Abstract
Although growth factor receptors are generally thought to carry out their role in signal transduction at the cell surface, many of these transmembrane proteins translocate to the nucleus after ligand stimulation. Here, we show that the nuclear translocation of fibroblast growth factor receptor (FGFR)1 occurs via a mechanism distinct from classical nuclear import but dependent on importin β, a component of multiple nuclear import pathways. Furthermore, we show that nuclear FGFR1 induces c-Jun and is involved in the regulation of cell proliferation. These data are the first description of a nuclear import pathway for transmembrane growth factor receptors and elucidate a novel signal transduction pathway from the cell surface to the nucleus.
Keywords: FGF receptor, importin β, nuclear transport, c Jun, cyclin D1
Introduction
Growth factors regulate a diverse array of cellular functions, ranging from proliferation and apoptosis to migration and differentiation. Mutations in growth factor signaling pathways are the cause of many cancers. The vast majority of growth factors act on cells by binding to specific transmembrane receptors at the cell surface, which activate ligand-dependent intracellular signaling networks, typically via the interaction of effector proteins with the activated receptor. Ultimately, these pathways exert their effects on cells by regulating gene expression, often via the nuclear translocation of a downstream kinase and the subsequent phosphorylation of nuclear substrates.
In addition to events occurring at the plasma membrane, evidence is accumulating for growth factor receptor function after internalization. For example, internalized EGF receptor has been reported to activate both phospholipase Cγ and Ras (Haugh et al. 1999a,Haugh et al. 1999b), and internalized TrkA regulates nerve growth factor–induced neuronal differentiation (Zhang et al. 2000). After internalization, many transmembrane receptors are translocated to the nucleus, including FGF receptor (FGFR) 1, TrkA, insulin receptor, growth hormone receptor, receptors for interferons and interleukins, angiotensin type 1 receptor, and TGF-β type I receptor (Jans and Hassan 1998; Lu et al. 1998; Zwaagstra et al. 2000). In many cases, including FGFR1, neither the ligand nor the receptor harbors a nuclear localization signal (NLS), and little is known about the mechanism of nuclear import of the receptor. Furthermore, no definitive function for nuclear growth factor receptors has yet been elucidated.
FGFR1 is activated by members of the FGF family, including basic FGF (FGF-2) (Bikfalvi et al. 1997). Multiple FGF-2 isoforms result from alternative translational initiation, giving rise to 21–24-kD forms with limited tissue distribution and a ubiquitously expressed 18-kD form (Vagner et al. 1996). The higher molecular weight isoforms contain functional NLSs, whereas the 18-kD isoform does not (Florkiewicz et al. 1991). However, treatment of cells with 18 kD FGF-2 results in the nuclear translocation of FGFR1 (Maher 1996b) despite the lack of an NLS in either the ligand or the receptor. We undertook the present study to define the functional roles of nuclear FGFR1 and determine the mechanism of its transport into the nucleus. We show that nuclear translocation of FGFR1 is mediated by importin β and that FGFR1 in the nucleus plays a role in the regulation of the cell cycle.
Materials and Methods
Cell Fractionation and Immunoblotting
Swiss 3T3 cells were grown as described previously (Maher 1996b) and treated with recombinant human FGF-2 (25 ng/ml). Cellular stores of ATP were depleted with oligomycin B (50 μM) and 2-deoxyglucose (6 mM) in glucose-free DME containing 0.5% dialyzed serum for the indicated times. Cytosolic and nuclear fractions were prepared as described previously (Schreiber et al. 1989; Maher 1996b). Western blotting and immunoprecipitation were as described previously (Reilly et al. 2000), using antibodies against the Xpress epitope tag, EGFR, c-Jun, c-Fos, c-Myc, cyclin E, and importin β (Santa Cruz Biotechnology, Inc.), phospho-c-Jun (New England Biolabs, Inc.), cyclin D1 and p27Kip1 (NeoMarkers), PDGFR (Upstate Biotechnology), and FGFR (mAb6) (Maher 1996b). Protein concentrations were determined using the bicinchoninic acid protein assay (Pierce Chemical Co.), and equal loading was verified by staining blots with Ponceau S and quantifying the eluted dye by spectrophotometry. ATP concentrations of cytosolic fractions were determined using a commercial assay kit (Sigma-Aldrich). Densitometry of blots was performed using NIH Image v1.62, and statistical comparisons were performed using Student's t test.
