Abstract
Assembly and maintenance of myofibrils require dynamic regulation of the actin cytoskeleton. In Caenorhabditis elegans, UNC-60B, a muscle-specific actin depolymerizing factor (ADF)/cofilin isoform, is required for proper actin filament assembly in body wall muscle (Ono, S., D.L. Baillie, and G.M. Benian. 1999. J. Cell Biol. 145:491–502). Here, I show that UNC-78 is a homologue of actin-interacting protein 1 (AIP1) and functions as a novel regulator of actin organization in myofibrils. In unc-78 mutants, the striated organization of actin filaments is disrupted, and large actin aggregates are formed in the body wall muscle cells, resulting in defects in their motility. Point mutations in unc-78 alleles change conserved residues within different WD repeats of the UNC-78 protein and cause less severe phenotypes than a deletion allele, suggesting that these mutations partially impair the function of UNC-78. UNC-60B is normally localized in the diffuse cytoplasm and to the myofibrils in wild type but mislocalized to the actin aggregates in unc-78 mutants. Similar Unc-78 phenotypes are observed in both embryonic and adult muscles. Thus, AIP1 is an important regulator of actin filament organization and localization of ADF/cofilin during development of myofibrils.
Keywords: myofibrils, AIP1, ADF/cofilin, WD repeats, actin filament dynamics
Introduction
Myofibrils in striated muscles are highly organized forms of actin cytoskeleton. Actin is the major component of the thin filaments, and their assembly and maintenance require regulation of filament dynamics (Littlefield and Fowler 1998); but, the mechanisms that controls these processes are not understood. Body wall muscle of the nematode Caenorhabditis elegans has obliquely striated myofibrils, which has provided opportunities to study their assembly and function (Waterston 1988; Moerman and Fire 1997). In the regulation of thin filament assembly, UNC-60B, a muscle-specific actin depolymerizing factor (ADF)/cofilin isoform, is required for enhancing actin dynamics and proper actin assembly into myofibrils (Ono et al. 1999). This study supports an important function of ADF/cofilin during myofibril assembly in vertebrates, which has been suggested by the facts that ADF/cofilin is the major G-actin binding protein in embryonic chicken skeletal muscle (Nagaoka et al. 1996) and a muscle-specific cofilin isoform exists in mammals (Ono et al. 1994).
ADF/cofilins are a family of actin regulatory proteins that promote rapid turnover of the actin cytoskeleton (Bamburg 1999). ADF/cofilin enhances actin filament turnover by increasing the rate of depolymerization from the pointed ends (Carlier et al. 1997) and by severing F-actin, thereby increasing the number of ends (Maciver et al. 1991). Cooperative binding of ADF/cofilin to F-actin changes the twist of the filament (McGough et al. 1997) and weakens lateral contacts in the filament (McGough and Chiu 1999), which is a likely cause of the severing activity, whereas the structural basis of the depolymerizing activity is not known. These two activities can be uncoupled by several mutations (Moriyama and Yahara 1999; Pope et al. 2000; Ono et al. 2001). Importantly, genetic studies have shown that mutations that abolish only severing or F-actin binding by ADF/cofilin cause abnormal actin assembly in C. elegans (Ono et al. 1999) or defects in actin turnover and viability in yeast (Lappalainen and Drubin 1997; Lappalainen et al. 1997), whereas a mutation that only impairs the depolymerizing activity causes no apparent phenotype in yeast (Moriyama and Yahara 1999). In addition, ADF/cofilin is localized in lamellipodia of motile cells (Bamburg and Bray 1987; Svitkina and Borisy 1999) and is involved in increase in the number of free barbed ends at the leading edge (Chan et al. 2000). Thus, the severing activity of ADF/cofilin is crucial for its cellular function. However, the severing activity of purified ADF/cofilin is weak and could be due to a spontaneous breakage of the structurally distorted ADF/cofilin-bound filaments.
Recently, actin-interacting protein 1 (AIP1) has been characterized as a factor that rapidly disassembles ADF/cofilin-bound actin filaments. AIP1 is a conserved WD repeat protein, and was originally identified in yeast as one of several actin-interacting proteins from a two-hybrid screen (Amberg et al. 1995). AIP1 itself is a weak F-actin binding protein, whereas, in the presence of ADF/cofilin, binding of AIP1 to F-actin is enhanced and rapid disassembly of the filaments is induced (Aizawa et al. 1999; Okada et al. 1999; Rodal et al. 1999). The filament disassembly is based on severing rather than depolymerization from the ends (Aizawa et al. 1999; Okada et al. 1999). Genetic studies in yeast agree with the biochemical data. The temperature-sensitive lethality of a COF1 (the yeast cofilin gene) allele is suppressed by a multicopy plasmid containing AIP1 (Iida and Yahara 1999). AIP1 is not essential for viability, but a deletion of AIP1 is synthetic lethal in combination with mutant COF1 alleles (Iida and Yahara 1999; Rodal et al. 1999). In addition, AIP1 has been implicated in stress response in Physarum (Matsumoto et al. 1998), early Xenopus development (Okada et al. 1999), response to acoustic damage in the avian auditory epithelium (Adler et al. 1999), and several actin-dependent processes in Dictyostelium (Konzok et al. 1999). However, in spite of these observations, the role of AIP1 in the regulation of actin cytoskeleton has not been clear because deletions of the AIP1 genes in yeast and Dictyostelium do not cause apparent phenotypes in the actin filament organization of the mutant cells (Iida and Yahara 1999; Konzok et al. 1999; Rodal et al. 1999).
