Abstract
T helper cells type 1 (Th1s) that produce interferon-γ predominantly mediate cellular immune responses and are involved in the development of chronic inflammatory conditions, whereas Th2s which produce large amounts of IL-4 and IL-5 upregulate IgE production and are prominent in the pathogenesis of allergic diseases. The precise factors determining whether Th1- or Th2-mediated immune responses preferentially occur at a peripheral site of antigen exposure are largely unknown. Chemokines, a superfamily of polypeptide mediators, are a key component of the leukocyte recruitment process. Here we report that among four CXC (CXCR1-4) and five CC (CCR1-5) chemokine receptors analyzed, CXCR3 and CCR5 are preferentially expressed in human Th1s. In contrast, Th2s preferentially express CCR4 and, to a lesser extent, CCR3. In agreement with the differential chemokine receptor expression, Th1s and Th2s selectively migrate in response to the corresponding chemokines. The differential expression of chemokine receptors may dictate, to a large extent, the migration and tissue homing of Th1s and Th2s. It may also determine different susceptibility of Th1s and Th2s to human immunodeficiency virus strains using different fusion coreceptors.
CD4+ T cells can be subdivided into different subsets based on the kind of lymphokines they produce (1). Th1s secrete IFN-γ and lymphotoxin, whereas Th2s secrete IL-4, -5, and -13. Th1s predominantly control cell-mediated immune responses and appear to be involved in chronic inflammatory conditions, whereas Th2s upregulate IgE production and are prominent in the pathogenesis of allergic diseases (2, 3). Both Th1s and Th2s can develop from naive, peripheral CD4+ T cell populations. The differentiation process is initiated by the ligation of the TCR; cytokines, present during the initiation of a T cell response, determine the development of the particular Th subset (4– 8). Polarization of the T cell subsets most likely occurs in the secondary lymphoid organs to which naive T cells preferentially migrate. Memory lymphocytes and effector precursor cells, in contrast, migrate to peripheral tissues (9, 10). It is likely that, given their different effector functions, Th1s and Th2s are differentially recruited to peripheral sites of inflammation (11). Indeed, it has been shown that Th1s, but not Th2s, express a functional ligand for P- and E-selectin and therefore are selectively recruited to sites where Th1 immune responses occur (12).
Chemokines, a superfamily of polypeptide mediators, are a key component of the leukocyte recruitment process (13–16). The relative position of a Cys tandem defines four structural motifs (CXC, CC, C, and CX3C). Five receptors for CC chemokines (named CCR1 through 5) and four for CXC chemokines (CXCR1 through 4) have been defined (13–16). Receptor expression in different leukocyte subsets dictates to a large extent the spectrum of action of chemokines, and differentiation or cellular activation modulates receptor expression (13–18). Here we report that human Th1s and Th2s differentially express chemokine receptors and, accordingly, differentially migrate in response to different chemokines. Therefore, chemokines are part of effector and amplification mechanisms of polarized Th1- and Th2-mediated immune responses and their receptors might serve as Th1 versus Th2 markers, as well as targets for selective modulation of T cell dependent immunity.
Materials and Methods
Media and Cytokines.
The medium used throughout was RPMI 1640 supplemented with 2 mM l-glutamine, 1% nonessential amino acids, 1% pyruvate, 50 μg/ml kanamicin, 5 × 10−5 M 2-mercaptoethanol (GIBCO BRL, Gaithersburg, MD) supplemented with 5% fetal clone serum (Hyclone Labs., Logan, UT). Human recombinant IL-2 and -12 were provided by Dr. Maurice Gately (Hoffmann-La Roche Inc., Nutley, NJ), IL-4 was purchased from PharMingen (San Diego, CA), and human recombinant monocyte chemotactic protein (MCP)-1, IFN-γ–inducible 10-kD protein (IP-10), macrophage inflammatory protein (MIP)- 1β and eosinophil chemoattractant protein (eotaxin) were from PeproTech Inc. (Rocky Hill, NJ). Human recombinant MCP-3 and human MIP-1α were a gift from Dr. A. Minty (Sanofi Elf Bio Recherches, Labège, France) and Dr. L. Czaplewski (British Bio-technology Limited, Cowley, UK), respectively. Macrophage-derived chemokine (MDC) was obtained as described (19). Cytokines were endotoxin free as assessed by Limulus amebocyte assay.
