Abstract
The thylakoid ΔpH-dependent/Tat pathway is a novel system with the remarkable ability to transport tightly folded precursor proteins using a transmembrane ΔpH as the sole energy source. Three known components of the transport machinery exist in two distinct subcomplexes. A cpTatC–Hcf106 complex serves as precursor receptor and a Tha4 complex is required after precursor recognition. Here we report that Tha4 assembles with cpTatC–Hcf106 during the translocation step. Interactions among components were examined by chemical cross-linking of intact thylakoids followed by immunoprecipitation and immunoblotting. cpTatC and Hcf106 were consistently associated under all conditions tested. In contrast, Tha4 was only associated with cpTatC and Hcf106 in the presence of a functional precursor and the ΔpH. Interestingly, a synthetic signal peptide could replace intact precursor in triggering assembly. The association of all three components was transient and dissipated upon the completion of protein translocation. Such an assembly–disassembly cycle could explain how the ΔpH/Tat system can assemble translocases to accommodate folded proteins of varied size. It also explains in part how the system can exist in the membrane without compromising its ion and proton permeability barrier.
Keywords: thylakoid protein transport; chloroplast; Tat protein transport; Sec independent; membrane protein assembly
Introduction
Chloroplast thylakoids and bacterial inner membranes share two pathways that transport precursor proteins with hydrophobic signal peptides (Mori and Cline, 2001). The first is called the Sec-dependent pathway. It employs the integral membrane SecYE complex and peripheral SecA ATPase to translocate proteins in an unfolded conformation (Manting and Driessen, 2000). A second pathway, called the ΔpH-dependent system (in chloroplasts) or the twin arginine translocation (Tat)* system (in bacteria and archaea), has several exceptional features that distinguish it from analogous transporters. It derives its energy solely from the transmembrane proton gradient, rather than NTP hydrolysis (Cline et al., 1992). Its precursors bear a required twin arginine in their signal peptides (Chaddock et al., 1995; Henry et al., 1997). And it transports tightly folded proteins, some larger than the thickness of the bilayer, without compromising the membrane permeability barrier (Clark and Theg, 1997; Hynds et al., 1998; Teter and Theg, 1998; Berks et al., 2000).
Biochemical and genetic studies have identified three components of the plant thylakoid ΔpH/Tat machinery: Hcf106, Tha4, and cpTatC (Settles et al., 1997; Mori et al., 1999, 2001; Walker et al., 1999). The bacterial orthologues are TatB, TatA/E, and TatC, respectively (Berks et al., 2000; Wu et al., 2000). Hcf106 and Tha4 are homologous membrane proteins with an amino-proximal membrane spanning domain followed by a stroma-facing predicted amphipathic helix and soluble carboxyl-proximal domain (Settles et al., 1997; Mori et al., 1999). cpTatC contains six predicted membrane spanning domains with amino and carboxyl termini exposed to the stromal (cis) side of the membrane (Mori et al., 2001). Each thylakoid component directly participates in the transport process because antibody against any single component specifically and completely disables ΔpH/Tat protein transport (Mori et al., 1999, 2001).
Several groups have reported on complexes of the known ΔpH/Tat-dependent components. Bolhuis et al. (2001) purified an ∼600-kD TatABC complex from digitonin-solubilized Escherichia coli membranes that contained equimolar amounts of TatB and TatC and variable amounts of TatA. Sargent et al. (2001) isolated an ∼600-kD complex from CHAPS-solubilized E. coli membranes that contained TatA and TatB in a ratio of 15:1 and formed ring-like structures (Sargent et al., 2001). Unfortunately, the functional relevance of these interesting complexes is unknown. We recently showed that the thylakoid components exist in distinct subcomplexes. Blue native gel electrophoresis and coimmunoprecipitation of digitonin-solubilized thylakoids revealed that cpTatC and Hcf106 are members of an ∼700-kD complex and that Tha4 is present in an independent complex that varies in size from 70 to 400 kD depending on solubilization conditions (Cline and Mori, 2001). Importantly, we showed that the ∼700-kD cpTatC–Hcf106 complex is a receptor that specifically binds precursors with the functional twin arginine motif (Cline and Mori, 2001). Tha4 functions after precursor binding, because antibody to Tha4, although having no effect on precursor binding, prevented the precursor from progressing past the receptor-bound state, even in the presence of a ΔpH (Cline and Mori, 2001). These observations prompted us to propose that Tha4 assembles with the receptor complex to build an active translocase when all requirements for translocation are satisfied.
