Abstract
Although excess fat mass is broadly linked to increased cardiovascular risk, the relation between vascular phenotype and degree of obesity in extreme weight categories is unknown. We examined brachial artery vasomotor responses using ultrasound in 203 consecutive patients mainly afflicted with severe obesity (mean age 44 ± 11 yr; body mass index (BMI) 46 ± 9 kg/m2, range 30–72 kg/m2; and body weight 128 ± 29kg, range 69–207 kg). We studied a unique population with >70% of subjects characterized as morbidly obese (BMI ≥ 40) including a 31% group of super-obese individuals (BMI ≥ 50). Brachial artery flow-mediated dilation (FMD) and nitroglycerin-mediated dilation (NMD) were examined as measures of endothelium-dependent and -independent dilation, respectively, in relation to clinical, hemodynamic, and metabolic parameters. Endothelial function was significantly impaired in the highest as compared to lowest tertile of body weight (FMD 6.5 ± 4.6 vs. 9.8 ± 4.8%, p<0.001), whereas NMD was similar in all groups. Univariate correlates of FMD were gender, weight, waist circumference, BMI, diastolic blood pressure, and creatinine. In multivariate analysis, weight was a strong independent significant predictor of FMD (β= −0.23, p=0.005) in addition to gender. Within an overweight population, cumulative weight burden remains strongly linked to progressive arterial dysfunction. In conclusion, these results suggest that cardiovascular risks intensify with escalating obesity, and underscore the importance of therapeutic weight loss interventions in the context of the expanding obesity epidemic.
Keywords: endothelium, obesity, vasculature
Introduction
While obesity is broadly linked to impaired vasoreactivity when compared to lean populations, the relation between degree of obesity and vascular phenotype across a wide range of individuals with excess fat remains unknown.1 In addition, whether vascular dysfunction progresses or reaches a plateau as body weight increases throughout the extremely obese range remains an open question. Thus, the purpose of this study was to examine the relationship between arterial dilator responses and overall weight burden in expressly overweight individuals.
Methods
We enrolled 216 consecutive obese men and women (BMI ≥ 30 kg/m2; range 30–72 kg/m2), age ≥18 years, from 2002 to 2006 receiving care at the Boston Medical Center Nutrition and Weight Management Center. This high-volume ambulatory center provides outpatient comprehensive dietary, medical, behavioral, or surgical treatments to promote lifestyle modification and weight loss. Patients with unstable medical conditions such as active coronary syndromes, heart failure, systemic infection, malignancy, or pregnancy were excluded. All subjects gave written, informed consent and the study was approved by the Boston Medical Center Institutional Review Board. A significant portion of individuals in this specialized ambulatory clinic exhibited extreme obesity, as 71% of the study population was morbidly obese (class 3 obese, BMI ≥ 40) and 31% of total subjects were super-obese (BMI ≥ 50).
Ultrasound studies were performed in a temperature-controlled room with subjects lying supine in a fasting state. Studies were performed during a weight-stable period prior to initiation of any weight loss intervention. Trained sonographers examined brachial artery vasomotor responses using a noninvasive, standardized method of ultrasound imaging as previously described, using a Toshiba Powervision 6000 system.2,3 Flow-mediated dilation (FMD) and nitroglycerin-mediated dilation (NMD) of the brachial artery were examined as measures of endothelium-dependent and -independent dilation, respectively. Brachial artery FMD responses were examined following a 5-minute cuff occlusion in an upper arm position above the antecubital crease. Pulsed-Doppler flow velocity signals at baseline and after cuff deflation provided measures of reactive hyperemia. Sublingual nitroglycerin (0.4 mg) was omitted if the subject declined or had a history if migraines, blood pressure <100 mmHg, previous adverse reaction to nitrates, or used phosphodiesterase type-5 medications. An investigator blinded to clinical information performed all off-line analyses of digitized end-diastolic images. 203 subjects had technically analyzable ultrasound data and were included in the analyses.
Concomitant with the vascular studies, clinical characteristics along with blood pressure, heart rate, height, weight, BMI, and waist circumference were recorded for each subject. Biochemical analyses included lipids, glucose, insulin, homeostasis model assessment of insulin resistance (HOMA-IR), hemoglobin A1c, and renal function were quantified from blood samples collected in a fasting state.
