Abstract
Reactive carbonyl compounds (RCCs) formed during lipid peroxidation and sugar glycoxidation, namely Advanced lipid peroxidation end products (ALEs) and Advanced Glycation end products (AGEs), accumulate with ageing and oxidative stress-related diseases, such as atherosclerosis, diabetes or neurodegenerative diseases. RCCs induce the ‘carbonyl stress' characterized by the formation of adducts and cross-links on proteins, which progressively leads to impaired protein function and damages in all tissues, and pathological consequences including cell dysfunction, inflammatory response and apoptosis. The prevention of carbonyl stress involves the use of free radical scavengers and antioxidants that prevent the generation of lipid peroxidation products, but are inefficient on pre-formed RCCs. Conversely, carbonyl scavengers prevent carbonyl stress by inhibiting the formation of protein cross-links. While a large variety of AGE inhibitors has been developed, only few carbonyl scavengers have been tested on ALE-mediated effects. This review summarizes the signalling properties of ALEs and ALE-precursors, their role in the pathogenesis of oxidative stress-associated diseases, and the different agents efficient in neutralizing ALEs effects in vitro and in vivo. The generation of drugs sharing both antioxidant and carbonyl scavenger properties represents a new therapeutic challenge in the treatment of carbonyl stress-associated diseases.
Keywords: ALE, atherosclerosis, cancer, carbonyl scavenger, carbonyl stress, diabetes, 4-hydroxynonenal, inflammation, lipid peroxidation, neurodegenerative diseases
Introduction
Reactive carbonyl compounds (RCCs) formed endogenously during lipid peroxidation and the glycoxidation of carbohydrates are precursors of advanced glycation end products (AGEs) and advanced lipid peroxidation end products (ALEs), which form cross-links on tissular proteins (carbonyl stress), and accumulate during ageing and in chronic diseases (Dalle-Donne et al., 2003; Smit and Lutgers, 2004). Carbonyl stress induces progressively protein dysfunctions and damages in all tissues, with pathological consequences such as inflammation and apoptosis contributing to the progression of diseases (Dalle-Donne et al., 2003; Petersen and Doorn, 2004). Therefore, inhibiting the chemical modification of tissue proteins may prevent the pathological consequences of carbonyl stress and may represent a new therapeutic strategy for patients. Most of the carbonyl stress inhibitors used so far have been developed to prevent the accumulation of AGEs in diabetes and its complications including accelerated atherosclerosis, nephropathy, cataract and neuropathies (Thomas et al., 2005; Peyroux and Sternberg, 2006). In contrast, except antioxidants that inhibit indirectly the generation of lipid peroxidation products, few carbonyl scavenger agents known to reduce in vitro the accumulation of ALEs precursors have been tested in vivo on the progression of ALE-related diseases. This review summarizes the mechanisms involved in the generation of ALE precursors, their targets and role in carbonyl stress and their consequences in ageing and in the pathogenesis of diseases. The review then focusses on carbonyl scavenger agents able to neutralize in vitro and in vivo protein modifications induced by ALE precursors, and their potential interest in pre-clinical and clinical studies, as new pharmacological approaches in carbonyl stress-related diseases.
Formation and signalling properties of ALEs and ALE precursors
Lipid peroxidation induced by oxidants and oxidative stress, generates a huge variety of lipid peroxidation products, including RCCs and more stable products such as ketones and alkanes (Figures 1 and 2). Moreover, the α-oxoaldehyde methylglyoxal may be generated during glycoxidation (Thornalley et al., 1999) and as a by-product of catabolism of lipids, glucose and amino acids (Figure 3) (Peyroux and Sternberg, 2006).
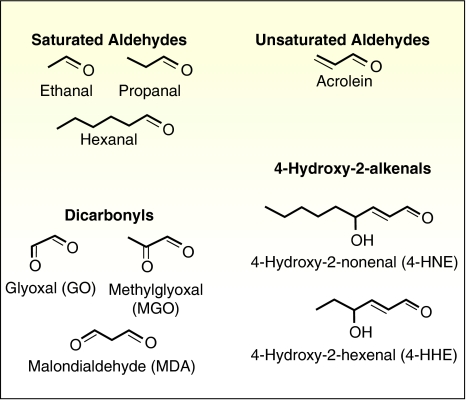
Figure 1.
