Abstract
The urokinase-type plasminogen activator receptor (uPAR) is involved in the regulation of cell motility in a variety of cell types. We show here that expression of human uPAR in growing murine fibroblasts leads to a dramatic reorganization of the actin cytoskeleton. uPAR expression induces multiple rapidly advancing protrusions that resemble the leading edge of migrating cells. The cytoskeletal changes are independent of uPA and activation of the RGD-binding activity of integrins but require uPAR binding to vitronectin (VN). The actin reorganization is blocked by coexpression of dominant negative versions of either Rac (N17Rac) or p130Cas, but not by inhibitors of Cdc42 or Rho, and is accompanied by a Rac-dependent increase in cell motility. In addition, a fourfold increase in the level of activated Rac is induced by uPAR expression. We conclude that uPAR interacts with VN both to initiate a p130Cas/Rac-dependent signaling pathway leading to actin reorganization and increased cell motility and to act as an adhesion receptor required for these responses. This mechanism may play a role in uPAR-mediated regulation of cell motility at sites where VN and uPAR are co-expressed, such as malignant tumors.
Keywords: cell migration, Rho family GTPases, vitronectin, cytoskeleton, urokinase-type plasminogen activator receptor
Introduction
Cell migration is involved in many normal physiological processes such as embryonic development, wound healing, and mobilization of host defense against infections, whereas temporal and spatial deregulation of migration plays a major role in diseases such as cancer and inflammation. The regulation of cell motility is complex and involves numerous intracellular pathways with a dynamic reorganization of the actin cytoskeleton playing a decisive part (for reviews see Lauffenburger and Horwitz 1996; Mitchison and Cramer 1996).
The glycosyl phosphatidylinositol–anchored urokinase-type plasminogen activator receptor (uPAR) has an important role in the regulation of cell motility (for review see Andreasen et al. 1997). It was initially identified as a cellular binding site for the serine protease uPA (Stoppelli et al. 1985; Vassalli et al. 1985), but in addition it binds the extracellular matrix molecule vitronectin (VN) (Wei et al. 1994; Kanse et al. 1996) and a cleaved form of high molecular mass kininogen (Colman et al. 1997). The receptor also associates with integrins (for reviews see Chapman et al. 1999; Ossowski and Aguirre-Ghiso 2000), a newly identified member of the macrophage mannose receptor family (Behrendt et al. 2000), and intracellularly with the multifunctional cation-independent mannose 6-phosphate receptor (Nykjær et al. 1998). uPAR is extensively glycosylated and composed of three homologous domains (D1, D2, and D3) (for review see Behrendt et al. 1995). The binding of both uPA and VN to uPAR involves direct interaction with D1 but requires the integrity of the full-length receptor (Ploug et al. 1994; Behrendt et al. 1996; Kanse et al. 1996; Høyer-Hansen et al. 1997; Sidenius and Blasi 2000). The ligation of uPAR with uPA enhances uPAR affinity for VN; however, it is not essential for uPAR–VN interaction (Wei et al. 1994; Kanse et al. 1996; Høyer-Hansen et al. 1997; Aguirre Ghiso et al. 1999; Carriero et al. 1999).
uPAR appears to have several roles in cell motility regulation. It serves to localize proteolytic activity on the surface during invasive processes (for review see Mignatti and Rifkin 1993), but it can also function as a signaling receptor since proteolytically inactive uPA variants induce both chemotaxis/chemokinesis and a wide range of signal transduction effects (for reviews see Blasi 1999; Koshelnick et al. 1999; Ossowski and Aguirre-Ghiso 2000). A uPA-independent role for the receptor in the migration of leukocytes and epithelial cells has also been reported (Gyetko et al. 1994, Gyetko et al. 2000; Nguyen et al. 1999; Wilson and Gibson 2000).
Though the activation of the src-type kinase p56/p59hck, PKC, extracellular signal–regulated kinase (ERK), and phosphatidylinositol 3-kinase (PI3K) (Busso et al. 1994; Resnati et al. 1996; Fibbi et al. 1998; Nguyen et al. 1999; Kusch et al. 2000) have each been linked to a uPA-induced increase of cell motility in different cell types, the mechanism by which uPAR transduces signals to regulate cell migration remains largely uncharacterized. uPAR has been coimmunoprecipitated with αVβ5 as well as β1 or β2 integrins from a variety of cell lines, and direct in vitro binding between the integrin αMβ2/CR3/Mac-1 and uPAR has been shown, raising the possibility that integrins might function as transmembrane adaptors (Bohuslav et al. 1995; Wei et al. 1996; Xue et al. 1997; Aguirre Ghiso et al. 1999; Carriero et al. 1999; Chapman et al. 1999; Preissner et al. 2000). In addition, uPAR can regulate integrin function by direct association with integrin-containing complexes (Simon et al. 1996; Sitrin et al. 1996; Wei et al. 1996; Aguirre Ghiso et al. 1999; Carriero et al. 1999; Yebra et al. 1999). An additional complication to the role of uPAR in cell adhesion and motility is its ability to bind VN. Binding of multimeric or surface-absorbed forms of VN to uPAR has been demonstrated both in vitro with purified components and in vivo where the uPAR–VN interaction mediates cellular adhesion of cytokine-stimulated monocytes as well as uPAR-transfected HEK293 and erythroid progenitor cells (Wei et al. 1994; Deng et al. 1996; Kanse et al. 1996; Preissner et al. 2000; Sidenius and Blasi 2000).
The interactions of uPAR with components normally associated with cytoskeletal structures such as integrins and extracellular matrix molecules and its colocalization with integrins and cytoskeletal components such as vinculin at sites of cell–matrix contact (Hebert and Baker 1988; Pollanen et al. 1988; Myohanen et al. 1993; Wilcox et al. 1996; Kindzelskii et al. 1997; Xue et al. 1997) suggest that its role in cell motility may involve regulation of the actin cytoskeleton. Supporting this, uPA can induce uPAR-mediated changes in vascular smooth muscle cell morphology via a pertussis toxin and herbimycin A–sensitive pathway as well as a pathway involving Tyk2 and PI3K (Degryse et al. 1999; Kusch et al. 2000). In addition, uPAR appears to be necessary for the induction of αVβ5-dependent actin-rich protrusions in HT-1080 cells (Carriero et al. 1999).
In the regulation of the actin cytoskeleton, small GTPases of the Rho family play a pivotal role (for reviews see Van Aelst and D'Souza-Schorey 1997; Hall 1998). The best characterized members of this family, Rho, Rac, and Cdc42, regulate the assembly of stress fibers, lamellipodia/ruffles, and filopodia, respectively (Ridley and Hall 1992; Ridley et al. 1992; Kozma et al. 1995; Nobes and Hall 1995). In cell migration, Rac appears to have a critical role in regulating protrusive activity at the leading edge of cells, Cdc42 controls directional cues, and Rho appears to regulate aspects of cell adhesion and contraction (Allen et al. 1998; Nobes and Hall 1999).
We have investigated the role of uPAR in actin cytoskeleton and cell motility regulation in fibroblasts. We find that the interaction of uPAR with VN induces protrusions and increased cell motility and that these effects are dependent on activation of Rac.
Materials and Methods
Reagents
Mouse monoclonal antibodies R2, R3, and R9 against human uPAR were a gift from Drs. Ebbe Rønne and Gunilla Høyer-Hansen (Finsen Laboratory, Copenhagen, Denmark). Mouse monoclonal anti-VN 13H1 and human VN purified as described previously (Preissner et al. 1985) were gifts from Dr. Klaus Preissner (Justus Liebig University, Giessen, Germany). Rabbit polyclonal anti–human IgG (Nykjær et al. 1998) was a gift from Dr. Anders Nykjær (University of Aarhus, Aarhus, Denmark). Human uPA and diisopropylfluorophosphate (DFP)-treated human uPA were gifts from Dr. Peter Andreasen (University of Aarhus, Denmark). Mouse monoclonal 9E10 against the myc epitope was produced in house, biotin-conjugated 9E10 was from Cymbus Biotechnology, mouse monoclonal anti-Rac (clone 23A8) and rabbit polyclonal anti–glutathione S-transferase (GST) were from Upstate Biotechnology, mouse monoclonals antivinculin (VIN-11-5), antiphosphotyrosine (PT 66), as well as VN, fibronectin (FN), the phosphatidylinositol-specific PLC (PIPLC), rhodamine-conjugated phalloidin, and pertussis toxin were from Sigma-Aldrich. Mouse monoclonal antipaxillin was from Zymed Laboratories. Hamster monoclonal anti–mouse β3 (2C9.G2) and mouse monoclonal anti–human CD11b (ICRF44) were from BD PharMingen. FITC-conjugated goat anti–rabbit, FITC-conjugated goat anti–mouse, and TRITC-conjugated donkey anti–rabbit antibodies were purchased from Jackson ImmunoResearch Laboratories. C3 transferase protein and GST–PAK–CRIB fusion protein were produced as described previously (Sander et al. 1998; Nobes and Hall 1999). The peptides GRGDdSP and GPenGRGDSPCA were from GIBCO BRL. Herbimycin A, bisindolylmaleimide I, Gö 6983, and U71322 were from Calbiochem.
Cell Culture
Swiss 3T3 and COS-7 cells were grown and passaged in DME containing 10% FCS and penicillin/streptomycin (100 IU/ml and 100 μg/ml). For culture of NIH 3T3 cells, FCS was replaced by donor calf serum. Swiss 3T3 cells were used for passages 7–14 and NIH 3T3 cells for passages 11–20. For passaging, cells were washed twice in sterile PBS, trypsinized with 0.5 ml trypsin (2.5 mg/ml) (GIBCO BRL) for 1 min, and resuspended in DME plus 10% FCS.
Immunocytochemistry
Cells on coverslips were permeabilized by incubation in 0.2% Triton X-100 in PBS for 5 min, then incubated for 10 min in sodium borohydride (0.5 mg/ml). For double immunofluorescence staining, cells were incubated with the primary antibody (5–10 μg/ml) in PBS for 30 min, followed by incubation with the secondary FITC-conjugated antibody (1:200) and rhodamine-conjugated phalloidin (0.1 μg/ml) in PBS for 30 min. Coverslips were exposed to the above solutions by placing them upside down in drops of 50 μl of liquid. Between incubations, cells were washed five times by immersion in PBS. For three-way immunofluorescence detection of uPAR expression, myc-tagged construct expression, and actin changes, coverslips were first incubated in a solution of rabbit anti–human uPAR IgG and biotin-conjugated mouse monoclonal anti-myc (9E10) antibodies, followed by a solution of FITC–goat anti–rabbit antibody, Alexa-conjugated streptavidin (Molecular Probes), and rhodamine-conjugated phalloidin. Coverslips were mounted, analyzed, and photographed as described previously (Nobes and Hall 1999).
