Abstract
Background
Activation of mast cells and the systemic release of histamine are common side effects of opiates such as codeine and morphine. In some individuals, codeine not only elicits a sizable early response due to mast cell degranulation, but can also lead to late cutaneous allergic inflammation possibly through the production of chemokines. However, individuals who exhibit a late phase reaction to codeine often do not react to its synthetic analog, meperidine. The goal of this study was to test whether codeine and meperidine induce secretion of inflammatory mediators in human mast cells.
Methods
To characterize opiate activation of human mast cells, we stimulated cultured human (LAD2 cell line and CD34+-derived) mast cells with codeine and meperidine and measured degranulation and chemokine production.
Results
Codeine, but not meperidine, activated human mast cell degranulation within 30 min in a dose-dependent manner. Degranulation was blocked by the phosphoinositol-3 kinase (PI3K) inhibitor, wortmannin, and pertussis toxin but not by Ro-31-8220, a PKC inhibitor or forskolin, a cyclic adenylyl cyclase activator. After 3 and 8 h of stimulation, codeine, but not meperidine, activated human mast cells to release monocyte chemoattractant protein-1 (CCL2), regulated on activation, normal T expressed and secreted (RANTES, CCL5) and interleukin-8 (CXCL 8) but not inducible protein-10 (CXCL10).
Conclusions
Codeine activates human mast cell degranulation and chemokine production by activating protein kinase A and PI3 kinase, possibly leading to NF-κB activation. Therefore, opiates may regulate late phase allergic inflammation by activating chemokine production by human mast cells.
Keywords: chemokines, codeine, human, innate immunity, mast cells, opiate receptors
Opiates and their synthetic counterparts, opioids, have important pharmacologic actions that provide relief for pain and effective management of disease. Unfortunately, anaphylactic reactions to opiates and opioids have been reported and continue to cause concern. In fact, it is believed that some heroin fatalities may be because of anaphylactic shock (1). Hypersensitivities to naturally occurring opiates are more common than hypersensitivities to opioids and are clinically manifested as urticaria, skin rash, and contact dermatitis at the injection site (2). These reactions occur when opiates activate skin mast cells to release histamine that can cause flushing, warming of the skin, sweating, itching, and postural hypotension. The opiate codeine has been used as a positive control for mast cell activation in skin prick testing for many years (3). Although degranulation and release of histamine by human skin mast cells can account for many of the symptoms associated with codeine allergy, it is possible that mast cells release other mediators that contribute to the clinical symptoms.
Opiates and synthetic opioids can cause direct rodent mast cell degranulation without the presence of specific immunoglobulin (Ig)E antibodies (4). However, IgE antibodies to morphine and codeine have been detected in the serum of at least one subject who experienced a life-threatening anaphylactic reaction following the administration of a papveretum–hyoscine combination (5). This suggests that opiate allergy may be IgE receptor (FcεRI) mediated in some rare cases. In cases where IgE is not involved, human mast cells are directly activated by another unknown mechanism. Skin biopsies taken from normal subjects after intradermal morphine injection have demonstrated mast cell degranulation when examined by electron microscopy, providing convincing evidence for a direct effect of opiates on skin mast cells. This effect is only partially inhibited by naloxone, an opiate receptor antagonist, which implies that the mechanism of mast cell activation may include a component that is independent of opiate receptors (6, 7). Furthermore, opiate sensitivity appears to be dependent on mast cell heterogeneity as human skin mast cells degranulate in vitro in the presence of morphine but human lung, intestinal, or heart mast cells do not release histamine in response to opiates (8).
Therefore, we hypothesized that opiates activate cultured human mast cell degranulation and mediator release and that naturally occurring opiates such as codeine would be more effective at stimulating human mast cells than synthetic opioids such as meperidine. The aims of our study were to (i) determine the relative potency of codeine and meperidine in stimulating human mast cell degranulation, (ii) determine if codeine and meperidine stimulate human mast cell cytokine and chemokine production, and (iii) determine whether opiate activation of human mast cells is opioid receptor-dependent.
Materials and methods
Growth of human mast cells
LAD2 were cultured in serum-free media (StemPro-34 SFM, Invitrogen, Carlsbad, CA, USA) supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 50 μg/ml streptomycin, and 100 ng/ml stem cell factor (SCF; 9). Human peripheral blood-derived CD34+ cells (HuMC) were cultured in StemPro-34 SFM supplemented with 2 mM l-glutamine, 50 μg/ml streptomycin, 100 IU/ml penicillin, 100 ng/ml SCF, and 100 ng/ml recombinant human interleukin (IL)-6 (PeproTech, Inc., Rocky Hill, NJ, USA). Recombinant human IL-3 (30 ng/ml) was added for the first week. At 8–10 weeks, cultures consisted of >99% HuMC (10).
Degranulation assay
Cells were stimulated with codeine (Sigma-Aldrich, St Louis, MO, USA) in HEPES buffer [10 mM HEPES, 137 mM NaCl, 2.7 mM KCl, 0.38 mM Na2HPO4·7H2O, 5.6 mM glucose, 1.8 mM CaCl2·2H2O, 1.3 mM MgSO4·7H2O, 0.04% bovine serum albumin (BSA), pH 7.4] or Ca2+-free HEPES buffer (containing all except CaCl2) and incubated at 37°C for 0.5 h. The β-hexosaminidase in the supernatants and cell lysates was quantified by hydrolysis of p-nitrophenyl-N-acetyl-β-d-glucosamide (Sigma-Aldrich) in 0.1 M sodium citrate buffer (pH 4.5) for 90 min at 37°C. The percentage of β-hexosaminidase release was calculated as a percent of total content. In some instances, the LAD2 cells were pretreated with 100 nM fluticasone propionate (Sigma) for 20 h. Fluticasone propionate was dissolved in dimethyl sulfoxide (DMSO) to produce a stock solution of 1 mM. Mast cell degranulation was unaffected by 0.01% or 0.1% DMSO (vehicle).