Here we provide evidence that a complex of cpTatC, Hcf106, and Tha4 transiently forms during the protein translocation cycle. The association of all three components occurred only under conditions of protein transport and dissipated upon the completion of protein translocation. These results describe cycles of component assembly and disassembly that coincide with the initiation and termination of protein translocation and imply that the active translocase consists of all three components. The significance of this finding in relation to models for operation of the thylakoid ΔpH/Tat translocase is discussed.
Results and discussion
Tha4 associates with cpTatC–Hcf106 in the presence of precursor and the ΔpH
To examine transient associations that might exist during protein transport, we used a chemical cross-linking approach with intact thylakoids (Fig. 1). Thylakoids were treated with the thiol-cleavable homobifunctional cross-linker dithiobis (succinimidyl propionate) (DSP) under a variety of conditions related to protein transport. After quenching of the reaction, thylakoids were solubilized and denatured with SDS and then subjected to immunoprecipitation with specific antibodies. Bound proteins were analyzed by SDS-PAGE and immunoblotting after cross-linker cleavage to release partners.
Figure 1.
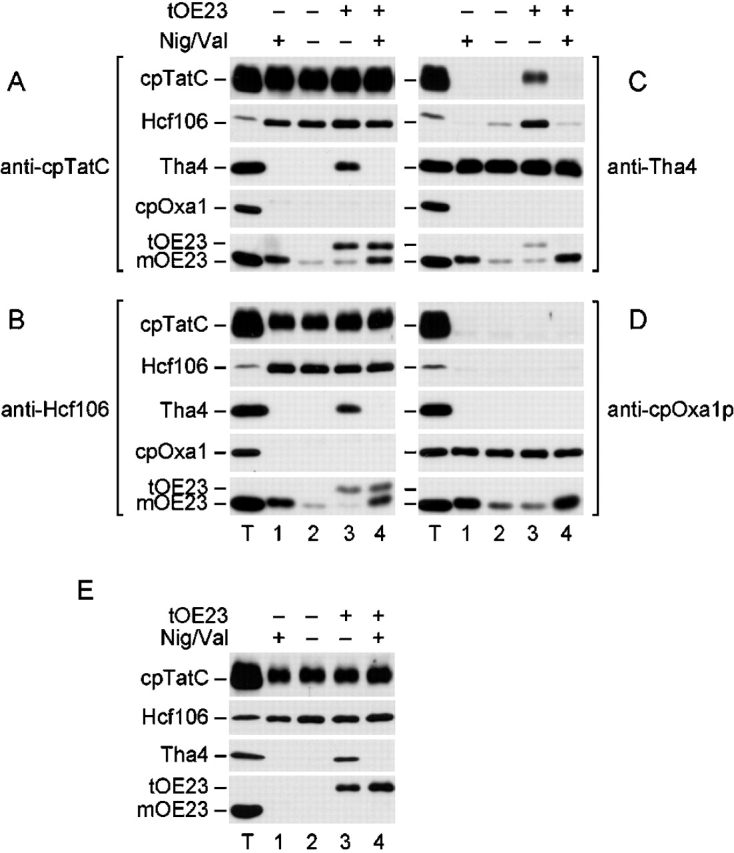
Tha4 associates with cpTatC and Hcf106 under transport conditions to form cpTatC–Hcf106–Tha4 complex. Thylakoids were incubated in the light to generate a pH gradient. Where indicated, reactions were supplemented with 0.5/1.0 μM nigericin/valinomycin (Nig/Val) to dissipate the ΔpH and/or with 1.0 μM of tOE23. Cross-linking reactions were performed with 1 mM DSP final concentration as in the Materials and methods. Cross-linked thylakoids were recovered by centrifugation, dissolved with SDS, and subjected to immunoprecipitation with anti-cpTatC (A), anti-Hcf106 (B), anti-Tha4 (C), or anti-cpOxa1p (D). Immunoprecipitated proteins, after release from cross-linked partners with β-mercaptoethanol, were analyzed by SDS-PAGE and immunoblotting. Antibodies used for immunoblotting are designated just to the left of immunoblots. Immunoprecipitates equivalent to 10 μg Chl thylakoids were loaded (lanes 1–4). A thylakoid control (lane T) contained 2.5 μg Chl of untreated thylakoids, except for the OE23 immunoblots, which contained 0.1 μg Chl. (E) Cross-linked and solubilized thylakoid proteins were first subjected to immunoprecipitation with anti-cpTatC. The resulting immunoprecipitates were eluted by incubating the antigen–IgG–protein A–Sepharose complexes with 4 M urea, 2% SDS, 125 mM Tris-HCl, pH 6.8, for 1 h at room temperature. Eluates, diluted eightfold with immunoprecipitation buffer lacking SDS, were then subjected to a second immunoprecipitation with anti-Hcf106. Immunoprecipitates were then analyzed by immunoblotting, as in A–D.