Confocal Microscopy
Swiss 3T3 cells were plated at low density on glass coverslips in DME containing 0.5% serum and treated with FGF-2 (25 ng/ml). Cellular stores of ATP were depleted as above. After treatment, cells were immunostained for FGFR1 as described previously (Maher 1996b), and nuclei were counterstained with TO-PRO-3 or SYTOX Green (Molecular Probes). Optical sections were collected using a Bio-Rad MRC1024 laser scanning confocal microscope attached to a ZEISS Axiovert 100TV. In all cases, optical sections were through the median plane of the nucleus, as determined by nuclear counterstaining.
Nuclear Import Assay
Assays were carried out using Swiss 3T3 cells essentially as described (Adam et al. 1990). Cells grown on coverslips were washed in transport buffer (20 mM Hepes, pH 7.3, 110 mM KOAc, 5 mM NaOAc, 2 mM Mg[OAc]2, 1 mM EGTA, 2 mM DTT, and 1 μg/ml each aprotinin, leupeptin, and pepstatin) and permeabilized with 75 μM digitonin (Calbiochem). Nuclear import assays were carried out at 30°C in transport buffer with an ATP regenerating system (1 mM ATP, 5 mM creatine phosphate, 20 U/ml creatine phosphokinase) (Calbiochem) and 50% rabbit reticulocyte lysate (Promega). FGF-2 was added at 50 ng/ml. Importin β was depleted from the rabbit reticulocyte lysate with mAb3E9 (Affinity Bioreagents, Inc.) and anti–mouse agarose (Sigma-Aldrich). Mock immunodepletion was performed with purified mouse IgG. For antibody inhibition of importin β, mAb3E9 ascites fluid was diluted 1:4 with transport buffer containing 5 mg/ml BSA and dialyzed against transport buffer. A fluorescent transport substrate (Cy5-NLS-BSA) was used to verify the effects of treatments on classical NLS-mediated nuclear import. Immunostaining for FGFR1 and confocal microscopy were as described above.
Plasmid Construction and Transfection
An expression vector encoding a COOH-terminal epitope tag was constructed from pcDNA3.1/HisA as follows: the vector was digested with HindIII and KpnI to excise the epitope tag and the ends were blunted then ligated. Bases 926–1013 of the original vector were amplified by PCR with an XbaI site appended to the 5′ end and a stop codon followed by an ApaI site appended to the 3′ end. After digestion, this fragment was ligated into the XbaI/ApaI-digested vector lacking the epitope tag.
Full-length wild-type FGFR1, NLS-R1, and ΔSP-R1 were generated by PCR with the following forward primers: FGFR1, 5′-GCGG-ATCCATGTGGAGCTGGAAGTGCCTCCTC-3′; NLS-R1, 5′-GCGG-ATCCATGGACCCAAAAAAGAAGAGAAAGGTAAGGCCGTCC- CCGACCTTG-3′; ΔSP-R1, 5′-CCGGATCCATATGAGGCCGTCC-CCGACCTTG-3′; and the reverse primer, 5′-CGCTCGAGGCG-GCGTTTGAGTCCCCGATTGGC-3′. All three were subcloned into pcDNA3.1/HisA/C-term. The NLS-R1 construct encodes a methionine followed by the SV-40 large T antigen NLS (DPKKKRKV) appended to the NH2 terminus of the mature FGFR1 protein (amino acids 22–822). The ΔSP-R1 construct encodes the mature FGFR1 protein (amino acids 22–822) with a methionine appended to the NH2 terminus. The NLS-R1kd construct was generated by subcloning the KpnI-Bsu36I fragment of CD-R1 K514M (Reilly et al. 2000) into the corresponding region of the NLS-R1 plasmid. The vector and constructs were verified by DNA sequencing.
For transient transfections, NIH 3T3 cells were seeded at 4 × 105 cells/cm2, grown for 18 h in DME supplemented with 10% calf serum, and then transfected using Lipofectamine 2000 (GIBCO BRL) according to the manufacturer's protocol. Cells were maintained in DME supplemented with 10% calf serum for 24 h, starved for 24 h, and then treated with recombinant human FGF-2.
