Abstract
Background and purpose:
Sphingosine 1-phosphate (S1P) selectively and potently constricts isolated cerebral arteries, but this response has not been pharmacologically characterized.
Experimental approach:
The receptor subtype(s) involved in S1P-induced cerebrovascular constriction were characterized using genetic (S1P2 and S1P3 receptor null mice) and pharmacological tools (phospho-FTY720, a S1P1/3/4/5 receptor agonist; SEW2871, a S1P1 receptor agonist, JTE-013, a S1P2 receptor antagonist, VPC23019, a S1P1/3 receptor antagonist). Isolated basilar or peripheral (femoral, mesenteric resistance) arteries, from either rat or mouse, were studied in a wire myograph.
Key results:
S1P concentration-dependently constricted basilar artery in rat, wild-type (WT) and S1P2 null mice, but barely affected vascular tone in S1P3 null mice. Vasoconstriction to U46619 (a thromboxane analogue) or to endothelin-1 did not differ between WT, S1P2 and S1P3 null mice. JTE-013 inhibited not only S1P-induced vasoconstriction, but also KCl-, U46619- and endothelin-1-induced constriction. This effect was observed in WT as well as in S1P2 null mice. VPC23019 increased the concentration-dependent vasoconstriction to S1P in both rat and mouse basilar arteries with intact endothelium, but not in rat basilar artery without endothelium. Phospho-FTY720 concentration-dependently constricted rat basilar arteries, but not femoral or mesenteric resistance arteries, while SEW2871 did not induce any response in the same arteries.
Conclusions and implications:
S1P constricts cerebral arteries through S1P3 receptors. The purported S1P2 receptor antagonist JTE-013 does not appear to be selective, at least in rodents. Enhancement of S1P-induced contraction by VPC23019 might be related to blockade of S1P1 receptors and NO generation.
Keywords: S1P, basilar artery, JTE-013, FTY720, VPC23019, SEW2871
Introduction
Sphingosine 1-phosphate (S1P) is an important signalling molecule in the cardiovascular system (Waeber et al., 2004). S1P derived from membrane sphingolipids and glycerophospholipids and/or released by activated platelets binds to high-affinity G-protein-coupled receptors (S1P1–S1P5; formerly EDG1,5,3,6,8; McGiffert et al., 2002; Anliker and Chun, 2004; Gardell et al., 2006). S1P receptor expression has been documented in endothelial cells as well as in vascular smooth muscle (Hla and Maciag, 1990; Okazaki et al., 1993).
Sphingosine 1-phosphate induces vasoconstriction in isolated arteries as well as in vivo. It constricts isolated cerebral arteries at submicromolar concentrations (Tosaka et al., 2001; Coussin et al., 2002; Salomone et al., 2003), but not peripheral arteries (Salomone et al., 2003). This effect on cerebral circulation has also been observed in in vivo experiments (Tosaka et al., 2001; Salomone et al., 2003). The fact that submicromolar S1P concentrations constrict cerebral arteries in vitro is likely to be physiologically relevant, because plasma S1P concentrations have been reported to be in this range (Yatomi et al., 1997; Murata et al., 2000). Moreover, the S1P concentration in the supernatant during clot formation has been shown to induce basilar artery spasm (Tosaka et al., 2001).
The identity of the receptor subtype mediating constriction to S1P is unclear. On the basis of the results of in vitro experiments using antisense gene delivery, we have previously proposed that the cerebrovascular constriction induced by S1P is mostly S1P3 receptor mediated (Salomone et al., 2003). However, the involvement of S1P2 in S1P-induced contraction of canine coronary smooth muscle cells has been suggested, based on the effect of JTE-013, an S1P2 receptor antagonist (Ohmori et al., 2003). Furthermore, at least part of S1P-induced vasoconstriction seems to occur through a Pertussis toxin-sensitive mechanism (Bischoff et al., 2000; Salomone et al., 2003), suggesting that S1P receptors coupled to Gi/Go proteins (possibly S1P1; Lee et al., 1996) may play a role in S1P-induced vasoconstriction.
In the present study, we took advantage of new pharmacological tools and, importantly, receptor knockout mice to identify the S1P receptor subtype(s) mediating cerebral vasoconstriction. On the basis of the lack of S1P-induced vasomotor tone in basilar artery isolated from S1P3 receptor null mice, the lack of vasoconstriction following an S1P1 receptor agonist (SEW2871) and the cerebrovascular selective vasoconstriction to phospho-FTY720 (an agonist for all S1P receptors but S1P2), we conclude that S1P-induced vasoconstriction of rat and mouse basilar artery occurs through S1P3 receptor stimulation.
Methods
Animals
All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee.