Fig. 1, A–D, depicts four panels of immunoblots; each panel resulted from immunoprecipitations with the antibody designated next to the panel, and each row represents an immunoblot with antibody to the protein specified next to the row. cpTatC and Hcf106 were consistently associated under all conditions tested, as determined by their combined presence in immunoprecipitates of either anti-cpTatC (Fig. 1 A) or anti-Hcf106 (Fig. 1 B). In contrast, Tha4 was only present in anti-cpTatC or anti-Hcf106 immunoprecipitates when thylakoids were cross-linked in the presence of tOE23, a ΔpH/Tat pathway precurser protein, and the thylakoidal ΔpH, i.e., the necessary conditions for transport (Fig. 1, A and B, lane 3). Correspondingly, cpTatC and Hcf106 were only present in anti-Tha4 immunoprecipitates when thylakoids were cross-linked under transport conditions (Fig. 1 C, lane 3). Hcf106 was frequently present in trace amounts in anti-Tha4 immunoprecipitates when either precursor or ΔpH was present alone (Fig. 1 C, lanes 2 and 4). The significance of this minor association is not known. No interactions were detected if cross-linking agent was omitted from the reaction (unpublished data; Cline and Mori, 2001).
Precursor (tOE23) was associated with cpTatC and Hcf106 either in the presence or absence of ΔpH (Fig. 1, A and B, lanes 3 and 4). Because strong thylakoid association of tOE23 (and several other authentic ΔpH/Tat substrates) was not detected previously (Ma and Cline, 2000), this result demonstrates a reversible interaction of precursor with cpTatC–Hcf106 and confirms that binding to the receptor complex is independent of the pH gradient (Cline and Mori, 2001). As expected, direct or indirect interaction of precursor with Tha4 was detected only in the presence of the ΔpH (Fig. 1 C, lane 3). Mature OE23 (mOE23) was present in varying amounts in immunoprecipitates of all the components. However, this most likely represents nonspecific association due to the abundant endogenous OE23 in the thylakoid lumen. Anti-cpOxa1p immunoprecipitation served as a control for nonspecific associations, as Oxa1p does not participate in ΔpH/Tat transport (Moore et al., 2000). mOE23 was found in all Oxa1p immunoprecipitates, consistent with nonspecific association (Fig. 1 D). In contrast, no interaction of cpOxa1p with any ΔpH/Tat pathway component was found under any conditions (Fig. 1 D, and A–C, cpOxa1p blot), confirming the specificity of associations among cpTatC, Hcf106, and Tha4.
The results in Fig. 1, A–C, demonstrate interactions between pairs of components, but fall short of showing that all components are in the same complex. To specifically address this question, sequential immunoprecipitations with anti-cpTatC followed by anti-Hcf106 were conducted before immunoblotting (Fig. 1 E), such that each cross-linked product contained both cpTatC and Hcf106. Tha4 was detected in the sequential immunoprecipitates only under transport conditions (Fig. 1 E, lane 3). This indicates that a cpTatC–Hcf106–Tha4 complex forms during protein transport. In sequential immunoprecipitates, tOE23 was detected as expected with or without the ΔpH. However, mOE23 was absent from immunoprecipitates, further supporting the interpretation that mOE23 presence in immunoprecipitates in Fig. 1, A–D, was due to nonspecific interactions. From this, we conclude that after processing to remove the signal peptide, the substrate no longer associates with components of the machinery.
