Abstract
At the developing neuromuscular junction the Agrin receptor MuSK is the central organizer of subsynaptic differentiation induced by Agrin from the nerve. The expression of musk itself is also regulated by the nerve, but the mechanisms involved are not known. Here, we analyzed the activation of a musk promoter reporter construct in muscle fibers in vivo and in cultured myotubes, using transfection of multiple combinations of expression vectors for potential signaling components. We show that neuronal Agrin by activating MuSK regulates the expression of musk via two pathways: the Agrin-induced assembly of muscle-derived neuregulin (NRG)-1/ErbB, the pathway thought to regulate acetylcholine receptor (AChR) expression at the synapse, and via a direct shunt involving Agrin-induced activation of Rac. Both pathways converge onto the same regulatory element in the musk promoter that is also thought to confer synapse-specific expression to AChR subunit genes. In this way, a positive feedback signaling loop is established that maintains musk expression at the synapse when impulse transmission becomes functional. The same pathways are used to regulate synaptic expression of AChRɛ . We propose that the novel pathway stabilizes the synapse early in development, whereas the NRG/ErbB pathway supports maintenance of the mature synapse.
Keywords: Agrin; MuSK; synapse formation; skeletal muscle; neuromuscular junction
Introduction
The formation of a subsynaptic apparatus at the neuromuscular junction is an example of extreme subcellular differentiation induced by the motor neuron in a small segment of the muscle fiber. Four key components have been identified in this process: Agrin, a heparansulfate proteoglycan secreted from the motor nerve; MuSK, a component of the Agrin receptor complex; acetylcholine receptors (AChRs);* and rapsyn, a peripheral membrane component required for the aggregation of the AChRs in the muscle membrane. Agrin/MuSK act upstream of AChRs and rapsyn, but deletion of any of these components prevents synapse formation (Schaeffer et al., 2001).
When a synapse forms, a group of muscle nuclei driven by signals from the nerve selectively begins to transcribe genes coding for proteins involved in synapse formation and maintenance, including AChR subunit genes. Two major factors expressed in motor nerves, neuregulin (NRG)-1 and Agrin, have been invoked in this process. NRG-1, by activating ErbB receptor tyrosine kinases in the muscle membrane, is thought to activate AChR genes (Burden and Yarden, 1997; Fischbach and Rosen, 1997). Agrin by activating the receptor tyrosine kinase MuSK aggregates the AChRs and MuSK in the membrane. In cultured myotubes, activation of ErbB receptors by NRG-1 activates AChRα, δ, and ɛ subunit gene transcription via the MAPK (extracellular signal–regulated kinase [ERK]) and the phosphatidylinositol 3′-kinase pathways (Tansey et al., 1996; Altiok et al., 1997). ERK in turn activates the Ets transcription factor GABP to bind to a regulatory element in the AChRɛ and δ promoters, termed N-box, thus stimulating the transcription of the respective genes. The same regulatory element also mediates the localization of AChRδ and ɛ subunit expression to the synapse (Koike et al., 1995; Duclert et al., 1996; Fromm and Burden, 1998; Schaeffer et al., 1998; Sapru, 2001). Together these data suggest that the nerve regulates synaptic AChR expression via NRG-1. However, NRG-1 from neurones is not required to induce AChR genes in muscle fibers. Specifically, activation of MuSK by Agrin induces the formation of a postsynaptic membrane including AChR expression in vivo in the absence of a nerve terminal and thus of neural NRG-1 (Cohen et al., 1997; Jones et al., 1997; Meier et al., 1997).
The mRNA for MuSK also accumulates at the synapse, suggesting synapse-specific activation of its gene by the nerve (Valenzuela et al., 1995), but the mechanisms involved are not known. The spatio-temporal expression pattern of musk is similar to that of the AChRs. In fetal muscles, musk mRNA is expressed constitutively along the entire length of the fibers. In adult fibers, a neural signal locally induces musk mRNA (Valenzuela et al., 1995; Bowen et al., 1998), perhaps through Agrin/MuSK (Moore et al., 2001). On the other hand, in cultured myotubes musk mRNA is also up-regulated by NRG-1 (Ip et al., 2000).
Here, we describe experiments aimed at elucidating the mechanism involved in the nerve-induced expression of MuSK at the synapse and the roles of Agrin and NRG-1 in this process. The results indicate that synaptic expression of musk depends on the presence of an N-box in the musk promoter. Similarly, musk is activated through this N-box by neuronal Agrin via the activation of MuSK. Agrin-induced musk expression is controlled in part by a secondary NRG/ErbB pathway organized by Agrin/MuSK and in part by a novel shunt path in which MuSK signals to the muscle nuclei more directly via Rac and c-Jun NH2-terminal kinase (JNK) but is independent of NRG/ErbB. Remarkably, AChRɛ subunit expression is regulated via the same pathways. Thus, the nerve uses identical pathways to regulate the expression of key components in neuromuscular synapse formation.
Results
Cloning of the nsk2/musk promoter sequence
We screened a mouse genomic phage library with a 500-bp 5′ cDNA fragment of nsk2, the mouse ortholog of rat and human musk, and isolated 10 kb of mouse genomic sequence containing 5 kb each upstream and downstream of the first exon of nsk2 (Ganju et al., 1995). We denote this a nsk2/musk fragment. The transcription start site (+1) in nsk2/musk was determined by 5′ RACE (Fig. 1 A). The upstream region is TATA-less and lacks an obvious initiator element but contains several potential binding sites for transcription factors. Specifically, eight E-boxes are contained within the 1.6 kb upstream from the transcription start site (Fig. 1 B). The two proximal E-boxes (−40/−45 and –147/–152) are conserved between mouse nsk2 and the human musk ortholog (Fig. 1 A). E-boxes are thought to mediate muscle-specific and activity-dependent expression of AChR genes (Tang et al., 1994; Angus et al., 2001) by binding basic helix–loop–helix transcription factors of the myoD family (Schaeffer et al., 2001). Another putative E-box was found in the first intron at position +920 (Fig. 1, A and B). Importantly, the first intron of the nsk2 gene also contains at +871 an N-box, 5′-GTACCGGAAATA-3′ (Fig. 1, A and B). Its sequence is identical to the N-box of the AChRɛ subunit gene promoter where it is thought to mediate nerve- and NRG-induced transcription of the AChRɛ subunit gene (Duclert et al., 1993, 1996; Schaeffer et al., 1998). When compared with the human sequence, the overall identity of the first intron in mouse and human nsk2/musk is 74%, with the N-box located in a very highly conserved (91%) stretch of 80 bp which also contains putative-binding sites for TEF-1 and AP-1 (Fig. 1 A).
Figure 1.
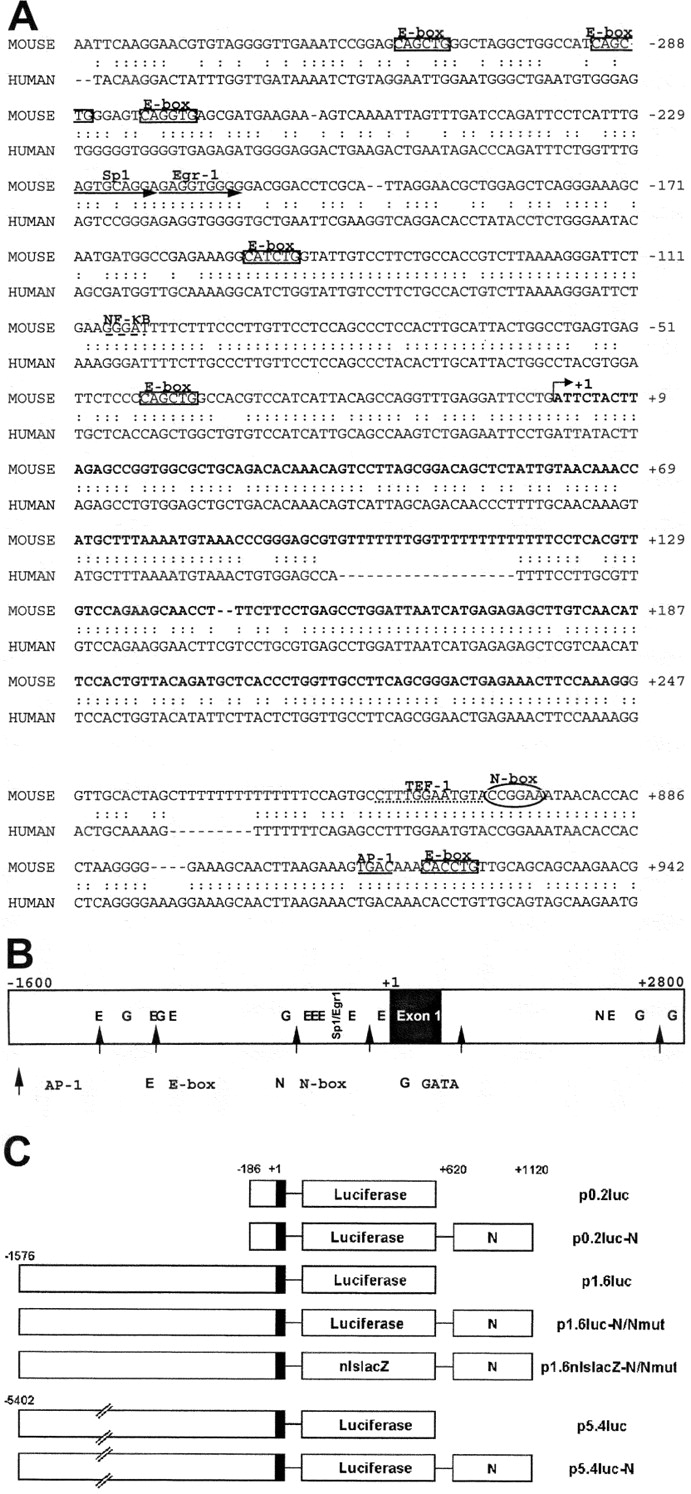
Regulatory sequences of the nsk2 gene. (A) Sequence comparison of the mouse (top) and the human (bottom) promoter regions with exon1 and part of the first intron of the nsk2/musk gene. The putative transcription start determined by 5′ RACE PCR is indicated as +1 for the mouse sequence. Boxes indicate E-boxes, the oval shows the N-box, black arrows show Sp1/Egr-1 putative binding sites, the large dotted line indicates a putative NF-κB binding site, the small dotted line indicates a putative TEF-1 binding site, and a putative AP-1 binding site is underlined. (B) Schematic representation of a larger region upstream and downstream the first exon. Potential binding sites for transcription factors are indicated. Prediction of the transcription factor binding site have been generated with the MatInspector software from Genomatix. (C) Schematic diagram of the different constructs used in vitro or in vivo experiments with either the luciferase gene or the lacZ gene as a reporter.
