Abstract
Background and purpose:
Hydrogen sulphide (H2S) is an endogenous gaseous mediator active in the multilevel regulation of pathophysiological functions in mammalian cardiovascular tissues.
Experimental approach:
This study investigated the pharmacological activity of a new H2S-releasing derivative of diclofenac, S-diclofenac (2-[(2,6-dichlorophenyl)amino]benzeneacetic acid 4-(3H-1,2-dithiole-3-thione-5-yl)-phenyl ester) in the isolated rabbit heart submitted to low-flow ischaemia-reperfusion damage.
Key results:
S-diclofenac (3, 10 and 30 μM), despite inhibiting prostacyclin generation by cardiac tissues, achieved dose-dependent normalization of coronary perfusion pressure, reducing left ventricular contracture during ischaemia and improving left ventricular developed pressure and ±dP/dtmax at reperfusion. Creatine kinase and lactate dehydrogenase activities in heart perfusates were significantly reduced during reperfusion. These effects were accompanied by substantial release of reduced glutathione (GSH), indicating that the H2S moiety may have up-regulated cysteine transport. The anti-ischaemic activities of S-diclofenac and the H2S-donor sodium hydro sulphide (NaHS) were partially prevented by the KATP channel antagonist glibenclamide, suggesting a mechanism similar to H2S-induced cardioprotection in metabolic ischaemic preconditioning. Perfusion with the nitric oxide (NO) synthase inhibitor NG-monomethyl-L-arginine worsened the myocardial ischaemia-reperfusion damage, but this was dose-dependently prevented by S-diclofenac and NaHS, suggesting that the released H2S may have overcome NO deficiency.
Conclusion and implications:
These data show that S-diclofenac had marked anti-ischaemic activity in ischaemic-reperfused rabbit hearts despite inhibition of prostaglandin generation. Increased GSH formation leading to activation of KATP channels may have contributed to this beneficial effect. The pharmacological profile of S-diclofenac and its anti-inflammatory activity, with diminished gastrointestinal side effects, offer therapeutic applications in cardiovascular disease.
Keywords: S-diclofenac, diclofenac, hydrogen sulphide, ischaemia–reperfusion, isolated rabbit heart, cardioprotection
Introduction
Hydrogen sulphide (H2S), like nitric oxide (NO) and carbon monoxide, is an endogenous gaseous mediator active in the multilevel regulation of physiological and pathological functions in mammalian tissues (Li et al., 2006; O'Sullivan, 2006). Synthesis of H2S from cysteine occurs naturally through the activity of two pyridoxal-5′-phosphate-dependent enzymes, cystathionine γ-lyase (CSE) and cystathionine β-synthase (CBS), although other sources cannot be ruled out (Boehning and Snyder, 2003; Moore et al., 2003; Stipanuk, 2004). The localization of the H2S synthesizing enzymes and their endogenous levels in tissues imply that the cardiovascular system is a source of endogenous H2S. CSE appears to be the main source of H2S in the vasculature and heart (Wang, 2002), but CBS predominates in control of the nervous system (Boehning and Snyder, 2003). mRNA for the H2S producing enzyme CSE is expressed in myocardial tissues and H2S might also be produced in these tissues where it acts as a physiological regulator of cardiac function, mediated by the ATP-dependent K+ channel (KATP) pathway (Geng et al., 2004). Johansen et al. (2006) have provided the first evidence that exogenous H2S protects against irreversible ischaemia–reperfusion injury in the rat myocardium, supporting the likelihood of KATP opening in the mechanism of action. Elrod et al. (2007), working with an in vivo murine model of myocardial ischaemia–reperfusion injury, reported that the H2S-donor sodium hydrosulphide (NaHS) achieved significant cardioprotection through reversible inhibition of mitochondrial respiration at the time of reperfusion, thereby reducing oxidant generation and cardiac apoptosis.
On the grounds that H2S may play a beneficial role in inflammation (Zanardo et al., 2006) and that the inhibition of the generation of this gaseous transmitter contributes to gastric injury caused by non-steroidal anti-inflammatory drugs (Fiorucci et al., 2005), a novel H2S-releasing derivative of diclofenac (S-diclofenac, 2-[(2,6-dichlorophenyl)amino]benzeneacetic acid 4-(3H-1,2-dithiole-3-thione-5-yl)-phenyl ester), has been recently synthesized (Bhatia et al., 2005; Li et al., 2007). S-diclofenac shows potent anti-inflammatory activity with less gastric intolerance that the parent compound, with a significant release of H2S (Li et al., 2007).
In the light of this information, and considering that H2S and NO may react together to form a molecule (possibly a nitrosothiol) that might regulate NO availability in the cardiovascular system (Ali et al., 2006), we compared the effects of S-diclofenac and diclofenac in the isolated rabbit heart submitted to low-flow ischaemia–reperfusion. To gain more information on the relationship between H2S and NO, we studied the cardioprotective activity of S-diclofenac in the perfused heart submitted to low-flow ischaemia–reperfusion in the presence of the NOS inhibitor, NG-monomethyl-L-arginine (L-NMMA) (Rossoni et al., 1995). Previous work in rats and rabbits with NO-releasing aspirin (Rossoni et al., 2000, 2001) and NO-releasing naproxen (Rossoni et al., 2004) indicated significant cardioprotection in ischaemia–reperfusion experiments compared to the parent compounds.
Materials and methods
Animals
All animal procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Male New Zealand White rabbits (Charles River Laboratories, Calco, Lecco, Italy) weighing 1.9–2.1 kg were used. The animals were housed in a conditioned environment (22±1 °C, 55±5% relative humidity, 12-h light/12-h dark cycle), with free access to food and tap water. At least 5 days were allowed for animals to acclimatize before any experimental manipulations.
