Abstract
The presence of melanin-concentrating hormone (MCH) containing processes, projecting from the lateral hypothalamus to the medial nucleus tractus solitarius (mNTS), has been reported in the rat. It was hypothesized that MCH acting within the mNTS may modulate the central regulation of cardiovascular function. This hypothesis was tested in urethane-anesthetized, artificially ventilated, adult male Wistar rats. Microinjections (100 nl) of MCH (0.25, 0.5, 0.75, and 1 mM) into the mNTS of anesthetized rats elicited decreases in mean arterial pressure (20.4 ± 1.6, 50.7 ± 3.3, 35.7 ± 2.8 and 30.0 ± 2.6 mmHg, respectively). The decreases in heart rate in response to these concentrations of MCH were 40.0 ± 8.7, 90.0 ± 13.0, 48.0 ± 7.3 and 48.0 ± 8.0 beats/min, respectively. Maximum cardiovascular responses were elicited by a 0.5 mM concentration of MCH. Cardiovascular responses to MCH were similar in unanesthetized mid-collicular decerebrate rats. Control microinjections of normal saline (100 nl) did not elicit any cardiovascular response. Ipsilateral or bilateral vagotomy significantly attenuated MCH-induced bradycardia. Prior microinjections of PMC-3881-PI (2 mM; MCH-1 receptor antagonist) into the mNTS blocked the cardiovascular responses to microinjections of MCH. Microinjection of MCH (0.5 mM) into the mNTS decreased efferent greater splanchnic nerve activity. Direct application of MCH (0.5 mM; 4 nl) to barosensitive NTS neurons increased their firing rate. These results indicate that: 1) MCH microinjections into the mNTS activate MCH-1 receptors and excite barosensitive NTS neurons, causing a decrease in efferent sympathetic activity and blood pressure, and 2) MCH-induced bradycardia is mediated via the activation of the vagus nerves.
INTRODUCTION
Melanin concentrating hormone (MCH) was initially isolated from salmon pituitaries (Kawauchi et al., 1983). Subsequently, an antiserum against salmon MCH was used for demonstrating the presence of MCH (Skofitsch et al., 1985; Zamir et al., 1986b) and for isolation and purification of this peptide from the rat hypothalamus (Vaughan et al., 1989). The rat hypothalamic MCH is a 19-aminoacid cyclic peptide that differs from the salmon MCH in that it has an N-terminal extension of two amino acids and two other substitutions (Vaughan et al., 1989). MCH is derived from post-translational cleavage of the C-terminal of a larger precursor molecule consisting of 165 amino acids called pre-proMCH (Presse et al., 1990).
In the rat brain, major groups of MCH containing neurons are located predominantly in the lateral hypothalamic area and zona incerta and MCH-containing fibers are distributed throughout the brain and spinal cord (Bittencourt et al., 1992; Skofitsch et al., 1985; Zamir et al., 1986a,b). Moderate density of MCH immunoreactive fibers has been reported in the nucleus tractus solitarius (NTS) and the medullary reticular formation including gigantocellular reticular nucleus of the rat (Skofitsch et al., 1985; Zamir et al., 1986a,b). Similar distribution of MCH neurons and fibers has been reported in the human brain (Bresson et al., 1989; Mouri et al., 1993).
MCH has been identified as a natural ligand for an orphan G-protein coupled receptor, called SLC-1 receptor because of its sequence similarity with somatostatin receptor (Bachner et al., 1999; Chambers et al., 1999; Lembo et al., 1999; Saito et al., 1999; Saito et al., 2000; Shimomura et al., 1999). The SLC-1 receptor, re-named as the MCH-1 receptor, has been cloned in the rat and mouse (Kokkotou et al., 2001; Lakaye et al., 1998). The distribution of MCH-1 receptor in the rat brain and spinal cord (Hervieu et al., 2000) overlaps the areas exhibiting MCH immunoreactivity (Bittencourt and Elias, 1998). A second MCH receptor, called the MCH-2 receptor, has also been identified (Hill et al., 2001; Mori et al., 2001; Rodriguez et al., 2001; Sailer et al., 2001; Songzhu et al., 2001; Wang et al., 2001). Non-primate species, including the rat, do not possess a functional MCH-2 receptor (Tan et al., 2002).
Information regarding the physiological role of MCH is still emerging (for reviews see: Boutin et al., 2002; Griffond and Baker, 2002; Hervieu, 2003; Nahon, 1994). In teleost fish MCH has been reported to regulate skin color (Kawauchi et al., 1983) while in mammals this peptide has been implicated in regulating feeding behavior and energy homeostasis; MCH increases food intake and decreases energy expenditure. For example, transgenic mice over-expressing MCH exhibit hyperphagia (Ludwig et al., 2001) and mice with genetic deletion of MCH are hypophagic, lean and have an increased rate of energy expenditure (Kokkotou et al., 2005; Shimada et al., 1998). Intracerebroventricular (i.c.v.) injection of MCH elicits an increase (Ludwig et al., 1998; Rossi et al., 1997) while pharmacological antagonism of MCH-1 receptor elicits a decrease in food intake in rats (Kowalski et al., 2004).
The location of MCH neurons in the lateral hypothalamus (Skofitsch et al., 1985; Zamir et al., 1986a,b), which is known to be involved in the regulation of cardiovascular and other autonomic functions, suggests that this peptide may play a role in the modulation of autonomic functions including cardiovascular regulation in addition to its well established role in feeding behavior and energy homeostasis. Indeed, there are reports in literature which suggest a role of MCH in cardiovascular regulation. For example, mice lacking MCH-1 receptor exhibit an increase in heart rate (Astrand et al., 2004) and i.c.v. administration of MCH elicits hypotension and bradycardia in rats (Messina and Overton, 2007).
The nucleus tractus solitarius (NTS) is one of the medullary structures that plays an important role in the central regulation of cardiovascular function (for reviews see: Coote, 2007; Gordon and Sved, 2002; Guyenet, 2006; Sapru, 2002, 2004; Talman et al., 1984). The presence of MCH containing fibers and MCH-1 receptors has been demonstrated in the NTS (Hervieu et al., 2000; Zamir et al., 1986a,b). Based on these reports, it was hypothesized that the NTS may mediate the depressor and bradycardic responses to centrally administered MCH via activation of MCH-1 receptors. In order to test this hypothesis, MCH was directly microinjected into the NTS in rats and the mechanism of cardiovascular effects studied.
EXPERIMENTAL PROCEDURES
General Procedures
Adult male Wistar rats (Charles River Laboratories, MA, USA), weighing 300–350 g, were used in this study. All animals were housed under controlled conditions with a 12-hour light/dark cycle. Food and water were available to the animals ad libitum. The experimental procedures were performed in accordance with NIH “Guide for the Care and Use of Laboratory Animals” (Publication No. 80-23, revised 1996). All protocols were approved by the Institutional Animal Care and Use Committee at New Jersey Medical School, Newark. The number of animals used was the minimum required for statistical analyses of the data and every effort was used to minimize suffering to the animals.
Surgery
The rats were initially anesthetized with isoflurane (2–3% in 100% oxygen) delivered via a nose-mask, and the femoral vein and artery on one side were cannulated (using polyethylene 50 tubing) for intravenous injections and for monitoring the blood pressure (BP), respectively. The BP was monitored via a pressure transducer (Grass Instruments, West Warwick, RI, USA, model P23 Db) and the heart rate (HR) was monitored by a tachograph (Grass Instruments, model 7P4) that was triggered by the BP waves. Mean arterial pressure (MAP) was derived electronically from BP waves. Urethane (1.2–1.4 gm/kg) was injected intravenously in 6–7 aliquots at 2-min intervals and the administration of isoflurane was discontinued. The animal remained artificially ventilated with room air throughout the experiment. The depth of anesthesia was periodically tested by pinching the hind paw of the rat; the absence of a pressor response and/or withdrawal of the limb indicated that the rat was adequately anesthetized. Rectal temperature was continuously monitored and maintained at 37 ± 0.5° C using an infrared lamp connected to a temperature controller. A polygraph (Grass Instruments, model 7D) was used for all recordings.
Vagotomy was necessary in experiments designed to investigate the role of parasympathetic innervation to the heart in mediating the bradycardic responses elicited by microinjections of MCH into the mNTS. For these experiments, silk sutures were placed loosely around the vagus nerves bilaterally for subsequent identification and sectioning of the nerves.
Decerebration
Anesthesia was induced and maintained by tracheal administration of isoflurane as described earlier. The external and internal carotid, and pterygopalatine arteries were ligated bilaterally. The rats were placed in a prone position in a stereotaxic instrument (David Kopf Instruments, Tujunga, CA, USA, model 1430 adapted for rat) with the bite bar 18 mm below the interaural line. The parietal bones were removed, the dura was incised, a transection was made at mid-collicular level, the portion of the brain rostral to the transection was removed by suction and the cranial cavity was loosely packed with cotton balls. Administration of isoflurane was terminated at this time and a stabilization period of 50–60 min was allowed after the decerebration.