Chemical formulae of some aldehydic compounds derived from lipid peroxidation, including saturated aldehydes, unsaturated aldehydes, 4-hydroxy-2-alkenals and dicarbonyls. These reactive carbonyl compounds are also advanced lipid peroxidation end product precursors.
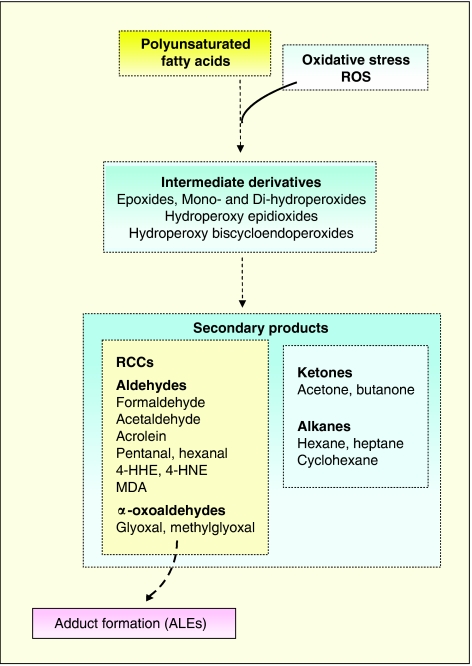
Figure 2.
Schematic steps of lipid peroxidation leading to the formation of secondary products and ALEs (Uchida, 2000; Aldini et al., 2006; Shibamoto, 2006). RCCs are able to react with proteins and other biological molecules, thereby forming ALEs. Stable products (such as alkanes) do not react with proteins. ALEs, advanced lipid peroxidation end products; RCCs, reactive carbonyl compounds.
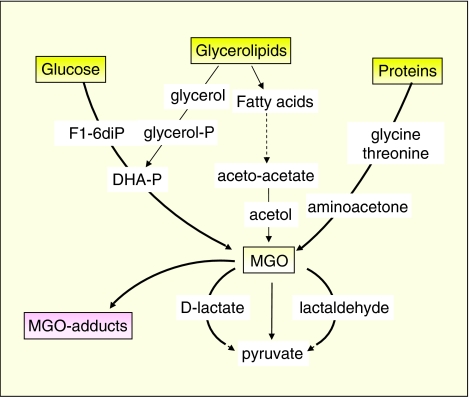
Figure 3.
Metabolic pathways leading to the formation of methylglyoxal (MGO) and of MGO adducts (Ramasamy et al., 2006). In addition to lipid peroxidation and glycoxidation, MGO can be formed as a by-product of glucose, glycerolipids and amino acids.
RCCs such as aldehydes and dicarbonyls, including hydroxyalkenals, acrolein, malondialdehyde (MDA), glyoxal and methylglyoxal, exhibit a large panel of biological properties. These aldehydes react on cellular and tissular proteins to form adducts (ALEs) that induce protein dysfunctions and alter cellular responses (Zarkovic, 2003; Petersen and Doorn, 2004). The rate of oxidation and aldehyde adduct formation is low under physiological conditions, but increases with ageing together with the decrease in antioxidant defences (McEwen et al., 2005; Voss and Siems, 2006). It is a slow process countered by the rapid turnover of short half-life cellular proteins, whereas modified long-life proteins accumulate in tissues with age (Lyons et al., 1991).
Formation of ALE precursors or RCCs
The oxidation of polyunsaturated fatty acids (PUFAs) generates RCCs, including highly reactive α,β-unsaturated hydroxyalkenals, such as 4-hydroxynonenal (4-HNE) and 4-hydroxyhexenal (4-HHE). Oxidation of n-6 PUFAs (mainly linoleic and arachidonic acids) leads to the formation of 4-HNE (Esterbauer, 1993), whereas oxidation of n-3 PUFAs (docosahexaenoic acid, eicosapentaenoic acid and linolenic acid) generates 4-HHE (Van Kuijk et al., 1990).
