Abstract
Background
Polychlorinated biphenyls (PCBs) are ubiquitous environmental toxicants, for which animal studies demonstrate immunotoxic effects, including thymic atrophy and suppressed immune responses; human investigations of similar end points are sparse. The thymus is essential for the differentiation and maturation of T-cell lymphocytes.
Objectives
The objective of this study was to examine the association between prenatal PCB exposures and estimated thymus volume in infants from eastern Slovakia, a region where PCBs were produced until 1984.
Methods
Mothers were enrolled at delivery, and maternal blood samples were collected for analysis of 15 PCB congeners, p,p′-DDT [1,1,1-trichloro-2,2′-bis(p-chlorophenyl)ethane], and p,p′-DDE [1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene]. Each mother was interviewed to obtain information on sociodemographic characteristics, past pregnancies, occupational history, medication history, and living environment. Neonatal thymus volume was estimated using ultrasound measurements on the third or fourth day after birth. Thymic index was calculated on 982 newborns from mothers with PCB measurements. We developed a predictive model of the natural log of the thymic index using multiple linear regression with covariates selected from the bivariate analyses.
Results
Prenatal PCB exposure was associated with a smaller thymic index at birth [β= −36 (natural log-transformed; nanograms per gram lipids); p = 0.047]. District of residence and delivery also predicted thymic index. Male sex, later gestational age, larger birth weight z-score, and Roma ethnicity were associated with a larger thymic index, whereas respiratory illness was associated with a lower thymic index.
Conclusions
This study provides the first evidence to date that PCB exposure in neonates is associated with a smaller thymic volume, suggesting possible impaired immunologic development.
Keywords: p,p′-DDE; p,p′-DDT; PCBs; thymic index; immune status; prenatal
Polychlorinated biphenyls (PCBs), a class of 209 congeners with two linked phenyl rings and variable chlorination in different sites, were introduced in 1929. They were widely used as dielectric fluids in electrical transformers and capacitors, and as heat exchangers or hydraulic fluids. Improper disposal has been a major means by which PCBs enter the environment. These chemicals are persistent in the environment and the human body because of their high lipophilicity and stability. Their production and use were banned in most industrialized countries in the late 1970s because of toxicity to wildlife (Elliott et al. 2000; Ross et al. 2004).
PCBs have shown toxic effects on various organs including tissue of the nervous, reproductive, and immunologic systems. Suppressed immune function can lead to increased susceptibility to infectious disease or certain types of cancers. A study in the Netherlands showed that prenatal or lactational exposures to PCBs were associated with changes in immune profiles in children (Weisglas-Kuperus et al. 1995, 2000).
The thymus is essential for the differentiation and maturation of T lymphocytes. Administration of PCBs in rodents, specifically dioxin-like PCBs, in vivo and in vitro induces thymic atrophy and immune suppression. In particular, thymic atrophy has been observed consistently in studies using various species after in vivo exposure to PCBs or dioxin (Beineke et al. 2005; DeCaprio et al. 1986; Goff et al. 2005; Silkworth and Antrim 1985). Some authors have suggested these effects might result from activation of the aryl hydrocarbon (Ah) receptor (Shimada 1987; Silkworth et al. 1986; Tryphonas et al. 2001). Of all tissues in the rat, the thymus has one of the highest expression levels of Ah-receptor mRNA (Carver et al. 1994). Other studies indicate that 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) or non-coplanar PCBs might induce immunotoxic effects through Ah-receptor independent mechanisms (Kerkvliet et al. 1990; Tryphonas et al. 2001). However, the mechanisms for immunotoxicity by PCBs are not fully understood, and given a broad range of interaction between the neurologic and immune systems, organs other than the thymus may be involved.
The thymic index, derived from sonographic measurements, has been used as an approximation of thymus size and is regarded as reliable, especially in young children (Hasselbalch et al. 1996). The thymic index is highly correlated with the actual volume and weight of the thymus (Hasselbalch et al. 1996). Previous studies have documented the development of the thymus in humans: The size and the thymic function increase to a maximum in infants (George and Ritter 1996; Steinmann et al. 1985) and then gradually decrease beginning at about 8 months (Hasselbalch et al. 1997). Some reports found breast-feeding, birth weight, birth length, and head circumference to be positively associated with thymic index (Benn et al. 2001; Hasselbalch et al. 1997; Iscan et al. 2000; Yekeler et al. 2004). Malnutrition, infection, short gestation, stress, and zinc depletion have been associated with a smaller thymic index (Dominguez-Gerpe and Rey-Mendez 2003; Jeppesen et al. 2003; Malpuech-Brugere 2001). However, we found no previous study that has directly addressed the thymic index as an outcome in humans in relation to hazardous chemical exposures in the environment. In this project, we studied thymus size in neonates as an indicator of dysregulated immunologic development in relation to prenatal exposure to PCBs.
Materials and Methods
Study population
Mothers were recruited for this study at the time of delivery from two districts in eastern Slovakia during 2002–2004: Michalovce, with high PCB contamination from a chemical manufacturing plant; and Svidnik, located around 70 km to the northwest, with substantially lower levels of PCBs. Each district has only one hospital, and the vast majority of births from these regions occur in these hospitals. For eligibility criteria, we excluded a) mothers with more than four previous births, b) mothers < 18 years of age, c) mothers who had resided < 5 years in their district, d) mothers with a major illness during pregnancy, and e) infants who had severe birth defects. The study subjects gave written informed consent. Of 1,134 eligible participants, PCB measurements in maternal serum were available on 1,076. Thymic index data from 982 were available after excluding subjects lacking thymic index measurements (n = 50), or radiologist information (n = 16), or who had implausible values of thymic index (n = 28). This study was approved by institutional review boards of the University of California, Davis, and the Slovak Medical University.
