Abstract
Members of the ciliary neurotrophic factor (CNTF)/leukemia inhibitory factor (LIF)/cardiotrophin gene family are potent survival factors for embryonic and lesioned motoneurons. These factors act via receptor complexes involving gp130 and LIFR-β and ligand binding leads to activation of various signaling pathways, including phosphorylation of Stat3. The role of Stat3 in neuronal survival was investigated in mice by Cre-mediated gene ablation in motoneurons. Cre is expressed under the neurofilament light chain (NF-L) promoter, starting around E12 when these neurons become dependent on neurotrophic support. Loss of motoneurons during the embryonic period of naturally occurring cell death is not enhanced in NF-L–Cre; Stat3flox/KO mice although motoneurons isolated from these mice need higher concentrations of CNTF for maximal survival in culture. In contrast, motoneuron survival is significantly reduced after facial nerve lesion in the adult. These neurons, however, can be rescued by the addition of neurotrophic factors, including CNTF. Stat3 is essential for upregulation of Reg-2 and Bcl-xl expression in lesioned motoneurons. Our data show that Stat3 activation plays an essential role for motoneuron survival after nerve lesion in postnatal life but not during embryonic development, indicating that signaling requirements for motoneuron survival change during maturation.
Keywords: CNTF; axotomy; lesion; bcl-xl; reg-2
Introduction
Neuronal cell death after trophic factor deprivation is regulated at several levels. Release of cytochrome C from mitochondria plays an important triggering role, and subsequent activation of caspases is modulated by proteins such as inhibitors of apoptosis (IAPs),* which are under transcriptional control of neurotrophic factors (Wiese et al., 1999a). Neurotrophic factors promote activation of kinase cascades, including the PI-3K/Akt pathway which leads to phosphorylation of Bad (Datta et al., 1997), Caspase 9 (Cardone et al., 1998), and FKRLH-1 (Brunet et al., 1999). In addition, activation of the mitogen-activated protein kinase (MAPK) pathway and subsequent CREB phosphorylation via RSK-2 (Riccio et al., 1999) participate in this complex scenario. Apoptosis of facial motoneurons after nerve lesion in neonatal mice is absent in Bax-deficient mice (Deckwerth et al., 1996). This indicates that Bax plays an essential role in lesion-mediated motoneuron death in postnatal rodents, and that the pronounced loss of motoneurons in ciliary neurotrophic factor (CNTF)/leukemia inhibitory factor (LIF) double knockout (KO) mice (Sendtner et al., 1996) after facial nerve lesion is mediated by cytochrome C release involving Bax. CNTF and LIF activate receptor complexes which include gp130 and LIFR-β receptor subunits. Binding of CNTF to its receptor activates the phosphatidylinositol 3 kinase (PI-3K) pathway, which leads to upregulation of IAP gene expression (Hirano et al., 1997; Wiese et al., 1999a). In contrast to the neurotrophins, CNTF and related ligands specifically activate Stat3 (Lutticken et al., 1994; Haas et al., 1999). Given that all neurotrophic factors activate the Ras pathway and Ras has been shown to fully support the survival of embryonic chick motoneurons (Weng et al., 1996), it remains open whether the survival response in motoneurons (Li et al., 1994) involves Stat3-regulated mechanisms. Antagonistic roles for MAPK and Stat3 signaling have been reported for neurite outgrowth in PC12 cells (Ihara et al., 1997). However, these experiments did not reveal any specific function of Stat3 in regulation of neuronal survival. Only few specific genes which are expressed in response to Stat3 in neurons are known so far. In particular, Stat3-dependent gene expression could differ from one to the other cell type so that findings with cell lines and nonneuronal cells might not necessarily apply to neurons. Stat3 is constitutively activated in fibroblasts which are transformed with v-src (Cao et al., 1996). The observation that Bcl-xl expression is upregulated in a Stat3-dependent manner in squamous cell carcinoma (Grad et al., 2000; Grandis et al., 2000) points to a role for Stat3 in antiapoptotic signaling, at least in tumor cells.
To assess the physiological function of Stat3 for motoneuron survival we have generated a Cre transgenic mouse line that expresses Cre under control of the human neurofilament light chain (NF-L) promoter and exhibits Cre expression in specific populations of neurons, including facial and spinal motoneurons. Neuron-specific Stat3 knockout mice were generated in which the Stat3 gene can be inactivated by Cre-mediated recombination (Takeda et al., 1998). These animals develop normally and do not reveal significant differences in motoneuron numbers in the facial nucleus and spinal cord, although isolated motoneurons from these animals need higher concentrations of CNTF for maximal survival in culture. However, after facial nerve transection in the adult, a dramatic loss of motoneurons is observed. We also report that lesion-induced expression of Reg-2 and Bcl-xl is reduced in mice in which Stat3 is eliminated by motoneuron-specific Cre expression. These data suggest that Stat3 plays an essential role in motoneuron survival after axotomy but not during embryonic development when motoneurons physiologically depend on neurotrophic factors for their survival.
Results
Cell type–specific inactivation of Stat3 by Cre expression under the NF-L promoter
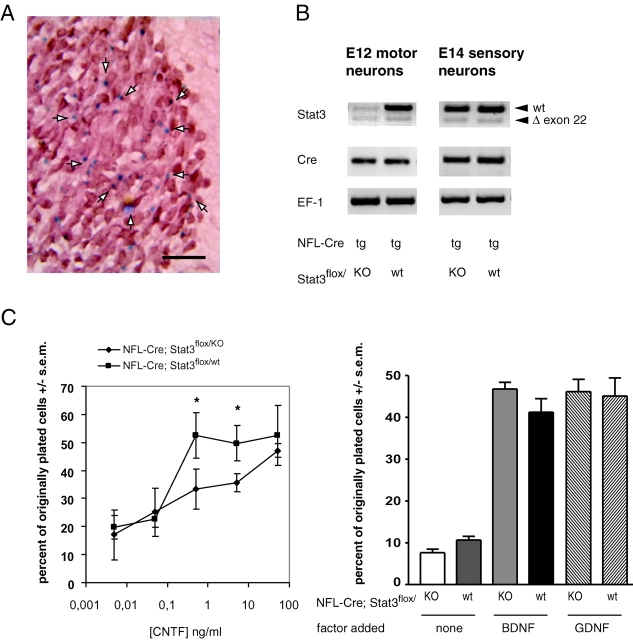
To study Stat3 function in postnatal motoneurons, we have generated a transgenic mouse line expressing the bacteriophage P1 Cre recombinase under control of the human NF-L promoter and intragenic sequences. Previous studies (Leconte et al., 1994; Akassoglou et al., 1997) have shown that the human NF-L promoter and its intragenic sequences can drive heterologous expression of transgenes in mice. Therefore, we have replaced the coding region of the first exon of the human NF-L gene by a CrepA cassette, leaving 2 kb of the 5′ promoter and 3 kb of downstream sequence intact (Fig. 1 A). Two lines of transgenic mice were established and various tissues were tested for Cre mRNA expression by reverse transcription (RT)-PCR. One line of transgenic mice exhibited Cre expression in brain and spinal cord but not in sciatic nerve or organs such as kidney, liver, and spleen (Fig. 1 B; unpublished data). To control whether Cre expressed under the NF-L promoter is functional, we cross-bred NF-L–Cre with a lacZ reporter strain (Akagi et al., 1997) and analyzed double transgenic mice at various embryonic and postnatal stages. Using a PCR-based assay (Akagi et al., 1997), we show that Cre expressed from the NF-L–Cre transgene is functional and recombines a loxP-flanked reporter gene specifically in neural tissues (Fig. 1 C). To test whether Cre expression is specific for neurons, we performed in situ hybridization with a probe specific for Cre mRNA. As shown in Fig. 1 D, the Cre transgene is expressed in motoneurons of the facial nucleus, but not in the surrounding glia. Cre expression was also detected in hippocampal and cortical neurons (unpublished data). We then tested the cell type specificity of Cre-mediated recombination by staining of brain slices and whole organs for β-galactosidase. Again, X-gal staining was confined to neurons, in particular spinal and brain stem motoneurons (Fig. 1 E and unpublished data). Neuron-specific inactivation of the Stat3 gene was achieved by cross-breeding NF-L–Cre transgenic mice with mice carrying a floxed Stat3 gene (Takeda et al., 1998) according to the scheme depicted in Fig. 1 F. In these mice, Cre-mediated recombination leads to removal of exon 22 of Stat3, which contains the functionally relevant tyrosine residue. Due to usage of a cryptic splice site, part of the following exon containing the regulatory serine phosphorylation site is also removed (Takeda et al., 1998). Inclusion of a Stat3 null allele resulted in 50% offspring devoid of functional Stat3 in a significant subpopulation of motoneurons (NF-L–Cre; Stat3flox/KO) and 50% offspring carrying one functional allele of Stat3 in motoneurons (NF-L–Cre; Stat3flox/wt).
Figure 1.