S1P2 and S1P3 null mice (Ishii et al., 2001, 2002; Yang et al., 2002) were bred and housed in our animal facility. Animals had free access to water and food. All experiments reported here were performed using 12- to 16-week-old (20–30 g) male mice. Both wild-type (WT) littermates and commercial C57BL6/j were used as controls. The genotype of each mouse was confirmed by PCR. Rats were male Sprague–Dawley, weighing 250–350 g.
Myograph experiments
Mice or rats were killed by fluothane anaesthesia followed by decapitation, and their brains were removed and immersed in physiological solution (composition (mM): NaCl, 118; KCl, 4.6; NaHCO3, 25; MgSO4, 1.2; KH2PO4, 1.2; CaCl2, 1.2; glucose, 10; EDTA, 0.025; pH 7.4 at 37 °C). The basilar artery was dissected, cut into 1.5- to 2-mm long segments and threaded onto 40-μm stainless steel wires (rat) or 15-μm tungsten wires (mice). Each segment was mounted in one of the four organ chambers of an isometric myograph (610 M; Danish Myo Technology, Aarhus, Denmark). For mice, an entire basilar artery was mounted in each organ chamber. Rat femoral and mesenteric arteries, as well as mouse aorta or carotid artery were threaded onto 40-μm stainless steel wires. In experiments with rat basilar artery, aimed at comparing the effect of intact functional endothelium on S1P and VPC23019, a 25-μm stainless steel wire was used instead of the 40-μm wire. After mounting, each preparation was equilibrated, unstretched, for 30 min, in physiological solution, maintained at 37 °C and aerated with a gas mixture of 95% O2 and 5% CO2. The normalized passive resting force and the corresponding diameter were then determined for each preparation from its own length–pressure curve, according to Mulvany and Halpern (1977). Contractile responses were recorded into a computer, by using a data acquisition and recording software (Myodaq and Myodata, Danish Myo Technology). After normalization and 30-min equilibration in physiological solution, the preparations were stimulated with isotonic depolarizing KCl solutions, in which part of NaCl had been replaced by an equimolar amount of KCl (composition (mM): NaCl, 22.6; KCl, 98.8; NaHCO3, 25; MgSO4, 1.2; KH2PO4, 1.2; CaCl2, 1.2; glucose, 10; EDTA, 0.025, pH 7.4 at 37 °C). After washout and 30-min recovery, the preparations were exposed to agonists (for example, S1P, phospho-FTY720 or SEW2871). In some experiments, the vasoconstriction to S1P was tested in the presence of 10 μM JTE-013, 10 μM VPC23019 or 0.1 mM N-ω-nitro-L-arginine methyl ester hydrochloride (L-NAME; 30-min preincubation) in rat or mouse basilar artery. To study endothelium-dependent vasodilating effects, preparations were preconstricted with 1–10 μM phenylephrine (rat femoral and mesenteric resistance arteries), 0.3–1 μM 5-hydroxytryptamine (5-HT; rat basilar artery) or 0.3 μM U46619 (mouse basilar artery). Mouse basilar arteries were also challenged with cumulative U46619 (10 nM–3 μM), a synthetic prostanoid analogue of PGF2α, and with endothelin-1 (ET-1; 10 pM–30 nM). In some preparations, endothelium was removed by perfusion with 0.03% Triton X-100. To assess the effectiveness of endothelium removal, preparations preconstricted by 0.3 μM 5-HT were challenged with 1 μM acetylcholine (Ach).
Statistical analysis
The negative logarithm (pD2) of the concentration producing 50% of the maximum effect (Emax) was calculated by linear regression from semi-logarithmic plots. Pharmacological parameters (pD2 and Emax), calculated from different animals and treatments, were averaged by group and treatment and compared by one-way ANOVA followed by Tukey's post hoc analysis. Nonlinear fits of concentration–contraction curves were also compared by F-test, by using the PRISM software (Graph Pad Software, San Diego, CA, USA). P-values less than 0.05 were considered statistically significant.
Drugs
Sphingosine 1-phosphate (Avanti Polar Lipids Inc., Alabaster, AL, USA) was solubilized as a millimolar stock solution in a buffer containing 100 mM Tris, 145 mM NaCl and 4 mg ml−1 fatty acid-free BSA, pH 9.0. This buffer did not produce changes in the tone of basilar arteries, except when its total amount added to the bath was 1 and 3% of the total 5-ml bath volume. The transient increase in tone observed in this case lasted only about 1 min (see Figure 1b), possibly because of a transient increase in the pH of the physiological bathing solution. Therefore, when measuring the effect of 10 and 30 μM S1P, we disregarded this transient peak and considered the ensuing steady-state tension. JTE-013 (Tocris, Ellisville, MO, USA) was dissolved in dimethyl sulphoxide as a 10-mM stock solution. VPC23019 (Avanti Polar Lipids) was dissolved in acidified dimethyl sulphoxide as a 10-mM stock solution. Phospho-FTY720 (Novartis Institutes of BioMedical Research, Basel, Switzerland) was dissolved in H2O/dimethyl sulphoxide (50:50) as a 5-mM stock solution. SEW2871 (Cayman Chemical Company, Ann Arbor, MI, USA) was dissolved in ethanol as a 10-mM stock solution. U46619 (Sigma-Aldrich, St Louis, MO, USA) was dissolved in ethanol as a 30-mM stock solution. ET-1 (Sigma) was dissolved in H2O as a 30-μM stock solution. FTY720 was from Cayman Chemical; PE, 5-HT, ACh, L-NAME were from Sigma; they were dissolved as 10-mM stock solution in H2O.
