Abstract
An important role of cell matrix adhesion receptors is to mediate transmembrane coupling between extracellular matrix attachment, actin reorganization, and cell spreading. Thrombospondin (TSP)-1 is a modulatory component of matrix expressed during development, immune response, or wound repair. Cell adhesion to TSP-1 involves formation of biochemically distinct matrix contacts based on stable fascin spikes. The cell surface adhesion receptors required have not been identified. We report here that antibody clustering of syndecan-1 proteoglycan specifically transduces organization of cortical actin and fascin bundles in several cell types. Transfection of COS-7 cells with syndecan-1 is sufficient to stimulate cell spreading, fascin spike assembly, and extensive protrusive lateral ruffling on TSP-1 or on syndecan-1 antibody. The underlying molecular mechanism depends on glycosaminoglycan (GAG) modification of the syndecan-1 core protein at residues S45 or S47 for cell membrane spreading and on the VC2 region of the cytoplasmic domain for spreading and fascin spike formation. Expression of the VC2 deletion mutant or GAG-negative syndecan-1 showed that syndecan-1 is necessary in spreading and fascin spike formation by C2C12 cells on TSP-1. These results establish a novel role for syndecan-1 protein in coupling a physiological matrix ligand to formation of a specific matrix contact structure.
Keywords: cell adhesion, extracellular matrix, proteoglycan, actin, protrusions
Introduction
The integrative effects of extracellular matrix on cell function depend on linkage from the matrix across the plasma membrane to the actin cytoskeleton. Such transmembrane coupling is mediated by numerous cell surface adhesion receptors which have specific matrix ligands (for review see Hynes 1999). The interactions support cell matrix adhesion and motility and also initiate signaling responses which regulate many aspects of cell behavior (for reviews see Clark and Brugge 1995; Schwartz et al. 1995; Yamada and Miyamoto 1995). Thrombospondin (TSP)-1 is a multifunctional glycoprotein component of extracellular matrix that is widely expressed during organogenesis and which is locally upregulated in adult tissues upon platelet activation, inflammatory response, or wound healing. Within the extracellular matrix, TSP-1 binds to other matrix glycoproteins, collagen V, perlecan, certain proteases, or cytokines and is associated with cell surfaces in nonfibrillar patches (for reviews see Bornstein 1995; Adams 1997a). TSP-1 functions as a cell adhesion molecule and also regulates cell adhesion to other matrix components (Murphy-Ullrich and Hook 1989). These properties have led TSP-1 to be considered with tenascins and SPARC as a regulatory component of extracellular matrix (for review see Chiquet-Ehrismann 1995). To understand the functions of these molecules in different tissue contexts, it is important to establish their cellular mechanisms of action.
This laboratory has shown that cell attachment and spreading on TSP-1 substrata is associated with organization of the cortical actin cytoskeleton to form stable, radial spikes which contain F-actin and the actin-binding protein fascin (Adams 1995, Adams 1997b). These spike structures and associated lamellae mediate adhesion to TSP-1 and are also needed in cell motile behavior on TSP-1 (Adams 1997b). Fascin spikes are assembled in the absence of focal contacts, and their assembly is dependent on the maintenance of a pool of the nonphosphorylated, actin-binding form of fascin and the regulated activities of Rac and Cdc42 small GTPases (Adams 1995; Adams et al. 1999; Adams and Schwartz 2000). Thus, the interactions of TSP-1 at the plasma membrane transduce signals into cells which have highly specific effects on the organization of F-actin and fascin within the cell cortex. To understand the molecular basis for this response, it is of obvious relevance to identify the necessary adhesive receptors.
Each subunit of the TSP-1 homotrimer contains four major domains that are involved in cell attachment to TSP-1: the NH2-terminal domain, the type 1 repeats, an arginine-glycine-aspartate motif in the last type 3 repeat, and the COOH-terminal globular domain (for review see Adams 1997a). Widespread analyses of many cell types have established that each domain interacts with different cell surface–binding partners. The NH2-terminal domain has a high affinity for heparin and heparan sulfate (HS)-glycosaminoglycans (GAGs) and can mediate endocytosis of TSP-1 in conjunction with low density lipoprotein receptor–related protein (Sun et al. 1989; Godyna et al. 1995; Mikhailenko et al. 1995). The type 1 repeats contain multiple binding sites for GAGs and a cell type–restricted receptor, CD36 (Asch et al. 1992; Guo et al. 1992a,Guo et al. 1992b; Pancake et al. 1992; Li et al. 1993; Gantt et al. 1997). The arginine-glycine-aspartate site, which is active in a conformation-dependent manner according to the nature of the disulfide pairings within the type 3 repeats, binds αvβ3 integrin on endothelial and smooth muscle cells and to some extent αIIbβ3 on platelets (Lawler et al. 1988; Lawler and Hynes 1989; Sun et al. 1992). Cell attachment involving other integrins has been reported in individual cell types, for example, a role for α4β1 and α5β1 integrins in attachment of activated T cells, but the binding sites for these interactions have not been mapped precisely (Yabkowitz et al. 1993). Activated α3β1 integrin binds a site within the NH2-terminal domain (Krutzsch et al. 1999). Two peptide motifs in the COOH-terminal globular domain act as binding sites for CD47/IAP (Gao et al. 1996).
The multiplicity of cell-binding sites presented by the intact TSP-1 molecule complicates analysis and interpretation of the activities of individual adhesive receptors in transmembrane coupling and organization of the actin cytoskeleton. Yet a knowledge of the mechanism involved is critical in understanding how TSP coordinates the assembly of fascin spikes in preference to other types of matrix contacts. Fascin spikes are also formed by cells adherent to Engelbreth-Holm-Swarm laminin and tenascin C and are localized at the leading edge of migratory cells where they have been functionally implicated in motile behavior (Adams 1997b; Fischer et al. 1997; Yamashiro-Matsumura et al. 1998). Thus, identification of the coupling mechanism for TSP-1 could prove of general significance in understanding how matrix contacts are coordinated by cells within complex, physiological extracellular matrices.
We hypothesized that ligation and clustering of individual adhesive receptors by specific antibodies could be used to identify roles for TSP-1–binding receptors in fascin spike assembly. We report here that, either by antibody clustering of syndecan-1 or by transfection of syndecan-1 expression constructs, ligation of syndecan-1 specifically coordinates the organization of fascin structures in several cell types. The molecular mechanism of these effects depends on the presence of GAG chains and on the cytoplasmic domain of the core protein. These novel results establish syndecan-1 as a functionally important transducer of fascin spike formation by TSP-1.
Materials and Methods
Cell Lines and Materials
C2C12 myoblastic cells were cultured in DME containing 20% FCS. Mouse embryo fibroblasts (MEF-1) and COS-7 green monkey kidney cells were obtained from American Type Culture Collection and cultured in DME containing 10% FCS. Human lung microvascular endothelial cells (HLMECs) were obtained from CLONTECH Laboratories, Inc. and cultured in the manufacturer's endothelial growth medium supplemented with 10 ng/ml EGF, 1 μg/ml hydrocortisone, 3 μg/ml bovine brain extract, and 10% FCS (BioWhittaker). Antibodies reactive with mouse syndecan-1 (CD138; rat monoclonal antibody 281-2) (Jalkanen et al. 1985), mouse β1 integrin subunit (CD29; antibody Ha2/5) (Kinashi and Springer 1994), mouse α5 integrin subunit (CD49e; antibody 5H10-27), and mouse β3 integrin subunit (CD61; hamster antibody 2C9.G2) were obtained from BD PharMingen. N-18 goat antibody to the NH2 terminus of mouse syndecan-1 was obtained from Santa Cruz Biotechnology, Inc. Rabbit antibody to CD36, reactive with human and mouse CD36, was from Research Diagnostics. Mouse monoclonal antibody miap 301 to mouse CD47/IAP and mouse hindlimb muscle from neonatal wild-type or CD47-null mice were gifts from Frederick Lindberg (University of Washington, St. Louis, MO) (Lindberg et al. 1996). Primary skeletal myoblasts were prepared from the dissected tissue by sequential trypsinization and replating, and their CD47 status was confirmed by immunostaining. Mouse monoclonal antibodies to human adhesion receptors included antibody SMO to CD36 (Serotec) (Hogg et al. 1984), BRIC 126, B6H12, and 2E11 to CD47 (Biodesign; AMS Biotechnology) (Avent et al. 1988; Brown et al. 1990), monoclonal antibody 13 to β1 integrin (Akiyama et al. 1989), LM609 to αvβ3 integrin (Cheresh 1987), and antibodies 1D4 and BB4 to syndecan-1 (Biodesign) (Dore et al. 1998). Purified nonimmune rat or mouse immunoglobulins were obtained from Sigma-Aldrich.
FACS® Analysis
Single cell suspensions of 106 C2C12 or COS-7 cells were incubated with antibodies to adhesion receptors diluted in PBS on ice for 30 min. Cells were washed three times in PBS at 4°C, resuspended in 1:50 dilutions of the appropriate FITC-conjugated secondary antibody, and incubated for 30 min on ice. The cells were washed again and passed through a Becton Dickinson FACScan™. 10,000 cells were analyzed per sample.