Microinjections
The anesthetized or decerebrate rats were fixed in a stereotaxic instrument in a prone position as described earlier. The medulla was exposed and the calamus scriptorius (reference point) was visually identified using an operating microscope (Carl Zeiss, Thornwood, NY, USA, model OPMI-1H). Four barreled glass-micropipettes (tip size 20–40 μm) were mounted on a micromanipulator (David Kopf Instruments, model 1460 with an AP slide 1262) and each barrel was connected via polyethylene tubing to one of the channels on a picospritzer (General Valve Corp, Fairfield, NJ, USA). The micropipettes included barrels containing L-glutamate (L-Glu), normal saline, MCH and MCH-1 receptor antagonist (see Drugs and Chemicals). The coordinates for the mNTS were: 0.5–0.6 mm rostral to the calamus scriptorius, 0.5–0.6 mm lateral to the midline and 0.5–0.6 mm deep from the dorsal medullary surface. The site eliciting depressor and bradycardic responses was identified by microinjections of L-Glu (5 mM) into the mNTS. A 100 nl volume was selected for all microinjections (unless indicated otherwise) because this volume elicited maximum cardiovascular responses (Brown et al., 2006). The duration of microinjection was 10 sec. Controls for microinjections consisted of 100 nl normal saline (pH 7.4).
Intracerebroventricular injections
Anesthetized rats were fixed in a prone position in the stereotaxic instrument as described earlier. The dorsal surface of the skull was exposed, bregma identified and parietal bones partially removed to create a window (4 × 4 mm) exposing the cortex. A triple barreled micropipette (one barrel filled with MCH, the second with normal saline and the third with India ink) was mounted on a micromanipulator and intracerebroventricular (i.c.v.) injections were made (coordinates: 0.3 mm caudal to Bregma, 1.5 mm lateral to the midline and 3 mm deep from the dorsal surface of the cortex). Diluted India ink (5 μl) was injected into the lateral ventricle and the location of the dye was examined postmortem under an operating microscope.
Greater splanchnic nerve recording
The greater splanchnic nerve (GSN) was exposed, using a retroperitoneal approach, in anesthetized rats. The segment of the GSN immediately proximal to the celiac ganglion was identified under an operating microscope sectioned at its junction with the celiac ganglion and a few mm of the central end of the nerve were desheathed (for a diagram, see Sapru et al., 1982). The exposed portion of the nerve was placed on a bipolar silver hook electrode and the nerve and the tips of the electrode were embedded in a silicone elastomer (WPI, Sarasota, FL, USA; Kwik-Sil) which was allowed to set for 5–10 min. The electrode was connected to a probe head-stage (CWE Inc., Ardmore, PA, USA, model Super-Z) and the whole nerve discharge was amplified (X10,000–20,000, using CWE, model BMA-830 amplifier) and filtered (100–5000 Hz). Amplified signals were digitized (22 kHz) using Neuro-Corder (Cygnus technologies, Delaware Water Gap, PA, USA), visualized on an oscilloscope (Tektronix Beaverton, OR, USA, model R5103N) and stored together with BP and HR on a video cassette recorder. The whole greater splanchnic nerve activity (GSNA) was full-wave rectified and a moving average (integrated) signal was obtained (CWE, model MA-821 moving averager, time-constant 100 msec). At the end of the experiment, the GSN was sectioned centrally and the remaining activity was considered to be the noise level which was subtracted from the GSNA amplitude. The amplitude of the GSNA was expressed as μV and the moving average signal was expressed as μV/100 msec.
Extracellular Neuronal Recording
A 5-barreled glass micropipette (Medical Systems, Greenvale, NY, USA) was pulled in a vertical pipette puller (Narishige, Tokyo, Japan, model PE-2) and the tip size was adjusted so that the resistance of the barrels was 4–8 mega Ohm. The micropipette was mounted on a micromanipulator (David Kopf Instruments, model 1460 with an AP slide model 1262). The barrel used for recording was filled with 4 M NaCl, the second barrel contained normal saline and other barrels contained L-Glu, MCH and MCH-1 receptor antagonist. The spontaneous extracellular activity of mNTS neurons was filtered (300–10,000 Hz) and amplified (X10,000–20,000; WPI, model DAM 80 amplifier). The signals were fed into a window discriminator (Frederick Haer, Brunswick, ME, USA, model 2503), visualized on an oscilloscope, and spike activity was monitored using a rate meter (CWE, model RIC-830). Ejection of the contents in different barrels on neurons was accomplished by application of pressure pulses (15 msec duration, 20 psig) and the volume of ejected solution (4 nl) was visually confirmed under a modified binocular horizontal microscope. Controls consisted of 4 nl pressure applications of normal saline (pH 7.4) to mNTS neurons.
Histology
The typical sites of microinjections were marked by a unilateral microinjection (100 nl) of diluted India ink. The animals were perfused with arterial administration of heparinized normal saline followed by 10% formalin, and the brains were removed and fixed in 10% formalin for 72 hrs. After the fixation procedure was completed, serial sections of the medulla were cut (30 μm) in a vibratome (The Vibratome Company, St Louis, MO, model 1000), mounted on slides, stained with cresyl violet and the microinjection site (marked with India ink) was identified under a microscope (Olympus Provis, Middlebush, NJ, USA, model AX70). The sections were photographed and compared with a standard atlas (Paxinos and Watson, 1986).
Drugs and chemicals
The following drugs and chemicals were used: L-Glu monosodium, isoflurane, MCH (Rat), PMC-3881-PI (Ac-Arg-[Cys-Met-Ava-Arg-Val-Tyr-Ava-Cys]-NH2) (antagonist for MCH-1 receptors) (Bednarek et al., 2002) and urethane. Rat MCH used in this study is identical to human MCH (Herviev, 2003). All solutions for microinjections were freshly prepared in normal saline (pH 7.4); the selection of normal saline as a vehicle instead of the artificial cerebrospinal fluid (aCSF) was prompted by better solubility of MCH in the normal saline. Where applicable, the concentrations of drugs injected into the mNTS refer to their salts. All drugs, except MCH, PMC-3881-PI, and isoflurane, were obtained from Sigma Chemicals (St. Louis, MO, USA). Isoflurane was purchased from Baxter Pharmaceutical Products (Deerfield, IL, USA), and MCH and PMC-3881-PI were purchased from Peptides International (Louisville, KY, USA).
Statistical analyses
For comparison of MAP and HR responses, the means and standard error of mean (SEM) were calculated for maximum changes in these values in response to microinjections of MCH or L-Glu into the mNTS. In dose-response and tachyphylaxis studies, comparisons of maximum decreases in MAP and HR in different groups of rats were made by using a one-way analysis of variance (ANOVA) followed by Tukey-Kramer’s multiple comparison test. Comparisons of the maximum decreases in MAP and HR elicited by microinjections of MCH or L-Glu into the mNTS, before and after the microinjections of an MCH-1 receptor antagonist (PMC-3881-PI) and also before and after bilateral vagotomy were made by using the Student’s paired t-test. For the analyses of nerve activities, control values of nerve activity represented the averages of GSNA amplitudes during 35 sec periods before the microinjections of various agents into the mNTS or intravenous injection of phenylephrine. Maximum inhibition in GSNA amplitudes, in response to different treatments, was averaged over a period of 35–60 sec and expressed as percent of the control value of GSNA amplitude. The mean values of the integrated signals before and after the administration of drugs were compared using Student’s paired t-test. For the analyses of neuronal activities, the mean values of the neuronal firing rate were determined after intravenous injections of phenylephrine, and microinjections of L-Glu, saline, MCH and PMC-3881-PI into the mNTS. The differences in neuronal firing in induced by microinjections of MCH into the mNTS, before and after the microinjections of PMC-3881-PI at the same site, were determined by ANOVA followed by Duncan’s multiple range test. In all cases, the differences were considered significant at P < 0.05.
RESULTS
Baseline values for MAP and HR in urethane-anesthetized rats used in this study were 107.5 ± 2.0 mmHg and 406.3 ± 7.4 bpm, respectively (n = 86). The values for baseline MAP and HR in the decerebrate rats (n = 8) were 91.4 ± 9.5 mmHg and 400 ± 20.0 bpm, respectively.
Dose-response of MCH
In this and other series of experiments, the mNTS was always identified by microinjections of L-Glu which stimulate neurons but not fibers of passage. Microinjections of L-Glu (5 mM) into the mNTS elicited depressor (46.4 ± 2.9 mmHg) and bradycardic (93.0 ± 12.3 bpm) responses (n = 28). As mentioned earlier, the volume of all microinjections was 100 nl unless indicated otherwise. The interval between microinjections of L-Glu and subsequent injections of other agents was at least 5 min.
Microinjections of MCH (0, 0.25, 0.5, 0.75, and 1 mM) were made into the mNTS; 0 mM concentration refers to normal saline (n = 28). A minimum of 5 rats was used for each concentration and no more than 2 concentrations were injected in each rat. The decreases in MAP elicited by these concentrations of MCH were 0 ± 0, 20.4 ± 1.6, 50.7 ± 3.3, 35.7 ± 2.8 and 30.0 ± 2.6 mmHg, respectively (Fig. 1A). Preliminary studies showed that smaller concentrations (e.g., 0.125 mM) elicited small and inconsistent responses; therefore, detailed dose-response was not studied for this concentration. ANOVA of MAP values was found to be highly significant (F = 22.5, df = 3,24, P < 0.0001). A Tukey Kramer post hoc multiple comparisons test showed that the depressor responses elicited by 0.5 mM concentration were significantly greater (P < 0.001) than those of 0.25, 0.75 and 1.0 mM concentrations (P < 0.001). The depressor responses to 0.5 mM and 0.75 mM concentrations were also significantly greater than those of 0.25 mM concentration (P < 0.001–0.01). The depressor responses elicited by 1 mM and 0.25 mM and 0.75 mM were not statistically different from each other (P > 0.05). The decreases in HR in response to the same concentrations of MCH (i.e., 0, 0.25, 0.5, 0.75, and 1 mM) were 0 ± 0, 40.0 ± 8.7, 90.0 ± 13.0, 48.0 ± 7.3 and 48.0 ± 8.0 beats/min (bpm), respectively (Fig. 1B). An ANOVA of HR values was also found to be significant (F = 15.68, df = 4,30, P < 0.0001). A Tukey Kramer post hoc multiple comparisons test showed that the effect of 0.5 mM concentration was significantly greater than that of 0.25, 0.75 and 1 mM concentration (P < 0.05). However, the differences between the effects of 0.25, 0.75 and 1.0 mM were not statistically significant (P > 0.05). Thus, maximal BP and HR responses were elicited by 0.5 mM concentration of MCH. The onset and duration of cardiovascular responses to microinjections of MCH (0.25–1 mM) were 2–4 sec and 30–80 sec, respectively. The peak effect was observed at 5–20 sec.