Rabbit heart perfusion
Rabbit hearts were perfused as described previously (Henry et al., 1977; Rossoni et al., 2000, 2004). In brief, the rabbits were anaesthetized with thiopentone sodium (Pentothal; Abbott, Campoverde, Latina, Italy, 60 mg per kg) given by i.v. injection. The chest was opened, and the heart was rapidly excised and placed in cold (4 °C) Krebs Henseleit solution (KHS) with the following composition (mM): NaCl 118, KCl 4.8, KH2PO4 1.2, CaCl2 1.6, MgSO4 1.2, NaHCO3 25 and glucose 11.5. The heart, mounted on the experimental setup, was perfused retrogradely through the aorta at 20 ml min−1 (Minipuls-3 peristaltic pump; Gilson, Villiers-Le Bel, France) with KHS, which was held at 37 °C and aerated with 95% O2+5% CO2 to maintain normal pH, pO2 and pCO2 parameters. Coronary perfusion pressure (CPP) and left ventricular pressure (LVP) were measured with two HP-1280C pressure transducers (Hewlett-Packard, Waltham, MA, USA) connected to a Hewlett-Packard dynograph (HP-7754A). LVP was recorded with a polyethylene catheter with a small latex balloon on the tip (Hugo Sachs Elektronik, March-Hugstetten, Germany), inserted in the left ventricular cavity through the mitral valve opening. The volume of the balloon was adjusted to give peak left ventricular systolic pressure (LVSP) 97–100 mm Hg and left ventricular end-diastolic pressure (LVEDP) of 3- to 5-mm Hg. Hearts that could not achieve this contractile performance (7–8% of the hearts) were excluded. Left ventricular developed pressure (LVDevP; peak LVSP minus LVEDP) and the maximum rate of rise and fall of left ventricular pressure (±dP/dtmax) were also calculated.
Myocardial ischaemia–reperfusion
After 15 min equilibration, hearts were paced at 180 beats min−1 (Henry et al., 1977) with an electrical stimulator (S-88; Grass Instr., Quincy, MA, USA) using two silver electrodes attached to the right atrium and an additional 30 min of perfusion was carried out (pre-ischaemic period). Ischaemia was induced by reducing the flow rate from 20 to 1 ml min−1 for 40 min (ischaemic period). Normal flow rate (20 ml min−1) was then restored and the perfusion was continued for another 20 min (reperfusion period). Throughout the experiment, a thermoregulated chamber kept the heart temperature at 37 °C to avoid hypothermia-induced cardioprotection. Each experiment did not last for more than 2 h, during which time the experimental preparation was stable.
Experimental design
After preliminary experiments, different molar concentrations of the compounds under investigation were selected and tested in groups of seven hearts each. In particular, S-diclofenac (3, 10 and 30 μM) or diclofenac (3, 10 and 30 μM) were perfused through the hearts for 20 min before coronary flow was reduced. In some experiments (n=6 hearts per group) we compared the effect of S-diclofenac (30 μM) with the H2S-donor NaHS (30 μM), infused through the hearts for 20 min before flow reduction. The KATP inhibitor glibenclamide (100 μM) was given through the hearts for 30 min during the pre-ischaemic period; this infusion started 10 min before treatment with S-diclofenac or NaHS.
Creatine kinase and lactate dehydrogenase activities in heart perfusates
The perfusates, eluted from the heart during pre-ischaemic and reperfusion periods, were collected in an ice-cooled beaker, as 5 min samples. Each sample was used for the determination of creatine kinase (CK) and lactate dehydrogenase (LDH) activities according to the original method of Bergmeyer et al. (1970) and Hohorst (1963), respectively. The total activity of these enzymes was measured spectrophotometrically (Lambda-16; Perkin Elmer Italia, Monza, Milan, Italy) at 37 °C using specific kits, according to the manufacturer's instructions. Data are expressed as mU min−1 per g wet tissue (w.t.).
Prostacyclin and reduced glutathione determinations in heart perfusates
Prostacyclin (PGI2) and reduced glutathione (GSH) were measured directly in the coronary effluent collected in an ice-cooled beaker for 2 min immediately before ischaemia and during the first 10 min of reperfusion. PGI2 was determined as its stable metabolite 6-keto-prostaglandin F1α (6-keto-PGF1α) with an ELISA kit (detection limit, 3 pg ml−1) as described by Pradelles et al. (1985). GSH formation was analysed using a commercial GSH enzymatic assay kit (Anderson, 1985). PGI2 and GSH were assayed in duplicate and the results were expressed as ng min−1 per g w.t. and nnol min−1 per g w.t., respectively.
Myocardial ischaemia–reperfusion experiments with NOS inhibition: effects of S-diclofenac, diclofenac and NaHS
In this set of experiments (n=5 hearts per group) hearts were perfused for 10 min immediately before ischaemia with the NOS inhibitor, L-NMMA (10 μM). Previous papers (Rossoni et al., 1995, 2000, 2004) reported that at this regimen L-NMMA increases CPP, aggravating post-ischaemic ventricular dysfunction. To evaluate cardioprotective activity, S-diclofenac, diclofenac and NaHS were perfused through the hearts at the concentration of 30 μM for 20 min (10 min before L-NMMA plus 10 min during L-NMMA).
Statistical analysis
Each value is the mean±s.e.mean. Statistical significance was established by ANOVA followed by Bonferroni's multiple comparisons. Differences with a probability of 5% or less were considered significant. The area under the curve (AUC) was estimated according to the trapezoid method (Purves, 1992) using the Microcal Origin 3.5 computer program (Microcal Software Inc., Northampton, MA, USA).
Drugs
The following drugs were used: S-diclofenac (CTG Pharma, Milan, Italy), diclofenac sodium salt, glibenclamide, NaHS and L-NMMA (Sigma-Aldrich, St Louis, MO, USA), assay kits for CK and LDH (Sentinel Diagnostic, Milan, Italy) and for 6-keto-PGF1α and GSH determinations (Cayman Chemical Co., Ann Arbor, MI, USA). NaHS (containing 28–32% H2O) was dissolved freshly in ultrapure water to provide a stock solution of 1 mM NaHS. S-diclofenac, diclofenac and glibenclamide, dissolved in dimethylsulphoxide (DMSO) at 0.5 M stock concentration and further diluted in KHS, were prepared daily. The final DMSO concentration (<0.1%) (vehicle) per se had no effects on the parameters tested. All other chemicals were of analytical or ultrapure grade.
