Abstract
Background
We previously conducted a study to assess whether household exposures to tap water increased an individual’s internal dose of trihalomethanes (THMs). Increases in blood THM levels among subjects who showered or bathed were variable, with increased levels tending to cluster in two groups.
Objectives
Our goal was to assess the importance of personal characteristics, previous exposures, genetic polymorphisms, and environmental exposures in determining THM concentrations in blood after showering.
Methods
One hundred study participants completed a health symptom questionnaire, a 48-hr food and water consumption diary, and took a 10-min shower in a controlled setting. We examined THM levels in blood samples collected at baseline and 10 and 30 min after the shower. We assessed the significance of personal characteristics, previous exposures to THMs, and specific gene polymorphisms in predicting postshower blood THM concentrations.
Results
We did not observe the clustering of blood THM concentrations observed in our earlier study. We found that environmental THM concentrations were important predictors of blood THM concentrations immediately after showering. For example, the chloroform concentration in the shower stall air was the most important predictor of blood chloroform levels 10 min after the shower (p < 0.001). Personal characteristics, previous exposures to THMs, and specific polymorphisms in CYP2D6 and GSTT1 genes were significant predictors of both baseline and postshowering blood THM concentrations as well as of changes in THM concentrations associated with showering.
Conclusion
The inclusion of information about individual physiologic characteristics and environmental measurements would be valuable in future studies to assess human health effects from exposures to THMs in tap water.
Keywords: CYP2D6, CYP2E1, disinfection by-products, drinking water disinfection, GSTT1, showering exposures, trihalomethanes
Epidemiologic studies have consistently found significant associations between exposure to drinking water disinfection byproducts (DBPs) and human cancers (Cantor et al. 1998; Freedman et al. 1997; Hildesheim et al 1998; King and Marrett 1996; McGeehin et al. 1993; Villanueva et al. 2006b; Zierler et al. 1988); however, the reported odds ratios were typically < 2.0 and quite variable. Studies of adverse reproductive outcomes associated with DBP exposures have yielded inconsistent results (e.g., King et al. 2000; Savitz et al. 1995, 2006; Waller et al. 1998). As suggested by Villanueva et al. (2006a), additional information, including individual physiologic and genetic characteristics, recent relevant exposures, and direct environmental measurements, may improve exposure assessment and thus the precision of disease risk estimates associated with human exposure to disinfection byproducts.
We previously conducted a study to assess whether, in addition to drinking water, household water uses that generate aerosols or release volatile chemicals are an important and measurable source of trihalomethane (THM) exposure (Backer et al. 2000). Increases in blood THM levels among the 10 subjects who showered or bathed were highly variable and fell into two significantly different groups, suggesting possible differences in THM metabolism. For example, the mean (± SD) increase in blood bromoform levels was 8.2 ± 2.97 pg/mL for one group of five participants and 21.2 ± 4.26 pg/mL for the remaining five participants. Miles et al. (2002) reported finding similar clustering of blood THM levels after various household water use activities.
The variation of blood THM levels may have been the result of differences in physiologic characteristics or recent related exposures. For example, THM blood levels depend not only on tap water quality, but also on the nature and frequency of water use activities (Nuckols et al. 2005).
Blood levels of THMs after environmental exposures may reflect individual differences in drug metabolizing enzyme gene polymorphisms. For example, brominated THMs are substrates for glutathione S-transferase theta (GSTT)–mediated glutathione conjugation reactions (Landi et al. 1999). Individuals with the active enzyme may clear brominated THMs from the blood more rapidly than similarly exposed individuals with the null genotype, and thus have lower levels (Landi et al. 1999).
Polymorphisms in metabolic genes may also mediate disease risks. GSTT1 polymorphisms may be important in determining an individual’s risk for non-Hodgkin lymphoma, stomach cancer, and liver cancer associated with exposure to dichloromethane (Kerridge et al. 2002; Lan et al. 2001; Setiawan et al. 2000). The cytochrome P450 gene CYP2D6 (cytochrome P450 2D6) is involved in metabolism of xenobiotic chemicals (Nebert and Russell 2002). It has 5–10 variants affecting metabolic activity to influence levels of parent compound and metabolites that may be responsible for adverse health effects. For CYP2E1 (cytochrome P450 2E1), Infante-Rivard (2004) found that exposure to high levels of THMs affected fetal growth only in individuals with a specific genetic polymorphism. In addition, specific variants of CYP2E1 could be involved in the metabolic activation of trihalomethane-related carcinogens (Gao and Zhang 1999).
The primary objective of this study was to determine the major contributors to variability in blood THM levels after showering, and particularly to evaluate whether the clustering of blood THM levels after showering that we observed in our previous study was a spurious finding because of our small sample size or was real based on physiologic and genetic differences among individuals.
Materials and Methods
Institutional review board approvals
The institutional review boards of the Centers for Disease Control and Prevention (CDC), the National Institutes of Health, the General Clinical Research Center (GCRC) at the University of Pittsburgh, and Battelle Memorial Institute approved this study protocol. We have complied with all applicable requirements for protection of human subjects. Study participants gave written informed consent before the study.
Study participants
This study was conducted at the GCRC clinical research laboratory during July–September 2004. We recruited potential study participants from a panel who volunteered to participate in research at the GCRC. Study participants provided blood samples for a complete blood count, standard blood chemistry panel, and enzyme activity and genotyping, including CYP2D6 (accession no. AY545216; GenBank; http://www.ncbi.nlm.nih.gov/GenBank), CYP2E1 (accession no. DQ515958; GenBank), GSTT1 [glutathione S-transferase (theta class); accession no. BC007065; GenBank], and GSTM1 [glutathione S-transferase (μ class); accession no. BC024005; GenBank]. Eligible subjects (18–45 years of age) had a normal blood screen, were not pregnant (verified for women with a pregnancy test on the day of the study), not nursing an infant, and physically able to take a shower and provide blood samples. All subjects were nonsmokers, did not drink an average of more than one alcoholic beverage per day, were willing to abstain from drinking alcoholic beverages for 2 days before the study, did not take acetaminophen five or more times a week, and were free from chronic lung disease and asthma.