Cell Morphology Assays
For cell morphology assays with growing Swiss 3T3 cells in the presence of 10% FCS, cells were trypsinized as described above and plated on 13-mm glass coverslips at a density of 3 × 103 cells/coverslip and used for injection 16 h later. For assays under serum-free conditions, coverslips were coated with VN (10 μg/ml) or FN (50 μg/ml) for 1 h at 37°C, washed once in PBS and once in serum-free medium, and overlaid with 800 μl serum-free medium. Cells were then trypsinized as described above, pelleted by centrifugation at 1,000 rpm for 2 min, washed two times in serum-free medium, and seeded on coverslips at a density of 6 × 103 cells/coverslip. 2 h later, cells were used for injection. For assays with quiescent serum-starved Swiss 3T3 cells, cells were plated at 2 × 105 in 60-mm dishes in DME plus 5% FCS and antibiotics. 8 d later, the overlaying medium was collected, cells were trypsinized as above, the trypsin was inhibited by addition of 10 ml serum-free medium containing 0.5 mg/ml soybean trypsin inhibitor, and cells were pelleted by centrifugation at 1,000 rpm for 2 min and resuspended in serum-free medium. 25–70 μl of the cell suspension was added to coverslips coated with VN or FN as described above, and collected medium was added at a 1:40 dilution. Cells were used for injection 40 h later. Cells were injected with the expression plasmids pRc/CMV–uPAR, pRK5–myc-V12Rac, pRK5–myc-N17Cdc42, pRK5–myc-N17Rac, pRK5–myc-Wiskott-Aldrich syndrome protein (WASP)(201–321), pRK5-CD11b, pRK5-CD18 (Caron and Hall 1998), pSSRα-p130CasΔSD, pEBG–GST-p130CasΔSD (Mayer et al. 1995; Cho and Klemke 2000), or C3 transferase protein as indicated in the text and figure legends. 4 or 8 h after injection, coverslips were washed once in PBS, fixed for 9 min in 4% paraformaldehyde at room temperature, and washed again in 2× PBS. For some experiments, DFP–uPA, VN, antibodies, or RGD peptides were added to the coverslips as indicated in the figure legends. For some experiments, cells were pretreated with inhibitors for the indicated concentrations and times: pertussis toxin (100 ng/ml, 16 h), bisindolylmaleimide I or Gö 6983 (1 μM, 30 min), herbimycin A (1 μM, 16 h), PD98059 (60 μM, 30 min), or wortmannin (100 μM, 30 min).
Determination of Rac Activity
NIH 3T3 cells at 50% confluency were trypsinized, washed twice in Hebs buffer (20 mM Hepes, 137 mM NaCl, 5 m KCl, 0.7 mM Na2HPO4, 6 mM D-glucose, pH 7.5), resuspended in 1 ml Hebs with 20 μg of the desired plasmid DNA, and electroporated with a single pulse at 500 μF, 274 V using a Bio-Rad Laboratories Gene Pulser. 4, 8, or 16 h after transfection, cells were washed twice in ice-cold TBS (50 mM Tris, 150 mM Nacl, pH 7.5), transferred to 4°C, and lysed in Ripa buffer (TBS plus 1% Triton X-100, 0.5% sodium deoxycholate, 0.l% SDS, 10 mM MgCl2, 0.2 mM PMSF, 10 μg/ml aprotinin/leupeptin). Lysates were cleared by centrifugation at 13,000 rpm for 2 min, samples were withdrawn for analysis of total Rac, and the remaining lysate was incubated for 30 min at 4°C with 20 μg of a 50% slurry of GST–PAK–CRIB fusion protein on glutathione-coupled agarose beads. Beads were then washed three times in TBS plus 1% Triton X-100, 10 mM MgCl2, 0.2 mM PMSF, 10 μg/ml aprotinin/leupeptin. Equal amounts of protein from total cell lysates and corresponding amounts of the beads solution were then analyzed for Rac content by SDS-PAGE and immunoblotting using mouse monoclonal anti-Rac clone 23A8.
Adhesion Assays
NIH 3T3 cells were transfected by electroporation with the desired plasmids as described above. The green fluorescent protein (GFP) expression vector pEGFP-C1 (CLONTECH Laboratories, Inc.) was included to allow detection of transfected cells. For detachment assays, cells were seeded directly after electroporation on glass coverslips in DME containing 10% FCS at a density of 104 cells/coverslip. After 16 h, cells were treated with 5 mM EDTA in the same medium for 10 min and shaken gently. Cells were then washed in PBS and fixed as described above. For attachment assays, cells were trypsinized 24 h after transfection, resuspended in DME with 10% FCS in sterile tubes (no. 2097; Falcon), and incubated for 2 h at 37°C. Cells were then seeded on glass coverslips covered with medium containing additions as indicated in the figure legends. After 60 min, the coverslips were washed and fixed as described above. For both types of assay, the number of transfected adherent cells was determined by counting the number of GFP-positive cells in five fields at 100× magnification.
Time-lapse Videomicroscopy and Cell Motility
For time-lapse videomicroscopy of protrusion dynamics, growing cells on coverslips prepared as described above were injected with pRc/CMV–uPAR (100 μg/ml), then placed in DME plus 10% FCS and antibiotics, and buffered with 5 mM Hepes without sodium bicarbonate. Cells were allowed to recover for 1 h at 37°C at the atmospheric level of CO2 before filming. For analysis of cell motility, cells were injected as described above and allowed to express for 3 h before filming was commenced. Cells were filmed as described (Nobes and Hall 1999) at a rate of two frames per 10 s. Images for publishing were produced by grabbing single frames using ImageGrabber 1.16 software and further processed in Adobe Photoshop®.
Results
Effect of uPAR on Cell Morphology and the Actin Cytoskeleton
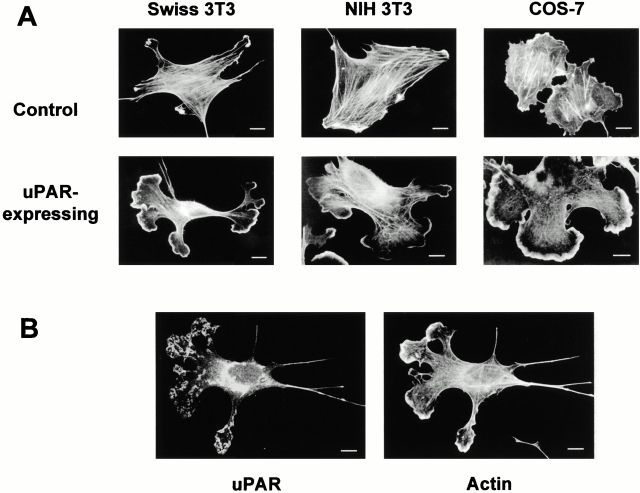
To analyze the effects of uPAR on the actin cytoskeleton, an expression vector for human uPAR was microinjected into murine fibroblasts. Since murine uPA does not bind to human uPAR (Appella et al. 1987; Estreicher et al. 1989; Quax et al. 1998), the specific effects of uPAR expression in the absence and presence of bound uPA could be analyzed. Surprisingly, uPAR expression by itself induced dramatic changes in the organization of the actin cytoskeleton in Swiss 3T3 cells growing in serum at subconfluent density. uPAR induced the appearance of multiple extending protrusions in 70–100% of the cells 4 h after injection (Fig. 1 A). The addition of human uPA (0.1–10 nM) had no observable effect on control or uPAR-expressing cells (data not shown). Time-course studies revealed that protrusions appeared 2 h after injection and were present for at least 16 h after injection. Control cells injected with FITC-dextran or an empty expression vector exhibited only a few minor protrusions similarly to uninjected cells. The actin reorganization induced by uPAR expression resembled that found at the leading edge of migrating cells, with an actin-dense lamellipodia at the leading edge of the protrusion and few actin structures in the rest of the protrusions (Fig. 1 A). uPAR itself was found in clusters partially colocalizing with the extending protrusions (Fig. 1 B). The observed effects were not due to cell type specificity since identical effects were observed in 70–100% of uPAR-expressing murine NIH 3T3 and african green monkey COS-7 cells (Fig. 1 A).
Figure 1.
Effect of uPAR expression on cell morphology. (A) Cells in growth medium (DME plus 10% FCS or donor calf serum) were injected with the expression plasmid pRc/CMV-uPAR (100 μg/ml) or with FITC-dextran for controls. As an additional control, cells were injected with an empty expression vector, and these cells were identical to FITC-dextran–injected cells (data not shown). 4 h after injection, the cells were fixed, uPAR-expressing cells were identified by double immunofluorescence with a rabbit polyclonal anti–human uPAR (Nykjær et al. 1998) followed by FITC goat anti–mouse IgG, and the actin cytoskeleton was visualized with rhodamine-phalloidin. Typical morphologies of the actin cytoskeleton in uPAR-expressing and control cells in three different cell lines are shown. (B) Localization of ectopically expressed uPAR. Swiss 3T3 cells injected with pRc/CMV-uPAR were fixed and stained as described in A. The localization of uPAR and the organization of the actin cytoskeleton are shown. Bars, 10 μm.
Since cells also reorganize their adhesion complexes during migration, we examined the effect of uPAR on these structures. In control cells, vinculin, a component of adhesion complexes, localized to classic focal adhesions (Fig. 2 A). uPAR expression resulted in its relocalization into smaller punctate complexes that appear at the leading edge of the extensions. These structures are similar to focal complexes described previously to be induced by activated Rac (Nobes and Hall 1995). Identical changes in the localization patterns were seen for other adhesion complex components such as paxillin and phosphotyrosine-containing proteins (data not shown).
Figure 2.
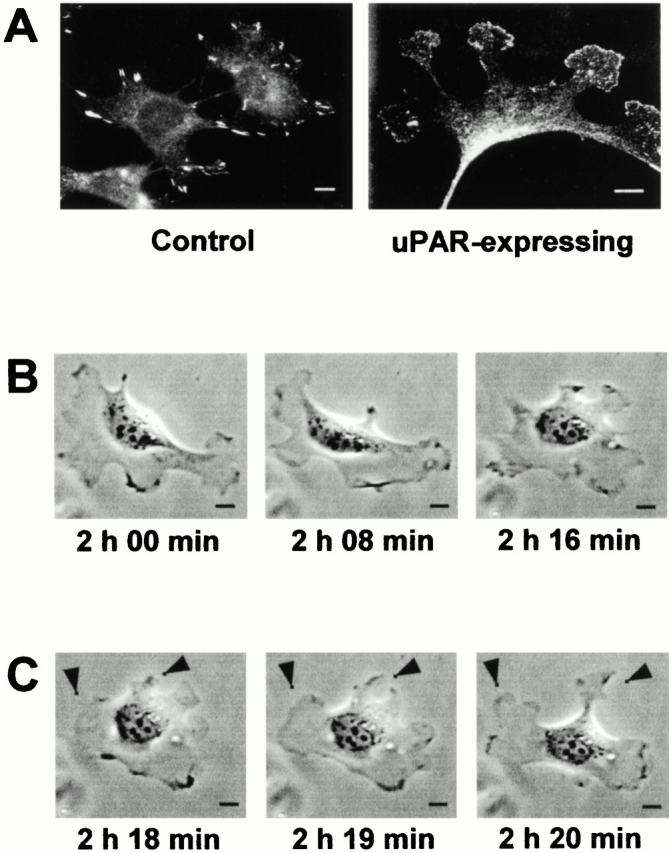
Effect of uPAR on vinculin localization and cell protrusion dynamics. (A) Swiss 3T3 cells were injected with pRc/CMV-uPAR (100 μg/ml) as described in the legend to Fig. 1. Vinculin localization was visualized by double immunofluorescence with mouse antivinculin (VIN-11-5) followed by FITC goat anti–mouse IgG. Representative examples of vinculin distribution in control and uPAR-expressing cells are shown. (B) Swiss 3T3 cells were injected with pRc/CMV-uPAR (100 μg/ml), allowed to recover for 1 h, and then analyzed by time-lapse videomicroscopy. A total of 15 individual cells were examined exhibiting identical responses. Images of a representative cell captured at the indicated times are shown. After time-lapse videomicroscopy, cells were fixed and stained to confirm uPAR expression. (C) Single protrusion dynamics in uPAR-expressing Swiss 3T3 cells. Cells were treated and analyzed as described in B. The extension of two separate protrusions within 2 min is indicated by arrowheads. Bars, 10 μm.
