Abstract
Fas ligand (FasL/CD95L) is best known for its role in delivering apoptotic signals through its receptor, Fas (APO-1/CD95). In this study, we present evidence that FasL has a second role as a signaling receptor. Alloantigen-specific proliferation by multiple FasL− murine CTL lines is depressed compared to that of FasL+ CTL lines. FasL− CTLs kill efficiently on a per recovered cell basis and can achieve wild-type levels of proliferation upon stimulation by optimal doses of anti-CD3, suggesting the lack of a costimulatory signal during antigen stimulation. To test this hypothesis directly, soluble FasIgG, a fusion protein of murine Fas and human IgG1, was added to FasL+ CTLs to demonstrate that blocking cell surface Fas–FasL interactions mimics the depression observed for FasL− CTLs. In addition, plate-bound FasIgG in conjunction with suboptimal anti-CD3 stimulation augments proliferative signals in FasL+ but not FasL− CTLs. In contrast to these results with CD8+ T cells, alloantigen-stimulated FasL− CD4+ T cells proliferate vigorously compared to FasL+ cells. These data demonstrate that reverse signaling through FasL is required for CTLs to achieve maximal proliferation and may provide clues to differences in the homeostatic regulation of activated CD4+ and CD8+ T cells during an immune response.
Fas ligand (L) belongs to the TNF superfamily, a group of type II transmembrane or secreted molecules whose interactions with their receptors, members of the TNF receptor superfamily, regulate important functions in immune cell proliferation, activation, differentiation, and survival (1–3). FasL exists mainly in membrane-bound form, is generally expressed on cells of the T cell lineage, and is induced during T cell activation (1). FasL and members of this ligand superfamily primarily interact with their receptors by direct cell–cell contact (3). This observation, coupled with the cross-species sequence conservation of the cytoplasmic domains of these ligands, has led to the suggestion that signaling occurs in both directions for this family of ligand–receptor pairs (3). In support of this argument, family members including CD27L, 4-1BBL, OX40L, CD30L, and CD40L have recently been shown to transduce internally costimulatory signals to activated peripheral lymphocytes (2, 4, 5). As of now, no data exist concerning the reverse signaling capacity of ligands with known negative regulatory functions, such as FasL. To explore this added twist to the picture of reverse signaling, we used CTL lines generated from B6 wildtype and B6.lpr mice, which both express FasL, and from B6.gld mice, which lack FasL function, to demonstrate that FasL shares this capacity for reverse signaling in CD8+ but not in CD4+ T cells. Our data reveal that FasL, a molecule long associated with the induction of apoptosis in Fas+ target cells (6) can also positively regulate the proliferative capacity of CD8+ T cells that express it. These data also offer some insight into how CD4+ and CD8+ T cells may be differentially regulated during an immune response.
Materials and Methods
Mice and Reagents.
C57BL/6 (B6), B6.MRL-Faslpr (B6.lpr), B6Smn.C3H-Faslgld (B6.gld), B6.C-H2bm1/By (B6.bm1), B6.C- H2bm12/KhEg (B6.bm12), C3H/HeJ (C3H), C3H/HeJ-Faslgld (C3H.gld), and C57BL/6-scid/SzJ (B6.SCID) mice were all purchased from The Jackson Laboratory (Bar Harbor, ME) and used at 6–9 wk of age. Reagents include rat antibodies specific for murine CD4 (RL172.4R6; reference 7) and for murine CD8 (3.168.8; reference 8), a hamster antibody specific for murine CD3-ε (145-2C11; PharMingen, San Diego, CA), and a mouse antibody specific for murine H-2Kk (16-3-1N; reference 9). NIH (Harvard Medical School, Boston, MA) 3T3 transfectants expressing the FasIgG fusion protein, which consists of the extracellular domain of murine Fas joined to the hinge and constant regions of human IgG1 (10), were derived by Dr. Ben Stanger and provided by Dr. A. Marshak-Rothstein (Boston University, Boston, MA). Experiments included either the supernatant of this cell line or sera from B6.SCID mice injected 5 wk previously with the transfectants.