Fig. 1.
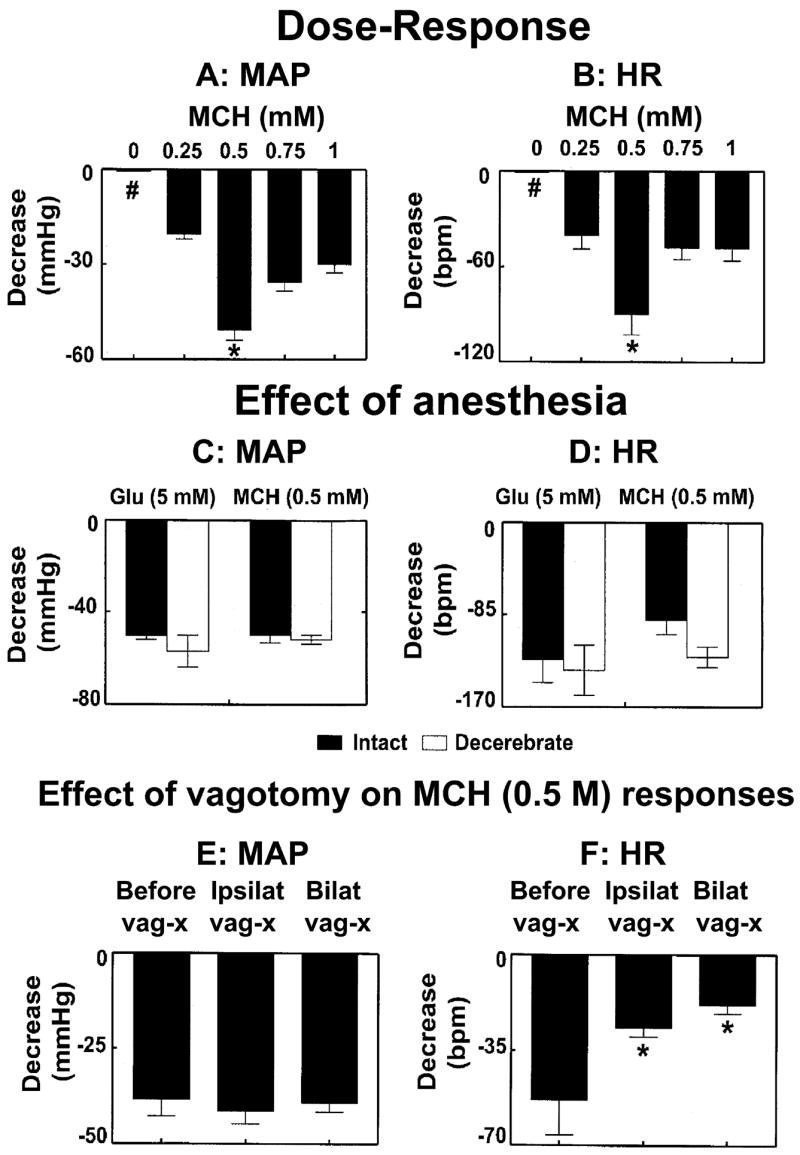
Cardiovascular effects of MCH. Dose-response for decreases in mean arterial pressure (MAP; A) and heart rate (HR; B) responses to microinjections (100 nl) of MCH (0, 0.25, 0.5, 0.75, and 1 mM) into the mNTS (n = 28); 0 mM refers to normal saline (pH 7.4) which elicited no responses (indicated by # symbol). The decreases in MAP and HR elicited by 0.5 mM concentration of MCH were significantly greater than those of other concentrations (*P < 0.001–0.01). Comparison of MAP (C) and HR (D) responses induced by microinjections to L-Glu (5 mM) and MCH (0.5 mM) into the mNTS of urethane-anesthetized (n = 9; dark bars) and unanesthetized decerebrate (n = 8; open bars) rats; L-Glu or MCH-induced decreases in MAP and HR were not statistically different (P > 0.05) in the two groups of rats. Effect of vagotomy on MCH-induced effects on MAP (E) and HR (F) (n = 7); ipsilateral (ipsilat) and bilateral (bilat) vagotomy did not significantly (P > 0.05) alter MCH-induced depressor responses but the HR responses were significantly (*P < 0.05) attenuated.
Diminution of responses to the repeated administration of the same concentration of the agonist (tachyphylaxis) was tested by making 3 consecutive microinjections of MCH (0.5 mM) into the mNTS at 20 min intervals (n = 5). The decreases in MAP in response to these microinjections were 43.9 ± 3.1, 41.2 ± 3.1, and 41.1 ± 3.0 mmHg, respectively; a repeated measure ANOVA showed that these values were not statistically different (P > 0.05). Likewise, the decreases in HR in response to 3 microinjections of the same concentration of MCH in the same group of rats were 70.0 ± 10.0, 61.0 ± 8.5 and 61.3 ± 15 bpm, respectively; these responses were also not statistically different from each other (P > 0.05). Since maximal MAP and HR responses were elicited by 0.5 mM concentration of MCH and repeated microinjections of this concentration did not exhibit tachyphylaxis, this concentration was used for other experiments.
Leakage of the MCH, if any, from the injection site in the mNTS into the systemic circulation or CSF was not responsible for the MCH-induced depressor and bradycardic responses. This conclusion was based on our observation that the concentrations of MCH (0.5 mM, 100 nl) that elicited depressor and bradycardic responses when microinjected into the mNTS, did not elicit a response when injected intravenously (n = 5) or i.c.v. (n = 4).
Experiments were carried out to exclude the possibility that MCH microinjected into the mNTS may have spread to adjacent regions and elicited depressor and bradycardic responses. In these experiments (n = 9), a site in the mNTS where microinjections of L-Glu elicited depressor and bradycardic responses, was first identified in each rat; microinjections of MCH (0.5 mM) at this site elicited decreases in MAP (50.7 ± 3.3 mmHg) and HR (90.0 ± 13.0 bpm). In the same group of rats, microinjections of L-Glu and MCH (0.5 mM) into an adjacent area (e.g., the cuneate nucleus) elicited no cardiovascular responses. Similar microinjections of L-Glu and MCH into the ventrolateral NTS of the same rats did not elicit cardiovascular responses. In another group of rats (n = 3), L-Glu (5 mM; 30 nl) was microinjected into the area postrema; pressor and tachycardic responses were elicited from this region. Microinjections of MCH (0.5 mM, 30 nl) at the same site elicited no responses. These results indicate that responses elicited by MCH in the mNTS are not mediated by spread of the injected peptide to brain regions adjacent to this nucleus.
Effect of urethane-anesthesia on MCH responses
In urethane-anesthetized (n = 9) and unanesthetized decerebrate (n = 8) rats, microinjections of L-Glu elicited MAP decreases of 50.4 ± 1.5 and 57.0 ± 7.3, respectively. The decreases in MAP induced by microinjections of MCH (0.5 mM) into the mNTS of the same groups of urethane-anesthetized and unanesthetized decerebrate rats were 50.7 ± 3.3 and 51.9 ± 2.0 mmHg, respectively. Thus, the L-Glu-induced decreases in MAP in urethane-anesthetized and unanesthetized decerebrate rats were not statistically different (P > 0.05) (Fig. 1C). The decrease in HR induced by L-Glu (5 mM) into the mNTS of the same group of urethane-anesthetized and unanesthetized decerebrate rats were 126.7 ± 21.0 and 136.3 ± 23.2 bpm, respectively. The decreases in HR induced by MCH (0.5 mM) into the mNTS of the same group of urethane-anesthetized and unanesthetized decerebrate rats were 90.0 ± 13.0 and 124.0 ± 9.3 bpm, respectively. Thus, the L-Glu-induced decreases in HR in urethane-anesthetized and unanesthetized decerebrate rats were not statistically different (P > 0.05) (Fig. 1D). These results indicated that the urethane did not significantly (P > 0.05) affect the cardiovascular responses to microinjections of L-Glu or MCH into the mNTS.
MCH-induced bradycardia: effect of vagotomy
The decrease in MAP induced by microinjections of MCH (0.5 mM) into the mNTS in another group of rats (n = 7) was 38.3 ± 4.4 mmHg. After ipsilateral and bilateral vagotomy MCH-induced decreases in MAP were 41.4 ± 3.3 and 39.2 ± 2.4 mmHg, respectively (P > 0.05) (Fig. 1E). In the same group of rats, the decrease in HR induced by microinjections of MCH into the mNTS was 53.3 ± 8.4 bpm. After ipsilateral and bilateral vagotomy in the same group of rats, the MCH-induced decreases in HR were 26.6 ± 3.3 and 18.3 ± 3.1 bpm, respectively (P < 0.05) (Fig. 1F). Thus, ipsilateral and bilateral vagotomy did not significantly alter MCH-induced depressor responses but these procedures significantly attenuated the HR responses to microinjections of MCH into the mNTS.