In Fig. 1, the pH gradient was generated with light and dissipated with a combination of the ionophores nigericin and valinomycin. In other experiments, we found that the protonophore nigericin, but not valinomycin, was responsible for preventing Tha4 association with cpTatC and Hcf106, thereby verifying that the ΔpH, but not the Δψ, was the critical component of the proton motive force for triggering Tha4 association (unpublished data).
A twin arginine signal peptide promotes ΔpH-triggered association of Tha4 with cpTatC–Hcf106
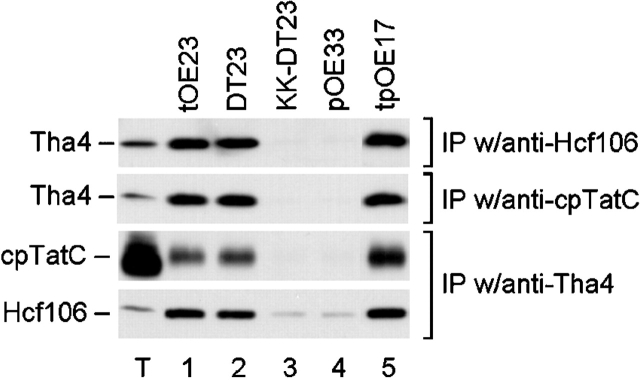
Thylakoid cross-linking assays were conducted with functional and nonfunctional precursors in the presence of the ΔpH in order to explore precursor requirements for Tha4 association with cpTatC–Hcf106 (Fig. 2). DT23 (lane 2) as well as tOE23 (lane 1) induced Tha4 association with cpTatC and Hcf106, as determined by reciprocal immunoprecipitations with antibodies to the three components. DT23 and tOE23 are both very efficient ΔpH/Tat pathway precursors (Henry et al., 1997). Neither KK-DT23, a nonfunctional precursor in which a twin lysine replaces the essential twin arginine (Cline and Mori, 2001), nor pOE33, a Sec pathway precursor protein induced Tha4 association (lanes 3 and 4). Remarkably, a 33-residue synthetic peptide (tpOE17) corresponding to the lumen-targeting signal peptide of maize OE17 precursor induced Tha4 interaction with cpTatC–Hcf106 (Fig. 2, lane 5). Interaction of tpOE17 with the translocation machinery was previously indicated by its ability to competitively inhibit protein transport on the ΔpH/Tat pathway (Teter and Theg, 1998). Taken together, these results suggest that binding of a twin arginine signal peptide and energizing the membrane with the proton gradient induce structural changes that promote Tha4 association with the cpTatC–Hcf106 receptor complex.
Figure 2.
A twin arginine signal peptide induces the association of Tha4 with cpTatC–Hcf106. Reactions included 1.0 μM of the functional precursors (tOE23 and DT23), the nonfunctional precursor (KK-DT23), the Sec pathway precursor (pOE33), or the 33-residue synthetic signal peptide (tpOE17), as indicated in the figure, and were conducted in the light as described in Fig. 1. Antibodies used for the immunoprecipitation are designated to the right of each panel and antibodies used for immunoblotting are to the left. IP, immunoprecipitation.
The cpTatC–Hcf106–Tha4 complex disassociates after protein transport
The above results imply that Tha4 dissociates from the cpTatC–Hcf106 complex upon completion of translocation. To directly test this possibility, we initiated protein transport with precursor-bound thylakoids and simultaneously monitored transport and component association as a function of time (Fig. 3 A for experimental schedule). For this experiment, we employed tOE17, a ΔpH/Tat pathway substrate that stably binds to the cpTatC–Hcf106 complex (Cline and Mori, 2001). Prebound tOE17 was transported to the lumen during the first 10 min of the transport reaction, as assessed by processing of precursor to mature form. After 10 min, no further transport occurred (Fig. 3, B and C). Association of components, assessed by immunoprecipitation with anti-Hcf106 and immunoblotting with anti-Tha4, rose to a peak at 2 min and was no longer detected after 10 min (Fig. 3). Addition of fresh tOE23 at 15 min resulted in restoration of protein transport and reassociation of Tha4 with Hcf106 (Fig. 3 B), indicating that Tha4 dissociation was not due to inactivation of the machinery or dissipation of the ΔpH. In a similar experiment, we verified that Tha4 also dissociated from cpTatC upon substrate depletion, but cpTatC and Hcf106 remained associated (unpublished data). These results demonstrate that Tha4 disassociates from the cpTatC–Hcf106 complex upon the completion of protein translocation, even in the presence of the ΔpH, and that the components then appear to return to their pretransport organization (Cline and Mori, 2001).