Results and Discussion
Nuclear Import of FGFR1
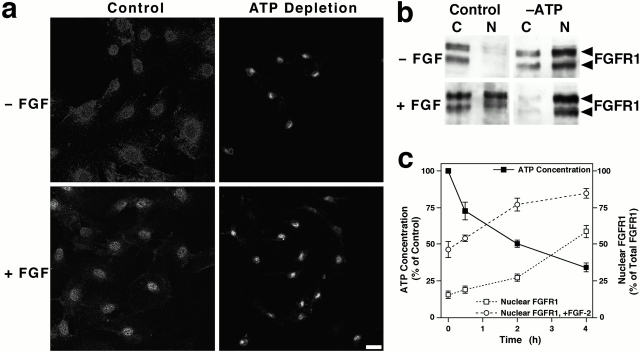
Participation of nuclear FGFR1 in FGF-induced signal transduction events requires regulated transport of the receptor into the nucleus. In examining the energy dependence of FGF-2–induced nuclear accumulation of FGFR1, we observed that ligand-induced nuclear translocation occurred in fibroblasts depleted of ATP using a combination of oligomycin B and 2′-deoxyglucose (Fig. 1). Interestingly, ATP depletion also induced the nuclear translocation of FGFR1 in the absence of FGF-2. Confocal microscopy demonstrated the localization of FGFR1 in untreated cells to the plasma membrane and in vesicle-like structures within the cytoplasm (Fig. 1 a; see Fig. 4 b for higher magnification). The low level of plasma membrane staining is a result of the technique used to permeabilize the cells (Maher 1996b), and cytoplasmic localization of FGFR1 has been described previously (Maher 1996a). In FGF-2–treated cells, FGFR1 was located within the nucleus and not associated with the nuclear envelope (Fig. 1 a). ATP depletion caused morphological changes in the cells, including shrinkage of the cell body and nucleus. Localization of FGFR1 in the nucleus of ATP-depleted cells was confirmed by differential interference contrast microscopy, and FGFR1 immunoreactivity was coincident with a fluorescent nuclear counterstain (data not shown). These data were confirmed by biochemical isolation of membrane-depleted nuclei (Fig. 1 b). As ATP concentrations within cells decreased, a progressive increase in the nuclear accumulation of FGFR1 was observed, and this effect was potentiated at all time points by pretreatment with FGF-2 (Fig. 1 c).
Figure 1.
FGF and depletion of ATP induce the nuclear translocation of FGFR1. (a) Swiss 3T3 fibroblasts were untreated or subjected to ATP depletion by treatment with oligomycin B and 2-deoxyglucose for 4 h and analyzed by FGFR1 immunostaining and confocal microscopy. The onset of ATP depletion and FGF-2 treatment was simultaneous. Nuclear localization was confirmed by fluorescent nuclear counterstaining (data not shown). (b) Cells treated as in “a” were separated into cytosolic (C) and nuclear (N) fractions, and equal amounts of protein were immunoblotted for FGFR1. FGFR1 is detected as bands migrating at 145 and 120 kD, representing alternative splice variants (Johnson and Williams 1993). (c) Cellular ATP concentration and percent of FGFR1 in the nuclear fraction during ATP depletion. Cells were pretreated with FGF-2 for 1 h before the onset of ATP depletion. Nuclear FGFR1 was determined by densitometry of FGFR1 immunoblots, and values represent the mean ± SEM of triplicate experiments. Bar, 30 μm.
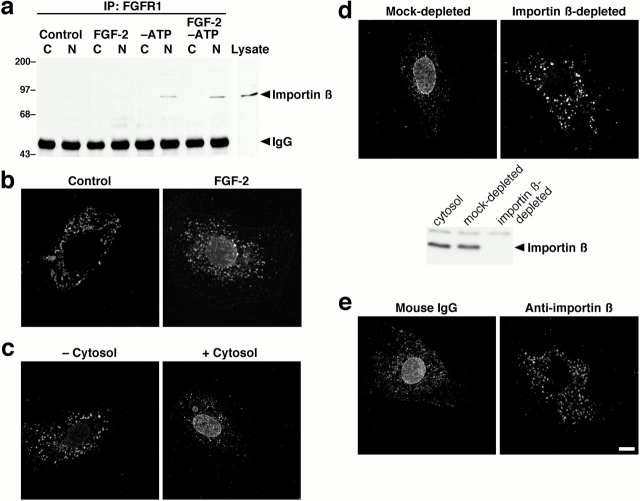
Figure 4.