Generation and Maintenance of T cell lines.
Alloreactive H-2k– specific CTLs were originally generated from age-matched B6 wild-type, B6.lpr, and B6.gld mice by incubating donor spleen and LN cells with an equal number of 3,000 rad C3H splenocytes in RPMI 1640 supplemented with 10% FCS (RP10). Lines were maintained by restimulation every 8–10 d for 1–12 rounds. RP10 was supplemented after the third stimulation with 50 mM α-methyl mannoside and 5% supernatant from rat cells stimulated for 2 d with 3 μg/ml Con A. CD8+ T cells for generating anti-Kbm1 CTLs were purified from spleen and LN cells from age-matched B6 wild-type and B6.gld mice by nonadherence to nylon wool, treatment with anti-CD4 plus complement, and adherence to plate-bound anti-CD8 antibodies. Purity as monitored by flow cytometry was >99%. All CTL lines were routinely monitored by flow cytometry and CTL assay. CD4+ T cells for generating primary anti–H-2bm12 responders were similarly nylon wool–purified, anti-CD8 killed, anti-CD4 panned spleen and LN cells, with >95% purity by flow cytometry.
Cytotoxicity and Proliferation Assays.
Serial dilutions of effector cells and 104 tumor targets labeled with 51Cr sodium chromate were plated in round-bottomed 96-well plates in a final volume of 200 μl. After a 4 h incubation at 37°C, 100 μl of supernatant was collected and specific lysis was determined as follows: percentage of specific lysis = 100 × [(cpm released by CTL − spontaneous release)/(cpm released by detergent − spontaneous release)]. Spontaneous release in the absence of CTL was <20% in all experiments. For antigen-specific proliferation, 2 × 104 CTL from long-term lines or 2 × 105 primary T cells were cultured with 5 × 105 irradiated allogeneic stimulator cells in a total volume of 200 μl of RP10. For long-term lines, media were supplemented with 5% rat Con A supernatant or 50 U/ml recombinant human IL-2. For optimal anti-CD3 stimulation, responders were added to wells coated at 4°C overnight with 15 μg/ml goat anti– hamster IgG (GahIg) in PBS and then for 4 h with 5 μg/ml anti-CD3 antibodies. For proliferation with FasL costimulation, responders were added to wells coated overnight at 4°C with both 15 μg/ml GahIg and 15 μg/ml goat anti–human IgG and then for 4 h with a mixture of 1.0 μg/ml anti-CD3 (suboptimal dose) with either 4.6 μg/ml FasIgG or isotype-matched human IgG1 (huIgG1). Proliferation levels were measured by thymidine incorporation 18 h after pulsing with 1 μCi [3H]TdR/well on day 3. Background counts (responders with only first stage antibodies) were subtracted from the values. Antigen-specific proliferation was also assayed for viable cells by trypan blue exclusion over the course of the culture period. Where appropriate, APCs were removed before counting viable cells by anti–H-2k plus complement. For the FasIgG blocking assay, FasIgG was added to cultures at 2.8–10 μg/ml. HuIgG1 and/or B6.SCID preimmune sera were used as negative controls.
Results and Discussion
Antigenic Stimulation of B6.gld CTL Lines Is Characterized by Depressed Proliferation and Cell Recoveries Relative to CTL Lines Expressing Functional FasL.