4-HNE can react with histidine (His), cysteine (Cys) or lysine (Lys) residues of proteins, leading to the formation of stable Michael adducts with a hemiacetal structure (Schaur, 2003). The chemical reactions involved in 4-HNE interactions with proteins are summarized in Figure 4, and include reactions between the C=C double bond with a nucleophile (Cys, glutathione (GSH) and amine) via 1,2- and 1,4-Michael addition (Nadkarni and Sayre, 1995). The 1,2-Michael addition involves the reaction of a primary amine (Lys) with the α,β-unsaturated carbonyl, resulting in the formation of a Schiff base at acidic pH. This step is reversible (Petersen and Doorn, 2004).
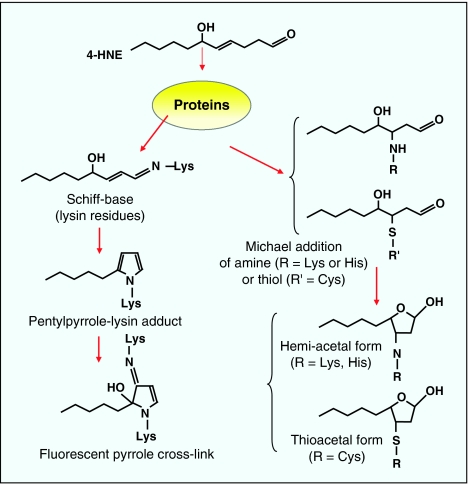
Figure 4.
Schematic reactions of 4-HNE with proteins leading to the formation of 4-HNE-protein adducts. (1) Schiff base formation on primary amine (for example, lysin) leading to more complex compounds, such as pyrrole-Lys adducts and fluorescent cross-links. (2) Michael addition of 4-HNE on amino groups (Lys and His) or thiols (Cys or GSH), followed by cyclization and hemi-acetal or hemi-thioacetal formation (Sayre et al., 1993; Uchida 2000; Carini et al., 2004). 4-HNE, 4-hydroxynonenal; Lys, lysine; His, histidine; Cys, cysteine; GSH, glutathione.
The 1,4-Michael addition of 4-HNE involves the reaction of the nucleophile with the β-carbon, resulting in the addition of the nucleophile and proton across the C=C double bond (Nadkarni and Sayre, 1995). Among the protein residues that react with 4-HNE, Cys exhibits the highest reactivity, followed by His and Lys (Petersen and Doorn, 2004), but Cys–HNE adducts could be less stable than His–HNE adducts (Uchida, 2003). Concerning the modification of apolipoprotein B, 4-HNE was reported to attack mainly the Lys and to a lesser extent His and Cys residues (Jürgens et al., 1986).
MDA and acrolein are formed during lipid peroxidation and bind to nucleophiles (Poli and Schaur, 2000). MDA is one of the most abundant aldehydes, resulting from peroxidation of arachidonic, eicosapentaenoic and docosahexaenoic acid (Esterbauer et al., 1991). MDA reacts with Lys residues by forming Schiff bases (Esterbauer, 1993), and plays a major role in low-density lipoprotein (LDL) modification and their metabolic deviation towards macrophages (Palinski et al., 1989; Steinberg, 1997). Protein modifications by bifunctional aldehydes can also lead to intramolecular or intermolecular protein cross-linking. The accumulation of MDA adducts on proteins is involved in the formation of the fluorescent pigment lipofuscin, which accumulates progressively during ageing (Chowdhury et al., 2004). Acrolein (CH2=CH–CHO) is also formed during lipid peroxidation and is a strong electrophile exhibiting high reactivity with Cys, His and Lys nucleophile residues (Uchida et al., 1998).
Another class of reactive ALE precursors is represented by α-oxoaldehydes (methylglyoxal and glyoxal) (Figure 1). These agents are basically generated during diabetes and hyperglycaemia, from the Maillard reaction, which involves the condensation of sugars (glucose) with proteins, leading to the formation of a Schiff base, followed by a rearrangement (non-enzymatic glycation) generating the Amadori product. During Amadori rearrangement, α-oxoaldehydes (methylglyoxal and glyoxal) are formed, and react with Lys and arginine residues to form AGEs. These α-oxoaldehydes are also generated from lipid peroxidation (glyoxal), the catabolism of ketone bodies and the fragmentation of triosephosphates (methylglyoxal) (Figure 3). α-Oxoaldehydes are increased in the early stages of glycation in hyperglycaemic conditions, and are detected in atherosclerotic lesions (Thornalley et al., 1999).
