Abstract
Protein phosphatase 1 (PP1, Glc7p) functions in the final stage of SNARE-mediated vesicle transport between docking and fusion. During this process, trans-SNARE complexes, formed between molecules in opposing membranes, convert to cis-complexes, with all participants in the same lipid bilayer. Here, we show that glc7 mutant cells accumulate SNARE complexes. These complexes are clearly different from those found in either wild-type or sec18–1 cells as the Sec1p/Munc18 (SM) protein Vps45p does not bind to them. Given that PP1 controls fusion, the SNARE complexes that accumulate in glc7 mutants likely represent trans-SNARE complexes. Vps45p dissociates from the membrane in the absence of PP1 activity, but rapidly reassociates after its reactivation. These data reveal that SM proteins cycle on and off membranes in a stage-specific manner during the vesicle transport reaction, and suggest that protein phosphorylation plays a key role in the regulation of this cycle.
Keywords: SNAREs; fusion; protein phosphatase 1; docking; membranes
Introduction
Intracellular membrane trafficking is a highly regulated process (Sudhof, 1995). SNAREs play a critical role in this process by regulating membrane docking and fusion (Sudhof, 1995; Weber et al., 1998). Transport vesicles contain membrane proteins (v-SNAREs), which specifically bind to cognate membrane proteins (t-SNAREs) in the appropriate target membrane. Large families of v- and t-SNARE proteins have been identified in eukaryotic cells with different members localizing to different compartments (Sudhof, 1995). The correct pairing of v- and t-SNAREs in opposing membranes is defined by the formation of a stable high affinity complex referred to as the trans- or SNARE core complex (Nichols et al., 1997; Sutton et al., 1998; Weber et al., 1998). v- and t-SNAREs contain α-helical domains that contribute to a four-helix bundle giving rise to the SNARE complex (Sutton et al., 1998).
SNARE complex assembly occurs in several discrete stages. Initially, in a priming step, cis-SNARE complexes in both donor and target membranes are disassembled through the ATPase action of NSF in conjunction with its attachment protein α-SNAP (Mayer et al., 1996). Once primed, tethering machinery may guide the SNAREs in each membrane into close proximity and a proofreading machinery likely ensures fidelity of the aligned v- and t-SNARE pairs (Zerial and McBride, 2001). Formation of the high affinity trans-SNARE complex represents the committed step of docking (Ungermann et al., 1998). This step is accompanied by conversion of trans- to cis-complexes (Lin and Scheller, 1997; Sutton et al., 1998). Although SNARE proteins are sufficient to catalyze docking and fusion of artificial membrane liposomes in vitro (Weber et al., 1998; McNew et al., 2000), other factors are essential in vivo, suggesting multiple levels of regulation.
The Sec1p/Munc18 (SM)* family plays an essential role in regulating membrane transport (Jahn, 2000). Disruption or deletion of any of the four SM proteins in Saccharomyces cerevisiae causes a block in vesicle transport (Jahn, 2000). SM proteins are peripheral membrane proteins. We have demonstrated previously that the SM protein Vps45p is dependent on its cognate t-SNARE Tlg2p for its membrane association (Bryant and James, 2001). Moreover, it has been shown that many SM proteins bind their cognate t-SNARE with high affinity in vitro (Pevsner et al., 1994b; Yamaguchi et al., 2002), suggesting that the stable association of SM proteins with membranes is mediated via this interaction. But how this interaction changes as the t-SNARE engages in core complex assembly during docking is not known. At the neuronal synapse, it is thought that the SM protein Munc18a is inhibitory to core complex assembly and that its dissociation from the t-SNARE is required to promote this process (Pevsner et al., 1994a). Neither the fate of the liberated SM protein, or the mechanism that underlies its dissociation is known. One possibility is that SM proteins dissociate from membranes before core assembly. Intriguingly, many SM proteins, including Vps45p are present as both cytosolic and membrane-bound forms (Cowles et al., 1994; Bryant and James, 2001). The cytosolic pool may represent newly synthesized protein or Vps45p that has been discharged from the membrane during vesicle transport. In either case, it is not clear at what stage SM proteins achieve their membrane association. Intriguingly, Sec1p is found stably associated with the cis-SNARE complex (Carr et al., 1999), suggesting that the terminal stage of the transport reaction may be the point at which SM proteins reassociate with the membrane in order to reactivate the t-SNARE for another round of vesicle transport.
Here, we have found that the SM protein Vps45p dissociates from membranes before fusion and reassociates after fusion probably by binding to a cis-SNARE complex. The cyclical membrane association of Vps45p is temporally linked to the docking–fusion stage of vesicle transport at a step controlled by the protein phosphatase Glc7p. We propose that the dissociation of SM proteins from the membrane may be controlled by phosphorylation and that this family of proteins may act as molecular switches to control SNARE complex assembly.