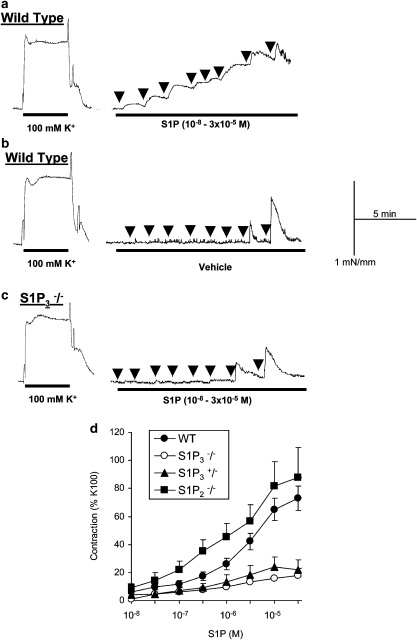
Figure 1.
Vasoconstriction to sphingosine 1-phosphate (S1P) in isolated basilar artery from wild-type (WT) or S1P2 or S1P3 receptor null mice. (a) Typical recording showing the contractile responses to cumulative concentrations of S1P in basilar artery isolated from WT mice; arrows indicate the addition of each concentration of S1P, from 10−8 to 3 × 10−5 M, in half-log steps, to the organ bath; contractile response to high (100 mM) K+-induced depolarization is shown for comparison. (b) Effect of the S1P solvent (transient vasoconstriction, when injecting 50 and 150 μl) in basilar artery isolated from WT mice. (c) Typical recording showing the contractile response to S1P in basilar artery isolated from S1P3−/− mice. (d) Averaged contractile responses to cumulative concentrations of S1P in WT, S1P2−/−, S1P3−/− and S1P3+/− mice, expressed in % of high (100 mM) K+-induced contractions in the same preparations: mean and s.e.mean. The corresponding pharmacological parameters are given in Table 1.
Results
S1P2 and S1P3 receptor null mice displayed morphologically normal basilar arteries. Their normalized diameter and vasoconstrictor responses to 100 mM KCl, the thromboxane receptor agonist U46619 and ET-1 did not differ from those of WT basilar arteries (Table 1).
Table 1.
Morphological and pharmacological parameters of isolated mouse basilar arteries
| Wild type (n=15) | S1P3−/− (n=6) | S1P3+/− (n=3) | S1P2−/− (n=6) | |
|---|---|---|---|---|
| Normalized diameter (μm) | 173±5 | 178±5 | 181±4 | 165±17 |
| Constriction to high (100 mM) K+ (mN mm−1) | 0.96±0.08 | 0.69±0.11 | 0.89±0.25 | 0.80±0.13 |
| Constriction to U46619 | ||||
| pD2 (EC50, μM) | 7.01±0.18 (0.18) | 7.22±0.14 (0.07) | 6.73±0.04 (0.19) | 7.09±0.30 (0.16) |
| Emax (% high K+) | 99±4 | 124±17 | 90±3 | 125±16 |
| Constriction to ET-1 | ||||
| pD2 (EC50, nM) | 9.86±0.26 (0.28) | 9.87±0.53 (0.32) | 9.57±0.23 (0.37) | 10.12±0.15 (0.09) |
| Emax (% high K+) | 78±8 | 97±22 | 53±7 | 84±19 |
| Constriction to S1P | ||||
| pD2 (EC50, μM) | 5.65±0.08 (2.8) | ND (ND) | ND (ND) | 6.16±0.17** (1.0) |
| Emax (% high K+) | 78±10 | 12±4** | 24±7* | 89±21 |
Abbreviations: Emax, maximal effect; ET-1, endothelin-1; ND, not determined; pD2, negative logarithm; SIP, sphingosine 1-phosphate.
*P< 0.05, **P< 0.01 versus wild type; one-way ANOVA and Tukey's HSD test.