Results
Ischaemia–reperfusion in isolated rabbit heart
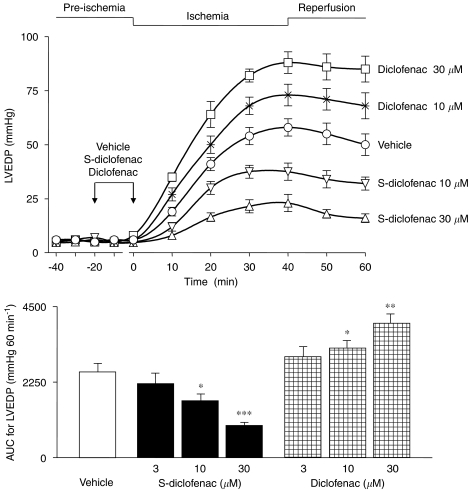
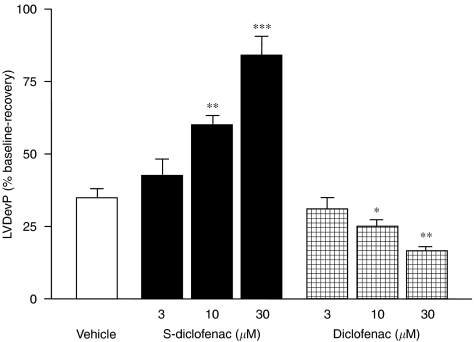
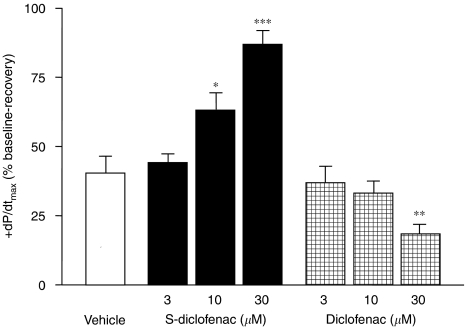
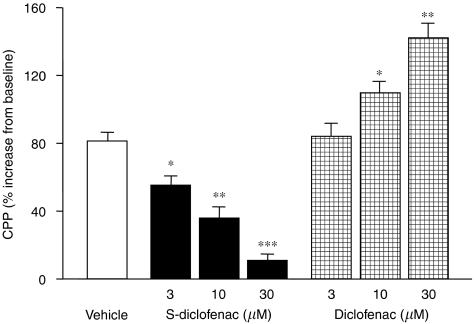
At baseline, the cardiac parameters were similar and not significantly different (P>0.05) in the seven experimental groups (Table 1). When the perfusion of electrically paced isovolumic left rabbit heart preparations was reduced from 20 to 1 ml min−1 for 40 min, LVEDP progressively rose indicating that, after the standstill, an ischaemic process was occurring. During reperfusion left ventricular function was impaired, LVDevP and +dP/dtmax being significantly reduced, and CPP considerably increased from baseline (Figures 1, 2, 3 and 4). Perfusion of the hearts with S-diclofenac (3, 10 and 30 μM) for 20 min before ischaemia gave dose-dependent myocardial protection against mechanical changes due to the ischaemia–reperfusion. The characteristic ventricular contracture observed during the 40 min of ischaemia was reduced, favouring a better recovery of LVDevP and +dP/dtmax at reperfusion (Figures 1, 2 and 3). At the same time, CPP fell in proportion to the S-diclofenac dose (Figure 4). In clear contrast with this picture, perfusion with diclofenac (3, 10 and 30 μM) severely worsened the myocardial ischaemic damage. At reperfusion, the LVEDP was significantly higher than in vehicle-treated preparations, and this was associated with a marked depression of LVDevP and +dP/dtmax, and an increase in CPP (Figures 1, 2, 3 and 4). S-diclofenac and diclofenac had no effects on LVEDP, LVDevP, +dP/dtmax and CPP values during the pre-ischaemic period (Table 1).
Table 1.
Cardiac parameters determined in isolated perfused rabbit hearts at baseline
| Treatment | CPP (mm Hg) | LVEDP (mm Hg) | LVDevP (mm Hg) | +dP/dtmax (mm Hg s−1) | −dP/dtmax (mm Hg s−1) |
|---|---|---|---|---|---|
| Vehicle | 58.2±6.3 | 3.6±0.5 | 93.4±5.7 | 2179±185 | 1672±215 |
| S-diclofenac (3 μM) | 56.7±6.1 | 3.3±0.4 | 95.2±4.8 | 2238±201 | 1698±185 |
| S-diclofenac (10 μM) | 61.8±4.9 | 3.7±0.3 | 88.7±7.0 | 1987±164 | 1592±149 |
| S-diclofenac (30 μM) | 59.1±5.5 | 3.3±0.3 | 91.0±5.1 | 2117±173 | 1613±201 |
| Diclofenac (3 μM) | 56.4±3.7 | 4.1±0.6 | 92.5±6.3 | 2091±204 | 1644±197 |
| Diclofenac (10 μM) | 62.0±4.3 | 3.7±0.3 | 89.1±7.1 | 2275±192 | 1780±180 |
| Diclofenac (30 μM) | 60.8±5.0 | 4.3±0.5 | 97.6±6.0 | 2256±217 | 1742±238 |
Abbreviations: CPP, coronary perfusion pressure; LVDevP, left ventricular developed pressure; LVEDP, left ventricular end-diastolic pressure; +dP/dtmax and −dP/dtmax, maximum rate of rise and fall of left ventricular pressure; S-diclofenac, 2-[(2,6-dichlorophenyl)amino]benzeneacetic acid 4-(3H-1,2-dithiole-3-thione-5-yl)-phenyl ester; w.t., wet tissue.
Data are mean±s.e.mean of seven heart preparations per group. Cardiac parameters were calculated immediately before ischaemia.
Figure 1.