Generation of Th1 and Th2 Lines from Cord Blood Leukocytes.
Human neonatal leukocytes were isolated from freshly collected, heparinized, neonatal cord blood by Ficoll-Paque (Pharmacia Biotech AB, Uppsala, Sweden) density gradient centrifugation. Th1 and Th2 lines were generated by stimulating cord blood leukocytes with 1 μg/ml PHA (Wellcome, Beckenham, UK) in the presence of 2 ng/ml IL-12 and 200 ng/ml neutralizing anti–IL-4 antibodies (PharMingen) for Th1 cultures, or 200 U/ml IL-4 (PharMingen) and 2 μg/ml neutralizing anti–IL-12 antibodies 17F7 and 20C2 (provided by M. Gately, Hoffmann-La Roche Inc.) for Th2 cultures, respectively. Cells were washed on day 3 and expanded in medium containing 100 U/ml IL-2. CD4+ cells were purified as described (20) and analyzed for chemokine receptor expression and chemotaxis between days 12 and 15.
Purification of CD45RA+ T Cells.
CD45RA+ T cells were purified from cord blood as previously described (8). After Ficoll density gradient centrifugation, monocytes were depleted by two rounds of plastic adherence and B cells were depleted by adherence to nylon wool. CD45RA+ T cells were isolated by two rounds of immunomagnetic negative selection with a mixture of the following monoclonal antibodies: anti-CD16 (B73.1; reference 21), anti-CD45RO (UCHL-1; reference 22), and anti– HLA-DR (1-1C4; reference 23). The suspension was incubated with goat anti–mouse IgG-coated Dynabeads (Dynal, Great Neck, NY) and exposed to a magnetic field using a magnetic particle concentrator (Dynal) according to the manufacturer's instructions. The purity of the CD3/CD45RA+ T cells using this procedure was typically >98% as determined by flow cytometry.
T Cell Clones.
Lolium perenne group I (Lol p1)–specific T cell clones were generated from peripheral blood mononuclear cells of two Lol p1 allergic subjects as described (24 and De Lalla, C., manuscript in preparation). The four Lol p1–specific T cell clones that we have selected for the study had a polarized cytokine profile. Two (E4.1 and D4.11) produced IL-4 but not IFN-γ and were categorized as Th2, and two (ET3.22 and ET3.20) able to produce IFN-γ but not IL-4 were categorized as Th1 clones. The two additional Th1 clones we have studied (GL93 and AC29) were specific for the hepatitis delta antigen and described in reference 25.
Single Cell Analysis of Cytokine Production.
Single cell analysis of cytokine production was performed as previously described (20). In brief, cord blood–derived Th1 and Th2 lines were collected after 10 d of culture with IL-2, washed, and restimulated with PMA (50 ng/ml) and ionomycin (1 μg/ml) for 4 h. Brefeldin A (10 μg/ml) was added during the last 2 h of culture. Then the cells were fixed with 4% paraformaldehyde and permeabilized with saponin. Fixed cells were stained with FITC–anti-IFN-γ and PE– anti-IL-4 mAbs (PharMingen) and analyzed by FACS® (Becton Dickinson, Mountain View, CA).
Migration Assay.
Cell migration was evaluated using a chemotaxis microchamber technique as previously described (26). 27 μl of chemoattractant solution or control medium (RPMI 1640 with 1% FCS) were added to the lower wells of a chemotaxis chamber (Neuroprobe, Pleasanton, CA). A nitrocellulose filter (Neuroprobe) was layered onto the wells, and covered with a silicon gasket and with the top plate. 50 μl of cell suspension (1.5 × 106/ml T cells) were seeded in the upper chamber. The chamber was incubated at 37°C in air with 5% CO2 for 60 min. At the end of the incubation, filters were removed, stained with Diff-Quik (Baxter S.p.A., Rome, Italy), and five high power oil-immersion fields were counted.