Nuclear import of FGFR1 is mediated by importin β. (a) Coimmunoprecipitation of importin β with FGFR1. Cells were untreated or subjected to ATP depletion by treatment with oligomycin B and 2-deoxyglucose for 2 h, in the absence or presence of FGF-2, and separated into cytosolic (C) and nuclear (N) fractions. FGFR1 was immunoprecipitated from equal amounts of protein, and complexes were separated by SDS-PAGE and immunoblotted for importin β. The whole cell lysate is shown for comparison, the immunoglobulin heavy chain (IgG) is indicated, and molecular weights are indicated in kD. (b–e) Nuclear translocation of FGFR1 in Swiss 3T3 fibroblasts was examined using an in vitro nuclear import assay (Adam et al. 1990), and cells were analyzed by FGFR1 immunostaining and confocal microscopy. Unpermeabilized cells (b) were untreated or incubated for 30 min with FGF-2. Digitonin-permeabilized cells (c–e) were incubated for 30 min with FGF-2 in the absence or presence of exogenous cytosol (c), with mock- or importin β–depleted exogenous cytosol (d) or with exogenous cytosol plus mouse IgG or a neutralizing antibody against importin β (e). Depletion of importin β from exogenous cytosol was confirmed by immunoblotting (d). Bar, 10 μm.
To rule out the possibility that the effects of ATP depletion on the nuclear localization of FGFR1 represent a nonspecific translocation of cell surface receptors, we examined the localization of PDGF receptor and EGF receptor in ATP-depleted cells (Fig. 2 a). Depletion of ATP did not induce the nuclear accumulation of either receptor, indicating that the observed effects are specific for FGFR1. Additionally, ATP depletion did not result in the nuclear accumulation of ERK1 and 2, proteins which normally translocate to the nucleus after growth factor stimulation of cells (data not shown). To rule out the possibility that the effects of ATP depletion on the nuclear localization of FGFR1 were due to a generalized response to cellular stress, we subjected cells to osmotic shock, which did not result in nuclear accumulation of FGFR1 (Fig. 2 b).
Figure 2.
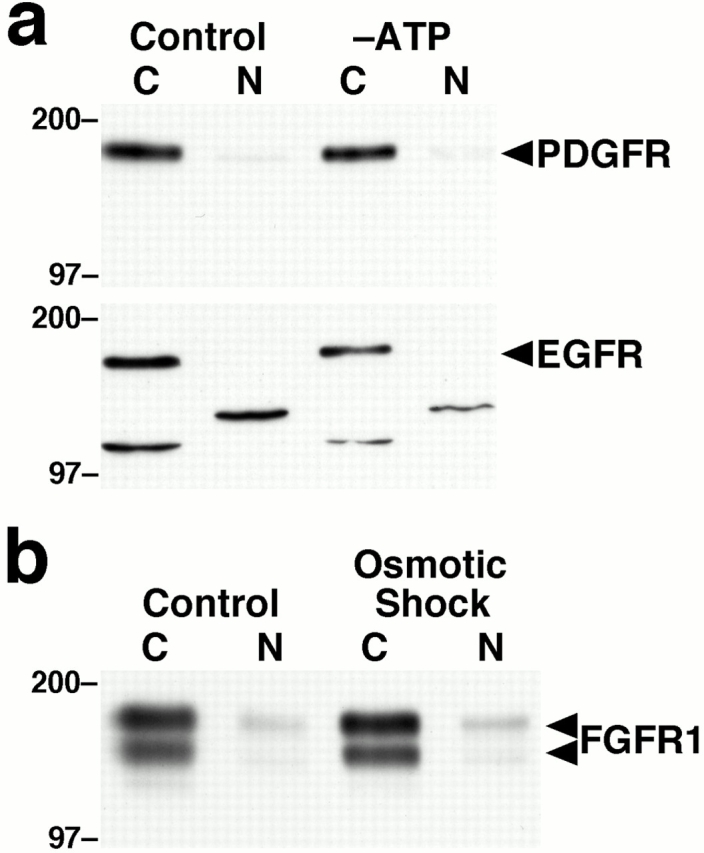
The effects of ATP depletion are specific for FGFR1. (a) Cells were untreated or subjected to ATP depletion by treatment with oligomycin B and 2-deoxyglucose for 2 h, separated into cytosolic (C) and nuclear (N) fractions, and equal amounts of protein were immunoblotted for PDGF receptor (PDGFR) or EGF receptor (EGFR). Molecular weights are indicated in kD. (b) Cells were untreated or subjected to osmotic shock (300 mM sorbitol, 1 h), separated into cytosolic (C) and nuclear (N) fractions, and equal amounts of protein were immunoblotted for FGFR1.