Estimates of the frequency of GSTT1 positive in the various populations range from 15 to 80% (El-Masri et al.1999; Garte et al. 2001; Raimondi et al. 2006). Therefore, we used the initial GCRC genotyping results to identify potential participants who were GSTT1 positive. We recruited 43 study participants with GSTT1 positive (GSTT1 wild type or GSTT1 heterozygous null) and 57 with GSTT1 homozygous null genotypes. We then assessed these individuals for their CYP2D6 and CYP2E1 genotypes.
Of 112 volunteers, 100 met eligibility criteria, agreed to participate, and completed study activities over a 6-week period. A random sample of nine subjects repeated the study for analysis of the repeatability of our results (analysis not shown here). There were five family groups, and 11 subjects were blood relatives of another study participant.
Questionnaire and diary
We collected questionnaire data for each participant just before conducting study activities. Specifically, we collected data on demographics (age, sex, race), height, weight, occupation, smoking, use of chloral hydrate, recent respiratory symptoms, and activities that were sources of THM exposure (bathing, swimming, using hot tub or sauna, washing clothes or dishes) or that might affect the activity of the enzymes of interest [alcohol consumption (Dupont et al. 2000), using solvents or cleaners containing volatile organic compounds (VOCs)].
Study participants recorded in a diary how much tap water they drank during the 48 hr preceding study activities. We estimated cold water intake as number of 8-oz cups. Weisel and Chen (1994) found that heating water increased THM concentrations and that household exposures calculated using the THM concentration in heated water were 50% higher than those calculated using the THM concentration in cold water. Thus, we estimated hot water intake as 2 × [number of bowls of soup made with water + 8-oz cups of hot coffee + 8-oz cups of hot tea + (servings of hot cereal × 0.66) + (servings of rice or pasta × 0.66) + (servings of vegetables cooked in water × 0.05)].
Certain foods induce synthesis of some of the metabolic enzymes of interest in this study. Subjects recorded in a diary how many servings of broccoli, cauliflower, lettuce, onions, garlic, watercress, and black tea they ate/drank during the 48 hr preceding study activities (Kall et al.1996; Leclercq et al. 1998; Le Marchand et al. 1999; Stupans et al. 2001). We estimated the intake of foods of interest as the number of servings of broccoli, cauliflower, and lettuce + (servings of onions × 0.2) + (servings of garlic, watercress, and black tea × 0.1).
Study activities
To minimize the influence of foods, beverages, alcohol consumption, and selected medications on study results, participants fasted overnight, did not drink any alcoholic beverages or take medications that affect the enzymes of interest (i.e., dextromethorphan, chlorpheniramine, chloral hydrate) during the 48 hr before study activities, and did not drink caffeinated beverages on the day of the study. Urine samples were collected immediately before and after study activities for future analysis of haloacetic acid concentrations and one extra blood sample was collected for future genotyping.
On the day of the study, subjects completed the questionnaire and provided two 10-mL blood samples, took a 10-min temperature-controlled shower, provided one 10-mL blood sample first 10 min and then 30 min after turning off the shower, and then ingested a 250-mg chlorzoxazone tablet (Lemmon Company, Sellersville, PA). One 10-mL blood sample was drawn 2 hr later for analysis of chlorzoxazone metabolism as a measure of CYP2E1 enzyme activity. Study participants remained at the study site for an additional 2 hr to ensure that no adverse reaction to chlorzoxazone occurred.
To limit study participants’ exposure to THMs unrelated to the shower, we required that they not flush the toilet or run tap water while in the study area, dry off and dress as quickly as possible, and stay in a separate room away from further exposure to THMs. We provided study participants with THM-free bottled drinking water.
Biological specimens
Whole blood samples for THM analysis were collected by a certified phlebotomist using Vacutainer tubes processed to remove VOC contamination (Cardinali et al. 1995). Samples were analyzed for THM levels using headspace solid phase microextraction (SPME) coupled with gas chromatography (GC) and high-resolution mass spectrometry (MS). Analyte quantification was based on stable isotope dilution (Bonin et al. 2005).
In vivo CYP2E1 activity.
CYP2E1 is inducible but the genotype does not predict the phenotype. We therefore measured CYP2E1 activity by the in vivo chlorzoxazone test (Frye et al. 1998; Scott et al. 1999). Serum concentrations of chlorzoxazone and its major metabolite, 6-hydroxychlorzoxazone, were measured by high performance liquid chromatography. We used the ratio of 6-hydroxychlorzoxazone to chlorzoxazone as a phenotypic measure of CYP2E1 activity.
GSTT1, CYP2D6, and CYP2E1 genotyping.
Genomic DNA was extracted from peripheral blood using the PureGene DNA Isolation Kit (Gentra Systems, Inc., Minneapolis, MN) according to the manufacturer’s instructions. We screened the CYP2D6*3 and 4 and CYP2E1*5 variant alleles with TaqMan allele discrimination-based assays using the Applied Biosystems 7700 system (Applied Biosystems, Foster City, CA). For the differential polymerase chain reaction (PCR) method for GSTT1 genotyping, we used a housekeeping gene (β-globin) as an internal control (Pemble et al. 1994).
Positive and negative PCR controls were included with each amplification reaction. For both genotyping analyses, we used previously sequenced genomic DNA samples as positive controls for the homozygous wild-type, heterozygous, and homozygous variant genotypes with every PCR analysis to verify reproducibility of the assay and to confirm accuracy of genotype classifications. Approximately 10% of randomly selected samples were repeated blindly for verification of genotyping assays. All results were interpreted independently by two laboratory staff members, and no discordant genotype classifications were identified.
Environmental samples
Air
We collected three air samples near the subject’s breathing zone: a preexposure bathroom sample, a 10-min time-integrated sample in the enclosed shower stall during the shower, and a 5-min time-integrated postshower sample in the unventilated bathroom. Air samples were collected using evacuated stainless steel canisters. Filled canisters were sealed and shipped to Battelle (Columbus, OH) for analysis. We analyzed the samples for THMs by automated GC/MS using a modified version of U.S. Environmental Protection Agency method TO-14 (Winberry et al. 1990). Full details of air sampling and analysis procedures are available elsewhere (Gordon et al. 2006).