Time-lapse videomicroscopy revealed that the protrusions were transient and highly motile and as seen in Fig. 2B and Fig. C, the cell outline can completely change within a period of 15 min and a single protrusion can extend from the cell periphery within 2 min. These observations suggest that uPAR expression leads to the generation of signals that alter cell morphology through dynamic regulation of the actin cytoskeleton.
Extracellular Interactions of uPAR in Cytoskeletal Regulation
To confirm that the uPAR-induced cytoskeletal changes and protrusions are due to surface expression of the receptor, PIPLC which removes GPI-linked proteins from the cell surface was included in the assay. The PIPLC treatment removed all surface-expressed human uPAR as assessed by immunofluorescence studies on nonpermeabilized cells (data not shown). PIPLC treatment blocked the appearance of protrusions induced by uPAR expression (Fig. 3 A). The effect of PIPLC was not due to a general effect on protrusive activity, since similar protrusions, induced by activated Rac, were not inhibited by PIPLC treatment (see below). Further evidence for cell surface activity of uPAR in the induction of protrusions was obtained by examining the effect of domain-specific monoclonal antibodies against uPAR. The monoclonal antibody R2, which recognizes an epitope within domains D2 and D3 (Rønne et al. 1991; Gårdsvoll et al. 1999), had no effect, whereas the antibodies R3 and R9, which both recognize the D1 domain, inhibited protrusion induction by 80–100% (Fig. 3 A). We conclude that uPAR-induced protrusions require cell surface expression of uPAR and availability of uPAR D1.
Figure 3.
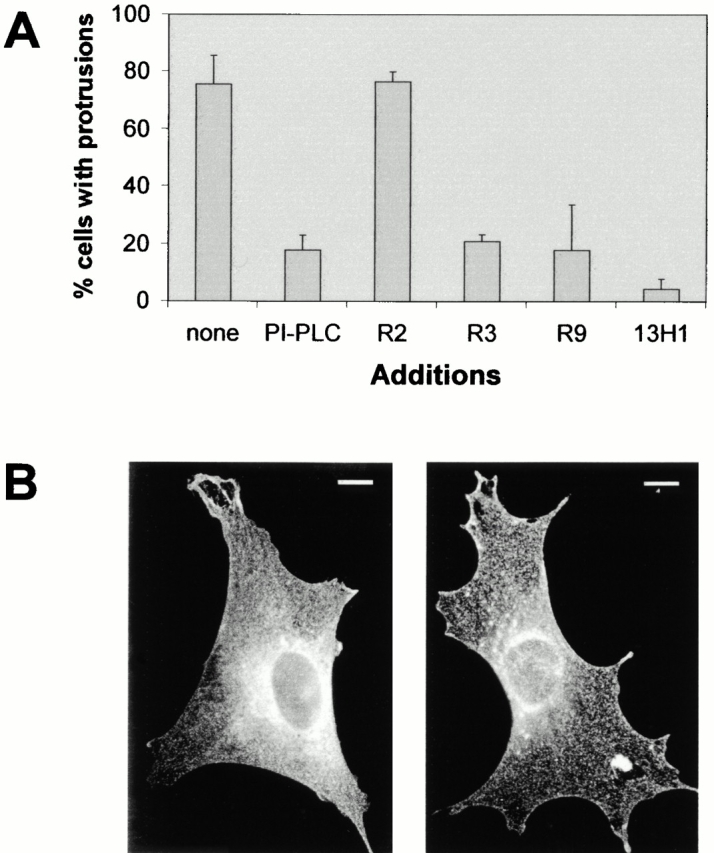
Role of uPAR interaction with VN in the induction of protrusions and uPAR localization. (A) Swiss 3T3 cells were injected with pRc/CMV-uPAR (100 μg/ml) and returned to growth medium without additions (none) or containing PIPLC (2 U/ml), mouse monoclonal antibody (Mab) against human uPAR clones R2, R3, and R9 (30 μg/ml), or mouse monoclonal antibody clone 13H1 (30 μg/ml) against VN as indicated. Cells were fixed and stained as described above and the number of uPAR-expressing cells exhibiting clearly identifiable protrusions was determined. Data are average ± SD for at least three experiments, each examining 100 injected cells. (B) uPAR-expressing cells treated with anti-uPAR R9 or anti-VN 13H1 were fixed, and uPAR localization was visualized as described in the legend to Fig. 1 A. Typical examples of uPAR distribution are shown. Bar, 10 μm.
Role of VN in uPAR-Mediated Cytoskeletal Rearrangement
The uPAR D1 domain has been reported to be essential for both VN and uPA binding (Ploug et al. 1994; Behrendt et al. 1996; Høyer-Hansen et al. 1997). Since murine uPA does not interact with the human receptor, the D1 dependence suggests a role for serum-derived VN in the uPAR-mediated induction of protrusions. The monoclonal anti–human VN antibody 13H1 has been shown previously to inhibit binding of human VN to uPAR (Kanse et al. 1996). Western blotting showed that this antibody also recognizes bovine VN (data not shown), and the 13H1 antibody totally abolished the uPAR-induced effects (Fig. 3 A). Interestingly, uPAR was localized in a homogenous punctate pattern throughout the cell when treated with antibodies against the uPAR D1 domain or VN (Fig. 3 B), indicating that VN binding affects uPAR localization.
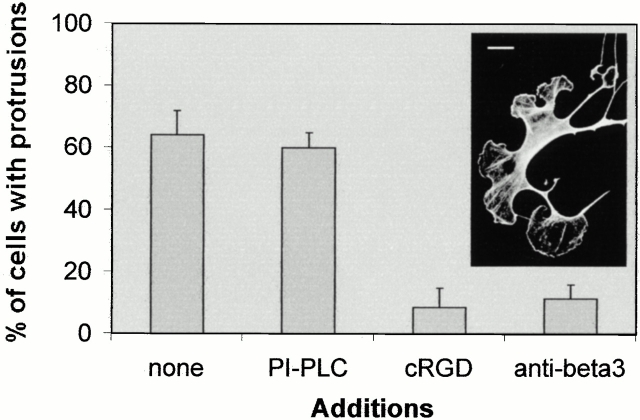
Since uPAR has been described to modulate integrin affinity (Chapman et al. 1999; Ossowski and Aguirre-Ghiso 2000; Preissner et al. 2000), we tested whether de novo binding of integrins to RGD-containing extracellular matrix proteins was necessary for the uPAR-induced response. The peptides GRGDdSP (RGDd) and GPenGRGDSPCA (cRGD), known to preferably interfere with integrin-mediated cell adhesion to FN and VN, respectively (Pierschbacher and Ruoslahti 1987), were added to cells before injection of the uPAR expression plasmid. Uninjected cells were less spread and contained fewer stress fibers than untreated cells (compare Fig. 4 B and Fig. 1 A), but the peptides did not inhibit the uPAR-mediated induction of protrusions, indicating the involvement of an adhesion receptor different from RGD-binding integrins (Fig. 4A and Fig. B). In addition, uPAR-induced protrusions were not affected by a function-blocking antibody to the integrin β3 subunit. In contrast, the cRGD and the anti-β3 antibody inhibited Rac-induced protrusions (see below).
Figure 4.
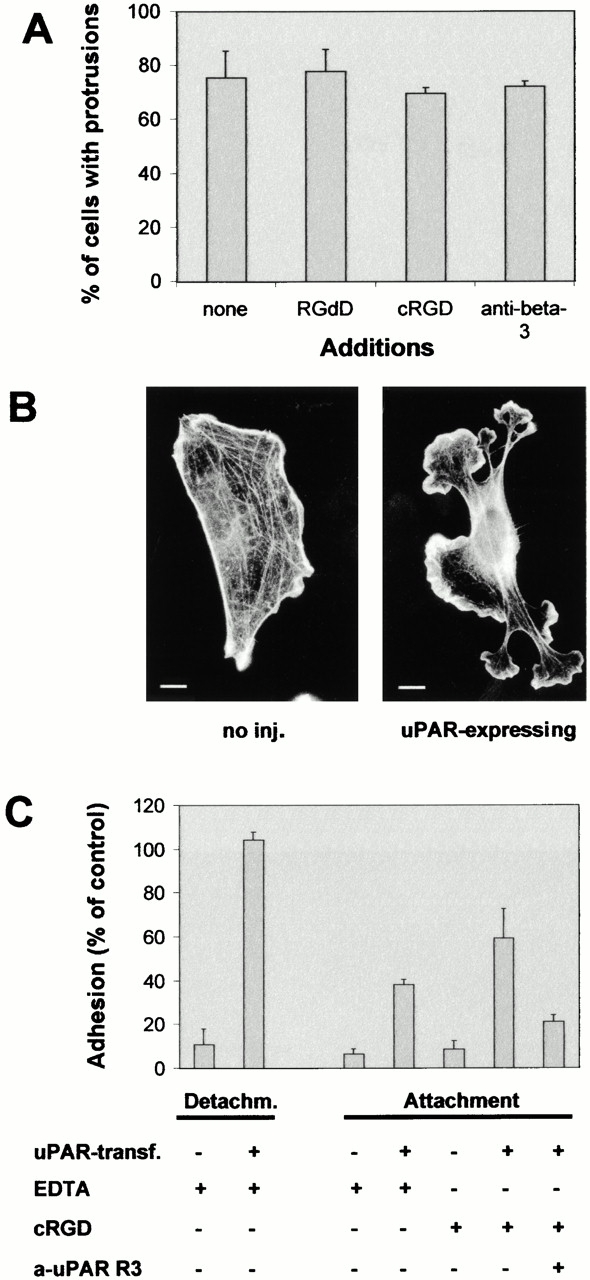
Role of RGD-binding integrins in uPAR-induced protrusions and adhesion. (A) Swiss 3T3 cells were injected with pRc/CMV-uPAR (100 μg/ml) and incubated in growth medium containing GRGDdSP (RGDd) (1.2 mM), GPenGRGDSPCA (cRGD) (0.05 mM), or hamster anti–mouse β3 integrin (clone 2C9.G2) (20 μg/ml) as indicated for 4 h before fixing and staining. The number of uPAR-expressing cells exhibiting clearly identifiable protrusions was determined. Data are average ± SD for at least three experiments, each examining 100 injected cells. (B) Swiss 3T3 cells were injected with FITC-dextran or pRC/CMV-uPAR and treated with cRGD (0.05 mM). The organization of the actin cytoskeleton was visualized as described above. Typical morphologies of uninjected (no inj.) or uPAR-expressing cells are shown. (C) NIH 3T3 cells transfected with pEGFP-C1 and empty vector or pRC/CMV-uPAR as indicated were subjected to detachment or attachment assays (see Materials and Methods for details) in the presence of 5 mM EDTA, 0.05 mM cRGD, and 20 μg/ml anti-uPAR R3 as indicated. Results are expressed as fraction of adherent cells relative to identically transfected cells in the absence of additions. In the absence of additions, the adhesion efficiencies of uPAR-transfected and control cells were indistinguishable (data not shown). Data are average ± SD for at least two experiments performed in triplicate. Bars, 10 μm.
uPAR has been demonstrated to mediate adhesion of different cell types to VN (Wei et al. 1994; Deng et al. 1996; Sidenius and Blasi 2000). To investigate if uPAR could function as an adhesion receptor in murine fibroblasts, NIH 3T3 cells were transfected with the expression plasmid for human uPAR. The uPAR-transfected NIH 3T3 cells were totally resistant to detachment from serum-coated coverslips with EDTA, whereas control cells were easily detached (Fig. 4 C). In addition, uPAR-expressing cells, but not parental NIH 3T3 cells, were able to attach to these coverslips in the presence of EDTA or the cRGD peptide. The attachment of uPAR-transfected cells in the presence of the cRGD peptide was inhibited by the uPAR R3 antibody that recognizes the uPAR D1 domain.