Inhibitors
Signaling molecule inhibitors were all obtained from Sigma-Aldrich and used at the indicated concentration; H89, 10 μM (protein kinase A inhibitor), wortmannin, 10 μM [phosphoinositol-3 kinase (PI3K) inhibitor], Ro-31-8220 methane-sulfonate salt, 10 μM (PKC inhibitor), forskolin, 5 μM (AC activator), SQ 22,536, 1.0 μM (AC blocker), pertussis toxin, 0.1 μM (G-protein inhibitor), and opioid receptor antagonists naloxone, 10 μM and naltrexone 10 μM were tested.
LAD2 cells were pretreated with inhibitors for 10 min or with pertussis toxin for 4 h. Cells were then stimulated with codeine (0, 0.01, 0.1, 1, 2.5, and 5 μg/ml) for 30 min and β-hexosaminidase release was measured (n = 3). Time-course studies of stimulation with codeine were also conducted with times ranging from 0, 5, 10, 20 to 30 min.
CBA assay for cytokines
The following chemokines, IL-8, regulated on activation, normal T expressed and secreted (RANTES), monokine induced by IFN-γ (MIG), monocyte chemoattractant protein (MCP)-1, and inducible protein (IP)-10, were measured, and the lower limit of detection was 10 pg/ml. Cells were washed with media and suspended at 1 × 106 cells per well, then stimulated with codeine for 3, 8, and 16 h. Cell-free supernatants were isolated and analyzed for human cytokine and chemokine expression using the Human Chemokine Kit I cytometric bead array kit (BD Biosciences, San Diego, CA, USA).
Enzyme-linked immunoassay for cytokines, chemokines, and cysteinyl leukotrienes
Cells were washed with media and suspended at 1 × 106 cells per well, then stimulated with codeine for 3, 8, and 16 h. Cell-free supernatants were isolated and analyzed for human cytokine and chemokine expression using the following commercial enzyme-linked immunoassay (ELISA) kits; granulocyte-macrophage colony-stimulating factor (GM-CSF), macrophage inflammatory protein–1α (MIP-1α), tumor necrosis factor (TNF)-α, IL-8, IL-6 (according to the manufacturer's instructions; R&D Systems, Minneapolis, MN, USA), and CysLT (Cayman Chemcial, Ann Arbor, MI, USA). The lower limits of detection, respectively, are 7.8, 31.2, 15.6, 31.2, 3.12, and 13 pg/ml. In some cases, cells were preincubated with signaling inhibitors UO126 [ERK mitogen-activated protein (MAP) kinase inhibitor, 1 μM], SB203580 (p38 inhibitor; 50 μM), or SP600125 (JNK inhibitor; 30 μM) for 30 min prior to activation (all inhibitors were purchased from Calbiochem, La Jolla, CA, USA).
Real-time polymerase chain reaction analysis
Total RNA was isolated from each preparation using the RNeasy Mini Kit (Qiagen Inc., Valencia, CA, USA). One microgram of total cellular RNA was reverse transcribed using Invitrogen Reverse Transcription reagents and oligo dT. Gene expression was analyzed using real-time polymerase chain reaction (RT-PCR) on an ABI7500 SDS system. About 100 ng of cDNA was used in each quantitative PCR assay. Primer sets for PCR amplifications were designed using the primer express software (Applied Biosystems, Foster City, CA, USA). Probes and primers were synthesized from Applied Biosystem and Integrated DNA Technologies (Coralville, IA, USA) (Table 1). All reactions were performed in triplicate for 40 cycles as per the manufacturer's recommendation. Samples were normalized using the geometric mean of GAPDH15 and data are reported as the ratio of treated cells/untreated control cells. Different stocks of cDNA were used for all amplifications to determine the relative quantities across multiple runs.
Table 1.