Results and discussion
Vps45p binds to monomeric Tlg2p and to the cis-SNARE–core complex
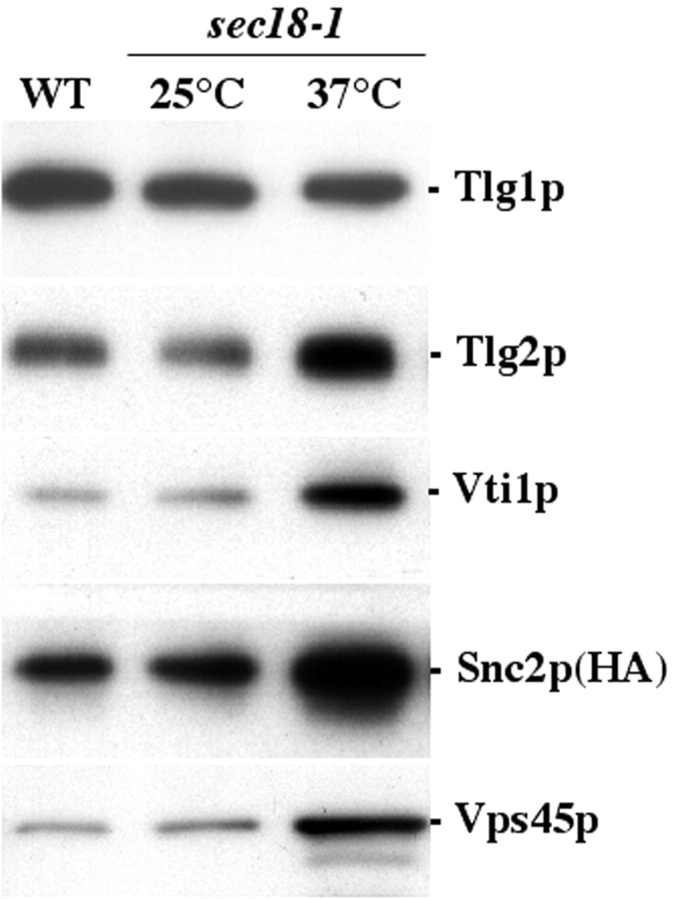
We have demonstrated previously that Vps45p binds to Tlg2p both in vitro and in vivo (Bryant and James, 2001). Other SM proteins, including Sly1p and Sec1p, bind to their respective SNARE complexes (Kosodo et al., 1998; Carr et al., 1999; Peng and Gallwitz, 2002). Importantly, Sec1p binds to the cis-core complex, indicating a role for SM proteins after vesicle fusion (Grote et al., 2000). To determine whether Vps45p binds to the Tlg2p-containing core complex, we took advantage of cells harboring the temperature-sensitive sec18–1 mutation. Sec18p is an ATPase that catalyses the disassembly of SNARE complexes so that they can be recycled for further rounds of vesicle transport (Mayer et al., 1996). At the restrictive temperature (37°C), the mutant Sec18–1p is deficient in this ATPase activity and sec18–1 cells, thus, accumulate cis-SNARE complexes (Grote et al., 2000). Consistent with this, we observed a significant increase in the accumulation of cis-Tlg2p–Tlg1p–Vti1p–Snc2p SNARE complexes in sec18–1 cells placed at the nonpermissive temperature (37°C). Fig. 1 demonstrates that there is a five- to sevenfold increase in the amount of Tlg2p, Vti1p, and Snc2p that coprecipitate with Tlg1p under these conditions. Commensurate with the increase in SNARE complexes, we observed a fivefold increase in the amount of Vps45p that coprecipitated with Tlg1p. As we have previously demonstrated that Vps45p does not bind directly to Tlg1p (Bryant and James, 2001), these data indicate that Vps45p, like Sec1p (Carr et al., 1999) and Sly1p (Kosodo et al., 2002; Peng and Gallwitz, 2002), binds to its cognate SNARE complex. Given that, we observed the association of Vps45p with SNARE complexes in sec18–1 cells, this likely corresponds to an association with cis-complexes, and is in agreement with previous studies (Carr et al., 1999; Grote et al., 2000).
Figure 1.
Vps45p binds to cis-Tlg1p–Tlg2p–Vti1p–Snc2p SNARE complexes that accumulate upon inactivation of SEC18 . Tlg1p-containing complexes were immunoprecipitated from the wild-type (WT) cells (NOzY21) or sec18–1 cells (NOzY22) grown at 25°C (25°C), or grown at 25°C, and then incubated at 37°C for 10 min before cell lysate preparation (37°C). Immunoblot analysis was used to detect the amount of Tlg1p, Tlg2p, Vti1p, HA-tagged Snc2p, and Vps45p in the immunoprecipitated complexes.