Figure 3.
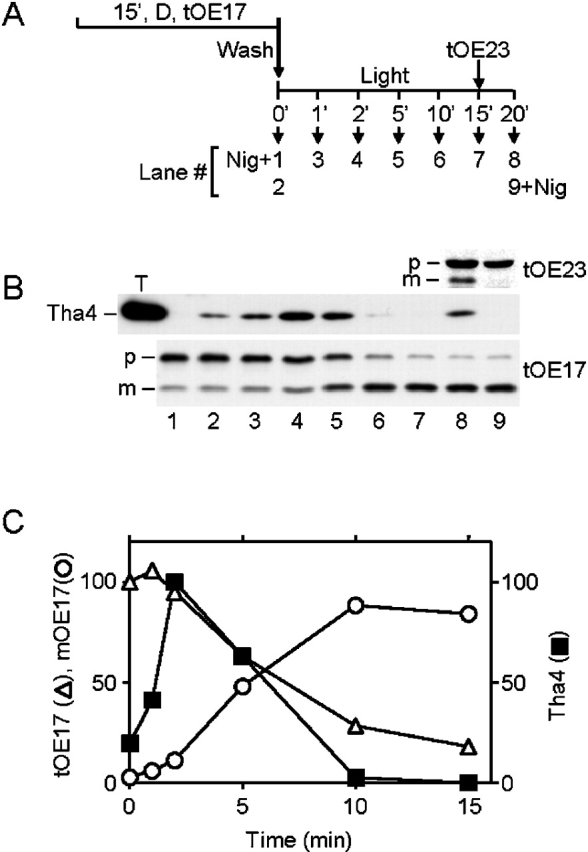
Tha4 dissociates from cpTatC–Hcf106 upon completion of translocation. Thylakoids were preincubated with radiolabeled tOE17 in the dark on ice for 15 min as previously described (Ma and Cline, 2000). After washing and resuspension in transport buffer at 0.33 mg Chl/ml, transport from the bound state was initiated by transfer of the thylakoids to a 25°C illuminated water bath. After a 15-min transport reaction, radiolabeled tOE23 was added to the reaction. 0.5 μM nigericin was added to the reactions shown in lanes 1 and 9 at the same time as transfer to light (lane 1) or addition of tOE23 (lane 9). Thylakoids were withdrawn at the indicated time points, recovered by centrifugation, and analyzed by SDS-PAGE/fluorography. Chemical cross-linking was performed on duplicate aliquots at the indicated time points with 0.75 mM DSP for 5 min followed by 5 min quenching with 50 mM Tris-HCl, pH 8.0. SDS-solubilized thylakoids were subjected to immunoprecipitation with anti-Hcf106, and immunoprecipitates were analyzed by immunoblotting with anti-Tha4. The reaction scheme is shown in A. (B) Immunoblot of anti-Hcf106 immunoprecipitates with anti-Tha4 and fluorograms of samples depicting tOE17 and tOE23 transport. The positions of precursor and mature forms are designated by p and m, respectively, on the left side of the blots. A thylakoid control (lane T) contained 2.5 μg Chl of untreated thylakoids. (C) Quantification of Tha4, tOE17, and mOE17 shown in B, lanes 2–7. The density of scanned Tha4 bands on X-ray film was determined using AlphaEase software (Alpha Innotech Corp.). tOE17 and mOE17 were quantified by scintillation counting of excised gel bands and adjusted for the different leucine contents of tOE17 and mOE17. Amounts of mOE17 were corrected by subtracting the amount of mOE17 present at zero time. The amounts displayed are relative quantities; the amount of tOE17 in lane 2 and the amount of Tha4 in lane 4 were arbitrarily set to 100%.