Importin β Mediates FGFR1 Nuclear Import
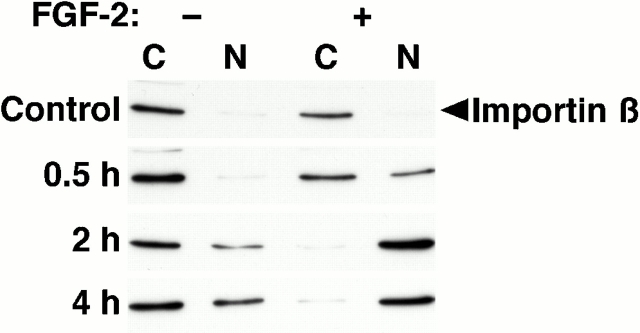
Importin β is a critical component of multiple nuclear import pathways (Nakielny and Dreyfuss 1999). Recently, it was reported that microinjected importin β accumulates in the nucleus of ATP-depleted cells, whereas export of importin β from the nucleus is energy-dependent (Kose et al. 1999), characteristics similar to those of FGFR1. To determine if importin β is involved in the nuclear import of FGFR1, we first examined the localization of endogenous importin β in ATP-depleted cells. In untreated cells and cells treated with FGF-2, importin β was localized almost exclusively in the cytosol. Depletion of ATP resulted in a progressive nuclear accumulation of importin β, and this effect was potentiated by FGF-2 (Fig. 3). These data are consistent with a model in which energy is required to export importin β from the nucleus. In the ATP-depleted state, importin β that enters the nucleus during endogenously occurring transport fails to be exported. The potentiation of importin β nuclear accumulation by FGF-2 suggests that importin β plays a role in the nuclear import of FGFR1. Alternatively, since activation of FGF-induced signaling pathways results in the nuclear translocation of transcription factors such as NF-κB (Byrd et al. 1999), importin β may accumulate in the nucleus of ATP-depleted, FGF-2–treated cells after import of NLS-bearing cargoes.
Figure 3.
ATP depletion induces the nuclear translocation of importin β. Cells were untreated or subjected to ATP depletion by treatment with oligomycin B and 2-deoxyglucose for the indicated times, in the absence or presence of FGF-2. Cells were separated into cytosolic (C) and nuclear (N) fractions, and equal amounts of protein were immunoblotted for importin β.
To establish a direct role for importin β in the nuclear import of FGFR1, we initially demonstrated the presence of a complex containing FGFR1 and importin β in the nucleus of ATP-depleted cells by coimmunoprecipitation of importin β with FGFR1 (Fig. 4 a). For treatment times up to 4 h, the amount of importin β which coprecipitates with FGFR1 is proportional to the amount of importin β detected in the nuclear fractions (data not shown). In cells treated with FGF-2 but not depleted of ATP, the complex most likely dissociates, followed by reexport of the importin β from the nucleus. Precipitation of importin β was not observed in control experiments using preimmune IgG (data not shown). The physical association of importin β with FGFR1 supports the hypothesis of a functional role in import. This role was confirmed using the well-characterized in vitro nuclear import assay in digitonin-permeabilized cells (Adam et al. 1990). In nonpermeabilized cells, treatment with FGF-2 resulted in the nuclear accumulation of FGFR1 (Fig. 4 b). Permeabilization of cells with digitonin has been shown to release ∼20% of the total cellular protein and to extract the soluble factors necessary for nuclear import (Adam et al. 1990), including ≥80% of the importin β (Chi et al. 1995). FGF-2–induced nuclear import of FGFR1 was inhibited in digitonin permeabilized fibroblasts and reconstituted by the addition of exogenous cytosol (Fig. 4 c), indicating that soluble factors are necessary for the nuclear import of FGFR1. Immunodepletion of importin β from the exogenous cytosol (Chi et al. 1995) blocked the import of a fluorescently labeled transport substrate (Cy5-NLS-BSA) (data not shown). This treatment also inhibited FGF-2–induced nuclear import of FGFR1 (Fig. 4 d), whereas mock-depletion of the exogenous cytosol with preimmune mouse IgG had no effect. In an alternate approach, addition of a monoclonal antibody against importin β to exogenous cytosol also blocked FGF-2–induced nuclear import of FGFR1 (Fig. 4 e). These data demonstrate that importin β mediates the nuclear import of FGFR1.
Our findings provide the first description of a mechanism for the nuclear import of a transmembrane receptor tyrosine kinase. The phenomenon of nuclear translocation of receptors is well established for steroid hormone receptors and orphan nuclear receptors but has met with some controversy concerning transmembrane growth factor receptors. Nuclear localization has been demonstrated by biochemical and microscopic means for many transmembrane receptors (Jans and Hassan 1998), including a seven transmembrane domain receptor (Lu et al. 1998). Arguments that nuclear localization is an artifact have been made based in part on the lack of a defined mechanism for nuclear import, since most transmembrane receptors lack NLSs. By providing direct evidence of importin β–mediated nuclear import of FGFR1, which does not contain an NLS, our data resolve this issue.