Northern Blot Analysis.
Total RNA was extracted by the guanidinium thiocyanate method, blotted, and hybridized as described (15, 17). Probes obtained as described (15, 17), were labeled by the Megaprime DNA labeling system (Amersham, Buckinghamshire, UK) with α-[32P]dCTP (3,000 Ci/mmol; Amersham). Membranes were prehybridized at 42°C in Hybrisol (Oncor, Inc., Gaithersburg, MD) and hybridized overnight with 106 cpm/ml of 32P-labeled probe. Membranes were then washed three times with 2× SSC (1× SSC = 0.15 M NaCI, 0.015 M sodium citrate, pH 7.0) at room temperature for 10 min, twice with 2× SSC, 1% SDS at 60°C for 20 min, and then with 0.1× SSC for 5 min, before being autoradiographed using Kodak (Rochester, NY) XAR-5 films and intensifier screens at −80°C. After autoradiography, filters were stripped and reprobed with a β-actin cDNA probe to assess for differences in RNA loading. Densitometric analysis of the β-actin band showed lane to lane variation in RNA loading <15%.
Results and Discussion
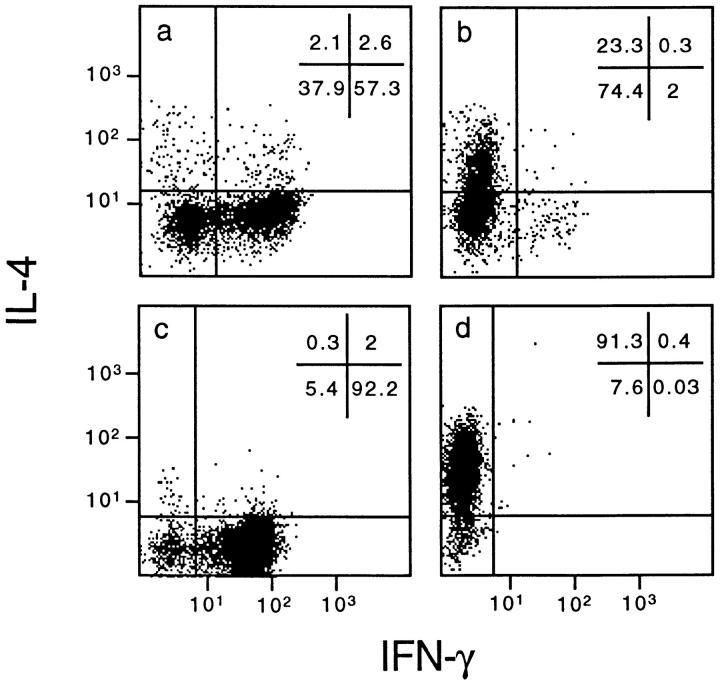
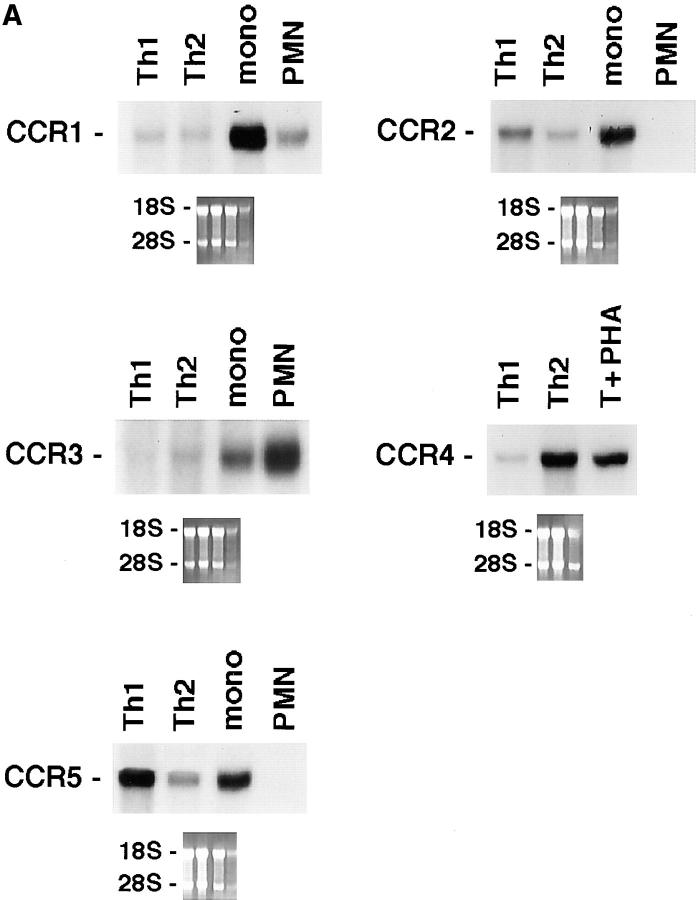
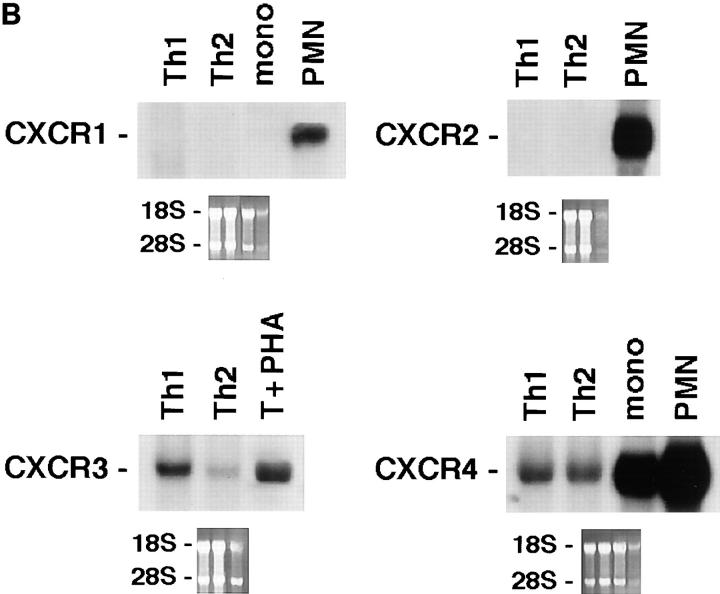
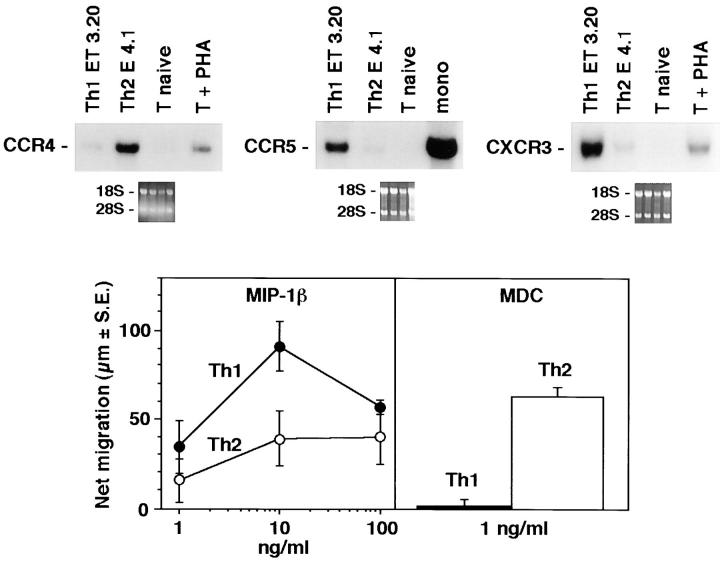
We generated Th1 and Th2 lines by stimulating human cord blood lymphocytes with mitogen in the presence of IL-12 and neutralizing anti–IL-4 mAb or IL-4 and neutralizing anti–IL-12 mAb, respectively. As previously reported, this protocol allows the establishment of human T cell lines with strongly polarized cytokine production (8). As shown at the single cell level by measuring intracellular cytokine production (Fig. 1), neonatal T cells primed under the Th1 conditions differentiated into T cells producing IFN-γ but little IL-4, whereas naive