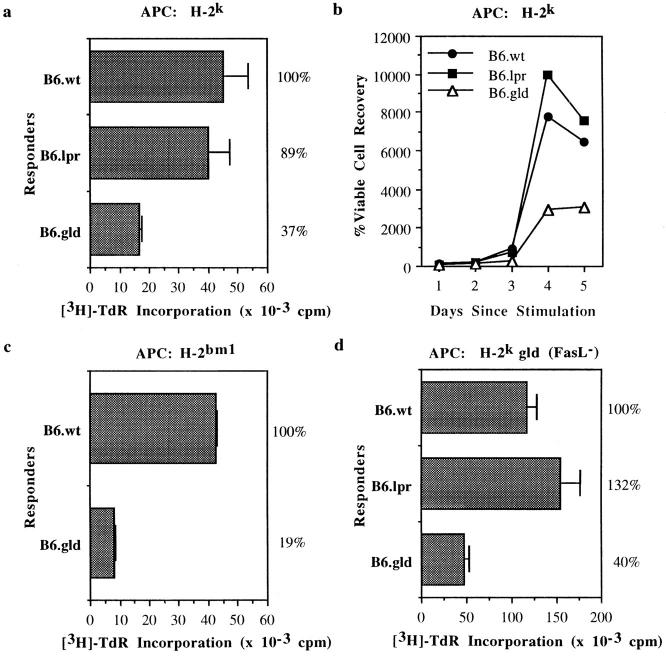
Proliferation assays using CTL lines derived from wild-type B6, B6.lpr, and B6.gld mice revealed that FasL+ CTLs proliferate better in response to allogeneic stimulation than do FasL− CTLs (Fig. 1, a and c). APC titration from 5 × 104 to 1 × 106 cells cocultured per well with CTLs did not alter the relative depression in proliferation of B6.gld CTL lines (data not shown). In addition, kinetic analysis of viable cell counts revealed that the lack of FasL function also influences the ability of CTLs to increase in number over the course of an MLC (Fig. 1 b), and that this expansion coincides with FasL expression by the responding T cells. As determined by flow cytometry, FasL expression on the CTL lines is upregulated by 3 h after antigenic stimulation, peaks on day 3, and begins to decrease by day 4 (data not shown). This pattern of depressed proliferative capacity and number of recovered cells was reproducible for three independent CTL lines and was not reversed by the addition of exogenous IL-2. The depressed proliferation and recovered cell counts were also seen upon coculture of B6.gld CTLs with APCs from C3H.gld mice, demonstrating that this phenomenon is not caused by Fas-mediated killing of gld CTLs by FasL expressed on the APCs (Fig. 1 d). Autocrine Fas-mediated suicide (10–12) can also be ruled out as a mechanism for depressed proliferation because gld CTLs do not express functional cell surface FasL. Analysis by flow cytometry (data not shown) also ensured that there is not an accumulation of Thy1+CD8−CD4− cells analogous to those found in vivo in gld and lpr mice (1).
Figure 1.
Depressed proliferation and cell recovery of FasL-deficient CTLs relative to FasL+ CTLs over the course of an MLC. (a and b) [3H]TdR uptake and viable cell counts over days 1–5 from long-term CTL lines from B6, B6.lpr, and B6.gld mice cultured with allogeneic H-2k splenocytes. APCs were removed before counting by antibody depletion. (c) CTL responders were purified CD8+ T cells derived from B6 and B6.gld mice cultured with H-2bm1 splenocytes. (d) [3H]TdR uptake by CTL responders used in a, cultured with C3H.gld splenocytes. Experiments were repeated two to seven times. All data are averages of triplicate wells and error bars represent the SD of the means. Percentages appearing to the right of the figures indicate normalized comparison to B6 wild-type.
FasL− CD4+ T Cells Vigorously Proliferate upon Antigenic Stimulation and Do Not Contribute to the Depressed Proliferation in FasL− CTL Lines.
To test the potential involvement of CD4+ T cells in initiating or maintaining the depressed proliferative capacity of FasL− compared to wild-type CTLs, purified populations of CD8+ T cells from B6 and B6.gld mice were tested for their response against the MHC class I molecule Kbm1. The same pattern of depression was observed for gld relative to wild-type CTLs in proliferation and expansion of cell numbers over the course of an MLC (Fig. 1 c). These data indicate that the submaximal proliferation by FasL− CTL lines is not initiated or maintained by the lack of CD4+ T cell help and further emphasize that the observed depression in proliferation is not merely an idiosyncracy of a single B6.gld-derived CTL line. In addition, this decreased proliferative capacity is not restricted to long-term lines, as primary cultures of anti-Kbm1 lines also showed the same pattern of proliferation (data not shown).