Sequences of primers and probes used for real-time PCR analysis of cytokine and chemokine expression
| Gene | Forward primer | Reverse primer | Probe |
|---|---|---|---|
| MCP-1 | 5′-GCTATAGAAGAATCACCAGCAGCAA-3′ | 5′-TGGCCACAATGGTCTTGAAG-3′ | TGTCCCAAAGAAGCTGT |
| MIG | 5′-GCAAGGAACCCCAGTAGTGAGA-3′ | 5′-CCCTTGGTTGGTGCTGATG-3′ | AGGGTCGCTGTTCC |
| IL-6 | 5′-CCAGTACCCCCAGGAGAAGATT-3′ | 5′-TCGAGGATGTACCGAATTTGTTT-3′ | ACACAGACAGCCACTCA |
| IP-10 | 5′-TGAAATTATTCCTGCAAGCCAAT-3′ | 5′-ATGGCCTTCGATTCTGGATTC-3′ | TCCACGTGTTGAGATC |
| IFN-γ | 5′-TGTCCAACGCAAAGCAATACA-3′ | 5′-GTTTTAGCTGCTGGCGACAGT-3′ | TCATCCAAGTGATGGCT |
| GM-CSF | 5′-AGGGCCCCTTGACCATGA-3′ | 5′-CAAAGGTGATAGTCTGGTTGCA-3′ | CAGCACTGCCCTCCAACCCCG |
| RANTES | 5′-TCGCTGTCATCCTCATTGCTA-3′ | 5′-GCACTTGCCACTGGTGTAGAAA-3′ | CTGGGACACCACACCCTGCTGC |
| IL-8 | 5′-CTGGCCGTGGCTCTCTTG-3′ | 5′-TTGGCAAAACTG TTTAGCACTCC-3′ | CAGCCTTCCTGATTTCTGCAGCTCTGTGT |
| MIP-1α | 5′-CTACACCTCCCGGCAGATTC-3′ | 5′-CCGGCTTCGCTTGGTTAG-3′ | CAGTGCTCCAAGCCCGGTGTCATC |
| GAPDH | 5′-GAGGTGAAGGTCGGAGTC-3′ | 5′-GAAGATGGTGATGGGATTTC-3′ | CAAGCTTCCCGTTCTCAGCC |
MCP, monocyte chemoattractant protein; IL, interleukin; IP, inducible protein; GM-CSF, granulocyte-macrophage colony-stimulating factor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IFN, interferon; RANTES, regulated on activation, normal T expressed and secreted; PCR, polymerase chain reaction.
Semi-quantitative PCR analysis
Total cellular RNA was isolated and reverse transcribed as above. PCR amplification of cDNA or genomic DNA was performed on a Peltier Thermal Cycler System (Model #PTC-220, Bio-Rad, Hercules, CA, USA). About 100 ng of cDNA was used in each PCR assay using Invitrogen reagents. Thirty-five cycles of PCR amplification were performed using optimized annealing temperatures. Opioid receptor primers μ, δ, and κ were designed using the primer express software (Perkin-Elmer Applied Biosystems). Primers were custom synthesized by Integrated DNA Technologies (Table 2). Amplification products were analyzed by agarose gel electrophoresis and visualized by ethidium bromide staining using Quantity 1-D analysis software/system (Bio-Rad).
Table 2.
Sequence of primers used for semi-quantitative polymerase chain reaction analysis of opioid receptor expression
| Opioid receptor – κ |
| Forward: 5′-GGCTTTGGCAGATGCTTTAG-3′ |
| Reverse: 5′-TGCAAGGAGCACTCAATGAC-3′ |
| Opioid receptor – δ |
| Forward: 5′-CATCCACATCTTCGTCATCG-3′ |
| Reverse: 5′-AAGCAGCGCTTGAAGTTCTC-3′ |
| Opioid receptor – μ |
| Forward: 5′-GAAAAGTCTCGGTGCTCCTG-3′ |
| Reverse: 5′-GATGGAGTAGAGGGCCATGA-3′ |
Preparation of protein lysates
Cells were washed with HEPES buffer containing BSA (0.04%) and resuspended at 5 × 105 cells/90 μl in the same buffer and activated at 37°C by the addition of 10 μl of codeine (2.5 μg/ml) for 0, 5, 10, 15, 20, 25, and 30 min. Cells were then lysed by the addition of 100 μl of protease inhibitor/phosphatase inhibitor mixture. The cell suspensions were passed through a 20-gauge needle.
Western blotting
Samples were loaded onto a 4–15% Tris-Hcl polyacrylamide gel (Bio-Rad) and transferred onto a nitrocellulose membrane (Bio-Rad). Membrane was blocked in blocking buffer (Bio-Rad) for an hour, then incubated with primary antibody pERK, pJNK, and pp38. Membranes were washed and incubated with Alexa Fluor 680 goat anti-rabbit IgG (H + L) from Invitrogen for an hour. The membranes were then washed three times with TBS-Tween and visualized with the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA).
Statistical analysis
Each experiment was performed at least three separate times and in quadruplicate and values displayed represents mean ± SEM. P-values were determined by Student's t-test (between groups).