Blockade of MCH-induced responses
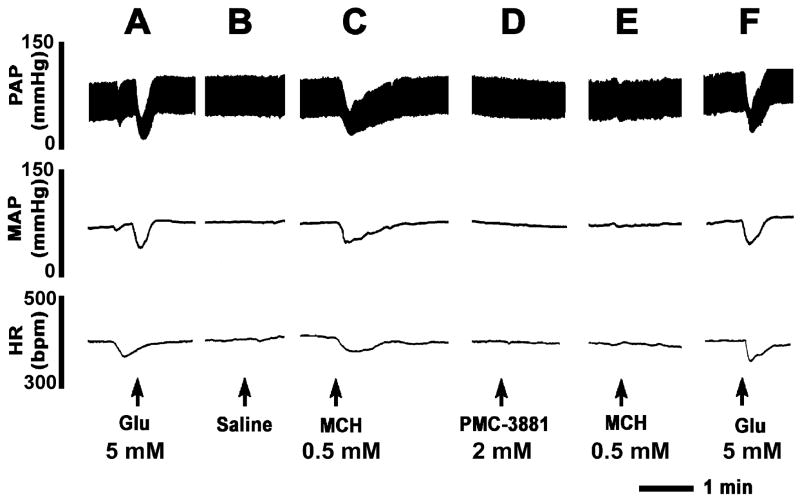
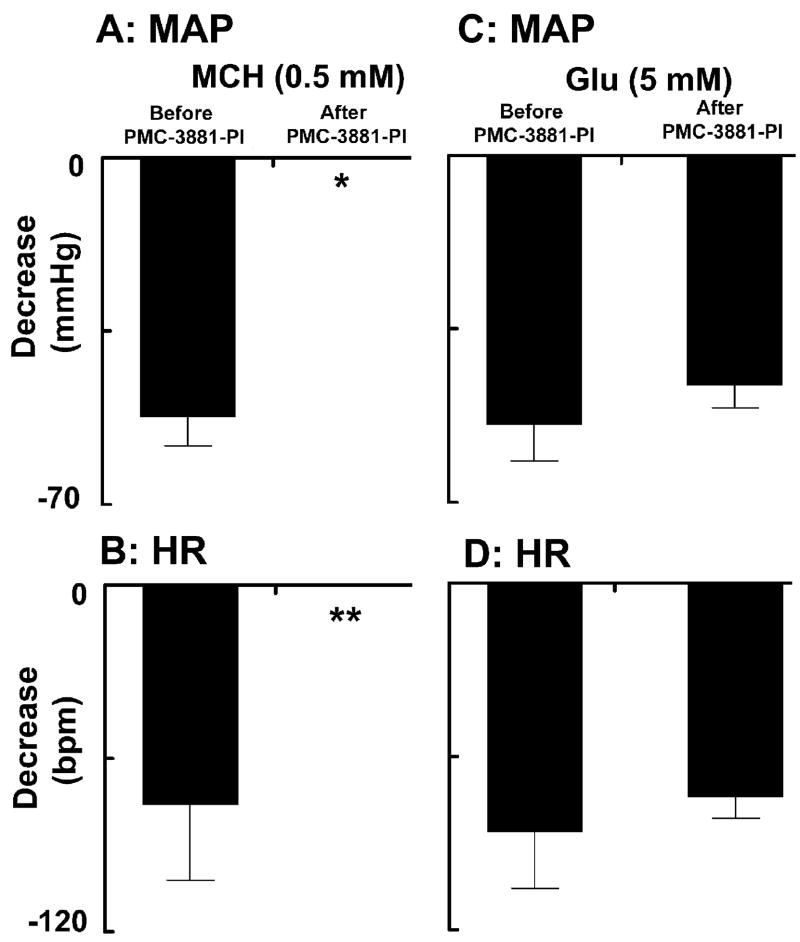
A typical recording from one rat is shown in Fig. 2. The mNTS-region was identified by a microinjection of L-Glu (5 mM) (Fig. 2A). About 5 min after the BP and HR returned to basal levels, a microinjection of normal saline (pH 7.4; 100 nl) at the same site elicited no responses (Fig. 2B). About 2 min after the microinjection of saline, microinjection of MCH (0.5 mM) at the same site elicited decreases in pulsatile arterial pressure (PAP), MAP and HR (Fig. 2C). About 20 min after recovery of the responses, PMC-3881-PI (2 mM; antagonist at MCH-1 receptors) was microinjected at the same site (20 min time interval was selected to avoid MCH-induced tachyphylaxis, if any). The MCH-1 receptor antagonist did not elicit a cardiovascular response by itself (Fig. 2D). Within 2 min, MCH (0.5 mM) was again microinjected at the same site; the responses to MCH were blocked (Fig. 2E) and the blockade persisted for at least 20 min. Microinjection of L-Glu (5 mM), 2 min after MCH injection, elicited a decrease in MAP and HR which was not significantly different than the initial L-Glu response (Fig. 2F). Group data for these experiments (n = 5) are shown graphically in Fig. 3. The decrease in MAP in response to microinjections of MCH (0.5 mM) into the mNTS (52.3 ± 5.8 mmHg) was completely blocked by prior microinjection of PMC-3881-PI (2 mM) at the same site (P < 0.001) (Fig. 3A). Similarly, the decrease in HR (76.0 ± 26.0 bpm) by the same microinjection of MCH in the same group of rats was also completely blocked by prior microinjection of PMC-3881-PI at the same site (P < 0.0001) (Fig. 3B). The lack of responses to MCH after microinjections of PMC-3881-PI was not due to tachyphylaxis because, as described earlier, repeated microinjections of MCH (0.5 mM) did not exhibit tachyphylaxis when the interval between injections was at least 20 min. The decreases in MAP elicited by microinjections of L-Glu (5 mM) into the mNTS before and after the microinjection of PMC-3881-PI (2.0 mM) were 54.3 ± 7.2 and 46.3 ± 4.6 mmHg, respectively (P > 0.05, Fig. 3C). Similarly, in the same group of rats, the decreases in HR elicited by the same concentration of L-Glu into the mNTS before and after the microinjection of the same concentration of PMC-3881-PI were 86.0 ± 20.0 and 74.0 ± 7.5 bpm, respectively (P > 0.05) (Fig. 3D). This experiment indicated that PMC-3881-PI did not exert any deleterious effects at the site of injection because the responses to an unrelated agonist (L-Glu) at the same site remained unaltered. The concentration of PMC-3881-PI used in this experiment (2 mM) for blocking MCH responses was selected based on the observation that smaller concentrations of this antagonist (e.g., 1 mM) were not sufficient to block the effects of MCH (0.5 mM).
Fig. 2.
Microinjections of MCH into the mNTS elicit depressor and bradycardic responses. Top trace: Pulsatile arterial pressure (PAP, mmHg), middle trace: Mean arterial pressure (MAP, mmHg), bottom trace: Heart rate (HR, beats/min). A: mNTS was identified by a microinjection of L-Glu (5 mM). B: 5 min later, microinjection of normal saline (pH 7.4) did not elicit any response. C: 2 min later, microinjection of MCH (0.5 mM) elicited a decrease in BP and HR. D: PMC-3881-PI (2.0 mM, MCH-1 receptor antagonist) was microinjected 20 min after the microinjection of MCH; no responses were elicited. E: Microinjection of MCH (0.5 mM) at this time failed to elicit a response. F: Microinjection of L-Glu (5 mM) 2 min later elicited the usual decrease in BP and HR.
Fig. 3.
Blockade of MCH responses by PMC-3881-PI. A: The decrease in MAP in response to microinjections of MCH (0.5 mM) into the mNTS was completely blocked after the microinjection of PMC-3881-PI (2.0 mM) at the same site (*P < 0.001) (n = 5). B: Decrease in HR in response to microinjections of MCH (0.5 mM) into the mNTS in the same group of rats was also completely blocked by prior microinjections of PMC-3881-PI at the same site (**P < 0.0001). The decreases in MAP (C) and HR (D) elicited by microinjections of L-Glu (5 mM) into the mNTS of the same group of rats were not significantly (P > 0.05) altered by prior microinjections of PMC-3881-PI at the same site.