Role of Nuclear FGFR1 in Cell Proliferation
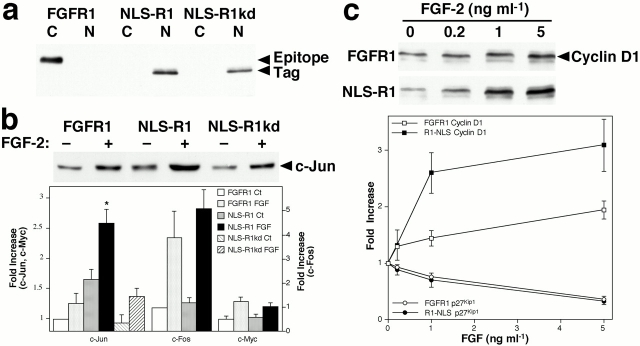
To examine the functional role of nuclear FGFR1, we transfected mouse fibroblasts with a construct encoding full-length FGFR1 with the signal peptide replaced by the SV-40 large T antigen NLS. This protein (NLS-R1) was constitutively localized to the nucleus (Fig. 5 a), as determined by biochemical fractionation of the cells. Cells expressing NLS-R1 showed elevated expression of c-Jun compared with cells transfected with wild-type FGFR1 (Fig. 5 b) or with a construct encoding FGFR1 lacking the signal peptide (ΔSP-R1) or with vector alone (data not shown). A construct encoding NLS-R1 with a point mutation that inactivates the tyrosine kinase (NLS-R1kd) failed to stimulate c-Jun expression, demonstrating that the observed responses depend on receptor kinase activity. The basal levels of two other immediate early gene products, c-Fos and c-Myc, were unaffected by NLS-R1 when compared with the expression in cells transfected with wild-type FGFR1 (Fig. 5 b). Treatment with FGF-2 induced the expression of c-Jun, c-Fos, and c-Myc in FGFR1 and NLS-R1–transfected cells, and FGF-2–induced c-Jun expression was potentiated in NLS-R1–transfected cells compared with cells transfected with wild-type FGFR1 (Fig. 5 b). The level of c-Jun expression in unstimulated FGFR1–transfected cells was similar to that in vector-transfected controls and most likely represents incomplete quiescence due to the transfection procedure or the duration of the serum deprivation. This increased baseline expression may be masking part of the stimulatory effects of NLS-R1. In addition to immediate early gene expression, several other FGF-induced signal transduction events were examined in cells transfected with vector, FGFR1, ΔSP-R1, or NLS-R1. No significant differences were observed in basal or FGF-stimulated phosphorylation of ERK1/2, p38 MAPK, CREB, ATF-2, Akt/PKB, or p70S6K (data not shown). This is consistent with the activation of these kinase modules and their downstream effectors by cell surface FGFR1 and indicates that the induction of c-Jun by NLS-R1 is a specific result of the nuclear localization of the receptor.
Figure 5.
Nuclear FGFR1 induces c-Jun expression and potentiates FGF-induced cyclin D1 expression. (a) NIH 3T3 fibroblasts were transfected with constructs encoding full-length wild-type FGFR1, FGFR1 with the signal peptide replaced by the SV-40 large T antigen NLS (NLS-R1), or with a construct encoding NLS-R1 with a kinase-inactivating point mutation (NLS-R1kd), each with an epitope tag at the COOH terminus. Cells were separated into cytosolic (C) and nuclear (N) fractions, and equal amounts of protein were immunoblotted for the epitope tag. The molecular weight of wild-type FGFR1 (140 kD) is greater than that of NLS-R1 (110 kD) due to glycosylation. (b) Basal and FGF-induced immediate early gene expression in transfected cells. Cells were transiently transfected with the indicated constructs, starved for 24 h in low-serum medium, and then treated for 1 h with 1 ng/ml FGF-2. Equal amounts of whole cell lysate protein were immunoblotted for c-Jun, c-Fos, and c-Myc. Expression levels were quantified by densitometry of immunoblots, and values are normalized to untreated FGFR1-transfected cells and represent the mean ± SEM of triplicate experiments. *P < 0.01 versus FGFR1-transfected cells treated with FGF-2. (c) FGF-2–induced cyclin D1 and p27Kip1 expression in transfected cells. Transiently transfected cells were starved for 24 h in low-serum medium and then treated for 16 h with FGF-2. Equal amounts of whole cell lysate protein were immunoblotted for cyclin D1 and p27Kip1. Expression levels were quantified by densitometry of immunoblots, and values are normalized to untreated cells and represent the mean ± SEM of triplicate experiments.