Water samples
The shower head was modified to allow remote water sampling. Duplicate samples were collected 5 min after each shower began. Participants were instructed to set the shower water temperature between 104 and 105°F (40–41°C). Shower water temperature was monitored by the participant and study staff via a digital thermometer in the shower stall and a remote radio thermometer outside the bathroom. Water samples were collected in borosilicate glass vials containing sodium thiosulfate to quench further THM formation and phosphate buffer to standardize pH between 6.0 and 6.5. We analyzed water samples for THM levels using headspace SPME–GC/MS with quantification based on stable isotope-dilution (Cardinali et al. 2004).
Statistical analyses
We conducted statistical analyses using SAS, version 9.0 (SAS Institute Inc., Cary, NC). We evaluated associations of predictor variables (e.g., demographics, genotype, THM concentrations in air, THM concentrations in water) with THM levels in blood 10 min after showering and THM level changes (10 min minus baseline) after showering. For changes in THM levels in blood, the logs of the differences (10 min minus baseline) were used as our outcome measure. The residuals from the final models were evaluated for normality; this assumption was satisfied for each model.
We used generalized estimating equations to model these data to account for the correlations caused by having multiple members of the same family in the analyses. Univariate analyses were run separately for each THM. We chose independent variables that had a p-value ≤ 0.10 for inclusion in initial multivariate models and used a backward selection approach to select a final model for each outcome measure. After choosing a final model for the change in blood levels of THMs at 10 min after exposure, we wished to investigate the contribution of individual variables to the total variability explained by all variables. The partial r2 measures the marginal contribution of one explanatory variable when all others are already included in the model. Partial r2 values were not available in the generalized estimating equations procedure, so our r2 results were based on a least-squares analysis.
Results
In this article, we present the results from the analysis of environmental samples and blood samples collected at baseline and at 10 min after the shower.
Approximately half (54) of our 100 study participants were women, and most (73) were white. Eighty-five study participants reported eating at least one of the foods of interest within 24 hr of the study, and about one-fifth reported having upper respiratory symptoms within 4 weeks of the study. Eleven subjects reported exposure to bleach, and five or fewer people reported exposures to specific solvents or other VOCs within 24 hr of the study (data not shown). Over half (57) of our study participants were GSTT1 null; 43 were GSTT1 positive (GSTT1 wild type or GSTT1 heterozygous null). Nearly three-fourths (74) of our study participants were CYP2D6 wild type (*1/*1), 21 were heterozygous (*1/*3 or *1/*4), pand 5 were homozygous recessive (*4/*4). The results of the chlorzoxazone assay for CYP2E1 enzyme activity were as follows: median, 0.54; interquartile range, 0.37–0.72; range, 0.21–1.72. Consistent with previous reports (Gurley et al 2002; Lucas et al 1998), the distribution of 6-hydroxychlorzoxazone/chlorzoxazone ratios was skewed toward lower values (i.e., lower metabolic activity).
Results from the analyses of THMs in shower water and shower stall air samples are presented in Table 1. Chloroform was present in the highest concentrations in both water and air samples, followed by concentrations of bromodichloromethane, dibromochloromethane, and bromoform. We prevented air circulation during study activities; thus, THM air concentrations in the 10-min integrated shower samples and the 5-min integrated postshower samples were similar.
Table 1.
THM concentrations [median (interquartile range)]a in environmental samples: tap water collected during showering and three integrated air samples (preexposure baseline, a time-integrated sample in the shower stall while the participant is showering, and a time-integrated sample to cover the 5-min postshower exposure period).
| Air (μg/mb)
|
||||
|---|---|---|---|---|
| THMc,d | Water (μg/L) | Preexposure baseline sample | 10-min integrated shower stall sample | 5-min integrated post-shower bathroom sample |
| Bromoform | 1.0 (0.4–1.5) | < LODe | 2.69 (1.46–4.13) | 2.69 (1.46–4.34) |
| Dibromochloromethane | 9.5 (6.2–13) | < LOD | 27.6 (20.5–38.8) | 29.5 (20.4–38.6) |
| Bromodichloromethane | 21 (18–24) | < LOD | 75.0 (59.1–86.2) | 78.7 (64.1–87.8) |
| Chloroform | 66 (56–72) | 1.31 (0.69–1.61) | 245 (212–279) | 248 (221–288) |
| Total THMs | 98 (91–102) | 1.31 (0.69–1.61) | 353 (314–394) | 362 (328–408) |
LOD, limit of detection.
Range is the 25th and 75th percentiles.
LOD for THM concentrations in air: bromoform, 2.1 μg/m3; dibromochloromethane, 1.7 μg/m3; bromodichloromethane, 1.3 μg/m3; chloroform, 0.98 μg/m3.
LOD for trihalomethanes in water: bromoform: 0.12 μg/L; dibromochloromethane: 0.24 μg/L; bromodichloromethane: 0.48 μg/L; chloroform: 0.92 μg/L.
When the concentration of an analyte was below the LOD, the concentration was replaced with the LOD/√2.
Except for chloroform, the THM concentrations in air were below the LOD. Only chloroform was included in the total THM value for air.
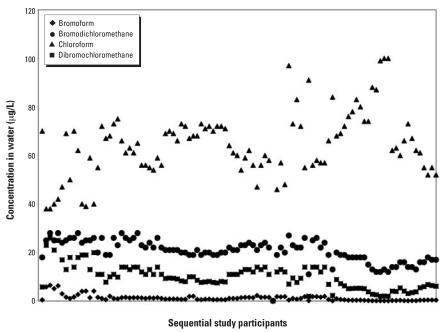
The distribution of THMs in water samples by consecutive study participant number over the 12-week study period is shown in Figure 1. Bromoform levels were > 6 μg/L for all but one of the first 12 participants and < 2 μg/L for the remaining participants. The drop in bromoform concentration during the study was likely the result of unusually high rainfall that occurred in Pittsburgh at the time of the study. It is likely that the unusually high water volume diluted the local water source, thus diluting tap-water bromine concentrations. Despite the very low levels of bromoform in the water, we were able to detect bromoform in 75 of the baseline blood samples and in 98 of the 10-min postshowering blood samples. The concentrations of mono-and dibrominated compounds also decreased over the study period. Over the study period, chloroform levels in shower water varied from < 40 μg/L to nearly 100 μg/L.
Figure 1.
THM concentrations in shower water. Data are presented by study ID number in numeric and chronologic order over the 12-week study period (n = 99).