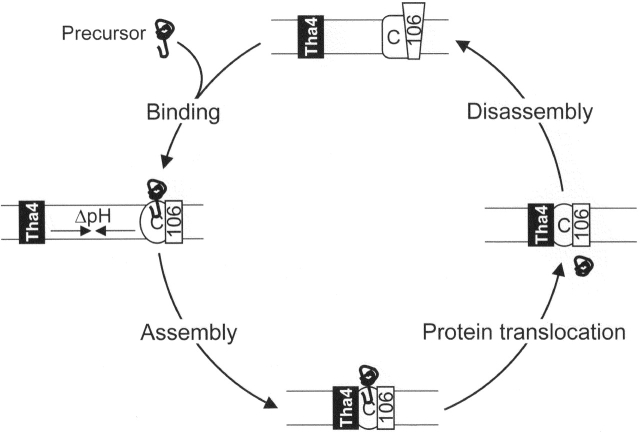
A model for cyclical assembly of the ΔpH-dependent translocase during protein transport
Here, we provided evidence for regulated assembly of the thylakoid ΔpH/Tat translocase. A working model for this process is presented in Fig. 4. The first step is reversible precursor binding to the cpTatC–Hcf106 receptor complex, which occurs in the presence or absence of the ΔpH (Fig. 4, binding step). Preliminary purification studies indicate that the precursor-bound receptor complex consists predominantly, if not entirely, of cpTatC, Hcf106, and precursor (unpublished data). Precursor binding may induce some structural alteration of this complex because binding is prerequisite for the second step, ΔpH-dependent recruitment of Tha4 to the receptor complex (Fig. 4, assembly step). Structural changes upon precursor binding have been shown for the translocase of the outer mitochondrial membrane (Rapaport et al., 1998). We also expect that the ΔpH induces a conformational change in some component(s) that triggers their association. Such a conformational change might result from protonation of lumen-proximal acidic residues of components, such as the conserved glutamate that is found in the transmembrane domain of both Hcf106 and Tha4 proteins (Mori and Cline, 2001). Conformational changes have been shown to accompany protonation and deprotonation of acidic residues in transmembrane domains of other proteins. An example is subunit c of the ATP synthase, where the induced structural change is related to the proton-driven rotation of the c12 disk (Rastogi and Girvin, 1999).
Figure 4.
Working model for formation of active translocase of thylakoid ΔpH-dependent/Tat pathway. See text for details.
The association of Tha4 with cpTatC–Hcf106 that occurs in the assembly step presumably creates an active translocase for translocation of the bound precursor protein (Fig. 4, translocation step). The structure of the ΔpH/Tat translocase is not known. Existing data do not allow conclusions regarding which components directly interact in the translocation complex. For example, it is even possible that the precursor serves as a bridge between the receptor complex and Tha4, although we find this less likely in view of the fact that the 33-residue synthetic signal peptide was sufficient to induce component association (Fig. 2). Current models suggest that the translocase consists of a cpTatC–Hcf106 core and multiples of Tha4 to form a translocation channel of varying diameter (Berks et al., 2000; Mori and Cline, 2001). This is interesting, considering that blue native gel analysis of thylakoids (Cline and Mori, 2001) and cross-linking analysis of E. coli membranes (DeLeeuw et al., 2001) suggest that Tha4 (TatA) exists as oligomers. Thus, merging of the two subcomplexes of components (Cline and Mori, 2001) might produce the translocase.
Finally, in our model, completion of translocation results in disassociation of Tha4 from the cpTatC–Hcf106 complex (Fig. 4, disassembly step). This may be an essential step depending on the precise nature of the active translocase and the manner by which it is assembled. For example, sensing of the size and shape of the substrate protein, which appears to be a feature of the ΔpH/Tat-dependent system, may be accomplished for each substrate during the building of the active translocase. If such were the case, disassembly after translocation would be necessary. Although many unanswered questions remain regarding the mechanism of the ΔpH/Tat system, the results presented here provide a framework for the overall process that may allow more detailed mechanisms to be described and tested for each step.
Materials and methods
Materials
Washed thylakoids were prepared from intact pea chloroplasts as previously described (Cline et al., 1993). In vitro transcription plasmids for tOE17 and tOE23 have been previously described (Henry et al., 1997). Radiolabeled tOE17 and tOE23 were translated in a wheat germ extract from capped RNA in the presence of [3H]leucine. E. coli-expressed pOE33 and tOE23 were prepared as previously described (Cline et al., 1993, Mori and Cline, 1998). DNA encoding DT23 and KK-DT23 (Henry et al., 1997), in which the twin arginine of DT23 in the signal peptide was replaced by a twin lysine, was cloned into the expression vector pETH3C; the proteins were expressed in E. coli and purified from inclusion bodies as described by Cline et al. (1993). DSP was from Pierce Chemical Co. Antibodies used here have been described elsewhere (Mori et al., 1999, 2001; Cline and Mori, 2001). The OE17 synthetic peptide (tpOE17) was provided by Steven Theng (University of California, Davis, CA).