To investigate whether VN was sufficient to support uPAR-induced protrusions under serum-free conditions, cells in “log phase” were trypsinized, washed extensively in serum-free medium, and replated on either VN- or FN-coated coverslips in the absence of serum. 2 h after replating, the cells were injected with the expression plasmid for human uPAR. Only uPAR-expressing cells plated on VN exhibited protrusive structures that were essentially identical to those seen in the presence of serum, whereas uPAR expression in cells plated on FN did not produce protrusions (Fig. 5A and Fig. B). To determine whether soluble VN could induce a cytoskeletal response through its interaction with uPAR, VN (in the presence of 0.05 mM cRGD) was added back to control and uPAR-injected cells plated on FN. A strong induction of lamellipodia was observed specifically in the uPAR-expressing cells. In contrast to the effect of uPAR expression in cells plated on VN, and thus exposed to a substrate-attached reservoir of the ligand, the effect of soluble VN was transient with a strong response after 5 min of treatment but no effect after 4 h. Short- or long-term treatment with uPA had no observable effect (Fig. 5A and Fig. B; data not shown).
Figure 5.
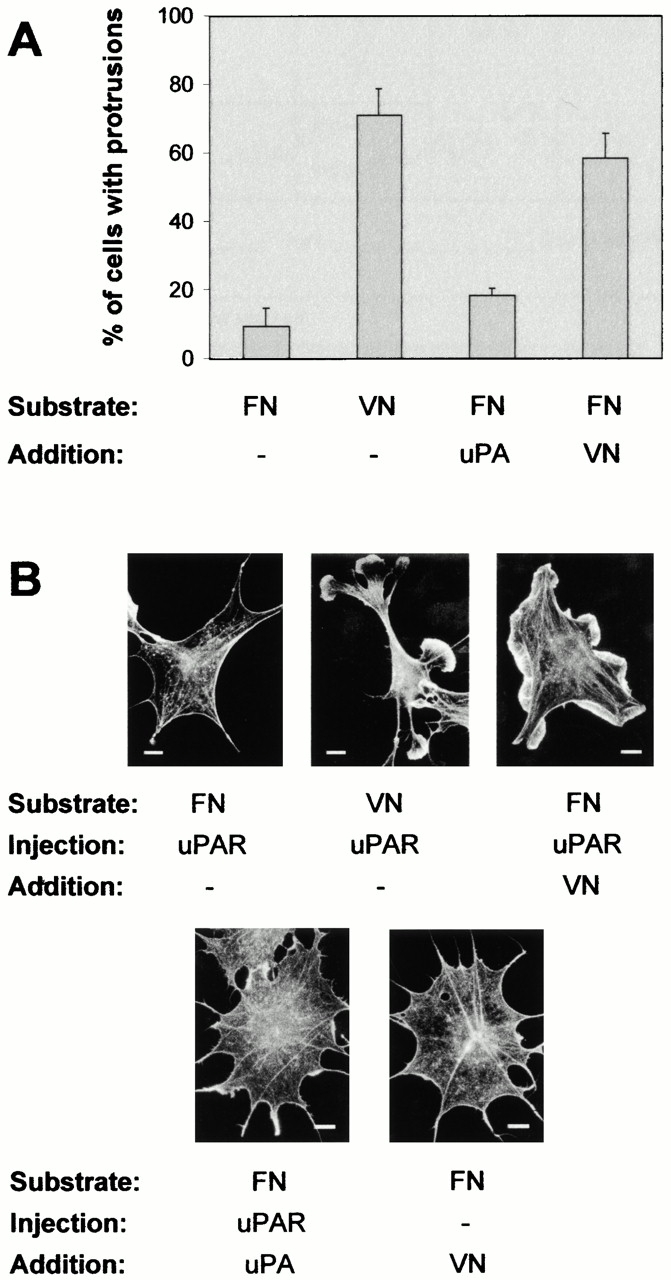
Role of extracellular matrix factors in uPAR-induced protrusions. (A) Growing Swiss 3T3 cells were trypsinized, washed two times in serum-free medium, and replated in serum-free medium on coverslips precoated with FN (50 μg/ml) or VN (10 μg/ml). 2 h after plating, cells were injected with pRc/CMV-uPAR. After 4 h of expression, cells were fixed, stained, and the number of uPAR-expressing cells with clearly identifiable protrusions/lamellipodia was determined as described above. In some cases, cells were treated with soluble VN (10 μg/ml) or DFP-uPA (10 nM) for 5 min in the presence of 0.05 mM cRGD before fixation. Data are average ± SD for at least three experiments, each examining 100 injected cells. (B) Cells plated on FN or VN as indicated were injected with pRC/CMV-uPAR, treated with soluble VN or DFP-uPA in the presence of 0.05 mM cRGD as indicated, then fixed and stained as described above. Typical morphologies of the actin cytoskeleton are shown. Bars, 10 μm.
Taken together, these results suggest that an interaction between uPAR domain D1 and VN is necessary and sufficient (a) to induce effects on the cytoskeleton in Swiss 3T3 cells and (b) to provide the adhesive force required for protrusions.
Role of Intracellular Signaling Pathways in uPAR Induction of Cytoskeletal Changes
Previous work has implicated pertussis toxin–inhibitable Src activation (Fazioli et al. 1997), PKC activation (Busso et al. 1994; Fibbi et al. 1998; Carriero et al. 1999), or ERK activation (Nguyen et al. 1999) as involved in uPAR-mediated effects on the actin cytoskeleton or cell motility. We tested the effect of pertussis toxin, the Src inhibitor herbimycin A, the PKC inhibitors bisindolylmaleimide I and Gö 6983, or the ERK inhibitors PD98059 and U0126 on the uPAR-induced changes. Herbimycin A treatment by itself induced cytoskeletal changes, making its effect on uPAR-induced changes difficult to interpret. None of the other treatments had any significant effects on the uPAR-induced response, although pertussis toxin did inhibit lysophosphatidic acid–induced ERK activation, bisindolylmaleimide I and Gö6983 inhibited PMA-induced ERK activation, and PD98059 and U0126 inhibited platelet-derived growth factor– and EGF-induced ERK activation (data not shown).
Since Rho GTPases play an instrumental role in actin cytoskeleton regulation, we analyzed their role in uPAR induction of protrusions. Coexpression of the dominant negative N17Rac led to complete inhibition of the induction of protrusions (Fig. 6 A). In addition, the reorganization of adhesion complexes was totally inhibited (data not shown). In contrast, coexpression of the dominant negative N17Cdc42 had little effect. To further analyze if Cdc42 activity was required for protrusion induction, the effect of a fragment of the Cdc42-specific effector WASP (WASP[201–321]), which has been demonstrated previously to inhibit Cdc42-induced responses (Nobes and Hall 1999), was also examined. WASP(201–321) had no significant effect on the induction of protrusions, even though it fully inhibited morphological changes induced by an activated mutant of Cdc42 (data not shown). Coinjection of recombinant C3 transferase protein to inhibit Rho activity had no effect on the ability of uPAR to induce protrusions, although it did inhibit stress fiber induction by activated Rho.
Figure 6.
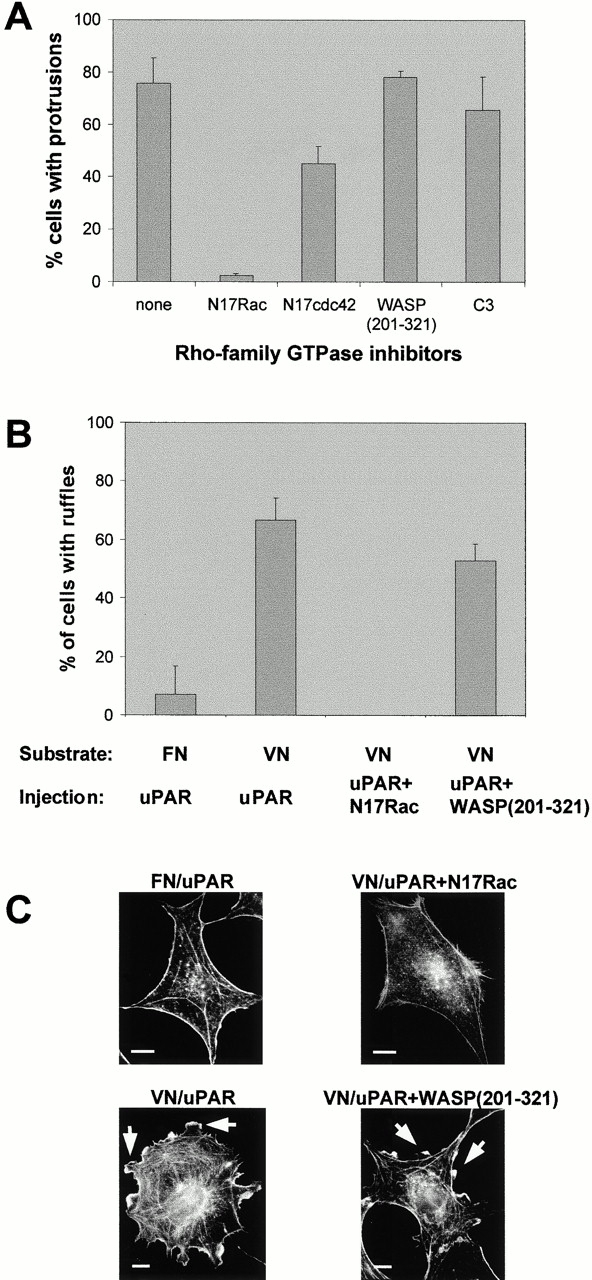
Role of Rho GTPases in uPAR-induced protrusions. (A) Swiss 3T3 cells in growth medium were coinjected with pRC/CMV-uPAR (100 μg/ml) and the expression plasmids pRK5–myc-N17Rac (20 μg/ml), pRK5–myc-N17cdc42 (100 μg/ml), or pRK5–myc-WASP(201–321) (40 μg/ml), or with 30 μg/ml of recombinant C3 transferase protein as indicated. After 4 h of expression, cells were fixed. uPAR expression was determined as described above. Expression of inhibitor constructs was verified by immunofluorescence staining with biotin-conjugated mouse anti-myc (clone 9E10) followed by Alexa-conjugated streptavidin. Data are average ± SD for at least three experiments, each examining 100 injected cells. (B) Subconfluent quiescent Swiss 3T3 cells plated on FN or VN were injected with pRC/CMV-uPAR (100 μg/ml) or coinjected with pRc/CMV-uPAR (100 μg/ml) and pRK5–myc-N17Rac (25 μg/ml) or pRK5–myc-WASP(201–321) (40 μg/ml) as indicated. After 3–8 h of expression, cells were fixed and stained as described above. The number of uPAR- or coexpressing cells with clearly identifiable lamellipodia was determined. Data are average ± SD for at least three experiments, each examining 100 injected cells. (C) Typical morphologies of quiescent, subconfluent Swiss 3T3 cells injected with expression plasmids as described in B. The morphology of the actin cytoskeleton as visualized by staining with rhodamine-phalloidin is shown. Typical lamellipodia are indicated by arrowheads. Bars, 10 μm.