Results
Codeine activates human mast cell degranulation
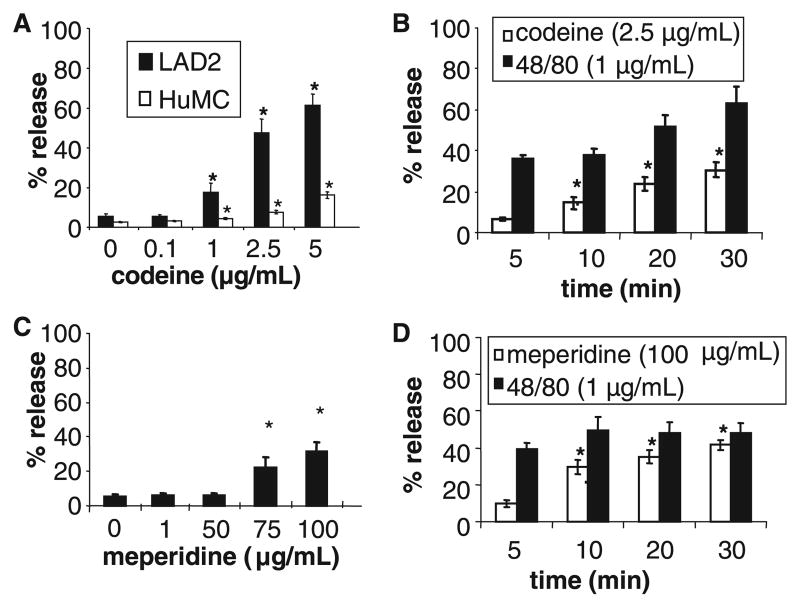
LAD2 human mast cells and HuMC CD34+ progenitor-derived mast cells were stimulated with codeine and meperidine and mast cell degranulation was measured. Codeine stimulated LAD2 mast cell degranulation in a concentration-dependent manner reaching maximal degranulation of 60% with 5.0 μg/ml of codeine (Fig. 1A). Codeine-induced degranulation was relatively slow compared to that induced by compound 48/80 (Fig. 1B). Whereas compound 48/80 induced 40% release after 5 min of stimulation, codeine induced only 7% release after 5 min of stimulation. The synthetic opioid, meperidine, did not induce significant LAD2 or HuMC degranulation at concentrations up to 50 μg/ml (Fig. 1C). At higher concentrations (75 and 100 μg/ml), meperidine induced over 30% degranulation by LAD2 cells. Higher concentrations of meperidine (100 μg/ml) induced maximum mast cell degranulation within 5 min of incubation (Fig. 1D).
Figure 1.
(A) Codeine causes degranulation by HuMC CD34+-derived cells and LAD2 cells. HuMC and LAD2 cells were stimulated with codeine for 30 min and β-hexosaminidase release was measured (n = 3). (B) LAD2 cells were stimulated with 2.5 μg/ml codeine or 1 μg/ml compound 48/80 for times indicated and β-hexosaminidase release was measured (n = 3). (C) LAD2 were stimulated with meperidine at indicated concentrations for 30 min and β-hexosaminidase release was measured (n = 3). (D) LAD2 were stimulated with 100 μg/ml meperidine or 1 μg/ml compound 48/80 for indicated times and β-hexosaminidase release was measured (n = 3, *P < 0.05).
Codeine activation of mast cell degranulation is partially calcium dependent and corticosteroid sensitive
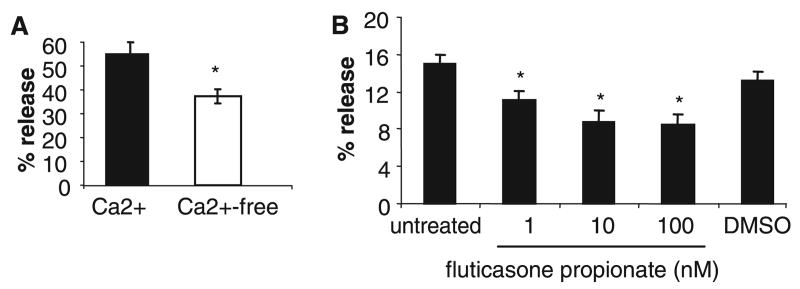
Some reports have suggested that codeine activation of human mast cells may be calcium independent. For example, the oral administration of calcium channel blockers does not affect the size of codeine-induced skin reactions in normal volunteers (11). Therefore, we activated human mast cells with codeine in Ca2+-free and Ca2+-containing (HEPES buffer; 1.8 mM Ca2+) buffer. Mast cells stimulated with codeine in Ca2+-free buffer degranulated only 37.4% compared with mast cells stimulated in Ca2+-containing buffer (54.3%; Fig. 2A).
Figure 2.
Codeine-induced mast cell degranulation is partially calcium and glucocorticoid sensitive. (A) LAD2 were stimulated with codeine (2.5 μg/ml) in Ca2+-free or Ca2+-containing (1.8 mM) media for 30 min and β-hexosaminidase release was measured (n = 3). LAD2 were pretreated with 1–100 nM fluticasone propionate, or dimethyl sulfoxide (DMSO) control (0.1%) for 20 h then stimulated with codeine (1 μg/ml) and β-hexosaminidase release was measured (n = 3, *P < 0.05).
It has been reported that skin prick responses to codeine but not histamine are decreased in some patients with asthma who had been receiving prolonged corticosteroid therapy (12) implying that codeine activation of human mast cells may be corticosteroid sensitive. Therefore, we pretreated human mast cells with fluticasone propionate (1–100 nM) for 20 h, then stimulated with codeine. Fluticasone pretreatment reduced human mast cell degranulation dose dependently (Fig. 2B). At 1 nM, fluticasone inhibited degranulation by approximately 18% (Fig. 2B). By comparison, the DMSO (0.1%) vehicle control did not affect mast cell degranulation.