Extraction of Proteoglycans
Cells were plated at 107 cells per 90-mm dish and allowed to attach for 2 h. Cells were then lysed in 8 M urea, 0.2% Triton X-100, 10 mM Tris-HCl, pH 8.0, and sonicated on ice, using a Branson model 450 Sonifier in four 10-s bursts at amplitude setting 8 with 10 s cooling time allowed between each burst. The lysates were clarified by centrifugation, and lysate corresponding to 107 cells was combined with 100-μl packed volume of DEAE-Sephacel (Sigma-Aldrich) which had been prepared by washing in 8 M urea, 0.2% Triton X-100, 10 mM Tris-HCl, pH 8.0. The mixture was rotated end-over-end for 16 h at 4°C. The DEAE-Sephacel beads were washed in TBS and eluted with 1 M NaCl in lyase digestion buffer (100 mM Hepes, pH 6.5, 10 mM CaCl2, 0.5% CHAPS, 0.2 mg/ml BSA). Proteoglycans were collected as described by Sanderson et al. 1992. Enzymatic cleavage of chondroitin sulfate (CS)- and HS-GAG chains was performed by diluting the proteoglycan samples two times with dH2O followed by incubation with either 50 mU/ml chondroitinase ABC, 50 mU/ml heparitinase II (Seikagaku), or a mixture of both enzymes for 2 h at 37°C. A second addition of enzyme(s) was made and the reaction mixtures were incubated for a further 2 h at 37°C. Samples were then concentrated and desalted by centrifugation in Centricon 10 concentrators (Amicon) and combined with SDS-PAGE sample buffer containing 100 mM DTT as a reducing agent. Electrophoresis of samples was performed under reducing conditions as described by Koda et al. 1985, using 4–15% SDS-polyacrylamide gradient gels and Tris/borate buffer containing 40 mM Tris, 60 mM boric acid, 0.8 mM EDTA, 1 mM Na2SO4, and 0.1% SDS. Samples were electroblotted overnight at 60 V onto nitrocellulose (0.22-μm pore size; Bio-Rad Laboratories) and probed for syndecan-1 by incubation with 5 μg/ml monoclonal antibody 281.2 for 1 h, followed by ECL detection of alkaline phosphatase–conjugated secondary antibody (Tropix; PerkinElmer) using Hyperfilm ECL (Amersham Pharmacia Biotech).
Transfection and Cytoskeletal Organization Assays
Cells for transient transfection were plated at 30–40% confluency and transfected with plasmid DNA by use of Superfect reagent (QIAGEN) according to the manufacturer's instructions. The plasmids used included expression plasmids for enhanced green fluorescent protein (EGFP) (CLONTECH Laboratories, Inc.), EGFP-fascin, wild-type and GAG-addition site mutants of mouse syndecan-1 (Saunders et al. 1989; Langford et al. 1998), and wild-type rat syndecan-2 (Klass et al. 2000). Syndecan-1 mutants lacking the VC2 regions of the cytoplasmic domain were prepared by PCR-based mutagenesis using as a primer pair the forward primer 5′-TCTGGGCAGCATGAGACG and the reverse primer 5′-CTGGTAGGCACCGCCATTGGCT and Pfu polymerase (New England Biolabs, Inc.). The PCR product was cloned into the pCDNA3 expression vector by TOPO cloning kit (Invitrogen). The 5′ and 3′ junctions of the cDNA were confirmed by DNA sequencing, using the dideoxy chain-termination method and standard forward and reverse vector primers. Expression of cDNAs was confirmed by in vitro translation using the TNT kit (Promega).
Transiently transfected cells were used in adhesion assays 36–42 h after transfection. Glass coverslips were coated with 50 nM recombinant human TSP-1, prepared using the baculovirus system as described (Adams et al. 1998) or with the appropriate immunoglobulins overnight at 4°C. To orient antireceptor antibodies, dishes were first coated with 10 μg/ml avidin (Vector Laboratories) and then with 10 μg/ml biotin-conjugated antiimmunoglobulin antibodies of the appropriate species specificity (Vector Laboratories or ICN Biomedicals). Surfaces were then coated at 4°C overnight with 40 μg/ml solutions of the antireceptor antibodies. All coverslips were rinsed in TBS and blocked with 2 mg/ml heat-denatured BSA for 1 h. Single cell suspensions, prepared by release with 10 mM EDTA or by trypsinization, were allowed to attach for 1 h at 37°C in serum-free DME. Nonattached cells were removed by rinsing in TBS and the adherent cells were fixed before analysis of cytoskeletal organization by indirect immunofluorescence. For staining with rhodamine-phalloidin, antivinculin monoclonal VIN 11.5 (Sigma-Aldrich), or mouse monoclonal 4G10 to phosphotyrosine (Upstate Biotechnology) cells were fixed in 3.7% paraformaldehyde and processed as described previously (Adams 1995). For staining with monoclonal antibody to fascin (Dako), cells were fixed in absolute methanol and fascin localization was detected with an FITC-conjugated goat anti–mouse secondary antibody (ICN Biomedicals). For quantitation of spread area or length of fascin actin bundles either digital images were processed in ImagePro Plus or tracings from photographic enlargements were scanned into Adobe Photoshop® and then processed. Excel worksheets were used to calculate descriptive statistics and to determine significance using an unpaired two-tailed t test.
Cell Motility Measurements
Nunc slideflasks were coated with antibodies to adhesion receptors as described above or with 50 nM recombinant TSP-1 and blocked with heat-denatured BSA. EDTA-released cells were suspended at 3 × 105 cells/ml and plated for 30 min. Nonadherent cells were removed and the behavior of the attached cells was tracked over the next 1.5 h by time-lapse videomicroscopy as described (Adams 1997b). Cells were recorded at 10 frames/min and alterations in spread area, ruffling behavior, or cell locomotion were measured from traces. Ruffling behavior was scored as the extension of lateral, substratum-adherent ruffles at cell margins (lateral ruffles). Cell locomotion was scored as the displacement of the cell centroid over time. The extension of short, dynamic projections from the dorsal cell surface (apical projections) was also scored. At least 50 cells were traced for each transfection condition, and five replicate experiments were analyzed in total. For parameters that appeared altered between control or experimental transfections, statistical significance was determined in Microsoft Excel using a two-tailed t test.
Results
Expression of Adhesion Receptors for Thrombospondin-1 by C2C12 Myoblasts
C2C12 skeletal myoblasts attach to TSP-1 through predominant interactions with the type 1 repeats and COOH-terminal domain (Adams and Lawler 1994). With the aim of identifying the adhesive receptors which couple cell attachment to fascin spike organization, we first characterized the expression by C2C12 cells of receptors that are involved in cell attachment to TSP-1 in multiple cell types. FACS® analysis was carried out on live cell suspensions which had been disaggregated either by treatment with 10 mM EDTA or by trypsinization. EDTA-released C2C12 cells expressed β1 and β3 integrin subunits and also CD47 and syndecan-1 but did not express detectable CD36 (Fig. 1 A; data not shown). Direct analysis of integrin heterodimers by immunoprecipitation of surface-labeled cells established expression of α2β1, α3β1, α4β1, α5β1, α7β1, and αvβ1 but not α1β1 integrins. The cells also express the αvβ3 and αvβ5 heterodimers (Adams et al. 1998 and references therein; Adams, J.C., unpublished data). The expression profiles for CD47 and the integrin subunits were very similar in trypsinized or EDTA-treated cells (shown for β1 staining only; Fig. 1 A). However, after trypsin treatment for 2 min at 37°C expression of syndecan-1 was reduced and more heterogeneous (Fig. 1 A, panel Syn-1[T]). As expected, because of the known protease sensitivity of the syndecan-1 extracellular domain, trypsinization for 5 min at 37°C resulted in a complete loss of cell surface syndecan-1 (data not shown; Fitzgerald et al. 2000).
Figure 1.
Expression of TSP-1–binding receptors by C2C12 cells. (A) FACS® profiles. C2C12 cells were disaggregated with EDTA or with trypsin and stained with antibodies to the indicated adhesion receptors or nonimmune (NI) rat immunoglobulin. Similar profiles were obtained for EDTA (E) or trypsin-disaggregated (T) cells with the CD47, β1, and β3 antibodies (both profiles shown for β1 integrin only), whereas cells trypsinized for 2 min showed more heterogeneous syndecan-1 staining. (B) Syndecan-1 is expressed on C2C12 cells as a mixed proteoglycan. Proteoglycan extracts were prepared from C2C12 cells, resolved on gradient polyacrylamide gels under reducing conditions, transferred to nitrocellulose, and probed with antibody 281-2 to mouse syndecan-1. Lane 1, undigested extract; lane 2, + chondroitinase ABC digestion; lane 3, + heparitinase II digestion; lane 4, + digestion with both enzymes. Markers are indicated in kD. Results are representative of three experiments.
The syndecan-1 core protein contains multiple sites for addition of HS- and CS-GAGs, and these posttranslational modifications are made with a high degree of cell type specificity (Kokenyesi and Bernfield 1994; reviewed by Bernfield et al. 1999). To determine the nature of the GAG substitutions in C2C12 cells, we examined the apparent molecular mass of syndecan-1 after extraction of proteoglycans in combination with digestion with GAG lyases specific for either HS or CS. In control extracts, syndecan-1 resolved on immunoblots as bands of apparent molecular mass of ∼120 and 97 kD with associated smearing (Fig. 1 B, lane 1). Upon digestion with chondroitinase ABC, the 120-kD band was much reduced, and the majority of syndecan-1 appeared as a broad band in the range of 66–97 kD that comigrated with core protein (Fig. 1 B, lanes 2 and 4). The breadth of this band likely relates to the tendency of syndecan-1 cores to form SDS-resistant oligomers as well as the presence of various HS substitutions. Upon digestion with heparitinase II, the 120-kD band was lost, and syndecan-1 appeared as an extended higher molecular weight smear as well as the ∼97-kD species (Fig. 1 B, lane 3). Double digestion with both enzymes resulted in complete conversion of the diffuse higher molecule weight forms into the core protein band (Fig. 1 B, lane 4). Thus, in C2C12 cells, syndecan-1 is expressed as a mixed CS/HS proteoglycan.
Direct Ligation of Individual TSP-1 Adhesion Receptors Has Distinct Effects on F-Actin Organization
To dissect the effects of ligation and clustering of individual TSP-1 receptors on cell attachment and actin cytoskeletal organization, the panel of antibodies reactive with the extracellular domains of mouse β1 or β3 integrin subunits, CD47, or syndecan-1 were used as adhesive substrata for C2C12 cells. All the antibodies supported quantitatively increased cell attachment relative to nonimmune rat IgG. C2C12 underwent partial spreading on the β1 integrin subunit antibody and displayed organization of F-actin at cell margins (Fig. 2 and Table ). Cells on the β3 integrin subunit antibody also spread partially and developed concentrations of F-actin at points on the cell surface (Fig. 2). On the CD47 antibody, cells spread more extensively and displayed larger actin-containing ruffles at cell margins (Fig. 2). For all these antibodies, similar results were obtained using either trypsinized or EDTA-disaggregated cells (shown in Fig. 2 for trypsinized cells only).