PI3K is necessary for growth factor–mediated activation of Rac (Hawkins et al. 1995; Nobes et al. 1995), and Tyk2 and PI3K activation have recently been implicated in uPAR-mediated regulation of the actin cytoskeleton and cell motility (Kusch et al. 2000). Therefore, we have tested the effect of the PI3K inhibitor wortmannin on the induction of protrusions by uPAR. The inhibitor was shown to be active in inhibition of platelet-derived growth factor–induced ruffles in quiescent Swiss 3T3 cells, but it had no observable effect on the uPAR-induced protrusions (data not shown). We conclude that uPAR-induced protrusions are dependent on Rac but not on Cdc42 or Rho.
Effect of uPAR on Rac Activity
Activation of Rac in quiescent serum-starved Swiss 3T3 cells leads to the formation of lamellipodia and ruffles at the cell periphery (Ridley et al. 1992). To determine whether the interaction of VN with uPAR leads to Rac activation, quiescent serum-starved Swiss 3T3 cells were plated on FN or VN and injected with the uPAR expression plasmid. uPAR-expressing cells plated on VN exhibited a combination of patches of lamellipodia and small spikes at the circumference of the cell, whereas uPAR-expressing cells on FN were devoid of these actin structures (Fig. 6B and Fig. C). Time-lapse videomicroscopy revealed that the lamellipodia were actively extending structures, whereas the appearing spikes were due to a small retraction of the cell cortex (data not shown). The lamellipodia were dependent on activation of Rac since they were abolished by coexpression of N17Rac (Fig. 6B and Fig. C). In contrast, no significant reduction in lamellipodia was caused by WASP(201–321).
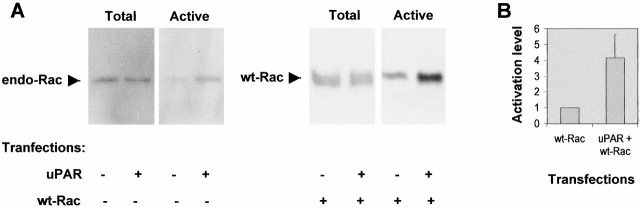
To investigate whether Rac activation could also be detected in growing cells where a strong protrusive activity was induced by uPAR, the level of GTP-bound Rac was determined. Growing NIH 3T3 cells were mock-transfected, transfected with uPAR, or cotransfected with uPAR and wild-type Rac. Activated Rac was precipitated from cell lysates by the Rac-binding domain of p21-activated kinase (PAK) fused to GST (Sander et al. 1998). As seen in Fig. 7 A, an increase in the activity of both endogenous Rac and cotransfected wild-type Rac could be detected in response to uPAR. In cells transfected with wild-type Rac, uPAR expression induced a fourfold activation of Rac when lysates were analyzed 16 h after transfection (Fig. 7 B). Assays performed 4, 8, and 16 h after transfection yielded similar results. We conclude that uPAR induces Rac activation.
Figure 7.
Effect of uPAR expression on Rac activity in growing NIH 3T3 cells. (A) NIH 3T3 cells were transfected by electroporation with pRC/CMV-uPAR and/or pRK5–myc-wtRac as indicated. PRK5-myc transfection was used as a control. The levels of total cellular Rac and GST–PAK–CRIB precipitated active Rac were determined by SDS-PAGE on 15% gels followed by immunoblotting for Rac. Results shown for endogenous Rac activation are for cells lysed 4 h after transfection. Results shown for cotransfected wtRac activation are for cells lysed 16 h after transfection. (B) The level of activated Rac from cells lysed 16 h after transfection with pRK5–myc-wtRac or pRK5–myc-wtRac and pRC/CMV-uPAR was quantified by density scanning using NIH Image software. The ratio of activated to total Rac was determined and results are expressed relative to the Rac activity in cells transfected with pRK5–myc-wtRac. Results are mean ± SD from three independent experiments.
To initiate an investigation into the mechanism by which uPAR activates Rac, we have focused on the possibility that an integrin might function as a transmembrane adaptor for uPAR signaling. The docking protein p130Cas is phosphorylated upon integrin activation and has been proposed to participate in Rac activation (for review see O'Neill et al. 2000). We therefore tested the effect of a dominant negative version of p130Cas (p130CasΔSD) on uPAR-induced protrusions. Coexpression of p130CasΔSD, which lacks the substrate domain for phosphorylation, strongly inhibited the uPAR-induced effect (Fig. 8 A), suggesting that it plays a role in the pathway leading to cytoskeletal changes.
Figure 8.
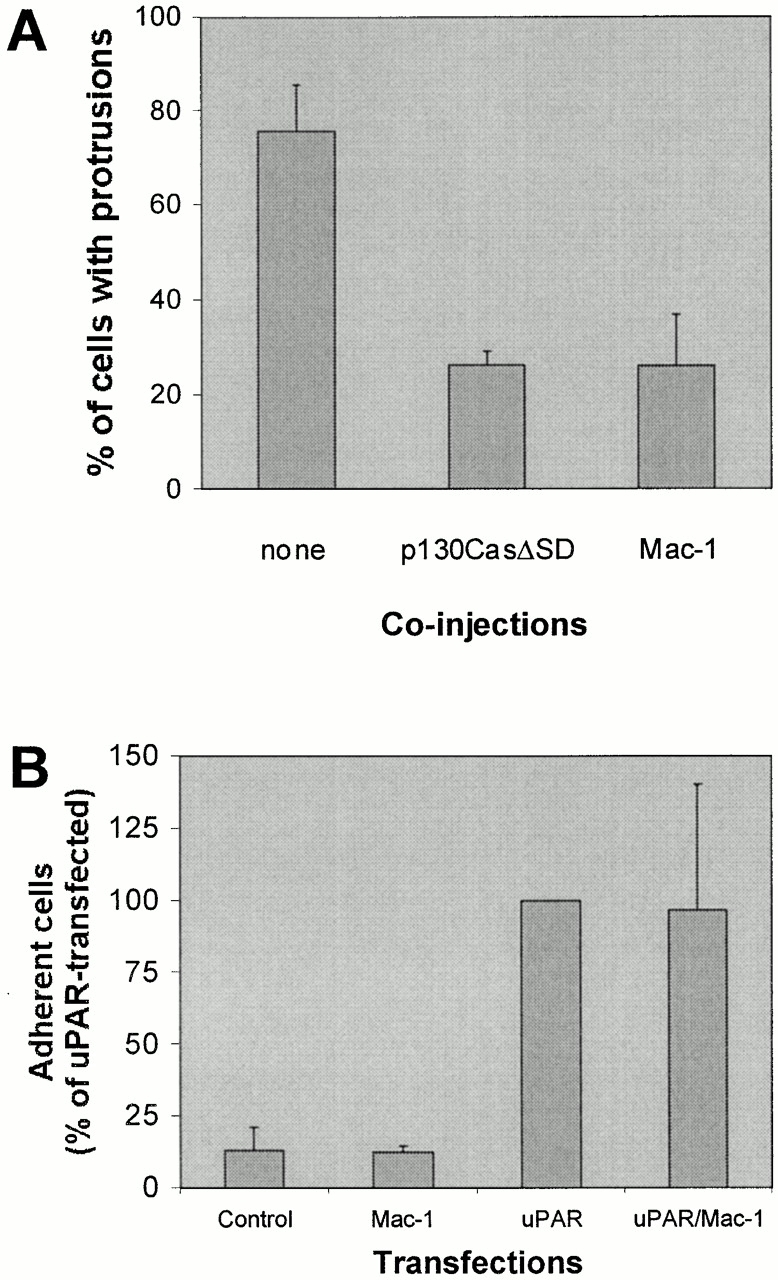
Effect of p130CasΔSD and Mac-1 on uPAR-induced protrusions. (A) Swiss 3T3 cells in growth medium were coinjected with pRC/CMV-uPAR (100 μg/ml) and either one of the expression plasmids pSSRα-p130CasΔSD/pEBG-GST-p130CasΔSD or both of the expression plasmids pRK5-CD11b and pRK5-CD18 (α- and β chain of Mac-1). After 4 h of expression, cells were fixed, stained, and the number of uPAR-expressing cells with clearly identifiable protrusions was determined as described above. Expression of inhibitor constructs was verified by immunofluorescence staining using anti-GST (rabbit polyclonal) or anti-CD11b (clone ICRF 44) as primary antibodies. Expression of untagged and GST-tagged p130CasΔSD gave identical results and the data were averaged for presentation. Data are average ± SD for at least three experiments, each examining 100 injected cells. (B) NIH 3T3 cells transfected with pEGFP-C1 and empty vector or pRC/CMV-uPAR and/or pRK5-CD11b and pRK5-CD18 as indicated were subjected to detachment assays (see Materials and Methods for details) in the presence of 5 mM EDTA. Results are expressed as the fraction of adherent cells relative to the number uPAR-transfected adherent cells under these conditions. Results are mean ± SD of three experiments each performed in triplicate.
The integrin Mac-1 has been shown to bind to uPAR (Wei et al. 1996; Simon et al. 2000), but it does not signal downstream to Rac (Caron and Hall 1998). To test whether Mac-1 could inhibit uPAR signaling to Rac, the effect of coexpression of Mac-1 with uPAR was examined. As seen in Fig. 8 A, Mac-1 strongly inhibited the uPAR-induced protrusions, whereas it did not affect the ability of uPAR to mediate adhesion to VN (Fig. 8 B). This suggests that it could block the interaction of uPAR with a membrane protein required for signaling.
Effect of Rac on Cell Morphology
We have found that expression of activated Rac in growing Swiss 3T3 cells also leads to the induction of protrusions (Fig. 9) similar to those induced by uPAR (although they have a less prominent actin meshwork at the leading edge and the cell body is almost totally devoid of stress fibers). Due to the morphological similarities between uPAR- and Rac-induced protrusions, we investigated whether they shared other characteristics. Rac-induced protrusions were not inhibited by p130CasΔSD or Mac-1 coexpression, indicating that these inhibitors function upstream of Rac (data not shown). In addition, Rac-induced protrusions were not sensitive to PIPLC treatment, but they were inhibited by treatment with the cyclic RGD peptide GPenSPRGDCA or by a function-blocking antibody to the integrin αVβ3 (Fig. 9). We conclude that in contrast to the uPAR-induced protrusions, where uPAR activates Rac and also acts as a direct adhesion receptor, the Rac-induced protrusions are dependent on αVβ3 binding to the extracellular matrix.