Generation and characterization of NF-L–Cre. (A) The coding region of exon 1 within a 6-kb human NF-L gene fragment was replaced by the open reading frame of bacteriophage P1 Cre recombinase leaving regulatory sequences within NF-L introns intact. (B) Cre recombinase is specifically expressed in brain and spinal cord. RT-PCR on total RNA derived from adult cerebral cortex, brainstem, spinal cord, and sciatic nerve detects Cre message only brain and spinal cord, but not in sciatic nerve, liver, spleen, and kidney (latter three not shown). (C) Transgenic Cre recombinase is enzymatically active and recombines a reporter allele in vivo. NF-L–Cre mice were cross-bred with a lac Z reporter strain. Cre-mediated recombination of the reporter gene was detected via PCR on genomic DNA by a 580-bp product as opposed to the 1,700-bp product of the unrecombined reporter allele. Cre- mediated recombination is therefore specific for brain and spinal cord. (D) Cre recombinase expression is neuron- specific. In situ hybridization detects Cre expression in the facial nucleus and other brain regions (unpublished data). The transgenic Cre allele is solely expressed in neurons but not in surrounding glia. (E) Cre-mediated site-specific recombination in facial motoneurons. Brain sections from mice transgenic for NF-L–Cre and for the lacZ reporter allele were stained with X-Gal for β-galactosidase activity reflecting Cre-mediated activation of the lacZ reporter gene. 53.7 ± 12.9% (mean ± SD; n = 3) of motoneurons in the adult facial nucleus appeared X-Gal positive. (F) Breeding scheme to produce mice with a neuron-specific Stat3 gene ablation. Mice with the indicated genotypes were crossed to produce mice lacking functional Stat3 in motoneurons (NF-L–Cre; Stat3flox/KO) or mice carrying at least one wild-type Stat3 allele (NF-L–Cre; Stat3flox/wt) at a 50% ratio. Bar, 100 μm.
To test whether Cre is active in embryonic motoneurons, we analyzed NF-L–Cre transgenic lacZ reporter mice at embryonic day 13 (Fig. 2 A). At this stage Cre-mediated activation of the lacZ reporter gene was detectable in many spinal motoneurons (Fig. 2 A). We then isolated motoneurons from 12-d-old NF-L–Cre; Stat3flox/wt and NF-L–Cre; Stat3flox/KO embryos. After 1 d in culture, a prominent signal for Cre expression and a clear band for the recombined Stat3 gene was detectable, whereas a reduction of the wild-type transcript by at least 50% was apparent (Fig. 2 B). We have also isolated sensory neurons from E14 NF-L–Cre; Stat3flox/KO mice to test for Cre-mediated recombination of the floxed Stat3 allele. Although the NF-L promoter is only active in a subpopulation of DRG neurons (unpublished data), a prominent band corresponding to the recombined Stat3 (Fig. 2 B) was detectable. These data demonstrate that Stat3 gene deletion has already started around E12 in motoneurons, 2 d before these cells were isolated for testing survival responses to various neurotrophic factors.
Figure 2.
Spinal motoneurons do not depend on Stat3 signaling during the phase of naturally occurring cell death. (A) Embryonic motoneurons express Cre under control of the human NF-L promoter. E13 embryos from NF-L–Cre transgenic lacZ reporter mice were processed for β-galactosidase activity. A section through the lumbar spinal cord shows β-gal positive neurons (blue, arrows) only in the ventrolateral motor columns. (B) RT-PCR shows the presence of the recombined shortened Stat3 transcript lacking exon 22 as early as E12. Motoneurons from NF-L–Cre; Stat3flox/wt and NF-L–Cre; Stat3flox/KO mice were isolated, kept overnight in the presence of GDNF to recover and reexpress genes before total RNA was isolated. Sensory neurons from NF-L–Cre; Stat3flox/KO and NF-L–Cre; Stat3flox/wt mice were isolated at E14 and total RNA was prepared after 4 h in culture. (C) Embryonic motoneurons from NF-L–Cre; Stat3flox/KO mice require higher doses of CNTF for maximal survival in vitro. Motoneurons from E14 spinal cord of NF-L–Cre; Stat3flox/wt and NF-L–Cre; Stat3flox/KO mice were cultivated in the presence of BDNF (1 ng/ml), GDNF (0.1 ng/ml), and various concentrations of CNTF for 5 d. After 5 d in vitro, surviving motoneurons were counted and numbers expressed in relation to originally plated cells. Motoneurons from NF-L–Cre; Stat3flox/KO mice exhibited the same maximal survival response toward neurotrophic factors including CNTF as those from NF-L–Cre; Stat3flox/wt animals, indicating that Stat3 is not required for motoneuron survival during this critical period of development (asterisk indicates P < 0.05 ANOVA). Bar, 200 μm.
Spinal motoneurons do not depend on Stat3 signaling during the developmental period of naturally occurring cell death
Isolation and quantification of motoneurons from E14 spinal cord revealed no difference in the number of motoneurons from NF-L–Cre; Stat3flox/wt and NF-L–Cre; Stat3flox/KO embryos (unpublished data). These motoneurons were cultured in the presence of BDNF, GDNF, or CNTF for 6 d. Motoneurons from NF-L–Cre; Stat3flox/KO embryos did not show any alteration in their survival response to BDNF and GDNF (Fig. 2 C). In contrast, a shift in the dose–response curve to CNTF was observed. Motoneurons lacking endogenous Stat-3 (NF-L–Cre; Stat3flox/KO) require higher concentrations for their survival. 50 ng/ml of CNTF supported maximal survival of neurons at a rate of 47.1 ± 2.3% of originally plated cells. This maximal survival rate did not differ from survival rates of wild-type motoneurons (52.5 ± 10.6%; Fig. 2 C). We then counted the numbers of facial motoneurons in E19 embryos and could not detect a significant reduction in NF-L–Cre; Stat3flox/KO versus NF-L–Cre; Stat3flox/wt mice. Similarly, no reduction was observed in the number of motoneurons in the lumbar spinal cord at E19 (unpublished data). Accordingly, we have counted motoneuron numbers in the facial nucleus of 6-wk-old mice. No reduction of motoneuron numbers could be observed (Table I), indicating that the rate of physiologically occurring cell death of motoneurons during late embryonic development and the perinatal period is not enhanced. This supports the conclusion from our in vitro results that Stat3 is dispensable for motoneuron survival during the critical period of developmental motoneuron cell death.
Table I.
NF-L–Cre; Stat3 flox/KO mice do not display significant motoneuron loss compared to NF-L–Cre; Stat3 flox/wt littermates
| NF-L–Cre; Stat3flox/wt | NF-L–Cre; Stat3flox/KO | |||||
|---|---|---|---|---|---|---|
| Mean | SEM | n | Mean | SEM | n | |
| Facial motoneurons (6 wk)a | 2,206 | 88 | 4 | 2,281 | 89 | 4 |
| Facial motoneurons (1 yr)a | 2,243 | 100 | 8 | 2,201 | 56 | 6 |
| Lumbar spinal motoneurons (1 yr) | 2,506 | 34 | 3 | 2,184 | 187 | 3 |
Number of motoneurons per side.
Neuron-specific Stat3 null mice do not show enhanced neuronal degeneration during postnatal development
Mice devoid of functional Stat3 in motoneurons (NF-L–Cre; Stat3flox/KO) are born at the expected Mendelian ratio and develop normally. In contrast to mice with inactivation of Stat3 in a broad range of organs and tissues using the balancer-Cre transgenic strain (Alonzi et al., 2001), our mice are viable and fertile and do not show any obvious abnormalities. Using a motor performance test developed in our laboratory (Masu et al., 1993), we could not find any functional motor deficit in 1-yr-old NF-L–Cre; Stat3flox/KO mice (Table II). In addition, no reduction in motoneuron numbers could be detected by counting motoneurons in serial sections of facial nuclei and lumbar spinal cord of 1-yr-old NF-L–Cre; Stat3flox/KO mice (Table I).
Table II.
1-yr-old NF-L–Cre; Stat3flox/KO mice do not show impaired motor function or weight loss compared with their NF-L–Cre; Stat3 flox/wt littermates
| NF-L–Cre; Stat3flox/wt | NF-L–Cre; Stat3flox/KO | |||||
|---|---|---|---|---|---|---|
| Mean | SEM | n | Mean | SEM | n | |
| Body weight (g) | 43.6 | 2.3 | 7 | 44.0 | 3.1 | 4 |
| Running wheel (rounds)a | 16,032 | 4,172 | 7 | 24,650 | 3,347 | 4 |
| Motor performance scoreb | 19.5 | 0.41 | 7 | 18.3 | 0.63 | 4 |
Rounds in a running wheel within 7 d.
Not significant by Mann-Whitney U test.
Endogenous Stat3 is required for motoneuron survival after facial nerve transection
Endogenous CNTF and LIF play an important physiological role for motoneuron survival after nerve lesion in young adult mice (Sendtner et al., 1996, 1997). Moreover, it has been shown that Stat3 is phosphorylated in response to axotomy in adult mice and remains activated until regeneration has completed (Haas et al., 1999; Schwaiger et al., 2000). Therefore, we have investigated whether Stat3 is an essential mediator of the survival response under such pathophysiological conditions.