Specimen collection
After delivery, maternal blood specimens (30–35 mL) were collected by a nurse using venipuncture into heparinized, standard, and EDTA vacutainers. All tubes were refrigerated at 5–10°C immediately after collection of blood. Samples were transported to the Department of Biochemistry of each local hospital within 2 hr for processing. Serum was isolated by centrifugation (15 min at 3,000 rpm). Three milliliters of serum were placed into an 8-mL glass vial labeled with the identification code and stored frozen at −18°C. These were transported to the Slovak Medical University in Bratislava in thermo boxes with cooling cartridges to prevent thawing, and stored at −18°C for PCB analysis; a small amount (0.2 mL) was aliquoted for lipid measurement and stored.
PCB measurement
We determined the concentrations of 15 congeners of PCBs [PCB International Union of Pure and Applied Chemistry (IUPAC) nos. 28, 52, 101, 105, 114, 118, 123, 138, 153, 156, 157, 167, 170, 180, and 189), p,p′-DDT [1,1,1-trichloro-2,2′-bis(p-chlorophenyl)ethane], and p,p′-DDE [p,p′-DDE (1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene] in the maternal serum samples by high-resolution gas chromatography (GC) with electron capture detection (Conka et al. 2005; Kocan et al. 1994). Briefly, the procedure included standardized extraction, cleanup, and quantification. PCB-174 as an extraction standard had been added to the blood serum before the analytes were isolated using solid phase extraction. The SPE extract was concentrated and then cleaned by passing through a Florisil-H2SO4/silica gel column. The eluate was then evaporated to a small volume, and PCB-103 was added as a syringe standard to correct the final volume of samples before GC injection. An aliquot of the mixture was injected and analyzed on a chromatography system (HP 5890; Hewlett-Packard, Palo Alto, CA, USA) equipped with a Ni-63 electron capture detector using a 60-m DBP-5 capillary column (J&W Scientific, Folsom, MA, USA). Quantification was based on the calibration curve generated by authentic PCB standard solutions at five different concentration levels. Quality control activities consisted of analyses of samples in batches of 10 simultaneously with a blank sample and in-house reference material (spiked porcine serum). Response for a particular congener had to be in the range of 90–110% using the concentration of the middle point of the calibration curves for that congener. We determined the limit of detection for each analyte as the mean of background noise plus 3 standard deviations from five reagent blank samples.
Six of the individual PCB congeners were selected to be included into PCB sum (PCB IUPAC nos. 118, 138, 153, 156, 170, and 180) based on having < 20% of samples below the limit of detection (LOD). When one of these PCB congeners was below the limit of detection in an individual’s sample, we imputed by assigning the LOD value divided by the square root of 2 (Persky et al. 2001; Weisskopf et al. 2005). PCB-sum concentrations were determined on a wet weight basis (nanograms per milliliter); subsequently these PCB concentrations were adjusted for lipids (nanograms per gram).
The Department of Toxic Organic Pollutants at the Slovak Medical University in Bratislava (SMU) performed the laboratory analyses. The laboratory has participated in intercalibration studies organized by the World Health Organization (2000) and the German Agency for Occupational and Environmental Medicine (Deutsche Gesellschaft fuer Arbeitsmedizin und Umweltmedizin e.V.).
Lipids measurement
We estimated total serum lipids (TL) using the enzymatic summation method (Akins et al. 1989). We measured serum total cholesterol (TC) and triglyceride (TG) using a DuPont Automatic Clinical Analyzer III analyzer (Dupont, Jonesboro, AR, USA), and cholesterol oxidase without cholesterol esterase was used to detect free cholesterol (FC). The method by Takayama et al. (1977) was used to determine serum choline-containing phospholipids (PL). Total serum lipids were calculated from the formula
The lipids were measured at a biochemical laboratory accredited by the Slovak National Accreditation Service located at the Ministry of Defense Military hospital in Bratislava.
Thymus measurement
The thymus was measured on the 3rd or 4th day after birth in 982 neonates from October 2002 through December 2004. Four radiologists from Michalovce and two radiologists from Svidnik measured the thymus using a sonographic scanner [in Michalovce: Esaote 580 FD Caris plus (convex probe 7.5 MHz); Esaote SpA, Genova, Italy; in Svidnik: Esaote AU 5 Harmonic (convex probe 7.5 MHz); Esaote SpA, Firenze, Italy]. The thymus is located in the anterior mediastinum in front of the large vessels, and is well delineated by sonography. The infant was examined on his or her back with the neck extended and head fixed in an optimal position for measurement. A transsternal approach was used to measure the maximal transverse diameter (width) of the thymus. In the plane perpendicular to this width, the largest sagittal area (longitudinal scan plan) was also measured. These two measurements were multiplied to obtain the thymic index. The thymic index was used as an estimate of the thymus volume.
Data collection
The two major data sources for covariates were an interview with the mother conducted by trained staff during the 5-day hospital stay, and the newborn medical record. The interview obtained information on sociodemographic characteristics, past pregnancies, occupational history, medical conditions, medication history before and during the pregnancy, and living environment. Romani ethnicity was attributed to the mother if the ethnic origin of either of her parents was Romani, the Romani language was spoken at the home of the child, or the mother was planning to raise her child with the Romani language. This definition of Romani was subsequently independently confirmed by a Slovak member of the research team who used additional information such as the family’s last name to confirm the ethnicity. Ethnicity was categorized into two groups: Slovakian/other eastern European or Romani. Smoking history during or before pregnancy was extracted from the interview data. Alcohol consumption was defined as one or more drinks of beer, wine, or liquor per week during pregnancy or in the 3 months before pregnancy. Maternal illness history included respiratory infection, asthma, or allergy during the same time period. Parity was coded as an ordinal variable (0–4).
The abstraction of the newborn’s medical records included birth weight and gestational age (weeks). The estimate of gestational age was based on last menstrual period (LMP) reported in the medical records and the clinical judgment made by the woman’s physician.