Figure 9.
Effect of V12Rac expression in growing Swiss 3T3 cells. Swiss 3T3 cells in growth medium were injected with pRK5–myc-V12Rac (100 μg/ml). Cells were then incubated in the absence or presence of GRGDdSP (RGDd) (1.2 mM), GPenGRGDSPCA (cRGD) (0.05 mM), or hamster anti–mouse β3 integrin (clone 2C9.G2) (20 μg/ml) as indicated for 4 h before fixing and staining. Rac-expressing cells were identified with mouse anti-myc (clone 9E10) followed by FITC goat anti–mouse antibodies, and the number of cells expressing clearly identifiable protrusions was determined. Data are average ± SD for at least three experiments, each examining 100 injected cells. The inset shows the typical morphology of the actin cytoskeleton in a V12Rac-expressing cell. Bar, 10 μm.
Effect of uPAR Expression on Cell Motility
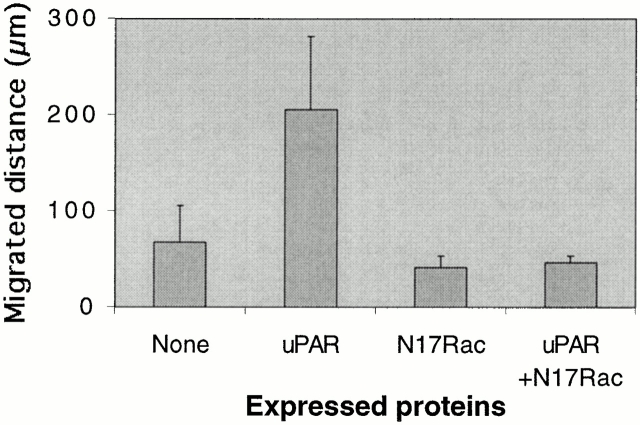
The effect of uPAR on cell motility was analyzed by time-lapse videomicroscopy. Cells were injected with expression plasmids as indicated in the legend to Fig. 10. 3 h after injection, cells were filmed for 3 h, fixed, and protein expression in the observed cells was confirmed by immunofluorescence. For quantification of motility, the position of the center of the cell nucleus was manually tracked at 2-min intervals throughout the 3-h period and the distance of its movement was calculated. uPAR expression induced a threefold increase in cell migration which was completely inhibited by coexpression of N17Rac. Examination of the migration paths revealed that both basal and uPAR-induced cell migration occurred in a nondirectional “random” manner with cells changing their course several times during the assay (data not shown). We conclude that the cytoskeletal changes induced by uPAR-mediated Rac activation are associated with an increase in cell motility.
Figure 10.
Effect of uPAR expression on cell motility. Swiss 3T3 cells in growth medium were injected with pRc/CMV-uPAR (100 μg/ml) and/or pRK5–myc-N17Rac (20 mg/ml), and the average distance migrated in 3 h was calculated. The effects of uPAR expression and the effect of N17Rac expression on the uPAR-induced increase in migration were found to be statistically significant by Student's t test (P < 0.0001). Results are mean ± SD from examination of at least 12 individual cells.
Discussion
Many studies have implicated uPAR as an important regulator of cell motility (Andreasen et al. 1997). Its effects extend beyond the localization of proteolytically active uPA at the cell surface to encompass both the induction of signaling events after ligation with proteolytically inactive uPA variants and a poorly characterized uPA-independent role (Gyetko et al. 1994; Blasi 1999; Chapman et al. 1999; Koshelnick et al. 1999; Gyetko et al. 2000; Ossowski and Aguirre-Ghiso 2000; Preissner et al. 2000; Wilson and Gibson 2000). Here we show that uPAR expression has major effects on the actin cytoskeleton leading to the induction of membrane protrusive activity and increased cell motility. Since murine uPA, produced by the fibroblasts used here, is unable to bind the expressed human uPAR (Appella et al. 1987; Estreicher et al. 1989; Quax et al. 1998), it appears that these effects are uPA independent. However, inhibition of uPAR binding to VN does block the uPAR-induced effects on the actin cytoskeleton and the addition of VN, but not uPA, to cells under serum-free conditions induces cytoskeletal changes. Furthermore, the uPAR-induced effects are independent of integrin binding to VN. We conclude that interaction of uPAR with VN is the extracellular event causing the observed effects on the actin cytoskeleton.
Intracellularly, we find that inhibition of pathways reported previously to be involved in uPA-induced morphology changes or cell motility such as activation of pertussis toxin–sensitive G proteins, PKC, ERK, or PI3K (Busso et al. 1994; Resnati et al. 1996; Fazioli et al. 1997; Carriero et al. 1999; Degryse et al. 1999; Nguyen et al. 1999; Kusch et al. 2000), has no effect on uPAR-induced cytoskeletal changes in Swiss 3T3 cells. In contrast, uPAR-induced actin reorganization and cell motility are completely inhibited by inhibition of the small GTPase Rac. Since uPAR expression also leads to Rac activation in quiescent and growing cells, we conclude that activation of Rac is a key event in the signaling cascade by which uPAR–VN interaction induces cytoskeletal rearrangement and increased cell motility.
Constitutively activated Rac induces morphological changes similar to those of uPAR. Intriguingly, both uPAR and Rac expression have very different effects depending on whether the cells are growing in serum or are quiescent and serum-starved. Both uPAR and Rac induce lamellipodia in quiescent Swiss 3T3 cells (Ridley et al. 1992; Fig. 7), but they give rise to advancing protrusions in growing Swiss 3T3 cells (Fig. 1 and Fig. 10). In uPAR-expressing cells, protrusions are inhibited by N17Rac, suggesting that Rac controls not only lamellipodia formation at the leading edge but also an activity responsible for membrane protrusion. We find that quiescent cells require pretreatment with serum for ∼12–18 h before uPAR or Rac expression can induce protrusions (data not shown). A similar dependence on serum or lysophosphatidic acid has been reported for the induction of cell surface protrusions and invasion by Cdc42 or Rac expression in T lymphoma cells (Stam et al. 1998). Those authors found that the effects were coupled to a requirement for Rho and PLC activity. Although we could not test the effect of the PLC inhibitor U73122 due to a toxic effect on subconfluent Swiss 3T3 cells, we found no requirement for Rho in the induction of protrusions. This indicates that in different cell types Rac can cooperate with different signaling pathways to induce membrane protrusive activity.
We also noted some important differences between Rac- and uPAR-induced protrusions. Extracellularly, the Rac-induced response is dependent on an integrin, in this case αvβ3, similarly to the previously reported requirement for α4β1 and α5β1 in Rac-induced T lymphocyte spreading on FN (D'Souza-Schorey et al. 1998). In contrast, the uPAR-induced cytoskeletal changes as well as uPAR-mediated adhesion occur in the presence of antagonists of RGD-binding integrins. Therefore, it appears that uPAR functions both as a mediator of signaling to the cytoskeleton and as an adhesion receptor through its interaction with VN. Such a dual function is well known for other adhesion receptors such as integrins (Giancotti and Ruoslahti 1999). Intracellularly, the uPAR-induced response is inhibited by p130CasΔSD but Rac-induced effects are not, suggesting that p130Cas participates in the uPAR-initiated pathway leading to Rac activation. Considering the nature of the intracellular pathway downstream of Rac, it is interesting to note that expression of a kinase-dead mutant of the Rac effector PAK1 leads to cell shape changes and motility patterns in NIH 3T3 cells (Sells et al. 1997, Sells et al. 1999) that are similar to those we observe after uPAR expression in these cells. Therefore, it is likely that PAK could play a role in uPAR-initiated signaling to the cytoskeleton that is independent of its kinase activity.
How does the uPAR–VN complex induce signaling to the cytoskeleton? VN-dependent clustering of uPAR in HT 1080 cells has been reported previously (Ciambrone and McKeown-Longo 1992), and we have observed that inhibition of the uPAR–VN interaction, by monoclonal antibodies or by plating the cells on FN, resulted in a change in uPAR distribution from large clusters into smaller punctate structures. Since addition of uPA or clustering of uPAR by antibodies has been reported to induce several different signaling events (Resnati et al. 1996; Koshelnick et al. 1997; Sitrin et al. 1999), it is possible that VN could induce signaling through uPAR clustering.
VN is also known to promote colocalization of VN-binding integrins with uPAR (Xue et al. 1997), and uPAR has been shown to modulate integrin affinity by a mechanism that might involve direct interaction (Simon et al. 1996; Sitrin et al. 1996; Wei et al. 1996; Aguirre Ghiso et al. 1999). The inability of RGD peptides to block the uPAR-induced response and the fact that uPAR does not affect adhesion complexes in the presence of N17Rac argue against a direct activation or inactivation of the matrix-binding function of integrins as the cause for the cytoskeletal rearrangements shown here. However, it is possible that one or more integrins could function as transmembrane adaptors to propagate a uPAR-induced signal. The ability of p130CasΔSD and Mac-1 to inhibit the induction of protrusions is consistent with this model. Blasi and coworkers have shown that exposure of a peptide sequence corresponding to amino acids 84–95 (between domains D1 and D2) of uPAR can induce chemotaxis as well as cytoskeletal changes in monocytic and smooth muscle cells (Resnati et al. 1996; Degryse et al. 1999), leading to the suggestion that this sequence might interact with a transmembrane adaptor. It is possible that uPAR binding to VN induces a conformational change that either directly exposes that sequence or makes the region between uPAR domains D1 and D2 accessible to protease cleavage and subsequent exposure. The identification of the molecular mechanism propagating VN–uPAR-induced signaling across the membrane to the cytoskeleton is an important task for the future.
Previously, the interaction of uPAR with VN has been suggested to play both positive and negative roles in cell motility regulation. In some cases, increased uPAR-mediated adhesion to VN induced by uPA was found to inhibit cell migration on VN (Stahl and Mueller 1997; Waltz et al. 1997). However, uPAR expression also induces haptotactic migration of HEK293 or MCF-7 cells onto VN- or serum-coated surfaces in a uPA-independent manner (Wei et al. 1996; Nguyen et al. 1999). In addition, endogenous uPAR is required for uPA-independent monocyte chemotaxis, neutrophil recruitment, and epithelial cell wound healing (Gyetko et al. 1994, Gyetko et al. 2000; Wilson and Gibson 2000). It is tempting to speculate that some or all of these effects might involve activation of an intracellular pathway similar to the one we describe here. In line with this suggestion, the uPAR-induced effects in MCF-7 cells are insensitive to the inhibition of ERK activation similarly to our observation in fibroblasts, whereas a uPA-induced increase in MCF-7 motility is not (Nguyen et al. 1999).
Overexpression of uPAR has been widely demonstrated in several types of tumors and is often associated with poor prognosis (for review see Andreasen et al. 1997). Furthermore, evidence that VN is expressed at relatively high levels in at least some types of cancer, such as hepatocellular carcinoma and gliomas, is accumulating (Gladson and Cheresh 1991; Gladson et al. 1995; Maenpaa et al. 1997; Kondoh et al. 1999). Since Rac is essential for cellular invasion in several model systems (Michiels et al. 1995; Keely et al. 1997; Shaw et al. 1997; Stam et al. 1998; Banyard et al. 2000; Cho and Klemke 2000), this raises the possibility that uPAR–VN-induced activation of Rac could play a role in tumor invasion and cancer metastasis. It will now be important to determine the role of VN–uPAR-induced Rac activation in a physiological context and to characterize further the transmembrane proteins and intracellular signaling molecules mediating this activation.