In this study, I demonstrate that the C. elegans unc-78 gene encodes an AIP1 homologue and is required for actin filament organization in muscle cells. Mutations in unc-78 cause accumulation of microfilaments in the muscle cells and slow movement of the mutant animals (Waterston et al. 1980; Zengel and Epstein 1980), which has suggested that unc-78 is important for myofibril organization. The results presented here provide the first genetic evidence that an AIP1-encoding gene is required for organized actin filament assembly in vivo.
Materials and Methods
Nematode Strains
Nematodes were grown at 20°C as described (Brenner 1974). The wild-type strain is N2. unc-78 alleles used are e1217, e1221, st43 (provided by Drs. P. Hippe and B. Waterston, Washington University, St. Louis, MO; Waterston et al. 1980), su135, su187 (provided by Dr. H. Epstein, Baylor College of Medicine, Houston, TX; Zengel and Epstein 1980), and gk27 (provided by Dr. E. Gilchrist, C. elegans Reverse Genetics Core Facility at the University of British Columbia, Vancouver, Canada). The original strain VC34 carrying the gk27 deletion was outcrossed four times with N2. unc-60 (s1309) was provided by Dr. D. Baille (Simon Fraser University, Burnaby, Canada). All strains used in this study are homozygous for each allele unless specified.
Sequencing of Genomic DNA
Genomic DNA fragments for C04F6.4 were amplified from the total DNA with Taq DNA polymerase as two pieces: a 3.0-kb fragment (exons 1–3) and a 1.1-kb fragment (exons 4–6). All the exons were sequenced with an ABI PRIZM dye terminator cycle sequencing kit and an ABI310 genetic analyzer (Applied Biosystems). Sequencing was performed on at least two independent PCR products to insure that there were no PCR-induced errors.
Phylogenetic Analysis
Sequence alignment and phylogenetic analysis of AIP1 proteins were performed by MEGALIGN (DNASTAR Inc.) with the Clustal method, using the PAM250 matrix. Sequence data are available from GenBank/EMBL/DDBJ under the following accession numbers: human WDR1 (AF020056), mouse WDR1 (AF020055), chicken WDR1 (AF020054), Xenopus AIP1 (AF124140), Drosophila (AAF49822), C. elegans K08F9.2 (CAB03187), Dictyostelium DAIP1 (U36936), Physarum p66 (U86011), Arabidopsis (AAD14533), Schizosaccharomyces pombe (CAB11489), Saccharomyces cerevisiae AIP1 (U35666).
Motility Assay
A motility assay was performed as described (Epstein and Thomson 1974) under specific conditions used previously (Ono et al. 1999). A t test was performed on the data of wild type and each mutant.
Phalloidin Staining
Nematodes were collected and washed with M9 buffer, fixed with 4% formaldehyde in 1x cytoskeleton buffer (10 mM MES-KOH, pH 6.1, 138 mM KCl, 3 mM MgCl2, 2 mM EGTA) containing 0.32 M sucrose (Cramer and Mitchison 1993) for 15 min, permeabilized with acetone at −20°C for 5 min, washed with PBS containing 0.5% Triton X-100 and 30 mM glycine (PBS-TG) for 10 min, and stained with 0.2 μg/ml tetramethylrhodamine-phalloidin (Sigma-Aldrich) in PBS-TG for 30 min. After washing with PBS-TG three times for 10 min each, they were mounted with ProLong Antifade (Molecular Probes).
Immunofluorescence Microscopy
Immunofluorescent staining of adult nematodes was performed as described (Finney and Ruvkun 1990). Embryos were obtained by a hypochlorite treatment of gravid adults (Epstein et al. 1993), fixed with 4% formaldehyde in 1x cytoskeleton buffer containing 0.32 M sucrose for 15 min, permeabilized with methanol at −20°C for 5 min, washed with PBS-TG for 10 min, and stained with antibodies diluted in 1% BSA in PBS-TG. Primary antibodies used are antiactin monoclonal antibody (C4; ICN Biomedicals) and anti–UNC-60B (Ono et al. 1999). They were visualized by labeling with secondary antibodies, Alexa488-labeled goat anti–mouse IgG (Molecular Probes) and Cy3-labeled donkey anti–rabbit IgG (Jackson ImmunoResearch Laboratories), respectively.