After facial nerve transection in 4-wk-old mice, a significant loss of facial motoneurons in NF-L–Cre; Stat3flox/KO mice as opposed to NF-L–Cre; Stat3flox/wt littermates (Fig. 3) was observed. 2 wk after lesion, 57 ± 6% (n = 6, P < 0.001 versus control side) of the axotomized facial motoneurons survived in NF-L–Cre; Stat3flox/KO mice (Fig. 3 B). This corresponds to approximately the same loss of motoneurons observed in CNTF−/−/LIF−/− mice after nerve lesion (Sendtner et al., 1996).
Figure 3.
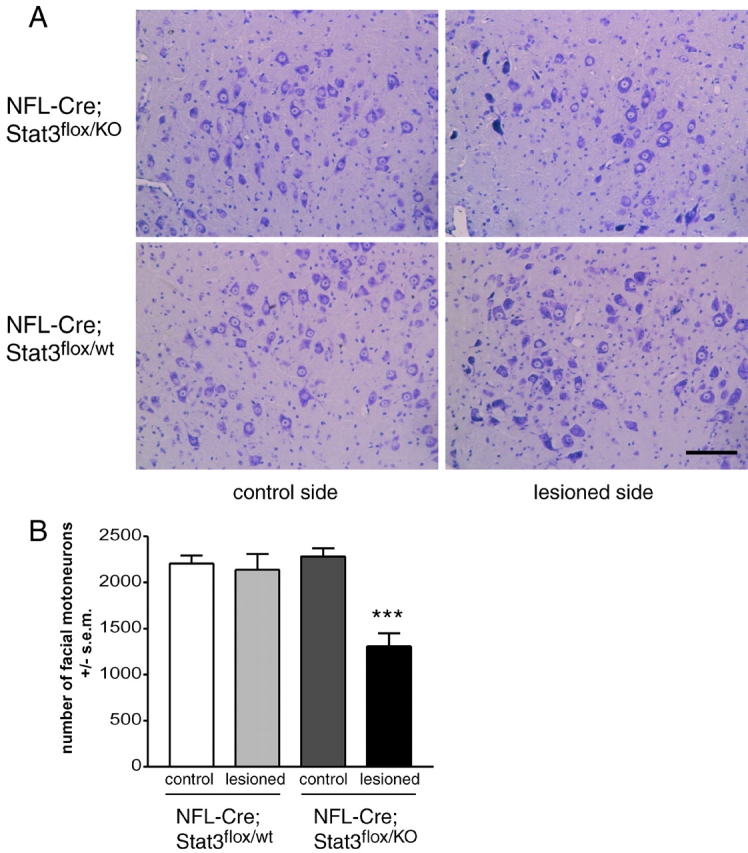
Lack of functional Stat3 in motoneurons leads to axotomy-induced motoneuron cell death in the adult. (A) Photomicrographs from Nissl-stained sections through the facial nucleus. Mice lacking functional Stat3 (NF-L–Cre; Stat3flox/KO) in motoneurons exhibit a pronounced loss of motoneurons at 2 wk after axotomy, while no loss of motoneurons is observed in littermates retaining a wild-type Stat3 allele (NF-L–Cre; Stat3flox/wt). Interestingly, the facial nuclei on the control sides of NF-L–Cre; Stat3flox/KO mice appear normal and indistinguishable from facial nuclei of NF-L–Cre; Statflox/wt mice. (B) Quantification of facial motoneurons by counting serial sections in brain stem covering the facial nuclei both on the lesioned and unlesioned side reveals a 43% loss of motoneurons in NF-L–Cre; Stat3flox/KO mice, whereas no significant motoneuron loss is observed in NF-L–Cre; Stat3flox/wt mice. Statistical analysis was performed using ANOVA with Bonferroni's post hoc comparison. Asterisks indicate P < 0.001. Bar, 100 μm.
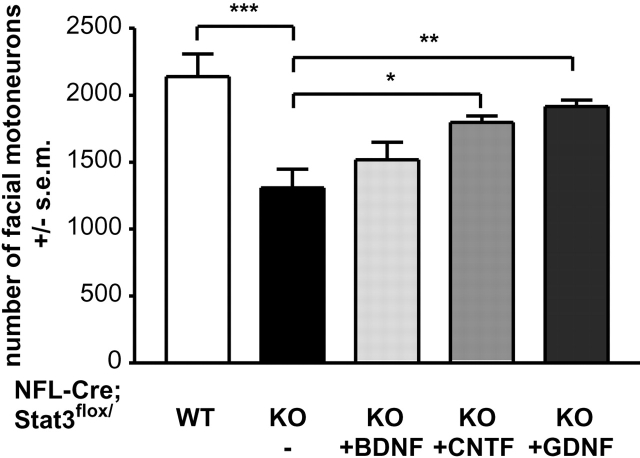
Motoneurons lacking Stat3 can be rescued from lesion-induced cell death by neurotrophic factors
Transection of the facial nerve in newborn mice leads to degeneration of more than 80% of the corresponding motoneurons. These neurons can be rescued by local administration of neurotrophic factors (Sendtner et al., 1990, 1992; Henderson et al., 1994). To test whether this survival response is impaired in the absence of Stat3, GDNF and BDNF were locally administered to the lesioned facial nerve in 4-wk-old NF-L–Cre; Stat3flox/KO and NF-L–Cre; Stat3flox/wt mice. GDNF was most potent in NF-L–Cre; Stat3flox/KO animals and increased motoneuron survival to 86 ± 2% (n = 4, P < 0.01 compared with untreated control) at 2 wk after axotomy (Fig. 4) . BDNF application led to an increase in the number of surviving motoneurons from 57 ± 6% (n = 6) to 67 ± 6% (n = 5) that did not reach statistical significance (Fig. 4). We also applied CNTF to the proximal nerve stump in NF-L–Cre; Stat3flox/KO mice. In this case, a significant increase in the number of surviving motoneurons was found (79 ± 2%, n = 4, P < 0.01 versus untreated control), indicating that CNTF-mediated survival after axotomy does not solely rely on Stat3 signaling (Fig. 4).
Figure 4.
Beneficial effect of GDNF and CNTF on the survival of axotomized NF-L–Cre; Stat3flox/KO motoneurons. CNTF and GDNF rescued >50% of otherwise degenerated motoneurons, whereas the effect of BDNF was not significant. Motoneurons were counted in serial sections through the brain stem and statistical analysis was performed using ANOVA with Bonferroni's post hoc comparison. Single asterisk indicates P < 0.05; double asterisk indicates P < 0.01; triple asterisk indicates P < 0.001.
Reg-2 and Bcl-xl are induced in a Stat3-dependent manner in axotomized motoneurons
It has been shown previously that Reg-2, a Schwann cell mitogen (Livesey et al., 1997) and motoneuron survival factor (Nishimune et al., 2000), is expressed in spinal motoneurons upon CNTF addition via LIFR-β–mediated signal transduction. Reg-2 acts on isolated motoneurons in an autocrine and paracrine mode to enhance survival (Nishimune et al., 2000). The Reg-2 promoter contains three Stat3 binding sites and is up-regulated in response to Stat3 activation (Dusetti et al., 1995). Therefore, Reg-2 produced by Stat3 bearing motoneurons could enhance the survival of Stat3 null motoneurons (that cannot make Reg-2 on their own) in a paracrine manner. Stat3 phosphorylation in facial motoneurons peaks at 2 d after axotomy (Schwaiger et al., 2000). Therefore, we have chosen this time point to measure expression of Reg-2 in the facial nucleus by semiquantitative RT-PCR. After facial nerve lesion in NF-L–Cre; Stat3flox/wt mice, Reg-2 mRNA is induced 11.1 ± 4.67-fold in the facial nucleus on the lesioned side, demonstrating that Reg-2 expression is induced by axotomy in facial motoneurons as shown previously for spinal motoneurons (Livesey et al., 1997). In NF-L–Cre; Stat3flox/KO mice Reg-2 expression increases 3.82 ± 2.6-fold compared with the unlesioned control (means calculated from three experiments). Reg-2 mRNA levels in NF-L–Cre; Stat3flox/KO mice are only 34% of those observed in NF-L–Cre; Stat3flox/wt mice (n = 4–5, P < 0.05; Fig. 5 A). Therefore, Stat3 is a likely regulator of Reg-2 expression in vivo. In situ hybridization on sections including both facial nuclei on the lesioned and the control side showed that Reg-2 expression was confined to motoneurons (Fig. 5 B).
Figure 5.