Sphingosine 1-phosphate concentration-dependently constricted basilar arteries isolated from either WT or S1P2−/− mice (Figure 1). S1P did not constrict other mouse arteries (aorta, carotid artery; not shown). S1P was significantly more potent in basilar arteries from S1P2−/− mice than in basilar arteries from WT mice (Table 1). In most basilar arteries from S1P3−/− mice, S1P barely affected the vascular tone (⩽10% of 100 mM KCl-induced contraction). In two preparations, however, S1P induced a weak vasoconstriction (about 30% of 100 mM KCl-induced contraction). S1P-induced vasoconstriction was also significantly impaired in S1P3+/− (heterozygous) mice (Figure 1 and Table 1).
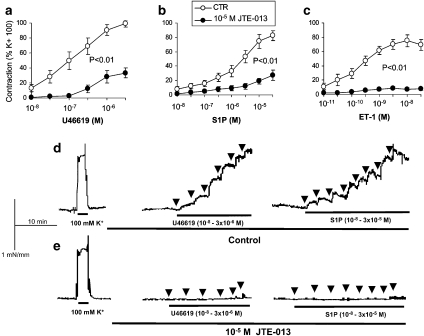
The higher potency of S1P in arteries from S1P2−/− may suggest that S1P2 receptor activation inhibits S1P-induced constriction in WT mice. To test this hypothesis, we used JTE-013, a purported inhibitor of S1P2 receptors (Ohmori et al., 2003). As shown in Figure 2, 10 μM JTE-013 strongly inhibited S1P-induced contraction in basilar arteries from WT. However, JTE-013 also inhibited the contraction induced by 100 mM KCl (by 64.0±5.5%, n=5, P<0.01), U46619 and ET-1 (Figure 2). Furthermore, the inhibitory effects of JTE-013 on vascular contraction were also present in basilar arteries from S1P2−/− (Figure 2), suggesting that the effects of JTE-013 are unrelated to S1P2 receptor antagonism.
Figure 2.
Effect of JTE-013 on the responsiveness of mouse basilar artery to sphingosine 1-phosphate (S1P) and other agonists. (a–c) Averaged contractile responses to cumulative concentrations of U46619, S1P or endothelin-1 (ET-1) in basilar arteries from wild-type (WT) mice, in the presence or absence of 10 μM JTE-013. Contractile responses are expressed in % of high (100 mM) K+-induced contractions in the same preparations in the absence of JTE-013. Values are mean and s.e.mean; n=5. (d, e) Typical recordings (experiment repeated twice with similar results) showing the contractile responses to cumulative concentrations of U46619 and S1P, in the absence (d) or presence of 10 μM JTE-013 (e), in basilar artery isolated from S1P2−/− mice; arrows indicate the addition of each concentration of U46619 (10−8 to 3 × 10−6 M) or S1P (10−8 to 3 × 10−5 M), in half-log steps, to the organ bath; contractile responses to high (100 mM) K+-induced depolarization in the same preparations are shown for comparison. When used, JTE-013 was preincubated for 30 min before challenging with U46619, S1P or ET-1.
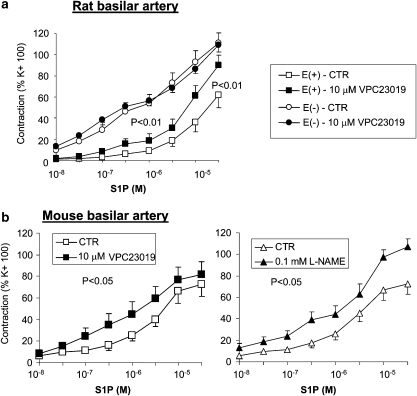
VPC23019 has been described as an S1P1/3 receptor antagonist in stable transfectants expressing either human S1P1 or S1P3 receptors (Davis et al., 2005). In a guanosine-5′-O-(3-[35S]thio)triphosphate assay, their pKb values (−log M) for S1P1 and S1P3 receptors were, respectively, 7.49±0.15 and 5.98±0.08. S1P1 receptors have been described as stimulating endothelial release of nitric oxide, which produces vasodilatation (Igarashi et al., 2003). We therefore tested the effect of VPC23019 in rat basilar arteries either with intact endothelium or without functional endothelium. As shown in Figure 3a, S1P-induced contractile responses were significantly weaker (P<0.01) in basilar arteries with intact endothelium (relaxation to ACh, 71.0±4.6%, n=16) than in basilar arteries without endothelium (relaxation to ACh, 9.2±3.3%, n=16). VPC23019 significantly increased the S1P-induced vasoconstriction in preparations with intact endothelium (P<0.01), but did not change it in preparations without endothelium. In intact mouse basilar artery (relaxation to ACh, 45.6±7.2%, n=16), VPC23019 shifted to the left the concentration–contraction curve to S1P by a half log (Figure 3b); preincubation with 0.1 mM L-NAME produced a similar leftward shift (Figure 3b). VPC23019 did not modify the contractile responses to KCl, 5-HT or U46619 (not shown).
Figure 3.