Results and Discussion
The unc-78 Gene Encodes a Homologue of AIP1
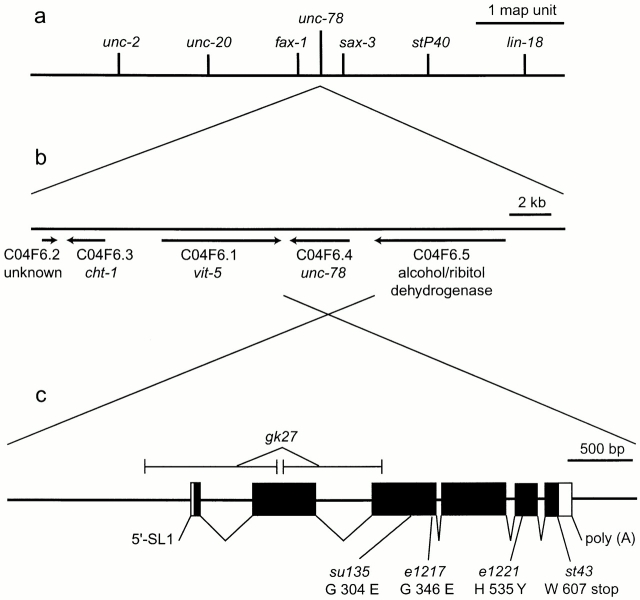
The unc-78 gene has been mapped to a position between fax-1 and sax-3 on the left arm of the X chromosome (Zallen et al. 1998; Much et al. 2000) (Fig. 1 a). Within this interval, a gene encoding a homologue of AIP1 (C04F6.4) has been predicted by the C. elegans Sequencing Consortium (Fig. 1 b). By sequencing the genomic DNA from five unc-78 alleles, I identified sequence alterations in C04F6.4 (Fig. 1 c). Furthermore, unc-78(gk27), a deletion allele of the C04F6.4 gene (Fig. 1 c), caused a similar but stronger Unc-78 phenotype than other unc-78 alleles (Fig. 2) and failed to complement unc-78(e1217). These results demonstrate that C04F6.4 is the unc-78 gene.
Figure 1.
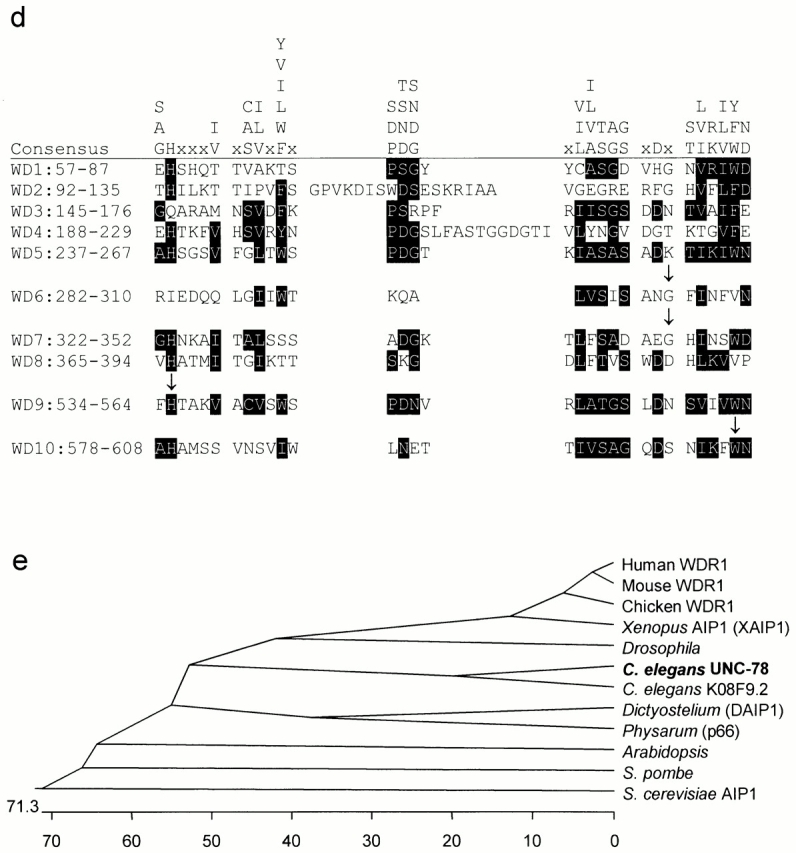
Genetic map and the structure of the unc-78 gene. (a) A part of the left arm of the X chromosome. (b) Arrangement of genes in the cosmid C04F6 (total 25 kb) predicted by the C. elegans Sequencing Consortium. (c) Exon–intron structure of the unc-78 gene (C06F6.4). Exons are indicated by boxes. The coding regions are represented by black filled regions. SL1 indicates a trans-splicing acceptor site. Locations of deletions and point mutations in unc-78 alleles are shown. (d) Putative WD repeats in UNC-78. Residues that match the consensus sequence (Smith et al. 1999) are indicated by black boxes. Arrows indicate residues that are altered by unc-78 mutations. (e) Phylogenetic analysis of AIP1 proteins. The scale shows the distance between sequences; units indicate the number of residue substitutions.
Figure 2.