Analyses were completed using SPSS for Windows, version 12.1 (SPSS Inc.). Data are presented as mean ± SD, unless otherwise indicated. Correlations between vascular parameters and clinical or biochemical data were examined using linear regression analysis. Examined variables included age, gender, weight, BMI, waist circumference, heart rate, blood pressure, lipids, glucose, insulin, HOMA-IR, hemoglobin A1c, creatinine, FMD, NMD, hyperemic flow, history of diabetes, hypertension, coronary artery dieasease, and smoking. Univariate correlates of vascular function (p<0.05) were entered into a stepwise multiple regression analysis to identify independent predictors of FMD. Clinical and vascular parameters were compared among tertiles of body weight using analysis of variance (ANOVA) or chi-square tests, as appropriate. Data were categorized and presented in tertiles of body weight based on independent correlations between weight and flow-mediated dilation.
Results
A total of 203 patients (mean age 44 ± 11 yr, 80% female) completed the study. All subjects were obese with average BMI 46 ± 9 kg/m2 (range 30–72 kg/m2), total body weight 128 ± 29 kg (69–207 kg) and waist circumference 130 ± 19 cm (94–180 cm). As expected in this demographic group, nearly half of the patients had hypertension and approximately one third was diabetic. Using a cut-point HOMA-IR value of 1.7, 74% of the study population exhibited insulin resistance.4,5 Patient characteristics displayed in tertiles of body weight categories are shown in Table 1. Adiposity measures including BMI and waist circumference increased with rising tertiles of weight status (p<0.001 by ANOVA). Other clinical parameters that varied significantly between groups included blood pressure, plasma insulin, gender, and smoking status.
Table 1.
Subjects characteristics by weight tertile
| Variable | <113 kg (n = 67) | 113–137 kg (n = 67) | >137 kg (n = 69) | P |
|---|---|---|---|---|
| Age (years) | 46 ± 12 | 45 ± 11 | 41 ± 11 | 0.055 |
| Weight (kg) | 98 ± 11 | 124 ± 7 | 161 ± 17 | <0.001 |
| Body Mass Index (kg/m2) | 38 ± 4 | 45 ± 5 | 55 ± 7 | <0.001 |
| Waist circumference (cm) | 114 ± 10 | 129 ± 9 | 150 ± 15 | <0.001 |
| Systolic blood pressure (mm Hg) | 127 ± 14 | 135 ± 14 | 127 ± 15 | 0.002 |
| Diastolic blood pressure (mm Hg) | 71 ± 9 | 75 ± 11 | 72 ± 12 | 0.103 |
| Total cholesterol (mg/dl) | 193 ± 38 | 194 ± 37 | 188 ± 41 | 0.636 |
| Low density lipoprotein cholesterol (mg/dl) | 115 ± 32 | 112 ± 31 | 112 ± 37 | 0.883 |
| High density lipoprotein cholesterol (mg/dl) | 51 ± 12 | 50 ± 16 | 46 ± 11 | 0.058 |
| Triglycerides (mg/dl) | 134 ± 61 | 163 ± 103 | 152 ± 104 | 0.197 |
| Glucose (mg/dl) | 109 ± 37 | 113 ± 47 | 104 ± 21 | 0.428 |
| Insulin (μU/mL) | 16 ± 14 | 15 ± 12 | 21 ± 11 | 0.030 |