We, and others, have shown that Vps45p binds to monomeric Tlg2p in vitro (Bryant and James, 2001; Dulubova et al., 2002; Yamaguchi et al., 2002). The observation that Sec1p binds to Ssop-containing SNARE complexes, but not to the monomeric t-SNARE (Carr et al., 1999), raises the possibility that the association observed between Tlg2p and Vps45p in vivo does not reflect the SM protein binding to the monomeric t-SNARE, but rather to the cis-SNARE complex. This is the case for the interaction between Sec1p and Ssop (Carr et al., 1999), as demonstrated by the lack of interaction between these two proteins in sec4–8 cells (Carr et al., 1999). SEC4 encodes the Rab protein involved in the fusion of secretory vesicles with the plasma membrane, and sec4–8 cells are defective in assembly of Ssop SNARE complexes (Grote and Novick, 1999). The Rab protein involved in VPS45-dependent membrane transport is encoded by VPS21 (Gerrard et al., 2000). Fig. 2 shows that Tlg1p was not found in complex with its SNARE binding partners in vps21Δ mutant cells. Under these conditions, Tlg1p no longer coprecipitated Vps45p (Fig. 2 A). Importantly, Vps45p was still found in complex with Tlg2p in vps21Δ cells (Fig. 2 B). These data demonstrate that Vps45p binds to the monomeric t-SNARE Tlg2p, and to the cis-Tlg2p–Tlg1p–Vti1p–Snc2p SNARE complex in vivo.
Figure 2.

Vps45p binds to Tlg2p in isolation from its SNARE binding partners. (A) Tlg1p- and (B) Tlg2p-containing complexes were immunoprecipitated from the wild-type (WT) (NOzY21) or vps21Δ (NOzY24) cells. Immunoblot analysis was used to detect the amount of Tlg1p, Tlg2p, Vti1p, HA-tagged Snc2p, and Vps45p in the immunoprecipitated complexes as indicated.
Our finding that Vps45p binds to both monomeric Tlg2p and to the cis-core complex may indicate that the interaction between this SM protein and the t-SNARE is maintained throughout the SNARE cycle. To test this model, and further characterize the interaction between Vps45p and its cognate SNARE complex, we set out to assess the ability of Vps45p to bind to a trans-SNARE complex. To generate trans-SNARE complexes in vivo we endeavored to find a mutant that blocks membrane transport between vesicle docking and bilayer fusion. One protein that has been implicated at this stage is protein phosphatase 1 (PP1). PP1 (Glc7p) has been shown to function at a late stage of vacuole fusion, likely during the bilayer mixing phase (Peters et al., 1999). A cell-free assay that reconstitutes homotypic vacuolar fusion has been used to define discrete stages in this reaction (Wickner, 2002). Inhibition of either Rab or SNARE function blocks at an early stage in this assay (Wickner, 2002). In contrast, inhibition of PP1 using either microcystin or temperature-sensitive alleles of GLC7 block at a very late stage after docking (Peters et al., 1999). In addition to vacuolar fusion, it has also been shown that PP1 is involved in multiple intracellular fusion steps, including ER to Golgi complex transport and endocytic traffic (Peters et al., 1999). This suggests that PP1 is a general component of the membrane fusion machinery that, like Sec18p, operates in all vesicle transport reactions (Peters et al., 1999). One prediction from the model that PP1 (Glc7p) is involved at a late, postdocking stage of membrane fusion is that inhibition of PP1 action results in an accumulation of trans-SNARE complexes (Peters et al., 1999).
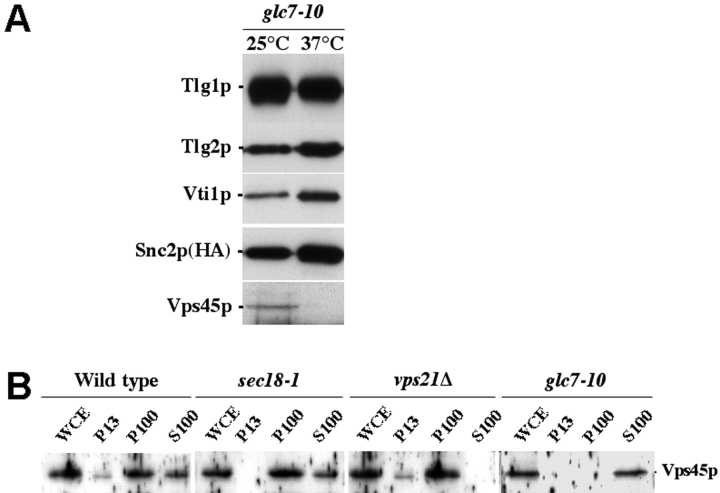
Fig. 3 demonstrates that there is a significant three- to fivefold increase in the amount of Tlg2p, Vti1p, and Snc2p that coprecipitate with Tlg1p when cells harboring a temperature-sensitive allele of GLC7 are incubated at the nonpermissive temperature (Fig. 3 A). Intriguingly, no Vps45p was found associated with the SNARE complexes that accumulate in these cells (Fig. 3 A, 37°C). This is in contrast to the cis-SNARE complexes that accumulate in sec18–1 cells (Fig. 1). The observation that Vps45p binds to SNARE complexes present in wild-type cells, and also to those detected in sec18–1 cells at the permissive temperature, indicates that these are cis-SNARE complexes, whereas those that accumulate in glc7–10 cells at 37°C are in the trans-configuration.