Effect of VPC23019 on the responsiveness of basilar artery from rat (a) and mouse (b) to sphingosine 1-phosphate (S1P). Contractile responses to cumulative concentrations of S1P, expressed in % of high (100 mM) K+-induced contractions in the same preparations, are shown as mean and s.e.mean (n=7–9). Isolated basilar arteries were incubated for 30 min with VPC23019 (10 μM), vehicle (5 μl dimethyl sulphoxide; final concentration: 0.1%) or 0.1 mM N-ω-nitro-L-arginine methyl ester hydrochloride (L-NAME) before being challenged with cumulative concentrations of S1P. Nonlinear fits for each individual data set were compared by F-test.
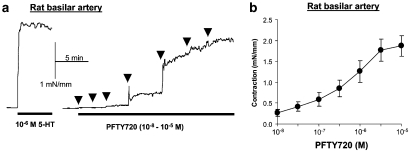
Phospho-FTY720 is the active form of the prodrug FTY720. It specifically interacts with S1P1,3,4,5 but not with S1P2 receptors (Mandala et al., 2002). FTY720 has also been shown to inhibit sphingosine phospholyase (the enzyme responsible for irreversible S1P degradation) in vitro in a dose-dependent fashion, with effects occurring at concentrations as low as 300 nM, while phospho-FTY720 is inhibitory only at 30 μM (Bandhuvula et al., 2005). In rat basilar artery, FTY720 (10 nM–0.1 mM) did not significantly affect the resting tone, or the tone induced by 5-HT or high K+ (not shown). In contrast, phospho-FTY720 concentration-dependently induced a vasoconstriction (EC50 0.6±0.2 μM, Emax 2.04±0.33 mN mm−1, n=11) comparable in amplitude to S1P-induced constriction (Figure 4). Phospho-FTY720 did not induce any significant contraction or relaxation in peripheral arteries such as femoral and mesenteric resistance arteries (not shown).
Figure 4.
Vasoconstriction to phospho-FTY720 in isolated rat basilar artery. (a) Typical recording showing the contractile responses to cumulative concentrations of phospho-FTY720; arrows indicate the addition of each concentration of phospho-FTY720, from 10−8 to 10−5 M, in half-log steps, to the organ bath; the contractile response to 5-hydroxytryptamine (5-HT, 1 μM) is shown for comparison. (b) Averaged contractile responses to cumulative concentrations of phospho-FTY720: mean and s.e.mean (n=12). The corresponding pharmacological parameters are given in the text.
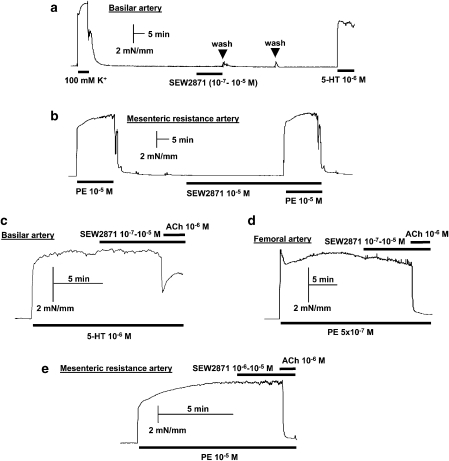
SEW2871, an S1P1 receptor agonist (Jo et al., 2005), did not induce any contraction in rat basilar or mesenteric resistance arteries when tested at concentrations up to 10 μM (Figure 5). Because S1P1 receptor activation has been linked to endothelial nitric oxide synthase (eNOS) activation (Igarashi and Michel, 2000), we tested the potential vasodilatory effect of SEW2871 on basilar, femoral and mesenteric resistance arteries preconstricted with either 1 μM 5-HT or 1–10 μM phenylephrine. In these preparations (Figure 5), SEW2871 did not elicit any significant vasodilatation. The functional integrity of endothelium was confirmed by challenging the preparations with ACh.
Figure 5.
Absence of vasoconstriction (a, b) or vasodilatation (c–e) to SEW2871 in different arteries isolated from rat. Typical recordings show the lack of effect of SEW2871, either as single concentration or as cumulative concentrations (by half-log steps), in comparison with positive controls for vasoconstriction, such as high (100 mM) K+-induced depolarization and 5-hydroxytryptamine (5-HT, 1 μM) in basilar artery (a) and phenylephrine (PE, 10 μM) in mesenteric resistance artery (b). For endothelium-dependent vasodilatation, acetylcholine (ACh; 1 μM) was used as a positive control in basilar (c), femoral (d) and mesenteric resistance arteries (e). Each preparation/condition was tested 3–4 times with similar results.