Data analysis
Univariate distributions were examined and implausible values were investigated and resolved by communication with field staff. The thymic index was highly skewed (test of normality by Shapiro-Wilk, p < 0.0001), so we used the logarithmic transformation (loge) of the thymic index in the analysis as the outcome variable. As the primary predictor variable of interest, the total maternal PCB concentration based on the six most abundant congeners was adjusted for serum lipid concentration and expressed as nanograms per milligram (equivalent to micrograms per gram) total lipid. Because the PCB distribution was also skewed (p < 0.0001), these values were log-transformed as well to reduce the influence of extreme values. Birth weight was expressed as a Z-score standardized for sex, parity, and gestational age based on all births in Slovakia in 2004.
As a first step in the data analysis, we conducted simple linear regressions (bivariate analyses) with the thymic index as the outcome. Possible confounders such as sex, socioeconomic status (parental education), smoking, alcohol consumption, ethnicity, and maternal medical history including such ailments as diabetes, hypertension, hypothyroidism, allergy, asthma, and respiratory disease were evaluated. Then a multiple linear regression model was developed with covariates selected from the bivariate analyses. Variables with low or no association with the thymic index at p > 0.3 were excluded from final multiple linear regression models. We used a backward selection approach to develop a final predictive model. After exponentiation from the final regression model, expected thymic indices were calculated; results are presented as percent change in thymic index across the interquartile range and for the 10th–90th increase in PCBs respectively.
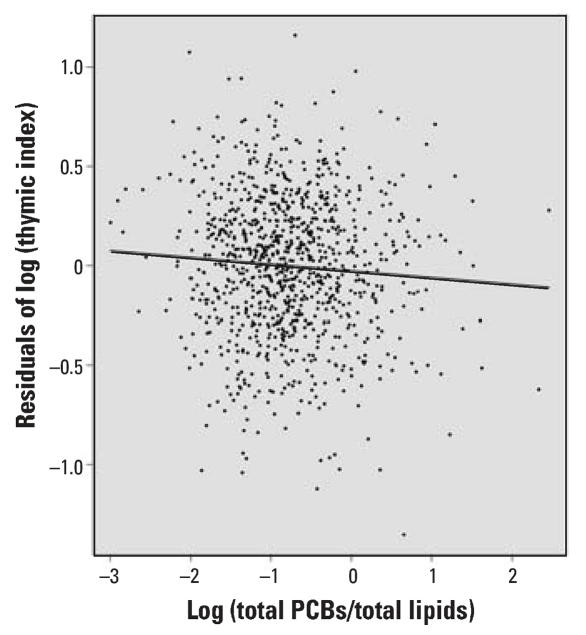
Scatterplots of residuals (the difference between the observed thymic index values and those predicted by the regression equation) for thymic index were plotted from the multivariate model that included all variables in the final model except PCBs. Figure 1 shows the partial residuals from a model that included only the covariates as predictors, as well as the best-fitting line after inclusion of log-transformed PCBs, demonstrating the decreasing trend of the log-transformed thymic index with increasing exposure. The SAS statistical package was used for analysis (version 9.1; SAS Institute Inc., Cary, NC, USA).
Figure 1.
Scatterplot of residuals for thymic index in relation to log maternal serum PCBs after adjusting for other predictors.
Results
Table 1 describes characteristics of the cohort. About one-fifth of the mothers was > 30 years of age and around 7% had a university degree. In this study population, 92% of participants were married or living with their partners, 36% were smokers, and 30% consumed alcohol during either the pregnancy or the 3 months before conception. 59% of women were multiparous and 8% had more than two previous children. One percent and 4% of mothers reported asthma and allergy, respectively, and 20% reported respiratory infections during the period from 3 months before conception through the end of pregnancy.
Table 1.
Characteristics of the study cohort consisting of 982 mother–infant pairs with deliveries 2002–2004 in two districts of eastern Slovakia.
| Characteristic | No. (%) | Characteristic | No. (%) |
|---|---|---|---|
| District | Maternal smoking | ||
| Michalovce | 702 (71.5) | No | 613 (62.4) |
| Svidnik | 280 (28.5) | Yes | 357 (36.4) |
| Maternal age (years) | Missing | 12 (1.2) | |
| ≤19 | 84 (8.6) | Maternal alcohol consumption | |
| 20–29 | 689 (70.1) | No | 673 (68.5) |
| ≥30 | 209 (21.3) | Yes | 298 (30.4) |
| Maternal education | Missing | 11 (1.1) | |
| Basic schooling | 204 (20.8) | Parity | |
| High school without graduation | 260 (26.5) | 0 | 404 (41.2) |
| High school with graduation | 443 (45.1) | 1 | 328 (33.4) |
| More than college/university | 72 (7.3) | 2 | 171 (17.4) |
| Missing | 3 (0.3) | 3 | 75 (7.6) |
| Sex of child | 4 | 2 (0.2) | |
| Male | 503 (51.2) | Missing | 2 (0.2) |
| Female | 479 (48.8) | Maternal allergy history | |
| Ethnicity | No | 937 (95.4) | |
| Slovakian/other eastern European | 757 (77.1) | Yes | 35 (3.6) |
| Romani | 215 (21.9) | Missing | 10 (1.0) |
| Missing | 10 (1.0) | Maternal asthma history | |
| Marital status | No | 973 (99.1) | |
| Married or living with partner | 905 (92.2) | Yes | 9 (0.9) |
| Never married | 59 (6.0) | Maternal respiratory history | |
| Divorced | 7 (0.7) | No | 774 (78.8) |
| Missing | 11 (1.1) | Yes | 198 (20.2) |
| Missing | 10 (1.0) | ||
The median thymic index was 8.7 (mean, 9.6; range, 2.1–32.5), and 97% were < 20. The distribution of thymic index was skewed to the right. Table 2 reports the information on thymic index from six radiologists in two districts. The measurements of radiologists 5 and 6, both from Svidnik area, seemed to be larger and had wider variation than those of radiologists from Michalovce. Radiologist 5 was the most experienced of all of them.
Table 2.