Effects of S-diclofenac and diclofenac on LVEDP in paced isovolumic left rabbit heart preparations subjected to low-flow ischaemia–reperfusion. The upper panel show the time-course of activity for two drug concentrations, and the bar graph (lower panel) shows the AUC for all concentrations of each drug. Each point/bar represents the mean±s.e.mean of seven experiments. *P<0.05, **P<0.01 and ***P<0.001 versus vehicle group. AUC, area under the curve; LVEDP, left ventricular end-diastolic pressure; S-diclofenac, 2-[(2,6-dichlorophenyl)amino]benzeneacetic acid 4-(3H-1,2-dithiole-3-thione-5-yl)-phenyl ester.
Figure 2.
Effects of S-diclofenac and diclofenac on LVDevP in paced isovolumic left rabbit heart preparations subjected to low-flow ischaemia and reperfusion. LVDevP was calculated at the end of the 20-min reperfusion and expressed as percentage of the pre-ischaemic values (see Table 1). Each bar represents the mean±s.e.mean of seven experiments.*P<0.05, **P<0.01 and ***P<0.001 versus vehicle group. LVDevP, left ventricular developed pressure; S-diclofenac, 2-[(2,6-dichlorophenyl)amino]benzeneacetic acid 4-(3H-1,2-dithiole-3-thione-5-yl)-phenyl ester.
Figure 3.
Effects of S-diclofenac and diclofenac on +dP/dtmax in paced isovolumic left rabbit heart preparations subjected to low-flow ischaemia and reperfusion. +dP/dtmax was calculated at the end of the 20-min reperfusion, and expressed as percentage of the pre-ischaemic values (see Table 1). Each bar represents the mean±s.e.mean of seven experiments. *P<0.05, **P<0.01 and ***P<0.001 versus vehicle group. +dP/dtmax, maximum rate of rise of left ventricular pressure; S-diclofenac, 2-[(2,6-dichlorophenyl)amino]benzeneacetic acid 4-(3H-1,2-dithiole-3-thione-5-yl)-phenyl ester.
Figure 4.
Effects of S-diclofenac and diclofenac on CPP in paced isovolumic left rabbit heart preparations subjected to low-flow ischaemia–reperfusion. CPP was calculated at the end of the 20-min reperfusion, and expressed as percentage of the pre-ischaemic values (see Table 1). Each bar represents the mean±s.e.mean of seven experiments. *P<0.05, **P<0.01 and ***P<0.001 versus vehicle group. CPP, coronary perfusion pressure; S-diclofenac, 2-[(2,6-dichlorophenyl)amino]benzeneacetic acid 4-(3H-1,2-dithiole-3-thione-5-yl)-phenyl ester.
CK and LDH activities in heart perfusates
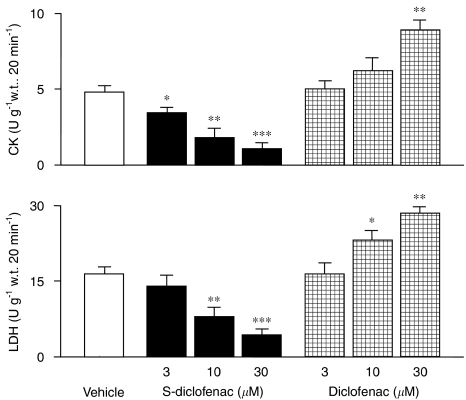
CK and LDH, as indicators of myocardial damage, were determined in the coronary effluent collected from each heart during the pre-ischaemic and reperfusion periods. There were no differences in the pre-ischaemic period (data not shown). During reperfusion, CK and LDH activities in the vehicle-treated group were 5.2 times (P<0.001) and 9.3 times (P<0.001) those in the pre-ischaemic period (CK, 0.92±0.16 U per g w.t. per 20 min; LDH, 1.75±0.33 U per g w.t. per 20 min) (data not shown). S-diclofenac (3, 10 and 30 μM), perfused for 20 min before ischaemia, reduced the release of CK and LDH in a concentration-dependent manner at reperfusion, compared to vehicle-treated hearts (Figure 5). Unlike the effects of S-diclofenac, the increased severity of post-ischaemic ventricular dysfunction caused by diclofenac was associated with a marked increase of both CK and LDH activities in heart effluents (Figure 5).
Figure 5.
Effects of S-diclofenac and diclofenac on CK and LDH activities in paced isovolumic left rabbit heart preparations submitted to low-flow ischaemia–reperfusion. CK and LDH were calculated during the 20-min reperfusion and expressed as the increase from pre-ischaemic values (CK, 0.92±0.16 U per g w.t. per 20 min; LDH, 1.75±0.33 U per g w.t. per 20 min). Each bar represents the mean±s.e.mean of seven experiments. *P<0.05, **P<0.01 and ***P<0.001 versus vehicle group. CK, creatine kinase; LDH, lactate dehydrogenase; S-diclofenac, 2-[(2,6-dichlorophenyl)amino]benzeneacetic acid 4-(3H-1,2-dithiole-3-thione-5-yl)-phenyl ester.
PGI2 release in heart perfusates
PGI2 is the major eicosanoid produced by jeopardized myocardium (Van Bilsen et al., 1989) and its rate of formation increases particularly during the first 5–10 min of reperfusion, declining rapidly thereafter (Berti et al., 1988; Engels et al., 1990). In the present study, in vehicle-treated hearts the generation of 6-keto-PGF1α (the stable metabolite of PGI2) during reperfusion was enhanced 4.2 times (P<0.001) compared to the pre-ischaemic period (3.15±0.53 ng min−1 per g w.t.) (Table 2). When the hearts were perfused with S-diclofenac or diclofenac, 6-keto-PGF1α release was inhibited in a concentration-dependent manner in both the pre-ischaemic and reperfusion periods. Diclofenac inhibited 6-keto-PGF1α release at a concentration approximately three times lower than that required to obtain a similar inhibition with S-diclofenac (Table 2).
Table 2.