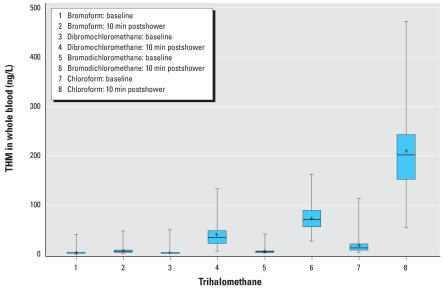
THM concentrations in blood samples and the changes in concentration (10 min postshower minus baseline) are presented in Table 2. The blood THM data for one study participant seemed anomalous (> 2 SDs from the sample means at baseline and after showering) and thus were removed from these analyses. Blood THM levels are presented in Figure 2. THM concentrations measured 10 min after the shower were log-normally distributed.
Table 2.
THM concentrations (ng/L) in blood samples collected from 99 study participants immediately before showering and blood samples collected from the same participants 10 min after completing the shower, and the changes in THM concentrations (10 min postshower minus baseline).
| Median concentration (interquartile rangea)
|
Median difference (interquartile range)
|
||
|---|---|---|---|
| THMb | Baseline postshower | 10 min minus baseline | 10 min postshower |
| Bromoform | 0.91 (0.7–1.2) | 4.0 (2.2–6.2) | 2.9 (1.5–4.5) |
| Dibromochloromethane | 1.2 (0.71–2.1) | 32 (21–46) | 31 (20–42) |
| Bromodichloromethane | 2.2 (1.4–3.5) | 69 (54–88) | 64 (49–84) |
| Chloroform | 10 (6.7–18) | 200 (150–240) | 187 (144–230) |
Range is the 25th–75th percentiles.
Limit of detection (LOD) for THMs in blood: bromoform: 0.55 ng/L; dibromochloromethane: 0.23 ng/L; bromodichloromethane: 0.24 ng/L; chloroform: 2.4 ng/L. When the concentration of an analyte was below the LOD, the concentration was replaced with LOD/√2.
Figure 2.
THM concentrations in whole blood at baseline and 10 min postshowering. The capped bars are the minimum and maximum, the box extents indicate the 25th and 75th percentiles, the line inside the box marks the 50th percentile, and the + indicates the mean (n = 99).
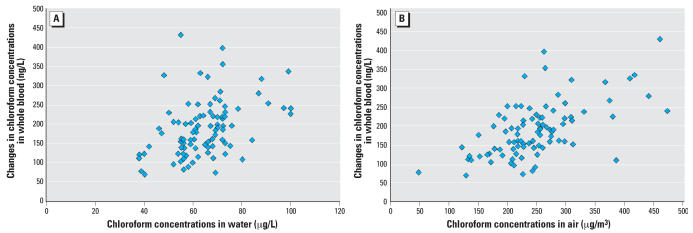
The magnitude of postshower increases in blood THMs reflected THM concentrations in shower water and air. The data for chloroform are presented in Figure 3. The blood concentrations of bromoform reflected water concentrations of bromoform.
Figure 3.
Associations between the change in chloroform concentrations in whole blood (10 min postshower minus baseline) and THM concentrations in shower water (A) and the 10-min integrated shower air sample (B). The correlation (r2) between changes in chloroform concentrations in whole blood and in shower water was 0.60 (p < 0.001). The correlation between chloroform concentrations in water and the 10-min integrated shower air sample was 0.63 (p < 0.001) (n = 99).
Variables that were statistically significant (p ≤ 0.10) in univariate analyses were included in initial models for THM levels in blood. We included water and air (10-min integrated air sample) THM levels; height; weight; body mass index (BMI); sex; race; age; alcohol consumption; and recent swimming, sauna use, and water-related household activities (doing laundry, washing dishes). We also included genotype (for CYP2D6 and GSTT1), and the ratio of 6-hydroxychlorzoxazone to chlorzoxazone (for CYP2E1) in our models. A summary of statistics from GENMOD procedure (SAS Institute Inc.) modeling blood levels of THMs 10 min postshowering and the change in THM concentrations (10 min postshower minus baseline) is presented in Table 3.
Table 3.
Summary of statistics from the multivariate generalized linear models procedure modeling the log of blood levels of THMs at 10 min postshower and the log of the differences in blood THMs (10 min post-shower minus baseline).
| Trihalomethane | Parameter | Estimate | 95% CIs |
|---|---|---|---|
| 10 min postshower | |||
| Bromoform | Intercept | 1.21# | 0.77 to 1.64 |
| BMIa | −0.02** | −0.04 to −0.01 | |
| Swamb | 1.43# | 1.29 to 1.56 | |
| Water concentrationc | 0.56# | 0.46 to 0.65 | |
| Dibromochloromethane | Intercept | 2.63# | 2.28 to 2.99 |
| BMI | −0.02** | −0.03 to −0.01 | |
| Water concentration | 0.08# | 0.06 to 0.11 | |
| Air concentrationd | 0.01** | 0.003 to 0.02 | |
| Bromodichloromethane | Intercept | 3.18# | 2.84 to 3.52 |
| BMI | −0.01* | −0.02 to −0.003 | |
| Saunae | 0.14* | 0.02 to 0.25 | |
| Hot water intake | −0.002# | −0.003 to −0.001 | |
| CYP2D6 *1/*4f | −0.11 | −0.26 to 0.04 | |
| CYP2D6 *4/*4 | 0.20* | 0.005 to 0.39 | |
| Water concentration | 0.03* | 0.01 to 0.05 | |
| Air concentration | 0.01* | 0.001 to 0.01 | |
| Chloroform | Intercept | 4.58# | 4.37 to 4.80 |
| Chlorine cleanersg | −0.15* | −0.27 to −0.02 | |
| Hot water intake | −0.002# | −0.003 to −0.001 | |
| CYP2D6 *1/*4 | −0.08 | −0.20 to 0.04 | |
| CYP2D6 *4/*4 | 0.31** | 0.08 to 0.54 | |
| GSTT1 nullh | 0.10 | 0.00 to 0.20 | |
| Air concentration | 0.003# | 0.002 to 0.004 | |
| 10 min postshower minus baseline | |||
| Bromoform | Intercept | 1.91# | 1.47 to 2.36 |
| BMI | −0.03** | −0.05 to −0.01 | |
| Hot water intake | −0.003# | −0.004 to −0.002 | |
| Log water concentrationi | 1.01# | 0.92 to 1.10 | |
| Dibromochloromethane | Intercept | 1.62# | 1.23 to 1.98 |
| BMI | −0.01** | −0.02 to 0.005 | |
| Hot water intake | −0.003# | −0.004 to −0.002 | |
| CYP2D6 *1/*4 | −0.11 | −0.25 to 0.03 | |
| CYP2D6 *4/*4 | 0.23* | 0.06 to 0.40 | |
| Air concentration | 0.01* | 0.001 to 0.013 | |
| Log water concentration | 0.88# | 0.70 to 1.07 | |
| Bromodichloromethane | Intercept | 1.21* | 0.001 to 2.41 |
| BMI | −0.01* | −0.02 to −0.002 | |
| Hot water intake | −0.003# | −0.004 to −0.002 | |
| CYP2D6 *1/*4 | −0.10 | −0.25 to 0.05 | |
| CYP2D6 *4/*4 | 0.2* | 0.005 to 0.40 | |
| Log air concentrationj | 0.76# | 0.47 to 1.06 | |
| Chloroform | Intercept | 1.21 | 0.22 to 2.20 |
| BMI | −0.01** | −0.02 to −0.004 | |
| Swam | −0.13* | −0.24 to −0.01 | |
| Hot water intake | −0.003# | −0.004 to −0.002 | |
| GSTT1 null | 0.13* | 0.02 to 0.24 | |
| Log air concentration | 0.43** | 0.12 to 0.75 | |
| Log water concentration | 0.45* | 0.03 to 0.86 | |
Values represent the convergence of the algorithm developed with GENMOD (SAS Institute Inc.) and are parameter estimates and 95% confidence intervals (CIs) of variables with p < 0.05. n = 99.