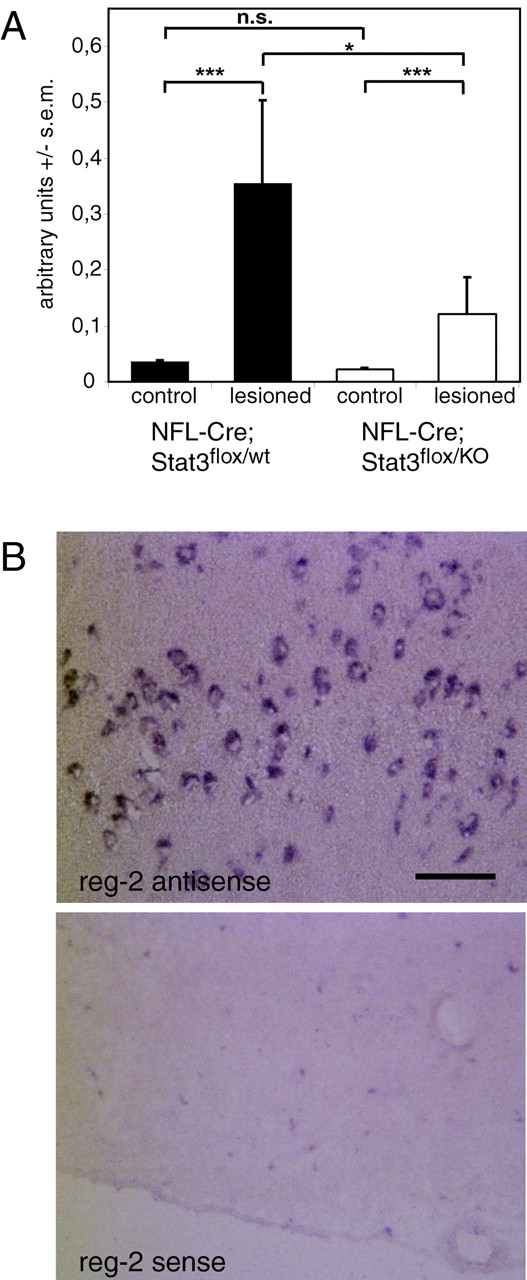
Reg-2 expression is induced in axotomized facial motoneurons in a Stat3-dependent manner. (A) RT-PCR analysis using total RNA derived from acutely isolated facial nuclei revealed an increase of Reg-2 mRNA 2 d after facial nerve transection. In NF-L–Cre; Stat3flox/KO mice, Reg-2 mRNA levels are only 34% of the amount in the NF-L–Cre; Stat3flox/wt littermates. This indicates that Stat3 is involved in the upregulation of Reg-2 in motoneurons after axotomy (n = 5, P < 0.05, ANOVA with Bonferroni's post hoc comparison). Results shown are from one representative experiment of three experiments with similar results. (B) In situ hybridization reveals that Reg-2 is specifically expressed in facial motoneurons, but not in surrounding glial cells. The bottom panel shows an adjacent section hybridized with the sense probe. Bar, 100 μm.
We have also investigated other Stat3-regulated genes which modulate neuronal survival. Bcl-xl is highly expressed in various regions of the nervous system (Motoyama et al., 1995), including the facial nucleus (Hamner et al., 1999; Fig. 6 B). Using semiquantitative RT-PCR we found that Bcl-xl expression is induced 8.13 ± 2.02-fold at 48 h after axotomy in the facial nucleus of control NF-L–Cre; Stat3flox/wt mice. In the facial nucleus of NF-L–Cre; Stat3flox/KO littermates, Bcl-xl expression is induced 5.3 ± 0.46-fold (means calculated from three experiments). Thus, in NF-L–Cre; Stat3flox/KO mice Bcl-xl expression in the facial nucleus is only 65% of that observed in NF-L–Cre; Stat3flox/wt (n = 3–5; P < 0.05), indicating that Stat3 significantly contributes to the upregulation of Bcl-xl in lesioned motoneurons (Fig. 6 A). In addition, we tested the expression of Bcl-2 and Bcl-w in the facial nucleus on the lesioned and the control side of NF-L–Cre; Stat3flox/KO and NF-L–Cre; Stat3flox/wt mice. None of these two genes was significantly induced after axotomy, independent of the presence or absence of Stat3 (Table III). Similarly, expression of Bax, a proapoptotic member of this family was similar in NF-L–Cre; Stat3flox/wt and NF-L–Cre; Stat3flox/KO mice, and no up-regulation was observed on the lesioned side on the mRNA level.
Figure 6.
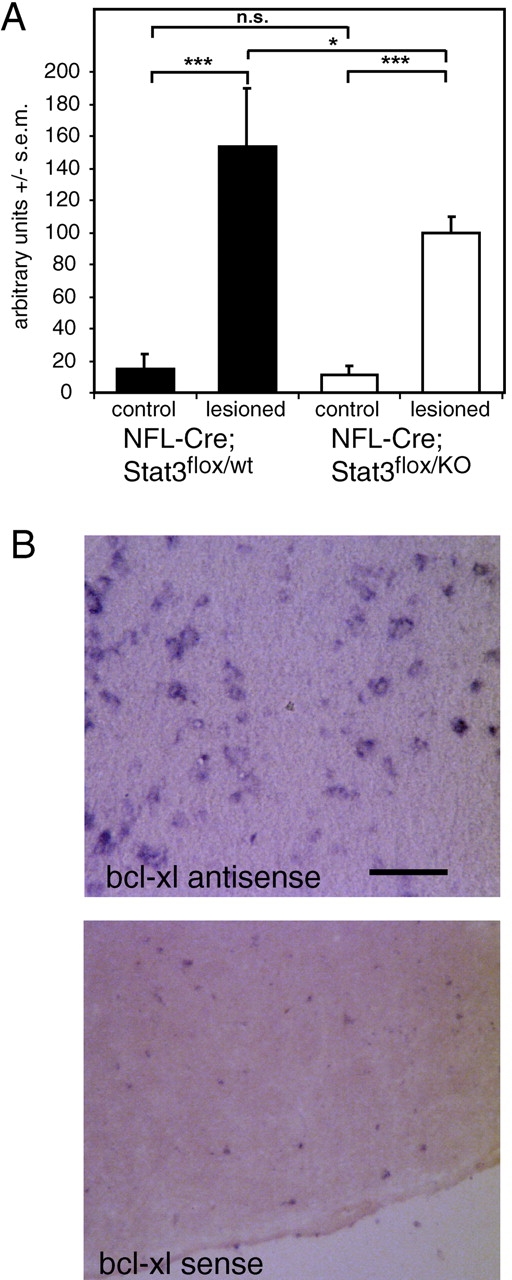
Bcl-xl expression in axotomized facial motoneurons depends on Stat3. (A) RT-PCR analysis using total RNA derived from acutely isolated facial nuclei revealed an increase of Bcl-xl mRNA 2 d after facial nerve transection. In NF-L–Cre; Stat3flox/KO mice, Bcl-xl mRNA levels are only 65% of the amount in the NF-L–Cre; Stat3flox/wt littermates. This indicates that Stat3 is involved in the upregulation of Bcl-xl in motoneurons after axotomy (n = 5, P < 0.05, ANOVA with Bonferroni's post hoc comparison). Results shown are from one representative experiment of three experiments with similar results. (B) In situ hybridization reveals that Bcl-xl is specifically expressed in murine facial motoneurons, but not in surrounding glial cells. The bottom panel shows an adjacent section hybridized with the sense probe. Bar, 100 μm.
Table III. Summary of gene expression profiles for Bcl-2 and IAP gene families in NF-L–Cre; floxed Stat3 mice. Fold change in gene expression after axotomy relative to control side.
| NF-L–Cre; Stat3flox/wt | NF-L–Cre; Stat3flox/KO | |
|---|---|---|
| Bcl-2 | 1.0 ± 0.30 | 0.75 ± 0.45 |
| Bcl-w | 1.1 ± 0.34 | 1.1 ± 0.32 |
| Bax | 1.0 ± 0.09 | 1.0 ± 0.10 |
| c-IAP-1 | 1.0 ± 0.24 | 1.2 ± 0.34 |
| c-IAP-2 | 1.2 ± 0.34 | 1.5 ± 0.38 |
| XIAP | 0.9 ± 0.16 | 1.5 ± 0.20 |
IAP gene expression is not induced after facial nerve lesion
In embryonic neurons, IAP proteins (Deveraux et al., 1998) that function as protein inhibitors of caspases are essential components of the signaling pathways of neurotrophic factors (Wiese et al., 1999a, 2001). To test whether these molecules are involved in axotomy-induced cell death of NF-L–Cre; Stat3flox/KO motoneurons, we performed semiquantitative RT-PCR specific for c-IAP1, c-IAP-2, and XIAP using RNA from facial nuclei of adult NF-L–Cre; Stat3flox/wt and NF-L–Cre; Stat3flox/KO mice as templates. No change in IAP expression was observed at 48 h after axotomy. Moreover, no difference was found between NF-L–Cre; Stat3flox/wt and NF-L–Cre; Stat3flox/KO mice, indicating that Stat3 does not play a role in regulation of IAP gene expression. (Table III).