Figure 2.
F-actin organization in C2C12 cells adherent on adhesion receptor antibodies. Cells were plated onto surfaces, coated with 50 μg/ml of the indicated antibodies (see Materials and Methods) for 1 h in serum-free medium, then fixed and stained with TRITC-phalloidin. Bar, 15 μm.
Table 1.
Effects of Adhesion Receptor Antibodies on Fascin Distribution in C2C12 Cells
| Antibody substratum | Input cells attached | Attached cells with fascin spikes | Attached cells with localized vinculin |
|---|---|---|---|
| % | % | % | |
| Nonimmune IgG | 6 ± 3.8 | 0 | 0 |
| Anti–β1 integrin | 81 ± 5.3 | 0 | 78 ± 4.2 |
| Anti–β3 integrin | 77 ± 6.6 | 0 | 64 ± 6.5 |
| Anti-CD47 | 58 ± 3.7 | 10 ± 5.8 | 0 |
| Anti–syndecan-1, EDTA release | 72 ± 6.3 | 88 ± 4.3 | 0 |
| Anti–syndecan-1, 2 min treatment with trypsin | 56 ± 4.9 | 68 ± 3.2 | 0 |
| Anti–syndecan-1, 5 min treatment with trypsin | 11 ± 2.3 | 0 | 0 |
Cells were plated for 1 h in serum-free medium on antibody-coated surfaces, then fixed and stained for fascin or vinculin. Results are given as mean ± SEM from five experiments.
In contrast, EDTA-disaggregated C2C12 cells spread fully on 281-2 antibody to syndecan-1 and displayed zones of F-actin ruffles and small projections at cell margins (Fig. 2). Cells trypsinized for 2 min, many of which retained cell surface syndecan-1 (Fig. 1 A), showed a less uniform response (Table and Fig. 3). Extensively trypsinized cells which lacked syndecan-1 attached at only background levels to the antibody and did not spread (data not shown). To establish that these responses were not due to differing affinities or avidities of individual antibody/antigen binding interactions, F-actin organization was examined on surfaces coated with different concentrations of the antibodies to β1 integrin, β3 integrin, CD47, or syndecan-1. The cells spread poorly on any antibody at 5 μg/ml coating concentration. Actin spikes and ruffles were organized by EDTA-dissociated cells on the 281-2 antibody in the concentration range 30–100 μg/ml and were not organized by cells adherent to the β1 or β3 antibodies even when they were coated at 100 μg/ml. Cells adherent on the CD47 antibody formed small F-actin ruffles on surfaces coated with ≥50 μg/ ml of the antibody (data not shown).
Figure 3.

Organization of fascin and vinculin in C2C12 cells adherent on adhesion receptor antibodies. (A) Cells were plated on surfaces coated with 50 μg/ml of the indicated anti–mouse antibodies for 1 h in serum-free medium and then fixed and stained for fascin or vinculin. (B) Fascin spike formation by primary mouse skeletal myoblasts on TSP-1. Wild-type or CD47-null skeletal myoblasts were plated on 50 nM TSP-1 for 1 h and then stained for fascin. Both populations formed extensive fascin spikes. Bar: (A, panels 1–6) 15 μm; (A, vinculin-stained panel) 8 μm; (B) 10 μm.
Effects of Antibody Ligation of Adhesion Receptors on the Distribution of Fascin and other Actin-associated Proteins
To establish whether F-actin organization by antibody ligation of any of the adhesion receptors resulted in localized actin bundling by fascin, the antibody-adherent C2C12 cells were stained for fascin. In the very few cells which attached to rat IgG, fascin was diffuse (not shown). On the β1 or β3 integrin antibodies, fascin was also diffuse and the cell margins did not show fascin projections (Fig. 3 A). On the CD47 antibody, fascin appeared diffuse, and in 90% of the cells staining was uniform throughout the irregular, actin-rich marginal regions of the cells. The occasional cell showed small fascin-containing structures (one such cell is shown in Fig. 3). In striking contrast, fascin localized to radial spike structures in the cortex of EDTA-released cells on the syndecan-1 antibody (Fig. 3). C2C12 cells trypsinized for 2 min were more heterogeneous in spreading and spike formation than the EDTA-treated cells, and ∼68% of the cells showed fascin spikes or less well-organized fascin-containing projections (Fig. 3). Quantification of these results confirmed that the syndecan-1 antibody strongly and specifically evoked the formation of fascin spikes (Table ). Because antibodies to different epitopes on CD47 vary in their ability to perturb CD47-mediated functions (Brown et al. 1990), we further examined the role of CD47 in fascin spike formation by comparing the ability of wild-type and CD47-null primary skeletal myoblasts to form fascin spikes on TSP-1. The two cell populations were indistinguishable with regard to adhesion and the organization of fascin spikes (Fig. 3 B).
We extended the analysis of receptor ligation by antibodies to additional cell types which show different degrees of spreading on TSP-1 substrata. MEF-1 mouse fibroblasts spread and form fascin spikes and filopodia on TSP-1 (Fig. 4 A). Cells remained rounded with diffuse fascin when plated on the anti–mouse β1, β3, or CD47 antibodies (shown for β1 only, Fig. 4 A). When plated on the 281-2 antibody to mouse syndecan-1, MEF-1 formed broad circumferential lamellae containing radial F-actin ribs which corresponded to sites where fascin and actin were bundled (Fig. 4 A). HLMECs express fascin and syndecan-1 but as reported for endothelial cells from other blood vessel sources, do not spread on TSP-1 (Fig. 4 B; Lawler et al. 1988; Murphy-Ullrich and Hook 1989; Mertens et al. 1992; Adams, J.C., unpublished data). The cells show specific enrichment of F-actin and fascin structures at cell margins when attached to TSP-1 (Fig. 4 B). HLMECs attached to surfaces coated with antibodies to human β1, β3, CD47, or syndecan-1 and showed most microfilament organization on the β1 antibody. However, fascin was completely diffuse in these cells and was not enriched at cell margins (Fig. 4 B; data not shown). On either of two antibodies to human syndecan-1, HLMECs formed short marginal structures which contained F-actin and fascin (shown for BB4 antibody only, Fig. 4 B). We also examined fascin localization in HLMECs plated on surfaces coated with SMO antibody to human CD36 (Hogg et al. 1984). The cells remained rounded and smooth edged and did not show specific localization of fascin (data not shown). Neither the BRIC 126, B6H12, nor 2E11 antibodies to human CD47 caused fascin–actin bundling (Fig. 4 B; data not shown). Thus, in several cell types from different tissue sources, ligation of syndecan-1 specifically promoted the organization of cortical fascin independently of the degree of cell spreading.
Figure 4.
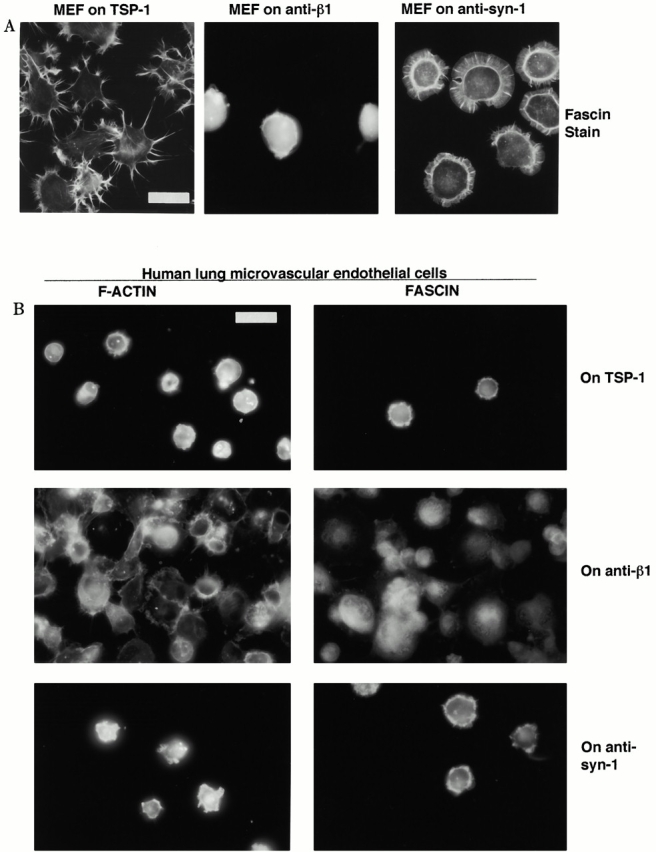
Organization of F-actin and fascin in cells adherent on TSP-1 or adhesion receptor antibodies. (A) EDTA-released mouse embryonic fibroblast cells (MEF) were plated on 50 nM TSP-1 or surfaces coated with 50 μg/ ml of the indicated antibodies as used in Fig. 1 and Fig. 2 for 1 h in serum-free medium and then fixed and stained for fascin. (B) EDTA-released HLMECs were plated on 50 nM TSP-1 or surfaces coated with 50 μg/ ml of antibodies to human adhesion receptors for 1 h in serum-free medium and then fixed and stained for F-actin or fascin. Bars, 20 μm.