Codeine activates human mast cell cytokine and chemokine production
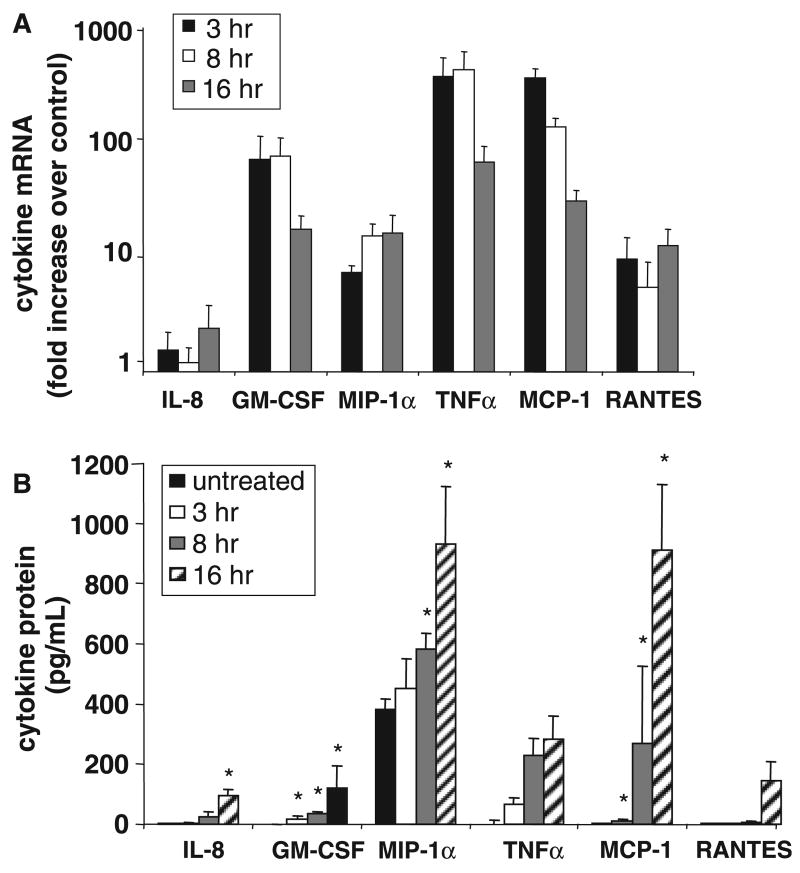
We further measured the expression of cytokines and chemokines after 3, 8, and 16 h of codeine stimulation by RT-PCR (Fig. 3A). Expression of TNF, GM-CSF, RAN-TES, and MIP-1α increased considerably following codeine stimulation but expression of IL-8 and IL-6 increased only modestly with codeine stimulation. Protein production of these mediators was confirmed using cytometric bead array. Codeine induced production of IL-8, GM-CSF, TNF, MCP-1, and RANTES. LAD2 cells constitutively made high levels of MIP-1α and increased production further after codeine stimulation (Fig. 3B).
Figure 3.
Codeine stimulation activates cytokine and chemokine production. (A) LAD2 cells were stimulated with codeine (1.0 μg/ml) for 3, 8, and 16 h and interleukin (IL)-6, IL-8, tumor necrosis factor (TNF), interferon-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), regulated on activation, normal T expressed and secreted (RANTES), and MIP-1α expression was measured by real-time polymerase chain reaction (n = 3). Data were normalized to glyceraldehyde-3-phosphate dehydrogenase mRNA levels and is expressed as fold increase over unstimulated control. (B) LAD2 cells were stimulated with codeine (1.0 μg/ml) for 3, 8, and 16 h and IL-8, GM-CSF, MIP-1α, IL-6, TNF, inducible protein-10, monocyte chemoattractant protein-1, MIG, and RANTES production was measured by cytometric bead array (CBA) and ELISA (n = 3, P < 0.05). Cytokine production by unstimulated (vehicle control) cells at each time point was the same as spontaneous release at time zero (untreated).
Codeine activation of mast cell degranulation is G-protein and PI3 kinase dependent
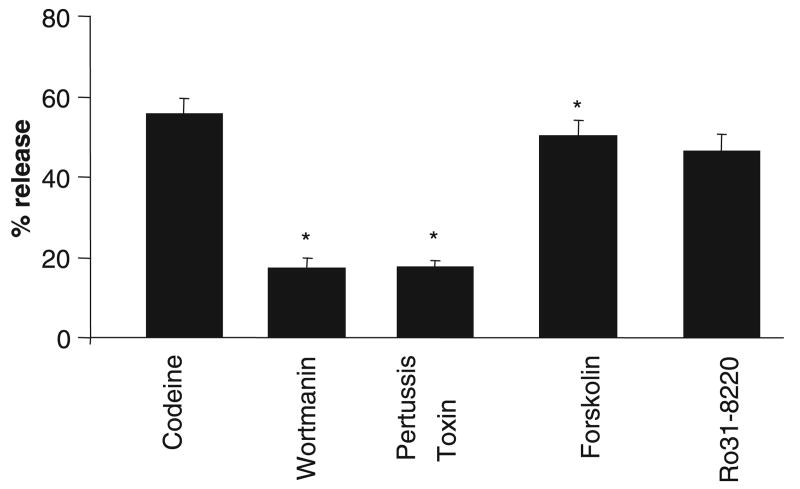
To determine the pathways involved in codeine-induced human mast cell degranulation, LAD2 cells were pretreated with wortmannin (PI3K inhibitor), pertussis toxin (G-protein inhibitor), forskolin (adenylate cyclase activator), or Ro-31-8220 (PKC inhibitor) prior to activation with codeine. Wortmannin and pertussis toxin inhibited mast cell degranulation by more than 60%, whereas forskolin and Ro-31-8220 had no effect (Fig. 4).
Figure 4.
Codeine-induced mast cell degranulation is protein kinase A and PI3K dependent. LAD2 cells were pretreated with vehicle (0.1% dimethyl sulfoxide), H89 (1 μM), SQ222536 (10 μM), Ro-31-8220 (100 nM), forskolin (10 μM), wortmannin (10 μM), or pertussis toxin (3.5 nM), stimulated with codeine (5 μg/ml) for 30 min and β-hexosaminidase release was measured (n = 3, P < 0.05).
