Abstract
Background and purpose.
Based on their proven ability, in animal models of stroke, to reduce damage to brain grey matter, many drugs have been tested in clinical trials but without success. Failure to save axons from injury and to protect functional outcome has been proposed as the major reason for this lack of success. We have previously demonstrated in two rodent models of cerebral ischaemia, that AS601245 (1,3-benzothiazol-2-yl (2-{[2-(3-pyridinyl) ethyl] amino}-4 pyrimidinyl) acetonitrile), an inhibitor of the c-Jun NH2-terminal kinase (JNK), has neuroprotective properties. The aim of the present study was to further investigate if AS601245 in addition to its ability to protect neurons also could protect neurites and preserve memory after cerebral ischaemia, in gerbils.
Experimental approach.
Using immunohistochemical techniques and a behavioural test, we studied the effect of the compound AS601245 on neurodegeneration and cognitive deficits after global cerebral ischaemia in gerbils.
Key results.
At a dose of 80 mg kg−1, i.p., AS601245 reduced damage to neurites by 67% (P<0.001 versus controls) and activation of astrocytes by 84% (P<0.001 versus controls). In addition, AS601245 (80 mg kg−1, i.p.) prevented ischaemia-induced impairment of memory in the inhibitory avoidance task model.
Conclusions and implications.
The present results suggest that AS601245 reduced damage to neurites and decreased astrogliosis following global ischaemia and also improved long-term memory, supporting JNK inhibition as a promising therapeutic strategy for ischaemic insults to the CNS.
Keywords: global cerebral ischaemia, JNK, neuroprotection, functional recovery, memory
Introduction
Ischaemic stroke is the third leading cause of death and of long-term disability in the United States and in Europe. The interruption of cerebral blood flow induces a sudden drop in adenosine triphosphate production in the brain, which results in generation of oxygen radicals, inflammation, excitotoxicity and, finally, axonal damage and neuronal cell death. Therapeutic strategies for ischaemic stroke target restoration of the cerebral blood flow and protection from neuronal death, thus reducing lesion volumes. Up to now, the only therapy to be approved by health authorities for ischaemic stroke is the intravenous administration of recombinant tissue plasminogen activator as a thrombolytic agent, to restore as early as possible cerebral blood flow in infarcting brain tissues (The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group, 1995). In contrast, several neuroprotective therapies that were able to reduce ischaemic injury in preclinical models of stroke failed when tested in clinical trials. Several explanations for this failure have been offered (De Keyser et al., 1999; Liebeskind and Kasner, 2001; Ovbiagele et al., 2003), including the timing of the intervention, the selection of the appropriate dose and duration of the therapy, and the choice of the appropriate patient population. In addition, several neuroprotective strategies for compound development protected only grey matter from damage in animal models, ignoring the axonal injury that also contributes to neurological damage and functional deficits (Gladstone et al., 2002). Relevant to this issue is the observation that ebselen, a lipid-soluble seleno-organic compound, significantly improved the outcome of patients with ischaemic stroke in a phase 3 clinical trial (Yamaguchi et al., 1998) and also significantly reduced grey and white matter damage in a rodent model of focal cerebral ischaemia (Imai et al., 2001). On the contrary, many drugs targeting N-methyl-D-aspartate receptors failed in clinical trials, even if they were able to protect grey matter from ischaemic insults (McCulloch, 1992). Dewar et al. (1999) suggested that the lack of efficacy of such compounds could be linked to their inability to also protect axons and oligodendrocytes.