A 2.1-kb nsk2/musk promoter fragment mediates synapse-specific expression
Next, we examined whether a 2.1-kb nsk2/musk fragment contained regulatory elements conferring the physiological expression pattern to nsk2/musk, i.e., synapse-specific expression in electrically active fibers and an increase in expression in nonsynaptic regions upon muscle denervation (Valenzuela et al., 1995).
To test for synapse-specific activation, the proximal 1.6-kb upstream region was linked to nlslacZ as a reporter, followed by 0.5 kb of intronic sequence containing the N-box (Fig. 1 C, p1.6nlslacZ-N). Innervated, i.e., electrically active rat soleus muscles were then transfected in vivo by electroporation with p1.6nlslacZ-N, and synapse-specific expression of nlslacZ was tested 10–14 d later in the microscope by examining the location of muscle nuclei expressing β-galactosidase (β-Gal)–positive nuclei with respect to the location of synapses. The latter were marked by histochemical staining for acetylcholinesterase (AChE). Indeed, in some fibers β-Gal–positive nuclei were tightly colocalized with synapses (Fig. 2 A); in other fibers they were also seen in nonsynaptic regions. To see whether the AChE/β-Gal colocalization reflected preferential activation of the nsk2/musk promoter fragment at the synapse, we compared the number of β-Gal/AChE colocalizations expected for a random process with the number of colocalizations actually observed. For this purpose, fiber bundles were dissected, and the lengths of fiber segments occupied by β-Gal–positive nuclei relative to the length of the dissected fibers were determined. This ratio multiplied by the number of β-Gal–positive fibers examined gives the number of β-Gal–positive synapses expected for random colocalization. Comparison with the number of β-Gal/AChE colocalizations actually observed showed that β-Gal–positive synapses occurred five to six times more frequently than expected by chance (Fig. 2 B).
Figure 2.
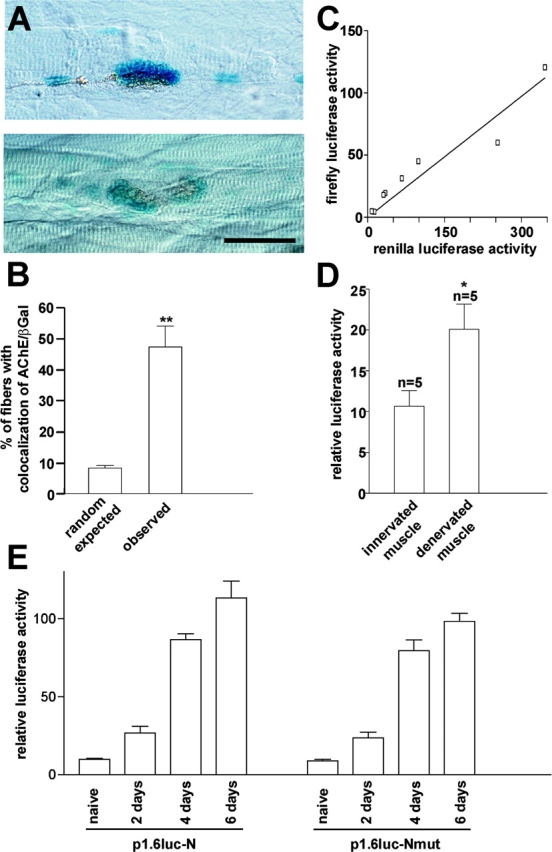
A 2.1-kb nsk2/musk promoter fragment confers spatio-temporal expression patterns to reporters comparable to those of endogenous musk. (A) Examples of endplates marked by histochemical staining for AChE and expressing colocalized β-Gal–positive nuclei upon transfection with p(1.6nlslacZ-N). Nuclei are in blue and AChE is in brown. Bar, 40 μm. (B) The percentage of β-Gal–positive fibers with colocalizations of β-Gal–positive nuclei and synaptic AChE observed (as illustrated in A) is significantly higher than expected for a random process. Means ± SE are given (n = 4 muscles, 17–56 β-Gal–positive fibers [170 fibers total] examined per muscle). (C) The level of firefly luciferase (luc) activity expressed upon transfection with p(1.6luc-N) increases linearly with the activity of a cotransfected standard, p(RL-TK), of Renilla luciferase, indicating that normalization to RL-TK expression to account for differences in transfection efficiency between muscles as performed in D is appropriate. (D) The 2.1-kb nsk2/musk promoter fragment responds to muscle denervation (4 d) by increasing expression of luciferase activity (means ± SE, n = 5). In the same experiments, the levels of RL-TK were not significantly different in innervated control and in denervated fibers (data not depicted). (E) Activation of p1.6luc-N and p1.6luc-Nmut in C2C12 myoblasts and in differentiating myotubes (n = 3 parallel cultures for each transfected plasmid). *P < 0.05 and **P < 0.01 in two-tailed t test. Bars ± SEM.
To test the nsk2/musk promoter fragment for its activation by muscle denervation, nlslacZ in p1.6nlslacZ-N was replaced by the cDNA encoding firefly luciferase (luc) as a reporter (Fig. 1 C, p1.6luc-N). Innervated, i.e., electrically active rat soleus muscles were then electroporated in vivo with a mixture of p1.6luc-N and an expression vector for Renilla luciferase, pRL-TK, as a reference. 10–13 d later, the soleus nerve was cut. After another 4 d, luciferase activities normalized to RL-TK activity in muscle extracts were compared with those in transfected control fibers that had been left innervated. The comparison revealed a significant twofold increase in normalized luciferase activities upon denervation (Fig. 2 D). A similar increase was seen when the N-box was mutated as described in the next paragraph, indicating that up-regulation by denervation was not dependent on the N-box. Finally, the expression profile of p1.6luc-N during in vitro differentiation of C2C12 muscle cells was similar to that of endogenous musk mRNA in these cells, i.e., a low level in myoblasts followed by a strong increase in differentiating myotubes (Valenzuela et al., 1995), and it was not affected by mutations in the N-box (Fig. 2, E and F). Thus, the isolated 2.1-kb promoter fragment of nsk2/musk confers expression patterns to the reporters as they are observed for endogenous musk.
Agrin activates the 2.1-kb nsk2/musk fragment conferring synapse-specific expression in vivo via an N-box
Expression of endogenous musk can be induced by Agrin/MuSK (Moore et al., 2001). Therefore, we next tested whether the 2.1-kb nsk2/musk promoter fragment conferring synaptic expression could be activated specifically by neuronal Agrin. For this purpose, we injected expression plasmids coding for full-length chicken neuronal Agrin, pcAgrin748, p1.6luc-N, and pRL-TK as a reference along with pnlsGFP-S6 intracellularly into extrasynaptic regions of innervated soleus muscles. As a control, pcAgrin748 was replaced with an expression vector for the nonneuronal isoform of Agrin, pcAgrin700 (Ruegg et al., 1992). 2 wk later, in fiber regions containing GFP-positive nuclei, luciferase activity measured in fibers expressing neuronal Agrin was ∼3.5 times higher than in fibers expressing muscle Agrin (Fig. 3 A). On a “per nucleus” basis, this is a lower estimate because the fiber segments dissected (2–3 mm in length) contained many more GFP-positive nuclei outside the Agrin-induced ectopic, postsynaptic membranes (and thus not activated by Agrin) than nuclei colocalized with such sites. Thus, neuronal Agrin but not muscle Agrin activates the same 2.1-kb nsk2/musk promoter fragment, (1.6 N) as does the nerve.
Figure 3.