Univariate distribution of thymic index by six radiologists.
| Radiologists
|
||||||
|---|---|---|---|---|---|---|
| Michalovce
|
Svidnik
|
|||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| No. | 363 | 155 | 89 | 95 | 187 | 93 |
| Mean | 8.1 | 8.7 | 8.3 | 8.1 | 12.8 | 13.2 |
| Median | 7.6 | 8.3 | 8.0 | 8.0 | 11.6 | 13.2 |
| SD | 3.3 | 3.0 | 2.7 | 2.8 | 5.3 | 4.2 |
| 10th–90th | 4.5–12.2 | 5.0–12.3 | 5.3–11.0 | 4.8–12.3 | 7.3–20.2 | 8.3–18.5 |
The mean, median, and 10th and 90th percentiles of the PCB sum were 620, 440, 190, and 1,170 ng/g serum lipids respectively. Table 3 shows the final multiple regression model predicting the log thymic index as a function of the lipid-adjusted PCB sum, adjusting for sex of infant, maternal smoking and alcohol history, Romani ethnicity, gestational age, Z-score of birth weight, district of residence, and maternal respiratory infection history during pregnancy or the 3 months before pregnancy. Higher serum PCB concentrations were associated with a smaller thymic index. For an increase in PCB across the interquartile range [280–700 ng/g (ug/g) serum lipids], the thymic index decreased by 3%, (p = 0.047). An increase from the 10th to 90th percentile [190–1,170 ng/g serum lipids] was associated with a 7% reduced thymic index. Female infants had a smaller thymic index by 10% in comparison with male infants. Maternal smoking, alcohol, and respiratory infection histories were associated with a smaller thymic index by 3%, 5%, and 6% respectively; however, smoking and alcohol consumption were not statistically significant. The district differences observed in the crude data were upheld after adjusting for covariates. Each unit of Z-score of birth weight adjusted for gestational age (1 SD increase) and each week of gestational age increased thymic index by 13% and 3%, respectively.
Table 3.
Final multiple linear regression model of log thymic index with log PCBs and other covariates as predictors (n = 982).
| Predictor variable | β | SE | p-Value |
|---|---|---|---|
| PCB [natural log-transformed (ng/mg serum lipids)]a | −0.036 | 0.018 | 0.047 |
| Sex (male vs. female) | −0.102 | 0.023 | < 0.0001 |
| Smoking | −0.032 | 0.024 | 0.181 |
| Alcohol consumption | −0.051 | 0.026 | 0.050 |
| Ethnicity (Romani vs. Slovakian/other Eastern European) | 0.104 | 0.030 | < 0.001 |
| Gestational age at delivery (weeks) | 0.031 | 0.010 | 0.003 |
| Z-score of birth weight | 0.126 | 0.012 | < 0.0001 |
| District (Michalovce vs. Svidnik) | 0.417 | 0.031 | < 0.0001 |
| Respiratory infection histories | −0.066 | 0.029 | 0.023 |
For units of ng/g, this coefficient is multiplied by 10−3.
We tested p,p′-DDE and p,p′-DDT in the multiple linear regression models. Although the same direction of effect was observed as was seen in PCBs, the coefficients were small and not significant (results not shown).
Table 4 presents a separate final multiple linear regression model in which we focused on the measurements (n = 187) from the radiologist who is the most experienced. Applying the beta coefficient from this model to the population interquartile range of PCBs yielded a decrease in the thymic index of 8.9% (p = 0.033). If PCBs increased from the 10th to the 90th percentile, the thymic index was reduced by 16.9%.
Table 4.
Final multiple linear regression model of log thymic index for the most experienced radiologist, no. 5 from Svidnik, with log PCBs and other covariates as predictors (n = 187).
| Predictor variable | β | SE | p-Value |
|---|---|---|---|
| PCB [natural log-transformed (ng/mg serum lipids)]a | −0.102 | 0.047 | 0.033 |
| Sex (male vs. female) | −0.097 | 0.058 | 0.100 |
| Smoking | −0.067 | 0.060 | 0.263 |
| Alcohol consumption | −0.085 | 0.059 | 0.153 |
| Ethnicity (Romani vs. Slovakian/other Eastern European) | 0.135 | 0.079 | 0.092 |
| Gestational age at delivery (weeks) | 0.053 | 0.023 | 0.022 |
| Z-score of birth weight | 0.113 | 0.032 | < 0.001 |
| Respiratory infection histories | −0.181 | 0.090 | 0.046 |
For units of ng/g, this coefficient is multiplied by 10−3.
Figure 1 shows the scatterplot of the log-transformed maternal serum PCBs against the residuals of the log thymic index from a multiple linear regression model with all predictor variables except PCBs. This plot visually presents the degree of negative slope explained by PCBs alone.
Discussion
The thymus is a flat, bi-lobed organ located above the heart. It plays a critical role in the differentiation and maturation of T-cell lymphocytes in immune system. These T lymphocytes are mainly responsible for cell-mediated immunity, which does not involve antibodies but protects against cancer cells, intracellular bacteria, and viruses. T lymphocytes secrete cytokines that can contribute to activating other immune cells or can serve a cytotoxic or regulatory function (Goldsby et al. 2003).
It has been reported that certain risk factors such as malnutrition, zinc depletion, stress, HIV or other infection, preterm birth, and seasonality [e.g., birth during hungry season, July–December, in Gambia (Collinson et al. 2003)] are associated with a smaller thymus size (Collinson et al. 2003; Dominguez-Gerpe and Rey-Mendez 2003; Jeppesen et al. 2003; Malpuech-Brugere 2001). Information is more limited on possible mechanisms to support these reported associations. Suggested hypotheses (Dominguez-Gerpe and Rey-Mendez 2003) include enhanced apoptosis in thymocytes, or reduced migration of precursor T lymphocytes from bone marrow to thymus. A small thymic index at birth was associated with reduced interleukin-7 in breast milk of the mother.