Recent evidences suggest the use of c-Jun NH2-terminal kinase (JNK) inhibitors as an effective therapy to prevent neuronal cell death (Zhang and Zhang, 2005). We have previously demonstrated that the benzothiazole derivative AS601245 (1,3-benzothiazol-2-yl (2-{[2-(3-pyridinyl) ethyl] amino}-4 pyrimidinyl) acetonitrile), a new potent adenosine triphosphate-competitive JNK inhibitor, provided significant protection against the delayed loss of hippocampal CA1 neurons in a gerbil model of transient global ischaemia and was also neuroprotective in rats after focal cerebral ischaemia (Carboni et al., 2004). The ischaemia-induced phospho-c-Jun expression was attenuated by AS601245, providing a mechanism through which AS601245 blocks the loss of neuronal cells. These data, together with toxicological studies showing that AS601245 does not interact with physiological parameters, especially arterial pressure, blood glucose and temperature, suggest that the neuroprotective properties of AS601245 are likely to be related to its ability to block JNK, its downstream molecule c-Jun and dependent caspase signalling pathways (Carboni et al., 2005). Although AS601245 is selective for JNK against many other kinases (Carboni et al., 2004), it is however not possible to formally rule out from our previous studies that other signalling pathways might also contribute to the neuroprotection. Our previous findings suggested that AS601245 could be a promising neuroprotective treatment for stroke. However, functional outcomes and protection of axons in addition to neuronal cell bodies were not investigated.
Hunter et al. (1998) have suggested that any successful strategy to evaluate the neuroprotective efficacy of anti-ischaemic agents in animal models of cerebral ischaemia should focus on combining both histopathological and neurobehavioural studies. Thus, the aim of the present study was to determine whether after transient global ischaemia in gerbils, AS601245 was able to reduce injury to neurites and astrogliosis and to prevent ischaemia-induced impairment of long-term memory.
Methods
Cerebral ischaemia
All animal procedures and the experimental protocol were designed in accordance with the European Communities Council Directive (86/609/EEC), which complies with the Swiss code of practice for the care and use of animals for scientific purposes. Sixty-eight adult male Mongolian gerbils (Elevage Janvier, Le Genest St Isle, France) were kept in a temperature- (20±1 °C) and light/dark cycle-controlled environment (lights on at 0700 hours, off at 1900 hours). Standard laboratory chow (UAR, Villemoisson-sur-Orge, France) and water were available ad libitum.
Gerbils were anaesthetized with 4% isoflurane (Baxter, Volketswil, Switzerland) in air (medical grade), administered via facemask. Anaesthesia was then maintained using 3% isoflurane until the end of the surgery. Bilateral common carotid arteries were dissected and occluded with bulldog clamps for 5 min. In the sham-operated group, the same procedure was performed without carotid occlusion. AS601245 in saline was administered intraperitoneally 15 min and 24 h after restoration of carotid blood flow. For behavioural testing and histology, the doses used were 40, 60 and 80 mg kg−1. They were known from previous studies to be devoid of effect on locomotor activity in rodents. For immunohistochemistry, only the dose of 80 mg kg−1 was used. Control animals received the same volume of saline.
Histology
After behavioural testing (10 days after cerebral ischaemia), the animals were killed by decapitation, their brains frozen at −20 °C in 2-methylbutane and then cut in 20-μm-thick sections in a cryocut (Microm HM 500 OM, Walldorf, Germany). The hippocampus was examined for histopathological changes (data not shown). The sections were stained with cresyl violet acetate and examined under the microscope. Damage in the whole hippocampus was scored on four sections with a 0–4 scale (Gronborg et al., 1999). The total score was obtained as the sum of scores in the right and the left hippocampi.
Immunohistochemistry for glial fibrillary acidic protein and non-phosphorylated neurofilaments
Seven days following transient ischaemia, the animals were deeply anaesthetized with 60 mg kg−1 intraperitoneal pentobarbital (Veterinaria, Zurich, Switzerland) and were perfused transcardially with cold 0.1 M phosphate-buffered saline, followed by cold 4% phosphate-buffered saline-buffered paraformaldehyde (Sigma-Aldrich, Schweiz, Switzerland). Brains were removed and postfixed for 24 h at 4 °C and then transferred to 20% sucrose (Fluka, Steinheim, Germany) in phosphate-buffered saline at 4 °C overnight. Frozen coronal sections (40 μm in thickness) of the brains were prepared using a cryocut and processed by the free-floating method. After quenching endogenous peroxidase in 2% H2O2 (Fluka) and blocking with 30% bovine serum albumin, the sections were incubated overnight at 4 °C with a rabbit anti-glial fibrillary acidic protein (GFAP) polyclonal antibody (Chemicon, Temecula, USA) or a mouse anti-non-phosphorylated neurofilament monoclonal antibody (clone SMI32; Sternberger Monoclonal Incorporated, Baltimore, USA) at 1:1000 dilution. On the second day, the sections were incubated in biotinylated goat anti-rabbit secondary antibody and Vector avidin-biotin-peroxidase complex (Vector Laboratories, Burlingame, USA). The immunoreactive staining was visualized with a DAB substrate kit (Sigma-Aldrich). Positive (primary antibody isotype) and negative (omission of the primary antibody) controls for each antibody were included in the protocol and minimal staining was detected (data not shown). Each section was mounted, dehydrated and coverslipped.