Effect of MCH on sympathetic nerve activity
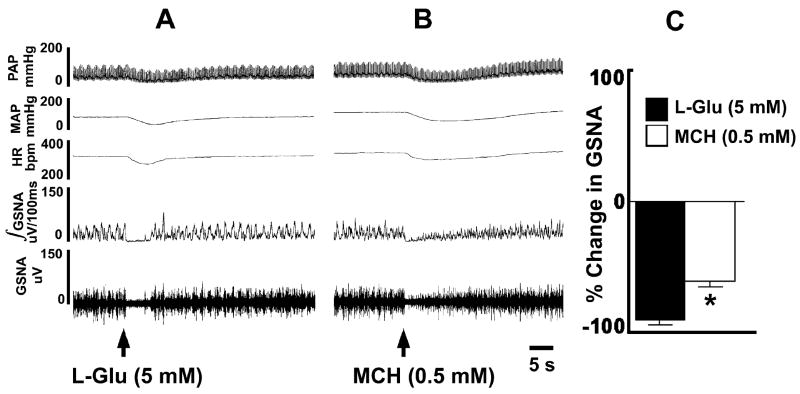
A recording showing the effects of microinjections of MCH into the mNTS on efferent greater splanchnic nerve discharge is presented in Fig. 4. Efferent discharge was recorded from a segment of the nerve rostral to the celiac ganglion. An intravenous bolus injection of phenylephrine (PE; 10 μg/kg) increased BP which, in turn, elicited reflex bradycardia and inhibition of efferent sympathetic nerve discharge (not shown). About 5 min later, when the effects of PE subsided, microinjection of L-Glu (5 mM) into the mNTS inhibited the nerve discharge which lasted for 6–12 sec (Fig. 4A). After 5 min, microinjections of saline (100 nl) into the mNTS did not alter the sympathetic nerve activity (not shown). Within 5 min, MCH (0.5 mM) was microinjected at the same site in the mNTS; a decrease in efferent nerve discharge was elicited which lasted for 15–50 sec (Fig. 4B). Group data (n = 6) for this experiment are shown in Fig. 4C. Microinjections of L-Glu (5 mM) and MCH (0.5 mM) into the mNTS elicited significant (P < 0.0001) decreases in the greater splanchnic nerve activity (89.8 ± 0.8 and 60.5 ± 4.1 %, respectively) when compared to basal nerve activity.
Fig. 4.
Effect of MCH on sympathetic nerve activity. In each panel, top trace: PAP (mmHg), 2nd trace: MAP (mmHg), 3rd trace HR (bpm), 4th trace: Integrated greater splanchnic nerve activity (GSNA; μV/100 msec), and 5th trace: whole GSNA. Inhibition of GSNA by a pressor response induced by phenylephrine (10 μg/kg, i.v.) indicated that the GSNA was barosensitive (not shown). A: Microinjection of L-Glu (5 mM) into the mNTS decreased PAP, MAP, HR, integrated GSNA and whole GSNA. B: Microinjection of MCH (0.5 mM) into the NTS also decreased PAP, MAP, HR, integrated GSNA and whole GSNA. C: Group data (n = 6) for this experiment. Microinjections of L-Glu (5 mM; dark bar) and MCH (0.5 mM; open bar) into the mNTS elicited significant (*P < 0.0001) decreases in the GSNA when compared to basal nerve activity.
Neuronal Recording
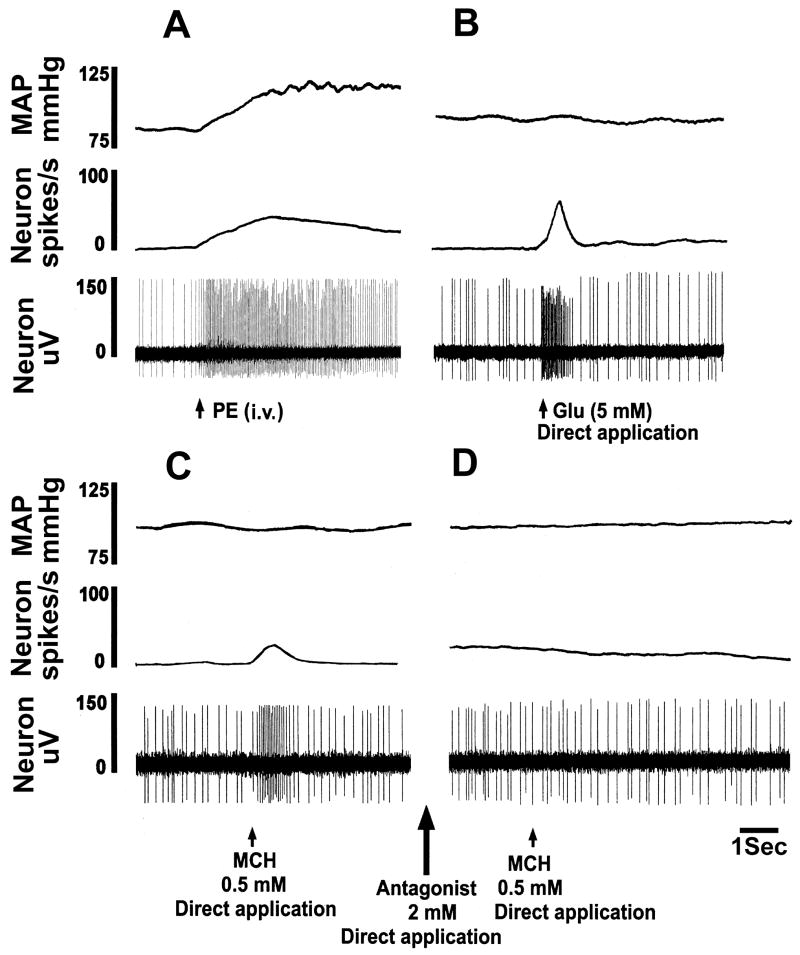
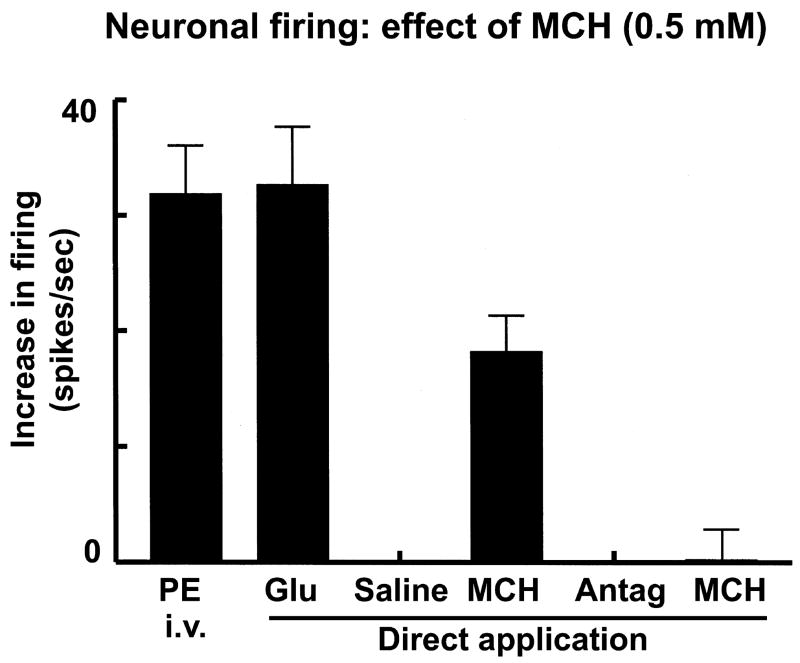
The results obtained in microinjection studies were confirmed by single mNTS neuronal recordings as follows. A typical tracing is shown in Fig. 5. An intravenous bolus injection of phenylephrine (3 μg/kg) increased the NTS-neuronal firing rate from the basal rate of 8 to 46 spikes/sec) indicating that the neuron was involved in baroreflex and, therefore, cardiovascular regulation (Fig. 5A). Smaller doses of phenylephrine (i.e., 3 μg/kg, instead of 10 μg/kg used in sympathetic nerve recording experiments) were used in neuronal recording experiments in order to avoid the possibility of losing the neurons due to large increases in BP. Pressure micro-application (4 nl) of L-Glu (5 mM) elicited an increase in neuronal firing (from a basal rate of 8 to 58 spikes/sec) which lasted for 0.8 sec (Fig. 5B). Pressure micro-application of normal saline (pH 7.4) elicited no response (not shown) indicating that pressure micro-application alone was not responsible for the changes in neuronal firing. MCH (0.5 mM) was pressure micro-applied to the mNTS neuron 2–3 sec after the saline; an increase in the neuronal firing (from the basal rate of 10 to 28 spikes/sec) was elicited which lasted for 0.8 sec (Fig. 5C). The duration of the effects of L-Glu and MCH was short because the ejection volumes were very small volume (4 nl) in all pressure micro-applications to the mNTS neurons. PMC-3881-PI (2 mM) was applied to the neuron 2–3 sec after the application of MCH; the firing of the neuron was not altered (not shown). Within 2–3 sec, MCH was again applied to the neuron. PMC-3881-PI blocked the excitatory effect of MCH (Fig. 5D). Group data of neuronal recording experiments are shown in Fig. 6. The basal firing rate of mNTS neurons (25 neurons) in this group of rats (n = 5) was 10.4 ± 3.3 spikes/sec (not shown). An intravenous bolus injection of phenylephrine (PE; 3 μg/kg) increased the NTS-neuronal firing rate; the peak increase (31.9 ± 4.2 spikes/sec from the basal neuronal firing, P < 0.001) was reached within 3.5 sec. The firing of the neuron returned to basal level within 3.4 ± 0.4 sec. When the BP and neuronal firing returned to basal level, L-Glu (5 mM) was directly applied to the neurons by pressure micro-application. L-Glu increased the neuronal firing; the peak increase (32.7 ± 5.0 spikes/sec, P < 0.01) was reached within 0.7 sec and lasted for 1.12 ± 0.4 sec. Pressure micro-application of normal saline (pH 7.4) did not elicit a response. Subsequent pressure micro-application of MCH (0.5 mM) increased the firing of the mNTS neurons; the peak increase (8.2 ± 3.1 spikes/sec, P < 0.05) was reached within 0.5 sec and lasted for 0.7 ± 0.3 sec. PMC-3881-PI (MCH-1 receptor antagonist; 2 mM) was directly applied to the neuron 2–3 sec after the application of MCH; no significant change in the neuronal firing compared to the basal firing rate was observed. Within 2–3 sec, MCH was again applied to the neurons; PMC-3881-PI blocked the excitatory effect of MCH (a small increase of 0.25 ± 0.4 spikes/sec was observed).