In vivo rodent experiments and in vitro studies have shown loss of thymocytes or thymic atrophy induced by certain PCB congeners (Beineke et al. 2005; Robertson et al. 1984; Tan et al. 2003). Other studies suggest this occurs by enhanced differentiation with impaired proliferation of thymocytes (Esser et al. 1994; Kremer et al. 1994; Lai et al. 1994). PCBs may interfere with the interaction of stromal cells that interact with the developing thymocytes rather than acting directly on thymocytes themselves (Esser et al. 1994; Kremer et al. 1994; Lai et al. 1994). Thymocytes develop in a three-dimensional stromal-cell network that consists of epithelial cells, dendritic cells, and macrophages (Goldsby et al. 2003). The interaction between stromal cells and thymocytes leads to proliferation and maturation of a repertoire of T cells (Van 1991). Fine et al. (1989, 1990) reported from an in vivo study with BALB/c mice that TCDD, chemical compounds structurally similar to PCBs, caused thymic atrophy through changes in lymphocyte precursors in the bone marrow and in the fetal liver. Camacho et al. (2004) showed that in C57BL/6 mice after perinatal exposure, TCDD induced increased apoptosis causing thymic atrophy.
Before sonographic examination became available, thymus size measurements were possible only on autopsy. Several earlier studies using sonographic measurements were either case reports or used their own estimates of thymus size such as thickness. Since Hasselbalch et al. (1996) suggested the thymic index as an estimate of the size of thymus, this approach has been adopted by several researchers (Aaby et al. 2002; Iscan et al. 2000; Jeppesen et al. 2003; Yekeler et al. 2004).
Our findings are consistent with previous studies showing the thymic index to be positively correlated with birth weight (Hasselbalch et al. 1997; Iscan et al. 2000; Yekeler et al. 2004) and gestational age (Jeppesen et al. 2003). Male infants had larger thymic index even after adjustment for birth weight in our study; Aaby et al. (2002) also reported a larger thymic index in boys, though this study did not adjust for birth weight. We also observed that maternal smoking, alcohol consumption, and respiratory infections during pregnancy or the 3 months before conception were associated with a lower thymic index, although smoking and alcohol consumption were not statistically significant in our data.
We found increased thymic index among infants with Romani ethnicity. Considering the deficient nutrition (e.g., the estimated average daily intake of vitamin C is 44% of recommended daily allowance) (Brazdova et al. 1998); social conditions for the Romani in eastern Slovakia including low socioeconomic status, slum housing, and suboptimal hygienic conditions; and a high prevalence of smoking and alcohol consumption (Brazdova et al. 1998; Koupilova et al. 2001; Krajcovicova-Kudlackova et al. 2004; Vivian and Dundes 2004), a reduced thymic index might have been expected. Because we adjusted for smoking and alcohol consumption (as well as birth weight, which is also lower in Romani children), the mechanism leading to increased thymic index remains unclear. Interestingly, in our data, the Romani group showed lower prevalence, compared with non-Romani, of maternal illness history such as respiratory infections (17.2 vs. 21.3%), asthma (0.5 vs. 1.1%), or allergy (0 vs. 4.6%) during pregnancy or in the 3 months before pregnancy. Therefore, the possibility of selection bias (e.g., participation of more healthy subjects from Romani background) cannot be excluded in this study. In utero exposures to unmeasured environmental factors/pollutants during critical windows of gestation in the Romani ethnic group might play a role in the increased thymic index as well.
Despite the long time period since their arrival to Europe from Asia, the Romani maintain significantly smaller stature in terms of body height and weight and percentage of body fat, and a trend toward lower concentrations of the thyroid hormone thyroxine (Ginter et al. 2001); thus genetic differences cannot be excluded. Olesen et al. (2005) observed a larger thymic index was associated with children 0–6 years of age having atopic dermatitis compared with healthy children, and suggested that this positive association might be related to an unbalanced establishment of the peripheral T-lymphocyte system.
Six radiologists measured the thymic index from the two districts where different instruments were used. Inter- or intrareliability comparisons among these six were not performed. Inclusion of a set of indicator variables for radiologist in the final model did not influence the parameter estimates for PCBs or for other covariates. We also tested models for the PCB association with thymic index within radiologists, and our finding was most strongly confirmed with infants examined by the most experienced radiologist (no. 5, from Svidnik).
p,p′-DDE and p,p′-DDT were also evaluated in the multiple linear regression models, but were not significant. When we summed PCBs, p,p′-DDT, and p,p′-DDE, the parameter estimate was very close to that for PCBs alone.
Zinc is an essential trace mineral in humans and is known as an inhibitor of apoptosis. Zinc deficiency is associated with thymic atrophy and lymphopenia (Fraker et al. 2000), and zinc supplements resulted in faster recovery of thymus tissue in malnourished children (Chevalier et al. 1996). Venkataraman et al. (2004a, 2004b) found that serum zinc concentration was significantly reduced in PCB-exposed rats. A possible mechanism for the observed reduced thymic size in the present study might be zinc deficiency induced by PCB exposure.
Our results provide the first evidence to date that higher prenatal PCB exposures are associated with a smaller thymic index at birth, which may imply impaired or delayed immune development in utero. Other researchers have noted that PCBs can alter immunologic function. Weisglas-Kuperus et al. (1995, 2000) reported increased numbers of CD8+ T lymphocytes and lower antibody tiers in response to rubella and mumps vaccines in association with higher prenatal PCB exposure in infants. Also, they observed that significant recurrent otitis media and higher prevalence of chicken pox were associated with a higher PCB body burden at 42 months of age. The median level of the sum of PCBs 118, 138, 153, and 180 in the Dutch study (Weisglas-Kuperus et al. 1995, 2000) was 2.07 μg/L, whereas in our study population it was 3.67 μg/L. If we compare the median concentration of PCB-153, the major congener, among different studies, it ranged from 30 to 140 ng/g lipid (Longnecker et al. 2003) in general populations from the United States, the Netherlands, Germany, and Canada, and in our study the concentration was 140 ng/g lipids. Considering half-lives of PCBs and the general decline in body burdens in most parts of the world (Cerna et al. 2007; Noren and Meironyte 2000), and noting the fact that the specimens from the Netherlands study were measured in the early 1990s, the residents in eastern Slovakia are currently exposed to high levels of PCBs.