To further evaluate effects on the actin-based cytoskeletal organization and compare these with the effects of TSP-1, we stained C2C12 cells on antibody substrata for components of focal contacts. Whereas cells on the β1 or β3 antibodies assembled vinculin into small, focal contact–like structures (shown for the β1 antibody only, Fig. 3), vinculin was diffuse in the cells adherent on the CD47 or syndecan-1 antibodies. We also found that cells adherent on the syndecan-1 antibody showed very low and diffuse staining for phosphotyrosine (data not shown). Thus, antibody ligation of syndecan-1 specifically induces the organization of actin and fascin bundles in the absence of focal contact assembly. Furthermore, these results demonstrate that the antiintegrin antibodies used in these experiments had appropriate integrin-activating activities in that they induced cytoskeletal coupling resulting in the focal localization of vinculin (Table ).
Syndecan-1 Is Sufficient for Fascin Spike Formation
To establish whether syndecan-1 is sufficient to mediate assembly of fascin and actin-containing spikes, we examined the effects of transient expression of mouse syndecan-1 in a heterologous cell system, green monkey COS-7 cells. COS-7 cells contain appropriate endogenous coupling mechanisms for spike formation because they express fascin and undergo limited spike formation when attached to TSP-1 (Adams 1997b). FACS® analysis established that the 281-2 antibody to mouse syndecan-1 did not stain the surface of live COS-7 cells. COS-7 cells did not attach over background to surfaces coated with syndecan-1 antibody (data not shown). COS-7 cells transiently cotransfected with control, empty expression plasmid, and EFGP-fascin or expression plasmid encoding syndecan-1 plus EGFP-fascin were disaggregated by EDTA treatment and used in adhesion assays on TSP-1. Indirect immunofluorescent staining of live cells was used to confirm the cell surface expression of syndecan-1 by transfected cells (data not shown).
The vector-transfected cells attached to TSP-1 remained rounded, and showed concentrations of actin and fascin at cell margins indistinguishable from those of untransfected cells (Fig. 5). In marked contrast, syndecan-1 transfectants showed greatly enhanced spreading on TSP-1. The cells spread to form adherent lamellipodial sheets with marginal F-actin and fascin-containing projections of varying lengths (Fig. 5). The syndecan-1 transfectants attached on the syndecan-1 antibody also showed greatly increased spreading which involved the formation of flatly spread lamellae in which radial projections containing F-actin and fascin were present at spread margins or extended as individual projections beyond the edge of the lamellae (Fig. 5). This effect involved a dramatic increase in the number of fascin spikes per cell (see Fig. 7 A). Cell membrane spreading involved a threefold increase in cell area (statistically significant at P = 0.0001; see Fig. 7 A). To examine the specificity of this effect, the syndecan-1 transfectants were plated on another matrix ligand, fibronectin. No increase in cell area or alteration in major F-actin structures was observed (Fig. 5; data not shown). Thus, the effects of syndecan-1 depend on its ligation by specific ligands and are not simply a consequence of the surface expression of syndecan-1.
Figure 5.
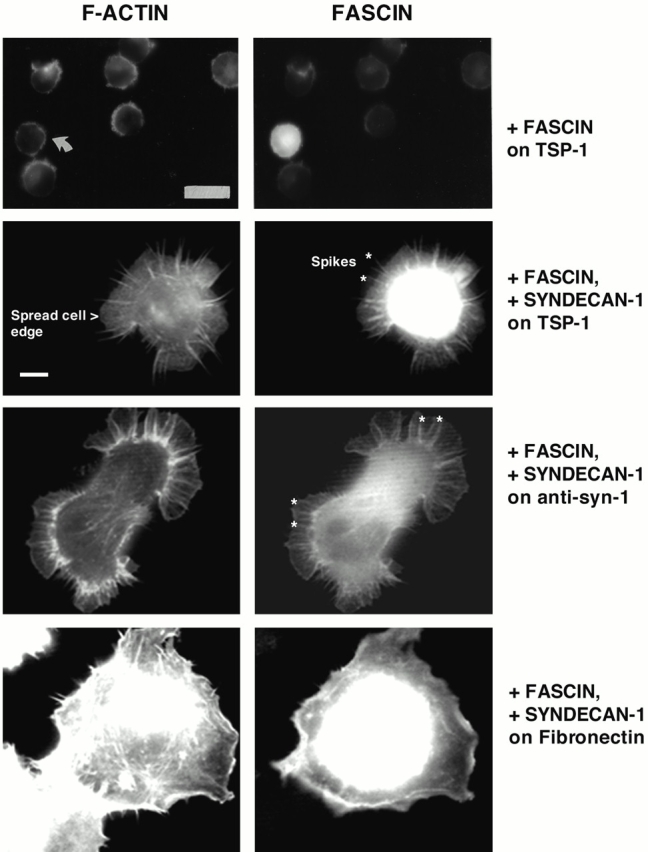
Expression of syndecan-1 specifically promotes cell spreading and formation of fascin spikes in TSP-1–adherent COS-7 cells. COS-7 cells were transfected with syndecan-1 and EGFP-fascin expression plasmids for 48 h. EDTA-released cells were plated on 50 nM TSP-1, fibro nectin, or 40 μg/ml anti–mouse syndecan-1 antibody in serum-free medium for 1 h and then fixed and stained for F-actin. Results shown are representative of three independent experiments, and ≥100 transfected cells were scored per experiment. The morphological features used for quantitation are also indicated. These were cell area measured by spread cell edge, and numbers and lengths of the radial fascin–actin bundles that form spikes and ribs. Examples of these bundles are marked with asteriks in two of the panels. Bars: (top two panels) 16 μm; (bottom six panels) 5 μm.
Figure 7.
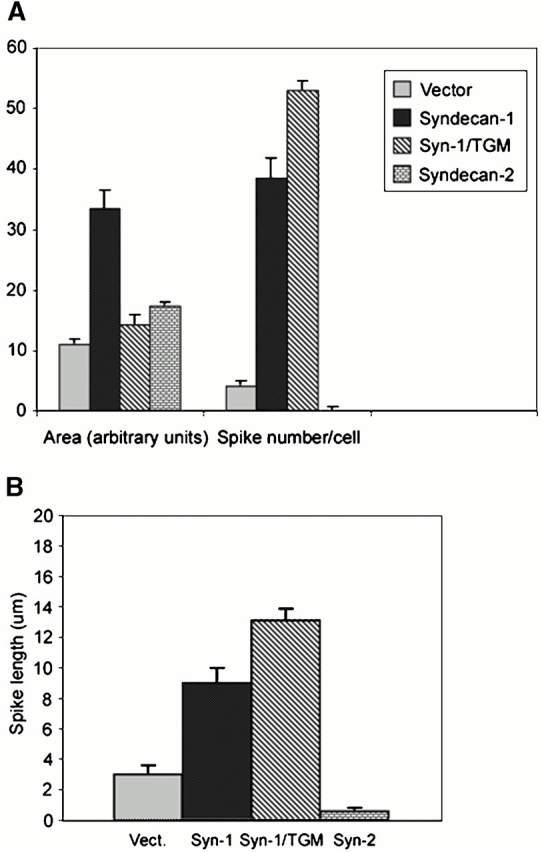
Expression of wild-type syndecan-1 promotes cell spreading and fascin spike assembly in TSP-1–adherent COS-7 cells. COS-7 cells transfected with empty expression vector or syndecan constructs were plated on 50 nM TSP-1 for 40 min in serum-free medium and then tracked by time-lapse videomicroscopy for 1 h. Cell areas and numbers of spikes (A) or spike lengths (B) were calculated from traces of spread cell margins after 1 h of adhesion to TSP-1. See Fig. 5 for examples of the morphological features. Bars show mean ± SEM for data from three experiments.
GAG Substitutions and the Syndecan-1 Cytoplasmic Domain Have Distinct Roles in the Mechanism of Syndecan-1–mediated Cell Spreading and Fascin Spike Organization on Thrombospondin-1
The extracellular domain of syndecan-1 proteoglycan contains three conserved sites for GAG substitution near the NH2 terminus. These are sites of addition for HS-GAGs in many cell types but can also be substituted by CS-GAGs (Kokenyesi and Bernfield 1994). HS-GAG chains have well-established functional roles in the activities of syndecans, particularly in binding growth factors such as FGF (for reviews see Rapraeger and Ott 1998; Bernfield et al. 1999). To determine whether GAGs also contribute to cytoskeletal coupling by syndecan-1, COS-7 cells were transiently cotransfected with EGFP-fascin and a syndecan-1 expression construct in which the three sites of GAG addition, serines 37, 45, and 47, had been mutated to alanines, syn-1/TGM (Langford et al. 1998). Strikingly, these cells spread poorly on TSP-1 compared with the wild-type syndecan-1 transfectants, and lamellipodia were not observed. Instead, the cells formed actin- and fascin-containing projections that appeared long in comparison to the wild-type syndecan-1 transfectants (Fig. 6). These parameters were quantitated by image analysis measurements. The mean cell area of syn-1/TGM transfectants was not significantly increased compared with vector control transfectants. However, the number of spikes per cell and their length were strongly increased (significant at P = 0.001; Fig. 7A and Fig. B). Compared with cells expressing wild-type syndecan-1, the number of spikes per cell was somewhat increased (significant at P = 0.04), and the mean length of spikes was increased from 8.5 to 13.5 μm (significant at P = 0.01; Fig. 7 B).
Figure 6.

GAG modifications of syndecan-1 and regions of the cytoplasmic domain are required for fascin spike formation on TSP-1. COS-7 cells were transfected with the indicated syndecan-1 mutants and EGFP-fascin expression plasmids, and 48 h later EDTA-released cells were plated on 50 nM TSP-1 in serum-free medium for 1 h and then fixed and stained for F-actin. Results shown are representative of three experiments, and ≥100 transfected cells were scored per experiment. Bar, 16 μm.
COS-7 cells were next transfected with constructs in which the GAG addition sites were individually mutated. Expression of the syn-1/ S37A mutant caused increased cell spreading and spike formation in a similar manner to wild-type syndecan-1 in cells adherent on TSP-1 (not shown). Expression of either the syn-1/S45A or the syn-1/S47A constructs correlated with enhanced formation of elongated spikes in poorly spread cells, and thus resembled the effects of the syn-1/TGM mutant (Fig. 6). Thus, GAG substitution at either S45 or S47 was needed in lamellar spreading on TSP-1 but was not required for the organization of filopodial-like fascin-containing projections.