Calculated from height and weight data (CDC 2005).
Swam in a chlorinated pool within 48 hr of doing study activities.
Concentration of the analyte in water (μg/L).
Concentration of analyte in air (μg/m3).
Used sauna within 48 hr of doing study activities.
Genotype groups: *1/*1 = wild type is the comparison group; *1/*4 and *1/*3 = heterozygous extensive metabolizers (should have high ratio); *4/*4, *1/*5,*5B/*5B (also referred to as *5/*5) = genetic variants with significantly decreased metabolizing activity (should have low ratio).
Participant used household bleach or cleaners with bleach within 48 hr of doing study activities.
GSTT1 null, compared to GSTT1 positive.
Log [concentration of analyte in water (μg/L)].
Log [concentration of analyte in air (μg/m3)].
p < 0.05.
p < 0.01.
p < 0.001.
Ten minutes after the shower, shower water concentration was an important predictor of individual blood levels of all the THMs except chloroform. For bromodichloromethane and chloroform, which are more volatile than the other THMs, air concentrations were important in determining blood levels.
Previous exposures and personal characteristics also affected blood THM levels at 10 min postshowering. Swimming was the most important predictor of blood bromoform levels, and higher BMI was associated with lower blood levels of all THMs except chloroform. CYP2D6 genotypes with decreased metabolizing activity were significant predictors of increased blood bromodichloromethane and chloroform levels, and the GSTT1 null (inactive enzyme) genotype was associated with an increase in chloroform blood levels.
The important predictor variables in the models for the changes (10 min postshower minus baseline) in blood THM concentrations were similar to those that were important in predicting 10-min postshower blood THM concentrations (Table 3). For example, the CYP2D6 enzyme variant with decreased metabolizing activity was associated with higher 10-min postshower blood concentrations of bromodichloromethane and chloroform and with higher postshower changes in blood concentrations of bromodichloromethane and dibromochloromethane. Similar to the 10-min postshower concentrations, the GSTT1 null genotype was associated with greater changes in chloroform concentrations 10 min postshower. Environmental concentrations, hot water intake, and BMI were important in determining the postshower changes in blood levels of all THMs.
The importance of the environmental concentrations of THMs in determining the increase in blood levels was confirmed when we examined the partial r2. For chloroform and bromoform, the partial r2 for variables in the final model included 0.3841 (p < 0.001) for log(chloroform concentration in air) and 0.3434 (p < 0.0001) for log(bromodichloromethane concentration in air), respectively. r2 for the other variables in these models were smaller by an order of magnitude. For bromoform and dibromochloromethane, the partial r2 for variables in the final model included 0.86 (p < 0.0001) for log(bromoform concentration in water) and 0.80 (p < 0.0001) for log(dibromochloromethane concentration in water), respectively. Again, r2 for other variables in the model were smaller by at least an order of magnitude.
Discussion
In this study, we found that environmental concentrations (water, air) were the most important predictors for increases in blood THM levels from a showering exposure. We also found that personal characteristics, previous exposures, and metabolic enzyme polymorphisms were significant modulators of shower-related increases in blood concentrations for some THMs.
In assessing the effects of personal characteristics on internal levels of an environmental contaminant, it is important not only to carefully measure exposure, but also to limit exposure variability. In the present study, we limited experimental dose variability by controlling the duration and temperature of the shower. We also enhanced exposure measurements by including concentrations of THMs in shower air as well as water in our analyses. We did not see the clustering of blood THM concentrations that we observed in our previous study (Backer et al. 2000), suggesting that the earlier results were likely a spurious effect attributed to a small (n = 10) sample size. However, consistent with the earlier study, we found an association between shower water and postshowering blood THM concentrations. In the previous study, the average chloroform concentration in shower water was 31 μg/L and the average change in blood concentration (10 min post-shower minus baseline) was approximately 90 ng/L. Here, the geometric mean chloroform concentration in shower water was approximately 64 μg/L, and the average change in blood concentration (10 min postshower minus baseline) was approximately 190 ng/L. Our results demonstrate that blood chloroform and other THM levels are related to tap-water levels in a dose–response manner and that showering is an important source of THM exposure that should not be overlooked in conducting studies of disease risk.