The area occupied by GFAP-positive cells was determined using AnalySIS image software (Soft Imaging System, Munster, Germany) in the right and left hippocampi of each animal and expressed as percentage of total hippocampus area (100%) on the right or left side, respectively. Results were given as the GFAP-positive cell indexes in both the right and left hippocampi of each gerbil. For SMI32 staining, positive fibres were counted in a 300 × 300 μm2 area, applied at the centre of the CA1 region. Measures were repeated four times in each animal.
Behavioural evaluation
Long-term memory ability was evaluated by assessing the latency of a step-down inhibitory avoidance task (Karasawa et al., 1994). The box (20 cm long, 22 cm wide with 30 cm high walls; Letica Scientific Instruments, Barcelona, Spain) was divided into an electrified grid floor (20 cm long and 22 cm wide) and a safe platform (10 cm long, 10 cm wide, 3 cm high). Training was carried out 7 days after ischaemia. Each animal was placed initially on the safety platform. When the animal stepped down, it received a foot shock (0.6 mA). This training session lasted 10 min. Twenty-four hours later, the animal was placed again on the safe platform and the response latency (the time until it stepped down to the grid floor) was measured. If the animal did not step down to the grid floor within 3 min, a score of 180 s was assigned and the experiment was stopped. The test was repeated during two other consecutive days.
Statistical analysis
Data obtained from experiments were expressed as the mean of independent values±s.e.m. and were analysed using one-way analysis of variance (ANOVA) followed by Dunnett's or Bonferroni t-test. Values of P<0.05 were considered to show statistical significance.
Results
AS601245 protects neuronal cell bodies and neurites from ischaemic insult
The hippocampus is selectively vulnerable to global ischaemia, and neuronal death occurs in a delayed manner (Kirino, 1982, 2000). To confirm the neuroprotective properties of AS601245 (Carboni et al., 2004), damage to the cell bodies was evaluated by histological analysis using cresyl violet staining (data not shown). We assessed the effect of three AS601245 doses (80, 60 and 40 mg kg−1 intraperitoneally) using a histological ischaemic score and found a significant and dose-dependent reduction (P<0.01) in hippocampal damage (55±11, 40±15 and 24±9% of controls, respectively) as previously reported.
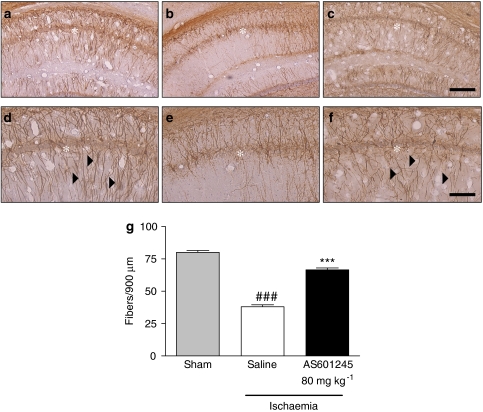
We further investigated the efficacy of AS601245 to protect the neurites from hippocampal CA1 neurons, using AS601245 only at the highest dose of 80 mg kg−1 intraperitoneally. One immunohistochemical marker that we used was SMI32, a monoclonal antibody directed against non-phosphorylated neurofilament H, described to label neuronal cell bodies, dendrites and some thick axons in the central and peripheral nervous systems (Sternberger and Sternberger, 1983). Interestingly, in gerbils, this antibody stained a number of dendrites in the control samples (Figures 1a and d). This observation is in accordance with the reports of Gai et al. (1994) showing SMI32 staining in substantia nigra of normal human brain and loss of staining in the substantia nigra of patients with Parkinson's disease. In our experiments, SMI32-stained neurites in the hippocampus were lost after ischaemic insult (Figures 1b and e). Treatment with AS601245 increased the number of immunopositive neurites 7 days after ischaemia (Figures 1c and f). We quantified the loss of SMI32-positive fibres in CA1 area of saline-treated ischaemic gerbils and showed a pronounced reduction of SMI32-labelled fibres compared to sham-operated gerbils (Figure 1g). Neurites expressing the non-phosphorylated form of neurofilaments were more abundant in AS601245-treated ischaemic gerbils than in respective controls (Figure 1g).