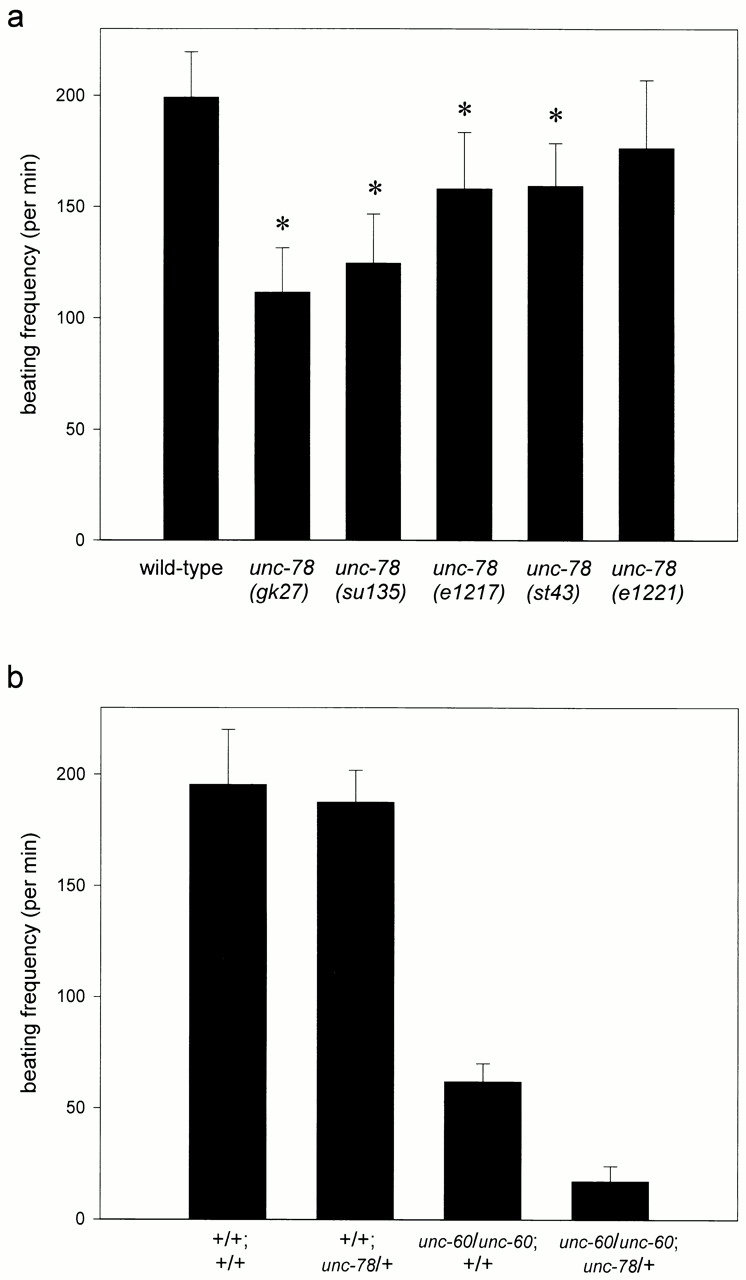
Motility defects by unc-78 mutations. (a) Motility of adult wild-type and unc-78 animals. *p < 0.05 by a t test. (b) Enhancement of the motility defect of unc-60(s1309) by the heterozygous unc-78(gk27) deletion. unc-78(gk27) was balanced by an X chromosome carrying lon-2 as a recessive marker. As controls, all other animals used in this assay are heterozygous for lon-2. Values are the means ± SD; n = 10.
The sequence of a full-length cDNA clone yk185g6 (provided by Y. Kohara, National Institute of Genetics, Mishima, Japan) of UNC-78 was determined (sequence data available from GenBank/EMBL/DDBJ under accession number AF324437) to verify the predicted exon–intron structure by the Sequencing Consortium and characterize the 5′- and 3′-untranslated regions. The UNC-78 protein (611 amino acids, 65 kD) contains 10 putative WD repeats (Fig. 1 d) and is 22–67% identical to those of AIP1 proteins (Fig. 1 e). The point mutations in the unc-78 alleles alter the sequence of WD repeats (Fig. 1c and Fig. d): they are located in WD6 (G304E; su135), WD7 (G346E; e1217), WD9 (H535Y; e1221 and su187), and WD10 (W607 to stop; st43). These mutations caused variable defects in motility of the homozygous animals (Fig. 2) in the order of su135, e1217, st43, and e1221 from the strongest to weakest, although the defects were no worse than those of the deletion mutant, unc-78(gk27). These residues are conserved not only in AIP1 proteins but also in WD repeats of other proteins (Smith et al. 1999), suggesting that the WD repeats are functionally important modules of UNC-78.
Searching through the C. elegans genome sequence, I found a second AIP1 isoform, K08F9.2, on chromosome V. This isoform is 67% identical in the amino acid sequence with UNC-78 and exhibits the highest homology with UNC-78 of all the AIP1 proteins (Fig. 1 e). So far, C. elegans is the only organism in which multiple AIP1 genes have been found. Drosophila, another multicellular organism for which we have a complete genome sequence, has only a single AIP1 gene (Goldstein and Gunawardena 2000).
Unc-78 Mutations Disrupt Actin Filament Organization in Body Wall Muscle
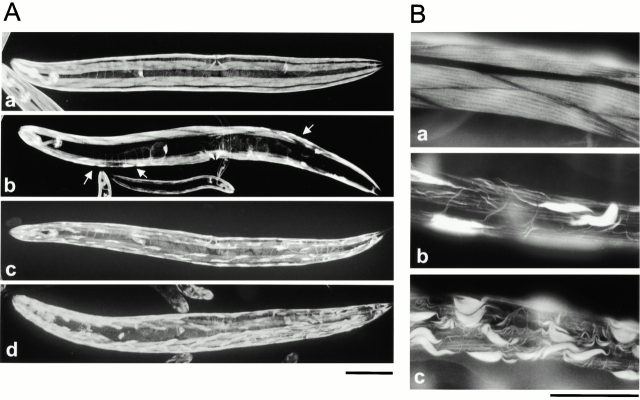
Waterston et al. 1980 reported that unc-78 mutants have large accumulations of thin filaments by electron microscopy. Phalloidin staining revealed that these aggregates contain F-actin (Fig. 3). In unc-78(e1217), striated organization of actin filaments was disorganized and marked aggregates were found in most of the body wall muscle cells (Fig. 3 A, c), whereas, in a weak mutant, unc-78(e1221), the phenotype appeared only in a subset of muscle cells, which randomly occurred throughout the body (Fig. 3 A, b, arrows). The deletion mutant, unc-78(gk27), exhibited a more severe phenotype having more actin aggregates in muscle cells than unc-78(e1217) (Fig. 3 A, d). At the light microscopic level, the phenotype was detected only in the body wall muscle. However, the promoter region of the unc-78 gene was active not only in body wall muscle but also in the pharynx and the spermatheca (data not shown), suggesting that the second AIP1 gene has a redundant function in some tissues.