Methods for the detection of ALE precursors and ALEs modified protein (or RCC/protein adducts)
Determination of ALEs precursors
Several analytical methods have been used to determine RCCs ranging from whole evaluation of RCCs by poorly specific methods, such as TBA reactive substances assay, to highly specific methods allowing a precise structural determination and quantitation.
As ALE precursors are formed in lipid fractions, a first difficulty is because RCCs are chemically reactive compounds that may react during sample preparation. It is therefore necessary to prepare RCCs derivatives, generally as dinitrophenylhydrazine (DNPH) derivatives (Esterbauer et al., 1991). More recently, RCCs derivatives of pentafluorophenylhydrazine, N-methylhydrazine, cysteamine and of o-phenylene diamine have been used successfully and exhibit several analytical advantages, as reviewed recently by Shibamoto (2006).
Another difficulty is the isolation and extraction of these compounds from lipid-rich samples. The classical extraction by solvent partition is difficult because of the amphiphilic character of numerous lipid peroxidation products. This led to develop new and more sophisticated methods, utilizing solid phase extraction cartridge, or ‘simultaneous purging and solvent extraction apparatus' (see review by Shibamoto, 2006).
DNPH derivatives converted into their corresponding hydrazones can be separated and analysed by various methods including gas chromatography, gas chromatography/mass spectrometry, HPLC/UV (high-performance liquid chromatography/ultraviolet) and HPLC/MS (mass spectometry) (Shibamoto, 2006).
Detection of ALEs modified proteins
Classical methods to detect and evaluate carbonyl content of modified protein (ALEs modified proteins) are based on the reaction of DNPH with carbonyl groups to form DNP-hydrazone derivatives that are measured by spectrophotometry. An alternative enzyme-linked immunosorbent assay (ELISA) method is the detection of the DNP moiety of the proteins by specific antibodies (Buss et al., 1997).
Recently, proteomics (mass spectrometry) methods have been used to identify ALEs modified proteins (RCC/protein adducts) after proteolytic digestion and analysis of peptide mass fingerprints by MALDI-TOF-MS or LC-ESI-MS/MS (Carini et al., 2004; Aldini et al., 2006; Sayre et al., 2006).
Polyclonal and specific monoclonal antibodies against RCC–protein adducts (including anti-4-HNE and anti-acrolein antibodies) allowed to visualize the presence of such modified proteins in various tissues, under physiological and pathophysiological conditions (Jurgens et al., 1990; Uchida et al., 1995; Waeg et al., 1996; Xu et al., 2000). Recently, this also allowed developing ELISA techniques for quantitative determination of oxidatively modified LDL and circulating proteins (Satoh et al., 1999; Borovic et al., 2006). Moreover, modification of specific cellular or tissular protein can be evaluated by western blot of the immunoprecipitated protein revealed with specific antibodies (for example, anti-HNE and anti-CML), and the modification can be compared to functional changes (Suc et al., 1998; Vindis et al., 2006).
Molecular and cellular targets and signalling properties of ALEs and ALE precursors
ALEs precursors play an active role in signal transduction by altering progressively the structure and function of circulating and tissular proteins, with consequences on the inflammatory status, cell proliferation and viability (Petersen and Doorn, 2004). The biological effects of ALE precursors are modulated by their local concentration, their availability depending on the presence of cellular detoxifying or metabolizing systems, for instance the conjugation of 4-HNE with cellular GSH catalysed by glutathione S-transferase (GST) and the functionality of proteolytic systems involved in the degradation of modified proteins (Petersen and Doorn, 2004).