Figure 1.
Effects of AS601245 administration on SMI32 immunostaining in the hippocampus after global ischaemia. (a–f) Representative microphotographs showing SMI32 immunostaining in the CA1 layer of the hippocampus in sham-operated gerbils ((a) low magnification; (d) high magnification), or in gerbils exposed to 5 min of ischaemia followed by 7 days of reperfusion in the absence of ((b) low magnification; (e) high magnification) or in the presence of AS601245 (80 mg kg−1, intraperitoneally) ((c) low magnification; (f) high magnification). Asterisks indicate pyramidal cell layer and arrowheads indicate vertical fine fibres. Scale bar, 200 μm (a–c) and 100 μm (d–f). (g) Quantification of SMI32 immunostaining in the CA1 layer of the hippocampus in sham-operated, saline-treated and AS601245-treated ischaemic animals. Each bar represents the mean±s.e.m. results from six animals. ###P<0.001 versus sham group and ***P<0.001 versus saline-treated group.
AS601245 reduces astrogliosis in the hippocampus after cerebral ischaemia
Glial fibrillary acidic protein is used as a marker of proliferation or activation of astrocytes after various stimuli, including global cerebral ischaemia (Petito et al., 1990; Petito and Halaby, 1993). In our study, GFAP immunoreactivity was rare in the hippocampus of sham-operated animals, representing 6% of the hippocampal area (Figure 2a). In contrast, the hippocampus from ischaemic animals receiving saline showed a strong immunoreactivity, which covered the entire area of GFAP-positive cells (Figure 2b). This staining was significantly reduced in AS601245-treated ischaemic gerbils (by 83±3%; P<0.001 versus saline-treated gerbils; Figures 2c and d).
Figure 2.
Effects of AS601245 administration on glial fibrillary acidic protein (GFAP) immunostaining in the hippocampus after global ischaemia. (a–c) Representative microphotographs showing GFAP immunostaining in the hippocampus in sham-operated (a), saline-treated (b) and AS601245-treated ischaemic animals (80 mg kg−1, intraperitoneally (i.p)) (c). Asterisks indicate pyramidal cell layer. Scale bar, 500 μm. (d) Quantification of GFAP immunostaining in the hippocampus of sham-operated animals or gerbils exposed to 5 min ischaemia followed by 7 days of reperfusion and treated by either saline or AS601245 (80 mg kg−1, i.p.). Area occupied by GFAP-positive cells was determined by image analysis, independently in the right and left hippocampi of each animal and expressed as percentage of total hippocampus area (100%) on the right or left side, respectively. Results are the GFAP-positive cell indexes in both the right and left hippocampi of each gerbil, and each bar represents the mean±s.e.m. of six animals per group. ***P<0.001 versus sham group and ###P<0.001 versus saline-treated group.
AS601245 preserves memory function after ischaemia
Seven days after cerebral ischaemia, animals were trained in a step-down type of inhibitory avoidance task apparatus. Response latency was measured 8, 9 and 10 days after ischaemia (Table 1). At day 8, the response latency exceeded the experimental limit (180 s) in sham-operated gerbils and decreased significantly in ischaemic saline-treated animals (P<0.01). The response latency time decreased further during the following 2 days (P<0.01). AS601245 dose-dependently improved the response latency, but only the highest dose (80 mg kg−1) caused a statistically significant effect in the response latency time at days 8, 9 and 10 after ischaemia induction (P<0.01 versus ischaemic vehicle-treated animals). The dose of 60 mg kg−1 induced a significant effect (P<0.05 versus ischaemic vehicle-treated animals) only at day 9, while the dose of 40 mg kg−1 did not change the latency time on any of the days of testing (P>0.05 versus ischaemic vehicle-treated animals).