Figure 3.
Vps45p loses membrane association upon inactivation of PP1. (A) Tlg1p-containing complexes were immunoprecipitated from cells harboring the glc7–10 mutation (NOzY23) grown at 25°C (25°C), or grown at 25°C and then incubated at 37°C for 10 min before cell lysate preparation (37°C). Immunoblot analysis was used to detect the amount of Tlg1p, Tlg2p, Vti1p, HA-tagged Snc2p, and Vps45p in the immunoprecipitated complexes. (B) wild-type (SF838–9D), sec18–1 (NOzY22), vps21Δ (SGY79), and glc7–10 (PAY704–1) cells were incubated at 37°C for 10 min before fractionation by differential centrifugation to yield a whole cell extract (WCE), a low-speed membrane pellet (P13), a high-speed membrane pellet (P100), and a soluble, cytosolic fraction (S100). The amount of Vps45p in each fraction was assessed using immunoblot analysis.
The above data raises important questions concerning SM protein function. If Vps45p dissociates from the trans-SNARE complex upon vesicle docking, does it remain membrane associated during this stage? To address this, we assessed the membrane association of Vps45p in wild-type, vps21Δ, sec18–1, and glc7–10 cells (Fig. 3 B). Consistent with previous studies, the majority of Vps45p was associated with membranes in wild-type cells (Cowles et al., 1994; Bryant and James, 2001). Vps45p retained its membrane localization in cells lacking VPS21, which are defective in SNARE complex assembly, presumably through its interaction with monomeric Tlg2p (Bryant and James, 2001; Fig. 2). Similarly, the SM protein was associated with membranes in sec18–1 cells at 37°C (Fig. 3 B). However, strikingly, Vps45p was not found associated with membranes in glc7–10 cells at 37°C, but rather localized to the cytosol (Fig. 3 B). These data are consistent with our observation that Vps45p binds to the SNARE complexes that accumulate in sec18–1 cells (Fig. 1), but not to those that accumulate in glc7–10 cells at 37°C (Fig. 3 A).
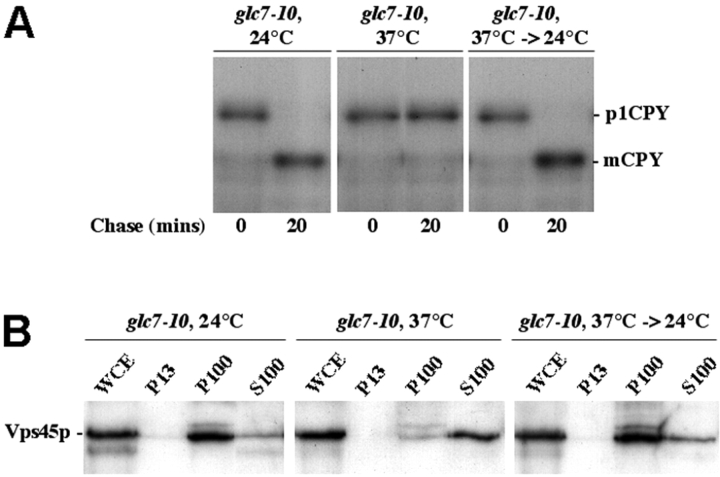
To assess the physiological relevance of the loss of membrane association upon inactivation of PP1, we sought to ascertain whether the dissociation observed upon inactivation of PP1 was reversible upon reactivation of the phosphatase. Fig. 4 A demonstrates that the block in membrane trafficking caused by incubation of glc7–10 cells is reversible. It has been shown previously that the transit of the vacuolar hydrolase carboxypeptidase Y (CPY) through the secretory pathway is blocked in PP1 temperature-sensitive cells at 37°C (Peters et al., 1999). After its synthesis, CPY is translocated into the lumen of the ER, where it is core glycosylated into the p1 form. CPY receives further modifications as it transits through the Golgi apparatus. In the trans-Golgi network, the p2 form of CPY is recognized by its receptor Vps10p, which delivers the protein to the vacuole where it is cleaved into the mature form of the protease (Bryant and Stevens, 1998). Fig. 4 A demonstrates that incubation of glc7–10 cells at 37°C blocks the processing of CPY. Concomitant with this block in vesicle fusion is the dissociation of Vps45p from membranes, as demonstrated by the observation that the SM protein shifts from the P100 (membrane) to the S100 (cytosol) fraction obtained from these cells after 10 min at 37°C (Fig. 4 B). The block in processing of CPY was reversed by returning the cells to 24°C (Fig. 4 A). Importantly, with this recovery in vesicular transport, Vps45p reassociated with membranes (Fig. 4 B). These data indicate that the redistribution of Vps45p from membranes to the cytosol, observed in glc7–10 cells (Figs. 3 and 4) represents a physiological intermediate.