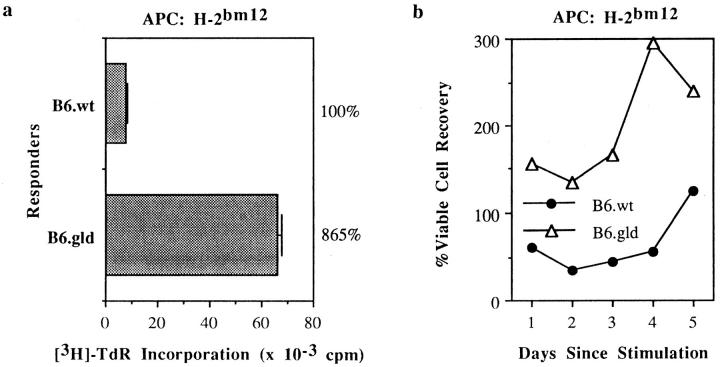
In contrast to their CD8+ counterparts, CD4+ T cells lacking functional FasL proliferate vigorously upon alloantigenic stimulation. Purified CD4+ cells from B6.gld mice cultured with class II different B6.bm12 stimulators proliferated better (Fig. 2 a) and generated more cells over the course of the 5 d of MLC (Fig. 2 b) than did CD4+ cells from wild-type B6 mice. This observation demonstrates the differential requirement for functional FasL expression in the promotion of maximal proliferation by CD8+ but not CD4+ T cells.
Figure 2.
Increased proliferation and cell recovery of primary FasL− CD4+ T cells relative to FasL+ CD4+ T cells over the course of an MLC. Purified CD4+ T cells from B6 and B6.gld mice were cultured (in the absence of rIL-2) with H-2bm12 splenocytes, and [3H]TdR uptake and viable cell counts were measured over days 1–5 of culture. All data are presented as in Fig. 1.
FasL− CTL Lines Proliferate to Wild-type Levels upon Stimulation with an Optimal Dose of Anti-CD3 and Can Efficiently Lyse Target Cells.
Despite their submaximal proliferation in response to antigenic stimulation, gld CTLs do not differ from wild-type CTLs in their ability to proliferate to an optimal dose of anti-CD3 antibody (Fig. 3 a) or to lyse target cells (Fig. 3 b). Therefore, the depression seen with antigenic stimulation of gld CTLs cannot simply be explained as a characteristic of a weak line or a general defect in TCR signaling. Instead, it implies that gld CTLs are missing a costimulatory signal required to achieve maximal proliferation during an antigenic response.
Figure 3.
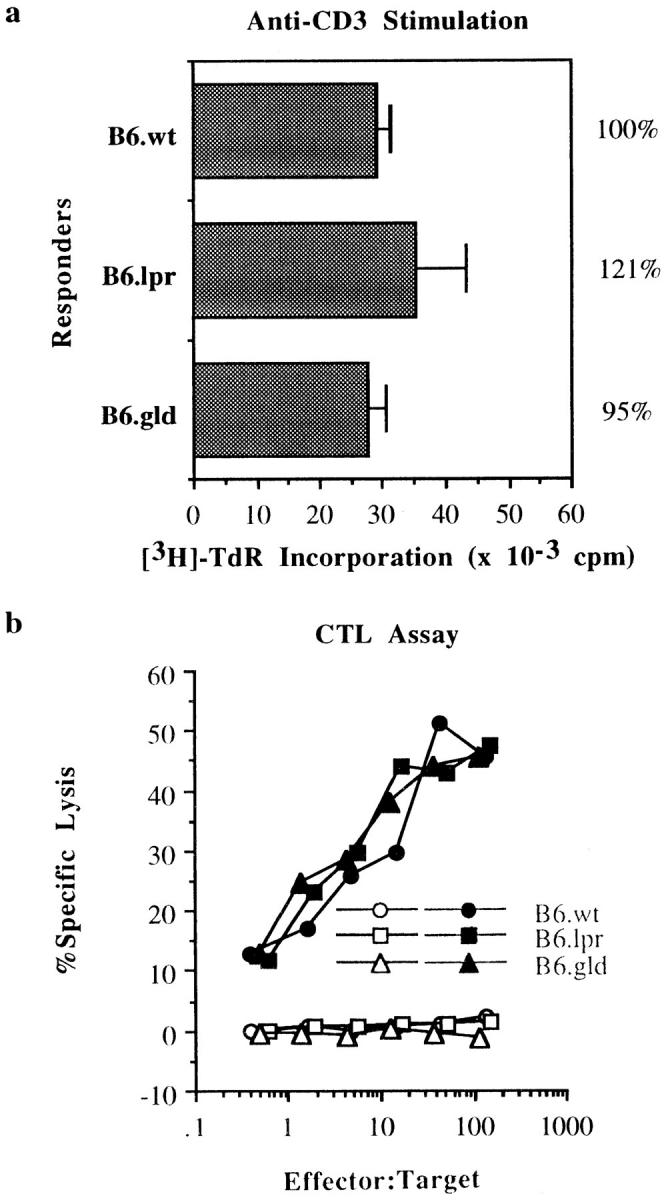
Lack of FasL influences neither the ability of gld CTLs to proliferate to wild-type levels after stimulation by optimal levels of plate-bound anti-CD3 antibodies nor their ability to perform cytolytic activity on a per recovered cell basis. (a) [3H]TdR incorporation by the long-term CTL lines used in Fig. 1 a, cultured over plate-bound anti-CD3 antibodies. Data are presented as in Fig. 1. (b) The same CTL responders were assayed for their ability to lyse 51Cr-labeled EL4 (H-2b, open symbols) and R1.1 (H-2k, filled symbols) tumor targets in a 4 h chromium release assay.