Discussion
This study used S1P receptor null mice (Ishii et al., 2001, 2002) to characterize unequivocally the S1P receptors responsible for cerebrovascular smooth muscle contraction. These data demonstrate that basilar arteries from S1P3−/− mice show very weak or no vasoconstriction to S1P. Furthermore, S1P is a more potent vasoconstrictor in basilar arteries from S1P2−/− mice than in basilar arteries from WT mice. On the basis of this evidence, we rule out the involvement of S1P2 receptors and suggest that the S1P3 subtype is the major contributor to S1P-induced vasoconstriction in cerebral circulation. However, some redundancy may exist in the signal-transduction pathways downstream of S1P receptor activation (for review see Waeber et al., 2004). For example, analysis of Rho activation in S1P3−/− mouse embryonic fibroblasts showed that S1P activated Rho to an extent similar to that observed in WT cells (Ishii et al., 2001; Liu et al., 2003), an effect attributed to S1P2 receptor stimulation, while PLC activation by S1P was clearly blunted in S1P3−/− mouse embryonic fibroblasts. Considering that both Rho-Rho kinase and PLC are involved in the vasoconstriction induced by S1P in cerebral arteries (Salomone et al., 2003), an S1P2/S1P3 receptor redundancy may explain the weak contraction to S1P (about 30% of high K+-induced tone) that we observed in two out of six S1P3−/− mice. Furthermore, homeostatic compensatory mechanisms could occur in S1P3−/− mice and may include receptor-independent effects of S1P, as recently implicated in smooth muscle contraction (Leiber et al., 2007).
The genetic evidence for a major role of S1P3 receptors in S1P-induced vasoconstriction of cerebral arteries is reinforced by the observation that S1P-induced vasoconstriction in heterozygous S1P3+/− mice was also blunted. It is worthy of note that basilar arteries from both S1P3−/− and S1P3+/− exhibited normal responses to other vasoconstrictors, such as high K+-induced depolarization, U46619 and ET-1.
Because we observed a higher potency of S1P in arteries from S1P2−/−, we attempted to determine whether this receptor subtype inhibits vascular smooth muscle contraction by using JTE-013, a compound previously shown to inhibit contraction of coronary artery smooth muscle cells via S1P2 receptor blockade (Ohmori et al., 2003). In our hands, however, JTE-013 not only inhibited S1P-induced contraction in basilar arteries but also inhibited the contraction induced by 100 mM KCl, U46619 and ET-1, suggesting that this effect was not specifically related to S1P receptor antagonism. Furthermore, the inhibitory effect of JTE-013 was also present in basilar arteries from S1P2−/− mice, indicating that it was completely unrelated to antagonism of S1P2 receptors. It is worth mentioning that the JTE-013 concentration used in our study (10 μM) is similar to the concentrations used in previous studies (Osada et al., 2002; Ohmori et al., 2003), and we found significant inhibition of KCl- and U46619-induced contraction even when using 1 μM JTE-013 (data not shown).
VPC23019 has been described as an S1P1/3 receptor antagonist, exhibiting pKb values of 7.5 and 6.0 for the S1P1 and S1P3 receptors, respectively (Davis et al., 2005); that is, the reported affinity of VPC23019 for S1P1 receptor subtype is 30 times higher than that for S1P3 receptors (Davis et al., 2005). S1P1 receptors have been linked to activation of eNOS (Yatomi et al., 2000; Igarashi et al., 2001), which produces vasodilatation (Igarashi et al., 2003). Conceivably, S1P1 receptor antagonism could increase S1P-induced vasoconstriction, by inhibiting a relaxing effect of S1P via eNOS activation. We observed that VPC23019 potentiated S1P-induced contractile response in both rat and mouse basilar arteries with intact endothelium; however, it failed to do so in preparations without endothelium. Our interpretation is therefore that VPC23019 might shift the S1P contraction curve to the left by inhibiting S1P1 receptor-induced NO release, while it only weakly inhibited the S1P3-mediated contraction, even at the highest concentration we tested. This is not unexpected, considering its published 30-fold lower affinity for the S1P3 subtype. The observation that removing endothelium or incubating with the NOS inhibitor L-NAME shifts to the left the curve to S1P is consistent with an endothelial pathway of vasodilatation sensitive to S1P, presumably acting at S1P1 receptor. It is also important to mention that VPC23019 binding to S1P receptors has been characterized in an HEK293T cell line overexpressing human S1P receptors (Davis et al., 2005), a system that may differ from our experimental system; for instance, minor species-specific differences in the primary sequence of human versus rodent S1P receptors may critically affect the drug-binding profile of S1P3 receptors, such that the affinity of VPC23019 for rodent S1P3 might be even lower than that for human S1P3.