Figure 4.
Vps45p cycles on and off membranes during membrane transport. Cells harboring the glc7–10 mutation (PAY704–1) were grown overnight at 24°C. Cells were then either left at 24°C, shifted to 37°C for 10 min or shifted to 37°C for 10 min and moved back to 24°C for 1 h. (A) The fate of newly synthesized CPY in these cells was assessed by immunoprecipitation of the protein after metabolic labeling of cells. CPY was processed at the permissive (24°C), but not at the nonpermissive (37°C) temperature. Shown are cells incubated at 37°C for 10 min, but identical results were observed at longer incubation times (up to 60 min). CPY processing was restored when cells previously incubated at 37°C for 10 min were returned to the 25°C. (B) The membrane association of Vps45p in these cells was assessed by subcellular fractionation by differential centrifugation followed by immunoblot analysis.
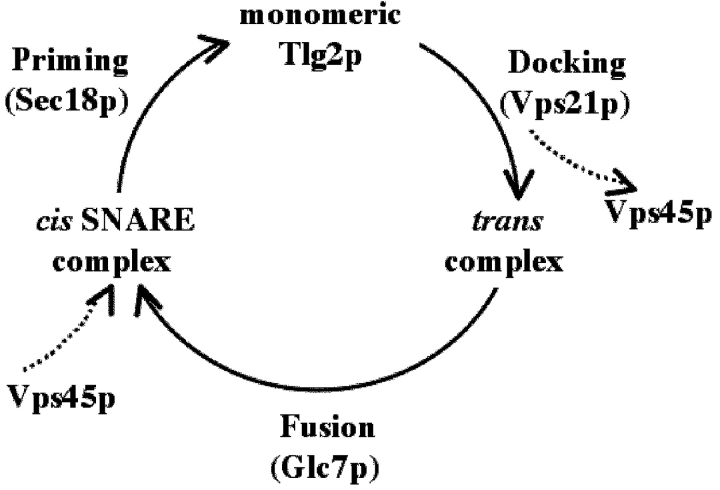
The SNARE assembly cycle depicted in Fig. 5 illustrates the dynamic equilibrium between the t-SNARE Tlg2p in its monomeric state, and as part of trans- and cis-complexes. Each one of these states defines an intermediate in the vesicle docking–fusion process. We have demonstrated that Vps45p binds to Tlg2p in this state before the formation of the trans-SNARE complex in a Rab (Vps21p)-dependent process (Lupashin and Waters, 1997; Grote and Novick, 1999). We show that Vps45p dissociates at this stage of the cycle and is released into the cytosol. The conversion of trans-SNARE complexes to the cis-configuration defines membrane fusion (Ungermann et al., 1998), and also represents the starting point for another round of vesicle transport. It is here that Vps45p reenters the cycle by binding to the cis-complex. The cycle is completed after disassembly of cis-SNARE complexes with Vps45p bound by Sec18p presumably resulting in the Vps45p–Tlg2p complex that is then ready to receive incoming vesicles in the process of docking.
Figure 5.
Model for the interaction of Vps45p with Tlg2p during SNARE complex assembly–disassembly. Model depicting the association and dissociation of Vps45p with membranes at different stages of the SNARE assembly–disassembly cycle. The cycle begins with the t-SNARE/SM heterodimer (Tlg2p/Vps45p), which precedes the docking reaction involving the Rab protein Vps21p. Docking results in the formation of a trans-SNARE complex involving the v-SNARE on the transport vesicle and the t-SNAREs in the target membrane. The SNARE complex is found in a cis-configuration following merging of the two lipid bilayers during the fusion reaction (involving the protein phosphatase, Glc7p), and is subsequently disassembled by the ATPase, Sec18p. Vps45p dissociates from membranes either before or during formation of the trans-complex in concert with the action of the Rab protein and/or the phosphatase and then rebinds to the cis-SNARE complex.
The formation of unifying models concerning SM protein function has been made difficult by conflicting observations concerning their binding properties: Munc18a binds to monomeric Syntaxin1a, but not to SNARE complexes (Yang et al., 2000). Sec1p binds to Ssop–SNARE complexes, but not to the monomeric t-SNARE (Carr et al., 1999); although others, such as Sly1p, Munc18c, and now Vps45p, bind both to the monomeric t-SNARE and to the SNARE complex (Kosodo et al., 2002; Peng and Gallwitz, 2002; unpublished data). Some of these differences may reflect the different conformations undertaken by these different proteins in vitro, but they may also represent kinetic differences at various stages of the cycle outlined in Fig. 5 for different SNARE pairs. For example, in neurones there is an accumulation of predocked vesicles (Sudhof, 1995), and therefore, presumably an accumulation of trans-SNARE complexes, whereas it is likely that the Sso-containing SNARE complexes observed in wild-type yeast cells are in the cis-configuration (Grote et al., 2000).