The clinical implication of this reduced thymic index remains to be clarified. Nonetheless, the reduction in thymic index associated with an increase across the interquartile range of PCBs was comparable to the reduction associated with a 1-week premature delivery: both were −3%. Thus, the magnitude of the impact of this level of PCBs might be equivalent to a one-week delay in thymic maturation, although the actual mechanism and consequences are unknown. Because we are following the children in this birth cohort, in future analyses, we will examine longitudinal data for thymic index measured at 6 and 16 months to determine whether prenatal or postnatal PCB exposures influence the trajectory of this organ, and to evaluate associations of PCBs with other immune parameters as well as with clinical outcomes.
Footnotes
We gratefully thank P. Ashwood for his specific advice on quality control and interpretation of this paper. We also acknowledge the ongoing assistance from our Scientific Advisory Board, including M. Alavanja, A. Bergman, A. Silverstone, J. Vena, D. Patterson, and G. Winneke. We thank Z. Yu and other field staff, and we also thank the mothers and children for participating in the study.
This project has been funded by the U.S. National Institutes of Health, National Cancer Institute, grant R01-CA96525, with additional support from the National Institute of Environmental Health Sciences, 1P01-ES11269 and the U.S. Environmental Protection Agency Science to Achieve Results (STAR) program R829388.
References
- Aaby P, Marx C, Trautner S, Rudaa D, Hasselbalch H, Jensen H, et al. Thymus size at birth is associated with infant mortality: a community study from Guinea-Bissau. Acta Paediatr. 2002;91(6):698–703. doi: 10.1080/080352502760069142. [DOI] [PubMed] [Google Scholar]
- Akins JR, Waldrep K, Bernert JT., Jr The estimation of total serum lipids by a completely enzymatic ‘summation’ method. Clin Chim Acta. 1989;184(3):219–226. doi: 10.1016/0009-8981(89)90054-5. [DOI] [PubMed] [Google Scholar]
- Beineke A, Siebert U, McLachlan M, Bruhn R, Thron K, Failing K, et al. Investigations of the potential influence of environmental contaminants on the thymus and spleen of harbor porpoises (Phocoena phocoena) Environ Sci Technol. 2005;39(11):3933–3938. doi: 10.1021/es048709j. [DOI] [PubMed] [Google Scholar]
- Benn CS, Jeppesen DL, Hasselbalch H, Olesen AB, Nielsen J, Bjorksten B, et al. Thymus size and head circumference at birth and the development of allergic diseases. Clin Exp Allergy. 2001;31(12):1862–1866. doi: 10.1046/j.1365-2222.2001.01128.x. [DOI] [PubMed] [Google Scholar]
- Brazdova Z, Fiala J, Hrstkova H. Dietary habits of Romany children [in Czech] Cs Pediatrie. 1998;53:419–423. [Google Scholar]
- Camacho IA, Nagarkatti M, Nagarkatti PS. Evidence for induction of apoptosis in T cells from murine fetal thymus following perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Toxicol Sci. 2004;78(1):96–106. doi: 10.1093/toxsci/kfh048. [DOI] [PubMed] [Google Scholar]
- Carver LA, Hogenesch JB, Bradfield CA. Tissue specific expression of the rat Ah-receptor and ARNT mRNAs. Nucleic Acids Res. 1994;22(15):3038–3044. doi: 10.1093/nar/22.15.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerna M, Spe vackova V, Batariova A, Smid J, Cejchanova M, Ocadlikova D, et al. Human biomonitoring system in the Czech Republic. Int J Hyg Environ Health. 2007;210(3–4):495–499. doi: 10.1016/j.ijheh.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Chevalier P, Sevilla R, Zalles L, Sejas E, Belmonte G, Parent G, et al. Immuno-nutritional recovery of children with severe malnutrition. Sante. 1996;6(4):201–208. [PubMed] [Google Scholar]
- Collinson AC, Moore SE, Cole TJ, Prentice AM. Birth season and environmental influences on patterns of thymic growth in rural Gambian infants. Acta Paediatr. 2003;92(9):1014–1020. [PubMed] [Google Scholar]
- Conka K, Drobna B, Kocan A, Petrik J. Simple solid-phase extraction method for determination of polychlorinated biphenyls and selected organochlorine pesticides in human serum. J Chromatogr A. 2005;1084(1–2):33–38. doi: 10.1016/j.chroma.2004.11.029. [DOI] [PubMed] [Google Scholar]
- DeCaprio AP, McMartin DN, O’Keefe PW, Rej R, Silkworth JB, Kaminsky LS. Subchronic oral toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in the guinea pig: comparisons with a PCB-containing transformer fluid pyrolysate. Fundam Appl Toxicol. 1986;6(3):454–463. doi: 10.1016/0272-0590(86)90219-8. [DOI] [PubMed] [Google Scholar]
- Dominguez-Gerpe L, Rey-Mendez M. Evolution of the thymus size in response to physiological and random events throughout life. Microsc Res Tech. 2003;62(6):464–476. doi: 10.1002/jemt.10408. [DOI] [PubMed] [Google Scholar]
- Elliott JE, Machmer MM, Wilson LK, Henny CJ. Contaminants in ospreys from the Pacific Northwest: II. Organochlorine pesticides, polychlorinated biphenyls, and mercury, 1991–1997. Arch Environ Contam Toxicol. 2000;38(1):93–106. doi: 10.1007/s002449910012. [DOI] [PubMed] [Google Scholar]