Table 1.
Effects of treatment with AS601245 on the response latency time in a passive avoidance task, after cerebral ischaemia
| Groups | Latency time (s), day 8 after ischaemia | Latency time (s), day 9 after ischaemia | Latency time (s), day 10 after ischaemia |
|---|---|---|---|
| Sham-operated | 180±0 | 169±7 | 160±15 |
| Vehicle-treated ischaemic | 139±20a | 90±23a | 68±23a |
| AS601245 (40 mg kg−1)-treated ischaemic | 132±20a | 82±21a | 83±23a |
| AS601245 (60 mg kg−1)-treated ischaemic | 140±19a | 114±21a,b | 90±23a |
| AS601245 (80 mg kg−1)-treated ischaemic | 173±6c | 151±1c | 136±17c |
The compound or vehicle was given intraperitoneally 15 min and 24 h after the restoration of carotid blood flow, following 5 min global cerebral ischaemia. Data are means±s.e.m. (n=10 animals).
P<0.01 versus the sham-operated group.
P<0.05 versus vehicle-treated ischaemic group.
P<0.01 versus vehicle-treated ischaemic group.
Discussion
The present study shows that the small molecule JNK inhibitor AS601245, in addition to its ability to protect neuronal cell bodies (Carboni et al., 2004), also prevents loss of neurites, decreases astrogliosis and improves long-term memory deficits induced by cerebral ischaemia.
Preclinical models of stroke have been developed in many species using different procedures (Ginsberg and Busto, 1989; Hossmann, 1998). Among the existing models, many suffer from poor reproducibility and standardization. One model, which is more satisfactory in this respect, is experimental global transient ischaemia in gerbils because of the vulnerability of the CA1 neurons of the hippocampus. The global model of cerebral ischaemia is therefore one of the most popular models for evaluating neuroprotective agents (Small and Buchan, 2000). In fact, in the Mongolian gerbil, transient bilateral carotid occlusion induces neuronal death, mainly in the CA1 and CA2 pyramidal area of the hippocampus, as this species lacks functional posterior communicating arteries that are necessary to complete the circle of Willis. The areas of neuronal degeneration in the hippocampus can be evaluated quite accurately in individual animals and is highly reproducible in all animals. Nevertheless, although grey matter lesions arising from bilateral carotid occlusion in gerbils have been extensively studied, the accompanying lesions to axons and dendrites have not been thoroughly investigated. Damage to axons results in cytoskeletal breakdown involving both microtubules and neurofilament proteins. Neurofilaments are the main constituent of the axonal cytoskeleton (Hoffman et al. 1992; Mukhopadhyay et al., 2004; Chan et al., 2005), and their phosphorylation states are dynamically regulated by myelination, demyelination and pathological changes in the axon. In animal models, neurodegeneration causes loss of neurofilament immunoreactivity (Gai et al., 1994). The marker SMI32 was used in the present study to recognize non-phosphorylated neurofilaments. While most neurons and neurites in the CA1 region of the hippocampus from animals of the saline-treated ischaemic group failed to label with SMI32, the perikaryon and processes of neurons from gerbils of the sham group labelled intensely. Previous studies have shown that SMI32 identified a subset of cortical neurons preferentially lost in neurodegenerative diseases (Ang et al., 1991; Campbell et al., 1991; Hof and Morrison, 1995). Furthermore, Gai et al. (1994) suggested that in Parkinson's disease, loss of neurofilament immunoreactivity might be a sensitive marker for characterization of degenerating neurons. These data together with our results would suggest that in global cerebral ischaemia in gerbils, SMI32 could label a population of hippocampal neurons with their processes that would be preferentially lost following transient cerebral ischaemia. The fact that the loss of SMI32 staining so closely parallels the loss of the majority of the CA1 neurons is consistent with this hypothesis and would deserve further analysis.