In a manner similar to the finding that Sec1p binds to SNARE complexes (Carr et al., 1999), we observed a robust interaction between Vps45p and Tlg2p in sec18–1 cells (Fig. 1). It is noteworthy that in a previous study, Lupashin and Waters (1997) reported that there was no significant interaction between Sly1p- and Sed5p-containing SNARE complexes that accumulate in sec18–1 cells, despite the fact that Sly1p has subsequently been shown to interact with Sed5p-containing SNARE complexes formed in vitro (Kosodo et al., 2002; Peng and Gallwitz, 2002). However, in that study (Lupashin and Waters, 1997), sec18–1 mutants were incubated for times significantly longer than those used here, and we have found that the period of incubation of these cells at the nonpermissive temperature is critical in these experiments (unpublished data).
Our model raises a number of important questions that provide a framework for further investigation directed at understanding the role of SM proteins. Why does Vps45p dissociate from the membrane during the docking–fusion stage? Do SM proteins play a role, similar to Rab proteins, and cycle on and off membranes in synchrony with the arrival of vesicles at the docking site? In addition, our paper raises questions concerning the role of PP1 in docking–fusion events. What is the role of phosphorylation that appears to be so intimately linked to the docking–fusion transition of vesicle transport; and is the dissociation of the SM protein during this phase the cause, or an effect, of this? Intriguingly, a role for the phosphorylation of Tlg2p has been implicated in modulating endocytosis (Gurunathan et al., 2002), and phosphorylation of Munc18a may be important in regulating its interaction with Syntaxin1a (Fujita et al., 1996). Finally, with our finding that Vps45p binds selectively to certain SNARE intermediates, could it be that there is a consensus in SM function that has been thus far difficult to realize? If phosphorylation plays an important role in the SM–SNARE interaction, replicating the conditions necessary to establish these interactions may not be trivial. Notably, Carr et al. (1999) stressed the importance of eliminating ATP from their cells before isolation of intact SNARE complexes. Answers to the above, and other, questions will further our understanding of this important family of proteins.
Materials and methods
Antibodies
Rabbit polyclonal antibodies against Tlg2p and Vps45p have been described previously (Bryant and James, 2001). Antibodies that specifically recognize Tlg1p and Vti1p were a gift from J. Coe (IMCB, Singapore) and W. Hong (IMCB, Singapore). The monoclonal antibody 16B12, which recognizes the influenza hemagglutinin (HA) epitope, was purchased from BabCo.
Plasmids
All DNA manipulations were performed using routine procedures. Plasmid pEG111, provided by E. Grote (Yale University, New Haven, CT) is an integrating vector encoding an HA-epitope–tagged version of Snc2p (Abeliovich et al., 1998). pNOz10 was constructed by subcloning a BamHI-HindII fragment encompassing the tagged Snc2p coding sequence from pEG111 into pRS306 (Sikorski and Hieter, 1989).
Strains
All yeast strains used in these studies are described in Table I. Yeast strains were constructed using standard genetic techniques and grown in rich media (YEPD; 1% yeast extract, 1% peptone, 2% dextrose) or standard minimal medium using either glucose, or raffinose and galactose as carbon sources. NOzY21, 22, 23, and 24 all harbor an allele of SNC2 encoding an HA-tagged version of the v-SNARE (Abeliovich et al., 1998). NOzY21, NOzY23, and NOzY24 were derived from SF838–9D (Rothman and Stevens, 1986), PAY704–1, provided by M. Stark (University of Dundee, Dundee, UK; Peters et al., 1999), and SGY79 (Gerrard et al., 2000), respectively, using pEGIII (Abeliovich et al., 1998). NOzY22 was constructed from SEY5186α, provided by S. Emr (University of California, San Diego, La Jolla, CA), using pNOz10.