Codeine activates MAP kinases
Cytokine and chemokine production by human mast cells induced by IgE and antigen is dependent upon activation of MAP kinases (13). We measured MAP kinase phosphorylation in human mast cells following codeine stimulation to determine the role of MAP kinases. LAD2 cells were stimulated with 2.5 μg/ml of codeine for 5–30 min and phosphorylation of ERK, JNK, and p38 was measured using Western blot. After 5 min of stimulation, codeine activated ERK and JNK phosphorylation (Fig. 5A). However, p38 phosphorylation could not be detected even after 30 min of stimulation with codeine. By comparison, interferon-γ (10 ng/ml) treatment activated p38 phosphorylation (data not shown; 14). We further determined whether codeine-induced MIP-1α production was sensitive to inhibitors of MAP kinases. The ERK kinase inhibitor, U0126, inhibited codeine-induced MIP-1α production by approximately 20%, whereas p38 and JNK inhibitors (SB203580 and SP6) had no effect (Fig. 5B). The inhibitors alone had no effect on MIP-1α production. These data suggest that ERK is involved in codeine activation on human mast cells.
Figure 5.
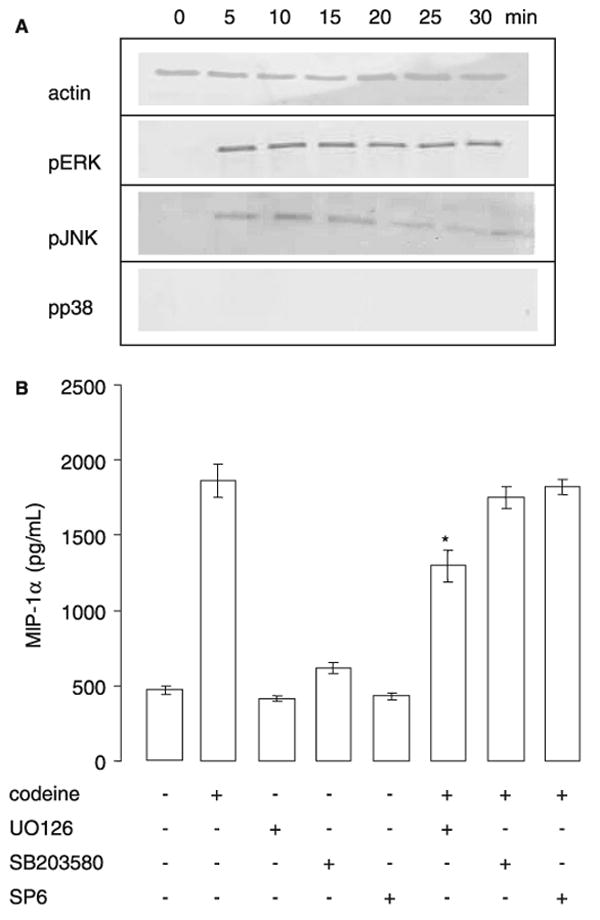
Codeine activates mitogen-activated protein kinase activation. (A) LAD2 cells were stimulated with codeine 2.5 μg/ml for 5, 10, 15, 20, 25, or 30 min and cell lysates were examined for the presence of phosphorylated ERK, JNK, and p38. Blots were stripped and reprobed with anti-actin mAb to monitor loading (representative of n = 3). (B) LAD2 cells were pretreated with U0126 (1 μM), SB203580 (50 μM), or SP600125 (30 μM) for 30 min, then stimulated with codeine and β-hexosaminidase release was measured (n = 3).
Human mast cells express opioid receptors
To characterize opioid receptor expression by human mast cells, we tested cells for the presence of opioid receptor mRNA by semi-quantitative RT-PCR. HuMC and LAD2 cells expressed mRNA for μ- and δ-opioid receptors (Fig. 6A). However, neither mast cell type expressed mRNA for the κ-opioid receptor.
Figure 6.
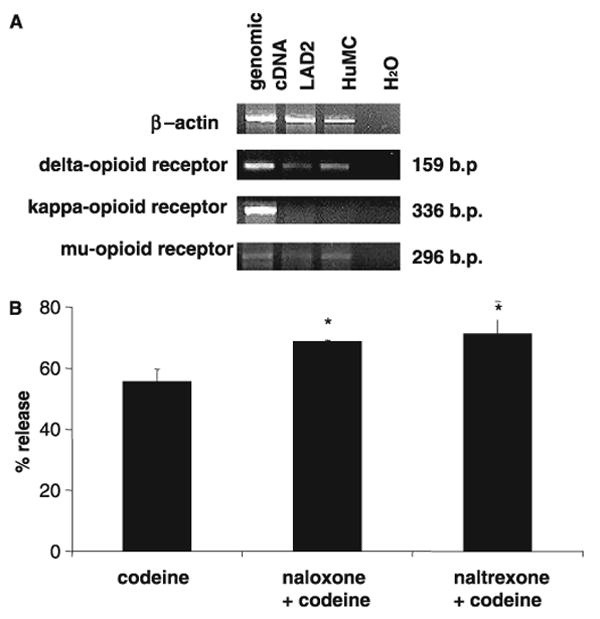
Human mast cells express opioid receptors. (A) Total RNA was isolated from LAD2 and HuMC and δ-, κ- and μ-opioid receptors expression was evaluated by semi-quantitative real time polymerase chain reaction. β-Actin is shown as a positive control. Total human genomic cDNA was used as a positive control and H2O was used as a negative control of receptor expression. (B) Laboratory of Allergic Diseases were pretreated with naloxone (10 μM) or naltrexone (10 μM) for 30 min, then stimulated with codeine (2.5 μg/ml) for 30 min and β-hexosaminidase release was measured (n = 3).