Discussion
We have investigated the role of the Stat3 gene in neuronal survival by Cre-mediated conditional gene inactivation. We find that Stat3 is dispensable for survival of motoneurons during the period of naturally occurring cell death (Fig. 2). Moreover, at least under physiological conditions, motoneuron survival is not reduced in postnatal NF-L–Cre; Stat3flox/KO animals (Fig. 3 and Table I), and no functional defect can be observed in mice which lack Stat3 expression in motoneurons (Table II). However, deficiency of Stat3 leads to the requirement of higher doses of CNTF for maximal survival of isolated embryonic motoneurons in cell culture. Stat3 is also required for motoneuron survival after nerve lesion in 6-wk-old mice (Fig. 3). The loss of axotomized motoneurons in mice with neuron-specific gene ablation of Stat3 can be significantly reduced by GDNF and high dose locally applied CNTF (Fig. 4). We have further demonstrated that upregulation of Reg-2 and Bcl-xl expression is reduced in axotomized motoneurons in NF-L–Cre; Stat3flox/KO mice (Figs. 5 and 6), whereas other antiapoptotic genes, such as IAP-1, IAP-2, XIAP, and Bcl-2 are not (Table III).
The signaling pathways used by neurotrophic cytokines of the LIF/CNTF/CT-1 family to promote neuronal survival are still not fully understood. Recent studies have shown that CNTF and NGF promote neuronal survival via upregulation of ITA gene expression in isolated chick sensory and sympathetic neurons (Wiese et al., 1999a). Members of the mammalian IAP family are highly upregulated by NF-κB activation, and it is thought that NF-κB is part of the signaling cascades that promote neuronal survival downstream of neurotrophic factor receptors, B-Raf, and PI-3K/Akt activation (Wiese et al., 2001). This is in agreement with recent findings that inhibition of NF-κB activation by I-κB expression or p65 gene targeting in primary neurons (Middleton et al., 2000) and inhibition of the PI-3K/Akt pathway (Alonzi et al., 2001) reduces survival in response to CNTF. However, survival in response to BDNF appeared unaffected by NF-κB inhibition (Middleton et al., 2000). It will be extremely interesting to find out whether survival by neurotrophins is mediated via other complementary pathways which upregulate IAPs under these experimental conditions. Alternatively, specific CNTF-signaling mediators such as Stat3 could inhibit such complementary pathways and thus interfere with signals after neurotrophin receptor activation. Indeed, it has been shown that Stat3 interferes with neurotrophin-activated signaling pathways, and thus inhibits NGF-mediated differentiation of PC-12 cells (Ihara et al., 1997). Another example how LIFR-β activation interferes with neurotrophin-mediated signaling pathways are experiments in which LIF promotes cell death of sympathetic neurons which depend on NGF for their survival (Kessler et al., 1993). Along these lines of evidence, Stat3 is not expected as an essential mediator of neuronal survival. It could even suppress survival of neurons in response to other classes of neurotrophic factors, in particular neurotrophins. In vivo models, in particular gene knockout mice, are therefore an ideal tool to study direct and indirect effects of gene inactivation under physiological conditions where these factors play together in promoting neuronal survival. Gene ablation studies have shown that GDNF (Moore et al., 1996; Sanchez et al., 1996; Oppenheim et al., 2000), CT-1 (Oppenheim et al., 2001), and corresponding receptor subunits such as LIFR-β (Li et al., 1995), gp130 (Nakashima et al., 1999), and CNTFR-α (DeChiara et al., 1995) are necessary for motoneuron survival during development, at least in subpopulations. In contrast, no enhanced motoneuron cell death could be observed in mice which lack receptors for neurotrophins (Smeyne et al., 1994; Klein et al., 1994; DeChiara et al., 1995), thus pointing to an important role of ligands for gp 130/LIFR-β as modulators of motoneuron survival during development. Gp130 and LIFR-β activate several signaling pathways, including Stat3 as a downstream effector. Therefore, it was suggested that Stat3 contributes to the survival effect of CNTF and related cytokines on motoneurons (Stahl and Yancopoulos, 1994). This hypothesis was substantiated by the finding that Reg-2, a Stat3-dependent gene, is involved in CNTF-mediated survival of isolated embryonic rat motoneurons (Nishimune et al., 2000). Reg-2 influences survival of embryonic motoneurons via NF-κB, which is in agreement with the hypothesis that NF-κB mediates neuronal survival via upregulation of IAP proteins.
In NF-L–Cre; Stat-3flox/KO mice, axotomized motoneurons can be rescued from cell death by the application of the neurotrophic factors BDNF, GDNF and, interestingly, also by CNTF. Thus, the effect of Stat3 deficiency could be overcome by other signaling pathways which are activated by CNTF when added locally at high concentrations to lesioned motoneurons. This finding corresponds to our in vitro data with embryonic motoneurons from NF-L–Cre; Stat3flox/KO mice showing reduced survival responses to CNTF at low doses. However, maximal survival of motoneurons could be achieved with high dose recombinant rat CNTF. Nevertheless, our data suggest that the signaling pathways which support motoneuron survival during development and after lesion differ from each other. In developing sensory and sympathetic neurons, CNTF and NGF lead to upregulation of ITA, the chick homologue of mammalian IAP-2 and/or XIAP. Interestingly, lesion of the facial nerve in adult mice does not lead to upregulation of IAP-1, IAP-2, and XIAP after binding of endogenous neurotrophic factors to their receptors on motoneurons. Therefore, it will be interesting to know whether other molecules such as NAIPs become important during postnatal life (Perrelet et al., 2000). Indeed, expression of all known members of the NAIP family is low in the nervous system of embryonic mice but upregulated after birth (Götz et al., 2000). At least for members of the NAIP family, it has been shown that they can inhibit neurite outgrowth (Götz et al., 2000). It is not known whether members of the IAP family exert similar effects. This, however, would counteract regeneration and regrowth of nerve fibers to their denervated targets. In addition, Bcl-2 overexpression promotes axonal regeneration of retinal ganglion cells (Chen et al., 1997). Therefore, signaling effects of IAPs and members of the Bcl-2 family could lead to additional cellular responses besides preventing apoptosis, and thus play an important role in the cellular programs leading to regeneration of motoneurons after lesion. In this study, we have found that Bcl-xl is highly upregulated in motoneurons after lesion. Previous studies have shown that Bcl-xl is an important mediator of neuronal survival (Gonzalez-Garcia et al., 1995). Gene ablation of Bcl-xl leads to massive neuronal cell death around embryonic day 13 (Motoyama et al., 1995). Mice overexpressing Bcl-xl (Parsadanian et al., 1998) are resistant to lesion-induced cell death. Thus, the upregulation of Bcl-xl by Stat3 appears as an essential component of neurotrophic signaling leading to survival of adult axotomized motoneurons.
NF-L–Cre; Stat3flox/KO mice do not exhibit enhanced motoneuron loss during postnatal development up to 1 yr of age. This finding indicates that Stat3 is dispensable for CNTF/LIF/CT-1/CLC-mediated survival of motoneurons under physiological conditions. In adult control mice, facial nerve transection leads only to minor loss of corresponding motoneurons. Under such conditions, endogenous neurotrophic factors such as CNTF, LIF, BDNF, and others become available to the axotomized neurons and promote their survival (Sendtner et al., 1997). In mice carrying null mutations for both CNTF and LIF, ∼40% of facial motoneurons are lost, indicating that factors of this gene family act as essential lesion factors (Sendtner et al., 1996). Our results indicate that these survival effects involve Stat3. We do not know whether the population of neurons which degenerates after axotomy in CNTF/LIF double knockout mice is the same as that in the NF-L–Cre; Stat3flox/KOmice. Given that not all motoneurons show recombination of the Stat3 gene in our study, it is likely that the rate of motoneuron cell death would even be higher under such ideal experimental conditions and thus exceed the loss observed in CNTF/LIF double knockout mice. This suggests that additional factors of the CNTF family such as CLF/CLC (Elson et al., 2000) act together with CNTF and LIF on lesioned motoneurons, and thus support their survival after nerve lesion in the adult.
In conclusion, our data reveal an essential function of Stat3 signaling for survival of adult motoneurons after axotomy, but not for survival of motoneurons during the developmental period of physiologically occurring cell death. This indicates that the contribution of various signaling cascades, which are activated in response to neurotrophic factors in order to support motoneuron survival, changes during development and that Stat3 becomes increasingly important after pathophysiological conditions.