T cells primed in the presence of IL-4 and anti–IL-12 resulted in a population of T cells producing mainly IL-4. To assess the selective expression of chemokine receptors in the different T cell subsets, we have analyzed the messenger RNA (mRNA) expression levels for the different chemokine receptors in both Th1 and Th2 lines, as well as in naive cells. As shown in Fig. 2, polarized Th1s and Th2s showed differential expression of chemokine receptors. Naive T cells had a very low level of all chemokine receptor mRNAs examined except for CXCR4, the receptor for stromal cell–derived factor 1 (SDF-1; data not shown). This finding is consistent with the view that SDF-1, constitutively expressed in a broad range of tissues, is involved in basal trafficking of naive lymphocytes (27). The receptor for MCP-1 through 4 (CCR2) was expressed at high levels in both Th1s and Th2s, with somewhat higher levels for Th1s and considerable variation among different cell preparations. CCR1 (regulated on activation, normal T cell expressed and secreted [RANTES], MIP-1α, MCP-3 receptor) was equally expressed in Th1s and Th2s. In contrast, CCR4 (thymus- and activation-regulated chemokine [TARC] and MDC receptor; references 19, 28) was expressed at much higher levels in Th2s versus Th1s (14.6-fold difference by densitometry). CCR3 (eotaxin and MCP-3 receptor) was expressed only in Th2s, though at very low level in bulk cultures, which required extremely long (7 d) exposure of the blots. In contrast, the MIP-1β receptor CCR5 was preferentially (4.9-fold) expressed in Th1s versus Th2s. When CXC chemokine receptors were studied, Th1 and Th2 had no detectable CXCR1 and CXCR2 (IL-8 receptors) and equal amounts of CXCR4 (SDF-1 receptor). Moreover, expression of CXCR3, the receptor for IP-10, I-TAC, and Mig (29) was selective for Th1s (6.7-fold difference). The results described are representative of four different preparations of Th1 and Th2 lines. The preferential expression of CXCR3 and CCR5 in Th1s and CCR4 in Th2s was further confirmed on two fully differentiated Th1 (ET3.22 and ET3.20) and two Th2 (E4.1 and D4.11) clones specific for the Lol p1 antigen, and on two Th1 clones (GL93 and AC29) specific for the hepatitis delta antigen (Fig. 3 and data not shown).
Figure 1.
Intracellular cytokine production of Th1 and Th2 lines and clones. Neonatal T cells were stimulated as described in Materials and Methods with PHA in the presence of IL-12 and neutralizing anti–IL-4 antibodies for Th1 cultures or IL-4 and neutralizing anti–IL-12 antibodies for Th2 cultures, respectively. Cells were washed on day 3 and expanded in a medium containing 100 U/ml IL-2. After 10 d, cells were washed, stimulated with PMA and ionomycin, and stained for intracellular IFN-γ and IL-4. Cells were then analyzed by FACS® analysis. The staining of two representative Th1 and Th2 lines (a and b, respectively) and Th1 (ET3.20) and Th2 (E4.1) clones (c and d, respectively) is shown.
Figure 2.
Differential expression of chemokine receptors in Th1s versus Th2s. 10 μg of total mRNA purified from Th1s and Th2s were used in Northern blots analysis. Autoradiographies were obtained after 12 h of exposure, except for CCR3 which required 7 d. Lane to lane variation in RNA loading was <15%, as assessed by densitometric analysis of β-actin expression. Results are representative of three experiments. (A) CCR1 through 5. (B) CXCR1 through 4.
Figure 3.