Astrogliosis, as revealed by reactive changes in GFAP immunostaining, has been shown to occur in the hippocampus after global ischaemia. It has been reported that changes in GFAP immunoreactivity correlated with neuronal degeneration (Petito and Halaby, 1993; Loos et al., 2003; Soltys et al., 2003). Consistent with these observations, in our study we found an increase in GFAP immunostaining in the hippocampus of saline-treated ischaemic gerbils when compared to sham-operated animals. In addition, AS601245 at the highest dose (80 mg kg−1) prevented, at least partially, this increase in GFAP immunostaining.
These results suggest that AS601245 ameliorated ischaemic damage in the hippocampus not only by saving cell bodies (Carboni et al., 2004) but also by preventing gliosis (Figure 2) and axonal degeneration (Figure 1). That AS601245 does not interact with physiological parameters (arterial pressure, blood glucose and temperature) strongly suggests that these results are likely to be related to the neuroprotective properties of this compound. This situation is in contrast to that reported with compounds like MK-801 (dizocilpine) where perikarya were protected against ischaemia-induced degeneration (Park et al., 1988), while axons and oligodendrocytes were still damaged (Yam et al., 2000). Thus, protection of the perikarya does not necessarily correlate with protection of axons and oligodendrocytes, suggesting that the results of our study are specific features of the neuroprotective effect with the JNK inhibitor AS601245, even if axonal protection is a consequence of the cell body preservation, a hypothesis that cannot be ruled out from our data. Also protection of the neurites and prevention of the gliosis by AS601245 cannot be directly related to its putative mechanism of action, that is, inhibition of the JNK signalling pathway and subsequent caspases (Carboni et al., 2005), as the molecular mechanisms leading to ischaemic brain damage are complex, with possible overlaps, and involve excitotoxicity, ionic imbalance, oxidative and nitrosative stress as well as apoptosis-like phenomena (Lo et al., 2003).
With regard to the possibility that not only was the cellular structure of neurons preserved but also the neurons remained functionally active, we evaluated the effects of AS601245 on the ischaemia-induced long-term memory impairment using a step-down inhibitory avoidance task. It is well known that functions like learning and memory could be affected after hippocampal damage (Olton et al., 1978; Volpe et al., 1985), including ischaemic insult, as shown by lower performance in an inhibitory avoidance task (Karasawa et al., 1994; Block, 1999). In our study, ischaemic animals treated with 80 mg kg−1 AS601245 performed better than saline-treated animals, in the same inhibitory avoidance paradigm, at a dose where there was also protection of neuritis. Together, these data corroborate the hypothesis that AS601245 treatment is not only neuroprotective, but it also has a functional relevance.
In conclusion, this study demonstrated that a small molecule inhibitor of the JNK pathway was not only able to reduce damage to neurites induced by global ischaemia in gerbils but also improve the associated long-term memory impairment, supporting JNK inhibition as a promising therapeutic strategy for treatment of ischaemic insults to the brain.
Abbreviations
- AS601245
(1,3-benzothiazol-2-yl (2-{[2-(3-pyridinyl) ethyl] amino}-4 pyrimidinyl) acetonitrile)
- GFAP
glial fibrillary acidic protein
- JNK
c-Jun NH2-terminal kinase
- MK-801
dizocilpine
Conflicts of interest
The authors state no conflict of interest. However, all the authors were/are employed by Merck Serono.