Figure 3.
Actin filament organization in wild-type and unc-78 mutants were visualized by staining adult animals with phalloidin. (A) Micrographs at a low magnification of wild type (a), unc-78(e1221) (b), unc-78(e1217) (c), and unc-78(gk27) (d). Arrows in b indicate cells that contain actin aggregates. The difference in the body size is due to slightly different growth stages. unc-78 mutants were not significantly different in body size from wild type. (B) High magnification view of body wall muscle of wild type (a), unc-78(e1217) (b), and unc-78(gk27) (c). These micrographs show that actin filaments are disorganized in unc-78 mutants and that unc-78(gk27) causes a more severe phenotype than unc-78(e1217). Bars: (A) 0.1 mm; (B) 20 μm.
Under a higher magnification, the phenotype in unc-78 mutants was more noticeable (Fig. 3 B). In unc-78(e1217), actin filaments in the striated myofibrils were diminished, and large aggregates and small bundles were detected in the cytoplasm (Fig. 3 B, b), whereas most of the actin filaments were assembled in myofibrils in wild type (Fig. 3 B, a). unc-78(gk27) had more severe phenotype than other unc-78 mutants: actin aggregates and small bundles were more numerous (Fig. 3 B, c).
The extent of the phenotype in actin organization (Fig. 3) correlates well with the severity of motility defects (Fig. 2). This strongly suggests that regulation of actin filament assembly by UNC-78 is an important mechanism in the formation of myofibrils that are able to execute effective contraction. Biochemical analysis of wild-type and mutant UNC-78 proteins should reveal how UNC-78 regulates actin filament dynamics and contributes to the development and maintenance of myofibrils.
UNC-60B (ADF/Cofilin) Is Mislocalized in unc-78 Mutants
Accumulation of actin aggregates in unc-78 mutants resembles the phenotypes of ADF/cofilin mutants (unc-60) (Waterston et al. 1980; Ono et al. 1999), suggesting that the two genes are involved in the same genetic pathway. Therefore, I tested for a genetic interaction between unc-78 and unc-60. The unc-60(s1309) mutation reduces the actin-depolymerizing activity of UNC-60B, a muscle-specific ADF/cofilin isoform, and is homozygous viable (Ono, et al. 1999). However, the unc-60(s1309);unc-78(gk27) homozygotes were lethal at a late larval stage. In addition, heterozygous deletion of unc-78 strongly enhanced the motility defect of unc-60(s1309) (Fig. 2 b). This genetic interaction suggests that ADF/cofilin and AIP1 function together in muscle cells.
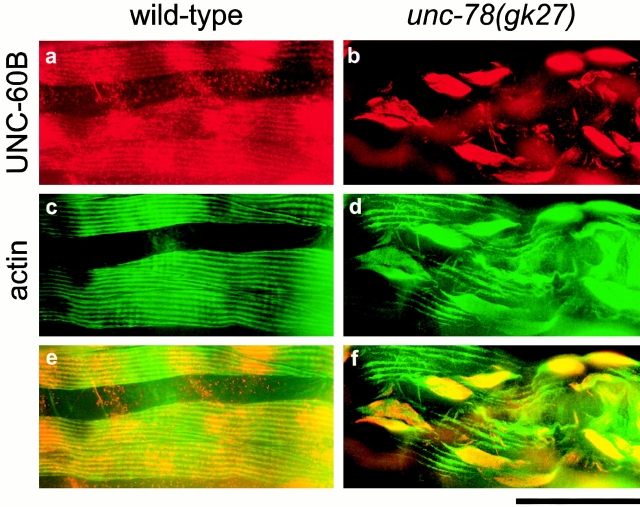
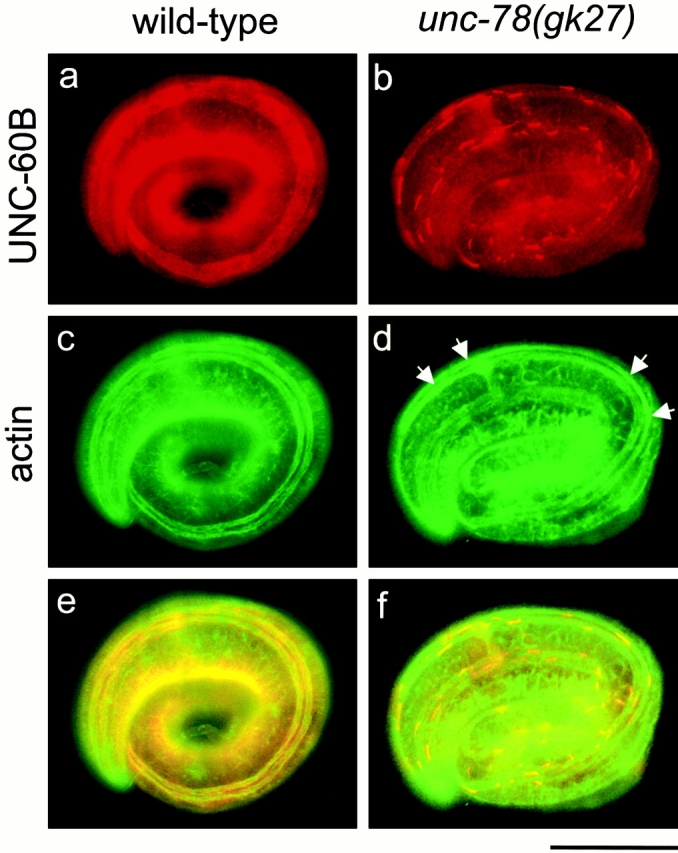
The localization of cofilin is altered in AIP1-null yeast cells (Iida and Yahara 1999; Rodal et al. 1999), but this is not the case in AIP1-null Dictyostelium cells (Konzok et al. 1999). In C. elegans, the localization of UNC-60B was altered in unc-78 mutants (Fig. 4). In wild type, UNC-60B was localized in the diffuse cytoplasm and to the myofibrils (Ono et al. 1999) (Fig. 4 a). However, in unc-78(gk27), the majority of UNC-60B was concentrated in the actin aggregates, and its diffuse and myofibrillar localization was reduced (Fig. 4 b). It should be noted that UNC-60B was associated with only part of the regions in the actin aggregates, which were often in the central core of the aggregates (Fig. 4 f). This may be due to the cooperative binding of UNC-60B with F-actin (Ono et al. 2001).
Figure 4.