LDL modification
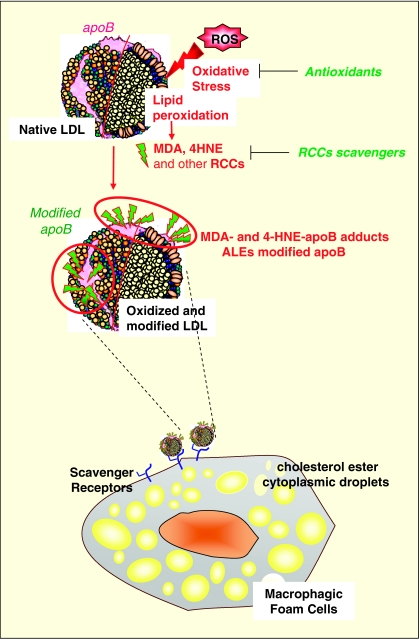
One of the best-known effects of ALEs precursors is their role in the modification of LDLs which is involved in the formation of early atherosclerotic lesions, according to the oxidative theory for atherosclerosis (Chisolm and Steinberg, 2000). LDL oxidation is a slow process, which occurs in the sub-intimal space, by contact of LDLs with reactive oxygen species (ROS) generated by vascular cells. Oxidized LDLs (oxLDLs) can be obtained in vitro by incubating LDLs with oxidants or cultured vascular cells (Jurgens et al., 1987; Steinberg, 1997). The oxidation process generates a huge variety of lipid peroxidation products among them MDA, 4-HNE, acrolein or glyoxal (Esterbauer et al., 1991; Esterbauer, 1993). As summarized in Figure 5, these aldehydes react with the Lys residues of apoB, which are required for LDL recognition by its specific apoB/E receptor expressed on most cell types except macrophages. LDL modification alters their affinity for the apoB/E receptor and deviates their metabolism towards scavenger receptor-bearing cells (macrophages and smooth muscle cells), which are progressively transformed into foam cells (Steinbrecher, 1999). The accumulation of foam cells leads to the formation of fatty streaks that are characteristic of the early atherosclerotic lesions (Ross, 1993). Like 4-HNE and MDA, methylglyoxal and glyoxal may directly modify LDLs and deviate their metabolism towards macrophages (Brown et al., 2005).
Figure 5.
Modification of LDL and formation of foam cells. ROS induce the oxidation of PUFA in LDLs. Lipid peroxidation leads to the formation of aldehydic compounds such as 4-HNE and MDA. These RCCs are able to react with lysin residues of apoB leading to modified apoB. The affinity of modified LDL for the apoB/E receptor decreases when apoB modification increases. Modified oxidized LDL are deviated towards the scavenger receptor pathway of macrophages, which are loaded with cholesterol esters and are transformed into foam cells, the signature of the initial step of atherosclerosis (Brown and Goldstein, 1983; Steinberg et al., 1989, 1997; Esterbauer et al., 1990, 1991). LDL, low-density lipoprotein, ROS, reactive oxygen species; PUFAs, polyunsaturated fatty acids; 4-HNE, 4-hydroxynonenal; MDA, malondialdehyde; RCCs, reactive carbonyl compounds.
Moreover, the degradation of oxLDLs in lysosomes is slowed down, possibly because 4-HNE is able to modify and inactivate the lysosomal cathepsin B (Hoppe et al., 1994).
Beside their metabolic deviation, oxLDLs exhibit a large variety of biological and atherogenic properties involved in the activation of inflammatory, mitogenic or proapoptotic pathways (Hajjar and Haberland, 1997; Uchida, 2000). The biological activity of oxLDLs depends on the presence of lipid peroxidation products such as oxysterols, lipoperoxides, lysophospholipids and reactive aldehydes (4-HNE, MDA, acrolein or methylglyoxal). These agents can mimic the toxic, inflammatory or mitogenic signalling mediated by oxLDLs (Leonarduzzi et al., 2005).
Modification of tyrosine kinase receptors and cell cycle
4-HNE added to cultured cells exhibits a clear dose-dependent effect, since physiological concentration of 4-HNE has growth-regulating effect, whereas higher concentration is primarily cytotoxic (Zarkovic et al., 1993).