References
- Ang LC, Munoz DG, Shul D, George DH. SMI-32 immunoreactivity in human striate cortex during postnatal development. Brain Res Dev Brain Res. 1991;61:103–109. doi: 10.1016/0165-3806(91)90119-4. [DOI] [PubMed] [Google Scholar]
- Block F. Global ischemia and behavioural deficits. Prog Neurobiol. 1999;58:279–295. doi: 10.1016/s0301-0082(98)00085-9. [DOI] [PubMed] [Google Scholar]
- Campbell MJ, Hof PR, Morrison JH. A subpopulation of primate corticocortical neurons is distinguished by somatodendritic distribution of neurofilament protein. Brain Res. 1991;539:133–136. doi: 10.1016/0006-8993(91)90695-r. [DOI] [PubMed] [Google Scholar]
- Carboni S, Antonsson B, Gaillard P, Gotteland JP, Gillon JY, Vitte PA. Control of death receptor and mitochondrial-dependent apoptosis by c-Jun N-terminal kinase in hippocampal CA1 neurones following global transient ischaemia. J Neurochem. 2005;92:1054–1060. doi: 10.1111/j.1471-4159.2004.02925.x. [DOI] [PubMed] [Google Scholar]
- Carboni S, Hiver A, Szyndralewiez C, Gaillard P, Gotteland JP, Vitte PA. AS601245 (1,3-benzothiazol-2-yl (2-[[2-(3-pyridinyl) ethyl] amino]-4 pyrimidinyl) acetonitrile): a c-Jun NH2-terminal protein kinase inhibitor with neuroprotective properties. J Pharmacol Exp Ther. 2004;310:25–32. doi: 10.1124/jpet.103.064246. [DOI] [PubMed] [Google Scholar]
- Chan WK, Yabe JT, Pimenta AF, Ortiz D, Shea TB. Neurofilaments can undergo axonal transport and cytoskeletal incorporation in a discontinuous manner. Cell Motil Cytoskeleton. 2005;62:166–179. doi: 10.1002/cm.20089. [DOI] [PubMed] [Google Scholar]
- De Keyser J, Sulter G, Luiten PG. Clinical trials with neuroprotective drugs in acute ischaemic stroke: are we doing the right thing. Trends Neurosci. 1999;22:535–540. doi: 10.1016/s0166-2236(99)01463-0. [DOI] [PubMed] [Google Scholar]
- Dewar D, Yam P, McCulloch J. Drug development for stroke: importance of protecting cerebral white matter. Eur J Pharmacol. 1999;375:41–50. doi: 10.1016/s0014-2999(99)00280-0. [DOI] [PubMed] [Google Scholar]
- Gai WP, Vickers JC, Blumbergs PC, Blessing WW. Loss of non-phosphorylated neurofilament immunoreactivity, with preservation of tyrosine hydroxylase, in surviving substantia nigra neurons in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1994;57:1039–1046. doi: 10.1136/jnnp.57.9.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MD, Busto R. Rodent models of cerebral ischemia. Stroke. 1989;20:1627–1642. doi: 10.1161/01.str.20.12.1627. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- Gronborg M, Johansen TE, Peters D, Ahring PK, Drejer J, Moller A, et al. Neuroprotection by a novel compound, NS521. J Pharmacol Exp Ther. 1999;290:348–353. [PubMed] [Google Scholar]
- Hof PR, Morrison JH. Neurofilament protein defines regional patterns of cortical organization in the macaque monkey visual system: a quantitative immunohistochemical analysis. J Comp Neurol. 1995;352:161–186. doi: 10.1002/cne.903520202. [DOI] [PubMed] [Google Scholar]
- Hoffman PN, Lopata MA, Watson DF, Luduena RF. Axonal transport of class II and III beta-tubulin: evidence that the slow component wave represents the movement of only a small fraction of the tubulin in mature motor axons. J Cell Biol. 1992;119:595–604. doi: 10.1083/jcb.119.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossmann KA. Experimental models for the investigation of brain ischemia. Cardiovasc Res. 1998;39:106–120. doi: 10.1016/s0008-6363(98)00075-3. [DOI] [PubMed] [Google Scholar]
- Hunter AJ, Mackay KB, Rogers DC. To what extent have functional studies of ischaemia in animals been useful in the assessment of potential neuroprotective agents. Trends Pharmacol Sci. 1998;19:59–66. doi: 10.1016/s0165-6147(97)01157-7. [DOI] [PubMed] [Google Scholar]
- Imai H, Masayasu H, Dewar D, Graham DI, Macrae IM. Ebselen protects both gray and white matter in a rodent model of focal cerebral ischemia. Stroke. 2001;32:2149–2154. doi: 10.1161/hs0901.095725. [DOI] [PubMed] [Google Scholar]