To determine if codeine activation was opioid receptor dependent, we pretreated LAD2 cells with naloxone and naltrexone (μ-receptor antagonists) prior to activation with codeine. Neither of these antagonists inhibited codeine-induced human mast cell degranulation (Fig. 6B) and, in fact, codeine stimulated degranulation was enhanced by both naloxone and naltrexone.
Discussion
Codeine and its close relative morphine are classified as natural products and have been found to be more likely to cause an allergic reaction than synthetic analogs such as meperidine (15). Our study shows that codeine activated human mast cell degranulation and chemokine and cytokine production, whereas meperidine had no effect. Previous literature and case reports have suggested that codeine activation of mast cells is IgE-mediated (5). However, as we used cells that were not sensitized, our study has shown that codeine activation of human mast cells did not require IgE and activated signaling pathways that are FcεRI independent.
The more frequent adverse effects of codeine administration are ataxia, miosis, swelling, nausea and vomiting, drowsiness, and dizziness. Pruritus and urticaria associated with codeine administration are thought to be caused by non-IgE-mediated induction of histamine release (15). Codeine exposure has also been related to maculopapular eruptions, angioneurotic edema, fixed drug eruption, bullous eruption, erythema multiforme, erythema nodosum, scarlatiniform rashes, or toxic epidermal necrolysis (2). Contact dermatitis from opium derivatives in pharmaceutical workers, nurses, and physicians has also been described (2). These symptoms imply that codeine may not only activate mast cell degranulation, but may also induce the recruitment of other inflammatory cells. Our data demonstrate that codeine activates acute mast cell degranulation as well as a more prolonged activation that activates the production of chemokines and cytokines such as IL-8, GM-CSF, TNF, MCP-1, and RANTES. These cytokines and chemokines can recruit and activate inflammatory cells such as T cells, dendritic cells, and neutrophils, all of which may contribute to the pathology of codeine sensitivity. Although codeine activated the phosphorylation of both JNK and ERK MAP kinases, the production of MIP-1α in response to codeine was only partially dependent on ERK MAP kinase activation.
Although the opiate codeine was a potent inducer of mast cell degranulation and cytokine/chemokine production, the opioid meperidine was not. Cross-sensitization among opium alkaloids due to delayed-type hypersensitivity has been described by de Groot and Conemans (16). In some patients, cross-reactivity occurs between codeine and morphine as these two opiates have a very similar chemical structure (15). Meperidine is chemically unrelated to codeine and, as such, patients sensitized to codeine do not react to meperidine. The responses that we describe were not IgE-dependent and therefore not dependent on IgE recognition of an epitope on the codeine or meperidine molecules. Our data show that opiates can activate human mast cells in the absence of IgE, suggesting another mechanism of activation, possibly via the opioid receptor.
Opiates/opioids bind to three types of opioid receptors: mu (υ), delta (δ), and kappa (κ). Receptor ligation activates intracellular signaling via G-proteins, ion channel modulation, and MAP kinase activation (17). Activation of all three receptors lead to downstream decreases in neurotransmitter release and nociceptive impulse propagation in nerve cells. Codeine preferentially binds to the μ-receptor. Our study has shown that human mast cells express mRNA for δ- and μ- but not the κ-opioid receptor. Furthermore, human mast cell degranulation is not blocked by either naloxone or naltrexone suggesting that codeine activation of human mast cells is not mediated by the μ-opioid receptor. Activation of MC degranulation may therefore occur via the δ-opioid receptor. It is also possible that codeine directly activates G-proteins as has been demonstrated for other mast cell basic secretagogues such as substance P (18) or compound 48/80 (19).
In conclusion, our studies show that codeine activation of human mast cells not only activates degranulation and release of preformed granule contained mediators, but can also induce the production of cytokines and chemokines including GM-CSF, TNF, MCP-1, MIP-1α, RANTES, and IL-8. In comparison, meperidine, a synthetic analog of codeine, did not activate human mast cell mediator production at the same doses. Codeine-induced release of MC mediators can recruit and activate T lymphocytes, eosinophils, and other inflammatory cells possibly contributing to some of the nonurticarial opioid reactions. These activation pathways were dependent on ERK MAP kinase activation and were partially blocked by ERK kinase inhibitors. These data may prove useful in the treatment of individuals sensitive to codeine and its derivatives.
Acknowledgments
We would like to thank Leslie C. Grammer for her assistance in the preparation of this manuscript.
This work was supported by The Ernest S. Bazely Trust and grants R01HL068546 and R01HL078860 from the National Institutes of Health.