Localization of UNC-60B and actin in adult body wall muscle. Wild type (a, c, and e) or unc-78(gk27) (b, d, and f) were double stained with anti–UNC-60B antibody (a and b) and antiactin antibody (c and d). Merged images of a and c (e) and b and d (f) are shown. Staining of UNC-60B in wild type often results in an uneven cytoplasmic staining pattern (a), which probably reflects insufficient fixation of the protein in the diffuse cytoplasm. Bar, 20 μm.
UNC-60B was mislocalized in unc-78 mutants during embryonic stages (Fig. 5) when myofibril assembly is very active. Accumulation of actin into striated myofibrils becomes evident after the threefold stage (∼520 min after the first cleavage) (Hresko et al. 1994) (Fig. 5 c). UNC-60B was mostly localized in the diffuse cytoplasm (Fig. 5 a). In unc-78(gk27) embryos, actin was assembled into myofibrils in a normal pattern, but slightly dense accumulations were formed along the myofibrils (Fig. 5 d, arrows). Remarkably, the localization of UNC-60B in the mutant was predominant in aggregated forms (Fig. 5 b). Some aggregates of UNC-60B were colocalized with the actin accumulations (Fig. 5b and Fig. f). However, many of them were associated with actin in the myofibrils without aggregates of actin, implying that these are the sites where abnormal actin filaments are built up later in development. These mutant phenotypes suggest that the role of UNC-78 is to disassemble UNC-60B–bound actin filaments, prevent formation of the aggregates, and maintain the dynamic state of their interactions.
Figure 5.
Localization of UNC-60B and actin in embryos. Wild type (a, c, and e) or unc-78(gk27) (b, d, and f) were double stained with anti–UNC-60B antibody (a and b) and antiactin antibody (c and d). Merged images of a and c (e) and b and d (f) are shown. Arrows in d indicate slightly dense accumulations of actin. Bar, 10 μm.
Conclusion
The results presented here demonstrate that UNC-78 is required for organized assembly of actin filaments into myofibrils and proper localization of UNC-60B in body wall muscle cells. This is the first genetic evidence that AIP1 regulates actin filament organization in vivo. In yeast and slime mold, AIP1-null cells do not show apparent disorganization of the actin cytoskeleton (Iida and Yahara 1999; Konzok et al. 1999; Rodal et al. 1999), although AIP1-null Dictyostelium cells are partially defective in cytokinesis, endocytosis, phagocytosis, and motility, which require actin dynamics (Konzok et al. 1999). Since myofibrils are highly organized forms of actin cytoskeleton, the processes of assembly and maintenance probably require tightly regulated actin filament dynamics, in which UNC-78/AIP1 is a critical factor.
Mislocalization of UNC-60B to actin aggregates in unc-78 mutants suggests that UNC-60B requires the activity of UNC-78 to enhance actin filament turnover. AIP1 and ADF/cofilin cooperatively disassemble actin filaments by severing in vitro (Introduction). UNC-60B itself is able to depolymerize and sever actin filaments (Ono and Benian 1998; Ono et al. 1999), but these activities are very weak. Therefore, aggregated forms of actin and UNC-60B in unc-78 mutants might be a consequence of insufficient actin turnover in the absence of the UNC-78 activity. Biochemical and cell biological analyses of wild-type and mutant UNC-78 will be needed to further characterize specific functions of UNC-78/AIP1 in the regulation of actin filament assembly.
Acknowledgments
The author thanks S. Langley for DNA sequencing, G. White for communicating data, and G. Benian for comments on the manuscript. Some nematode strains were provided by Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources.
This work was supported by grants from the American Heart Association to S. Ono.