Once exposure to exogenous chemicals occurs, genetic variability in metabolic enzymes may play a role in determining the relative internal dose of parent compound and metabolites. In our previous study (Backer et al. 2000), we noted that THM concentrations in blood samples collected 10 min after a shower appeared to cluster in two groups. In this study, we did not replicate this observation; however, we found that enzyme gene polymorphisms were significant predictors for levels of the most highly chlorinated THMs in blood 10 min after the shower. When compared with the wild type, the reduced activity genotypes CYP2D6 (*4/*4) or GSTT1 null were associated with increased chloroform (and thus presumably decreased metabolites) in the blood 10 min after exposure. Although not significant, the heterozygous extensive metabolizing CYP2D6 (*1/*4) genotype suppressed the magnitude of the increases in chloroform and bromodichloromethane in the blood 10 min after exposure. The phenotypic variation in CYP2E1 did not affect blood THM levels in our study. Our results provide support for the idea that individual genotypic variation can modulate systemic exposure, and thus health risks, associated with exposure to THMs by affecting the relative concentrations of parent THMs and detoxified or activated metabolites present in an individual’s blood.
We were particularly interested in the effects of genetic polymorphisms on bromoform metabolism. Our choice of study site was based on historical data indicating we could expect tap-water levels of all THMs to be high enough to be detectable in blood after a 10-min shower. However, as noted, tap-water bromoform concentrations decreased to concentrations < 2 μg/L after the first 12 subjects. Consequently, blood levels of bromoform 10 min postshowering were consistently low (often below detection) and not highly variable, and we were unable to address the effects of genetic polymorphisms on bromoform levels in blood.
Although evaluating individual genotypes is likely to be useful in interpreting the results of epidemiologic studies of environmental exposures, it requires collecting a biologic specimen from each study participant, thus increasing the resources needed for a given study. To enrich our study populations with the genotypes of interest without making the sample size prohibitively large, we prescreened study participants for specific genotypes. Even with this enrichment, we were able to consistently detect differential effects associated with enzyme polymorphisms only for chloroform.
Overall, our study results indicate that the most important predictors of blood THM concentrations after showering are the environmental exposures themselves. This is particularly evident in the decrease in blood bromoform levels that paralleled the decrease in water levels over the study period. Using the geometric mean concentration of bromoform as the reference, the ratios of bromodichloromethane and dibromochloromethane were similar in the shower water (20.1 and 8.5, respectively) and in the air during the shower (25.2 and 9.2, respectively). Chloroform is the most volatile of the THMs, and the ratio of chloroform to bromoform was of the same magnitude but higher in shower air (99.7) than in shower water (63.5). Again using the geometric mean concentration of bromoform as a reference, the ratios of bromodichloromethane and chloroform were very similar when comparing the levels in blood 10 min after the shower. Thus, immediately after showering, the relative amounts of the different THMs were consistent with what was in shower water and air.
Environmental exposure levels in both water and air were also significant predictors of the changes in blood THMs between baseline and 10 min postshowering. This is consistent with reports by Xu and Weisel (2005a, 2005b) that uptake of chloroform during showering occurs through both dermal and inhalation exposure routes, respectively.
Increased BMI was a predictor for smaller changes in blood THM levels. Compared with subjects with lower BMIs, subjects with higher BMIs tended to have lower blood THM levels at 10 min postshowering; this association may be attributed to THMs partitioning into lipid (Batterman et al. 2002). These results suggest that, given a specific exposure and a baseline measurement, personal characteristics such as BMI can modulate the distribution of these lipophilic DBPs within the body.
Of interest is the association of swimming with blood levels of bromoform and chloroform. Swimming within 48 hr of study activities was the most important predictor of blood bromoform levels 10 min after exposure. This may be because the bromoform levels were very low in our study showers compared with levels present in swimming pool water and in poolside air (Fantuzze et al. 2001). Because bromoform is a carcinogen (National Toxicology Program 1989), studies assessing health risks from THM exposures should query subjects about chlorinated pool use for a more complete assessment of brominated THM exposure. From our data, it is not clear why swimming would have a negative association with the postshower change in blood chloroform concentration. This association disappears with adjustment for baseline values; however, the pros and cons of such adjustment are not always clear (Glymour et al. 2005).
In summary, our analyses indicate that individual polymorphisms in GSTT1 and CYP2D6 genes were significant but minor predictors of blood trihalomethane levels 10 min after showering and of the differences between blood levels 10 min after showering and those at baseline. We demonstrated that it is important to quantify environmental contaminant levels when defining dermal and inhalation exposure to THMs. We also found that personal characteristics, such as BMI, and recent exposures, such as swimming and drinking hot tap water–based beverages, affected postshowering blood THM levels. Our study suggests that information about individual susceptibility factors, such as enzyme gene polymorphisms, together with information about individual physiologic characteristics and environmental measurements, should be included as a component of exposure assessment in future studies of human health risks from exposure to THMs.
Footnotes
We thank L. Thabrew, Y. Canon, J. Huwe, J. Faircloth, and C. Kilcoyne, at the General Clinical Research Center for their assistance in conducting this study.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
Funding for this study was provided by the National Center for Environmental Health, Centers for Disease Control and Prevention, NCI 5R01 CA059834 (Bladder Carcinogenesis), NCI 5 R01 DK 059 519 (Drug Metabolism in Liver Disease) and NCI 5M01-RR-000056 (General Clinical Research Center), and the Intramural Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics (contract NCI 5MO1-RR-000056).
References
- Backer LC, Ashley DL, Bonin MA, Cardinali FL, Kieszak SL, Wooten JV. Household exposures to drinking water disinfection byproducts: whole blood trihalomethane levels. J Expo Sci Environ Epidemiol. 2000;10:321–326. doi: 10.1038/sj.jea.7500098. [DOI] [PubMed] [Google Scholar]
- Batterman S, Zhang L, Wang S, Franzblau A. Partition coefficients for the trihalomethanes among blood, urine, water, milk, and air. Sci Total Environ. 2002;283:237–247. doi: 10.1016/s0048-9697(01)00890-7. [DOI] [PubMed] [Google Scholar]
- Bonin MA, Silva LK, Smith MM, Ashley DL, Blount BC. Measurement of trihalomethanes and methyl tert-butyl ether in whole blood using gas chromatography with high resolution mass spectrometry. J Anal Toxicol. 2005;29(2):81–89. doi: 10.1093/jat/29.2.81. [DOI] [PubMed] [Google Scholar]
- Cantor KP, Lynch CF, Hildesheim ME, Dosemeci M, Lubin J, Alavanja M, et al. Drinking water source and chlorination byproducts. I. Risk of bladder cancer. Epidemiology. 1998;9:21–28. [PubMed] [Google Scholar]
- Cardinali FL, Ashley DL, Morrow JC, Moll DM, Blount BC. Measurement of trihalomethanes and methyl tertiary-butyl ether in tap water using solid-phase microextraction GC/MS. J Chromatogr Sci. 2004;42:200–206. doi: 10.1093/chromsci/42.4.200. [DOI] [PubMed] [Google Scholar]
- Cardinali FL, McCraw JM, Ashley DL, Bonin MA. Treatment of vacutainers for use in the analysis of volatile organic compounds in human blood at the low parts-pertrillion level. J Chromatogr Sci. 1995;33:557–560. doi: 10.1093/chromsci/33.10.557. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) BMI—Body Mass Index: BMI for Adults. 2005. [[accessed 12 July 2007]]. Available: http://www.cdc.gov/nccdphp/dnpa/bmi/bmi-adult.htm.