Materials and methods
Generation of NF-L–Cre mice
The plasmid dNF-L containing a fragment of the human NF-L gene and promoter was a gift from J.P. Julien (McGill University, Montreal, Canada). A 6-kb EcoRI fragment containing the NF-L promoter and the NF-L gene up to the poly A-signal was subcloned in pBluescript II KS(+), resulting in a deletion of 3.4 kb of 3′ untranslated region (covered by EMBL/GenBank/DDBJ accession nos. X05608 and L04147). This vector, pKS.NF-L, was digested with ClaI and XhoI (to delete the coding region of exon 1), end-filled, and ligated to a blunt-ended CrepA fragment from our lab derived from pMC-Cre (a gift from H. Gu and K. Rajewsky, University of Cologne, Germany) to generate pKS.NF-L–Cre. pKS.neo was obtained by inserting a NheI-BamHI fragment containing the HSV-TK promoter neomycin resistance gene from pGH1 (Gu et al., 1993) into BamHI-XbaI–digested pBluescript II KS(+). A SmaI fragment from pKS.NF-L–Cre containing ∼2 kb of NF-L promoter sequence, Cre cDNA, polyadenylation signal, and the NF-L downstream region (intron 1 to endogenous polyadenylation signal) was subsequently ligated into the SmaI site of pKS.neo placing the NF-L–Cre sequences 3′ to the neomycin resistance cassette. The neo–NF-L–Cre fragment was isolated from this vector by KpnI-NotI digestion and gel-purified before electroporation into R1 embryonic stem cells (provided by A. Nagy, Toronto, Canada).
Transgenic G418-resistant clones were subjected to Southern blot analysis. Genomic DNA was digested with XbaI and probed with a CrecDNA (491bp MluI-EcoRV fragment; probe A). Chimeric mice were obtained by morula aggregation, and two transgenic clones (#50 and #13) gave rise to germline transmitting chimeric mice. Offspring from line #50 and #13 was bred onto CD-1 and C57Bl/6 genetic backgrounds, respectively. Tail DNA was also tested by Southern Blot (digested with EcoRI, probed with the Cre cDNA probe A), whereas PCR was used for routine genotyping of transgenic offspring. All data in this study were derived from animals of the NF-L–Cre line #50. Line #13 did not express Cre activity and was not maintained.
PCR protocols for genomic DNA
Inheritance of the NF-L–Cre transgene was checked by PCR on tail tip biopsies using the primers NF-L–SEQ 5′-TCG CAG GCT GCG TCA GGA G-3′ and pMC-Cre 5′-GGT ATG CTC AGA AAA CGC C-3′ that detect the unique combination of the human NF-L promoter and Cre cDNA. The product size is 250 bp. For genotyping of Stat3 transgenic animals we used the primers mStat3e22fwd 5′-CCT GAA GAC CAA GTT CAT CTG TGT GAC-3′ and mStat3e23rev 5′-CAC ACA AGC CAT CAA ACT CTG GTC TCC-3′ that yield products of 250 bp and 350 bp for wild-type and floxed Stat3, respectively. To detect the recombined lacZ reporter gene, the following primers were used: AG2 5′-CTG CTA ACC ATG TTC ATG CC-3′ and Z3 5′-GGC CTC TTC GCT ATT ACG-3′. The recombined allele yields a product of 580 bp. To detect Cre-mediated recombination of the floxed Stat3 gene we used the following primers: mStat3loxP 5′-GAT TTG AGT CAG GGA TCC ATA ACT TCG-3′ and mStat3e23rev. The recombined Stat3 allele yields a product of 150 bp.
Cross-breeding with lacZ-reporter mice and β-galactosidase histochemistry
Doubly transgenic NF-L–Cre/lacZ animals were killed by ether inhalation and perfused pericardially with 2% paraformaldehyde (PFA) in 0.1 M sodium phosphate buffer at pH 7.4. E13 embryos were immersion fixed in the same fixative for 4 h. Organs were removed and postfixed for 3 h in the same fixative and cryo-protected with 30% sucrose overnight. Tissues were embedded in Tissue-Tek (Sakura), cryo-sectioned at 20 μm, and stained for β-galactosidase activity overnight at 30°C in 0.1 M sodium phosphate buffer, pH 7.4, supplemented with 5 mM potassium hexacyanoferrate (I), 5 mM potassium hexacyanoferrate (III), 2 mM MgCl2, and 0.1% X-Gal (5-bromo-4-chloro-3-indolyl-galactoside). After X-gal staining sections were counterstained with 1% neutral red in 4 mM sodium acetate buffer, pH 3.5. Whole organs and embryos were immersed into X-Gal staining solution over night.
Motoneuron culture
Motoneurons were isolated from E14 spinal cord from individual embryos and cultured for at least 5 d (Wiese et al., 1999b, 2001) in the presence of BDNF (1 ng/ml), GDNF (0.1 ng/ml) or various concentrations of CNTF ranging from 0.005 ng/ml to 50 ng/ml. Genotyping of embryos was done after counting was complete to avoid observer bias.
Facial nerve lesion, application of factors and histological staining
In 4–5 week old anesthetized mice, the right facial nerve was exposed as it exits from the foramen stylomastoideum and transected with microscissors. Neurotrophic factors (5 μg) were applied in collagen foam. 2 wk later, the animals were perfused with 4% paraformaldehyde, brain stems were removed, and paraffin sections of 7 μm were Nissl stained. Motoneurons in the facial nucleus were counted in every fifth section as described (Sendtner et al., 1996).
Semiquantitative RT-PCR analysis
Extraction of total RNA was done using TriZol (Life Technologies) reagent. Using Superscript II reverse transcriptase (Life Technologies), 100 ng of total RNA were was transcribed per 10 μl with random hexamer primers following the supplied protocol including RNase H digestion. Cycle numbers of PCR reactions were optimized to yield a linear relationship between cDNA and signal intensity. PCR products were either visualized directly in ethidium bromide–stained agarose gels or transferred by Southern blotting on nitrocellulose membranes followed by hybridization with random priming labeled probes (for Bcl-xl and Reg-2). Quantification was performed using the Fuji BAS Reader and AIDA image analysis software (Raytest) and resulting values were normalized to EF1 signals. Primer sequences are given below followed by product size, cycle numbers, and annealing temperature. Cre mRNA: Cre2f 5′-ACG ACC AAG TGA CAG CAA TGC-3′; Cre1r 5′-CTC CCA CCG TCA GTA CGT GAG ATA-3′; 265 bp; 30; 58°C. β-Actin mRNA: Act-f 5′-GTG GGC CGC CCT AGG CAC CAG-3′; Act-r 5′-CTC TTT AAT GTC ACG CAC GAT TTC-3′; 539 bp; 30; 58°C. Stat3 mRNA: mStat3e22fwd 5′-CCT GAA GAC CAA GTT CAT CTG TGT GAC-3′; mStat3e23rev2 5′-CTG AGG GCT CAG CAC CTT; 150 bp; 30–35; 56°C. Stat3 mRNA Δ exon 22: mStat3e21fwd 5′-GTC TCC ACT TGT CTA CCT CTA-3′; mStat3e23rev2; 241 bp (wt), 148 bp (Δ 22); 35; 56°C. EF-1 mRNA: mefs 5′-ACA CGT AGA TTC CGG CAA GTC-3′; mefas 5′-CAA CAA TCA GGA CAG CAC ACT C-3′; 350 bp; 25–30; 55°C. Reg-2 mRNA: mReg-2fwd 5′-AGG AGA AGA CTC TCC GAA G-3′; mReg-2rev 5′-TTA ACC AGT AAA TTT GCA G-3′; 750 bp; 23–30; 53°C. Bcl-2 mRNA: bcl2fwd 5′-CTT TGT GGA ACT GTA CGG CCC CAG CAT GCG-3′; bcl2rev 5′-ACA GCC TGC AGC TTT GTT TCA TGG TAC ATC-3′; 250 bp; 22; 55°C. Bax mRNA: baxfwd 5′-TGG AGC TGC AGA GGA TGA TT-3′; baxrev 5′-AAG TTG CCA TCA GCA AAC AT; 95 bp; 35; 55°C (Greenlund et al., 1995). Bcl-xl mRNA: bcl-xlsense 5′-AGG CTG GCG ATG AGT TTG AA-3′; bcl-xlantisense 5′-CGG CTC TCG GCT GCT GCA TT-3′; 336 bp; 24; 60°C (Imaizumi et al., 1999). Bcl-w mRNA: bcl-wfwd 5′-GAA TTC ATG GCG ACC CCA GC-3′; bcl-wrev 5′-TAG ACT TTC TCA CTT GCT AGC-3′; 780 bp; 22; 55°C. Primer sequences and PCR conditions for c-IAP1, c-IAP2, and XIAP were as published previously (Wiese et al., 2001).