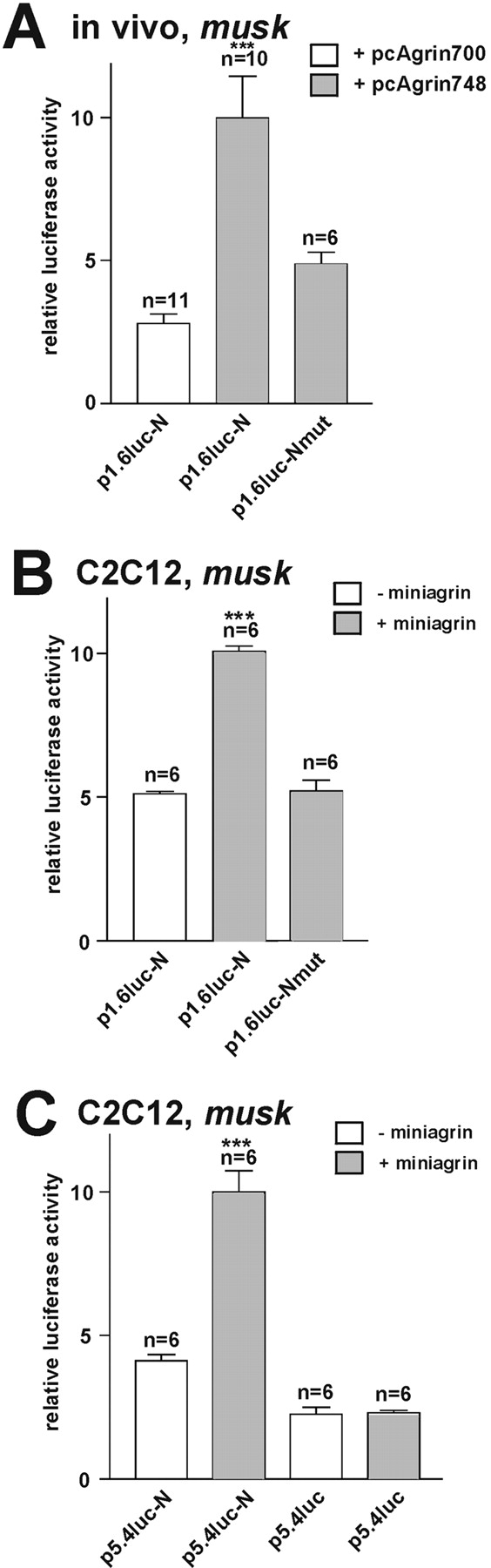
Neuronal but not muscle agrin activates nsk2/musk promoter fragments, depending on an N-box in the first intron. (A) Expression of luciferase activity under the control of the (1.6-N) promoter fragment in adult soleus muscle fibers in vivo is promoted by neuronal but not my muscle Agrin and is blocked upon mutation of the N-box. Muscle fibers were injected intracellularly with the expression plasmids indicated and with pRL-TK for normalization. (B) Agrin-induced expression of luciferase under the control of various nsk2/musk promoter fragments depends on the presence of the N-box in C2C12 myotubes. C2C12 myoblasts were transfected with pRL-TK and the plasmids indicated and grown and differentiated on a laminin substrate with or without mini-Agrin N257c21B8 attached. (C) Agrin-induced activation of a 5.4-kb nsk2/musk promoter fragment in C2C12 myotubes is blocked by removal of the intronic sequence containing the N-box. ***P < 0.001 in two-tailed t test. Bars ± SEM.
Unlike during myotube differentiation and upon denervation, the N-box of musk was essential for the activation of the musk promoter by Agrin. When two nucleotides in the N-box were mutated (CCGGAA → TTGGAA) to produce p1.6luc-Nmut, agrin-induced luciferase activity was significantly reduced (Fig. 3 A).
Neuronal Agrin triggers N-box–dependent musk expression in C2C12 myotubes
For further characterization of the nsk2/musk promoter, the responsiveness to Agrin of different promoter fragments of various lengths was examined by transactivation assays in C2C12 myotubes. In these experiments, a minimal variant of recombinant neuronal Agrin, cAgrinNtAc21B8 (Meier et al., 1998a), was presented to the myotubes in a form attached to the laminin culture substrate. Indeed, p1.6luc-N showing physiological activation in vivo also responded to neuronal mini-Agrin in myotubes but only when the N-box was intact (Fig. 3 B). Similar results were obtained when, immediately upstream from the transcription start site, the promoter was reduced to 0.2 kb (p0.2luc-N; unpublished data) or when it was extended to contain 5.4 kb of upstream sequence (p5.4luc-N; Fig. 1 C), except that basal luciferase activities and the response to Agrin increased somewhat as the upstream fragment length was increased (unpublished data). However, in all cases constructs required the presence of the intronic N-box to respond to Agrin (Fig. 3 C).
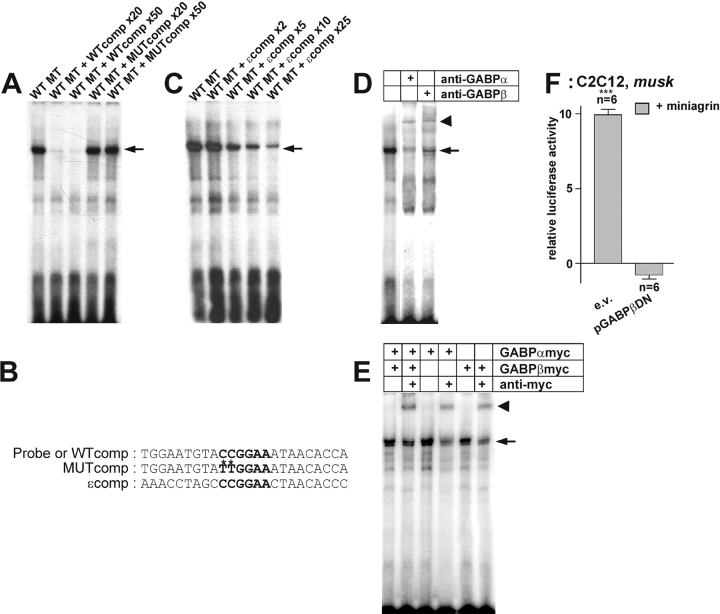
Binding of inactive GABP to the N-box blocks activation of nsk2/musk by Agrin
In cultured myotubes, musk, like AChR, is also activated by NRG-1 (Ip et al., 2000). The NRG-dependent activation of AChR genes depends on the binding of active transcription factor GABP to the N-box (Koike et al., 1995; Duclert et al., 1996; Fromm and Burden, 1998; Schaeffer et al., 1998). Therefore, we next tested in mobility shift assays whether the transcription factor complex GABP, composed of GABPα- and β subunits (Batchelor et al., 1998), binds to the N-box of the nsk2/musk promoter fragment, using a radiolabeled 24-base fragment of the first intron containing the N-box motif as a probe. Exposure of the probe to nuclear extracts from C2C12 myotubes revealed binding activity in the extract (Fig. 4 A). Adding nonlabeled probe eliminated the detectable shifted complex, indicating specificity of binding. Integrity of the N-box was necessary, since the nonradiolabeled probe with the N-box mutated was ineffective in competing for binding of the nuclear extract (Fig. 4, A and C). We next examined whether addition of a 24-nucleotide fragment of the rat AChRɛ subunit promoter, which also contains an N-box, ɛ-comp (Fig. 4 B), could compete for binding activity from C2C12 nuclear extracts. Indeed, a 25-fold molar excess of ɛ-comp prevented the retardation of the labeled N-box probe from nsk2/musk that had been produced by the addition of C2C12 nuclear extract (Fig. 4 C). Furthermore, a supershift was produced by binding of anti-GABPα or anti-GABPβ antibodies (Fig. 4 D). Likewise, when the labeled probe was exposed to an extract from HEK293 cells transiently expressing myc-tagged GABPα and GABPβ, respectively, supershifts were observed in the presence of anti-myc antibody (Fig. 4 E). These data combined indicate that the N-box motif of the MuSK promoter specifically binds GABP.
Figure 4.
GABP binds to the N-box of a nsk2/musk promoter fragment and activates it. (A) Binding activity of wild-type nuclear extracts to the N-box present in the probe. The specificity of the signal is eliminated by competition with an unlabeled wild-type probe (WTcomp), whereas an unlabeled mutant competitor (MUTcomp) leaves the signal unaffected. (B) Sequences of the oligonucleotides used as probes or competitors. (C) Unlabeled oligonucleotide containing the N-box motif of the AChRɛ subunit promoter (ɛ-comp) competes with N-box of musk for binding of nuclear extract. (D) The N-box binding activity produced by nuclear extracts from C2C12 cells (lane 1) is supershifted (bold arrow) by antibodies directed against GABPα (lane 2) and GABPβ (lane 3). (E) Nuclear extracts of transiently transfected HEK293 cells expressing GABPα-myc and GABPβ-myc (lanes 1 and 2) or only GABPα-myc (lanes 3 and 4) or GABPβ-myc (lanes 5 and 6) possess an N-box binding activity (arrow), which is supershifted (bold arrow) in the presence of an anti-myc antibody (Ab, lanes 2, 4, and 6). The second band seen in the shift may originate from binding activity specific for HEK293 cells. (F) Agrin-induced activation of the 2.1-kb nsk2/musk promoter fragment in C2C12 myotubes is blocked by overexpression of GABPβDN. ***P < 0.001 in two-tailed t test. Bars ± SEM. Note that for better resolution of the agrin-specific induction, luciferase activities in parallel control cultures (not depicted) are subtracted.