Proliferation by FasL+ CTL Lines in the Presence of Soluble FasIgG Mimics the Depression Observed for FasL− CTLs, whereas Costimulatory Signals Are Provided by Plate-bound FasIgG.
To pinpoint FasL as the source of the proliferative signal, soluble FasIgG fusion protein was added in an effort to block cell surface FasL–Fas interactions. A dose-dependent decrease in the proliferation of B6 wild-type CTLs was observed upon the addition to the culture of increasing amounts of soluble FasIgG but not isotype-matched huIgG1 (Fig. 4 a). Kinetic analyses also revealed that recovered cell numbers of both FasL+ CTLs (B6 wild-type and B6.lpr) were reduced to those of FasL− CTLs over the course of the MLC upon the addition of soluble FasIgG but not huIgG1 (Fig. 4 b). The fact that Fas− CTL lines are susceptible to blocking by soluble FasIgG demonstrates that Fas-mediated killing is not involved, which implicates FasL in positive signaling. When plate-bound FasIgG fusion protein was used in conjunction with suboptimal amounts of anti-CD3, a costimulatory signal was delivered for proliferation by FasL+ but not by FasL− CTLs (Fig. 4 c). These data demonstrate that the source of this positive signal is the FasL expressed on the wild-type and lpr CTL lines. Although it is important to note that the molecules mediating these signals have yet to be identified, the striking sequence conservation across species of the cytoplasmic domain of FasL suggests the presence of important functional domains. A proline-rich region of the cytoplasmic tail, in which 25 out of the 78 residues are prolines, including a stretch of 7 sequential proline residues (13, 14), is reminiscent of an SH3 binding site (15).
Figure 4.