c-Jun and c-Fos are components of the heterodimeric transcription factor AP-1, and both are required for cell proliferation (Kovary and Bravo 1991). c-Jun is also active as a homodimer, and its role in cell proliferation is mediated at least in part by direct activation of the cyclin D1 promoter (Bakiri et al. 2000). Cyclins and their cyclin-dependent kinase partners control cell cycle progression (Johnson and Walker 1999). Cyclin D1 is the principal G1-phase cyclin and is induced by multiple growth factors including FGF-2. In NLS-R1–transfected cells, FGF-induced cyclin D1 expression was potentiated compared with cells transfected with FGFR1 (Fig. 5 c), demonstrating a role for nuclear FGFR1 in the regulation of cell proliferation. However, despite the increase in basal c-Jun expression, no increase in the basal expression of cyclin D1 was observed in NLS-R1–transfected cells. c-Jun activity is regulated by both expression level and NH2-terminal phosphorylation, and phosphorylation is required for the full activity of c-Jun, induction of cyclin D1 expression, and cell proliferation (Behrens et al. 1999; Bakiri et al. 2000). Treatment of cells with FGF results in the activation of c-Jun NH2-terminal kinase (data not shown). However, the c-Jun expressed in untreated NLS-R1–transfected cells is not strongly phosphorylated, as determined by immunoblotting with a phosphospecific antibody, whereas the NLS-R1–potentiated increase in c-Jun expression in FGF-treated cells is accompanied by an increase in c-Jun phosphorylation (data not shown). Our data suggest that c-Jun activity represents a convergence point for FGFR1 signaling, with nuclear localization of the receptor inducing c-Jun expression and cell surface FGFR1-mediated c-Jun NH2-terminal kinase activation resulting in c-Jun phosphorylation.
Induction of cyclin D1 is only one of the required events in G1-phase progression and is accompanied by degradation of the cyclin-dependent kinase inhibitor p27Kip1 and the induction of cyclin E (Johnson and Walker 1999). FGF-induced degradation of p27Kip1 was not affected by nuclear FGFR1 (Fig. 5 c), nor was induction of cyclin E (data not shown). Consistent with these observations, no potentiation of basal or FGF-induced DNA synthesis was seen in NLS-R1–transfected cells (data not shown). These data support a model in which activation of FGFR1 at the cell surface initiates a set of signals required for proliferation, and these events are followed by translocation of FGFR1 to the nucleus and initiation of a subsequent set of events, including c-Jun induction and increased expression of cyclin D1, that are also required for proliferation.
Our data define a specific role for importin β–mediated nuclear translocation of FGFR1 in immediate early gene induction and cell proliferation, providing direct evidence for a nuclear function of a cell surface growth factor receptor. These studies suggest that regulated nuclear import of transmembrane receptors represents an additional mode of signal transduction, complementing the well characterized pathways of cytoplasmic kinase cascades.
Acknowledgments
We thank Larry Gerace for Cy5-NLS-BSA, Greg Mickey for construction of pcDNA3.1/HisA/C-term and CD-R1 K514M, and Shawndra Martinez and David Schubert for critical reading of the manuscript.
This work was supported by National Institutes of Health grants GM54604 and NS28121.
Footnotes
J.F. Reilly's present address is Neurome, Inc., 11149 N. Torrey Pines Rd., La Jolla, CA 92037. Tel.: (858) 677-0466. Fax: (858) 677-0458. E-mail: jreilly@neurome.com
Abbreviations used in this paper: FGFR, FGF receptor; NLS, nuclear localization signal.