- Karasawa Y, Araki H, Otomo S. Changes in locomotor activity and passive avoidance task performance induced by cerebral ischemia in Mongolian gerbils. Stroke. 1994;25:645–650. doi: 10.1161/01.str.25.3.645. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death. Neuropathology. 2000;20 Suppl:S95–S97. doi: 10.1046/j.1440-1789.2000.00306.x. [DOI] [PubMed] [Google Scholar]
- Liebeskind DS, Kasner SE. Neuroprotection for ischaemic stroke: an unattainable goal. CNS Drugs. 2001;15:165–174. doi: 10.2165/00023210-200115030-00001. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Loos M, Dihne M, Block F. Tumor necrosis factor-alpha expression in areas of remote degeneration following middle cerebral artery occlusion of the rat. Neuroscience. 2003;122:373–380. doi: 10.1016/s0306-4522(03)00498-6. [DOI] [PubMed] [Google Scholar]
- McCulloch J. Excitatory amino acid antagonists and their potential for the treatment of ischaemic brain damage in man. Br J Clin Pharmacol. 1992;34:106–114. doi: 10.1111/j.1365-2125.1992.tb04118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay R, Kumar S, Hoh JH. Molecular mechanisms for organizing the neuronal cytoskeleton. Bioessays. 2004;26:1017–1025. doi: 10.1002/bies.20088. [DOI] [PubMed] [Google Scholar]
- Olton DS, Walker JA, Gage FH. Hippocampal connections and spatial discrimination. Brain Res. 1978;139:295–308. doi: 10.1016/0006-8993(78)90930-7. [DOI] [PubMed] [Google Scholar]
- Ovbiagele B, Kidwell CS, Starkman S, Saver JL. Neuroprotective agents for the treatment of acute ischemic stroke. Curr Neurol Neurosci Rep. 2003;3:9–20. doi: 10.1007/s11910-003-0031-z. [DOI] [PubMed] [Google Scholar]
- Park CK, Nehls DG, Graham DI, Teasdale GM, McCulloch J. The glutamate antagonist MK-801 reduces focal ischemic brain damage in the rat. Ann Neurol. 1988;24:543–551. doi: 10.1002/ana.410240411. [DOI] [PubMed] [Google Scholar]
- Petito CK, Halaby IA. Relationship between ischemia and ischemic neuronal necrosis to astrocyte expression of glial fibrillary acidic protein. Int J Dev Neurosci. 1993;11:239–247. doi: 10.1016/0736-5748(93)90082-o. [DOI] [PubMed] [Google Scholar]
- Petito CK, Morgello S, Felix JC, Lesser ML. The two patterns of reactive astrocytosis in postischemic rat brain. J Cereb Blood Flow Metab. 1990;10:850–859. doi: 10.1038/jcbfm.1990.141. [DOI] [PubMed] [Google Scholar]
- Small DL, Buchan AM. Animal models. Br Med Bull. 2000;56:307–317. doi: 10.1258/0007142001903238. [DOI] [PubMed] [Google Scholar]
- Soltys Z, Janeczko K, Orzylowska-Sliwinska O, Zaremba M, Januszewski S, Oderfeld-Nowak B. Morphological transformations of cells immunopositive for GFAP, TrkA or p75 in the CA1 hippocampal area following transient global ischemia in the rat. A quantitative study. Brain Res. 2003;987:186–193. doi: 10.1016/s0006-8993(03)03327-4. [DOI] [PubMed] [Google Scholar]
- Sternberger LA, Sternberger NH. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci USA. 1983;80:6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Volpe BT, Pulsinelli WA, Davis HP. Amnesia in humans and animals after ischemic cerebral injury. Ann NY Acad Sci. 1985;444:492–493. doi: 10.1111/j.1749-6632.1985.tb37621.x. [DOI] [PubMed] [Google Scholar]
- Yam PS, Dunn LT, Graham DI, Dewar D, McCulloch J. NMDA receptor blockade fails to alter axonal injury in focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20:772–779. doi: 10.1097/00004647-200005000-00003. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, et al. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke. 1998;29:12–17. doi: 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- Zhang GY, Zhang QG. Agents targeting c-Jun N-terminal kinase pathway as potential neuroprotectants. Expert Opin Investig Drugs. 2005;14:1373–1383. doi: 10.1517/13543784.14.11.1373. [DOI] [PubMed] [Google Scholar]