To establish whether the cytoplasmic domain of the syndecan-1 core protein was critical for the formation of fascin spikes, COS-7 cells were transfected with a cytoplasmic deletion mutant of syndecan-1. All syndecans have short, 34-residue cytoplasmic domains that contain highly conserved membrane-proximal and COOH-terminal sequences, the C1 and C2 regions, respectively, separated by variable (V) regions which are unique to each family member. The C1 region is important for membrane translocation of syndecan-1 (Miettinen et al. 1994). Expression of syndecan-1 in which the V and C2 regions of the cytoplasmic domain had been deleted, syn-1/ΔVC2, did not promote either fascin spikes or lamellar cell spreading on TSP-1 (Fig. 6). Expression of the equivalent deletion mutant lacking the three GAG substitution sites, syn-1/TGM/ΔVC2, had similar effects (Fig. 6).
We also compared the effects of syndecan-2, a syndecan family member that is expressed by many mesenchymal cells, on cytoskeletal organization by cells adherent to TSP-1. The extracellular domain of syndecan-2 contains three analogous GAG addition sites, but the polypeptide sequence of the extracellular domain and of the cytoplasmic V region are unrelated to that of syndecan-1 (for review see Bernfield et al. 1999). Expression of syndecan-2 in COS-7 cells stimulated cell spreading on TSP-1 by 50% (significant at P = 0.04). However, the spread cells were completely smooth edged and lacked fascin spikes or cortical lamellipodia (Fig. 6 and Fig. 7A and Fig. B). Cumulatively, these results demonstrate that the syndecan-1 cytoplasmic domain in combination with effects from GAG chains are jointly needed in coupling extracellular TSP-1 to membrane spreading and fascin cytoskeletal organization.
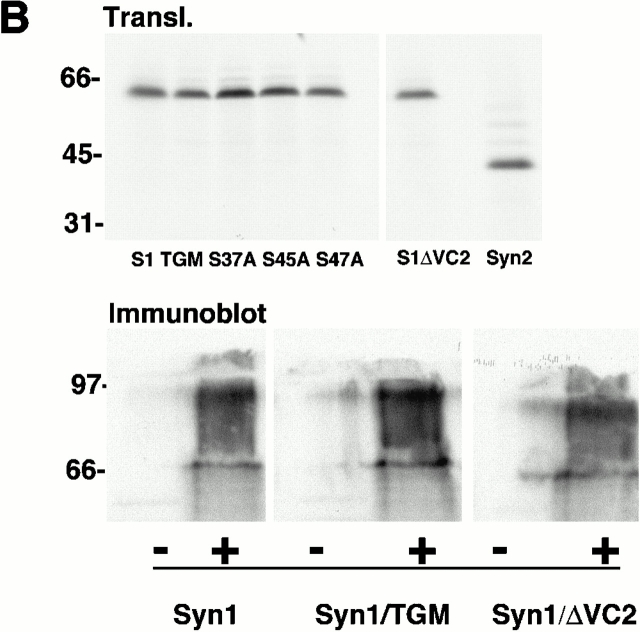
To determine whether these effects were specific to TSP-1 ligation of syndecan-1, we examined the responses of the transfectants to plating on syndecan antibody or on fibronectin. On the syndecan-1 antibody, the syn-1/TGM transfectants spread and organized fascin–actin bundles, whereas the syn-1/ΔVC2 transfectants remained round as they did on TSP-1 (Fig. 8 A). Thus, even upon direct, monospecific ligation of the core protein the V and C2 cytoplasmic regions are needed to transduce cytoskeletal organization. To further confirm that the effects of syndecan-1 expression cells resulted from specific ligation of syndecan-1 and not as an immediate consequence of syndecan overexpression, we examined cell behavior on fibronectin. No alterations in cell spreading or fascin distribution were apparent in cells transfected with syn-1/TGM, syn-1/ΔVC2, or syndecan-2 (Fig. 8 A). These cDNAs used were all translatable with equivalent efficiencies (Fig. 8 B), and both Syn1/TGM and Syn1/ΔVC2 proteins were expressed at comparable levels to the wild-type syndecan-1 as determined by FACS® analysis or immunoblot (Fig. 8 B; data not shown). Thus, the differing effects of the various syndecan-1 proteins on fascin organization most likely result from ligation of syndecan-1 by appropriate specific ligands.
Figure 8.
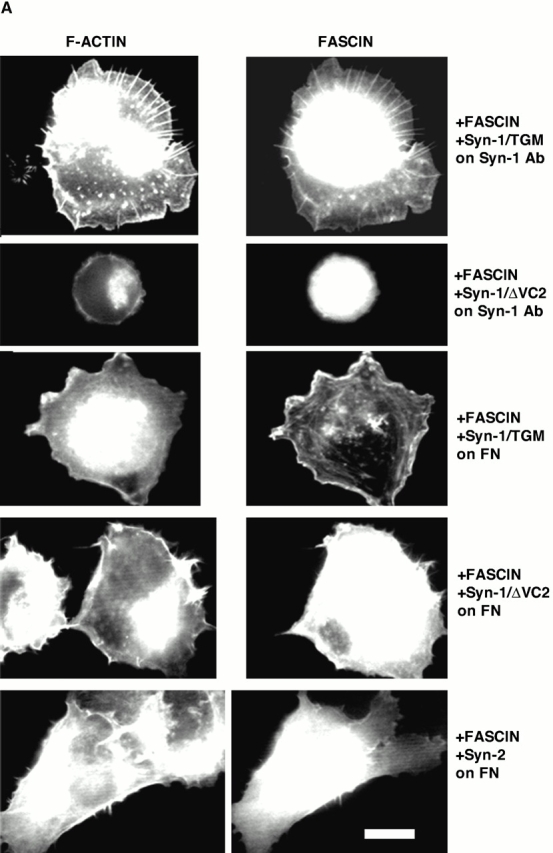
The effects of GAG removal from syndecan-1 are specific to TSP-1 adhesion. (A) COS-7 cells transfected for 48 h with the indicated syndecan constructs and EGFP-fascin were EDTA-released for 1-h adhesion assays on antibody to mouse syndecan-1 or 50 nM fibronectin. Actin organization was compared with EGFP-fascin distribution in the transfected cells. Results shown are representative of three experiments, and ≥50 transfected cells were scored per experiment. (B) Relative expression levels of syndecans used in this study. Top, in vitro translation of 1 μg of each syndecan construct detected by [35S]methionine incorporation; bottom, immunoblot of double heparitinase and chondroitinase–digested syndecan-1 proteins after expression and extraction from COS-7 cells detected by 281-2 antibody. Molecular weight markers are given in kD. Bar, 10 μm.
To determine the functional role of endogenous syndecan-1 in TSP-1–initiated formation of fascin spikes in C2C12 cells, we first examined the effect of prolonged trypsinization on fascin spike organization by C2C12 cells on TSP-1. Whereas EDTA-released cells spread and formed regions of fascin spikes, <20% of cells trypsinized for 5 min attached, and these remained completely round (Fig. 9A and Fig. B). These cells lack cell surface syndecan-1 (Table ). To examine the specific role of syndecan-1, the syn-1/ΔVC2 or syn-1/TGM constructs were transiently transfected into C2C12 cells. The syn-1/ΔVC2 molecule acted as an effective dominant negative, in that, transfected cells which attached to TSP-1 did not spread or form fascin spikes (Fig. 9 C). Cells expressing the syn-1/TGM construct were altered in morphology on TSP-1, yet formed fascin projections (Fig. 9 D). Thus, in C2C12 cells as in COS-7 cells, GAG substitution of syndecan-1 is important for cell spreading on TSP-1 but does not appear to be required in fascin spike organization.
Figure 9.
Role of endogenous syndecan-1 in fascin spike formation by C2C12 cells adherent on TSP-1. In a separate protocol, C2C12 cotransfected with Syn-1/ΔVC2 or Syn-1/TGM and EGFP-fascin expression plasmids were identified by EGFP-fascin expression after 1 h adhesion on TSP-1. Bar, 12 μm.
Syndecan-1–mediated Cytoskeletal Coupling Stimulates Protrusive Cortical Membrane Ruffling
In addition to their role in cell adhesion to TSP-1, fascin spikes support cell motility on TSP-1 matrix (Adams 1997a,Adams 1997b; Adams and Schwartz 2000). We undertook time-lapse experiments to examine the motile behavior of the syndecan-1–transfected COS-7 cells on TSP-1 or on syndecan-1 antibody-coated surfaces. Cells were scored for two aspects of motility: protrusive lateral ruffling activity and locomotion over the substratum. When attached to TSP-1, few of the vector transfectant cells showed extension of spikes in contact with the substratum, there was little ruffling activity at lateral cell margins, and all cells were nonlocomotary (Fig. 10). In marked contrast, the syndecan-1 transfectant cells showed greatly increased ruffling at their lateral margins (Fig. 10; significant at P > 0.001). These lateral ruffles appeared as dynamic phase dark regions that protruded rapidly along the cell margin or moved centripetally across the spread lamellae. These were also apparent in syndecan-1 transfectants adherent on syndecan-1 antibody (data not shown). Cells expressing Syn-1/TGM also showed highly significant increases in lateral ruffling (Fig. 10). In contrast, cells transfected with the syn-1/ΔVC2 construct did not show lateral ruffles and thus appeared similar to vector transfectants (data not shown). To examine the specificity of the ruffling response, we compared the motility of cells transfected with syndecan-2. These cells showed no lateral ruffling. Lateral ruffling thus specifically correlated with the formation of fascin spikes (Fig. 7 B and Fig. 10). The alterations in lateral ruffling occurred in the absence of any change in extension of apical projections, and thus related specifically to adhesion-dependent motility and not to a general change in membrane dynamics (Fig. 10). Interestingly, despite the large increases in cell spreading and lateral ruffling activity, syndecan-1 transfectants did not translocate over the substratum. For adhesion to TSP-1, this result was obtained using coating concentrations of 25 or 50 nM. For adhesion to the syndecan-1 antibody, this result was confirmed at a range of antibody-coating concentrations from 5 to 50 μg/ml. For all concentrations that supported cell spreading, no stimulation of cell locomotion was observed (data not shown). Thus, under these experimental conditions, the most dramatic effect of syndecan-1 ligation on motile behavior is the stimulation of active ruffling protrusions at lateral cell margins (Fig. 10).