Chemical cross-linking
Reaction mixtures containing 0.33 mg of chlorophyll (Chl) per ml of washed pea thylakoids in transport buffer (330 mM sorbitol, 50 mM Hepes-KOH, 3.33 mM MgCl2, pH 8.0) were preincubated for 5 min at 25°C under the indicated conditions. Cross-linking was initiated with DSP, added at 1/100 vol from 50–100 mM stock solutions in DMSO. After 5 min, cross-linking reactions were quenched with 50 mM Tris-HCl for an additional 5 min. Cross-linking and quenching was performed under the same conditions as the preincubation. Precursors and the synthetic OE17 signal peptide were solubilized in 8 M urea, 0.8 mM DTT for >3 h at 25°C and then added directly to reaction mixtures. Final concentrations of urea and DTT in the reaction were <80 mM and 8 μM, respectively. Preliminary experiments showed that urea at this concentration did not influence cross-linking or component associations.
Immunoprecipitation and immunoblotting
Immunoprecipitation was performed as previously described with IgG covalently cross-linked to protein A–Sepharose (Cline and Mori, 2001), except that the immunoprecipitation was conducted in 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCl, 150 mM NaCl, pH 7.4. Bound proteins were eluted by incubating the beads with 8 M urea, 5% SDS, 125 mM Tris-HCl, pH 6.8, 0.001% bromophenol blue for 1 h at 25°C. β-Mercaptoethanol was then added to the eluates at 5%. Eluted proteins were analyzed by SDS-PAGE and immunoblotting with specific antibodies. HRP-conjugated goat anti–rabbit IgG Fc was used as secondary antibody.
Acknowledgments
We thank Steve Theg for providing the OE17 synthetic peptide, Mike McCaffery for excellent technical assistance, and Mike McCaffery and Carole Dabney-Smith (University of Florida, Gainesville, FL) for critical review of the manuscript.
This work was supported in part by National Institutes of Health grant R01 GM46951 to K. Cline. This manuscript is Florida Agricultural Experiment Station journal series no. R-08713.
Footnotes
Abbreviations used in this article: Chl, chlorophyll; DSP, dithiobis (succinimidyl propionate); mOE23 or mOE17, mature form of OE23 or OE17; Tat, twin arginine translocation; tpOE17, 33-residue synthetic peptide corresponding to the lumen-targeting signal peptide of the OE17 precursor.
References
- Berks, B.C., F. Sargent, and T. Palmer. 2000. The Tat protein export pathway. Mol. Microbiol. 35:260–274. [DOI] [PubMed] [Google Scholar]
- Bolhuis, A., J.E. Mathers, J.D. Thomas, C.M. Barrett, and C. Robinson. 2001. TatB and TatC form a functional and structural unit of the twin-arginine translocase from Escherichia coli. J. Biol. Chem. 276:20213–20219. [DOI] [PubMed] [Google Scholar]
- Chaddock, A.M., A. Mant, I. Karnauchov, S. Brink, R.G. Herrmann, R.B. Klösgen, and C. Robinson. 1995. A new type of signal peptide: central role of a twin-arginine motif in transfer signals for the ΔpH-dependent thylakoidal protein translocase. EMBO J. 14:2715–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, S.A., and S.M. Theg. 1997. A folded protein can be transported across the chloroplast envelope and thylakoid membranes. Mol. Biol. Cell. 8:923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline, K., and H. Mori. 2001. Thylakoid ΔpH-dependent precursor proteins bind to a cpTatC–Hcf106 complex before Tha4-dependent transport. J. Cell Biol. 154:719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline, K., W.F. Ettinger, and S.M. Theg. 1992. Protein-specific energy requirements for protein transport across or into thylakoid membranes. Two lumenal proteins are transported in the absence of ATP. J. Biol. Chem. 267:2688–2696. [PubMed] [Google Scholar]
- Cline, K., R. Henry, C. Li, and J. Yuan. 1993. Multiple pathways for protein transport into or across the thylakoid membrane. EMBO J. 12:4105–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leeuw, E., I. Porcelli, F. Sargent, T. Palmer, and B.C. Berks. 2001. Membrane interactions and self-association of the TatA and TatB components of the twin-arginine translocation pathway. FEBS Lett. 506:143–148. [DOI] [PubMed] [Google Scholar]