Effects of S-diclofenac and diclofenac on 6-keto-PGF1α formation in paced isovolumic left rabbit heart preparations subjected to low-flow ischaemia and reperfusion
| Treatment |
6-keto-PGF1α (ng min−1 per g w.t.) |
||
|---|---|---|---|
| Pre-ischaemia | Reperfusiona | ||
| Vehicle | 3.15±0.53 | 13.22±1.42 | |
| S-diclofenac | 3 μM | 2.38±0.37 (24) | 10.81±1.05 (18) |
| S-diclofenac | 10 μM | 1.48±0.29 ** (53) | 7.13±0.94 * (46) |
| S-diclofenac | 30 μM | 0.51±0.12 *** (84) | 3.21±0.51 *** (76) |
| Diclofenac | 3 μM | 1.61±0.32 ** (49) | 8.33±1.04 * (37) |
| Diclofenac | 10 μM | 0.57±0.10 *** (82) | 3.59±0.18 *** (73) |
| Diclofenac | 30 μM | n.d. | 0.96±0.23 *** (93) |
Abbreviations: 6-keto-PGF1α, 6-keto-prostaglandin F1α; n.d., not detectable (detection limit 3 pg ml−1); S-diclofenac, 2-[(2,6-dichlorophenyl)amino]benzeneacetic acid 4-(3H-1,2-dithiole-3-thione-5-yl)-phenyl ester; w.t., wet tissue.
Data are mean±s.e.mean of seven heart preparations per group. Values within parentheses show, percentage inhibition compared to vehicle-treated hearts. Drugs were infused for 20 min before flow rate reduction.
Data refer to the first 10 min of reperfusion.
*P<0.05, **P<0.01 and ***P<0.001 versus vehicle-treated hearts.
GSH release in heart perfusates
The results for GSH in heart perfusates are shown in Table 3. In vehicle-treated hearts, the GSH was 9.8 times the pre-ischaemic level at reperfusion. Perfusion with S-diclofenac caused a concentration-dependent increase of GSH in the perfusate. This was particularly marked at 30 μM, when S-diclofenac significantly raised the rate of formation of GSH in both the pre-ischaemic and reperfusion periods. In contrast, diclofenac did not affect GSH release at any concentration (Table 3).
Table 3.
Effects of S-diclofenac and diclofenac on reduced GSH formation in paced isovolumic left rabbit heart preparations subjected to low-flow ischaemia and reperfusion
| Treatment |
GSH (nmol min−1 per g w.t.) |
||
|---|---|---|---|
| Pre-ischaemia | Reperfusiona | ||
| Vehicle | 0.53±0.06 | 5.18±0.67 | |
| S-diclofenac | 3 μM | 0.49±0.06 (−8) | 5.53±0.44 (+7) |
| S-diclofenac | 10 μM | 0.66±0.11 (+24) | 7.15±0.62* (+38) |
| S-diclofenac | 30 μM | 0.81±0.09* (+52) | 8.29±0.49** (+60) |
| Diclofenac | 3 μM | 0.57±0.10 (+7) | 5.07±0.30 (−2) |
| Diclofenac | 10 μM | 0.52±0.06 (−2) | 4.61±0.56 (−11) |
| Diclofenac | 30 μM | 0.46±0.05 (−13) | 3.98±0.47 (−23) |
Abbreviations: GSH, glutathione; S-diclofenac, 2-[(2,6-dichlorophenyl)amino]benzeneacetic acid 4-(3H-1,2-dithiole-3-thione-5-yl)-phenyl ester; w.t., wet tissue.
Data are mean±s.e.mean of seven heart preparations per group. Values in the parentheses show, percentage from vehicle-treated hearts. Drugs were infused for 20 min before flow rate reduction.
Data refer to the first 10 min of reperfusion.
*P<0.05 and **P<0.01 versus vehicle-treated hearts.
S-diclofenac and NaHS activity in myocardial ischaemia–reperfusion experiments: effect of glibenclamide
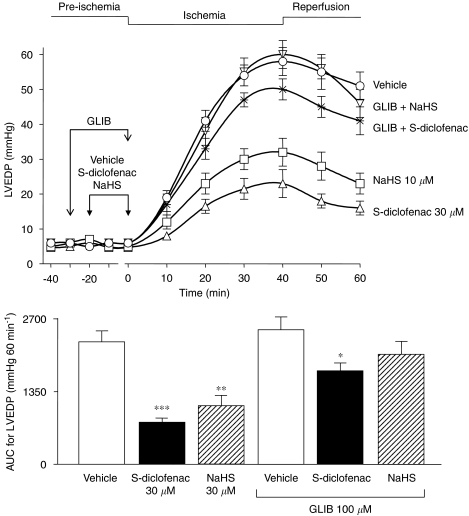
In these experiments, perfusion of the hearts with S-diclofenac (30 μM) and NaHS (30 μM) for 20 min before ischaemia clearly protected the myocardium, and the AUC for LVEDP were respectively 66% (P<0.001) and 52% (P<0.001) lower for S-diclofenac and NaHS than for vehicle-treated preparations (AUC=2265±196 mm Hg 60 min−1) (Figure 6). Pretreatment with 100 μM glibenclamide, which per se did not affect LVEDP, significantly antagonized the cardioprotecting activity of S-diclofenac and NaHS. The AUC for LVEDP in preparations treated with S-diclofenac and NaHS plus glibenclamide were only 23% (P<0.05) and 14% lower than the vehicle-treated preparations (Figure 6).
Figure 6.
Glibenclamide (GLIB) antagonized the cardioprotective effects of both S-diclofenac and NaHS on LVEDP in paced isovolumic left rabbit heart preparations subjected to low-flow ischaemia–reperfusion. The upper panel shows the time-course of activity of the drugs, and the bar graph (lower panel) the AUC. Each point/bar represents the mean±s.e.mean of six experiments. *P<0.05, **P<0.01 and ***P<0.001 versus the corresponding vehicle group. AUC, area under the curve; LVEDP, left ventricular end-diastolic pressure; NaHS, sodium hydrosulphide; S-diclofenac, 2-[(2,6-dichlorophenyl)amino]benzeneacetic acid 4-(3H-1,2-dithiole-3-thione-5-yl)-phenyl ester.