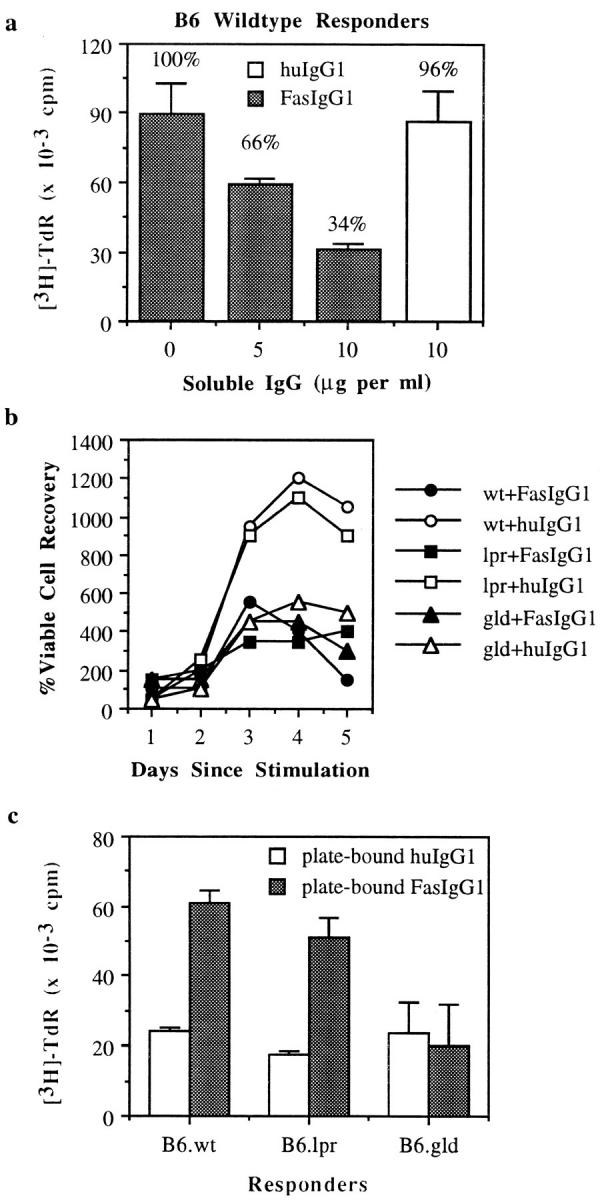
Soluble FasIgG blocks, whereas plate-bound FasIgG augments, proliferation of FasL+ CTLs. (a) [3H]TdR uptake of CTL responders from wild-type B6 mice cultured with allogeneic H-2k splenocytes alone or with either soluble huIgG1 or soluble FasIgG1. Data are normalized to proliferation levels in the absence of soluble IgG. (b) Viable cell counts of CTL responders from B6, B6.lpr, and B6.gld mice cultured with H-2k splenocytes and either soluble FasIgG1 or huIgG1. (c) [3H]TdR uptake of CTL responders seeded over suboptimal amounts of plate-bound anti-CD3 antibodies with either plate-bound FasIgG1 or huIgG1. Experiments were repeated three times. All data are averages of triplicate wells and error bars represent the SD of the means.
These results provide insights into the observed differences in the regulation of activated CD8+ and CD4+ T cells. Apoptosis of CD4+ T cells is primarily mediated through Fas (16), possibly both by fratricide and autocrine suicide (10–12). On the other hand, CD8+ T cells are less sensitive to Fas-mediated death (16, 17) and primarily undergo a slower death induced by the interaction of TNF with its receptor (16). However, neither mechanism operates exclusively in one T cell subpopulation or the other; TNFR-mediated apoptosis is measurable in CD4+ T cells in the absence of Fas expression (18) and CD8+ T cells are not completely resistant to Fas-mediated death (16). FasL− CD4+ T cells proliferate more extensively to superantigenic (19, 20) and class II–specific (Fig. 2) stimulation than do wild-type cells, while FasL− CD8+ T cells proliferate less vigorously than do wild-type cells (Fig. 1). The difference in proliferation observed for these T cell subpopulations may be the sum of two separate events, positive reverse signaling through FasL in CD8+ T cells and the sensitivity of CD4+ T cells to Fas-mediated death. In addition, FasL and Fas are both found on the surface of CTLs, leading to a scenario in which both positive and negative signals may be received by the same cell. The perceived “reduced sensitivity” of CD8+ T cells to Fas-mediated death may then be explained by the maintenance of a balance in the CD8+ T cell population in which cell proliferation induced by the FasL signal offsets the number of cells undergoing Fas-mediated death. Survival of each cell may depend upon which signal occurs first, or the FasL signal may be able to override the Fas negative signal, for example through intracellular competition for signaling molecules.