Table I. Yeast strains used in this study.
| Strain | Genotype | Source |
|---|---|---|
| SF838-9D | MATα ura3-52 leu2-3,112 his4-519 ade6 gal2 pep4-3 | Rothman and Stevens, 1986 |
| SGY79 | MATα ura3-52 leu2-3,112 his4-519 ade6 gal2 pep4-3 vps21Δ::Kan | Gerrard et al., 2000 |
| NOzY24 | MATα ura3-52 leu2-3,112 his4-519 ade6 gal2 pep4-3 vps21Δ::Kan SNC2::[LEU2, GAL1-SNC2-HA] | This study |
| NOzY21 | MATα ura3-52 leu2-3,112 his4-519 ade6 gal2 pep4-3 SNC2::[LEU2, GAL1-SNC2-HA] | This study |
| NOzY22 | MATα ura3-52 leu2-3,112 sec18-1 gal2 SNC2::[LEU2, GAL1-SNC2-HA] | This study |
| PAY704-1 | MATa ade2-1 his3-11,15 leu2-3,112 ura3-1 glc7::LEU2 trp1-1::glc7-10::TRP1 can1-100 ssd1-d2 | Peters et al., 1999 |
| NOzY23 | MATa ade2-1 his3-11,15 leu2-3,112 ura3-1 glc7::LEU2 trp1-1::glc7-10::TRP1 can1-100 ssd1-d2 SNC2::[URA3, GAL1-SNC2-HA] | This study |
Subcellular fractionation
Cells were fractionated using differential centrifugation after osmotic lysis as described previously (Bryant and James, 2001). Before any described temperature shifts, cells were grown in 10 ml YEPD to mid-log phase. The amount of Vps45p in each fraction was assessed using immunoblot analysis. Band intensities were quantified by densitometry using a scanning densitometer (Bio-Rad Laboratories) and NIH Image software.
SNARE complex immunoprecipitations
Tlg1p- and Tlg2p-containing complexes were immunoprecipitated from solubilized membranes as described previously (Abeliovich et al., 1998; Bryant and James, 2001). Precipitated proteins were detected using immunoblot analysis. Band intensities were quantified by densitometry using a scanning densitometer (Bio-Rad Laboratories) and NIH Image software.
Metabolic labeling and pulse-chase immunoprecipitation of CPY
The fate of newly synthesized CPY in glc7–10 cells was followed by immunoprecipitation of the protein as described previously (Bryant and James, 2001).
Acknowledgments
We would like to thank Mike Stark for advice on use of the glc7 strain, and Drs. Emr, Grote, Coe, and Hong for providing reagents.
This research was supported by the National Health and Medical Research Council of Australia.
N.J. Bryant's present address is Division of Biochemistry and Molecular Biology, Institute of Biomedical and Life Sciences, Davidson Building, University of Glasgow, Glasgow G12 8QQ, UK. E-mail: n.bryant@bio.gla.ac.uk
Footnotes
Abbreviations used in this paper: CPY, carboxypeptidase Y; HA, hemagglutinin; PP1, protein phosphatase 1; SM, Sec1p/Munc18.
References
- Abeliovich, H., E. Grote, P. Novick, and S. Ferro-Novick. 1998. Tlg2p, a yeast syntaxin homolog that resides on the Golgi and endocytic structures. J. Biol. Chem. 273:11719–11727. [DOI] [PubMed] [Google Scholar]
- Bryant, N.J., and D.E. James. 2001. Vps45p stabilizes the syntaxin homologue Tlg2p and positively regulates SNARE complex formation. EMBO J. 20:3380–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant, N.J., and T.H. Stevens. 1998. Vacuole biogenesis in Saccharomyces cerevisiae: protein transport pathways to the yeast vacuole. Microbiol. Mol. Biol. Rev. 62:230–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr, C.M., E. Grote, M. Munson, F.M. Hughson, and P.J. Novick. 1999. Sec1p binds to SNARE complexes and concentrates at sites of secretion. J. Cell Biol. 146:333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles, C.R., S.D. Emr, and B.F. Horazdovsky. 1994. Mutations in the VPS45 gene, a SEC1 homologue, result in vacuolar protein sorting defects and accumulation of membrane vesicles. J. Cell Sci. 107:3449–3459. [DOI] [PubMed] [Google Scholar]
- Dulubova, I., T. Yamaguchi, Y. Gao, S.W. Min, I. Huryeva, T.C. Sudhof, and J. Rizo. 2002. How Tlg2p/syntaxin 16 ‘snares’ Vps45. EMBO J. 21:3620–3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, Y., T. Sasaki, K. Fukui, H. Kotani, T. Kimura, Y. Hata, T.C. Sudhof, R.H. Scheller, and Y. Takai. 1996. Phosphorylation of Munc-18/n-Sec1/rbSec1 by protein kinase C: its implication in regulating the interaction of Munc-18/n-Sec1/rbSec1 with syntaxin. J. Biol. Chem. 271:7265–7268. [DOI] [PubMed] [Google Scholar]