Effects of S-diclofenac, diclofenac and NaHS in myocardial ischaemia–reperfusion experiments with NOS inhibition
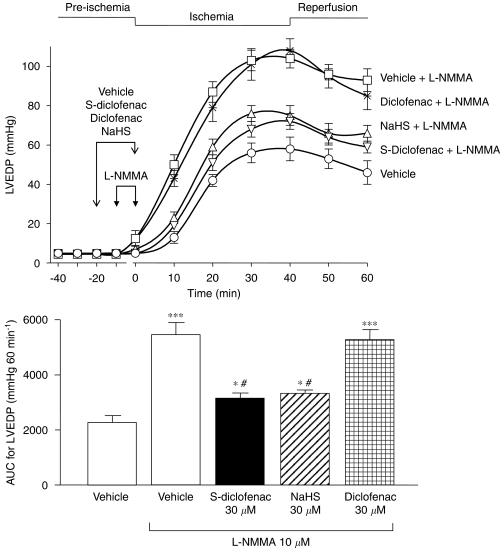
Perfusion of the hearts with L-NMMA (10 μM) for 10 min before flow reduction exacerbated ventricular dysfunction compared with vehicle-treated preparations. At the end of the ischaemic period, LVEDP rose to nearly twice the values in vehicle-treated hearts; P<0.001). At the end of reperfusion these values were still high (Figure 7) and cardiac contractility was severely depressed with a significant rise of CPP (data not shown). In these preparations, where the myocardial ischaemic damage was aggravated by L-NMMA, pretreatment with S-diclofenac (30 μM) or NaHS (30 μM) significantly prevented the worsening effects of NOS inhibition (Figure 7). S-diclofenac and NaHS reduced the AUC for the LVEDP increase by respectively 73% (P<0.01) and 67% (P<0.01) compared to L-NMMA-alone preparations (Figure 6). Diclofenac (30 μM) did not affect the increased myocardial–ischaemia injury due to L-NMMA (Figure 7).
Figure 7.
S-diclofenac and NaHS counteract the worsening induced by L-NMMA on LVEDP in paced isovolumic left rabbit heart preparations subjected to low-flow ischaemia–reperfusion. The upper panel shows the time-course of activity of the drugs, and the bar graph (lower panel) the AUC. Each point/bar represents the mean±s.e.m. of five experiments. *P<0.05, **P<0.01 and ***P<0.001 versus vehicle alone group. #P<0.01 versus L-NMMA alone group. AUC, area under the curve; L-NMMA, NG-monomethyl-L-arginine; LVEDP, left ventricular end-diastolic pressure; NaHS, sodium hydrosulphide; S-diclofenac, 2-[(2,6-dichlorophenyl)amino]benzeneacetic acid 4-(3H-1,2-dithiole-3-thione-5-yl)-phenyl ester.
Discussion
The main findings of this study indicate that the H2S-releasing derivative of diclofenac, S-diclofenac, provided marked cardioprotection in a well-characterized experimental model of myocardial ischaemia–reperfusion injury in the rabbit (Henry et al., 1977; Rossoni et al., 2000, 2004). In this model, the major determinants of myocardial performance are under control, and changes in +dP/dtmax and LVEDP are used as indexes of cardiac contractility and diastolic elastic stiffness (‘compliance'), respectively (Henry et al., 1977).
Unlike diclofenac, S-diclofenac reduced LVEDP, consequently improving LVDevP and +dP/dtmax, and lowering CPP at reperfusion. In spite of dose-related impairment of PGI2 formation by cardiac endothelial cells, the beneficial effects of S-diclofenac were accompanied by dose-dependent reduction of both CK and LDH activities in the cardiac perfusates, suggesting there was less loss of functional integrity of the sarcolemma. This may imply that H2S released by S-diclofenac is responsible for the drug's cardioprotective activity in the ischaemic–reperfused rabbit heart. However, we did not directly determine H2S released by S-diclofenac in cardiac tissues or in heart perfusates and this is now under investigation. It has been reported that in vitro incubation of 100 μM S-diclofenac with rat liver homogenate resulted in H2S release into the medium which peaked at 15 min (2.6±0.1% of substrate added) and remained elevated for a further 75 min (Li et al., 2007). Indirect evidence for H2S release from S-diclofenac appears to be the enhanced formation of GSH, particularly marked when S-diclofenac was perfused through the hearts at the maximal cardioprotective dose of 30 μM. It is worth noting that, in a well-studied model of oxidative stress caused by glutamate, H2S protected primary cultures of neurons from death by raising glutathione levels through upregulation of cystine transport, cystine being the rate-limiting substrate of glutathione synthesis (Kimura and Kimura, 2004).
In the present experiments, the increased formation of GSH may have contributed to S-diclofenac's beneficial effect against ischaemia–reperfusion damage. GSH, with its important antioxidant properties, plays a pivotal role in myocardial protection against ischaemia–reperfusion (Pan et al., 2006). Under conditions of oxidative stress, GSH reacts either as an electron donor to neutralize hydrogen peroxides and lipoperoxides or as a direct free radical scavenger (Leichtweis and Ji, 2001). Interestingly, in vehicle-treated preparations GSH generation was several times higher than the basal values during reperfusion, suggesting a protective mechanism against free radical production generated by cardiac tissues. In these conditions, diclofenac did not change the basal rate of GSH formation, and the worsening of myocardial ischaemia–reperfusion injury caused by this anti-inflammatory drug appears to be related to the inhibition of COX activity, with impaired formation of PGI2.
Prostaglandin formation, namely PGI2, is involved in a critical cytoprotective mechanism against myocardial damage caused by ischaemia (Ogletree et al., 1979) and the rate of PGI2 formation in the ischaemic–reperfused rabbit heart increases with the severity of the ischaemic process (Berti et al., 1988). Stabilization of cardiac lysosomes by normal PGI2 generation is of paramount importance in the ischaemic myocardium, because leakage of lysosomal enzymes (proteases and phospholipases) may contribute to the generation of irreversible damage in cardiomyocytes (Wildenthal et al., 1978).