The role of TCR engagement in conjunction with the FasL signal remains unclear. Other molecules known for their positive signaling capabilities have recently been implicated in the death of cells in the absence of a concomitant antigen receptor signal. For example, signaling through CD40 without concurrent engagement of the B cell receptor leads to Fas-mediated cell death (21, 22), and may serve an immunoregulatory role by removing nonspecific B cells. It will be interesting to determine if FasL can still signal without engagement of the CD3/TCR complex and to analyze the consequences of such uncoupled signaling. Should FasL fail to deliver a positive signal without the coengagement of the TCR, the death signal through Fas may serve to remove activated T cells after antigen has been eliminated. In light of the discovery that CD40 signals can direct germinal center B cells to become memory B cells (23), one could speculate on the role of FasL in the clonal expansion of antigen-specific CTLs and the generation of memory T cells. Adoptive transfer of OT-1 (reference 24; class I–restricted OVA-specific TCR transgenic B6) spleen and LN cells into B6 wild-type, B6.lpr, and B6.gld mice followed by intraperitoneal injection of OVA peptide daily for 3 d revealed that antigen-specific donor cells injected into B6.lpr mice were defective in their expansion compared to that demonstrated in the other two recipients (Martin, S., and M. Bevan, unpublished data). These data are consistent with the inability of the Fas− B6.lpr host to deliver a positive signal through FasL for clonal expansion. It will be interesting to determine whether this difference in expansion is related to the development of memory cells and if the FasL signal during an immune response influences the entry of CTLs into the memory pathway.
Several models that allow arrangement of the positive/ negative cell surface receptors into three categories have recently emerged: (a) surface receptors capable of both positive (survival or proliferation) and negative (death) signals such as Fas (25, 26) and TNFR1 (27); (b) positively signaling receptors that augment the death receptor signal (28, 29); and (c) induced receptors paired with constitutive receptors with the opposite signaling capacity, such as CD28 and CTLA-4 (30). Our data place Fas–FasL signaling into this third category. FasL, induced upon activation of CD8+ T cells, sends a negative signal outward through its constitutively expressed receptor while also receiving a positive signal.
Acknowledgments
We thank Drs. M. Bevan and S. Martin for sharing unpublished observations, our colleagues for helpful comments on the manuscript, and Dr. A. Marshak-Rothstein for FasIgG transfectants.
This work was supported by the following grants from the National Institutes of Health: research grant AG-13078 and Basic Immunology training grant CA-09537.
References
- 1.Nagata S, Golstein P. The fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 2.Gruss H-J, Dower SK. Tumor necrosis factor ligand superfamily: involvement in the pathology of malignant lymphomas. Blood. 1995;85:3378–3404. [PubMed] [Google Scholar]
- 3.Smith CG, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 4.Stuber E, Neurath M, Calderhead D, Fell HP, Strober W. Cross-linking of OX40 ligand, a member of the TNF/NGF cytokine family, induces proliferation and differentiation in murine splenic B cells. Immunity. 1995;2:507–521. doi: 10.1016/1074-7613(95)90031-4. [DOI] [PubMed] [Google Scholar]
- 5.Wiley SR, Goodwin RG, Smith CA. Reverse signaling viaCD30 ligand. J Immunol. 1996;157:3635–3639. [PubMed] [Google Scholar]
- 6.Rouvier E, Luciani M-F, Golstein P. Fas involvement in Ca2+-independent T cell–mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceredig R, Lowenthal JW, Nabholz M, MacDonald HR. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985;314:98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- 8.Sarmiento M, Glasebrook AL, Fitch FW. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt-2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980;125:2665–2672. [PubMed] [Google Scholar]