When using the S1P1 receptor agonist SEW2871 (Jo et al., 2005; Wei et al., 2005), up to 10 μM, we did not observe any relaxation in rat basilar, femoral or mesenteric resistance arteries (Figure 5), where the presence of a functional endothelium was routinely confirmed by the relaxing effect of ACh. In our experimental system, SEW2871 appears therefore ineffective as a vasodilator in these vessels, suggesting that it does not have sufficient affinity and/or efficacy at the rodent endothelial S1P1 receptors. In some vessels, however, S1P receptor subtypes other than S1P1 may be involved in S1P-induced endothelium-dependent vasodilatation. For example, S1P3 receptors have been shown to mediate vasodilatation in mouse aortas (Nofer et al., 2004; Tolle et al., 2005) when challenged with phospho-FTY720, an observation that we reproduced in our laboratory (result not shown), confirming previous reports.
In conclusion, because phospho-FTY720 is an agonist for all S1P receptors except S1P2 (Brinkmann et al., 2002; Mandala et al., 2002), the fact that phospho-FTY720 potently constricted rat cerebral arteries, taken together with our observations in basilar arteries from S1P2−/− mice, further reinforces the notion that S1P2 receptors are not involved in cerebrovascular constriction. The purported S1P2 receptor antagonist JTE-013 does not appear to be selective, at least in rodents. Our data show that S1P-induced vasoconstriction in cerebral arteries is mediated by S1P3 receptors. Enhancement of S1P-induced contraction by VPC23019 might be related to blockade of S1P1 receptors and NO generation.
Acknowledgments
This study was supported by NIH Grants NS052195 and NS049263 (to CW) and NS048478, DA019674 and MH51699 (to JC).
Abbreviations
- Emax
maximal effect
- ET-1
endothelin-1
- eNOS
endothelial nitric oxide synthase
- L-NAME
N-ω-nitro-L-arginine methyl ester hydrochloride
- S1P
sphingosine 1-phosphate
- WT
wild type
Conflicts of interest
The authors state no conflict of interest.
References
- Anliker B, Chun J. Lysophospholipid G protein-coupled receptors. J Biol Chem. 2004;279:20555–20558. doi: 10.1074/jbc.R400013200. [DOI] [PubMed] [Google Scholar]
- Bandhuvula P, Tam YY, Oskouian B, Saba JD. The immune modulator FTY720 inhibits sphingosine-1-phosphate lyase activity. J Biol Chem. 2005;280:33697–33700. doi: 10.1074/jbc.C500294200. [DOI] [PubMed] [Google Scholar]
- Bischoff A, Czyborra P, Meyer Zu Heringdorf D, Jakobs KH, Michel MC. Sphingosine-1-phosphate reduces rat renal and mesenteric blood flow in vivo in a pertussis toxin-sensitive manner. Br J Pharmacol. 2000;130:1878–1883. doi: 10.1038/sj.bjp.0703516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- Coussin F, Scott RH, Wise A, Nixon GF. Comparison of sphingosine 1-phosphate-induced intracellular signaling pathways in vascular smooth muscles: differential role in vasoconstriction. Circ Res. 2002;91:151–157. doi: 10.1161/01.res.0000028150.51130.36. [DOI] [PubMed] [Google Scholar]
- Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem. 2005;280:9833–9841. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- Gardell SE, Dubin AE, Chun J. Emerging medicinal roles for lysophospholipid signaling. Trends Mol Med. 2006;12:65–75. doi: 10.1016/j.molmed.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Hla T, Maciag T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J Biol Chem. 1990;265:9308–9313. [PubMed] [Google Scholar]
- Igarashi J, Bernier SG, Michel T. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J Biol Chem. 2001;276:12420–12426. doi: 10.1074/jbc.M008375200. [DOI] [PubMed] [Google Scholar]
- Igarashi J, Erwin PA, Dantas AP, Chen H, Michel T. VEGF induces S1P1 receptors in endothelial cells: implications for cross-talk between sphingolipid and growth factor receptors. Proc Natl Acad Sci USA. 2003;100:10664–10669. doi: 10.1073/pnas.1934494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi J, Michel T. Agonist-modulated targeting of the EDG-1 receptor to plasmalemmal caveolae. eNOS activation by sphingosine 1-phosphate and the role of caveolin-1 in sphingolipid signal transduction. J Biol Chem. 2000;275:32363–32370. doi: 10.1074/jbc.M003075200. [DOI] [PubMed] [Google Scholar]
- Ishii I, Friedman B, Ye X, Kawamura S, McGiffert C, Contos JJ, et al. Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LP(B3)/EDG-3. J Biol Chem. 2001;276:33697–33704. doi: 10.1074/jbc.M104441200. [DOI] [PubMed] [Google Scholar]