Figure 10.
Effects of syndecan expression on aspects of cell motile behavior. Apical projections and lateral protrusive ruffling activity of cells adherent on 50 nM TSP-1 were scored from phase–contrast time-lapse videos that tracked vector or syndecan transfectant cells from 45 min to 1.5 h of adhesion. Locomotion was scored as the displacement of cell centroids between 45 min and 2 h of adhesion. Bars show mean ± SEM for data from five experiments. Shade code same as in Fig. 7.
Discussion
Our results demonstrate a novel role for syndecan-1 in coupling the organization of fascin spikes in response to a physiological extracellular ligand, thrombospondin-1. We show by several independent approaches that syndecan-1 has a crucial role in this process. First, antibody ligation of syndecan specifically causes the formation of cortical actin- and fascin-containing bundles in several cell types. Second, overexpression of syndecan-1 in a heterologous cell type is sufficient to cause dramatic enhancement of cell spreading and the formation of fascin spikes in response to TSP-1. Third, treatments which deplete C2C12 cells of endogenous syndecan-1 inhibit cell spreading and fascin spike formation on TSP-1 substrata. Fourth, expression of a cytoplasmic domain deletion mutant of syndecan-1 had dominant negative effects on cell spreading on TSP-1. With regard to the molecular mechanism of this process, we demonstrate that there are distinct requirements for GAG substitution and the COOH-terminal region of the cytoplasmic domain in transducing cell spreading and fascin spike formation.
Syndecan-1 and thrombospondin-1 are coexpressed in many tissues, including skeletal muscle, during development (Corless et al. 1992). TSP-1 is known as a heparin-binding protein, and each subunit of the trimer contains multiple GAG-binding motifs within the NH2-terminal domain and type 1 repeats (for review see Adams 1997a). Dependent on the tissue source and structural characteristics of the GAGs, TSP-1 binds both HS- and CS-GAGs (Pancake et al. 1992; Herndon et al. 1999). GAG-dependent cell attachment or adhesion to TSP-1 has been reported in many cell types (for example, Roberts et al. 1987; Kaesberg et al. 1989; Adams and Lawler 1993; Gantt et al. 1997; Wilson et al. 1999). Disruption of focal contacts by soluble TSP-1 and the endocytosis of TSP-1 by low density lipoprotein receptor–related protein are also heparin-inhibitable processes (Murphy-Ullrich and Hook 1989; Godyna et al. 1995; Mikhailenko et al. 1995; Chen et al. 1996). However, the core proteins involved in GAG-mediated adhesion have remained unknown. By affinity chromatography, syndecan-1 was identified as the major thrombospondin-binding HS proteoglycan in mammary epithelial cell extracts (Sun et al. 1989). Our data now provide evidence for a role of syndecan-1 in cell spreading and major cytoskeletal responses to TSP-1.
Fascin spikes identify a specific matrix contact structure formed during cell spreading on TSP-1 which has functional roles in cell adhesion and motility on matrix substrata (Adams 1995, Adams 1997b). Interestingly, fascin spikes are also formed upon cell adhesion to tenascin-C and laminin-1, both of which are also ligands for syndecan-1 (Adams 1997b; Fischer et al. 1997; for review see Bernfield et al. 1999). Although syndecan-1 has a peripheral distribution in freshly spreading cells, it is not present in focal contacts and colocalizes with actin microfilaments when clustered and in long-term adherent cells (Rapraeger et al. 1986; Carey et al. 1994a). Antibody-mediated clustering of syndecan-1 also promotes actin reorganization in preadherent Schwann cells and Raji cells (Carey et al. 1994b; Lebakken and Rapraeger 1996). Our results link the binding of syndecan-1 by a physiological extracellular matrix ligand, TSP-1, to a specific effect on the actin cytoskeleton, that is, the formation of fascin spikes and ribs in adherent lamellipodia. Furthermore, this effect of syndecan-1 ligation compared with the effects of ligation of CD36, CD47, β1, or β3 integrins adhesive receptors implies a central role for syndecan-1 in extracellular coordination of actin bundling by fascin.
Several cell types show quantitatively high attachment to TSP-1 but do not undergo spreading (for example, Asch et al. 1991; Adams and Lawler 1993). This property does not show a simple correlation with recognition of a particular adhesive domain of TSP-1 (Adams and Lawler 1993) nor does it correlate in all cell types with a lack of fascin expression (Adams 1997a,Adams 1997b). We examined HLMECs as a example of a fascin-positive cell type that does not spread on TSP-1 yet is functionally responsive to TSP-1 (Good et al. 1990). Interestingly, these cells did not spread on antisyndecan antibodies, although cortical enrichments of actin and fascin were apparent on both TSP-1– or antibody-coated surfaces. Several possible mechanisms could underlie this effect. A certain level of syndecan-1 expression could be necessary to promote cell spreading and cytoskeletal organization on TSP-1. However, although the microvascular endothelial cells express lower levels of syndecan-1 than do epidermal keratinocytes, as determined by FACS® analysis, epidermal keratinocytes also do not spread on TSP-1 (Adams and Lawler 1993; Adams, J.C., unpublished data). In C2C12 cells, overexpression of either dominant negative or constitutively active forms of Cdc42 or Rac small GTPases correlates with cell rounding on TSP-1 (Adams and Schwartz 2000). An interesting speculation is that the constitutive lack of spreading of certain cell types on TSP-1 could result from differences in the sizes of the active pools of these signaling mediators.
Syndecans have principally been characterized as coreceptors with FGF receptors or integrin-adhesive receptors (for reviews see Bernfield et al. 1999; Woods and Couchman 2000). In particular, syndecan-4 binds the major heparin-binding site of fibronectin and acts coordinately with integrins such as α5β1 in the assembly of focal adhesions (Woods et al. 1993; Couchman and Woods 1999; Longley et al. 1999; Saoncella et al. 1999). Ligation of α5β1 integrin leads to activation of protein kinase C. By binding and activating protein kinase C, syndecan-4 appears to potentiate this signal for focal adhesion assembly (Vuori and Ruoslahti 1993; Oh et al. 1997a,Oh et al. 1997b). Fibronectin supports only limited and transient formation of fascin spikes and ruffles during the initial stages of cell spreading because α5β1-dependent activation of protein kinase C promotes phosphorylation of fascin and thereby downregulates its actin-binding activity (Adams 1995; Adams et al. 1999). Thus, the molecular process we have defined for syndecan-1 as a transducer of fascin spike formation appears distinct from the mechanisms by which syndecan-4 participates in focal contact assembly.
In examining the mechanism by which syndecan-1 mediates this effect on fascin, we have uncovered a dualistic activity of syndecan-1. Whereas the effect of direct antibody ligation of the syndecan-1 extracellular domain or transfection of wild-type syndecan-1 stimulated both lamellipodial cell spreading and associated formation of fascin- and actin-containing spikes and ribs, GAG modifications at specific sites on the extracellular domain were needed to support lamellipodial spreading on TSP-1. Cells expressing GAG-negative syndecan-1 or loss-of-function mutants at the S45 or S47 attachment sites showed florid formation of fascin spikes on TSP-1 and poor membrane spreading. This effect was separable from a necessity for the VC2 portion of the cytoplasmic domain in both fascin spike formation and cell spreading.
As a model to explain these results, we propose two separate activities of the extracellular domain: (a) an interaction of TSP-1 with the core protein that is transduced through the cytoplasmic domain, which principally couples spike formation and is permissive for cell spreading; and (b) a GAG-mediated receptor-clustering effect which acts to promotes cell membrane spreading. Although a protein–protein interaction of syndecan-1 with TSP-1 has not been reported, a separate role for the core protein would not be entirely unprecedented within the syndecan family. A region in the extracellular domain of syndecans is involved in lateral homophilic association (for reviews see Rapraeger and Ott 1998; Zimmermann and David 1999). The extracellular domain of syndecan-4 presented as a recombinant, monomeric, unglycosylated protein specifically supports fibroblast attachment and binds cell surfaces with nanomolar affinity (McFall and Rapraeger 1997, McFall and Rapraeger 1998). There are several reports of effects of syndecan-1 on cell morphology which do not require the cytoplasmic domain, and thus a separable role of the GAG chains appears plausible (Lebakken and Rapraeger 1996; Liu et al. 1998). Effects on F-actin distribution that depend on the cytoplasmic domain have also been reported (Carey et al. 1996).
Members of the PDZ family of proteins are known binding partners for the C2 COOH-terminal region of syndecans (Grootjans et al. 1997; for reviews see Rapraeger and Ott 1998; Bernfield et al. 1999; Zimmermann and David 1999). However, transfection of syndecan-2, which has similarly positioned GAG attachment sites and shares the common C1 and C2 cytoplasmic regions with syndecan-1, promoted cell spreading but did not lead to organization of fascin spikes on TSP-1. The V region of syndecan-4 uniquely binds and activates protein kinase C (Oh et al. 1997a; for reviews see Rapraeger and Ott 1998; Woods and Couchman 1998). The V regions of other syndecans might have other unique binding partners. Our results define a novel and specific requirement for syndecan-1 in coupling between TSP-1 and fascin spike formation. Further studies will be directed towards defining the molecular interactions responsible for the recruitment of fascin and actin into these cortical structures.