- 9.Ozato K, Mayer N, Sachs DH. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980;124:533–540. [PubMed] [Google Scholar]
- 10.Ju S-T, Panka DJ, Cui H, Ettinger R, El-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 11.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, Green DR. Cell-autonomous Fas(CD95)/ Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 12.Dhein J, Walczak H, Baumier C, Debatin K-M, Krammer PH. Autocrine T-cell suicide mediated by APO-1/ (Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 13.Lynch DH, Watson ML, Alderson MR, Baum PR, Miller RE, Tough T, Gibson M, Davis-Smith T, Smith CA, Hunter K, et al. The mouse Fas-ligand gene is mutated in gldmice and is part of a TNF family gene cluster. Immunity. 1994;1:131–136. doi: 10.1016/1074-7613(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 15.Ren R, Mayer BJ, Cicchetti P, Baltimore D. Identification of a ten–amino acid proline-rich SH3 binding site. Science. 1993;259:1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- 16.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 17.Ehl S, Hoffmann-Rohrer U, Nagata S, Hengartner H, Zinkernagel R. Different susceptibility of cytotoxic T cells to CD95 (Fas/Apo-1) ligand–mediated cell death after activation in vitro versus in vivo. . J Immunol. 1996;156:2357–2360. [PubMed] [Google Scholar]
- 18.Sytwu H-K, Liblau RS, McDevitt HO. The roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity. 1996;5:17–30. doi: 10.1016/s1074-7613(00)80306-4. [DOI] [PubMed] [Google Scholar]
- 19.Ettinger R, Panka DJ, Wang JK, Stanger BZ, Ju ST, Marshak-Rothstein A. Fas ligand–mediated cytotoxicity is directly responsible for apoptosis of normal CD4+T cells responding to a bacterial superantigen. J Immunol. 1995;154:4302–4308. [PubMed] [Google Scholar]
- 20.Russell JH, Wang R. Autoimmune gldmutation uncouples suicide and cytokine/proliferation pathways in activated, mature T cells. Eur J Immunol. 1993;23:2379–2382. doi: 10.1002/eji.1830230951. [DOI] [PubMed] [Google Scholar]
- 21.Rathmell JC, Townsend SE, Xu JC, Flavell RA, Goodnow CC. Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas (CD95)-ligands modulated by the B cell antigen receptor. Cell. 1996;87:319–329. doi: 10.1016/s0092-8674(00)81349-5. [DOI] [PubMed] [Google Scholar]
- 22.Rothstein TL, Wang JKM, Panka DJ, Foote LC, Wang Z, Stanger B, Cui H, Ju S-T, Marshak-Rothstein A. Protection against Fas-dependent Th1-mediated apoptosis by antigen receptor engagement in B cells. Nature. 1995;374:163–165. doi: 10.1038/374163a0. [DOI] [PubMed] [Google Scholar]
- 23.Arpin C, Dechanet J, Van Kooten C, Merville P, Brouard G, Briere F, Banchereau J, Liu Y-J. Generation of memory B cells and plasma cells in vitro. . Science. 1995;268:720–722. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- 24.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 25.Alderson MR, Armitage RJ, Maraskovsky E, Tough TW, Roux E, Schooley K, Ramsdell F, Lynch DH. Fas transduces activation signals in normal human T lymphocytes. J Exp Med. 1993;178:2231–2235. doi: 10.1084/jem.178.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mapara MY, Bargou R, Zugck C, Dohmer H, Ustaoglu F, Jonker RR, Krammer PH, Dorken B. APO-1 mediated apoptosis or proliferation in human chronic B lymphocytic leukemia: correlation with bcl-2 oncogene expression. Eur J Immunol. 1993;3:702–708. doi: 10.1002/eji.1830230320. [DOI] [PubMed] [Google Scholar]
- 27.Tewari M, Dixit VM. Recent advances in tumor necrosis factor and CD40 signaling. Curr Opin Genet Dev. 1996;6:39–44. doi: 10.1016/s0959-437x(96)90008-8. [DOI] [PubMed] [Google Scholar]
- 28.Tartaglia LA, Goeddel DV. Two TNF receptors. Immunol Today. 1992;13:151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- 29.Duckett CS, Gedrich RW, Gilfillan MC, Thompson CB. Induction of nuclear factor κB by the CD30 receptor is mediated by TRAF1 and TRAF2. Mol Cell Biol. 1997;17:1535–1542. doi: 10.1128/mcb.17.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]