Fig. 5.
Excitation of mNTS neurons by MCH. In each panel, top trace: MAP (mmHg), 2nd trace: neuronal firing rate (spikes/sec), and 3rd trace: neuronal action potentials (μV). A: Basal firing rate of this neuron was 8 spikes/sec. Increase in MAP induced by phenylephrine (PE; 3 μg/kg, i.v.) elicited an increase in the neuronal activity (46 spikes/sec) indicating that the neuron was barosensitive. B: When the neuronal firing returned close to basal level (8 spikes/sec), pressure micro-application (4 nl) of L-Glu (5 mM) directly to the neuron increased its firing (58 spikes/sec) which lasted for about 0.8 sec. C: When the neuronal firing returned close to the basal level (10 spikes/sec), pressure micro-application (4 nl) of MCH (0.5 mM) increased the neuronal firing (28 spikes/sec) which lasted for about 0.8 sec. When the neuronal firing returned to the basal level (10 spikes/sec), MCH-1 receptor antagonist (2 mM) was applied (4 nl) (large arrow between panels C and D); no change in neuronal firing was elicited (not shown). D: Subsequent pressure micro-application (4 nl) of MCH (0.5 mM) to the neuron failed to elicit an increase in neuronal firing.
Fig. 6.
Graphical representation of neuronal group data (n = 5; 25 neurons). An intravenous bolus injection of phenylephrine (PE; 3 μg/kg) increased the NTS-neuronal firing rate indicating that the neuron was involved in cardiovascular regulation. Pressure micro-application (4 nl) of L-Glu (5 mM) elicited an increase in neuronal firing. Micro-application of normal saline (pH 7.4) elicited no response. Micro-application of MCH (0.5 mM) to the neuron elicited an increase in its firing. Application of PMC-3881-PI (2 mM; MCH-1 receptor antagonist) to the neuron did not alter its firing but blocked the effect of subsequent (within 2–3 sec) pressure micro-application of MCH (0.5 mM).
Histology
The mNTS sites, where microinjections of MCH elicited depressor and bradycardic responses, were marked in 12 rats. A typical mNTS-site marked with India ink (100 nl) is shown in Fig. 7A (arrow); the site was 0.5 mm rostral to the calamus scriptorius, 0.57 mm lateral to the midline and 0.65 mm deep from the dorsal medullary surface. Composite diagrams of these sites are shown in Fig. 7B and C in which each spot represents one site of microinjection. The sites were located in mNTS, 0.5–0.6 mm rostral to the CS, 0.47–0.65 mm lateral to the midline and 0.43–0.65 mm deep from the dorsal medullary surface.
Fig. 7.
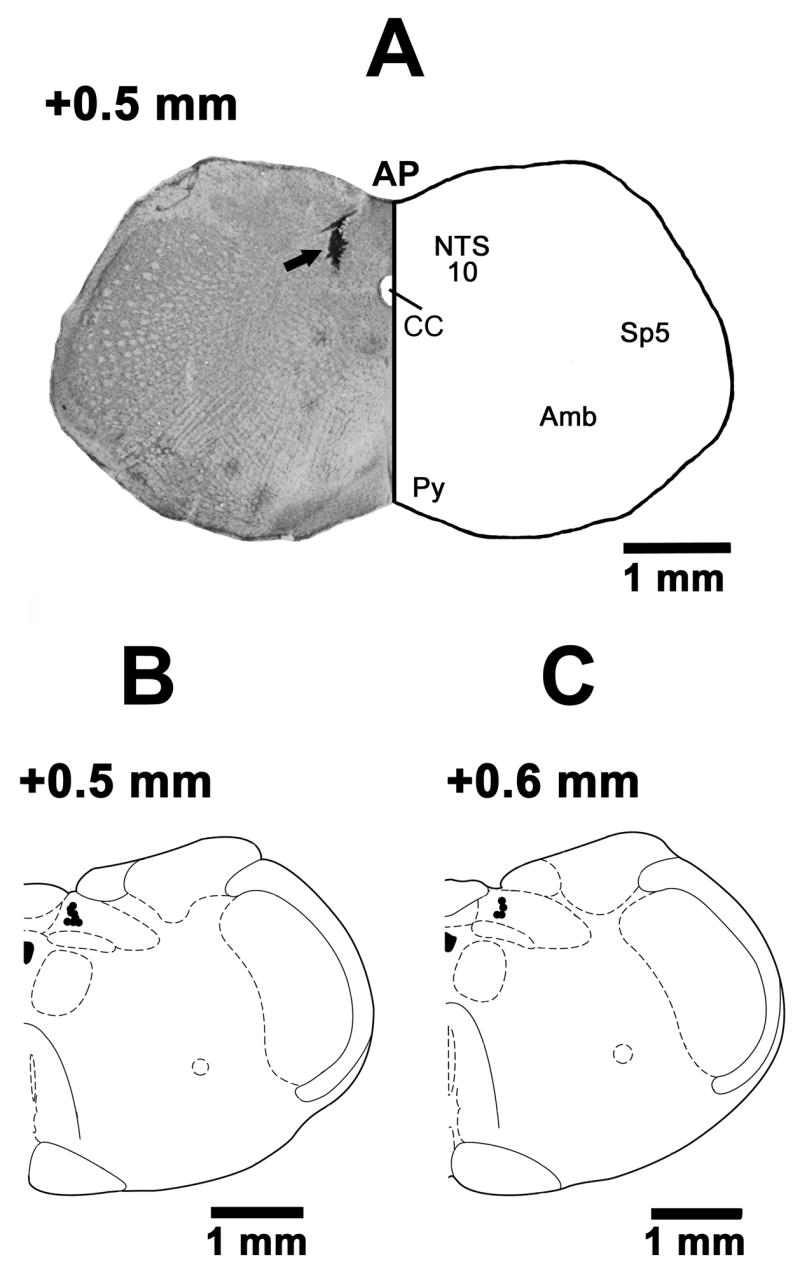
Histological identification of microinjection sites. A: Coronal section of the medulla at 0.5 mm rostral to the calamus scriptorius (CS) showing a typical mNTS microinjection site marked with India ink (100 nl; arrow). The center of the spot was 0.57 mm lateral to the midline and 0.65 mm deep from the dorsal medullary surface. B: A drawing of a coronal section at a level 0.5 mm rostral to the CS showing the mNTS microinjection sites as dark spots. Each dark spot corresponds to a microinjection site in one animal. C: A drawing of a coronal section at a level 0.6 mm rostral to the CS showing mNTS microinjection sites. The microinjection sites in B and C were located in the NTS, 0.5–0.6 mm rostral to the CS, 0.47–0.65 mm lateral to the midline and 0.43–0.65 mm deep from the dorsal medullary surface. Abbreviations: 10, dorsal motor nucleus of vagus; Amb, nucleus ambiguus; AP, area postrema; CC, central canal; NTS, nucleus tractus solitarius; Py, pyramidal tract; Sp5, spinal trigeminal nucleus.
DISCUSSION
In this study we have demonstrated for the first time that MCH microinjections into the mNTS elicit depressor and bradycardic responses. Although an earlier report (Messina and Overton, 2007) indicated that MCH elicits decreases in BP and HR, the peptide was injected i.c.v. in that study. Since substances injected by i.c.v. route can reach several central cardiovascular regulatory areas, it became necessary to investigate the possible central site of action of MCH. In our study, microinjection of normal saline into the mNTS did not elicit any responses, thus local distortion of brain tissue or other non-specific effects can be disregarded. Leakage, if any, of MCH into the peripheral circulation from the microinjection site was also excluded because the doses of MCH that elicited depressor and bradycardic responses when microinjected into the mNTS, did not elicit responses when injected intravenously. This observation is important considering that the presence of fenestrated capillaries and perivascular spaces in the caudal NTS may permit entry of solutes into the brain parenchyma in the commissural subnucleus of NTS and vice versa. The site-specificity of MCH-induced cardiovascular responses was established by the lack of responses to MCH microinjections into the areas located adjacent to mNTS such as the cuneate nucleus and area postrema. Moreover, the doses of MCH that elicited depressor and bradycardic responses when microinjected into the mNTS did not elicit cardiovascular responses when injected into the lateral ventricle. Microinjections of MCH into the mNTS showed a non-linear bell-shaped dose-response. This type of dose-response has been reported for several enzymes, peptides, and hormones and has been explained by homotropic allostery in which the agonist at higher concentrations binds to a modulator site, which is different from the primary binding site, and thereby affects the function of the receptor resulting in attenuated responses (Bindslev, 2004).
Anesthesia did not alter responses to MCH microinjections because the responses observed in unanesthetized mid-collicular decerebrate rats were qualitatively similar to those elicited in anesthetized rats. Contribution, if any, of structures rostral to the superior colliculi (e.g., hypothalamus) in mediating the responses to microinjections of MCH into the mNTS was also excluded based on the lack of alteration of cardiovascular responses by mid-collicular decerebration. Thus, MCH receptors in the mNTS may independently play a role in cardiovascular regulation. However, our results do not exclude the possibility that direct microinjections of MCH into other areas implicated in the central regulation of cardiovascular function, such as the lateral hypothalamic area, can elicit cardiovascular responses. These possibilities remain to be investigated in future studies in this and other laboratories.