| HOMA-IR | 3.5 ± 3.3 | 3.9 ± 6.6 | 4.3 ± 3.0 | 0.725 |
| Heart rate (beats per minute) | 71 ± 10 | 73 ± 11 | 73 ± 13 | 0.351 |
| Creatinine (mg/dl) | 0.7 ± 0.1 | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.134 |
| Hemoglobin A1c (%) | 6.2 ± 1.1 | 6.5 ± 1.6 | 6.1 ± 1.0 | 0.283 |
| Men | 8% | 15% | 38% | <0.001 |
| Smoker | 48% | 24% | 48% | 0.011 |
| Hypertension | 36% | 48% | 48% | 0.469 |
| Diabetes mellitus | 30% | 30% | 29% | 0.993 |
| Angiotensin converting enzyme inhibitor use | 13% | 19% | 17% | 0.642 |
| HMGCo-A reductase inhibitor use | 27% | 21% | 17% | 0.400 |
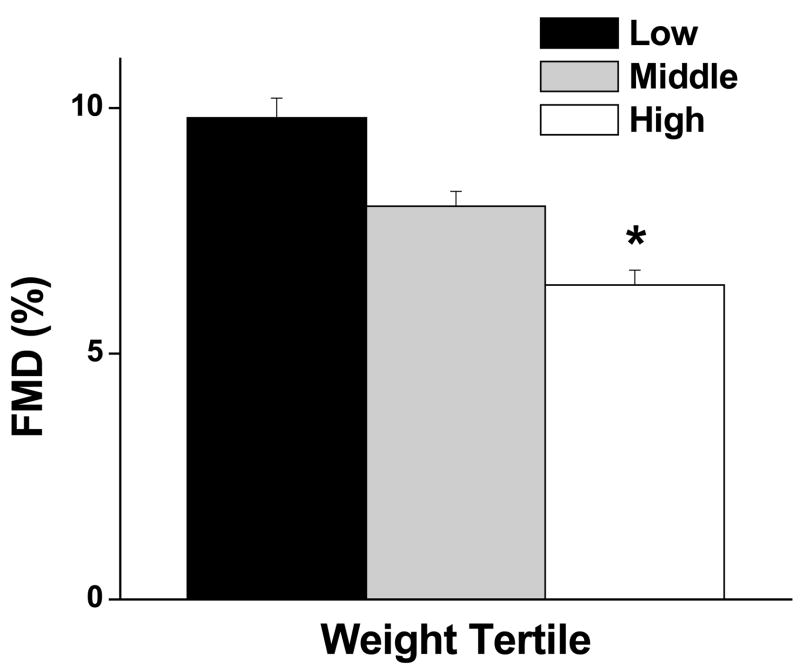
For the entire population, average flow-mediated dilation was 8.1 ± 4.8% (n=203) and nitroglycerin-mediated dilation was 11.6 ± 6.2% (n=95). As shown in the Figure, brachial artery FMD was significantly impaired in the highest vs. lowest tertile of body weight (6.5 ± 4.6% vs. 9.8 ± 4.8% respectively, p<0.001) lending support for graded vascular impairment with escalating obesity status. Additional brachial artery parameters are displayed in Table 2. In addition to impaired endothelium-dependent FMD, the heavier groups also exhibited lower reactive hyperemia, a marker of microvascular dilator dysfunction. In contrast, nitroglycerin-mediated, endothelium-independent dilator responses were similar between all groups.
Figure.
Brachial artery flow-mediated dilation (FMD) stratified according to body weight tertiles (low < 113 kg, middle 113–137 kg, and high >137 kg). As shown, FMD was significantly impaired in the high vs. low tertile (*p < 0.001). Data are presented as mean ± SE.
Table 2.
Brachial artery parameters by weight tertile
| Variable | <113 kg (n = 67) | 113–137 kg (n = 67) | >137 kg (n = 69) | P |
|---|---|---|---|---|
| Flow-mediated dilation (%) | 9.8 ± 4.8 | 8.0 ± 4.5 | 6.5 ± 4.6 | <0.001 |
| Flow-mediated diameter increase (mm) | 0.36 ± 0.16 | 0.32 ± 0.17 | 0.27 ± 0.18 | 0.011 |
| Reactive hyperemia (% increase) | 694 ± 352 | 520 ± 298 | 518 ± 398 | 0.011 |
| Nitroglycerin-mediated dilation (%) | 12.6 ± 5.3 | 11.5 ± 7.3 | 11.1 ± 5.8 | 0.664 |
As displayed in Table 3, univariate correlates of flow-mediated dilation were gender, weight, BMI, waist circumference, diastolic blood pressure, and creatinine. Multivariate analysis identified body weight (β = −0.23, p=0.005) as a significant independent predictor of FMD whereas other adiposity measures of waist circumference or BMI were not significant determinants of flow-mediated dilation when total weight was included as a variable in the analysis. Gender was also independently associated with endothelial function (β = 0.21, p=0.009). Waist circumference was weakly independently linked to arterial dilation (p=0.01) only if weight was excluded from the multivariate model.
Table 3.