Footnotes
Abbreviations used in this paper: ADF, actin depolymerizing factor; AIP1, actin-interacting protein 1; PBS-TG, PBS containing 0.5% Triton X-100 and 30 mM glycine.
References
- Adler H.J., Winnicki R.S., Gong T.W., Lomax M.I. A gene upregulated in the acoustically damaged chick basilar papilla encodes a novel WD40 repeat protein. Genomics. 1999;56:59–69. doi: 10.1006/geno.1998.5672. [DOI] [PubMed] [Google Scholar]
- Aizawa H., Katadae M., Maruya M., Sameshima M., Murakami-Murofushi K., Yahara I. Hyperosmotic stress-induced reorganization of actin bundles in Dictyostelium cells over-expressing cofilin. Genes Cells. 1999;4:311–324. doi: 10.1046/j.1365-2443.1999.00262.x. [DOI] [PubMed] [Google Scholar]
- Amberg D.C., Basart E., Botstein D. Defining protein interactions with yeast actin in vivo. Nat. Struct. Biol. 1995;2:28–35. doi: 10.1038/nsb0195-28. [DOI] [PubMed] [Google Scholar]
- Bamburg J.R. Proteins of the ADF/cofilin familyessential regulators of actin dynamics. Annu. Rev. Cell Dev. Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- Bamburg J.R., Bray D. Distribution and cellular localization of actin depolymerizing factor. J. Cell Biol. 1987;105:2817–2825. doi: 10.1083/jcb.105.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans . Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M.F., Laurent V., Santolini J., Melki R., Didry D., Xia G.X., Hong Y., Chua N.H., Pantaloni D. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnoverimplication in actin-based motility. J. Cell Biol. 1997;136:1307–1322. doi: 10.1083/jcb.136.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A.Y., Bailly M., Zebda N., Segall J.E., Condeelis J.S. Role of cofilin in epidermal growth factor-stimulated actin polymerization and lamellipod protrusion. J. Cell Biol. 2000;148:531–542. doi: 10.1083/jcb.148.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer L., Mitchison T.J. Moving and stationary actin filaments are involved in spreading of postmitotic PtK2 cells. J. Cell Biol. 1993;122:833–843. doi: 10.1083/jcb.122.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein H.F., Thomson J.N. Temperature-sensitive mutation affecting myofilament assembly in Caenorhabditis elegans . Nature. 1974;250:579–580. doi: 10.1038/250579a0. [DOI] [PubMed] [Google Scholar]
- Epstein H.F., Casey D.L., Ortiz I. Myosin and paramyosin of Caenorhabditis elegans embryos assemble into nascent structures distinct from thick filaments and multi-filament assemblages. J. Cell Biol. 1993;122:845–858. doi: 10.1083/jcb.122.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney M., Ruvkun G. The unc-86 gene product couples cell lineage and cell identity in C. elegans . Cell. 1990;63:895–905. doi: 10.1016/0092-8674(90)90493-x. [DOI] [PubMed] [Google Scholar]
- Goldstein L.S., Gunawardena S. Flying through the Drosophila cytoskeletal genome. J. Cell Biol. 2000;150:F63–F68. doi: 10.1083/jcb.150.2.f63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hresko M.C., Williams B.D., Waterston R.H. Assembly of body wall muscle and muscle cell attachment structures in Caenorhabditis elegans . J. Cell Biol. 1994;124:491–506. doi: 10.1083/jcb.124.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K., Yahara I. Cooperation of two actin-binding proteins, cofilin and Aip1, in Saccharomyces cerevisiae . Genes Cells. 1999;4:21–32. doi: 10.1046/j.1365-2443.1999.00235.x. [DOI] [PubMed] [Google Scholar]
- Konzok A., Weber I., Simmeth E., Hacker U., Maniak M., Müller-Taubenberger A. DAip1, a Dictyostelium homologue of the yeast actin-interacting protein 1, is involved in endocytosis, cytokinesis, and motility. J. Cell Biol. 1999;146:453–464. doi: 10.1083/jcb.146.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen P., Drubin D.G. Cofilin promotes rapid actin filament turnover in vivo. Nature. 1997;388:78–82. doi: 10.1038/40418. [DOI] [PubMed] [Google Scholar]
- Lappalainen P., Fedorov E.V., Fedorov A.A., Almo S.C., Drubin D.G. Essential functions and actin-binding surfaces of yeast cofilin revealed by systematic mutagenesis. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:5520–5530. doi: 10.1093/emboj/16.18.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield R., Fowler V.M. Defining actin filament length in striated musclerulers and caps or dynamic stability? Annu. Rev. Cell Dev. Biol. 1998;14:487–525. doi: 10.1146/annurev.cellbio.14.1.487. [DOI] [PubMed] [Google Scholar]
- Maciver S.K., Zot H.G., Pollard T.D. Characterization of actin filament severing by actophorin from Acanthamoeba castellanii . J. Cell Biol. 1991;115:1611–1620. doi: 10.1083/jcb.115.6.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S., Ogawa M., Kasakura T., Shimada Y., Mitsui M., Maruya M., Isohata M., Yahara I., Murakami-Murofushi K. A novel 66-kDa stress protein, p66, associated with the process of cyst formation of Physarum polycephalum is a Physarum homologue of a yeast actin-interacting protein, AIP1. J. Biochem. 1998;124:326–331. doi: 10.1093/oxfordjournals.jbchem.a022115. [DOI] [PubMed] [Google Scholar]