Abbreviations
- DMSO
dimethyl sulfoxide
- FcεRI
Fc epsilon receptor 1
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HuMC
human CD34+-derived mast cells
- IL-8
interleukin-8
- IP-10
inducible protein-10
- LAD
Laboratory of Allergic Diseases cells
- MAP
mitogen-activated protein
- MCP-1
monocyte chemoattractant protein-1
- PI3 kinase
phosphoinositide-3 kinase
- PKA
protein kinase A
- RANTES
regulated on activation, normal T expressed and secreted
- TNF
tumor necrosis factor
References
- 1.Edston E, Hage-Hamsten M. Anaphylactoid shock – a common cause of death in heroin addicts? Allergy. 1997;52:950–954. doi: 10.1111/j.1398-9995.1997.tb01256.x. [DOI] [PubMed] [Google Scholar]
- 2.Golembiewski JA. Allergic reactions to drugs: implications for perioperative care. J Perianesth Nurs. 2002;17:393–398. doi: 10.1053/jpan.2002.36669. [DOI] [PubMed] [Google Scholar]
- 3.Lin RY, Erlich ER, Don PC. Skin prick test responses to codeine, histamine, and ragweed utilizing the multitest device. Ann Allergy. 1990;65:222–226. [PubMed] [Google Scholar]
- 4.Grosman N. Histamine release from isolated rat mast cells: effect of morphine and related drugs and their interaction with compound 48/80. Agents Actions. 1981;11:196–203. doi: 10.1007/BF01967614. [DOI] [PubMed] [Google Scholar]
- 5.Harle DG, Baldo BA, Coroneos NJ, Fisher MM. Anaphylaxis following administration of papaveretum. Case report: implication of IgE antibodies that react with morphine and codeine, and identification of an allergenic determinant. Anesthesiology. 1989;71:489–494. [PubMed] [Google Scholar]
- 6.Casale TB, Bowman S, Kaliner M. Induction of human cutaneous mast cell degranulation by opiates and endogenous opioid peptides: evidence for opiate and nonopiate receptor participation. J Allergy Clin Immunol. 1984;73:775–781. doi: 10.1016/0091-6749(84)90447-0. [DOI] [PubMed] [Google Scholar]
- 7.Blunk JA, Schmelz M, Zeck S, Skov P, Likar R, Koppert W. Opioid-induced mast cell activation and vascular responses is not mediated by mu-opioid receptors: an in vivo microdialysis study in human skin. Anesth Analg. 2004;98:364–370. doi: 10.1213/01.ANE.0000097168.32472.0D. [DOI] [PubMed] [Google Scholar]
- 8.Tharp MD, Kagey-Sobotka A, Fox CC, Marone G, Lichtenstein LM, Sullivan TJ. Functional heterogeneity of human mast cells from different anatomic sites: in vitro responses to morphine sulfate. J Allergy Clin Immunol. 1987;79:646–653. doi: 10.1016/s0091-6749(87)80162-8. [DOI] [PubMed] [Google Scholar]
- 9.Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, et al. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res. 2003;27:677–682. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 10.Kulka M, Metcalfe DD. High-resolution tracking of cell division demonstrates differential effects of TH1 and TH2 cytokines on SCF-dependent human mast cell production in vitro: correlation with apoptosis and Kit expression. Blood. 2005;105:592–599. doi: 10.1182/blood-2004-07-2838. [DOI] [PubMed] [Google Scholar]
- 11.Shalit M, Rubinow A, Granit L, Bar-Sela S. Codeine-induced mast cell degranulation in human skin: effect of calcium channel blockers. Ann Allergy. 1987;59:461–463. [PubMed] [Google Scholar]
- 12.Olson R, Karpink MH, Shelanski S, Atkins PC, Zweiman B. Skin reactivity to codeine and histamine during prolonged corticosteroid therapy. J Allergy Clin Immunol. 1990;86:153–159. doi: 10.1016/s0091-6749(05)80060-0. [DOI] [PubMed] [Google Scholar]
- 13.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Kulka M, Dery R, Nahirney D, Duszyk M, Befus D. Differential regulation of CFTR by interferon-{gamma} in mast cells and epithelial cells. J Pharmacol Exp Ther. 2005;315:563–570. doi: 10.1124/jpet.105.087528. [DOI] [PubMed] [Google Scholar]
- 15.Nasser SM, Ewan PW. Opiate-sensitivity: clinical characteristics and the role of skin prick testing. Clin Exp Allergy. 2001;31:1014–1020. doi: 10.1046/j.1365-2222.2001.01090.x. [DOI] [PubMed] [Google Scholar]
- 16.de Groot AC, Conemans J. Allergic urticarial rash from oral codeine. Contact Dermatitis. 1986;14:209–214. doi: 10.1111/j.1600-0536.1986.tb01226.x. [DOI] [PubMed] [Google Scholar]
- 17.Sharp BM. Multiple opioid receptors on immune cells modulate intracellular signaling. Brain Behav Immun. 2006;20:9–14. doi: 10.1016/j.bbi.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Mousli M, Bronner C, Landry Y, Bockaert J, Rouot B. Direct activation of GTP-binding regulatory proteins (G-proteins) by substance P and compound 48/80. FEBS Lett. 1990;259:260–262. doi: 10.1016/0014-5793(90)80023-c. [DOI] [PubMed] [Google Scholar]
- 19.Palomaki VA, Laitinen JT. The basic secretagogue compound 48/80 activates G proteins indirectly via stimulation of phospholipase D-lysophosphatidic acid receptor axis and 5-HT1A receptors in rat brain sections. Br J Pharmacol. 2006;147:596–606. doi: 10.1038/sj.bjp.0706671. [DOI] [PMC free article] [PubMed] [Google Scholar]