Univariate correlates of flow-mediated dilation
| Regression Coefficient | P | |
|---|---|---|
| Gender | 0.31 | <0.001 |
| Weight (kg) | −0.28 | <0.001 |
| Body Mass Index (kg/m2) | −0.14 | 0.048 |
| Waist circumference (cm) | −0.24 | 0.001 |
| Systolic blood pressure (mm Hg) | −0.13 | 0.058 |
| Diastolic blood pressure (mm Hg) | −0.17 | 0.015 |
| Total cholesterol (mg/dl) | 0.05 | 0.530 |
| Low density lipoprotein cholesterol (mg/dl) | 0.11 | 0.121 |
| High density lipoprotein cholesterol (mg/dl) | −0.04 | 0.547 |
| Creatinine (mg/dl) | −0.16 | 0.030 |
| Triglycerides (mg/dl) | −0.08 | 0.271 |
| Hemoglobin A1c (%) | −0.08 | 0.306 |
| Glucose (mg/dl) | −0.05 | 0.466 |
| Insulin (μU/mL) | −0.05 | 0.584 |
| HOMA-IR | −0.08 | 0.388 |
Discussion
In the present study which included a large number of severely obese patients, we demonstrated that body weight is a significant predictor of vascular dysfunction after adjusting for covariates in a population afflicted with morbid obesity. Most importantly, weight burden was strongly linked to arterial dysfunction without evidence for a threshold cut-point in relation to degree of vascular impairment, lending strong support to our growing recognition that cardiovascular risk continues to rise with escalating obesity. Brachial artery flow-mediated dilation correlated with body weight, but not independently with BMI in severely obese subjects, suggesting that this conventional measure of adiposity may be limited in its discriminatory power with regard to cardiovascular risk in extreme obesity.
Our findings provide a clinical opportunity to gain insight into the pathophysiological link between morbid obesity and vascular function, as prior population studies examined vascular physiology in primarily mildly obese subjects. The Framingham database demonstrated an inverse association between brachial artery flow-mediated dilation and BMI, although the relation to other anthropometric measures was not reported.6 A similar study in Hispanics observed a gender difference noting a link between BMI and impaired vascular responses in women.7 Smaller cross-sectional studies suggest that weight distribution, in particular central localization of fat, may be a more important determinant of vascular phenotype. For example, in 2 studies of overweight adults, flow-mediated dilation correlated with waist-to-hip ratio, but not BMI or metabolic parameters.8,9 In other studies, accumulation of visceral fat quantified by abdominal computed tomography (CT)10 or ultrasound imaging11 was linked with impaired vasoreactivity, though a report in older men failed to confirm this relationship.12 Collectively, these studies brought to our attention that various adiposity measures relate to vascular diathesis, but very few studies to date examined vasomotor responses in expressly severe obesity (BMI ≥ 40) and a significant gap in knowledge exists with regard to this issue.
We offer novel data by providing evidence for progressive loss of vascular homeostasis with advancing weight burden into the extreme range, and from a clinical standpoint refute any potential notion that added increase in fat mass beyond a specific threshold cut-point fails to contribute to further health risks. Our results are in agreement with recent clinical data by McTigue et al demonstrating rising mortality across weight groups ranging from normal to the extremely obese.13 These findings combined with a growing population in highest weight categories underscore the danger in simplifying obesity as a homogeneous condition. Our present data suggest that progressive impairment in endothelial function may represent a pathophysiological mechanism linking obesity to cardiovascular risk. Although adiposity is associated with insulin resistance and dyslipidemia, we did not identify a specific relation between these conventional metabolic parameters and vasomotor function, an observation that has been fairly consistent across other studies that examined this issue in the obese.9,10 This can be partly explained by the already high prevalence of abnormal HOMA-IR in these subjects thus limiting its discriminatory power. Overall, since variables beyond traditional metabolic risk factors were determinants of vascular diathesis across weight tertiles, our identification of a specific impairment in endothelial function with progressive weight assumes even greater functional significance, and lends further support to the growing recognition that excess fat burden itself may be detrimental to vascular homeostasis.