4-HNE present in oxLDLs or exogenously added to the culture medium induces both modification and dysfunction of tyrosine kinase receptors (TKRs) such as epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor (PDGFR) in a biphasic manner. 4-HNE, at physiological and moderate concentrations (<1 μM and 1–10 μM, respectively) triggers a sustained activation of TKRs EGFR and PDGFR (Suc et al., 1997; Escargueil-Blanc et al., 2001). 4-HNE involved in TKRs modification originates from oxLDLs (as evidenced by the use of oxLDLs radiolabelled with [3H] hydroxynonenal) and from the lipid peroxidation of cell membrane (Escargueil-Blanc et al., 2001). The mechanism of TKRs activation involves the formation of 4-HNE adducts on the receptor, which triggers TKR autophosphorylation and the activation of the downstream signalling pathway, extracellular signal-regulated kinase (ERK)1/2 phosphorylation and cell cycle progression (Suc et al., 1997; Escargueil-Blanc et al., 2001). Modification of purified TKRs by 4-HNE in vitro triggers the activation of the receptor, thereby demonstrating that TKR modification and activation are causally related (Suc et al., 1997; Escargueil-Blanc et al., 2001). From these observations it can be speculated that, at low (physiological ?) concentrations, 4-HNE can act as a growth factor and promotes cell proliferation. However, high concentrations of 4-HNE inhibit cell proliferation mediated by growth factor receptors EGFR and PDGFR (Liu et al., 1999; Vindis et al., 2006), though a two-fold increase of the c-fos mRNA level was observed as a protective but abortive stress-induced response (Kreuzer et al., 1998). This inhibitory effect of 4-HNE on growth factor-mediated cell proliferation is in agreement with the progressive desensitization of PDGFRβ to its own ligand PDGF-BB, observed in smooth muscle cell in prolonged contact with oxLDL or 4-HNE (at concentrations>10 μM). The desensitization of PDGFRβ is characterized by an inhibition of the PDGF-induced PDGFRβ autophosphorylation, of the signalling cascade and of cell proliferation (Vindis et al., 2006). Inhibition of cell cycle progression, reported in leukaemic cells, is mediated by a decrease in expression of cyclin D1, D2 and A and an increase in the expression of the cycline kinase inhibitor p21, thereby inducing an accumulation of cells in the G0/G1 phase of the cell cycle (Barrera et al., 2004). 4-HNE induces an accumulation of the hypophosphorylated form of the retinoblastoma tumour-suppressor gene product hyperphosphorylated retinoblastoma, which binds and inactivates the E2F transcription factors, represses the transcription and induces cell cycle arrest (Barrera et al., 2004). Moreover, 4-HNE inhibits the expression of the protooncogene c-myc in HL-60, without affecting the expression of c-fos (Barrera et al., 1996). Higher toxic concentrations of 4-HNE (50 μM) enhanced c-fos transcription while cell proliferation is inhibited (Kreuzer et al., 1997). 4-HNE and acrolein adducts on PDGFR occur also in vivo in atherosclerotic lesions from hypercholesterolemic rabbits, apoE−/− mice and human patients (Vindis et al., 2006), which suggests that 4-HNE may impair receptor tyrosine kinase (RTK) signalling (PDGFR) within the vascular wall, and may potentially disturb PDGFR-mediated responses (proliferation, cell migration and wound healing). These biological effects are not restricted to 4-HNE, since methylglyoxal is also able to inhibit various RTKs, including EGFR, PDGFR and insulin receptor, suggesting a role for this modification in accelerated atherosclerosis in diabetes (Portero-Otin et al., 2002; Riboulet-Chavey et al., 2006; Cantero et al., 2007).
Other signalling protein kinases
Sub-cytotoxic concentrations of 4-HNE are known to directly or indirectly alter a variety of cell signalling kinases (Leonarduzzi et al., 2004). Only few reports provide information on the molecular mechanisms and on the identity of proteins that are targeted by 4-HNE. Sub-toxic concentrations of 4-HNE induce the expression of antioxidant genes such as heme-oxygenase and thioredoxine-1 via the activation of the mitogen-activated protein kinase (MAPK) pathway and the transcription factor Nrf2 (Chen et al., 2005; Siow et al., 2007), suggesting that 4-HNE may promote adaptive response to oxidative stress. Low 4-HNE concentrations induce the release of monocyte chemotactic protein-1 by macrophages, through an activation of PKCβ (Nitti et al., 2002), whereas the activation of PKCδ and Jun N-terminal kinase is rather associated with apoptosis via an upregulation of the activator protein-1 DNA binding transcription factor (Castello et al., 2005). More generally, the activation of c-Jun N-terminal kinase (JNK) and p38 kinase pathways by 4-HNE is associated with the activation of transcription factors initiating cellular responses including cell proliferation, inflammatory responses, proteasomal-mediated protein degradation and apoptosis (Petersen and Doorn, 2004; Leonarduzzi et al., 2005).