Differential expression of chemokine receptors in Th1 versus Th2 T cell clones. (Top) Expression of CCR4, CCR5, and CXCR3 was examined on four antigen-specific Th1 clones derived from three different donors and on two antigen-specific Th2 clones derived from two different donors with similar results. Northern analysis from one representative Th1 (ET3.20) and one Th2 (E4.1) clone is shown. (Bottom) Chemotactic responsiveness to MDC and MIP-1β of T cell clones ET3.20 (Th1) and E4.1 (Th2). For IP-10, see Fig. 4.
We and others have recently shown that the IL-12 receptor β2 chain is selectively expressed by activated Th1s (8, 30). Together with the IL-12Rβ2, the selective expression of CXCR3 and CCR5 in Th1s and of CCR4 in Th2s may have practical implications in monitoring Th1 and Th2 populations, particularly in immunopathological conditions.
The eotaxin receptor CCR3 deserves a particular mention. Sallusto et al. have recently reported the selective expression of the eotaxin receptor on human Th2s (31). Our data confirm the selective expression of CCR3 by cells of the Th2 subset. However, the extremely low level of expression, as compared for example with CCR4, may reflect a minority of positive clones in the Th2 population. This possibility is consistent with recent findings by Gerber et al. showing a high frequency of CCR3+ clones only among the allergen-specific T cells, but not among the IL-4– and IL-5–producing T cell clones specific for either the tetanus toxoid or the sulfamethoxazole antigen (32).
Having established that chemokine receptors are differentially expressed in Th1s versus Th2s, we wanted to evaluate the functional significance of this observation. As shown in Fig. 4, consistent with receptor expression, MIP-1α (CCR1 agonist) and MCP-1 (a selective CCR2 agonist) showed comparable chemotactic activity for Th1s and Th2s. In contrast, MDC (19; a selective CCR4 agonist) was at least 10 times more active on Th2s versus Th1s, whereas MIP-1β (CCR5) and IP-10 (CXCR3) were more active on Th1s. Eotaxin (a selective CCR3 agonist) was inactive or weakly active only on Th2s. Thus, as expected on the basis of receptor expression, certain chemokines show differential action on Th1s and Th2s.
Figure 4.

Migratory response of Th1s and Th2s to chemokines. Migration was assessed in nitrocellulose filters as described (26). Spontaneous migration was subtracted. Results are the mean of three (MDC, MCP-1), two (eotaxin, IP-10), or one (MIP-1α, MIP-1β) experiment. The IP-10 dose response was obtained with one Th1 (ET3.20) and one Th2 (E4.1) clone; bulk Th1 and Th2 lines were only tested at one concentration, with similar results.
The prototypic inducer of IP-10 and Mig in cell types such as macrophages or endothelial cells is IFN-γ (33, 34), a cytokine of central importance in the generation and expression of Th1 responses (35, 36). It is therefore logical to assume that CXCR3 ligands are important components of the trafficking and recruitment of Th1s. Similarly, eotaxin, which recruits eosinophils (37), crucial effectors of Th2 responses, may also direct the trafficking of some Th2s that induce growth and activation of basophils and eosinophils through local production of Th2 cytokines (31, 32). Although the precise pathophysiological role of the differential chemokine receptor expression on the two T cell subsets needs to be further clarified, it is likely to play an important role in the selective recruitment of effector cells to inflammatory sites. Whether the differential expression of chemokine receptors may influence functional cellular responses other than chemotaxis is under investigation. In this context, there is evidence that MCP-1 (a CCR2 ligand) may be preferentially associated with Th2 responses by costimulating IL-4 and blocking IL-12 production (38, 39).
In conclusion, the results presented here indicate that chemokine receptors differentially expressed in Th1s versus Th2s could serve as markers of these responses and tools to modulate polarized versions of T cell–dependent immunity. Moreover Th1s and Th2s, because of their different chemokine receptors' expression pattern, are likely to have different susceptibility to HIV strains using different fusion coreceptors.
Acknowledgments
We thank Vincenzo Barnaba (University of Rome, Rome, Italy) for the kind gift of T cell clones GL93 and AC29.