- McGough A., Chiu W. ADF/cofilin weakens lateral contacts in the actin filament. J. Mol. Biol. 1999;291:513–519. doi: 10.1006/jmbi.1999.2968. [DOI] [PubMed] [Google Scholar]
- McGough A., Pope B., Chiu W., Weeds A. Cofilin changes the twist of F-actinimplications for actin filament dynamics and cellular function. J. Cell Biol. 1997;138:771–781. doi: 10.1083/jcb.138.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman D.G., Fire A. Musclestructure, function, and development. In: Riddle D.L., Blumenthal T., Meyer B.J., Priess J.R., editors. C. elegans II. Cold Spring Harbor Laboratory Press; Plainview, NY: 1997. pp. 417–470. [PubMed] [Google Scholar]
- Moriyama K., Yahara I. Two activities of cofilin, severing and accelerating directional depolymerization of actin filaments, are affected differentially by mutations around the actin-binding helix. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:6752–6761. doi: 10.1093/emboj/18.23.6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Much J.W., Slade D.J., Klampert K., Garriga G., Wightman B. The fax-1 nuclear hormone receptor regulates axon pathfinding and neurotransmitter expression. Development. 2000;127:703–712. doi: 10.1242/dev.127.4.703. [DOI] [PubMed] [Google Scholar]
- Nagaoka R., Minami N., Hayakawa K., Abe H., Obinata T. Quantitative analysis of low molecular weight G-actin-binding proteins, cofilin, ADF and profilin, expressed in developing and degenerating chicken skeletal muscles. J. Muscle Res. Cell Motil. 1996;17:463–473. doi: 10.1007/BF00123362. [DOI] [PubMed] [Google Scholar]
- Okada K., Obinata T., Abe H. XAIP1a Xenopus homologue of yeast actin interacting protein 1 (AIP1), which induces disassembly of actin filaments cooperatively with ADF/cofilin family proteins. J. Cell Sci. 1999;112:1553–1565. doi: 10.1242/jcs.112.10.1553. [DOI] [PubMed] [Google Scholar]
- Ono S., Benian G.M. Two Caenorhabditis elegans actin depolymerizing factor/cofilin proteins, encoded by the unc-60 gene, differentially regulate actin filament dynamics. J. Biol. Chem. 1998;273:3778–3783. doi: 10.1074/jbc.273.6.3778. [DOI] [PubMed] [Google Scholar]
- Ono S., Minami N., Abe H., Obinata T. Characterization of a novel cofilin isoform that is predominantly expressed in mammalian skeletal muscle. J. Biol. Chem. 1994;269:15280–15286. [PubMed] [Google Scholar]
- Ono S., Baillie D.L., Benian G.M. UNC-60B, an ADF/cofilin family protein, is required for proper assembly of actin into myofibrils in Caenorhabditis elegans body wall muscle. J. Cell Biol. 1999;145:491–502. doi: 10.1083/jcb.145.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S., McGough A., Pope B.J., Tolbert V.T., Bui A., Pohl J., Benian G.M., Gernert K.M., Weeds A.G. The C-terminal tail of UNC-60B (ADF/cofilin) is critical for maintaining its stable association with F-actin and is implicated in the second actin-binding site. J. Biol. Chem. 2001;276:5952–5958. doi: 10.1074/jbc.M007563200. [DOI] [PubMed] [Google Scholar]
- Pope B.J., Gonsior S.M., Yeoh S., McGough A., Weeds A.G. Uncoupling actin filament fragmentation by cofilin from increased subunit turnover. J. Mol. Biol. 2000;298:649–661. doi: 10.1006/jmbi.2000.3688. [DOI] [PubMed] [Google Scholar]
- Rodal A.A., Tetreault J.W., Lappalainen P., Drubin D.G., Amberg D.C. Aip1p interacts with cofilin to disassemble actin filaments. J. Cell Biol. 1999;145:1251–1264. doi: 10.1083/jcb.145.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T.F., Gaitatzes C., Saxena K., Neer E.J. The WD repeata common architecture for diverse functions. Trends Biochem. Sci. 1999;24:181–185. doi: 10.1016/s0968-0004(99)01384-5. [DOI] [PubMed] [Google Scholar]
- Svitkina T.M., Borisy G.G. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell Biol. 1999;145:1009–1026. doi: 10.1083/jcb.145.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterston R.H. Muscle. In: Wood W.B., editor. The Nematode C. elegans. Cold Spring Harbor Laboratory Press; Plainview, NY: 1988. pp. 281–335. [Google Scholar]
- Waterston R.H., Thomson J.N., Brenner S. Mutants with altered muscle structure of Caenorhabditis elegans . Dev. Biol. 1980;77:271–302. doi: 10.1016/0012-1606(80)90475-3. [DOI] [PubMed] [Google Scholar]
- Zallen J.A., Yi B.A., Bargmann C.I. The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans . Cell. 1998;92:217–227. doi: 10.1016/s0092-8674(00)80916-2. [DOI] [PubMed] [Google Scholar]
- Zengel J.M., Epstein H.F. Identification of genetic elements associated with muscle structure in the nematode Caenorhabditis elegans . Cell Motil. 1980;1:73–97. doi: 10.1002/cm.970010107. [DOI] [PubMed] [Google Scholar]