Next, we tested whether Agrin-induced musk activation was abolished by binding of an inactive mutant of GABP. For this purpose, we compared in C2C12 myotubes the response of p1.6luc-N to Agrin in the presence and in the absence of pGABPβDN, an inactive mutant of GABPβ, which blocks activation of AChR genes in cultured myotubes (Schaeffer et al., 1998) and Agrin-induced postsynaptic differentiation in vivo (Briguet and Rüegg, 2000). Indeed, overexpression of GABPβDN abolished the induction of luciferase activity under the 1.6-N promoter fragment by mini-Agrin (Fig. 4 F). In fact, luciferase activity in Agrin-exposed, GABPβDN-overexpressing cultures were below those in Agrin-free control cultures, suggesting some constitutive activity of GABP or a similar factor in the absence of Agrin. Thus, induction of musk expression by neuronal Agrin involves binding of GABP or a related factor to the intronic N-box.
Agrin can activate the nsk2/musk promoter via two pathways converging on the N-box
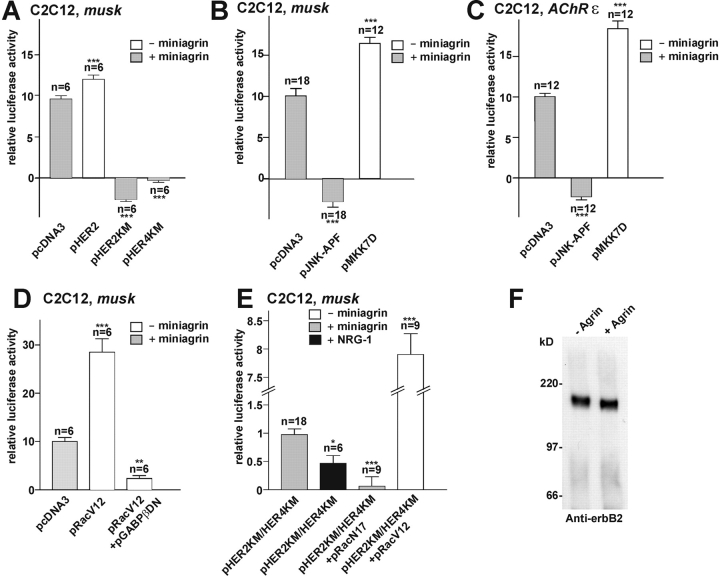
In cultured myotubes, NRG-1/ErbB-induced activation of AChRδ and ɛ is mediated by ERK and JNK MAPKs (Tansey et al., 1996; Altiok et al., 1997; Si et al., 1999). Therefore, we examined whether nsk2/musk was induced indirectly by Agrin, i.e., via the organization of an NRG-1/ErbB pathway with NRG derived from the myotubes, as we had observed previously for the induction of AChRɛ by Agrin (Meier et al., 1998b). As predicted for such a mechanism, activation of p1.6luc-N by mini-Agrin in C2C12 myotubes was abolished by overexpression of pHER2KM or pHER4KM, dominant-negative mutants of ErbB2 and ErbB4 (Fig. 5 A). Activation of nsk2/musk by Agrin was also blocked by overexpression of a dominant-negative mutant of JNK, pJNK-APF (Fig. 5 B). Conversely, a constitutively active mutant of MKK7, pMKK7D, which activates JNK, induced nsk2/musk in the absence of Agrin. A promoter–reporter construct of AChRɛ (Jones et al., 1996) was regulated similarly (Fig. 5 C). These data suggest that Agrin organizes a secondary NRG-1/ErbB pathway, which in turn activates musk and AChRɛ via ERK and JNK.
Figure 5.
Agrin uses two pathways for the activation of the 2.1-kb nsk2/musk promoter fragment in cultured myotubes. (A) In C2C12 myotubes, activation of p1.6luc-N by Agrin is fully blocked by HER2KM and HER4KM, dominant-negative mutants of human ErbB2 and ErbB4, respectively, whereas wild-type HER2 mimics the induction by Agrin. (B) Same as in A but blockade by dominant-negative JNK, JNK-APF; conversely, overexpression of MKK7D, a constitutively active kinase activating JNK, activates the fragment in the absence of Agrin. (C) Same as in B but with an AChRɛ promoter fragment (pLCF216ɛ). Note similar activation patterns of nsk2/musk and AChRɛ promoter fragments. (D) Constitutively active Rac, RacV12, activates p1.6luc-N; induction by Rac is completely blocked by GABPβDN. (E) With ErbB signaling blocked, p1.6luc-N is activated more strongly by agrin than by saturating (2 nM) NRG-1, and responses are fully blocked by dominant- negative Rac, RacN17. Constitutively active Rac, RacV12, strongly activates the promoter in spite of blockade of ErbB signaling. All experiments were performed on C2C12 cells transfected with p1.6luc-N. (F) Agrin does not discernibly increase ErbB2 levels in C2C12 myotubes. Experiments performed as in Fig. 3 with plasmids transfected as indicated. Responses are normalized to control values in the left-hand columns, and nonspecific background luciferase activities as observed in myotubes on agrin-free substrate are subtracted (C). *P < 0.05, **P < 0.01, and ***P < 0.001, respectively, in two-tailed t test. Bars ± SEM.
Next, we asked whether Agrin could also activate musk via a direct shunt from MuSK. For example, an alternative way for Agrin to activate JNK in C2C12 cells is via the Rho family GTPases Rac and Cdc42 (Weston et al., 2000; Luo et al., 2002). Consistent with such a mechanism, constitutively active Rac, RacV12, strongly activated p(1.6luc-N) in the absence of Agrin. This effect was abolished by GABPβDN (Fig. 5 D), suggesting that Rac activates nsk2/musk via the N-box only. If Agrin-induced activation of musk via Rac occurs independently of NRG/ErbB, Agrin will induce p1.6luc-N more strongly than saturating concentrations of NRG-1, and induction will be blocked by a dominant-negative mutant of Rac, RacN17. In normal C2C12 myotubes, no difference in the activation of p1.6luc-N by Agrin and by NRG-1 was resolved (unpublished data). However, when ErbB signaling was blocked by overexpression of inactive ErbB mutants, Agrin activated p(1.6luc-N) more strongly than saturating concentrations of NRG-1 (2 nM), which was not due to increased levels of ErbB2 (Fig. 5 F). Furthermore, activation was completely blocked by RacN17 (Fig. 5 E). Together, these data suggest that Agrin/MuSK can activate nsk2/musk via two pathways (Fig. 7): (a) via a direct shunt activated by MuSK involving Rac and (b) via the organization of a secondary NRG-1/ErbB pathway. Both pathways converge onto GABP. In C2C12 myotubes, the secondary NRG-1/ErbB pathway occludes the MuSK-induced shunt.
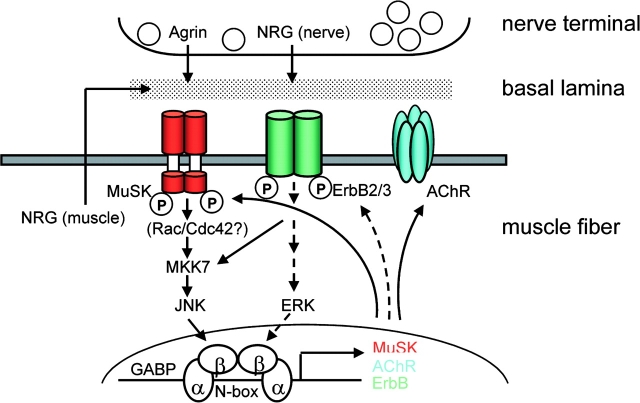
Figure 7.
Model for stabilization of synaptic gene expression through stabilization of musk expression by agrin from motor nerve terminal. Agrin secreted from nerve terminal activates preexisting MuSK to induce expression of musk via its N-box (i) by organizing an NRG/ErbB pathway, involving MuSK-induced recruitment of ErbB receptors and of muscle-derived NRG and (ii) by MuSK-induced activation of JNK (via Rac/Cdc42). With musk expression stabilized, the same pathways are used for AChR and erbB expression. Expression may be strengthened by NRG-1 secreted from nerve terminal. Complete inhibition of Agrin-induced musk transcription in C2C12 cells by overexpression of inactive ErbBs (HER2KM and HER4KM) and by dominant-negative JNK suggests that the two pathways are connected. The model is based on present data (solid arrows) and references cited (broken arrows).
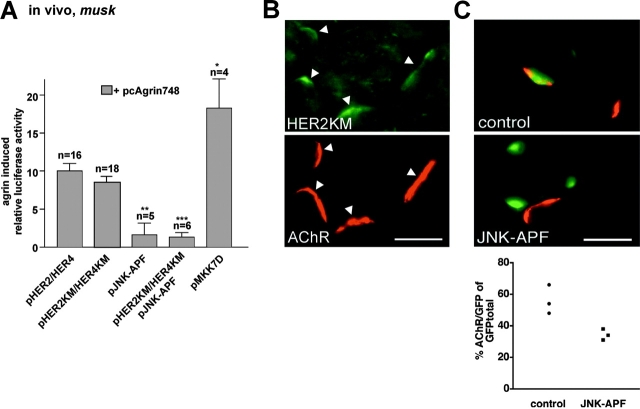
Next, we examined whether these pathways could be resolved in muscle fibers in vivo. Unlike in C2C12 cells, activation of nsk2/musk by Agrin was not blocked in adult fibers by overexpression of the inactive ErbB mutants. Specifically, upon injection with pHER2KM and pHER4KM into muscle fibers in vivo, activation of p1.6luc-N by neuronal Agrin was not significantly reduced compared with fibers injected with wild-type pHER2 and pHER4 (Fig. 6 A). Expression of pHERKM and accumulation of HER2KM at ectopic sites was ascertained by immunocytochemistry (Fig. 6 B). Similar results were obtained with the AChRɛ promoter–reporter construct (unpublished data).
