J Immunol. 1997;158:5083–5086. [PubMed] [Google Scholar]
- 72.Shiue L, Zoller MJ, Brugge JS. Syk is activated by phosphotyrosine-containing peptides representing the tyrosine-based activation motifs of the high affinity receptor for IgE. J Biol Chem. 1995;270:10498–10502. doi: 10.1074/jbc.270.18.10498. [DOI] [PubMed] [Google Scholar]
- 73.Kimura T, Sakamoto H, Appella E, Siraganian RP. Conformational changes induced in the protein tyrosine kinase p72sykby tyrosine phosphorylation or by binding of phosphorylated immunoreceptor tyrosine-based activation motif peptides. Mol Cell Biol. 1996;16:1471–1478. doi: 10.1128/mcb.16.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.LoGrasso PV, Hawkins J, Frank LJ, Wisniewski D, Marcy A. Mechanism of activation for Zap-70 catalytic activity. Proc Natl Acad Sci USA. 1996;93:12165–12170. doi: 10.1073/pnas.93.22.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neumeister EN, Zhu Y, Richard S, Terhorst C, Chan AC, Shaw AS. Binding of ZAP-70 to phosphorylated T-cell receptor ζ and η enhances its autophosphorylation and generates specific binding sites for SH2 domain– containing proteins. Mol Cell Biol. 1995;15:3171–3178. doi: 10.1128/mcb.15.6.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Isakov N, Wange RL, Wats JD, Aebersold R, Samelson LE. Purification and characterization of human ZAP-70 protein-tyrosine kinase from a baculovirus expression system. J Biol Chem. 1996;271:15753–15761. doi: 10.1074/jbc.271.26.15753. [DOI] [PubMed] [Google Scholar]
- 77.Couture C, Baier G, Altman A, Mustelin T. p56lck-independent activation and tyrosine phosphorylation of p72sykby T-cell antigen receptor/CD3 stimulation. Proc Natl Acad Sci USA. 1994;91:5301–5305. doi: 10.1073/pnas.91.12.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Couture C, Baier G, Oetken C, Williams S, Telford D, Marie-Cardine A, Baier-Bitterlich G, Gischer S, Burn P, Altman A, Mustelin T. Activation of p56lck by p72sykthrough physical association and N-terminal tyrosine phosphorylation. Mol Cell Biol. 1994;14:5249–5258. doi: 10.1128/mcb.14.8.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Latour S, Chow LML, Veillette A. Differential intrinsic enzymatic activity of Syk and Zap-70 protein-tyrosine kinases. J Biol Chem. 1996;271:22782–22790. doi: 10.1074/jbc.271.37.22782. [DOI] [PubMed] [Google Scholar]
- 80.Burshtyn DN, Scharenberg AM, Wagtmann N, Rajagopalan S, Berrada K, Yi T, Kinet J-P, Long EO. Recruitment of tyrosine phosphatase HCP by the killer cell inhibitory receptor. Immunity. 1996;4:77–85. doi: 10.1016/s1074-7613(00)80300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Olcese L, Lang P, Vely F, Cambiaggi A, Marguet D, Blery M, Hippen KL, Biassoni R, Moretta A, Moretta L, Cambier JC, Vivier E. Human and mouse killer-cell inhibitory receptors recruit PTP1C and PTP1D protein tyrosine phosphatases. J Immunol. 1996;156:4531–4534. [PubMed] [Google Scholar]
- 82.Campbell KS, Dessing M, López-Botet M, Cella M, Colonna M. Tyrosine phosphorylation of a human killer inhibitory receptor recruits protein tyrosine phosphatase 1C. J Exp Med. 1996;184:93–100. doi: 10.1084/jem.184.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valiante NM, Phillips JH, Lanier LL, Parham P. Killer cell inhibitory receptor recognition of human leukocyte antigen (HLA) class I blocks formation of a pp36/ PLC-γ signaling complex in human natural killer (NK) cells. J Exp Med. 1996;184:2243–2250. doi: 10.1084/jem.184.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]