Acknowledgments
We thank Drs. Peter Andreasen, Gunilla Høyer-Hansen, Richard L. Klemke, Anders Nykjær, Jayesh Patel, Klaus Preissner and Ebbe Rønne for their gifts of reagents and Dr. Richard F. Lamb for helpful discussions and suggestions.
L. Kjøller was supported by a fellowship from the European Molecular Biology Organization and by the Danish Cancer Society. A. Hall and L. Kjøller thank the Cancer Research Campaign for its generous support.
Footnotes
L. Kjøller's present address is The Finsen Laboratory, Rigshospitalet, Strandboulevarden 49, Bldg. 7.2., DK-2100 Copenhagen Ø, Denmark. Tel.: 45-3545-5615. Fax: 45-3538-5450.
Abbreviations used in this paper: DFP, diisopropylfluorophosphate; ERK, extracellular signal–regulated kinase; FN, fibronectin; GFP, green fluorescent protein; GST, glutathione S-transferase; PAK, p21-activated kinase; PI3K, phosphatidylinositol 3-kinase; PIPLC, phosphatidylinositol-specific PLC; uPA, urokinase-type plasminogen activator; uPAR, uPA receptor; VN, vitronectin; WASP, Wiskott-Aldrich syndrome protein.
References
- Aguirre Ghiso J.A., Kovalski K., Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J. Cell Biol. 1999;147:89–104. doi: 10.1083/jcb.147.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen W.E., Zicha D., Ridley A.J., Jones G.E. A role for Cdc42 in macrophage chemotaxis. J. Cell Biol. 1998;141:1147–1157. doi: 10.1083/jcb.141.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen P.A., Kjøller L., Christensen L., Duffy M.J. The urokinase-type plasminogen activator system in cancer metastasisa review. Int. J. Cancer. 1997;72:1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Appella E., Robinson E.A., Ullrich S.J., Stoppelli M.P., Corti A., Cassani G., Blasi F. The receptor-binding sequence of urokinase. A biological function for the growth-factor module of proteases. J. Biol. Chem. 1987;262:4437–4440. [PubMed] [Google Scholar]
- Banyard J., Anand-Apte B., Symons M., Zetter B.R. Motility and invasion are differentially modulated by Rho family GTPases. Oncogene. 2000;19:580–591. doi: 10.1038/sj.onc.1203338. [DOI] [PubMed] [Google Scholar]
- Behrendt N., Ronne E., Danø K. The structure and function of the urokinase receptor, a membrane protein governing plasminogen activation on the cell surface. Biol. Chem. Hoppe Seyler. 1995;376:269–279. [PubMed] [Google Scholar]
- Behrendt N., Ronne E., Danø K. Domain interplay in the urokinase receptor. Requirement for the third domain in high affinity ligand binding and demonstration of ligand contact sites in distinct receptor domains. J. Biol. Chem. 1996;271:22885–22894. doi: 10.1074/jbc.271.37.22885. [DOI] [PubMed] [Google Scholar]
- Behrendt N., Jensen O.N., Engelholm L.H., Mørtz E., Mann M., Danø K. A urokinase receptor-associated protein with specific collagen binding properties. J. Biol. Chem. 2000;275:1993–2002. doi: 10.1074/jbc.275.3.1993. [DOI] [PubMed] [Google Scholar]
- Blasi F. The urokinase receptor. A cell surface, regulated chemokine. APMIS. 1999;107:96–101. doi: 10.1111/j.1699-0463.1999.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Bohuslav J., Horejsi V., Hansmann C., Stockl J., Weidle U.H., Majdic O., Bartke I., Knapp W., Stockinger H. Urokinase plasminogen activator receptor, beta 2-integrins, and Src-kinases within a single receptor complex of human monocytes. J. Exp. Med. 1995;181:1381–1390. doi: 10.1084/jem.181.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busso N., Masur S.K., Lazega D., Waxman S., Ossowski L. Induction of cell migration by pro-urokinase binding to its receptorpossible mechanism for signal transduction in human epithelial cells. J. Cell Biol. 1994;126:259–270. doi: 10.1083/jcb.126.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron E., Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- Carriero M.V., Del Vecchio S., Capozzoli M., Franco P., Fontana L., Zannetti A., Botti G., D'Aiuto G., Salvatore M., Stoppelli M.P. Urokinase receptor interacts with alpha(v)beta5 vitronectin receptor, promoting urokinase-dependent cell migration in breast cancer. Cancer Res. 1999;59:5307–5314. [PubMed] [Google Scholar]
- Chapman H.A., Wei Y., Simon D.I., Waltz D.A. Role of urokinase receptor and caveolin in regulation of integrin signaling. Thromb. Haemost. 1999;82:291–297. [PubMed] [Google Scholar]
- Cho S.Y., Klemke R.L. Extracellular-regulated kinase activation and CAS/Crk coupling regulate cell migration and suppress apoptosis during invasion of the extracellular matrix. J. Cell Biol. 2000;149:223–236. doi: 10.1083/jcb.149.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciambrone G.J., McKeown-Longo P.J. Vitronectin regulates the synthesis and localization of urokinase-type plasminogen activator in HT-1080 cells. J. Biol. Chem. 1992;267:13617–13622. [PubMed] [Google Scholar]
- Colman R.W., Pixley R.A., Najamunnisa S., Yan W., Wang J., Mazar A., McCrae K.R. Binding of high molecular weight kininogen to human endothelial cells is mediated via a site within domains 2 and 3 of the urokinase receptor. J. Clin. Invest. 1997;100:1481–1487. doi: 10.1172/JCI119669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degryse B., Resnati M., Rabbani S.A., Villa A., Fazioli F., Blasi F. Src-dependence and pertussis-toxin sensitivity of urokinase receptor-dependent chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. Blood. 1999;94:649–662. [PubMed] [Google Scholar]
- Deng G., Curriden S.A., Wang S., Rosenberg S., Loskutoff D.J. Is plasminogen activator inhibitor-1 the molecular switch that governs urokinase receptor–mediated cell adhesion and release? J. Cell Biol. 1996;134:1563–1571. doi: 10.1083/jcb.134.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza-Schorey C., Boettner B., Van Aelst L. Rac regulates integrin-mediated spreading and increased adhesion of T lymphocytes. Mol. Cell. Biol. 1998;18:3936–3946. doi: 10.1128/mcb.18.7.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estreicher A., Wohlwend A., Belin D., Schleuning W.D., Vassalli J.D. Characterization of the cellular binding site for the urokinase-type plasminogen activator. J. Biol. Chem. 1989;264:1180–1189. [PubMed] [Google Scholar]
- Fazioli F., Resnati M., Sidenius N., Higashimoto Y., Appella E., Blasi F. A urokinase-sensitive region of the human urokinase receptor is responsible for its chemotactic activity. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:7279–7286. doi: 10.1093/emboj/16.24.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibbi G., Caldini R., Chevanne M., Pucci M., Schiavone N., Morbidelli L., Parenti A., Granger H.J., Del Rosso M., Ziche M. Urokinase-dependent angiogenesis in vitro and diacylglycerol production are blocked by antisense oligonucleotides against the urokinase receptor. Lab. Invest. 1998;78:1109–1119. [PubMed] [Google Scholar]
- Gårdsvoll H., Dano K., Ploug M. Mapping part of the functional epitope for ligand binding on the receptor for urokinase-type plasminogen activator by site-directed mutagenesis. J. Biol. Chem. 1999;274:37995–40003. doi: 10.1074/jbc.274.53.37995. [DOI] [PubMed] [Google Scholar]
- Giancotti F.G., Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Gladson C.L., Cheresh D.A. Glioblastoma expression of vitronectin and the alpha v beta 3 integrin. Adhesion mechanism for transformed glial cells. J. Clin. Invest. 1991;88:1924–1932. doi: 10.1172/JCI115516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladson C.L., Wilcox J.N., Sanders L., Gillespie G.Y., Cheresh D.A. Cerebral microenvironment influences expression of the vitronectin gene in astrocytic tumors. J. Cell Sci. 1995;108:947–956. doi: 10.1242/jcs.108.3.947. [DOI] [PubMed] [Google Scholar]
- Gyetko M.R., Todd R.F., III, Wilkinson C.C., Sitrin R.G. The urokinase receptor is required for human monocyte chemotaxis in vitro. J. Clin. Invest. 1994;93:1380–1387. doi: 10.1172/JCI117114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyetko M.R., Sud S., Kendall T., Fuller J.A., Newstead M.W., Standiford T.J. Urokinase receptor-deficient mice have impaired neutrophil recruitment in response to pulmonary Pseudomonas aeruginosa infection. J. Immunol. 2000;165:1513–1519. doi: 10.4049/jimmunol.165.3.1513. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hawkins P.T., Eguinoa A., Qiu R.G., Stokoe D., Cooke F.T., Walters R., Wennstrom S., Claesson-Welsh L., Evans T., Symons M. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr. Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- Hebert C.A., Baker J.B. Linkage of extracellular plasminogen activator to the fibroblast cytoskeletoncolocalization of cell surface urokinase with vinculin. J. Cell Biol. 1988;106:1241–1247. doi: 10.1083/jcb.106.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høyer-Hansen G., Behrendt N., Ploug M., Danø K., Preissner K.T. The intact urokinase receptor is required for efficient vitronectin bindingreceptor cleavage prevents ligand interaction. FEBS Lett. 1997;420:79–85. doi: 10.1016/s0014-5793(97)01491-9. [DOI] [PubMed] [Google Scholar]
- Kanse S.M., Kost C., Wilhelm O.G., Andreasen P.A., Preissner K.T. The urokinase receptor is a major vitronectin-binding protein on endothelial cells. Exp. Cell Res. 1996;224:344–353. doi: 10.1006/excr.1996.0144. [DOI] [PubMed] [Google Scholar]
- Keely P.J., Westwick J.K., Whitehead I.P., Der C.J., Parise L.V. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- Kindzelskii A.L., Eszes M.M., Todd R.F., III, Petty H.R. Proximity oscillations of complement type 4 (alphaX beta2) and urokinase receptors on migrating neutrophils. Biophys. J. 1997;73:1777–1784. doi: 10.1016/S0006-3495(97)78208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh N., Wakatsuki T., Ryo A., Hada A., Aihara T., Horiuchi S., Goseki N., Matsubara O., Takenaka K., Shichita M. Identification and characterization of genes associated with human hepatocellular carcinogenesis. Cancer Res. 1999;59:4990–4996. [PubMed] [Google Scholar]
- Koshelnick Y., Ehart M., Hufnagl P., Heinrich P.C., Binder B.R. Urokinase receptor is associated with the components of the JAK1/STAT1 signaling pathway and leads to activation of this pathway upon receptor clustering in the human kidney epithelial tumor cell line TCL-598. J. Biol. Chem. 1997;272:28563–28567. doi: 10.1074/jbc.272.45.28563. [DOI] [PubMed] [Google Scholar]
- Koshelnick Y., Ehart M., Stockinger H., Binder B.R. Mechanisms of signaling through urokinase receptor and the cellular response. Thromb. Haemost. 1999;82:305–311. [PubMed] [Google Scholar]