Figure 6.
Agrin activates nsk2/musk via an ErbB- independent pathway in muscle fibers in vivo. (A) Activation of the nsk2/musk promoter fragment by Agrin in vivo is marginally affected by HER2KM and HER4KM but is fully blocked by JNK-APF. MKK7D activates the nsk2/musk fragment in the absence of Agrin. Nonspecific background luciferase activities as observed in muscle fibers injected with expression vector for muscle agrin (pcAgrin700; see Fig. 3 A) are subtracted. (B) Injection of pHERKM induces expression of HERKM in muscle fibers in vivo as myc immunoreactivity was observed at agrin-induced ectopic postsynaptic membranes upon injecting pmyc_HER2KM, a fusion of HER2KM and myc. Bar, 25 μm. (C) JNK-APF inhibits ectopic subsynaptic differentiation by Agrin. Muscle fibers were injected with pcAgrin748, pnslGFP, and pJNK-APF or equivalent amounts of empty vector (control); the formation of ectopic AChR clusters in GFP-positive fibers was then examined in cross sections, and injected fibers were identified by their expression of GFP. (Top) Control fiber (note the formation of AChR cluster [red] in GFP-positive control fiber [green nuclei]). (Middle) Agrin secreted induces AChR cluster on adjacent, GFP-negative (JNK-APF–negative) but not on GFP- (JNK-APF) expressing fiber. Bar, 25 μm. (Bottom) The percentage of GFP-positive fibers forming AChR clusters in response to Agrin is depressed in JNK-APF compared with control fibers. Each data point represents one muscle. 83–513 fiber profiles were examined per muscle.
In contrast, overexpression of the inactive JNK mutant, JNK-APF, did block musk activation by Agrin (Fig. 6 A). Furthermore, with JNK-APF overexpressed the presence of HER2KM and HER4KM did not inhibit the promoter activation any further. Conversely, coinjection of pMKK7D significantly increased musk activation by Agrin in muscle fibers. Therefore, in adult fibers Agrin/MuSK induces nsk2/musk primarily via a direct shunt involving Rac and JNK activation but not NRG/ErbB signaling.
If active JNK activation promotes gene expression at the synapse, then inactive JNK-APF is predicted to inhibit Agrin-induced expression of endogenous synaptic genes, such as musk and AChRɛ. We tested this prediction in vivo by analyzing whether JNK-APF blocked Agrin-induced ectopic subsynaptic differentiation. Indeed, injection of pJNK-APF significantly reduced the formation of ectopic AChR clusters in electrically active fibers (Fig. 6 C). Since overexpression of inactive JNK does not affect AChR clustering itself (Luo et al., 2002), the inhibition is due to down-regulation of expression of genes important in synapse formation, such as musk and AChR.
Discussion
To understand how the motor neuron regulates the formation of a subsynaptic muscle membrane, it is important to know how it regulates expression of MuSK, the central organizer of subsynaptic differentiation. Endogenous musk is stimulated in vivo by activated MuSK (Moore et al., 2001), but the pathways involved are not known. Here, we have used various nsk2/musk promoter–reporter constructs comprising up to 5.4 kb upstream and 0.5-kb sequence from the first intron to analyze the signaling pathways activated by the nerve and by Agrin in muscle fibers in vivo and in cultured myotubes.
Using these constructs, we show the following: (a) the nerve can induce activation of nsk2/musk promoter fragments via pathways converging on a regulatory sequence termed N-box in the first intron of musk; (b) Agrin activates the nsk2/musk promoter fragments solely via the binding of an Ets transcription factor to this N-box; (c) activation is mediated by two converging pathways, the organization of an NRG-1/ErbB pathway and a novel NRG-1/ErbB-independent pathway via MuSK/Rac; (d) the NRG-1/ErbB-mediated pathway predominates in cultured myotubes, the other in muscle fibers in vivo; and (e) these pathways are also used for the regulation of an AChRɛ promoter fragment.
Recently, it has been proposed that NRG may regulate AChRɛ and AChRδ subunit genes via Sp1 binding to a neuregulin response element (CCACCCCC) different from the N-box in their promoters (Alroy et al., 1999). The p5.4luc-N construct contains at approximately –3.4 kb a consensus sequence of this type. However, neither this putative neuregulin response element nor an Sp1-binding consensus sequence at position –112 in the musk promoter rescued the construct's responsiveness to Agrin when it was lacking the N-box, indicating that these sequences are not involved in the regulation of musk by Agrin.
An important aspect of the physiological relevance of the exogenous promoter fragment is whether it reflects the expression pattern of endogenous musk in vivo. The 2.1-kb nsk2/musk fragment used for most experiments here satisfies this criterion, except that the specificity of the reporter expression at the synapse and its relative increase upon denervation were less pronounced. This was likely due to nonspecific activation of the transfected promoter fragment in extrasynaptic fiber segments, perhaps caused by a high number of plasmid molecules introduced into the fibers or by the absence of regulatory elements that are located beyond this region.
In cultured myotubes, the organization of a secondary NRG-1/ErbB pathway by Agrin is suggested by the blockade of Agrin-induced nsk2/musk promoter activation upon inhibition of ErbB-mediated signaling as found previously for AChRɛ (Meier et al., 1998b). Induction of AChRɛ via NRG is mediated by ERK (Tansey et al., 1996; Altiok et al., 1997) and involves activation of JNK MAPKs (Si et al., 1999). Specifically, although inactive JNK blocks NRG-induced AChRɛ activation, overexpression of wild-type JNK has no effect, suggesting that JNK is necessary but not sufficient to induce AChRɛ, but requires ERK (Si et al., 1999). However, the present data suggest that JNK can activate musk independently of NRG/ErbB. (a) In spite of ErbB signaling blocked, musk was activated by constitutively active RacV12 (Fig. 5 E). Interestingly, JNK is activated by Agrin/MuSK in C2C12 myotubes via Rac (Weston et al., 2000) and via Dishevelled (Luo et al., 2002). (b) With ErbB signaling blocked, musk activation by Agrin was stronger than by saturating concentrations of NRG, and activation was completely blocked by dominant-negative RacN17. Therefore, these findings suggest that in addition to the NRG/ErbB pathway Agrin activates an ErbB-independent shunt via Rac/JNK to induce musk. Both converge on GABP because GABP binds to the N-box, and its inactive mutant GABPβDN completely blocks ErbB- and Rac-dependent activation of musk. One explanation for the prevalence of the ErbB-dependent pathway in C2C12 cells may be the high levels of constitutively expressed NRG-1 and ErbBs in these cells (Meier et al., 1998b) which could favor the organization of the NRG/ErbB pathway.
In contrast, in muscle fibers in vivo, musk induction by Agrin is only marginally mediated by NRG/ErbB because it is little affected by HER2KM and HER4KM. Thus, Agrin appears to activate musk primarily via the Rac/JNK-dependent shunt pathway: (a) only interfering with JNK but not abolishing NRG/ErbB signaling abolished Agrin-induced musk expression, and (b) forced overexpression of MKK7, which activates JNK but not ERK and p38 MAPKs (Davis, 2000), induced musk, again suggesting convergence of the NRG/ErbB and the shunt pathways.
JNK is a plausible candidate mediating Agrin's induction of musk. It can phosphorylate GABP in vitro (Fromm and Burden, 2001) and in T cells where it induces interleukin-2 (Hoffmeyer et al., 1998). Thus, Agrin-induced musk activation mediated by JNK will depend on the presence of the N-box in the musk promoter as observed here. Consistent with a role in Agrin-dependent gene transcription, JNK-APF inhibited ectopic subsynaptic differentiation as assessed by the inhibition of ectopic AChR cluster formation induced by Agrin. A similar blockade has been found by dominant-negative GABPβDN with the same type of assay (Briguet and Rüegg, 2000).
Our conclusion of an ErbB-independent, MuSK-induced shunt prevailing in vivo depends on the functional expression of injected pHERKM in the muscle fibers. Evidence for this is (a) the immunohistochemical demonstration of their expression; (b) that pHER2KM and pHER4KM have been functionally tested in HEK293 cells (Weiss et al., 1997); (c) that transfection of pHER2/4KM into C2C12 cells blocks musk and AChRɛ activation by Agrin, and thus, the constructs are functional in muscle cells; (d) that injection of pHER4KM into muscle fibers in vivo partially inhibits the induction of ectopic synaptic membranes by Agrin; inhibition was similar to that caused by ablation of the erbB2 gene (Moore et al., 2001). The inhibitions by erbB2 ablation and by pHER4KM injection were incomplete but similar in magnitude, and they did not add. Thus, injection of pHER4KM alone could fully block NRG/ErbB signaling in vivo. As predicted for an NRG/ErbB-independent pathway, the blocking effect by ErbB ablation and HER4KM overexpression was reduced as the level of Agrin was increased.