Our study has several limitations. Waist-to-hip ratio assessment was not available in our study subjects. In addition, we did not quantify fat percentage or distribution as physical size limitations pose significant challenges for advanced imaging with CT and MRI, or X-ray absorptiometry as many morbidly obese subjects do not fit into scanners. Lastly, only a subset of patients received NTG, thus we cannot exclude a change in endothelium-independent dilation, although no such effect was seen in previous similar investigations.8,10 Overall, these inherent study limitations are counterbalanced by the relatively large and unique study population, clinical relevance of examining vascular function in severe obesity, and importance of filling a gap in knowledge with regard to this issue.
Acknowledgments
Source of Funding: This work was supported by a National Institutes of Health grant to Dr. Gokce (R01 HL074097).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olson TP, Schmitz KH, Leon AS, Dengel DR. Vascular structure and function in women - Relationship with body mass index. Am J Prev Med. 2006;30:487–492. doi: 10.1016/j.amepre.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Vita JA. Nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2002;359:186–200. doi: 10.1016/s0076-6879(02)59183-7. [DOI] [PubMed] [Google Scholar]
- 3.Gokce N, Keaney JF, Jr, Frei B, Holbrook M, Olesiak M, Zachariah BJ, Leeuwenburgh C, Heinecke JW, Vita JA. Long-term ascorbic acid administration reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1999;99:3234–3240. doi: 10.1161/01.cir.99.25.3234. [DOI] [PubMed] [Google Scholar]
- 4.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 5.Meyer C, Pimenta W, Woerle HJ, Van Haeften T, Szoke E, Mitrakou A, Gerich J. Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care. 2006;29:1909–1914. doi: 10.2337/dc06-0438. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF, Jr, Lehman BT, Fan S, Osypiuk E, Vita JA. Clinical correlates and heritability of flow-mediated dilation in the community - The Framingham Heart Study. Circulation. 2004;109:613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 7.Pulerwitz T, Grahame-Clarke C, Rodriguez CJ, Miyake Y, Sciacca RR, Hirata K, DiTullio MR, Boden-Albala B, Sacco RL, Homma S. Association of increased body mass index and impaired endothelial function among Hispanic women. Am J Cardiol. 2006;97:68–70. doi: 10.1016/j.amjcard.2005.07.125. [DOI] [PubMed] [Google Scholar]
- 8.Brook RD, Bard RL, Rubenfire M, Ridker PM, Rajagopalan S. Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am J Cardiol. 2001;88:1264–1269. doi: 10.1016/s0002-9149(01)02088-4. [DOI] [PubMed] [Google Scholar]
- 9.Williams IL, Chowienczyk PJ, Wheatcroft SB, Patel A, Sherwood R, Momin A, Shah AM, Kearney MT. Effect of fat distribution on endothelial-dependent and endothelial-independent vasodilatation in healthy humans. Diabetes Obes Metab. 2006;8:296–301. doi: 10.1111/j.1463-1326.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 10.Arcaro G, Zamboni M, Rossi L, Turcato E, Covi G, Armellini F, Bosello O, Lechi A. Body fat distribution predicts the degree of endothelial dysfunction in uncomplicated obesity. Int J Obes Relat Metab Disord. 1999;23:936–942. doi: 10.1038/sj.ijo.0801022. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto M, Akishita M, Eto M, Kozaki K, Ako J, Sugimoto N, Yoshizumi M, Toba K, Ouchi Y. The impairment of flow-mediated vasodilatation in obese men with visceral fat accumulation. Int J Obes Relat Metab Disord. 1998;22:477–484. doi: 10.1038/sj.ijo.0800620. [DOI] [PubMed] [Google Scholar]
- 12.Joseph LJO, Ryan AS, Sorkin J, Mangano C, Brendle DC, Corretti MC, Gardner AW, Katzel LI. Body fat distribution and flow-mediated endothelium-dependent vasodilation in older men. Int J Obes Relat Metab Disord. 2002;26:663–669. doi: 10.1038/sj.ijo.0801972. [DOI] [PubMed] [Google Scholar]
- 13.McTigue K, Larson JC, Valoski A, Burke G, Kotchen J, Lewis CE, Stefanick ML, Van Horn L, Kuller L. Mortality and cardiac and vascular outcomes in extremely obese women. JAMA. 2006;296:79–86. doi: 10.1001/jama.296.1.79. [DOI] [PubMed] [Google Scholar]