In situ detection of gene expression
Mouse brains were freshly dissected, embedded in Tissue-Tek, and flash-frozen in isopentane and stored at –80°C. Serial cryosections of 10 μm were collected and every tenth section was stained with the Nissl method to identify brain stem nuclei. After fixation with 4% paraformaldehyde and washes in PBS, sections were acetylated and washed in PBS and DEPC water. After 1 h prehybridization at 60°C in hybridization buffer (600 mM NaCl, 10 mM Tris HCl, pH 7.5, 1 mM EDTA, 0.05% yeast tRNA, 1× Denhardt's solution, 50% dextransulfate, and 100 μg/ml salmon sperm DNA), hybridization was performed using DIG-labeled probes (Roche T7/SP6-DIG RNA labeling kit; Roche) overnight at the same conditions. The following washes of 20 min were performed at room temperature: 2× SSC, 1× SSC, RNase A 20 mg/l, 1× SSC, 0.2× SSC. Afterwards, the sections were washed at 60°C in 0.2× SSC for 1 h followed by a 20 min wash in water at room temperature. Samples were blocked over night in 100 mM Tris, pH 7.5, 150 mM NaCl, and 2% FCS at 4°C. Anti-DIG Fab-fragment conjugated to alkaline phosphatase (Roche) was used at 1:500 dilution in blocking buffer. After 4 h incubation at room temperature, the sections were washed twice in blocking buffer without serum (pH 7.5 then pH 9.5) and developed using the BCIP/NBT system (DAKO) in the presence of 0.2 mM levamisol. Probes used were the complete open reading frame of rat Reg-2 (GI254694, a gift from C.E. Henderson, Marseille), the Cre open reading frame (GI15135), and the 336 bp mouse Bcl-xl fragment (GI506647 position 275–611) used for RT-PCR analysis. Controls included the same sequences in the sense orientation and a negative control without RNA probe to check for unspecific, endogenous phosphatase activity.
Testing of motor function
According to published procedures (Masu et al., 1993; Holtmann et al., 1999).
Statistical analysis
Results were analyzed using Graph Pad Prism Software. Student's t test, Mann-Whitney U Test and one-way ANOVA, followed by Bonferroni's post-hoc comparison were applied where appropriate. The null hypothesis was rejected on the basis of P < 0.05. Results are given as mean ± SEM unless otherwise indicated.
Acknowledgments
The authors are grateful to J. Kara and H. Brünner for expert histological assistance and to L. Schomburg for help with ISH. We also thank C. Goridis for communicating results on Cre expression in NF-L–Cre mice and two anonymous reviewers for helpful comments.
This study was supported by grants from the Deutsche Forschungsgemeinschaft, SFB 487, TP C4.
Jennifer Gunnerson's present address is Developmental Biology, Howard Florey Institute, Victoria 3010, Melbourne, Australia.
Footnotes
Abbreviations used in this paper: CNTF, ciliary neurotrophic factor; IAP, inhibitor of apoptosis; KO, knockout; LIF, leukemia inhibitory factor; NF-L, neurofilament light chain; PI-3K, phosphatidylinositol 3 kinase; RT, reverse transcription.
References
- Akagi, K., V. Sandig, M. Vooijs, D. Van, V.M. Giovannini, M. Strauss, and A. Berns. 1997. Cre-mediated somatic site-specific recombination in mice. Nucleic Acids Res. 25:1766–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akassoglou, K., L. Probert, G. Kontogeorgos, and G. Kollias. 1997. Astrocyte-specific but not neuron-specific transmembrane TNF triggers inflammation and degeneration in the central nervous system of transgenic mice. J. Immunol. 158:438–445. [PubMed] [Google Scholar]
- Alonzi, T., G. Middleton, S. Wyatt, V. Buchman, U.A.K. Betz, W. Müller, P. Musani, V. Poli, and A.M. Davies. 2001. Role of Stat3 and PI 3-kinase/Akt in mediating the survival actions of cytokines on sensory neurons. Mol. Cell. Neurosci. 18:270–282. [DOI] [PubMed] [Google Scholar]
- Brunet, A., A. Bonni, M.J. Zigmond, M.Z. Lin, P. Juo, L.S. Hu, M.J. Anderson, K.C. Arden, J. Blenis, and M.E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 96:857–868. [DOI] [PubMed] [Google Scholar]
- Cao, X., A. Tay, G.R. Guy, and Y.H. Tan. 1996. Activation and association of Stat3 with Src in v-Src-transformed cell lines. Mol. Cell. Biol. 16:1595–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone, M.H., N. Roy, H.R. Stennicke, G.S. Salvesen, T.F. Franke, E. Stanbridge, S. Frisch, and J.C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science. 282:1318–1321. [DOI] [PubMed] [Google Scholar]
- Chen, D.F., G.E. Schneider, J.C. Martinou, and S. Tonegawa. 1997. Bcl-2 promotes regeneration of severed axons in mammalian CNS. Nature. 385:434–439. [DOI] [PubMed] [Google Scholar]
- Datta, S.R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M.E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 91:231–241. [DOI] [PubMed] [Google Scholar]
- DeChiara, T.M., R. Vejsada, W.T. Poueymirou, A. Acheson, C. Suri, J.C. Conover, B. Friedman, J. McClain, L. Pan, and N. Stahl. 1995. Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits at birth. Cell. 83:313–322. [DOI] [PubMed] [Google Scholar]
- Deckwerth, T.L., J.L. Elliott, C.M. Knudson, E.M. Johnson, W.D. Snider, and S.J. Korsmeyer. 1996. BAX is required for neuronal death after trophic factor deprivation and during development. Neuron. 17:401–411. [DOI] [PubMed] [Google Scholar]
- Deveraux, Q.L., N. Roy, H.R. Stennicke, T. Van Arsdale, Q. Zhou, S.M. Srinivasula, E.S. Alnemri, G.S. Salvesen, and J.C. Reed. 1998. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 17:2215–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusetti, N.J., E.M. Ortiz, G.V. Mallo, J.C. Dagorn, and J.L. Iovanna. 1995. Pancreatitis-associated protein I (PAP I), an acute phase protein induced by cytokines. Identification of two functional interleukin-6 response elements in the rat PAP I promoter region. J. Biol. Chem. 270:22417–22421. [DOI] [PubMed] [Google Scholar]
- Elson, G.C., E. Lelievre, C. Guillet, S. Chevalier, H. Plun-Favreau, J. Froger, I. Suard, A.B. de Coignac, Y. Delneste, J.Y. Bonnefoy, et al. 2000. CLF associates with CLC to form a functional heteromeric ligand for the CNTF receptor complex. Nat. Neurosci. 3:867–872. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garcia, M., I. Garcia, L. Ding, S. O'Shea, L.H. Boise, C.B. Thompson, and G. Nunez. 1995. bcl-x is expressed in embryonic and postnatal neural tissues and functions to prevent neuronal cell death. Proc. Natl. Acad. Sci. USA. 92:4304–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz, R., C. Karch, M.R. Digby, J. Troppmair, U.R. Rapp, and M. Sendtner. 2000. The neuronal apoptosis inhibitory protein suppresses neuronal differentiation and apoptosis in PC12 cells. Hum. Mol. Genet. 9:2479–2489. [DOI] [PubMed] [Google Scholar]
- Grad, J.M., X.R. Zeng, and L.H. Boise. 2000. Regulation of Bcl-xL: a little bit of this and a little bit of STAT. Curr. Opin. Oncol. 12:543–549. [DOI] [PubMed] [Google Scholar]
- Grandis, J.R., S.D. Drenning, Q. Zeng, S.C. Watkins, M.F. Melhem, S. Endo, D.E. Johnson, L. Huang, Y. He, and J.D. Kim. 2000. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc. Natl. Acad. Sci. USA. 97:4227–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlund, L.J., S.J. Korsmeyer, and E.M. Johnson. 1995. Role of BCL-2 in the survival and function of developing and mature sympathetic neurons. Neuron. 15:649–661. [DOI] [PubMed] [Google Scholar]
- Gu, H., Y.R. Zou, and K. Rajewsky. 1993. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 73:1155–1164. [DOI] [PubMed] [Google Scholar]
- Haas, C.A., H.D. Hofmann, and M. Kirsch. 1999. Expression of CNTF/LIF-receptor components and activation of STAT3 signaling in axotomized facial motoneurons: evidence for a sequential postlesional function of the cytokines. J. Neurobiol. 41:559–571. [DOI] [PubMed] [Google Scholar]
- Hamner, S., Y. Skoglosa, and D. Lindholm. 1999. Differential expression of bcl-w and bcl-x messenger RNA in the developing and adult rat nervous system. Neuroscience. 91:673–684. [DOI] [PubMed] [Google Scholar]
- Henderson, C.E., H.S. Phillips, R.A. Pollock, A.M. Davies, C. Lemeulle, M. Armanini, L. Simmons, B. Moffet, R.A. Vandlen, and L.C. Simpson. 1994. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 266:1062–1064. [DOI] [PubMed] [Google Scholar]