A direct shunt from MuSK to the nuclei is consistent with the only marginal reduction in AChR density that we observed recently at mature endplates of erbB2−/− muscle fibers (Leu et al., 2003). Finally, it can explain the observation that in neonatal rapsyn−/− mice elevated levels of musk and AChRγ and δ subunit genes are expressed in the synaptic muscle region (Apel et al., 1997), although MuSK but not ErbB receptors are aggregated (Moscoso et al., 1995).
Recently, de Kerchove D'Exaerde et al. (2002) reported that unlike AChRɛ subunit mRNA, the level of endogenous musk mRNA was not reduced in transgenic mouse muscle expressing an inactive mutant of the transcription factor Ets2. They concluded that musk is not or only marginally regulated by a mechanism involving Ets transcription factors, in contrast to the findings described here. However, alternative explanations are also possible (see Discussion in de Kerchove D'Exaerde et al., 2002).
By stabilizing musk and, as a consequence, AChR and other genes, Agrin can “stabilize” developing neuromuscular junctions: in fetal muscle, postsynaptic-like sites begin to develop in the absence of Agrin but depend on the presence of MuSK; later in development, they become dispersed, unless they are stabilized by Agrin from motor nerve terminals (Lin et al., 2001; Yang et al., 2001). Apparently, NRG from nerve terminals is not sufficient to stabilize MuSK. At mature synapses, NRG-1 secreted from the nerve is likely to contribute more substantially to the synaptic expression of musk because more NRG-1 will be available than can be recruited by Agrin from the muscle fibers alone (Loeb et al., 1999). Consistent with this, mice heterozygous for the relevant NRG-1 isoform contain fewer AChRs than wild-type mice in their endplates (Sandrock et al., 1997); similarly, in wild-type mice Agrin-induced ectopic AChR clusters have lower AChR density than endplates.
Finally, the regulation of musk by Agrin via binding of GAPB to the N-box is similar if not identical to that proposed for the localization of AChRɛ and δ subunit gene expression to the synapse (Schaeffer et al., 1998). The promoters of musk and of AChRɛ, when activated by Agrin, respond in a similar way to interfering with Agrin-dependent signaling components, e.g., ErbBs (Meier et al., 1998b) or JNK, and their N-boxes compete for the same components of nuclear extracts. Thus, Agrin-activated MuSK sets up signaling loops that are not only used to control its own expression but also that of AChRɛ (Fig. 7) and potentially of other genes regulated via an N-box, such as utrophin (Angus et al., 2001), AChRδ subunit (Koike et al., 1995; Duclert et al., 1996; Fromm and Burden, 1998; Schaeffer et al., 1998), and AChE genes (Gramolini et al., 1998; Chan et al., 1999). Induction of musk by agrin-activated MuSK and, via the same pathway, of major synaptic genes downstream of MuSK is a simple way for the nerve to orchestrate by the secretion of Agrin the formation of the postsynaptic membrane and to stabilize it when the synapse begins to transmit impulses.
Materials and methods
Screening of a mouse genomic library
The genomic library (packaged in Lambda FIX II Vector®; Stratagene) was screened with a dig-labeled 580-bp fragment corresponding to the 5′ part of the mouse nsk2/MuSK cDNA (Ganju et al., 1995). Approximately 3 × 106 plaques were screened, and two positive clones containing upstream and downstream regions from the first exon were further purified. 1.4 kb of upstream and 1 kb of downstream sequence, respectively, were subcloned into the pBluescript KS® vector (Stratagene) and sequenced by using the Dye-Deoxy Termination cycle sequencing method. The sequence data available from GenBank/EMBL/DDBJ under accession no. AY114512.
5′ RACE analysis
The transcription start site was determined by 5‘ RACE. RNA was extracted from C2C12 cells using the RNeasy Mini Kit (QIAGEN). 5′ RACE was performed using the 5′ RACE system version 2 (GIBCO BRL). The cDNA was amplified using the anchor primer and an internal primer R1, TCTTCAACCAAGGCATCTACAAG. The PCR products were cloned into pGEM-T vector® (Promega), and 20 independent clones were sequenced.
Construction of the reporter constructs
p1.6luc-N.
The 1.6-kb genomic fragment containing the region upstream of the first exon was amplified by PCR by using the oligonucleotides 1.6F, GAGAAGCTTTCCCTTTTCTTTGACTATAG and 1.6R, GAGAAGCTTACTGTTTGTGTCTGCAGCGC. This PCR product was cloned into the HindIII site of the pGL3 vector containing the luciferase gene as a reporter (Promega) to give p1.6luc. The same strategy was used to insert downstream of the luciferase gene into the BamHI site of pGL3 a 1-kb fragment of the first intron of nsk2/musk containing the N-box by using the NboxFB, GAGGGATTCTCCAGAGTGTTTTGCTCCTG and the NboxRB, GAGGGATTCTCTAGAACTCTGCATAACGC oligonucleotides, giving p1.6luc-N.
p1.6luc-Nmut.
Two mutations were introduced into the N-box at +871 (Fig. 1 A) of p1.6luc-N by PCR: 871C>T and 872C>T. A first amplification with, respectively, NboxFB, etsmutR, CTTAGGTGGTGTTATTTCCAATACATTCCAAAGGCA and NboxFR and etsmutF, TGCCTTTGGAATGTATTGGAAATAACACCACCTAAG was performed. The two products were mixed, denatured for 5 min at 95°C, annealed 10 min at 56°C, and the annealed product was submitted to a second PCR by using the NboxFB and NboxFR primers. After sequencing, the mutated fragment was cloned into the pGL3 vector as described above.
p1.6nlslacZ-Nmut.
For some experiments, luciferase in p1.6luc-N was replaced by nlslacZ, a reporter construct directing β-Gal expression to nuclei for easier microscopic observation. For this purpose, a KpnI-NcoI fragment was excised from a 1.6luc construct and subcloned in a pLacF plasmid (Sanes et al., 1991). The fragment containing the N-box was added downstream the lacZ at the SphI restriction site, using the same primers as described above, but using SphI instead of the BamHI restriction site. The plasmid DNA was resuspended either in Tris (5 mM) for cell transfection and in vivo intracellular injection or in Tris 5 mM/NaCl 0.9%, pH, 7.4, for in vivo electroporation.
Other constructs.
Other constructs used have been described as indicated: pnlsGFP-S6 (Hashemolhosseini et al., 2000); pHER2, pHER2KM, pHER4, pHER4KM (Weiss et al., 1997); pGABPβDN, pGABPα-myc, pGABPβ-myc (Briguet and Rüegg, 2000); pJNK-APF (Gupta et al., 1995), pMKK7D (Wang et al., 1998); pLCF216ɛ (Jones et al., 1996); pRacV12, pRacN17 (Joneson et al., 1996).
Cell culture and transfection
C2C12 cells were cultured according to Jones et al (1999). 8 × 104 cells were passaged onto 6-well plates that had been coated with either laminin alone or with laminin and mini-Agrin (N257c21B8) comprising the NtA laminin-binding site and the minimal c21AgrinB8 of chicken Agrin (Gesemann et al., 1996; Meier et al., 1998a). This mini-Agrin is sufficient to induce AChRɛ subunit gene expression in vivo. Unlike full-length Agrin, it does not carry glycosaminoglycan side chains and thus cannot bind and accumulate NRG-1 without signaling through MuSK (Jones et al., 1996; Meier et al., 1998a,b). Myoblasts were transfected with 800 ng of reporter plasmid, 80 ng of pRL-TK plasmid (Promega) for normalization, and 150 ng of the appropriate plasmid using 6 μl of Fugene6 reagent (Roche Diagnostics). Myotubes were analyzed 7 d after transfection using the dual luciferase reporter assay system (Promega). Light intensity was quantified with a Turner Designs Model 20e Luminometer. NRG-1 was from R&D Systems.
Nuclear extracts and electrophoretic mobility shift assays
Muscle nuclei were collected from differentiated C2C12 myotubes according to the protocol of Schreiber et al. (1989) with the exception that all buffers contained a cocktail of protease inhibitors (Roche Diagnostics).
For the mobility shift assay experiments, the oligonucleotides were as follows: etsF, TGGAATGTACCGGAAATAACACCA; etsR, TGGTGTTATTTCCGGTACATTCCA; etsmutF, TGGAATGTATTGGAAATAACACCA; etsmutR, TGGTGTTATTTCCAATACATTCCA; ɛcompF, AAACCTAGCCCGGAACTAACACCC; and ɛcompR, GGGTGTTAGTTCCGGGCT-AGGTTT. Annealed oligonucleotide probes were labeled with [γ-32P]ATP using T4 polynucleotide kinase. For the binding reaction, 2 ng of labeled probe, 2 μg of poly dIdC, and 3–4 μg of nuclear extract were mixed and incubated for 20 min at RT, loaded on a 5% polyacrylamide gel and run at 200 V for 40 min. The gel was dried and exposed in a Storm imaging system (Amersham Biosciences). Antibodies against GABPα and GABPβ used in supershift experiments have been characterized by Schaeffer et al. (1998).