Proteasome
Proteasome is activated by oxidative stress (Grune and Davies, 2003) and by low concentrations of oxLDLs (Robbesyn et al., 2003). In contrast, HNE-modified proteins are poorly degraded by proteasome and tend to inhibit the proteolytic activity (Grune and Davies, 2003). The extensive modification of cellular proteins by 4-HNE and by the related aldehydes leads to the formation of protein aggregates that accumulate in cells and are not degraded by the proteasome (Sitte et al., 2000; Grune and Davies, 2003). The decreased degradation of modified protein and their accumulation could result from a direct inhibition of proteasome by oxidized and cross-linked proteins (Sitte et al., 2000; Grune and Davies, 2003), and by 4-HNE-modified proteins (Friguet, 2006). Though the modification of proteasome is not observed at low or moderate 4-HNE concentrations (Grune and Davies, 2003), higher concentrations may directly form adducts on the three proteolytic activities of proteasome (trypsin, chymotrypsine and peptidylglutamyl peptide hydrolase) (Okada et al., 1999; Ferrington and Kapphahn, 2004), which readily inhibits the enzymatic activity of proteasome and contributes to the accumulation of modified proteins (Grune and Davies, 2003), and to apoptosis mediated by oxLDL (Vieira et al., 2000). For instance, the reduction in the chymotrypsin-like activity could be explained by the modification of Cys residues, as mimicked by treatment of purified proteasome sub-units with the sulfhydryl-reactive chemical N-ethylmaleimide (Kapphahn et al., 2007). The inhibition of proteasome contributes to the pathogenicity of diseases characterized by an accumulation of cross-linked proteins, such as Alzheimer's disease, in which HNE-modified amyloid β-peptide accumulates and efficiently inhibits the proteasome (Shringarpure et al., 2000). The accumulation of ubiquitinated modified proteins resulting from proteasome inhibition in neuronal cells triggers a proinflammatory response characterized by an upregulation of COX-2 and the production of prostaglandin PGE2, which contributes to neurodegeneration (Rockwell et al., 2000).
The ubiquitination conjugation process may be not necessary for the degradation of oxidized protein by proteasome, (Shringarpure et al., 2003), but is involved in the degradation of cellular proteins in contact with oxLDL or 4-HNE, since its inhibition potentiates apoptosis (Vieira et al., 2000). This ubiquitination process is not altered by 4-HNE (Vieira et al., 2000), and could occur at a faster rate than that of native proteins, as reported for 4-HNE-modified α,B-crystallin (Marques et al., 2004). In this paper, the authors describe another ubiquitin/lysosomal pathway that could degrade HNE-modified proteins independently of proteasome (Marques et al., 2004). However, other reports indicate that the lysosomal degradation of modified proteins by lysosomal cathepsin is impaired in aging and in pathological situations leading to the accumulation of modified proteins (Sitte et al., 2000).
NF-κB
The pro-inflammatory transcription factor NF-κB (nuclear factor-κB) is a direct regulator of proinflammatory and anti-inflammatory genes, cell survival and proliferation (de Winther et al., 2005). The activation of NF-κB requires the phosphorylation of its inhibitor κB (IκB), which is necessary for its degradation by the ubiquitin–proteasome pathway (Roff et al., 1996). 4-HNE and acrolein inhibit the activation of NF-κB, either via a direct inhibitory effect on proteasome or through the inhibition of an upstream step required for the phosphorylation of IκB as reported in human monocytic cells, in which 4-HNE inhibits the activation of NF-κB induced by lipopolysaccharide, interleukin-1β and phorbol ester (Page et al., 1999). Conversely, 4-HNE prevents the activation of NF-κB elicited by Chlamydia pneumoniae by inhibiting the phosphorylation of IκB and its subsequent proteolysis (Donath et al., 2002). A potential mechanism has been proposed recently by Valacchi et al. (2005), who report that the inhibition of interferon-α (TNFα)-induced activation by acrolein could be due to the modification of IKKβ-subunit by acrolein. In contrast, aldehydes may induce inflammation via an activation of NF-κB, as shown for 4-HHE that activates NF-κB via the IκB kinase/NF-κB inducing kinase pathway. This mechanism involves an upstream activation of p38 MAPK and ERK1/2 kinase (Je et al., 2004).