- Dupont I, Bodenez P, Berthou F, Simon B, Bardou LG, Lucas D. Cytotochrome P-450 activity and oxidative stress in alcoholic patients. Alcohol Alcohol. 2000;35:98–103. doi: 10.1093/alcalc/35.1.98. [DOI] [PubMed] [Google Scholar]
- El-Masri HA, Bell DA, Portier CJ. Effects of glutathione transferase theta polymorphisms on the risk estimates of dichloromethane to humans. Toxicol Applied Pharmacol. 1999;158:221–230. doi: 10.1006/taap.1999.8715. [DOI] [PubMed] [Google Scholar]
- Fantuzze G, Righi E, Predieri G, Ceppelli G, Fabriziomaria G, Aggazzotti G. Occupational exposure to trihalomethanes in indoor swimming pools. Sci Total Environ. 2001;264:257–265. doi: 10.1016/s0048-9697(00)00722-1. [DOI] [PubMed] [Google Scholar]
- Freedman DM, Cantor KP, Lee NL, Chen L-S, Hei H-H, Ruhl CE, et al. Bladder cancer and drinking water: a population-based case-control study in Washington County, Maryland (United States) Cancer Causes Control. 1997;8:738–744. doi: 10.1023/a:1018431421567. [DOI] [PubMed] [Google Scholar]
- Frye RF, Adedoyin A, Mauro K, Matxke GR, Branch RA. Use of chlorzoxazone as an in vivo probe of cytochrome P450 2E1: choice of dose and phenotypic trait measure. J Clin Pharmacol. 1998;38:82–89. doi: 10.1002/j.1552-4604.1998.tb04381.x. [DOI] [PubMed] [Google Scholar]
- Gao Y, Zhang Q. Polymorphisms of the GSTM1 and CYP2D2 genes associated with susceptibility to lung cancer in Chinese. Mutat Res. 1999;444:441–449. doi: 10.1016/s1383-5718(99)00092-3. [DOI] [PubMed] [Google Scholar]
- Garte S, Gaspari L, Alexandrie A-K, Ambrosone C, Autrup H, Autrup JL, et al. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomark Prev. 2001;10:1239–1248. [PubMed] [Google Scholar]
- Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005;162:267–278. doi: 10.1093/aje/kwi187. [DOI] [PubMed] [Google Scholar]
- Gordon SM, Brinkman MC, Ashley DL, Blount BC, Lyu C, Masters J, et al. Changes in breath trihalomethane levels resulting from household water-use activities. Environ Health Perspect. 2006;114:514–521. doi: 10.1289/ehp.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Cui Y, et al. Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clin Pharmacol Ther. 2002;72:276–287. doi: 10.1067/mcp.2002.126913. [DOI] [PubMed] [Google Scholar]
- Hildesheim ME, Cantor KP, Lynch CF, Dosemeci M, Lubin J, Alavanja M, et al. Drinking water source and chlorination byproducts. II. Risk of colon and rectal cancers. Epidemiology. 1998;9:29–35. [PubMed] [Google Scholar]
- Infante-Rivard C. Drinking-water contaminants, gene polymorphisms, and fetal growth. Environ Health Perspect. 2004;112:1213–1216. doi: 10.1289/ehp.7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kall MA, Vang O, Clause J. Effects of dietary broccoli on human in vivo drug metabolizing enzymes: evaluation of caffeine, oestrone, and chlorzoxazone metabolism. Carcinogenesis. 1996;17:793–799. doi: 10.1093/carcin/17.4.793. [DOI] [PubMed] [Google Scholar]
- Kerridge I, Lincz L, Scorgie F, Hickey D, Granter N, Spencer A. Association between xenobiotic gene polymorphisms and non-Hodgkin’s lymphoma risk. Br J Haematol. 2002;118:477–481. doi: 10.1046/j.1365-2141.2002.03606.x. [DOI] [PubMed] [Google Scholar]
- King WD, Dodds L, Allen AC. Relation between stillbirth and specific chlorination byproducts in public water supplies. Environ Health Perspect. 2000;108:883–886. doi: 10.1289/ehp.00108883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King WD, Marrett LD. Case-control study of bladder cancer and chlorination byproducts in treated water (Ontario, Canada) Cancer Causes Control. 1996;7:596–604. doi: 10.1007/BF00051702. [DOI] [PubMed] [Google Scholar]
- Lan Q, Chow WH, Lissowska J, Hein DW, Buetow K, Engel LS, et al. Glutathione S-transferase genotypes and stomach cancer in a population-based case-control study in Warsaw, Poland. Pharmacogenetics. 2001;11:655–661. doi: 10.1097/00008571-200111000-00003. [DOI] [PubMed] [Google Scholar]
- Landi S, Hanley NM, Warren SH, Pegram RA, DeMarini DM. Induction of genetic damage in human lymphocytes and mutations in Salmonella by trihalomethanes: role of red blood cells and GSTT1-1 polymorphism. Mutagenesis. 1999;14:479–482. doi: 10.1093/mutage/14.5.479. [DOI] [PubMed] [Google Scholar]
- Leclercq I, Desager J-P, Horsmans Y. Inhibition of chlorzoxazone metabolism, a clinical probe for CYP2E1, by a single ingestion of watercress. Clin Pharmacol Ther. 1998;64:144–149. doi: 10.1016/S0009-9236(98)90147-3. [DOI] [PubMed] [Google Scholar]
- Le Marchand LL, Wilkinson GR, Wilkens LR. Genetic and dietary predictors of CYP2E1 activity: a phenotyping study in Hawaii Japanese using chlorzoxazone. Cancer Epidemiol Biomark Prev. 1999;8:495–500. [PubMed] [Google Scholar]
- Lucas D, Farez C, Bardou LG, Vaisse J, Attali JR, Valensi P. Cytochrome P450 2E1 activity in diabetic and obese patients as assessed by chlorzoxazone hydroxylation. Fundam Clin Pharmacol. 1998;12:553–558. doi: 10.1111/j.1472-8206.1998.tb00985.x. [DOI] [PubMed] [Google Scholar]