ErbB2 immunoprecipitation and Western blotting
C2C12 myotubes cultured in 10 cm plates were washed twice with ice-cold PBS, and membrane proteins were extracted with 1 ml detergent extraction buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 5 mM EGTA, 50 mM NaF, 10 mM sodium molybdate, 1 mM sodium orthovanadate, 1% Triton X-100, and 1× protease inhibitor cocktail [Roche Diagnostics]). Extracts were cleared from cell debris by centrifugation at 4°C. For each sample, the similar amounts of total protein was subjected to ErbB2 immunoprecipitation with 0.1 μg/ml anti-erbB2 antibody (C18; Santa Cruz Biotechnology, Inc.) for 1 h at 4°C and incubated with protein A–Agarose beads (Roche Diagnostics) overnight at 4°C. After washing five times with 1 ml detergent extraction buffer, excess liquid was removed, and the beads were boiled in 50 μl SDS sample buffer. Samples were separated on a 5% acrylamide gel and transferred to Immobilon-P (Millipore) by electroblotting. ErbB2 protein was detected by a polyclonal rabbit anti-ErbB2 antibody (a gift from A. Badache, Friedrich Miescher Institute, Basel, Switzerland) and with peroxidase-conjugated anti–rabbit antibody. Immunocomplexes were visualized followed by ECL.
Injections of plasmids into muscle fibers in vivo
Male Wistar rats were anaesthetized with Nembutal (1 ml/kg). Single fibers of rat soleus were injected in their proximal end plate–free region as described (Jones et al., 1997) with 100 ng/μl of the reporter plasmid, 50 ng/μl of the pRL-TK plasmid, the plasmids indicated, and with 50 ng/μl of the pnlsGFP-S6 plasmid (Jones et al., 1999) for later identification of injected fibers. After 2 wk, rats were killed with CO2, and the soleus muscles were excised for analysis using the Dual-Luciferase® Reporter Assay System (Promega). Blockade of ectopic subsynaptic differentiation in vivo by JNK-APF was tested as described by Briguet and Rüegg (2000).
Electroporation of muscles
Rats were anaesthetized, and the left soleus muscle was surgically exposed. 30 μl of a DNA mixture containing 55 μg of the p1.6luc-N or p1.6nlslacZ-N plasmids and 35 μg of the pRL-TK plasmid were injected into the soleus with a Hamilton syringe (gauge 34). After suturing the surgical cut, two stainless steel plate electrodes, covered with a conductive gel allowing electrical contact with the skin, were placed at each side of the leg. Eight pulses of 20-ms duration were delivered at 1-s intervals from a T830 square wave electroporator (BTX) (Mir et al., 1999). Analysis of luciferase activities was as described above. Muscles electroporated with p1.6nlslacZ-N were stained for β-Gal activity and subsequently for acetylcholinesterase (Koelle and Friedenwald, 1949). Fiber bundles teased from the muscles were examined under the microscope at 400×.
Acknowledgments
We thank Drs. L. Schaeffer and J.P. Changeux, (Institut Pasteur Paris, France), for GABP antibodies, and Dr. A. Briguet (Biozentrum, Basel, Switzerland) for GABP expression plasmids, Annick Werner for excellent technical assistance and Drs. B. Bettler, M.A. Rüegg and P. Escher for comments on the manuscript.
This work was supported by the Swiss National Science Foundation and the Swiss Foundation for Research on Muscle Diseases.
N. Gajendran's present address is Max-Planck-Institut für Infektionsbiologie, Abt. Immunologie, Schumannstrasse 21/22, D-10117 Berlin, Germany.
Footnotes
Abbreviations used in this paper: AChE, acetylcholinesterase; AChR, acetylcholine receptor; β-Gal, β-galactosidase; ERK, extracellular signal–regulated kinase; JNK, c-Jun NH2-terminal kinase; NRG, neuregulin.
References
- Alroy, I., L. Soussan, R. Seger, and Y. Yarden. 1999. Neu differentiation factor stimulates phosphorylation and activation of the Sp1 transcription factor. Mol. Cell. Biol. 19:1961–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altiok, N., S. Altiok, and J.P. Changeux. 1997. Heregulin-stimulated acetylcholine receptor gene expression in muscle: requirement for MAP kinase and evidence for a parallel inhibitory pathway independent of electrical activity. EMBO J. 16:717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus, L.M., R.Y. Chan, and B.J. Jasmin. 2001. Role of intronic E- and N-box motifs in the transcriptional induction of the acetylcholinesterase gene during myogenic differentiation. J. Biol. Chem. 276:17603–17609. [DOI] [PubMed] [Google Scholar]
- Apel, E.D., D.J. Glass, L.M. Moscoso, G.D. Yancopoulos, and J.R. Sanes. 1997. Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron. 18:623–635. [DOI] [PubMed] [Google Scholar]
- Batchelor, A.H., D.E. Piper, F.C. de la Brousse, S.L. McKnight, and C. Wolberger. 1998. The structure of GABPalpha/beta: an ETS domain-ankyrin repeat heterodimer bound to DNA. Science. 279:1037–1041. [DOI] [PubMed] [Google Scholar]
- Bowen, D.C., J.S. Park, S. Bodine, J.L. Stark, D.M. Valenzuela, T.N. Stitt, G.D. Yancopoulos, R.M. Lindsay, D.J. Glass, and P.S. DiStefano. 1998. Localization and regulation of MuSK at the neuromuscular junction. Dev. Biol. 199:309–319. [DOI] [PubMed] [Google Scholar]
- Briguet, A., and M.A. Rüegg. 2000. The Ets transcription factor GABP is required for postsynaptic differentiation in vivo. J. Neurosci. 20:5989–5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden, S., and Y. Yarden. 1997. Neuregulins and their receptors: a versatile signaling module in organogenesis and oncogenesis. Neuron. 18:847–855. [DOI] [PubMed] [Google Scholar]
- Chan, R.Y., C. Boudreau-Lariviere, L.M. Angus, F.A. Mankal, and B.J. Jasmin. 1999. An intronic enhancer containing an N-box motif is required for synapse- and tissue-specific expression of the acetylcholinesterase gene in skeletal muscle fibers. Proc. Natl. Acad. Sci. USA. 96:4627–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, N.A., W.E. Kaufmann, P.F. Worley, and F. Rupp. 1997. Expression of Agrin in the developing and adult rat brain. Neuroscience. 76:581–596. [DOI] [PubMed] [Google Scholar]
- Davis, R.J. 2000. Signal transduction by the JNK group of MAP kinases. Cell. 103:239–252. [DOI] [PubMed] [Google Scholar]
- de Kerchove D'Exaerde, A., J.R. Cartaud, and L. Schaeffer. 2002. Expression of mutant Ets protein at the neuromuscular synapse causes alterations in morphology and gene expression. EMBO Rep. 3:1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclert, A., N. Savatier, and J.P. Changeux. 1993. An 83-nucleotide promoter of the acetylcholine receptor epsilon-subunit gene confers preferential synaptic expression in mouse muscle. Proc. Natl. Acad. Sci. USA. 90:3043–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclert, A., N. Savatier, L. Schaeffer, and J.P. Changeux. 1996. Identification of an element crucial for the sub-synaptic expression of the acetylcholine receptor epsilon-subunit gene. J. Biol. Chem. 271:17433–17438. [DOI] [PubMed] [Google Scholar]
- Fischbach, G.D., and K.M. Rosen. 1997. ARIA: a neuromuscular junction neuregulin. Annu. Rev. Neurosci. 20:429–458. [DOI] [PubMed] [Google Scholar]
- Fromm, L., and S.J. Burden. 1998. Synapse-specific and neuregulin-induced transcription require an ets site that binds GABPalpha/GABPbeta. Genes Dev. 12:3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm, L., and S.J. Burden. 2001. Neuregulin-1-stimulated phosphorylation of GABP in skeletal muscle cells. Biochemistry. 40:5306–5312. [DOI] [PubMed] [Google Scholar]
- Ganju, P., E. Walls, J. Brennan, and A.D. Reith. 1995. Cloning and developmental expression of Nsk2, a novel receptor tyrosine kinase implicated in skeletal myogenesis. Oncogene. 11:281–290. [PubMed] [Google Scholar]
- Gesemann, M., V. Cavalli, A.J. Denzer, A. Brancaccio, B. Schumacher, and M.A. Ruegg. 1996. Alternative splicing of agrin alters its binding to heparin, dystroglycan, and the putative agrin receptor. Neuron. 16:755–767. [DOI] [PubMed] [Google Scholar]
- Gramolini, A.O., E.A. Burton, J.M. Tinsley, M.J. Ferns, A. Cartaud, J. Cartaud, K.E. Davies, J.A. Lunde, and B.J. Jasmin. 1998. Muscle and neural isoforms of agrin increase utrophin expression in cultured myotubes via a transcriptional regulatory mechanism. J. Biol. Chem. 273:736–743. [DOI] [PubMed] [Google Scholar]
- Gupta, S., D. Campbell, B. Derijard, and R.J. Davis. 1995. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 267:389–393. [DOI] [PubMed] [Google Scholar]