Acknowledgments
We thank the following investigators for their gifts: Kevin Langford and Ralph Sanderson for syndecan-1 expression plasmids, Frederick Lindberg for reagents to mouse CD47, and John Couchman for syndecan-2 plasmid.
We thank the Wellcome Trust for their financial support (grant 038284).
Footnotes
Abbreviations used in this paper: CS, chondroitin sulfate; EGFP, enhanced green fluorescent protein; GAG, glycosaminoglycan; HLMEC, human lung microvascular endothelial cell; HS, heparan sulfate; TSP, thrombospondin.
References
- Adams J.C. Formation of stable microspikes containing actin and the 55 kDa actin bundling protein, fascin, is a consequence of cell adhesion to thrombospondin-1implications for the anti-adhesive activities of thrombospondin-1. J. Cell Sci. 1995;108:1977–1990. doi: 10.1242/jcs.108.5.1977. [DOI] [PubMed] [Google Scholar]
- Adams J.C. Thrombospondin-1 Int. J. Biochem. Cell Biol 29 1997. 861 865a [DOI] [PubMed] [Google Scholar]
- Adams J.C. Characterization of cell-matrix adhesion requirements for the formation of fascin microspikes Mol. Biol. Cell. 8 1997. 2345 2363b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J.C., Lawler J. Diverse mechanisms for cell attachment to platelet thrombospondin. J. Cell Sci. 1993;104:1061–1071. doi: 10.1242/jcs.104.4.1061. [DOI] [PubMed] [Google Scholar]
- Adams J.C., Lawler J. Cell-type specific adhesive interactions of skeletal myoblasts with thrombospondin-1. Mol. Biol. Cell. 1994;5:623–637. doi: 10.1091/mbc.5.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J.C., Schwartz M.A. Stimulation of fascin spikes by thrombospondin-1 is mediated by the GTPases Rac and Cdc42. J. Cell Biol. 2000;150:807–822. doi: 10.1083/jcb.150.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J.C., Seed B., Lawler J. Muskelin, a novel intracellular mediator of cell adhesive and cytoskeletal responses to thrombospondin-1. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:4964–4974. doi: 10.1093/emboj/17.17.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J.C., Clelland J.D., Collett G.D.M., Matsumura F., Yamashiro S., Zhang L. Cell-matrix adhesions differentially regulate fascin phosphorylation. Mol. Biol. Cell. 1999;10:4177–4190. doi: 10.1091/mbc.10.12.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama S.K., Yamada S.S., Chen W.T., Yamada K.M. Analysis of fibronectin receptor function with monoclonal antibodiesroles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J. Cell Biol. 1989;109:863–875. doi: 10.1083/jcb.109.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch A.S., Templer J., Silberger S. Cellular attachment to thrombospondincooperative interactions between receptor systems. J. Biol. Chem. 1991;266:1740–1745. [PubMed] [Google Scholar]
- Asch A.S., Silbiger S., Heimer E., Nachman R.L. Thrombospondin sequence motif (CSVTCG) is responsible for CD36 binding. Biochem. Biophys. Res. Commun. 1992;182:1208–1217. doi: 10.1016/0006-291x(92)91860-s. [DOI] [PubMed] [Google Scholar]
- Avent N., Judson P.A., Parsons S.F., Mallinson G., Anstee D.J., Tanner M.J., Evans P.R., Hodges E., Maciver A.G., Holmes C. Monoclonal antibodies that recognize different membrane proteins that are deficient in Rh-null human erythrocytes. One group of antibodies reacts with a variety of cells and tissues whereas the other group is erythroid-specific. Biochem. J. 1988;251:499–505. doi: 10.1042/bj2510499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M., Gotte M., Park P.W., Reizes O., Fitzgerald M.L., Lincecum J., Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Bornstein P. Diversity of function is inherent in matricellular proteinsan appraisal of thrombospondin-1. J. Cell Biol. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E., Hooper L., Ho T., Gresham H. Integrin-associated proteina 50 kD plasma-membrane antigen physically and functionally associated with integrins. J. Cell Biol. 1990;111:2785–2794. doi: 10.1083/jcb.111.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey D.J., Stahl R.C., Cizmeci-Smith G., Asundi V.K. Syndecan-1 expressed in Schwann cells causes morphological transformation and cytoskeletal reorganization and associates with actin during cell spreading J. Cell Biol 124 1994. 161 170a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey D.J., Stahl R.C., Tucker B., Bendt K.M., Cizmeci-Smith G. Aggregation-induced association of syndecan-1 with microfilaments mediated by the cytoplasmic domain Exp. Cell Res 214 1994. 12 21b [DOI] [PubMed] [Google Scholar]
- Carey D.J., Bendt K.M., Stahl R.C. The cytoplasmic domain of syndecan-1 is required for cytoskeleton association but not detergent insolubility. J. Biol. Chem. 1996;271:15253–15260. doi: 10.1074/jbc.271.25.15253. [DOI] [PubMed] [Google Scholar]
- Chen H., Sottile J., Strickland D.K., Mosher D.F. Binding and degradation of thrombospondin-1 mediated through heparan sulphate proteoglycans and low-density-lipoprotein receptor-related proteinlocalization of the functional activity to the trimeric N-terminal heparin-binding region of thrombospondin-1. Biochem. J. 1996;318:959–963. doi: 10.1042/bj3180959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D.A. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc. Natl. Acad. Sci. USA. 1987;84:6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R. Inhibition of cell adhesion by anti-adhesive molecules. Curr. Opin. Cell Biol. 1995;7:715–719. doi: 10.1016/0955-0674(95)80114-6. [DOI] [PubMed] [Google Scholar]
- Clark E.A., Brugge J.S. Integrins and signal transduction pathwaysthe road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Corless C.L., Mendoza A., Collins T., Lawler J. Colocalization of thrombospondin and syndecan during murine development. Dev. Dyn. 1992;193:346–358. doi: 10.1002/aja.1001930408. [DOI] [PubMed] [Google Scholar]
- Couchman J.R., Woods A. Syndecan-4 and integrinscombinatorial signaling in cell adhesion. J. Cell Sci. 1999;112:3415–3420. doi: 10.1242/jcs.112.20.3415. [DOI] [PubMed] [Google Scholar]
- Dore J.M., Morard F., Vita N., Wijdenes J. Identification and location on syndecan-1 core protein of the epitopes of B-B2 and B-B4 monoclonal antibodies. FEBS Lett. 1998;426:67–70. doi: 10.1016/s0014-5793(98)00310-x. [DOI] [PubMed] [Google Scholar]
- Fischer D., Tucker R.P., Chiquet-Ehrismann R., Adams J.C. Cell-adhesive responses to tenascin-C splice variants involve formation of fascin microspikes. Mol. Biol. Cell. 1997;8:2055–2075. doi: 10.1091/mbc.8.10.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M.L., Wang Z., Park P.W., Murphy G., Bernfield M. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3–sensitive metalloproteinase. J. Cell Biol. 2000;148:811–824. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt S.M., Clavijo P., Bai X., Esko J.D., Sinnis P. Cell adhesion to a motif shared by the malaria circumsporozoite protein and thrombospondin is mediated by its glycosaminoglycan-binding region and not by CSVTCG. J. Biol. Chem. 1997;272:19205–19213. doi: 10.1074/jbc.272.31.19205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao A.-G., Lindberg F.P., Dimitry J.M., Brown E.J., Frazier W.A. Thrombospondin modulates αvβ3 function through integrin-associated protein. J. Cell Biol. 1996;135:533–544. doi: 10.1083/jcb.135.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good D.J., Polverini P.J., Rastinejad F., LeBeau M.M., Lemons R.S., Frazier W.A., Bouck N.P. A tumor-suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc. Natl. Acad. Sci. USA. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godyna S., Liau G., Popa I., Stefansson S., Argraves W.S. Identification of the low density lipoprotein receptor–related protein (LRP) as an endocytic receptor for thrombospondin-1. J. Cell Biol. 1995;129:1403–1410. doi: 10.1083/jcb.129.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootjans J.J., Zimmermann P., Reekmans G., Smets A., Degeest G., Durr J., David G. Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc. Natl. Acad. Sci. USA. 1997;94:13683–13688. doi: 10.1073/pnas.94.25.13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N.H.M., Krutzsch H.C., Negre E., Vogel T., Blake D.A., Roberts D.D. Heparin- and sulfatide-binding peptides from the type I repeats of human thrombospondin promote melanoma cell adhesion Proc. Natl. Acad. Sci. USA 89 1992. 3040 3044a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N.H.M., Krutzsch H.C., Negre E., Zabrenetzky V.V., Roberts D.D. Heparin-binding peptides from the type I repeats of thrombospondin. Structural requirements for heparin binding and promotion of melanoma cell adhesion and chemotaxis J. Biol. Chem 267 1992. 19349 19355b [PubMed] [Google Scholar]
- Herndon M.E., Stipp C.S., Lander A.D. Interactions of neural glycosaminoglycans and proteoglycans with protein ligandsassessment of selectivity, heterogeneity and the participation of core proteins in binding. Glycobiology. 1999;9:143–155. doi: 10.1093/glycob/9.2.143. [DOI] [PubMed] [Google Scholar]
- Hogg N., MacDonald S., Slusarenko M., Beverley P.C. Monoclonal antibodies specific for human monocytes, granulocytes and endothelium. Immunology. 1984;53:753–767. [PMC free article] [PubMed] [Google Scholar]
- Hynes R.O. Cell adhesionold and new questions. Trends Cell Biol. 1999;9:M33–M37. [PubMed] [Google Scholar]