- Ishii I, Ye X, Friedman B, Kawamura S, Contos JJ, Kingsbury MA, et al. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P(2)/LP(B2)/EDG-5 and S1P(3)/LP(B3)/EDG-3. J Biol Chem. 2002;277:25152–25159. doi: 10.1074/jbc.M200137200. [DOI] [PubMed] [Google Scholar]
- Jo E, Sanna MG, Gonzalez-Cabrera PJ, Thangada S, Tigyi G, Osborne DA, et al. S1P1-selective in vivo-active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem Biol. 2005;12:703–715. doi: 10.1016/j.chembiol.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Evans M, Hla T. The inducible G protein-coupled receptor edg-1 signals via the G(i)/mitogen-activated protein kinase pathway. J Biol Chem. 1996;271:11272–11279. doi: 10.1074/jbc.271.19.11272. [DOI] [PubMed] [Google Scholar]
- Leiber D, Banno Y, Tanfin Z. Exogenous sphingosine 1-phosphate and sphingosine kinase activated by endothelin-1 induced myometrial contraction through differential mechanisms. Am J Physiol Cell Physiol. 2007;292:C240–C250. doi: 10.1152/ajpcell.00023.2006. [DOI] [PubMed] [Google Scholar]
- Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, et al. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem. 2003;278:40330–40336. doi: 10.1074/jbc.M304455200. [DOI] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- McGiffert C, Contos JJ, Friedman B, Chun J. Embryonic brain expression analysis of lysophospholipid receptor genes suggests roles for s1p(1) in neurogenesis and s1p(1–3) in angiogenesis. FEBS Lett. 2002;531:103–108. doi: 10.1016/s0014-5793(02)03404-x. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Murata N, Sato K, Kon J, Tomura H, Yanagita M, Kuwabara A, et al. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem J. 2000;352 Part 3:809–815. [PMC free article] [PubMed] [Google Scholar]
- Nofer JR, van der Giet M, Tolle M, Wolinska I, von Wnuck Lipinski K, Baba HA, et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest. 2004;113:569–581. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori T, Yatomi Y, Osada M, Kazama F, Takafuta T, Ikeda H, et al. Sphingosine 1-phosphate induces contraction of coronary artery smooth muscle cells via S1P2. Cardiovasc Res. 2003;58:170–177. doi: 10.1016/s0008-6363(03)00260-8. [DOI] [PubMed] [Google Scholar]
- Okazaki H, Ishizaka N, Sakurai T, Kurokawa K, Goto K, Kumada M, et al. Molecular cloning of a novel putative G protein-coupled receptor expressed in the cardiovascular system. Biochem Biophys Res Commun. 1993;190:1104–1109. doi: 10.1006/bbrc.1993.1163. [DOI] [PubMed] [Google Scholar]
- Osada M, Yatomi Y, Ohmori T, Ikeda H, Ozaki Y. Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an EDG-5 antagonist. Biochem Biophys Res Commun. 2002;299:483–487. doi: 10.1016/s0006-291x(02)02671-2. [DOI] [PubMed] [Google Scholar]
- Salomone S, Yoshimura S, Reuter U, Foley M, Thomas SS, Moskowitz MA, et al. S1P(3) receptors mediate the potent constriction of cerebral arteries by sphingosine-1-phosphate. Eur J Pharmacol. 2003;469:125–134. doi: 10.1016/s0014-2999(03)01731-x. [DOI] [PubMed] [Google Scholar]
- Tolle M, Levkau B, Keul P, Brinkmann V, Giebing G, Schonfelder G, et al. Immunomodulator FTY720 induces eNOS-dependent arterial vasodilatation via the lysophospholipid receptor S1P3. Circ Res. 2005;96:913–920. doi: 10.1161/01.RES.0000164321.91452.00. [DOI] [PubMed] [Google Scholar]
- Tosaka M, Okajima F, Hashiba Y, Saito N, Nagano T, Watanabe T, et al. Sphingosine 1-phosphate contracts canine basilar arteries in vitro and in vivo: possible role in pathogenesis of cerebral vasospasm. Stroke. 2001;32:2913–2919. doi: 10.1161/hs1201.099525. [DOI] [PubMed] [Google Scholar]
- Waeber C, Blondeau N, Salomone S. Vascular sphingosine-1-phosphate S1P1 and S1P3 receptors. Drug News Perspect. 2004;17:365–382. doi: 10.1358/dnp.2004.17.6.829028. [DOI] [PubMed] [Google Scholar]
- Wei SH, Rosen H, Matheu MP, Sanna MG, Wang SK, Jo E, et al. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat Immunol. 2005;6:1228–1235. doi: 10.1038/ni1269. [DOI] [PubMed] [Google Scholar]
- Yang AH, Ishii I, Chun J. In vivo roles of lysophospholipid receptors revealed by gene targeting studies in mice. Biochim Biophys Acta. 2002;1582:197–203. doi: 10.1016/s1388-1981(02)00172-5. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, Asazuma N, et al. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem (Tokyo) 1997;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Ohmori T, Rile G, Kazama F, Okamoto H, Sano T, et al. Sphingosine 1-phosphate as a major bioactive lysophospholipid that is released from platelets and interacts with endothelial cells. Blood. 2000;96:3431–3438. [PubMed] [Google Scholar]