- Hashemolhosseini, S., C. Moore, L. Landmann, A. Sander, H. Schwarz, V. Witzemann, B. Sakmann, and H.R. Brenner. 2000. Electrical activity and postsynapse formation in adult muscle: gamma-AChRs are not required. Mol. Cell. Neurosci. 16:697–707. [DOI] [PubMed] [Google Scholar]
- Hoffmeyer, A., A. Avots, E. Flory, C.K. Weber, E. Serfling, and U.R. Rapp. 1998. The GABP-responsive element of the interleukin-2 enhancer is regulated by JNK/SAPK-activating pathways in T lymphocytes. J. Biol. Chem. 273:10112–10119. [DOI] [PubMed] [Google Scholar]
- Ip, F.C., D.G. Glass, D.R. Gies, J. Cheung, K.O. Lai, A.K. Fu, G.D. Yancopoulos, and N.Y. Ip. 2000. Cloning and characterization of muscle-specific kinase in chicken. Mol. Cell. Neurosci. 16:661–673. [DOI] [PubMed] [Google Scholar]
- Jones, G., A. Herczeg, M.A. Ruegg, M. Lichtsteiner, S. Kroger, and H.R. Brenner. 1996. Substrate-bound agrin induces expression of acetylcholine receptor epsilon-subunit gene in cultured mammalian muscle cells. Proc. Natl. Acad. Sci. USA. 93:5985–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, G., T. Meier, M. Lichtsteiner, V. Witzemann, B. Sakmann, and H.R. Brenner. 1997. Induction by agrin of ectopic and functional postsynaptic-like membrane in innervated muscle. Proc. Natl. Acad. Sci. USA. 94:2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, G., C. Moore, S. Hashemolhosseini, and H.R. Brenner. 1999. Constitutively active MuSK is clustered in the absence of agrin and induces ectopic postsynaptic-like membranes in skeletal muscle fibers. J. Neurosci. 19:3376–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joneson, T., M. McDonough, D. Bar-Sagi, and L. Van Aelst. 1996. RAC regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science. 274:1374–1376. [DOI] [PubMed] [Google Scholar]
- Koelle, G.B., and J.S. Friedenwald. 1949. A histochemical method for localizing cholinesterase activity. Proc. Soc. Exp. Biol. Med. 70:617–622. [DOI] [PubMed] [Google Scholar]
- Koike, S., L. Schaeffer, and J.P. Changeux. 1995. Identification of a DNA element determining synaptic expression of the mouse acetylcholine receptor delta-subunit gene. Proc. Natl. Acad. Sci. USA. 92:10624–10628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu, M., E. Bellmunt, M. Schwander, I. Farinas, H.R. Brenner, and U. Muller. 2003. Erbb2 regulates neuromuscular synapse formation and is essential for muscle spindle development. Development. 130:2291–2301. [DOI] [PubMed] [Google Scholar]
- Lin, W., R.W. Burgess, B. Dominguez, S.L. Pfaff, J.R. Sanes, and K.F. Lee. 2001. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature. 410:1057–1064. [DOI] [PubMed] [Google Scholar]
- Loeb, J.A., T.S. Khurana, J.T. Robbins, A.G. Yee, and G.D. Fischbach. 1999. Expression patterns of transmembrane and released forms of neuregulin during spinal cord and neuromuscular synapse development. Development. 126:781–791. [DOI] [PubMed] [Google Scholar]
- Luo, Z., Q. Wang, J. Zhou, J. Wang, M. Liu, X. He, A. Wynshaw-Boris, W. Xiong, B. Lu, and L. Mei. 2002. Regulation of AChR clustering by dishevelled interacting with MuSK and PAK1. Neuron. 35:489–505. [DOI] [PubMed] [Google Scholar]
- Meier, T., D.M. Hauser, M. Chiquet, L. Landmann, M.A. Ruegg, and H.R. Brenner. 1997. Neural agrin induces ectopic postsynaptic specializations in innervated muscle fibers. J. Neurosci. 17:6534–6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier, T., P.A. Marangi, J. Moll, D.M. Hauser, H.R. Brenner, and M.A. Ruegg. 1998. a. A minigene of neural agrin encoding the laminin-binding and acetylcholine receptor-aggregating domains is sufficient to induce postsynaptic differentiation in muscle fibres. Eur. J. Neurosci. 10:3141–3152. [DOI] [PubMed] [Google Scholar]
- Meier, T., F. Masciulli, C. Moore, F. Schoumacher, U. Eppenberger, A.J. Denzer, G. Jones, and H.R. Brenner. 1998. b. Agrin can mediate acetylcholine receptor gene expression in muscle by aggregation of muscle-derived neuregulins. J. Cell Biol. 141:715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir, L.M., M.F. Bureau, J. Gehl, R. Rangara, D. Rouy, J.M. Caillaud, P. Delaere, D. Branellec, B. Schwartz, and D. Scherman. 1999. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc. Natl. Acad. Sci. USA. 96:4262–4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, C., M. Leu, U. Muller, and H.R. Brenner. 2001. Induction of multiple signaling loops by MuSK during neuromuscular synapse formation. Proc. Natl. Acad. Sci. USA. 98:14655–14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscoso, L.M., G.C. Chu, M. Gautam, P.G. Noakes, J.P. Merlie, and J.R. Sanes. 1995. Synapse-associated expression of an acetylcholine receptor-inducing protein, ARIA/heregulin, and its putative receptors, ErbB2 and ErbB3, in developing mammalian muscle. Dev. Biol. 172:158–169. [DOI] [PubMed] [Google Scholar]
- Ruegg, M.A., K.W. Tsim, S.E. Horton, S. Kroger, G. Escher, E.M. Gensch, and U.J. McMahan. 1992. The agrin gene codes for a family of basal lamina proteins that differ in function and distribution. Neuron. 8:691–699. [DOI] [PubMed] [Google Scholar]
- Sandrock, A.W., Jr., S.E. Dryer, K.M. Rosen, S.N. Gozani, R. Kramer, L.E. Theill, and G.D. Fischbach. 1997. Maintenance of acetylcholine receptor number by neuregulins at the neuromuscular junction in vivo. Science. 276:599–603. [DOI] [PubMed] [Google Scholar]
- Sanes, J.R., Y.R. Johnson, P.T. Kotzbauer, J. Mudd, T. Hanley, J.C. Martinou, and J.P. Merlie. 1991. Selective expression of an acetylcholine receptor-lacZ transgene in synaptic nuclei of adult muscle fibers. Development. 113:1181–1191. [DOI] [PubMed] [Google Scholar]
- Sapru, M.K. 2001. Neuregulin-1 regulates expression of the Ets-2 transcription factor. Life Sci. 69:2663–2674. [DOI] [PubMed] [Google Scholar]
- Schaeffer, L., N. Duclert, M. Huchet-Dymanus, and J.P. Changeux. 1998. Implication of a multisubunit Ets-related transcription factor in synaptic expression of the nicotinic acetylcholine receptor. EMBO J. 17:3078–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer, L., A. de Kerchove D'Exaerde, and J.P. Changeux. 2001. Targeting transcription to the neuromuscular synapse. Neuron. 31:15–22. [DOI] [PubMed] [Google Scholar]
- Schreiber, E., P. Matthias, M.M. Muller, and W. Schaffner. 1989. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si, J., Q. Wang, and L. Mei. 1999. Essential roles of c-JUN and c-JUN N-terminal kinase (JNK) in neuregulin-increased expression of the acetylcholine receptor epsilon-subunit. J. Neurosci. 19:8498–8508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, J., S.A. Jo, and S.J. Burden. 1994. Separate pathways for synapse-specific and electrical activity-dependent gene expression in skeletal muscle. Development. 120:1799–1804. [DOI] [PubMed] [Google Scholar]
- Tansey, M.G., G.C. Chu, and J.P. Merlie. 1996. ARIA/HRG regulates AChR epsilon subunit gene expression at the neuromuscular synapse via activation of phosphatidylinositol 3-kinase and Ras/MAPK pathway. J. Cell Biol. 134:465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela, D.M., T.N. Stitt, P.S. DiStefano, E. Rojas, K. Mattsson, D.L. Compton, L. Nunez, J.S. Park, J.L. Stark, D.R. Gies, et al. 1995. Receptor tyrosine kinase specific for the skeletal muscle lineage: expression in embryonic muscle, at the neuromuscular junction, and after injury. Neuron. 15:573–584. [DOI] [PubMed] [Google Scholar]
- Wang, Y., B. Su, V.P. Sah, J.H. Brown, J. Han, and K.R. Chien. 1998. Cardiac hypertrophy induced by mitogen-activated protein kinase kinase 7, a specific activator for c-Jun NH2-terminal kinase in ventricular muscle cells. J. Biol. Chem. 273:5423–5426. [DOI] [PubMed] [Google Scholar]
- Weiss, F.U., C. Wallasch, M. Campiglio, W. Issing, and A. Ullrich. 1997. Distinct characteristics of heregulin signals mediated by HER3 or HER4. J. Cell. Physiol. 173:187–195. [DOI] [PubMed] [Google Scholar]
- Weston, C., B. Yee, E. Hod, and J. Prives. 2000. Agrin-induced acetylcholine receptor clustering is mediated by the small guanosine triphosphatases Rac and Cdc42. J. Cell Biol. 150:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X., S. Arber, C. William, L. Li, Y. Tanabe, T.M. Jessell, C. Birchmeier, and S.J. Burden. 2001. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron. 30:399–410. [DOI] [PubMed] [Google Scholar]