- Henry, R., M. Carrigan, M. McCaffery, X. Ma, and K. Cline. 1997. Targeting determinants and proposed evolutionary basis for the Sec and the Delta pH protein transport systems in chloroplast thylakoid membranes. J. Cell Biol. 136:823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynds, P.J., D. Robinson, and C. Robinson. 1998. The sec-independent twin-arginine translocation system can transport both tightly folded and malfolded proteins across the thylakoid membrane. J. Biol. Chem. 273:34868–34874. [DOI] [PubMed] [Google Scholar]
- Ma, X., and K. Cline. 2000. Precursors bind to specific sites on thylakoid membranes prior to transport on the Delta pH protein translocation system. J. Biol. Chem. 275:10016–10022. [DOI] [PubMed] [Google Scholar]
- Manting, E.H., and A.J.M. Driessen. 2000. Escherichia coli translocase: the unraveling of a molecular machine. Mol. Microbiol. 37:226–238. [DOI] [PubMed] [Google Scholar]
- Moore, M., M.S. Harrison, E.C. Peterson, and R. Henry. 2000. Chloroplast Oxa1p homolog albino3 is required for post-translational integration of the light harvesting chlorophyll-binding protein into thylakoid membranes. J. Biol. Chem. 275:1529–1532. [DOI] [PubMed] [Google Scholar]
- Mori, H., and K. Cline. 1998. A signal peptide that directs non-Sec transport in bacteria also directs efficient and exclusive transport on the thylakoid Delta pH pathway. J. Biol. Chem. 273:11405–11408. [DOI] [PubMed] [Google Scholar]
- Mori, H., and K. Cline. 2001. Post-translational protein translocation into thylakoids by the Sec and Delta pH–dependent pathways. Biochim. Biophys. Acta. 1541:80–90. [DOI] [PubMed] [Google Scholar]
- Mori, H., E.J. Summer, X. Ma, and K. Cline. 1999. Component specificity for the thylakoid Sec and ΔpH-dependent protein transport pathways. J. Cell Biol. 146:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, H., E.J. Summer, and K. Cline. 2001. Chloroplast TatC plays a direct role in thylakoid ΔpH-dependent protein transport. FEBS Lett. 501:65–68. [DOI] [PubMed] [Google Scholar]
- Rapaport, D., K.-P. Künkele, M. Dembowski, U. Ahting, F.E. Nargang, W. Neupert, and R. Lill. 1998. Dynamics of the TOM complex of mitochondria during binding and translocation of preproteins. Mol. Cell. Biol. 18:5256–5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi, V.K., and M.E. Girvin. 1999. Structural changes linked to proton translocation by subunit c of the ATP synthase. Nature. 402:263–268. [DOI] [PubMed] [Google Scholar]
- Sargent, F., U. Gohlke, E. de Leeuw, N.R. Stanley, T. Palmer, H.R. Saibil, and B.C. Berks. 2001. Purified components of the Escherichia coli Tat protein transport system form a double-layered ring structure. Eur. J. Biochem. 268:3361–3367. [DOI] [PubMed] [Google Scholar]
- Settles, A.M., A. Yonetani, A. Baron, D.R. Bush, K. Cline, and R. Martienssen. 1997. Sec-independent protein translocation by the maize Hcf106 protein. Science. 278:1467–1470. [DOI] [PubMed] [Google Scholar]
- Teter, S.A., and S.M. Theg. 1998. Energy-transducing thylakoid membranes remain highly impermeable to ions during protein translocation. Proc. Natl. Acad. Sci. USA. 95:1590–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, M.B., L.M. Roy, E. Coleman, R. Voelker, and A. Barkan. 1999. The maize tha4 gene functions in Sec-independent protein transport in chloroplasts and is related to Hcf106, TatA, and TatB. J. Cell Biol. 147:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L.F., B. Ize, A. Chanal, Y. Quentin, and G. Fichant. 2000. Bacterial twin-arginine signal peptide-dependent protein translocation pathway: evolution and mechanism. J. Mol. Microbiol. Biotechnol. 2:179–189. [PubMed] [Google Scholar]