- Kozma R., Ahmed S., Best A., Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol. Cell. Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusch A., Tkachuk S., Haller H., Dietz R., Gulba D.C., Lipp M., Dumler I. Urokinase stimulates human vascular smooth muscle cell migration via a phosphatidyl inositol 3-kinase-Tyk2 interaction. J. Biol. Chem. 2000;275:39466–39473. doi: 10.1074/jbc.M003626200. [DOI] [PubMed] [Google Scholar]
- Lauffenburger D.A., Horwitz A.F. Cell migrationa physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Maenpaa A., Kovanen P.E., Paetau A., Jaaskelainen J., Timonen T. Lymphocyte adhesion molecule ligands and extracellular matrix proteins in gliomas and normal brainexpression of VCAM-1 in gliomas. Acta Neuropathol. (Berl.) 1997;94:216–225. doi: 10.1007/s004010050696. [DOI] [PubMed] [Google Scholar]
- Mayer B.J., Hirai H., Sakai R. Evidence that SH2 domains promote processive phosphorylation by protein-tyrosine kinases. Curr. Biol. 1995;5:296–305. doi: 10.1016/s0960-9822(95)00060-1. [DOI] [PubMed] [Google Scholar]
- Michiels F., Habets G.G., Stam J.C., van der Kammen R.A., Collard J.G. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature. 1995;375:338–340. doi: 10.1038/375338a0. [DOI] [PubMed] [Google Scholar]
- Mignatti P., Rifkin D.B. Biology and biochemistry of proteinases in tumor invasion. Physiol. Rev. 1993;73:161–195. doi: 10.1152/physrev.1993.73.1.161. [DOI] [PubMed] [Google Scholar]
- Mitchison T.J., Cramer L.P. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- Myohanen H.T., Stephens R.W., Hedman K., Tapiovaara H., Rønne E., Høyer-Hansen G., Dano K., Vaheri A. Distribution and lateral mobility of the urokinase-receptor complex at the cell surface. J. Histochem. Cytochem. 1993;41:1291–1301. doi: 10.1177/41.9.8394852. [DOI] [PubMed] [Google Scholar]
- Nguyen D.H., Catling A.D., Webb D.J., Sankovic M., Walker L.A., Somlyo A.V., Weber M.J., Gonias S.L. Myosin light chain kinase functions downstream of Ras/ERK to promote migration of urokinase-type plasminogen activator–stimulated cells in an integrin-selective manner. J. Cell Biol. 1999;146:149–164. doi: 10.1083/jcb.146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes C.D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nobes C.D., Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes C.D., Hawkins P., Stephens L., Hall A. Activation of the small GTP-binding proteins rho and rac by growth factor receptors. J. Cell Sci. 1995;108:225–233. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- Nykjær A., Christensen E.I., Vorum H., Hager H., Petersen C.M., Røigaard H., Min H.Y., Vilhardt F., Møller L.B., Kornfeld S. Mannose 6-phosphate/insulin-like growth factor-II receptor targets the urokinase receptor to lysosomes via a novel binding interaction. J. Cell Biol. 1998;141:815–828. doi: 10.1083/jcb.141.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill G.M., Fashena S.J., Golemis E.A. Integrin signallinga new Cas(t) of characters enters the stage. Trends Cell Biol. 2000;10:111–119. doi: 10.1016/s0962-8924(99)01714-6. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Aguirre-Ghiso J.A. Urokinase receptor and integrin partnershipcoordination of signaling for cell adhesion, migration and growth. Curr. Opin. Cell Biol. 2000;12:613–620. doi: 10.1016/s0955-0674(00)00140-x. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M.D., Ruoslahti E. Influence of stereochemistry of the sequence Arg-Gly-Asp-Xaa on binding specificity in cell adhesion. J. Biol. Chem. 1987;262:17294–17298. [PubMed] [Google Scholar]
- Ploug M., Ellis V., Danø K. Ligand interaction between urokinase-type plasminogen activator and its receptor probed with 8-anilino-1-naphthalenesulfonate. Evidence for a hydrophobic binding site exposed only on the intact receptor. Biochemistry. 1994;33:8991–8997. doi: 10.1021/bi00196a017. [DOI] [PubMed] [Google Scholar]
- Pollanen J., Hedman K., Nielsen L.S., Danø K., Vaheri A. Ultrastructural localization of plasma membrane–associated urokinase-type plasminogen activator at focal contacts. J. Cell Biol. 1988;106:87–95. doi: 10.1083/jcb.106.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preissner K.T., Wassmuth R., Muller-Berghaus G. Physicochemical characterization of human S-protein and its function in the blood coagulation system. Biochem. J. 1985;231:349–355. doi: 10.1042/bj2310349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preissner K.T., Kanse S.M., May A.E. Urokinase receptora molecular organizer in cellular communication. Curr. Opin. Cell Biol. 2000;12:621–628. doi: 10.1016/s0955-0674(00)00141-1. [DOI] [PubMed] [Google Scholar]
- Quax P.H., Grimbergen J.M., Lansink M., Bakker A.H., Blatter M.C., Belin D., van Hinsbergh V.W., Verheijen J.H. Binding of human urokinase-type plasminogen activator to its receptorresidues involved in species specificity and binding. Arterioscler. Thromb. Vasc. Biol. 1998;18:693–701. doi: 10.1161/01.atv.18.5.693. [DOI] [PubMed] [Google Scholar]
- Resnati M., Guttinger M., Valcamonica S., Sidenius N., Blasi F., Fazioli F. Proteolytic cleavage of the urokinase receptor substitutes for the agonist-induced chemotactic effect. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:1572–1582. [PMC free article] [PubMed] [Google Scholar]
- Ridley A.J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley A.J., Paterson H.F., Johnston C.L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rønne E., Behrendt N., Ellis V., Ploug M., Danø K., Høyer-Hansen G. Cell-induced potentiation of the plasminogen activation system is abolished by a monoclonal antibody that recognizes the NH2-terminal domain of the urokinase receptor. FEBS Lett. 1991;288:233–236. doi: 10.1016/0014-5793(91)81042-7. [DOI] [PubMed] [Google Scholar]
- Sander E.E., van Delft S., ten Klooster J.P., Reid T., van der Kammen R.A., Michiels F., Collard J.G. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell–cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J. Cell Biol. 1998;143:1385–1398. doi: 10.1083/jcb.143.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells M.A., Knaus U.G., Bagrodia S., Ambrose D.M., Bokoch G.M., Chernoff J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- Sells M.A., Boyd J.T., Chernoff J. p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J. Cell Biol. 1999;145:837–849. doi: 10.1083/jcb.145.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L.M., Rabinovitz I., Wang H.H., Toker A., Mercurio A.M. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- Sidenius N., Blasi F. Domain 1 of the urokinase receptor (uPAR) is required for uPAR-mediated cell binding to vitronectin. FEBS Lett. 2000;470:40–46. doi: 10.1016/s0014-5793(00)01282-5. [DOI] [PubMed] [Google Scholar]
- Simon D.I., Rao N.K., Xu H., Wei Y., Majdic O., Ronne E., Kobzik L., Chapman H.A. Mac-1 (CD11b/CD18) and the urokinase receptor (CD87) form a functional unit on monocytic cells. Blood. 1996;88:3185–3194. [PubMed] [Google Scholar]
- Simon D.I., Wei Y., Zhang L., Rao N.K., Xu H., Chen Z., Liu Q., Rosenberg S., Chapman H.A. Identification of a urokinase receptor-integrin interaction site. Promiscuous regulator of integrin function. J. Biol. Chem. 2000;275:10228–10234. doi: 10.1074/jbc.275.14.10228. [DOI] [PubMed] [Google Scholar]
- Sitrin R.G., Todd R.F., III, Albrecht E., Gyetko M.R. The urokinase receptor (CD87) facilitates CD11b/CD18-mediated adhesion of human monocytes. J. Clin. Invest. 1996;97:1942–1951. doi: 10.1172/JCI118626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitrin R.G., Pan P.M., Harper H.A., Blackwood R.A., Todd R.F., III. Urokinase receptor (CD87) aggregation triggers phosphoinositide hydrolysis and intracellular calcium mobilization in mononuclear phagocytes. J. Immunol. 1999;163:6193–6200. [PubMed] [Google Scholar]
- Stahl A., Mueller B.M. Melanoma cell migration on vitronectinregulation by components of the plasminogen activation system. Int. J. Cancer. 1997;71:116–122. doi: 10.1002/(sici)1097-0215(19970328)71:1<116::aid-ijc19>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Stam J.C., Michiels F., van der Kammen R.A., Moolenaar W.H., Collard J.G. Invasion of T-lymphoma cellscooperation between Rho family GTPases and lysophospholipid receptor signaling. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:4066–4074. doi: 10.1093/emboj/17.14.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppelli M.P., Corti A., Soffientini A., Cassani G., Blasi F., Assoian R.K. Differentiation-enhanced binding of the amino-terminal fragment of human urokinase plasminogen activator to a specific receptor on U937 monocytes. Proc. Natl. Acad. Sci. USA. 1985;82:4939–4943. doi: 10.1073/pnas.82.15.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aelst L., D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Vassalli J.D., Baccino D., Belin D. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J. Cell Biol. 1985;100:86–92. doi: 10.1083/jcb.100.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz D.A., Natkin L.R., Fujita R.M., Wei Y., Chapman H.A. Plasmin and plasminogen activator inhibitor type 1 promote cellular motility by regulating the interaction between the urokinase receptor and vitronectin. J. Clin. Invest. 1997;100:58–67. doi: 10.1172/JCI119521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Waltz D.A., Rao N., Drummond R.J., Rosenberg S., Chapman H.A. Identification of the urokinase receptor as an adhesion receptor for vitronectin. J. Biol. Chem. 1994;269:32380–32388. [PubMed] [Google Scholar]
- Wei Y., Lukashev M., Simon D.I., Bodary S.C., Rosenberg S., Doyle M.V., Chapman H.A. Regulation of integrin function by the urokinase receptor. Science. 1996;273:1551–1555. doi: 10.1126/science.273.5281.1551. [DOI] [PubMed] [Google Scholar]
- Wilcox S.A., Reho T., Higgins P.J., Tominna-Sebald E., McKeown-Longo P.J. Localization of urokinase to focal adhesions by human fibrosarcoma cells synthesizing recombinant vitronectin. Biochem. Cell Biol. 1996;74:899–910. doi: 10.1139/o96-095. [DOI] [PubMed] [Google Scholar]
- Wilson A.J., Gibson P.R. Role of urokinase and its receptor in basal and stimulated colonic epithelial cell migration in vitro. Gut. 2000;47:105–111. doi: 10.1136/gut.47.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W., Mizukami I., Todd R.F., III, Petty H.R. Urokinase-type plasminogen activator receptors associate with beta1 and beta3 integrins of fibrosarcoma cellsdependence on extracellular matrix components. Cancer Res. 1997;57:1682–1689. [PubMed] [Google Scholar]
- Yebra M., Goretzki L., Pfeifer M., Mueller B.M. Urokinase-type plasminogen activator binding to its receptor stimulates tumor cell migration by enhancing integrin-mediated signal transduction. Exp. Cell Res. 1999;250:231–240. doi: 10.1006/excr.1999.4510. [DOI] [PubMed] [Google Scholar]