- Esser C, Lai ZW, Gleichmann E. Proliferation inhibition and Cd4/Cd8 thymocyte subset skewing by in-vivo exposure of C57Bl/6 mice to Ah receptor-binding 3,3’,4,4’-tetra-chlorobiphenyl. Exp Clin Immunogenet. 1994;11(2–3):75–85. doi: 10.1159/000424196. [DOI] [PubMed] [Google Scholar]
- Fine JS, Gasiewicz TA, Silverstone AE. Lymphocyte stem cell alterations following perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol Pharmacol. 1989;35(1):18–25. [PubMed] [Google Scholar]
- Fine JS, Silverstone AE, Gasiewicz TA. Impairment of prothymocyte activity by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Immunol. 1990;144(4):1169–1176. [PubMed] [Google Scholar]
- Fraker PJ, King LE, Laakko T, Vollmer TL. The dynamic link between the integrity of the immune system and zinc status. J Nutr. 2000;130(5S Suppl):1399S–1406S. doi: 10.1093/jn/130.5.1399S. [DOI] [PubMed] [Google Scholar]
- George AJ, Ritter MA. Thymic involution with ageing: obsolescence or good housekeeping? Immunol Today. 1996;17(6):267–272. doi: 10.1016/0167-5699(96)80543-3. [DOI] [PubMed] [Google Scholar]
- Ginter E, Krajcovicova-Kudlackova M, Kacala O, Kovacic V, Valachovicova M. Health status of Romanies (Gypsies) in the Slovak Republic and in the neighbouring countries. Bratisl Lek Listy. 2001;102(10):479–484. [PubMed] [Google Scholar]
- Goff KF, Hull BE, Grasman KA. Effects of PCB 126 on primary immune organs and thymocyte apoptosis in chicken embryos. J Toxicol Environ Health A. 2005;68(6):485–500. doi: 10.1080/15287390590903720. [DOI] [PubMed] [Google Scholar]
- Goldsby RA, Kindt TJ, Osborne BA, Kuby J. Immunology. New York: W.H.Freeman and Company; 2003. [Google Scholar]
- Hasselbalch H, Jeppesen DL, Ersboll AK, Engelmann MD, Nielsen MB. Thymus size evaluated by sonography. A longitudinal study on infants during the first year of life. Acta Radiol. 1997;38(2):222–227. doi: 10.1080/02841859709172053. [DOI] [PubMed] [Google Scholar]
- Hasselbalch H, Nielsen MB, Jeppesen D, Pedersen JF, Karkov J. Sonographic measurement of the thymus in infants. Eur Radiol. 1996;6(5):700–703. doi: 10.1007/BF00187675. [DOI] [PubMed] [Google Scholar]
- Iscan A, Tarhan S, Guven H, Bilgi Y, Yuncu M. Sonographic measurement of the thymus in newborns: close association between thymus size and birth weight. Eur J Pediatr. 2000;159(3):223–224. doi: 10.1007/s004310050056. [DOI] [PubMed] [Google Scholar]
- Jeppesen DL, Hasselbalch H, Nielsen SD, Sorensen TU, Ersboll AK, Valerius NH, et al. Thymic size in preterm neonates: a sonographic study. Acta Paediatr. 2003;92(7):817–822. [PubMed] [Google Scholar]
- Kerkvliet NI, Steppan LB, Brauner JA, Deyo JA, Henderson MC, Tomar RS, et al. Influence of the Ah locus on the humoral immunotoxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin: evidence for Ah-receptor-dependent and Ah-receptor-independent mechanisms of immunosuppression. Toxicol Appl Pharmacol. 1990;105(1):26–36. doi: 10.1016/0041-008x(90)90356-y. [DOI] [PubMed] [Google Scholar]
- Kocan A, Petrik J, Drobna B, Chovancova J. Levels of PCBs and some organochlorine pesticides in the human population of selected areas of the Slovak Republic. I. Blood. Chemosphere. 1994;29(9–11):2315–2325. doi: 10.1016/0045-6535(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Koupilova I, Epstein H, Holcik J, Hajioff S, McKee M. ealth needs of the Roma population in the Czech and Slovak Republics. Soc Sci Med. 2001;53(9):1191–1204. doi: 10.1016/s0277-9536(00)00419-6. [DOI] [PubMed] [Google Scholar]
- Krajcovicova-Kudlackova M, Blazicek P, Spustova V, Valachovicova M, Ginter E. Cardiovascular risk factors in young Gypsy population. Bratisl Lek Listy. 2004;105(7–8):256–259. [PubMed] [Google Scholar]
- Kremer J, Gleichmann E, Esser C. Thymic stroma exposed to arylhydrocarbon receptor-binding xenobiotics fails to support proliferation of early thymocytes but induces differentiation. J Immunol. 1994;153(6):2778–2786. [PubMed] [Google Scholar]
- Lai ZW, Kremer J, Gleichmann E, Esser C. 3,3’,4,4’-Tetrachlorobiphenyl inhibits proliferation of immature thymocytes in fetal thymus organ-culture. Scand J Immunol. 1994;39(5):480–488. doi: 10.1111/j.1365-3083.1994.tb03403.x. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Wolff MS, Gladen BC, Brock JW, Grandjean P, Jacobson JL, et al. Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environ Health Perspect. 2003;111:65–70. doi: 10.1289/ehp.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpuech-Brugere C. Immunonutrition and the thymus. utrition. 2001;17(11–12):972–973. doi: 10.1016/s0899-9007(01)00698-0. [DOI] [PubMed] [Google Scholar]
- Noren K, Meironyte D. Certain organochlorine and organobromine contaminants in Swedish human milk in perspective of past 20–30 years. Chemosphere. 2000;40(9–11):1111–1123. doi: 10.1016/s0045-6535(99)00360-4. [DOI] [PubMed] [Google Scholar]
- Olesen AB, Andersen G, Jeppesen DL, Benn CS, Juul S, Thestrup-Pedersen K. Thymus is enlarged in children with current atopic dermatitis. A cross-sectional study. cta Derm Venereol. 2005;85(3):240–243. doi: 10.1080/00015550510026352. [DOI] [PubMed] [Google Scholar]