- Gerrard, S.R., N.J. Bryant, and T.H. Stevens. 2000. VPS21 controls entry of endocytosed and biosynthetic proteins into the yeast prevacuolar compartment. Mol. Biol. Cell. 11:613–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote, E., and P.J. Novick. 1999. Promiscuity in Rab-SNARE interactions. Mol. Biol. Cell. 10:4149–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote, E., C.M. Carr, and P.J. Novick. 2000. Ordering the final events in yeast exocytosis. J. Cell Biol. 151:439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunathan, S., M. Marash, A. Weinberger, and J.E. Gerst. 2002. t-SNARE phosphorylation regulates endocytosis in yeast. Mol. Biol. Cell. 13:1594–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn, R. 2000. Sec1/Munc18 proteins: mediators of membrane fusion moving to center stage. Neuron. 27:201–204. [DOI] [PubMed] [Google Scholar]
- Kosodo, Y., Y. Noda, and K. Yoda. 1998. Protein-protein interactions of the yeast Golgi t-SNARE Sed5 protein distinct from its neural plasma membrane cognate syntaxin 1. Biochem. Biophys. Res. Commun. 250:212–216. [DOI] [PubMed] [Google Scholar]
- Kosodo, Y., Y. Noda, H. Adachi, and K. Yoda. 2002. Binding of Sly1 to Sed5 enhances formation of the yeast early Golgi SNARE complex. J. Cell Sci. 115:3683–3691. [DOI] [PubMed] [Google Scholar]
- Lin, R.C., and R.H. Scheller. 1997. Structural organization of the synaptic exocytosis core complex. Neuron. 19:1087–1094. [DOI] [PubMed] [Google Scholar]
- Lupashin, V.V., and M.G. Waters. 1997. t-SNARE activation through transient interaction with a rab-like guanosine triphosphatase. Science. 276:1255–1258. [DOI] [PubMed] [Google Scholar]
- Mayer, A., W. Wickner, and A. Haas. 1996. Sec18p (NSF)-driven release of Sec17p (alpha-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 85:83–94. [DOI] [PubMed] [Google Scholar]
- McNew, J.A., F. Parlati, R. Fukuda, R.J. Johnston, K. Paz, F. Paumet, T.H. Sollner, and J.E. Rothman. 2000. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature. 407:153–159. [DOI] [PubMed] [Google Scholar]
- Nichols, B.J., C. Ungermann, H.R. Pelham, W.T. Wickner, and A. Haas. 1997. Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature. 387:199–202. [DOI] [PubMed] [Google Scholar]
- Peng, R., and D. Gallwitz. 2002. Sly1 protein bound to Golgi syntaxin Sed5p allows assembly and contributes to specificity of SNARE fusion complexes. J. Cell Biol. 157:645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, C., P.D. Andrews, M.J. Stark, S. Cesaro-Tadic, A. Glatz, A. Podtelejnikov, M. Mann, and A. Mayer. 1999. Control of the terminal step of intracellular membrane fusion by protein phosphatase 1. Science. 285:1084–1087. [DOI] [PubMed] [Google Scholar]
- Pevsner, J., S.C. Hsu, J.E. Braun, N. Calakos, A.E. Ting, M.K. Bennett, and R.H. Scheller. 1994. a. Specificity and regulation of a synaptic vesicle docking complex. Neuron. 13:353–361. [DOI] [PubMed] [Google Scholar]
- Pevsner, J., S.C. Hsu, and R.H. Scheller. 1994. b. n-Sec1: a neural-specific syntaxin-binding protein. Proc. Natl. Acad. Sci. USA. 91:1445–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman, J.H., and T.H. Stevens. 1986. Protein sorting in yeast: mutants defective in vacuole biogenesis mislocalize vacuolar proteins into the late secretory pathway. Cell. 47:1041–1051. [DOI] [PubMed] [Google Scholar]
- Sikorski, R.S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 122:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof, T.C. 1995. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 375:645–653. [DOI] [PubMed] [Google Scholar]
- Sutton, R.B., D. Fasshauer, R. Jahn, and A.T. Brunger. 1998. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 395:347–353. [DOI] [PubMed] [Google Scholar]
- Ungermann, C., K. Sato, and W. Wickner. 1998. Defining the functions of trans-SNARE pairs. Nature. 396:543–548. [DOI] [PubMed] [Google Scholar]
- Weber, T., B.V. Zemelman, J.A. McNew, B. Westermann, M. Gmachl, F. Parlati, T.H. Sollner, and J.E. Rothman. 1998. SNAREpins: minimal machinery for membrane fusion. Cell. 92:759–772. [DOI] [PubMed] [Google Scholar]
- Wickner, W. 2002. Yeast vacuoles and membrane fusion pathways. EMBO J. 21:1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, T., I. Dulubova, S.W. Min, X. Chen, J. Rizo, and T.C. Sudhof. 2002. Sly1 binds to Golgi and ER syntaxins via a conserved N-terminal peptide motif. Dev. Cell. 2:295–305. [DOI] [PubMed] [Google Scholar]
- Yang, B., M. Steegmaier, L.C. Gonzalez, Jr., and R.H. Scheller. 2000. nSec1 binds a closed conformation of syntaxin1A. J. Cell Biol. 148:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2:107–117. [DOI] [PubMed] [Google Scholar]