- Jalkanen M., Nguyen H., Rapraeger A., Kurn N., Bernfield M. Heparan sulphate proteoglycans from mouse mammary epithelial cellslocalization on the cell surface with a monoclonal antibody. J. Cell Biol. 1985;101:976–984. doi: 10.1083/jcb.101.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaesberg P.R., Ershler W.B., Esko J.D., Mosher D.F. Chinese hamster ovary cell adhesion to human platelet thrombospondin is dependent on cell surface heparan sulfate proteoglycan. J. Clin. Invest. 1989;83:994–1001. doi: 10.1172/JCI113986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinashi T., Springer T.A. Adhesion molecules in hematopoietic cells. Blood Cells. 1994;20:25–44. [PubMed] [Google Scholar]
- Klass C.M., Couchman J.R., Woods A. Control of extracellular matrix assembly by syndecan-2 proteoglycan. J. Cell Sci. 2000;113:493–506. doi: 10.1242/jcs.113.3.493. [DOI] [PubMed] [Google Scholar]
- Koda J.E., Rapraeger A., Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells. Cell surface proteoglycan as a receptor for interstitial collagens. J. Biol. Chem. 1985;260:8157–8162. [PubMed] [Google Scholar]
- Kokenyesi R., Bernfield M. Core protein structure and sequence determine the site and presence of heparan sulfate and chondroitin sulfate on syndecan-1. J. Biol. Chem. 1994;269:12304–12309. [PubMed] [Google Scholar]
- Krutzsch H.C., Choe B.J., Sipes J.M., Guo N., Roberts D.D. Identification of an α3β1 integrin recognition site in thormbospondin-1. J. Biol. Chem. 1999;274:24080–24086. doi: 10.1074/jbc.274.34.24080. [DOI] [PubMed] [Google Scholar]
- Langford J.K., Stanley M.J., Cao D., Sanderson R.D. Multiple heparan sulfate chains are required for optimal syndecan-1 function. J. Biol. Chem. 1998;273:29965–29971. doi: 10.1074/jbc.273.45.29965. [DOI] [PubMed] [Google Scholar]
- Lawler J., Hynes R.O. An integrin receptor on normal and thrombasthenic platelets that binds thrombospondin. Blood. 1989;74:2022–2027. [PubMed] [Google Scholar]
- Lawler J., Weinstein R., Hynes R.O. Cell attachment to thrombospondinthe role of ARG-GLY-ASP, calcium, and integrin receptors. J. Cell Biol. 1988;107:2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebakken C.S., Rapraeger A.C. Syndecan-1 mediates cell spreading in transfected human lymphoblastiod (Raji) cells. J. Cell Biol. 1996;132:1209–1221. doi: 10.1083/jcb.132.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.X., Howard R.J., Leung L.L. Identification of SVTCG in thrombospondin as the conformation-dependent, high affinity binding site for its receptor, CD36. J. Biol. Chem. 1993;268:16179–16184. [PubMed] [Google Scholar]
- Lindberg F.P., Bullard D.C., Caver T.E., Gresham H.D., Beaudet A.L., Brown E.J. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274:795–798. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- Liu W., Litwack E.D., Stanley M.J., Langford J.K., Lander A.D., Sanderson R.D. Heparan sulfate proteoglycans as adhesive and anti-invasive molecules. Syndecans and glypican have distinct functions. J. Biol. Chem. 1998;273:22825–22832. doi: 10.1074/jbc.273.35.22825. [DOI] [PubMed] [Google Scholar]
- Longley R.L., Woods A., Fleetwood A., Cowling G.J., Gallagher J.T., Couchman J.R. Control of morphology, cytoskeleton and migration by syndecan-4. J. Cell Sci. 1999;112:3421–3431. doi: 10.1242/jcs.112.20.3421. [DOI] [PubMed] [Google Scholar]
- McFall A.J., Rapraeger A.C. Identification of an adhesion site within the syndecan-4 extracellular protein domain. J. Biol. Chem. 1997;272:12901–12904. doi: 10.1074/jbc.272.20.12901. [DOI] [PubMed] [Google Scholar]
- McFall A.J., Rapraeger A.C. Characterization of the high affinity cell-binding domain in the cell surface proteoglycan syndecan-4. J. Biol. Chem. 1998;273:28270–28276. doi: 10.1074/jbc.273.43.28270. [DOI] [PubMed] [Google Scholar]
- Mertens G., Cassiman J.J., Van den Berghe H., Vermylen J., David G. Cell surface heparan sulfate proteoglycans from human vascular endothelial cells. Core protein characterization and antithrombin III binding properties. J. Biol. Chem. 1992;267:20435–20443. [PubMed] [Google Scholar]
- Miettinen H.M., Edwards S.N., Jalkanen M. Analysis of transport and targeting of syndecan-1effect of cytoplasmic tail deletions. Mol. Biol. Cell. 1994;5:1325–1339. doi: 10.1091/mbc.5.12.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailenko I., Kounnas M.Z., Strickland D.K. Low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor mediates the cellular internalization and degradation of thrombospondin. A process facilitated by cell-surface proteoglycans. J. Biol. Chem. 1995;270:9543–9549. doi: 10.1074/jbc.270.16.9543. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich J.E., Hook M. Thrombospondin modulates focal adhesions in endothelial cells. J. Cell Biol. 1989;109:1309–1319. doi: 10.1083/jcb.109.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E.-S., Couchman J.R., Woods A. Syndecan-4 proteoglycan regulates the distribution and activity of protein kinase C J. Biol. Chem 272 1997. 8133 8136a [DOI] [PubMed] [Google Scholar]
- Oh E.-S., Couchman J.R., Woods A. Multimerisation of the cytoplasmic domain of syndecan-4 is required for its ability to activate protein kinase C J. Biol. Chem 272 1997. 11805 11811b [DOI] [PubMed] [Google Scholar]
- Pancake S.J., Holt G.D., Mellouk S., Hoffman S.L. Malaria sporozoites and circumsporozoite proteins bind specifically to sulfated glycoconjugates. J. Cell Biol. 1992;117:1351–1357. doi: 10.1083/jcb.117.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger A., Jalkanen M., Bernfield M. Cell surface proteoglycan associates with the cytoskeleton at the basolateral cell surface of mouse mammary epithelial cells. J. Cell Biol. 1986;103:2683–2696. doi: 10.1083/jcb.103.6.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger A.C., Ott V.L. Molecular interactions of the syndecan core proteins. Curr. Opin. Cell Biol. 1998;10:620–628. doi: 10.1016/s0955-0674(98)80038-0. [DOI] [PubMed] [Google Scholar]
- Roberts D.D., Sherwood J.A., Ginsburg V. Platelet thrombospondin mediates attachment and spreading of human melanoma cells. J. Cell Biol. 1987;104:131–139. doi: 10.1083/jcb.104.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson R.D., Sneed T.B., Young L.A., Sullivan G.L., Lander A.D. Adhesion of B lymphoid (MPC-11) cells to type I collagen is mediated by integral membrane proteoglycan, syndecan. J. Immunol. 1992;148:3902–3911. [PubMed] [Google Scholar]
- Saoncella S., Echtermeyer F., Denhez F., Nowlen J.K., Mosher D.F., Robinson S.D., Hynes R.O., Goetinck P.F. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc. Natl. Acad. Sci. USA. 1999;96:2805–2810. doi: 10.1073/pnas.96.6.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders S.M., Jalkanen S., O'Farrell J., Bernfield M. Molecular cloning of syndecan, an integral membrane protein. J. Cell Biol. 1989;108:1547–1556. doi: 10.1083/jcb.108.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M.A., Schaller M.D., Ginsberg M.H. Integrinsemerging paradigms of signal transduction. Annu. Rev. Cell Dev. Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Sun X., Mosher D.F., Rapraeger A. Heparan sulfate-mediated binding of epithelial cell surface proteoglycan to thrombospondin. J. Biol. Chem. 1989;264:2885–2889. [PubMed] [Google Scholar]
- Sun X., Skorstengaard K., Mosher D.F. Disulfides modulate RGD-inhibitable cell adhesive activity of thrombospondin. J. Cell Biol. 1992;118:693–701. doi: 10.1083/jcb.118.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuori K., Ruoslahti E. Activation of protein kinase C precedes α5β1 integrin-mediated cell spreading on fibronectin. J. Biol. Chem. 1993;268:21459–21462. [PubMed] [Google Scholar]
- Wilson K.E., Li Z., Kara M., Gardner K.L., Roberts D.D. Beta 1 integrin- and proteoglycan-mediated stimulation of T lymphoma cell adhesion and mitogen-activated protein kinase signaling by thrombospondin-1 and thrombospondin-1 peptides. J. Immunol. 1999;163:3621–3628. [PubMed] [Google Scholar]
- Woods A., McCarthy J.B., Furcht L.T., Couchman J.R. A synthetic peptide from the COOH-terminal heparin-binding domain of fibronectin promotes focal adhesion formation. Mol. Biol. Cell. 1993;6:605–613. doi: 10.1091/mbc.4.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A., Couchman J.R. Syndecanssynergistic activators of cell adhesion. Trends Cell Biol. 1998;8:189–192. doi: 10.1016/s0962-8924(98)01244-6. [DOI] [PubMed] [Google Scholar]
- Woods A., Couchman J.R. Integrin modulation by lateral association. J. Biol. Chem. 2000;275:24233–24236. doi: 10.1074/jbc.R000001200. [DOI] [PubMed] [Google Scholar]
- Yabkowitz R., Dixit V.M., Guo N. Activated T-cell adhesion to thormbospondin is mediated by the VLA-4 and VLA-5 integrins. J. Immunol. 1993;151:149–158. [PubMed] [Google Scholar]
- Yamada K.M., Miyamoto S. Integrin transmembrane signaling and cytoskeletal control. Curr. Opin. Cell Biol. 1995;7:681–689. doi: 10.1016/0955-0674(95)80110-3. [DOI] [PubMed] [Google Scholar]
- Yamashiro-Matsumura S., Yamakita Y., Ono S., Matsumura F. Fascin, an actin-bundling protein, induces membrane protrusions and increases cell motility of epithelial cells. Mol. Biol. Cell. 1998;9:993–1006. doi: 10.1091/mbc.9.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P., David G. The syndecans, tuners of transmembrane signaling FASEB J 13Suppl.1999. S91 S100 [DOI] [PubMed] [Google Scholar]