- Persky V, Turyk M, Anderson HA, Hanrahan LP, Falk C, Steenport DN, et al. The effects of PCB exposure and fish consumption on endogenous hormones. Environ Health Perspect. 2001;109:1275–1283. doi: 10.1289/ehp.011091275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LW, Parkinson A, Bandiera S, Lambert I, Merrill J, Safe SH. PCBs and PBBs: biologic and toxic effects on C57BL/6J and DBA/2J inbred mice. Toxicology. 1984;31(3–4):191–206. doi: 10.1016/0300-483x(84)90101-x. [DOI] [PubMed] [Google Scholar]
- Ross PS, Jeffries SJ, Yunker MB, Addison RF, Ikonomou MG, Calambokidis JC. Harbor seals (Phoca vitulina) in British Columbia, Canada, and Washington State, USA, reveal a combination of local and global polychlorinated biphenyl, dioxin, and furan signals. Environ Toxicol Chem. 2004;23(1):157–165. doi: 10.1897/03-85. [DOI] [PubMed] [Google Scholar]
- Shimada T. Lack of correlation between formation of reactive metabolites and thymic atrophy caused by 3, 4, 3’, 4’-tetrachlorobiphenyl in C57BL/6N mice. Arch Toxicol. 1987;59(5):301–306. doi: 10.1007/BF00295079. [DOI] [PubMed] [Google Scholar]
- Silkworth JB, Antrim L. Relationship between Ah receptor-mediated polychlorinated biphenyl (PCB)-induced humoral immunosuppression and thymic atrophy. J Pharmacol Exp Ther. 1985;235(3):606–611. [PubMed] [Google Scholar]
- Silkworth JB, Antrim LA, Sack G. Ah receptor mediated suppression of the antibody response in mice is primarily dependent on the Ah phenotype of lymphoid tissue. Toxicol Appl Pharmacol. 1986;86(3):380–390. doi: 10.1016/0041-008x(86)90365-0. [DOI] [PubMed] [Google Scholar]
- Steinmann GG, Klaus B, Muller-Hermelink HK. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol. 1985;22(5):563–575. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Takayama M, Itoh S, Nagasaki T, Tanimizu I. A new enzymatic method for determination of serum choline-containing phospholipids. Clin Chim Acta. 1977;79(1):93–98. doi: 10.1016/0009-8981(77)90465-x. [DOI] [PubMed] [Google Scholar]
- Tan Y, Li D, Song R, Lawrence D, Carpenter DO. Ortho-substituted PCBs kill thymocytes. Toxicol Sci. 2003;76(2):328–337. doi: 10.1093/toxsci/kfg233. [DOI] [PubMed] [Google Scholar]
- Tryphonas H, Feeley M, Robertson LW, Hansen LG. PCBs: Recent Advances in the Environmental Toxicology and Health Effects. Lexington: University Press of Kentucky; 2001. Polychilorinated biphenyl-induced immunomodulation and human health effects; pp. 193–209. [Google Scholar]
- Van EW. T-cell differentiation is influenced by thymic microenvironments. Annu Rev Immunol. 1991;9:591–615. doi: 10.1146/annurev.iy.09.040191.003111. [DOI] [PubMed] [Google Scholar]
- Venkataraman P, Sridhar M, Dhanammal S, Vijayababu MR, Arunkumar A, Srinivasan N, et al. Effects of vitamin supplementation on PCB (Aroclor 1254)-induced changes in ventral prostatic androgen and estrogen receptors. Endocr Res. 2004a;30(3):469–480. doi: 10.1081/erc-200035959. [DOI] [PubMed] [Google Scholar]
- Venkataraman P, Sridhar M, Dhanammal S, Vijayababu MR, Srinivasan N, Arunakaran J. Antioxidant role of zinc in PCB (Aroclor 1254) exposed ventral prostate of albino rats. J Nutr Biochem. 2004b;15(10):608–613. doi: 10.1016/j.jnutbio.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Vivian C, Dundes L. The crossroads of culture and health among the Roma (Gypsies) J Nurs Scholarsh. 2004;36(1):86–91. doi: 10.1111/j.1547-5069.2004.04018.x. [DOI] [PubMed] [Google Scholar]
- Weisglas-Kuperus N, Patandin S, Berbers GA, Sas TC, Mulder PG, Sauer PJ, et al. Immunologic effects of background exposure to polychlorinated biphenyls and dioxins in Dutch preschool children. Environ Health Perspect. 2000;108:1203–1207. doi: 10.1289/ehp.001081203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisglas-Kuperus N, Sas TC, Koopman-Esseboom C, van der Zwan CW, de Ridder MA, Beishuizen A, et al. Immunologic effects of background prenatal and postnatal exposure to dioxins and polychlorinated biphenyls in Dutch infants. Pediatr Res. 1995;38(3):404–410. doi: 10.1203/00006450-199509000-00022. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Anderson HA, Hanrahan LP, Kanarek MS, Falk CM, Steenport DM, et al. Maternal exposure to Great Lakes sport-caught fish and dichlorodiphenyl dichloroethylene, but not polychlorinated biphenyls, is associated with reduced birth weight. Environ Res. 2005;97(2):149–162. doi: 10.1016/j.envres.2004.01.014. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Fourth Round of WHO-Coordinated Study. WHO Report EUR/00/5020352. Copenhagen: World Health Organization Regional Office for Europe; 2000. Interlaboratory Quality Assessment of Levels of PCBs, PCDDs and PCDFs in Human Milk and Blood Plasma. [Google Scholar]
- Yekeler E, Tambag A, Tunaci A, Genchellac H, Dursun M, Gokcay G, et al. Analysis of the thymus in 151 healthy infants from 0 to 2 years of age. J Ultrasound Med. 2004;23(10):1321–1326. doi: 10.7863/jum.2004.23.10.1321. [DOI] [PubMed] [Google Scholar]