References
- Adam S.A., Marr R.S., Gerace L. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J. Cell Biol. 1990;111:807–816. doi: 10.1083/jcb.111.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakiri L., Lallemand D., Bossy-Wetzel E., Yaniv M. Cell cycle-dependent variations in c-Jun and JunB phosphorylationa role in the control of cyclin D1 expression. EMBO (Eur. Mol. Biol. Organ.) J. 2000;19:2056–2068. doi: 10.1093/emboj/19.9.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens A., Sibilia M., Wagner E.F. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat. Genet. 1999;21:326–329. doi: 10.1038/6854. [DOI] [PubMed] [Google Scholar]
- Bikfalvi A., Klein S., Pintucci G., Rifkin D.B. Biological roles of fibroblast growth factor-2. Endocr. Rev. 1997;18:26–45. doi: 10.1210/edrv.18.1.0292. [DOI] [PubMed] [Google Scholar]
- Byrd V.M., Ballard D.W., Miller G.G., Thomas J.W. Fibroblast growth factor-1 (FGF-1) enhances IL-2 production and nuclear translocation of NF-κB in FGF receptor-bearing Jurkat T cells. J. Immunol. 1999;162:5853–5859. [PubMed] [Google Scholar]
- Chi N.C., Adam E.J.H., Adam S.A. Sequence and characterization of cytoplasmic nuclear protein import factor p97. J. Cell Biol. 1995;130:265–274. doi: 10.1083/jcb.130.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florkiewicz R.Z., Baird A., Gonzalez A.-M. Multiple forms of bFGFdifferential nuclear and cell surface localization. Growth Factors. 1991;4:265–275. doi: 10.3109/08977199109043912. [DOI] [PubMed] [Google Scholar]
- Haugh J.M., Huang A.C., Wiley H.S., Wells A., Lauffenburger D.A. Internalized epidermal growth factor receptors participate in the activation of p21ras in fibroblasts J. Biol. Chem. 274 1999. 34350 34360a [DOI] [PubMed] [Google Scholar]
- Haugh J.M., Schooler K., Wells A., Wiley H.S., Lauffenburger D.A. Effect of epidermal growth factor receptor internalization on regulation of the phospholipase C-γ1 signaling pathway J. Biol. Chem. 274 1999. 8958 8965b [DOI] [PubMed] [Google Scholar]
- Jans D.A., Hassan G. Nuclear targeting by growth factors, cytokines, and their receptorsa role in signaling? Bioessays. 1998;20:400–411. doi: 10.1002/(SICI)1521-1878(199805)20:5<400::AID-BIES7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Johnson D.E., Williams L.T. Structural and functional diversity in the FGF receptor multigene family. Adv. Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- Johnson D.G., Walker C.L. Cyclins and cell cycle checkpoints. Annu. Rev. Pharmacol. Toxicol. 1999;39:295–312. doi: 10.1146/annurev.pharmtox.39.1.295. [DOI] [PubMed] [Google Scholar]
- Kose S., Imamoto N., Yoneda Y. Distinct energy requirement for nuclear import and export of importin β in living cells. FEBS Lett. 1999;463:327–330. doi: 10.1016/s0014-5793(99)01641-5. [DOI] [PubMed] [Google Scholar]
- Kovary K., Bravo R. The Jun and Fos protein families are both required for cell cycle progression in fibroblasts. Mol. Cell. Biol. 1991;11:4466–4472. doi: 10.1128/mcb.11.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Yang H., Shaw G., Raizada M.K. Angiotensin II-induced nuclear targeting of the angiotensin type 1 (AT1) receptor in brain neurons. Endocrinology. 1998;139:365–375. doi: 10.1210/endo.139.1.5679. [DOI] [PubMed] [Google Scholar]
- Maher P.A. Identification and characterization of a novel, intracellular isoform of fibroblast growth factor receptor-1 (FGFR-1) J. Cell. Physiol. 169 1996. 380 390a [DOI] [PubMed] [Google Scholar]
- Maher P.A. Nuclear translocation of fibroblast growth factor (FGF) receptors in response to FGF-2 J. Cell Biol. 134 1996. 529 536b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakielny S., Dreyfuss G. Transport of proteins and RNAs in and out of the nucleus. Cell. 1999;99:677–690. doi: 10.1016/s0092-8674(00)81666-9. [DOI] [PubMed] [Google Scholar]
- Reilly J.F., Mickey G., Maher P.A. Association of fibroblast growth factor receptor 1 with the adaptor protein Grb14. Characterization of a new receptor binding partner. J. Biol. Chem. 2000;275:7771–7778. doi: 10.1074/jbc.275.11.7771. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Muller M.M., Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagner S., Touriol C., Galy B., Audigier S., Gensac M.C., Amalric F., Bayard F., Prats H., Prats A.C. Translation of CUG- but not AUG-initiated forms of human fibroblast growth factor 2 is activated in transformed and stressed cells. J. Cell Biol. 1996;135:1391–1402. doi: 10.1083/jcb.135.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Moheban D.B., Conway B.R., Bhattacharyya A., Segal R.A. Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J. Neurosci. 2000;20:5671–5678. doi: 10.1523/JNEUROSCI.20-15-05671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaagstra J.C., Guimond A., O'Connor-McCourt M.D. Predominant intracellular localization of the type I transforming growth factor-β receptor and increased nuclear accumulation after growth arrest. Exp. Cell Res. 2000;258:121–134. doi: 10.1006/excr.2000.4905. [DOI] [PubMed] [Google Scholar]