- McGeehin M, Reif J, Becher J, Mangione E. A case-control study of bladder cancer and water disinfection methods in Colorado. Am J Epidemiol. 1993;138:492–501. doi: 10.1093/oxfordjournals.aje.a116883. [DOI] [PubMed] [Google Scholar]
- Miles AM, Singer P, Ashley D, Lynberg MC, Mendola P, Langlois P, et al. Comparison of trihalomethanes in tap water and blood. Environ Sci Technol. 2002;36:1692–1698. doi: 10.1021/es001991j. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of Tribromomethane (Bromoform) (CAS No. 75–25–2) in F344/N Rats and B6C3F1 Mice (Gavage Studies) Research Triangle Park, NC: National Toxicology Program; 1989. [PubMed] [Google Scholar]
- Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet. 2002;360:1155–1162. doi: 10.1016/S0140-6736(02)11203-7. [DOI] [PubMed] [Google Scholar]
- Nuckols JR, Ashley DL, Lyu C, Gordon SM, Hinckley AF, Singer P. Influence of tap water quality and household water use activities on indoor air and internal dose levels of trihalomethanes. Environ Health Perspect. 2005;113:863–870. doi: 10.1289/ehp.7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemble S, Schroeder KR, Spencer SR, Meyer DJ, Hallier E, Bolt HM, et al. Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem J. 1994;300:271–276. doi: 10.1042/bj3000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi S, Paracchini V, Autrup H, Barros-Dios JM, Benhamou S, Boffetta P, et al. Meta- and pooled analysis of GSTT1 and lung cancer: a HuGE-GSEC review. Am J Epidemiol. 2006;164:1027–1042. doi: 10.1093/aje/kwj321. [DOI] [PubMed] [Google Scholar]
- Savitz DA, Andrews KW, Pastore LM. Drinking water and pregnancy outcome in central North Carolina: source, amount, and trihalomethane levels. Environ Health Perspect. 1995;103:592–596. doi: 10.1289/ehp.95103592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz DA, Singer PC, Herring AH, Hartmann KE, Weinberg HS, Makarushka C. Exposure to drinking water disinfection byproducts and pregnancy loss. Am J Epidemiol. 2006;164:1043–1051. doi: 10.1093/aje/kwj300. [DOI] [PubMed] [Google Scholar]
- Scott RJ, Palmer J, Lewis IA, Pleasance S. Determination of a ‘CW cocktail’ of cytochrome P450 probe substrates and their metabolites in plasma and urine using automated solid phase extraction and fast gradient liquid chromatography tandem mass spectrometry. Rapid Commun Mass Spectrom. 1999;13:2305–2309. doi: 10.1002/(SICI)1097-0231(19991215)13:23<2305::AID-RCM790>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Setiawan VW, Zhang ZF, Yu GP, Li YL, Lu ML, Tsai CJ, et al. GSTT1 and GSTM1 null genotypes and the risk of gastric cancer: a case-control study in a Chinese population. Cancer Epidemiol Biomarkers Prev. 2000;9:73–80. [PubMed] [Google Scholar]
- Stupans I, Murray M, Kirlich A, Tuch KL, Hayball PJ. Inactivation of cytochrome P450 by the food-derived complex phenol oleuropein. Food Chem Toxicol. 2001;39:1119–1124. doi: 10.1016/s0278-6915(01)00060-6. [DOI] [PubMed] [Google Scholar]
- Villanueva CM, Cantor KP, Grimalt JO, Castano-Vinyals G, Malats N, Silverman D, et al. Assessment of lifetime exposure to trihalomethanes through different routes. Occup Environ Med. 2006a;63:273–277. doi: 10.1136/oem.2005.023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva CM, Cantor KP, Grimalt JO, Malats N, Silverman D, Tardon A, et al. Bladder cancer and exposure to water disinfection byproducts through ingestion, bathing, showering and swimming pool attendance. Am J Epidemiol. 2006b;165:148–156. doi: 10.1093/aje/kwj364. [DOI] [PubMed] [Google Scholar]
- Waller K, Swan SH, DeLorenze G, Hopkins B. Trihalomethanes in drinking water and spontaneous abortion. Epidemiology. 1998;9:134–140. [PubMed] [Google Scholar]
- Weisel CP, Chen WJ. Exposure to chlorination byproducts from hot water uses. Risk Anal. 1994;14:101–106. doi: 10.1111/j.1539-6924.1994.tb00032.x. [DOI] [PubMed] [Google Scholar]
- Winberry WT, Jr, Murphy NT, Riggin RM. Methods for Determination of Toxic Organic Compounds in Air: EPA Methods. Park Ridge, NJ: Noyes Data Corporation; 1990. [Google Scholar]
- Xu X, Weisel CP. Dermal uptake of chloroform and haloketones during bathing. J Expo Anal Environ Epidemiol. 2005a;15:289–296. doi: 10.1038/sj.jea.7500404. [DOI] [PubMed] [Google Scholar]
- Xu X, Weisel CP. Human respiratory uptake of chloroform and haloketones during showering. J Expo Anal Environ Epidemiol. 2005b;15:6–16. doi: 10.1038/sj.jea.7500374. [DOI] [PubMed] [Google Scholar]
- Zierler S, Feingold L, Danley RA, Craun G. Bladder cancer in Massachusetts related to chlorinated and chloraminated drinking water: a case-control study. Arch Environ Health. 1988;43:195–200. doi: 10.1080/00039896.1988.9935853. [DOI] [PubMed] [Google Scholar]