Based on the available information regarding medullo-spinal cardiovascular regulatory areas (Coote, 2007; Gordon and Sved, 2002; Guyenet, 2006; Sapru, 2002, 2004; Talman et al., 1984), the following mechanism may be involved in mediating the cardiovascular responses elicited by MCH microinjections into the mNTS. MCH stimulates mNTS neurons as shown by direct pressure micro-applications of this peptide to these neurons. Involvement of these neurons in cardiovascular regulation was indicated by their excitation in response to baroreceptor input which was activated by pressor responses to intravenous bolus injections of phenylephrine. Activation of mNTS neurons by MCH results in the stimulation of a population of GABAergic neurons located in the caudal ventrolateral medullary depressor area (CVLM). As a result, GABA released in the rostral ventrolateral medullary pressor area (RVLM) causes a decrease in the activity of neurons located in this region. Consequently, the activity of excitatory pathways from the RVLM neurons to the sympathetic preganglionic neurons (SPGNs) located in the intermediolateral cell column of the thoraco-lumbar cord (IML) is decreased. Since the output of SPGNs to the arterioles is decreased, the blood pressure decreases. Indeed, in this study, sympathetic activity recorded from the central end of the greater splanchnic nerve was decreased by microinjections of MCH into the mNTS.
Bradycardia elicited by microinjections of MCH into the mNTS was primarily mediated via the activation of the vagal innervation to the heart because ipsilateral as well as bilateral vagotomy significantly reduced bradycardic responses. The events that lead to MCH-induced bradycardia can be speculated as follows. Microinjections of MCH into the mNTS activate secondary mNTS neurons which, in turn, stimulate the parasympathetic preganglionic neurons located in the nucleus ambiguus and the activity of vagal innervation to the heart is increased causing bradycardia. The presence of an excitatory projection from the mNTS to the nucleus ambiguus and the predominant role of this nucleus in the vagal control of heart are consistent with this conclusion. However, decrease in the activity of the sympathetic innervation to the heart must also contribute to the MCH-induced decrease in HR because bilateral vagotomy did not completely abolish bradycardia.
PMC-3881-PI has been reported to be a potent antagonist at MCH-1 receptors (Bednarek et al., 2002). This compound is an effective antagonist at MCH-1 receptors (KB 3.6 nM) and exhibits 1000 fold selectivity for MCH-1 receptor compared to MCH-2 receptor. It does not show agonist effects at MCH receptors even at micro-molar concentrations (Bednarek et al., 2002). Since the effects of microinjections of MCH into the mNTS were blocked by prior microinjections of this antagonist at the same site, it was concluded that MCH-induced responses were mediated via MCH-1 receptors. PMC-3881-PI did not exert any deleterious effects at the site of injection because it did not alter responses to another unrelated agonist, L-Glu. However, microinjections of PMC-3881-PI alone did not elicit any cardiovascular responses suggesting the MCH is not involved in the control of cardiovascular function under normal physiological situations. This observation prompts a hypothesis that the MCH system may be activated under specific circumstances. One such situation is stress. It is well known that initial autonomic, endocrine and behavioral responses to stress provide a short-term metabolic lift to an individual. However, prolonged and inappropriate stressful situations can perturb homeostasis which may lead to disease. Increase in BP is one of the consequences of prolonged stress. It is possible that MCH is released in the mNTS in order to cope with this adverse effect of stress, causing decreases in BP and HR to ameliorate the hypertensive response.
CONCLUSION
In summary, microinjections of MCH into the mNTS elicit depressor and bradycardic responses which are mediated via MCH-1 receptors. The decrease in BP is mediated via excitation of mNTS neurons which results in a decrease in sympathetic activity. Bradycardia is mediated predominantly via the vagus nerves, although decrease in sympathetic nerve activity also contributes to this effect. MCH may be released in the mNTS in response to prolonged stress so that the adverse effects of stress on cardiovascular function can be ameliorated.
Acknowledgments
This work was supported in part by N.I.H. grants HL024347 and HL076248 awarded to Dr. H. N. Sapru.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- ANOVA
analysis of variance
- CS
calamus scriptorius
- CNS
central nervous system
- CVLM
caudal ventrolateral medullary depressor area
- GSNA
greater splanchnic nerve activity
- i.c.v
intracerebroventricular
- IML
intermediolateral cell column of the thoraco-lumbar spinal cord
- L-Glu
L-glutamate
- MAP
mean arterial pressure
- MCH
melanin concentrating hormone
- mNTS
medial subnucleus of NTS
- NTS
nucleus tractus solitarius
- PAP
pulsatile arterial pressure
- SEM
standard error of the mean
- RVLM
rostral ventrolateral medullary pressor area
Footnotes
Section Editor: Dr. J.I. Morrell
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Astrand A, Bohlooly YM, Larsdotter S, Mahlapuu M, Andersen H, Tornell J, Ohlsson C, Snaith M, Morgan DG. Mice lacking melanin-concentrating hormone receptor 1 demonstrate increased heart rate associated with altered autonomic activity. Am J Physiol. 2004;287:R749–R758. doi: 10.1152/ajpregu.00134.2004. [DOI] [PubMed] [Google Scholar]
- Bachner D, Kreienkamp HJ, Weise C, Buck F, Richter D. Identification of melanin concentrating hormone(MCH) as the natural ligand for the orphan somatostatin-like receptor 1 (SLC-1) FEBS Lett. 1999;457:522–524. doi: 10.1016/s0014-5793(99)01092-3. [DOI] [PubMed] [Google Scholar]
- Bednarek MA, Hreniuk DL, Tan C, Palyha OC, MacNeil DJ, Van der Ploeg LH, Howard AD, Feighner SD. Synthesis and biological evaluation in vitro of selective, high affinity peptide antagonists of human melanin-concentrating hormone action at human melanin-concentrating hormone receptor 1. Biochemistry. 2002;41:6383–6390. doi: 10.1021/bi0200514. [DOI] [PubMed] [Google Scholar]
- Bindslev N. A homotropic two-state model and auto-antagonism. BMC Pharmacology. 2004;4:11. doi: 10.1186/1471-2210-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;19:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Elias CF. Melanin-concentrating hormone and neuropeptide EI projections from the lateral hypothalamic area and zona incerta to the medial septal nucleus and spinal cord: a study using multiple neuronal tracers. Brain Res. 1998;805:1–19. doi: 10.1016/s0006-8993(98)00598-8. [DOI] [PubMed] [Google Scholar]
- Boutin JA, Suply T, Audinot V, Rodriguez M, Beauverger P, Nicolas JP, Galizzi JP, Fauchere JL. Melanin-concentrating hormone and its receptors: state of the art. Can J Physiol Pharmacol. 2002;80:388–395. doi: 10.1139/y02-056. [DOI] [PubMed] [Google Scholar]
- Bresson JL, Clavequin MC, Fellmann D, Bugnon C. Human hypothalamic neuronal system revealed with a salmon melanin-concentrating hormone (MCH) antiserum. Neurosci Lett. 1989;102:39–43. doi: 10.1016/0304-3940(89)90304-2. [DOI] [PubMed] [Google Scholar]
- Brown S, Chitravanshi VC, Sapru HN. Cardiovascular actions of adrenocorticotropin microinjections into the nucleus tractus solitarius of the rat. Neuroscience. 2006;143:863–874. doi: 10.1016/j.neuroscience.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Chambers J, Ames RS, Bergsma D, Muir A, Fitzgerald LR, Hervieu G, Dytko GM, Foley JJ, Martin J, Liu WS, Park J, Ellis C, Ganguly S, Konchar S, Cluderay J, Leslie R, Wilson S, Sarau HM. Melanin-concentrating hormone is the cognate ligand for the orphan G-protein-coupled receptor SLC-1. Nature. 1999;400:261–265. doi: 10.1038/22313. [DOI] [PubMed] [Google Scholar]
- Coote JH. Landmarks in understanding the central nervous control of the cardiovascular system. Exp Physiol. 2007;92:3–18. doi: 10.1113/expphysiol.2006.035378. [DOI] [PubMed] [Google Scholar]
- Gordon FJ, Sved AF. Neurotransmitters in central cardiovascular regulation: glutamate and GABA. Clin Exp Pharmacol Physiol. 2002;29:522–524. doi: 10.1046/j.1440-1681.2002.03666.x. [DOI] [PubMed] [Google Scholar]
- Griffond B, Baker BI. Cell and molecular cell biology of melanin-concentrating hormone. Int Rev Cytol. 2002;213:233–277. doi: 10.1016/s0074-7696(02)13016-6. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Hervieu G. Melanin-concentrating hormone functions in the nervous system: food intake and stress. Expert Opin Ther Targets. 2003;7:495–511. doi: 10.1517/14728222.7.4.495. [DOI] [PubMed] [Google Scholar]