This work was supported by Istituto Superiore di Sanita' (Project AIDS and Italy US Program on Cancer Research), special project Biotechnology Consiglio Nazionale delle Ricerche (CNR), Project Biotechnology 5%, and by a 40% fund from MURST Italy. The generous contribution from the Italian Association for Cancer Research (AIRC) is gratefully acknowledged. R. Bonecchi and A. Borsatti are the recipients of a fellowship of Fondazione A. and A. Valenti and Alfredo Leonardi Fund and G.L. Pfeiffer Foundation, respectively.
References
- 1.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–257. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 3.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh CS, Heimberger AB, Gold JS, O'Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T cell–receptor transgenic system. Proc Natl Acad Sci USA. 1992;89:6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seder RA, Paul WE, Davis MM, Fazekas de St B, Groth The presence of interleukin-4 during in vitro priming determines the lymphokine-producing potential of CD4+T cells from T cell receptor transgenic mice. J Exp Med. 1992;176:1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsieh C-S, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 7.Manetti R, Gerosa F, Giudizi MG, Biagiotti R, Parronchi P, Piccinni M-P, Sampognaro S, Maggi E, Romagnani S, Trinchieri G. Interleukin 12 induces stable priming for interferon γ (IFN-γ) production during differentiation of human T helper (Th) and transient IFN-γ production in established Th2 cell clones. J Exp Med. 1994;179:1273–1283. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogge L, BarberisMaino L, Biffi M, Passini N, Presky DH, Gubler U, Sinigaglia F. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Picker LJ, Butcher EC. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- 10.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 11.Lichtman AH, Abbas AK. T-cell subsets: recruiting the right kind of help. Curr Biol. 1997;7:242–244. doi: 10.1016/s0960-9822(06)00111-4. [DOI] [PubMed] [Google Scholar]
- 12.Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper–1 but not T-helper–2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 13.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 14.Schall, T.J. 1994. The chemokines. In The Cytokine Handbook. A. Thomson, editor. Academic Press, London. 419–460.
- 15.Sozzani S, Locati M, Allavena P, Van Damme J, Mantovani A. Chemokines: a superfamily of chemotactic cytokines. Int J Clin Lab Res. 1996;26:69–82. doi: 10.1007/BF02592349. [DOI] [PubMed] [Google Scholar]
- 16.Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 17.Sica A, Saccani A, Borsani A, Power CA, Wells TNC, Luini W, Polentarutti N, Sozzani S, Mantovani A. Bacterial lipopolysaccharide rapidly inhibits expression of C-C chemokine receptors in human monocyets. J Exp Med. 1997;185:969–974. doi: 10.1084/jem.185.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sozzani, S., W. Luini, A. Borsatti, N. Polentarutti, D. Zhou, L. Piemonti, G. D'Amico, C.A. Power, T.N. Wells, M. Gobbi, et al. 1997. Receptor expression and responsiveness of human dendritic cells to a defined set of CC and CXC chemokines. J. Immunol. In press. [PubMed]
- 19.Godiska R, Chantry D, Raport CJ, Sozzani S, Allavena P, Leviten D, Mantovani A, Gray PW. Human macrophage derived chemokine (MDC) a novel chemoattractant for monocytes, monocyte derived dendritic cells, and natural killer cells. J Exp Med. 1997;185:1595–1604. doi: 10.1084/jem.185.9.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panina-Bordignon P, Mazzeo D, Di Lucia P, D'Ambrosio D, Lang R, Fabbri L, Self C, Sinigaglia F. β2-agonists prevent Th1 development by selective inhibition of IL-12. J Clin Invest. 1997;100:1513–1519. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perussia B, Starr S, Abraham S, Fanning V, Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. I. Characterization of the lymphocyte subset reactive with B73.1. J Immunol. 1983;130:2133–2139. [PubMed] [Google Scholar]