- Hirano, T., K. Nakajima, and M. Hibi. 1997. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev. 8:241–252. [DOI] [PubMed] [Google Scholar]
- Holtmann, B., J. Zielasek, K.V. Toyka, and M. Sendtner. 1999. Comparative analysis of motoneuron loss and functional deficits in PMN mice: implications for human motoneuron disease. J. Neurol. Sci. 169:140–147. [DOI] [PubMed] [Google Scholar]
- Ihara, S., K. Nakajima, T. Fukada, M. Hibi, S. Nagata, T. Hirano, and Y. Fukui. 1997. Dual control of neurite outgrowth by STAT3 and MAP kinase in PC12 cells stimulated with interleukin-6. EMBO J. 16:5345–5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi, K., T. Morihara, Y. Mori, T. Katayama, M. Tsuda, T. Furuyama, A. Wanaka, M. Takeda, and M. Tohyama. 1999. The cell death-promoting gene DP5, which interacts with the BCL2 family, is induced during neuronal apoptosis following exposure to amyloid beta protein. J. Biol. Chem. 274:7975–7981. [DOI] [PubMed] [Google Scholar]
- Kessler, J.A., W.H. Ludlam, M.M. Freidin, D.H. Hall, M.D. Michaelson, D.C. Spray, M. Dougherty, and D.K. Batter. 1993. Cytokine-induced programmed death of cultured sympathetic neurons. Neuron. 11:1123–1132. [DOI] [PubMed] [Google Scholar]
- Klein, R., I. Silos-Santiago, R.J. Smeyne, S.A. Lira, R. Brambilla, S. Bryant, L. Zhang, W.D. Snider, and M. Barbacid. 1994. Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature. 368:249–251. [DOI] [PubMed] [Google Scholar]
- Leconte, L., O. Semonin, A. Zvara, S. Boisseau, C. Poujeol, J.P. Julien, and M. Simonneau. 1994. Both upstream and intragenic sequences of the human neurofilament light gene direct expression of lacZ in neurons of transgenic mouse embryos. J. Mol. Neurosci. 5:273–295. [DOI] [PubMed] [Google Scholar]
- Li, L., R.W. Oppenheim, M. Lei, and L.J. Houenou. 1994. Neurotrophic agents prevent motoneuron death following sciatic nerve section in the neonatal mouse. J. Neurobiol. 25:759–766. [DOI] [PubMed] [Google Scholar]
- Li, M., M. Sendtner, and A. Smith. 1995. Essential function of LIF receptor in motor neurons. Nature. 378:724–727. [DOI] [PubMed] [Google Scholar]
- Livesey, F.J., J.A. O'Brien, M. Li, A.G. Smith, L.J. Murphy, and S.P. Hunt. 1997. A Schwann cell mitogen accompanying regeneration of motor neurons. Nature. 390:614–618. [DOI] [PubMed] [Google Scholar]
- Lutticken, C., U.M. Wegenka, J. Yuan, J. Buschmann, C. Schindler, A. Ziemiecki, A.G. Harpur, A.F. Wilks, K. Yasukawa, and T. Taga. 1994. Association of transcription factor APRF and protein kinase Jak1 with the interleukin-6 signal transducer gp130. Science. 263:89–92. [DOI] [PubMed] [Google Scholar]
- Masu, Y., E. Wolf, B. Holtmann, M. Sendtner, G. Brem, and H. Thoenen. 1993. Disruption of the CNTF gene results in motor neuron degeneration. Nature. 365:27–32. [DOI] [PubMed] [Google Scholar]
- Middleton, G., M. Hamanoue, Y. Enokido, S. Wyatt, D. Pennica, E. Jaffray, R.T. Hay, and A.M. Davies. 2000. Cytokine-induced nuclear factor kappa B activation promotes the survival of developing neurons. J. Cell Biol. 148:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, M.W., R.D. Klein, I. Farinas, H. Sauer, M. Armanini, H. Phillips, L.F. Reichardt, A.M. Ryan, K. Carver-Moore, and A. Rosenthal. 1996. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 382:76–79. [DOI] [PubMed] [Google Scholar]
- Motoyama, N., F. Wang, K.A. Roth, H. Sawa, K. Nakayama, I. Negishi, S. Senju, Q. Zhang, and S. Fujii. 1995. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 267:1506–1510. [DOI] [PubMed] [Google Scholar]
- Nakashima, K., S. Wiese, M. Yanagisawa, H. Arakawa, N. Kimura, T. Hisatsune, K. Yoshida, T. Kishimoto, M. Sendtner, and T. Taga. 1999. Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation. J. Neurosci. 19:5429–5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimune, H., S. Vasseur, S. Wiese, M.C. Birling, B. Holtmann, M. Sendtner, J.L. Iovanna, and C.E. Henderson. 2000. Reg-2 is a motoneuron neurotrophic factor and a signalling intermediate in the CNTF survival pathway. Nat. Cell Biol. 2:906–914. [DOI] [PubMed] [Google Scholar]
- Oppenheim, R.W., L.J. Houenou, A.S. Parsadanian, D. Prevette, W.D. Snider, and L. Shen. 2000. Glial cell line-derived neurotrophic factor and developing mammalian motoneurons: regulation of programmed cell death among motoneuron subtypes. J. Neurosci. 20:5001–5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim, R.W., S. Wiese, D. Prevette, M. Armanini, S. Wang, L.J. Houenou, B. Holtmann, R. Gotz, D. Pennica, and M. Sendtner. 2001. Cardiotrophin-1, a muscle-derived cytokine, is required for the survival of subpopulations of developing motoneurons. J. Neurosci. 21:1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsadanian, A.S., Y. Cheng, C.R. Keller-Peck, D.M. Holtzman, and W.D. Snider. 1998. Bcl-xL is an antiapoptotic regulator for postnatal CNS neurons. J. Neurosci. 18:1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrelet, D., A. Ferri, A.E. MacKenzie, G.M. Smith, R.G. Korneluk, P. Liston, Y. Sagot, J. Terrado, D. Monnier, and A.C. Kato. 2000. IAP family proteins delay motoneuron cell death in vivo. Eur. J. Neurosci. 12:2059–2067. [DOI] [PubMed] [Google Scholar]
- Riccio, A., S. Ahn, C.M. Davenport, J.A. Blendy, and D.D. Ginty. 1999. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 286:2358–2361. [DOI] [PubMed] [Google Scholar]
- Sanchez, M.P., I. Silos-Santiago, J. Frisen, B. He, S.A. Lira, and M. Barbacid. 1996. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 382:70–73. [DOI] [PubMed] [Google Scholar]
- Schwaiger, F.W., G.H. Schmitt, A. Horvat, G. Hager, R. Streif, C. Spitzer, S. Gamal, S. Breuer, G.A. Brook, W. Nacimiento, and G.W. Kreutzberg. 2000. Peripheral but not central axotomy induces changes in Janus kinases (JAK) and signal transducers and activators of transcription (STAT). Eur. J. Neurosci. 12:1165–1176. [DOI] [PubMed] [Google Scholar]
- Sendtner, M., R. Gotz, B. Holtmann, J.L. Escary, Y. Masu, P. Carroll, E. Wolf, G. Brem, P. Brulet, and H. Thoenen. 1996. Cryptic physiological trophic support of motoneurons by LIF revealed by double gene targeting of CNTF and LIF. Curr. Biol. 6:686–694. [DOI] [PubMed] [Google Scholar]
- Sendtner, M., R. Gotz, B. Holtmann, and H. Thoenen. 1997. Endogenous ciliary neurotrophic factor is a lesion factor for axotomized motoneurons in adult mice. J. Neurosci. 17:6999–7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendtner, M., B. Holtmann, R. Kolbeck, H. Thoenen, and Y.A. Barde. 1992. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature. 360:757–759. [DOI] [PubMed] [Google Scholar]
- Sendtner, M., G.W. Kreutzberg, and H. Thoenen. 1990. Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature. 345:440–441. [DOI] [PubMed] [Google Scholar]
- Smeyne, R.J., R. Klein, A. Schnapp, L.K. Long, S. Bryant, A. Lewin, S.A. Lira, and M. Barbacid. 1994. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 368:246–249. [DOI] [PubMed] [Google Scholar]
- Stahl, N., and G.D. Yancopoulos. 1994. The tripartite CNTF receptor complex: activation and signaling involves components shared with other cytokines. J. Neurobiol. 25:1454–1466. [DOI] [PubMed] [Google Scholar]
- Takeda, K., T. Kaisho, N. Yoshida, J. Takeda, T. Kishimoto, and S. Akira. 1998. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J. Immunol. 161:4652–4660. [PubMed] [Google Scholar]
- Weng, G., M.A. Markus, A. Markus, A. Winkler, and G.D. Borasio. 1996. p21ras supports the survival of chick embryonic motor neurones. Neuroreport. 7:1077–1081. [DOI] [PubMed] [Google Scholar]
- Wiese, S., M.R. Digby, J.M. Gunnersen, R. Gotz, G. Pei, B. Holtmann, J. Lowenthal, and M. Sendtner. 1999. a. The anti-apoptotic protein ITA is essential for NGF-mediated survival of embryonic chick neurons. Nat. Neurosci. 2: 978–983. [DOI] [PubMed] [Google Scholar]
- Wiese, S., F. Metzger, B. Holtmann, and M. Sendtner. 1999. b. The role of p75NTR in modulating neurotrophin survival effects in developing motoneurons. Eur. J. Neurosci. 11:1668–1676. [DOI] [PubMed] [Google Scholar]
- Wiese, S., G. Pei, C. Karch, J. Troppmair, B. Holtmann, U.R. Rapp, and M. Sendtner. 2001. Specific function of B-Raf in mediating survival of embryonic motoneurons and sensory neurons. Nat. Neurosci. 4:137–142. [DOI] [PubMed] [Google Scholar]