The non-selective KATP blocker glibenclamide partially antagonized the beneficial effects of both S-diclofenac and NaHS in myocardial ischaemia–reperfusion damage. This seems to indicate that H2S, released in sufficient amounts by both S-diclofenac and NaHS, not only may have overcome the reduced production of H2S in ischaemic cardiomyocytes (Lapenna et al., 1996), but may have also triggered a signalling mechanism similar to that described for metabolic ischaemic preconditioning, where activation of sarcolemmal (and not mitochondrial) KATP play an important role (Gross and Peart, 2003; Bian et al., 2006; Johansen et al., 2006). However, additional mechanism/s involved in the cardioprotection provided by S-diclofenac may not be ruled out.
Recent findings that NO may regulate H2S production (Zhao et al., 2003; Zhong et al., 2003) strongly suggest an interaction between NO and the H2S system in metabolic preconditioning ischaemia. The results from the present study clearly support the concept that both H2S and NO are also involved in post-ischaemic ventricular dysfunction. The aggravation of ischaemia–reperfusion damage in rabbit heart preparations, with impaired NO generation caused by L-NMMA, was prevented by both S-diclofenac and NaHS. Reports that H2S prevents the development of hypertension induced by NG-nitro-L-arginine methyl ester strongly indicate that the impairment of NOS activity may have caused dysfunction of the H2S synthase/H2S pathways leading to the increase in systemic blood pressure (Zhong et al., 2003). It has also been reported that in spontaneously hypertensive rats, H2S generation is impaired and that exogenous H2S might exert beneficial effects in the pathogenesis of spontaneous hypertension (Yan et al., 2004). In the light of these results it is reasonable to speculate that in the case of the aggravation of myocardial ischaemia–reperfusion injury by L-NMMA in the present experiments, H2S generation in cardiac endothelial cells may have been reduced. However, we have not yet fully determined CSE activity and H2S formation in hearts deprived of NO and submitted to ischaemia–reperfusion, and current experiments should verify this critical point.
In conclusion, the present results indicate that the H2S-releasing diclofenac derivative, in spite of a blockade of prostaglandin formation, has noteworthy anti-ischaemic activity in the reperfused rabbit heart. GSH generation and opening of the KATP appear to be part of its mode of action although other mechanisms cannot be ruled out. The previous observation that nitro-aspirin (Rossoni et al., 2000) and nitro-naproxen (Rossoni et al., 2004) have anti-ischaemic effects similar to S-diclofenac and NaHS further illustrates that gaseous molecules such as H2S and NO, albeit with different mechanism(s) and suggestion of ‘cross talk' (Moore et al., 2003) and synergy (Hosoki et al., 1997), provide some protection in cardiovascular pathology. The pharmacological profile of S-diclofenac and its documented anti-inflammatory activity, with reduced gastrointestinal side effects (Bhatia et al., 2005; Li et al., 2007; Wallace et al., 2007), open up the way to a range of therapeutic applications also in cardiovascular disease, for instance in the treatment of myocardial ischaemia and prevention of infarct progression.
Abbreviations
- 6-keto-PGF1α
6-keto-prostaglandin F1α
- AUC
area under the curve
- CBS
cystathionine β-synthase
- CK
creatine kinase
- CPP
coronary perfusion pressure
- CSE
cystathionine γ-lyase
- ±dP/dtmax
maximum rate of rise and fall of left ventricular pressure
- DMSO
dimethylsulphoxide
- GSH
reduced glutathione
- KHS
Krebs Henseleit solution
- KATP
ATP-dependent K+ channel
- LDH
lactate dehydrogenase
- L-NMMA
NG-monomethyl-L-arginine
- LVP
left ventricular pressure
- LVDevP
left ventricular developed pressure
- LVEDP
left ventricular end-diastolic pressure
- LVSP
left ventricular systolic pressure
- NaHS
sodium hydrosulphide
- PGI2
prostacyclin
- S-diclofenac
2-[(2,6-dichlorophenyl)amino]benzeneacetic acid 4-(3H-1,2-dithiole-3-thione-5-yl)-phenyl ester
Conflict of interest
Dr Piero Del Soldato is a shareholder of CTG Pharma, Milan, Italy. This company has patents on reagents used in this study. Professor Anna Sparatore received a grant from CTG Pharma.
References
- Ali MY, Ping CY, Mok Y-YP, Whiteman M, Bathia M, Moore PK. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide. Br J Pharmacol. 2006;149:625–634. doi: 10.1038/sj.bjp.0706906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Sidhapuriwala J, Moore PK. Treatment with H2S-releasing derivative of diclofenac reduces inflammation in carrageenan-induced hindpaw oedema. Inflammation Res. 2005. p. S185.