- 22.Norton AJ, Ramsay AD, Smith SH, Beverly PCL, Isaacson PG. Monoclonal antibody (UCHL-1) that recognizes normal and neoplastic T cells in fixed tissues. J Clin Pathol. 1986;39:399–405. doi: 10.1136/jcp.39.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cammarota G, Sheirle A, Takacs B, Doran DM, Knorr R, Bannwardt W, Guardiola J, Sinigaglia F. Identification of a CD4 binding site on the β2domain of HLA-DR molecules. Nature. 1992;356:799–801. doi: 10.1038/356799a0. [DOI] [PubMed] [Google Scholar]
- 24.Sinigaglia F, Romagnoli P, Guttinger M, Takacs B, Pink JRL. Selection of T cell epitopes and vaccine engineering. Methods Enzymol. 1991;203:370–386. doi: 10.1016/0076-6879(91)03021-8. [DOI] [PubMed] [Google Scholar]
- 25.Nisini R, Paroli M, Accapezzato D, Bonino F, Rosina F, Santantonio T, Sallusto F, Amoroso A, Houghton M, Barnaba V. Human CD4+ T-cell response to hepatitis delta virus: identification of multiple epitopes and characterization of T-helper cytokine profile. J Virol. 1997;71:2241–2251. doi: 10.1128/jvi.71.3.2241-2251.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allavena P, Bianchi G, Zhou D, Van Damme J, Jilek P, Sozzani S, Mantovani A. Induction of natural killer cell migration by monocyte chemotactic protein-1, -2 and -3. Eur J Immunol. 1994;24:3233–3236. doi: 10.1002/eji.1830241249. [DOI] [PubMed] [Google Scholar]
- 27.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell–derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1110. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imai T, Baba M, Nishimura M, Kakizaki M, Tagaki S, Yoshie O. The T cell–directed CC chemokine TARC is a highly specific biological ligand for a CC chemokine receptor 4. J Biol Chem. 1997;272:15036–15042. doi: 10.1074/jbc.272.23.15036. [DOI] [PubMed] [Google Scholar]
- 29.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clarklewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP-10 and Mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 32.Gerber BO, Zanni MP, Uguccioni M, Loetscher M, Mackay CR, Pichler WJ, Yawalkar N, Baggiolini M, Moser B. Functional expression of the eotaxin receptor CCR3 in T lymphocytes co-localizing with eosinophils. Curr Biol. 1997;7:836–843. doi: 10.1016/s0960-9822(06)00371-x. [DOI] [PubMed] [Google Scholar]
- 33.Luster AD, Unkeless JC, Ravetch JV. Gamma-intereferon transcriptionally regulates an early gene containing homology to platelet proteins. Nature. 1985;315:672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- 34.Ohmori Y, Hamilton TA. A macrophage LPS-inducible early gene encodes the murine homogue of IP-10. Biochem Biophys Res Commun. 1990;168:1261–1267. doi: 10.1016/0006-291x(90)91164-n. [DOI] [PubMed] [Google Scholar]
- 35.Farrar MA, Schreiber RD. The molecular biology of interferon-γ and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 36.Wenner CA, Guler ML, Macatonia SE, O'Garra A, Murphy KM. Roles of IFN-gamma and IFN-alpha in IL-12–induced T helper cell–1 development. J Immunol. 1996;156:1442–1447. [PubMed] [Google Scholar]
- 37.Ponath PD, Qin S, Post TW, Wang J, Wu L, Gerard NP, Newman W, Gerard C, Mackay CR. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med. 1996;183:2437–2448. doi: 10.1084/jem.183.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zisman DA, Kunkel SL, Strieter RM, Tsai WC, Bucknell K, Wilkowski J. MCP-1 protects mice in lethal endotoxemia. J Clin Invest. 1997;99:2832–2836. doi: 10.1172/JCI119475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karpus WJ, Lukacs NW, Kennedy KJ, Smith WH, Hurst SD, Barrett TA. Differential CC chemokine–induced enhancement of T helper cell cytokine production. J Immunol. 1997;158:4129–4136. [PubMed] [Google Scholar]