- Hervieu GJ, Cluderay JE, Harrison D, Meakin J, Maycox P, Nasir S, Leslie RA. The distribution of the mRNA and protein products of the melanin-concentrating hormone (MCH) receptor gene, slc-1, in the central nervous system of the rat. Eur J Neurosci. 2000;12:1194–1216. doi: 10.1046/j.1460-9568.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- Hill J, Duckworth M, Murdock P, Rennie G, Sabido-David C, Ames RS, Szekeres P, Wilson S, Bergsma DJ, Gloger IS, Levy DS, Chambers JK, Muir AI. Molecular cloning and functional characterization of MCH2, a novel human MCH receptor. J Biol Chem. 2001;276:20125–20129. doi: 10.1074/jbc.M102068200. [DOI] [PubMed] [Google Scholar]
- Kawauchi H, Kawazoe I, Tsubokawa M, Kishida M, Baker BI. Characterization of melanin-concentrating hormone in chum salmon pituitaries. Nature. 1983;305:321–323. doi: 10.1038/305321a0. [DOI] [PubMed] [Google Scholar]
- Kokkotou EG, Tritos NA, Mastaitis JW, Slieker L, Maratos-Flier E. Melanin-concentrating hormone receptor is a target of leptin action in the mouse brain. Endocrinology. 2001;142:680–686. doi: 10.1210/endo.142.2.7981. [DOI] [PubMed] [Google Scholar]
- Kokkotou EG, Jeon JY, Wang X, Marino FE, Carlson M, Trombly DJ, Maratos-Flier E. Mice with MCH ablation resist diet-induced obesity through strain-specific mechanisms. Am J Physiol. 2005;289:R117–R124. doi: 10.1152/ajpregu.00861.2004. [DOI] [PubMed] [Google Scholar]
- Kowalski TJ, Farley C, Cohen-Williams ME, Varty G, Spar BD. Melanin-concentrating hormone-1 receptor antagonism decreases feeding by reducing meal size. Eur J Pharmacol. 2004;497:41–47. doi: 10.1016/j.ejphar.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Lakaye B, Minet A, Zorzi W, Grisar T. Cloning of the rat brain cDNA encoding for the SLC-1 G protein-coupled receptor reveals the presence of an intron in the gene. Biochim Biophys Acta. 1998;1401:216–220. doi: 10.1016/s0167-4889(97)00135-3. [DOI] [PubMed] [Google Scholar]
- Lembo PM, Grazzini E, Cao J, Hubatsch DA, Pelletier M, Hoffert C, St-Onge S, Pou C, Labrecque J, Groblewski T, O’Donnell D, Payza K, Ahmad S, Walker P. The receptor for the orexigenic peptide melanin-concentrating hormone is a G-protein-coupled receptor. Nat Cell Biol. 1999;1:267–271. doi: 10.1038/12978. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Mountjoy KG, Tatro JB, Gillette JA, Frederich RC, Flier JS, Maratos-Flier E. Melanin-concentrating hormone: a functional melanocortin antagonist in the hypothalamus. Am J Physiol. 1998;274:E627–E633. doi: 10.1152/ajpendo.1998.274.4.E627. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, Lowell B, Flier JS, Maratos-Flier E. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107:379–386. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina MM, Overton JM. Cardiovascular effects of melanin-concentrating hormone. Regul Pept. 2007;139:23–30. doi: 10.1016/j.regpep.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Mori M, Harada M, Terao Y, Sugo T, Watanabe T, Shimomura Y, Abe M, Shintani Y, Onda H, Nishimura O, Fujino M. Cloning of a novel G protein-coupled receptor, SLT, a subtype of the melanin-concentrating hormone receptor. Biochem Biophys Res Commun. 2001;283:1013–1018. doi: 10.1006/bbrc.2001.4893. [DOI] [PubMed] [Google Scholar]
- Mouri T, Takahashi K, Kawauchi H, Sone M, Totsune K, Murakami O, Itoi K, Ohneda M, Sasano H, Sasano N. Melanin-concentrating hormone in the human brain. Peptides. 1993;14:643–646. doi: 10.1016/0196-9781(93)90158-d. [DOI] [PubMed] [Google Scholar]
- Nahon JL. The melanin-concentrating hormone: from the peptide to the gene. Crit Rev Neurobiol. 1994;8:221–262. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1986. [Google Scholar]
- Presse F, Nahon JL, Fischer WH, Vale W. Structure of the human melanin concentrating hormone mRNA. Mol Endocrinol. 1990;4:632–637. doi: 10.1210/mend-4-4-632. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Beauverger P, Naime I, Rique H, Ouvry C, Souchaud S, Dromaint S, Nagel N, Suply T, Audinot V, Boutin JA, Galizzi JP. Cloning and molecular characterization of the novel human melanin-concentrating hormone receptor MCH2. Mol Pharmacol. 2001;60:632–639. [PubMed] [Google Scholar]
- Rossi M, Choi SJ, O’Shea D, Miyoshi T, Ghatei MA, Bloom SR. Melanin-concentrating hormone acutely stimulates feeding, but chronic administration has no effect on body weight. Endocrinology. 1997;138:351–355. doi: 10.1210/endo.138.1.4887. [DOI] [PubMed] [Google Scholar]
- Sailer AW, Sano H, Zeng Z, McDonald TP, Pan J, Pong SS, Feighner SD, Tan CP, Fukami T, Iwaasa H, Hreniuk DL, Morin NR, Sadowski SJ, Ito M, Ito M, Bansal A, Ky B, Figueroa DJ, Jiang Q, Austin CP, MacNeil DJ, Ishihara A, Ihara M, Kanatani A, Van Der Ploeg LH, Howard AD, Liu Q. Identification and characterization of a second melanin-concentrating hormone receptor, MCH-2R. Proc Natl Acad Sci USA. 2001;98:7564–7569. doi: 10.1073/pnas.121170598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Nothacker HP, Wang Z, Lin SH, Leslie F, Civelli O. Molecular characterization of the melanin-concentrating-hormone receptor. Nature. 1999;400:265–269. doi: 10.1038/22321. [DOI] [PubMed] [Google Scholar]
- Saito Y, Nothacker HP, Civelli O. Melanin-concentrating hormone receptor: an orphan receptor fits the key. Trends Endocrinol Metab. 2000;11:299–303. doi: 10.1016/s1043-2760(00)00290-3. [DOI] [PubMed] [Google Scholar]
- Sapru HN. Glutamate circuits in selected medullo-spinal areas regulating cardiovascular function. Clin Exp Pharmacol Physiol. 2002;29:491–496. doi: 10.1046/j.1440-1681.2002.03661.x. [DOI] [PubMed] [Google Scholar]
- Sapru HN. Neurotransmitters in the nucleus tractus solitarius mediating cardiovascular function. In: Dun NJ, Machado BH, Pilowsky PM, editors. Neural mechanisms of cardiovascular regulation. Boston: Kluwer Academic Publishers; 2004. pp. 81–98. [Google Scholar]
- Sapru HN, Gonzalez ER, Krieger AJ. Greater splanchnic nerve activity in the rat. Brain Res Bull. 1982;8:267–272. doi: 10.1016/0361-9230(82)90058-2. [DOI] [PubMed] [Google Scholar]
- Shimada M, Tritos NA, Lowell BB, Fier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Mori M, Sugo T, Ishibashi Y, Abe M, Kurokawa T, Onda H, Nishimura O, Sumino Y, Fujino M. Isolation and identification of melanin-concentrating hormone as the endogenous ligand of the SLC-1 receptor. Biochem Biophys Res Commun. 1999;261:622–626. doi: 10.1006/bbrc.1999.1104. [DOI] [PubMed] [Google Scholar]
- Skofitsch G, Jacobowitz DM, Zamir N. Immunohistochemical localization of a melanin concentrating hormone-like peptide in the rat brain. Brain Res Bull. 1985;15:635–649. doi: 10.1016/0361-9230(85)90213-8. [DOI] [PubMed] [Google Scholar]
- Songzhu A, Cutler G, Zhao JJ, Huang SG, Tian H, Li W, Liang L, Rich M, Bakleh A, Du J, Chen JL, Dai K. Identification and characterization of a melanin-concentrating hormone receptor. Proc Natl Acad Sci USA. 2001;98:7576–7581. doi: 10.1073/pnas.131200698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talman WT, Granata AR, Reis DJ. Glutamatergic mechanisms in the nucleus tractus solitarius in blood pressure control. Fed Proc. 1984;43:39–44. [PubMed] [Google Scholar]
- Tan CP, Sano H, Iwaasa H, Pan J, Sailer AW, Hreniuk DL, Feighner SD, Palyha OC, Pong SS, Figueroa DJ, Austin CP, Jiang MM, Yu H, Ito J, Ito M, Ito M, Guan XM, MacNeil DJ, Kanatani A, Van Der Ploeg LH, Howard AD. Melanin-concentrating hormone receptor subtypes 1 and 2: species-specific gene expression. Genomics. 2002;79:785–792. doi: 10.1006/geno.2002.6771. [DOI] [PubMed] [Google Scholar]
- Vaughan JM, Fischer WH, Hoeger C, Rivier J, Vale W. Characterization of melanin-concentrating hormone from rat hypothalamus. Endocrinology. 1989;125:1660–1665. doi: 10.1210/endo-125-3-1660. [DOI] [PubMed] [Google Scholar]
- Wang S, Behan J, O’Neill K, Weig B, Fried S, Laz T, Bayne M, Gustafson E, Hawes BE. Identification and pharmacological characterization of a novel human melanin-concentrating hormone receptor, mch-r2. J Biol Chem. 2001;276:34664–34670. doi: 10.1074/jbc.M102601200. [DOI] [PubMed] [Google Scholar]
- Zamir N, Skofitsch G, Bannon MJ, Jacobowitz DM. Melanin-concentrating hormone: unique peptide neuronal system in the rat brain and pituitary gland. Proc Natl Acad Sci USA. 1986a;83:1528–1531. doi: 10.1073/pnas.83.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir N, Skofitsch G, Jacobowitz DM. Distribution of immunoreactive melanin-concentrating hormone in the central nervous system of the rat. Brain Res. 1986b;373:240–245. doi: 10.1016/0006-8993(86)90337-9. [DOI] [PubMed] [Google Scholar]