- Bergmeyer HU, Rich W, Butter H, Schmidt E, Hillman G, Kreuz FH, et al. Standardization of methods for estimation of enzyme activity in biological fluids. Z Klin Chem Klin Biochem. 1970;8:658–660. [Google Scholar]
- Berti F, Rossoni G, Magni F, Caruso D, Omini C, Puglisi L, et al. Non-steroidal anti-inflammatory drugs aggravate acute myocardial ischemia in the perfused rabbit heart: a role for prostacyclin. J Cardiovasc Pharmacol. 1988;12:438–444. doi: 10.1097/00005344-198810000-00009. [DOI] [PubMed] [Google Scholar]
- Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S, et al. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther. 2006;316:670–678. doi: 10.1124/jpet.105.092023. [DOI] [PubMed] [Google Scholar]
- Boehning D, Snyder SH. Novel neural modulators. Annu Rev Neurosci. 2003;26:105–131. doi: 10.1146/annurev.neuro.26.041002.131047. [DOI] [PubMed] [Google Scholar]
- Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels W, Van Bilsen M, De Groot MJ, Lemmens PJ, Willemsen PH, Reneman RS, et al. Ischemia and reperfusion induced formation of eicosanoids in isolated rat hearts. Am J Physiol. 1990;258:H1865–H1871. doi: 10.1152/ajpheart.1990.258.6.H1865. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, et al. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005;129:1210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- Geng B, Yang J, Qi Y, Zhao J, Pang Y, Du J, et al. H2S generated by heart in rat and its effects on cardiac function. Biochem Biophys Res Commun. 2004;313:362–368. doi: 10.1016/j.bbrc.2003.11.130. [DOI] [PubMed] [Google Scholar]
- Gross GJ, Peart JN. KATP channels and myocardial preconditioning: an update. Am J Physiol Heart Circ Physiol. 2003;285:H921–H930. doi: 10.1152/ajpheart.00421.2003. [DOI] [PubMed] [Google Scholar]
- Henry PD, Schuchleib R, Davis J, Weiss ES, Sobel BE. Myocardial contracture and accumulation of mitochondrial calcium in ischemic rabbit heart. Am J Physiol. 1977;233:H677–H684. doi: 10.1152/ajpheart.1977.233.6.H677. [DOI] [PubMed] [Google Scholar]
- Hohorst HJ.L-(+)-lactate Methods of Enzymatic Analysis 1963Academic Press Inc: New York, USA; 215–219.In: Bergmeyer HU (ed). [Google Scholar]
- Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- Johansen D, Ytrehus K, Baxter GF. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury. Evidence for a role of KATP channels. Basic Res Cardiol. 2006;101:53–60. doi: 10.1007/s00395-005-0569-9. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- Lapenna D, de Gioia S, Ciofani G, Mezzetti A, Pierdomenico SD, Di Ilio C, et al. Impaired glutathione biosynthesis in the ischemic-reperfused rabbit myocardium. FEBS Lett. 1996;391:76–78. doi: 10.1016/0014-5793(96)00705-3. [DOI] [PubMed] [Google Scholar]
- Leichtweis S, Ji LL. Glutathione deficiency intensifies ischaemia–reperfusion induced cardiac dysfunction and oxidative stress. Acta Physiol Scand. 2001;172:1–10. doi: 10.1046/j.1365-201X.2001.00820.x. [DOI] [PubMed] [Google Scholar]
- Li L, Bhatia M, Moore PK. Hydrogen sulphide: a novel mediator of inflammation. Curr Opin Pharmacol. 2006;6:1–5. doi: 10.1016/j.coph.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Li L, Rossoni G, Sparatore A, Lee LC, Del Soldato P, Moore PK. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic Biol Med. 2007;42:706–719. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Moore PK, Bathia M, Moochhala S. Hydrogen sulfide: from the smell of the past to the mediator of the future. Trends Pharmacol Sci. 2003;24:609–611. doi: 10.1016/j.tips.2003.10.007. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SE. What is the significance of vascular hydrogen sulphide (H2S) Br J Pharmacol. 2006;149:609–610. doi: 10.1038/sj.bjp.0706907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogletree ML, Lefer AM, Smith JB, Nicolau KC. Studies on the protective effect of prostacyclin in acute myocardial ischemia. Eur J Pharmacol. 1979;56:95–103. doi: 10.1016/0014-2999(79)90438-2. [DOI] [PubMed] [Google Scholar]
- Pan TT, Feng ZN, Lee SW, Moore PK, Bian JS. Endogenous hydrogen sulfide contributes to the cardioprotection by metabolic inhibition preconditioning in the rat ventricular myocytes. J Mol Cell Cardiol. 2006;40:119–130. doi: 10.1016/j.yjmcc.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Pradelles P, Grassi J, Maclouf J. Enzyme immunoassays of eicosanoids using acetylcholine esterase as label: an alternative to radioimmunoassay. Anal Chem. 1985;57:1170–1173. doi: 10.1021/ac00284a003. [DOI] [PubMed] [Google Scholar]
- Purves R. Optimum numerical integration methods for estimation of area-under-the-curve (AUC) and area-under-the-moment-curve (AUMC) J Pharmacokinet Biopharm. 1992;20:211–226. doi: 10.1007/BF01062525. [DOI] [PubMed] [Google Scholar]
- Rossoni G, Berti F, Bernareggi M, Villa L, Agozzino S, Cereda R, et al. Protective effects of ITF 296 in the isolated rabbit heart subjected to global ischemia. J Cardiovasc Pharmacol. 1995;26:S44–S52. [PubMed] [Google Scholar]
- Rossoni G, Berti M, De Gennaro Colonna V, Bernareggi M, Del Soldato P, Berti F. Myocardial protection by the nitroderivative of aspirin, NCX 4016: in vivo and in vitro experiments in the rabbit. Ital Heart J. 2000;1:146–155. [PubMed] [Google Scholar]
- Rossoni G, Manfredi B, De Gennaro Colonna V, Bernareggi M, Berti F. The nitroderivative of aspirin, NCX 4016, reduces infarct size caused by myocardial ischemia–reperfusion in the anesthetized rat. J Pharmacol Exp Ther. 2001;297:380–387. [PubMed] [Google Scholar]
- Rossoni G, Manfredi B, Del Soldato P, Berti F. The nitric oxide-releasing naproxen derivative displays cardioprotection in perfused rabbit heart submitted to ischemia–reperfusion. J Pharmacol Exp Ther. 2004;310:555–562. doi: 10.1124/jpet.104.067397. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- Van Bilsen M, Engels W, van der Vusse GJ, Reneman RS. Significance of myocardial eicosanoid production. Mol Cell Biochem. 1989;88:113–121. doi: 10.1007/BF00223432. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Caliendo G, Santagada V, Cirino G, Fiorucci S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology. 2007;132:261–271. doi: 10.1053/j.gastro.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitters. FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- Wildenthal K, Decker RS, Poole AR, Griffin EE, Dingle JT. Sequential lysosomal alterations during cardiac ischemia. I. Biochemical and immunohistochemical changes. Lab Invest. 1978;38:656–661. [PubMed] [Google Scholar]
- Yan H, Du J, Tang C. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun. 2004;313:22–27. doi: 10.1016/j.bbrc.2003.11.081. [DOI] [PubMed] [Google Scholar]
- Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:E1411–E1418. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- Zhao W, Ndisang JF, Wang R. Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol. 2003;81:848–853. doi: 10.1139/y03-077. [DOI] [PubMed] [Google Scholar]
- Zhong G, Chen F, Cheng Y, Tang C, Du J. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J Hypertens. 2003;21:1819–1820. doi: 10.1097/00004872-200310000-00015. [DOI] [PubMed] [Google Scholar]