Apoptosis signalling
Elevated concentrations of 4-HNE or acrolein (>20 μM) are highly toxic for most cell types. The mechanism of apoptosis elicited by ALE precursors involves various effects, including signalling or protein modification. The activation of JNK has been particularly investigated in the antiproliferative and apoptotic effect of 4-HNE (Yang et al., 2003). This JNK pathway plays a major role in the cooperative apoptotic effect of tissue growth factor β-1 (TGFβ-1) and 4-HNE on colon cancer cell lines (Biasi et al., 2006). Both acrolein and 4-HNE increase the levels of the phosphorylated form of transcription factors c-jun (which promotes apoptosis) and CRE-binding protein (CREB) (involved in survival), but decrease the activity of the CREB-responsive promoters (while increasing c-jun responsive promoter), which contributes to neuron degeneration and apoptosis (Pugazhenthi et al., 2006). Methylglyoxal and glyoxal are pro-apoptotic through mechanisms involving calcium deregulation (Jan et al., 2005), GSH depletion, oxidative stress, and the activation of stress kinases p38 and JNK (Fukunaga et al., 2005; Chan et al., 2007).
4-HNE increases the mRNA and protein expression of the pro-apoptotic adaptors/regulators FasR, FasL, Bax, and caspases-1, -2, -3 and -8 (Choudhary et al., 2002; de Villiers et al., 2007). In human lens cultured cells (HLE B-3), 4-HNE adducts are correlated with the induction of Fas, the activation of JNK and caspase 3, while the transfection of the α-class GST mGSTA4a (which neutralizes 4-HNE) inhibits Fas expression (Cheng et al., 2001). The mechanism of cell death evoked by these aldehydes could also involve the generation of peroxynitrite (ONOO−), as reported for 4-HHE (Lee et al., 2004) and for methylglyoxal (de Arriba et al., 2006).
4-HNE impairs the mitochondrial function, via the alteration of GSH metabolism and the induction of massive mitochondrial oxidative stress (Lee et al., 2006; Raza and John, 2006). More specifically, moderately elevated concentrations of 4-HNE or very low doses of 4-HHE trigger a calcium-mediated induction of the mitochondrial transition pore (Kristal et al., 1996). In addition, in vitro experiments on isolated mitochondria or reconstituted models for the adenine nucleotide translocator (ANT) pretreated with 4-HNE or 4-HHE indicate that the modification of ANT by these aldehydes impairs its function and activity (Chen et al., 1995). Lastly, 4-HNE alters mitochondrial calcium uptake and cytosolic calcium homoeostasis, which results in necrosis or apoptosis. This mechanism is involved in neuronal cell death (Kruman and Mattson, 1999).
ALEs and ALE precursors in ageing and diseases
Considerable evidence suggests the involvement for ALEs in ageing and age-related diseases. Ageing process is associated with an imbalance between increased oxidative stress and progressive decrease in antioxidant defences, as well as the accumulation of modified proteins, due to increased protein damage and/or decreased degradation by proteasome (Friguet, 2006). More than simple markers of lipid peroxidation, ALEs may participate in initiation and progression of diseases, including cardiovascular diseases, diabetes, cancer, inflammatory and neurodegenerative disorders.
ALEs in cardiovascular and related diseases
The oxidative theory of atherosclerosis proposed by Steinberg (1997) 25 years ago postulates that 4-HNE- and MDA-modified LDLs are directly involved in the mechanisms of fatty streak formation, an early step of atherogenesis. The detection of 4-HNE and MDA adducts, and oxLDLs within the plaque is an hallmark in atherosclerosis (Palinski et al., 1989; Torzewski et al., 1998). The presence of auto-antibodies recognizing MDA-modified LDLs has been reported in the plasma, but their role on the development of atherosclerosis is debated (Fredrikson et al., 2006). Nonetheless, the detection in human plasma and atherosclerotic lesions of auto-antibodies directed against 4-HNE- or MDA-modified LDLs is considered as a marker of evolution for vascular atherosclerotic process (Tsimikas, 2006). Circulating modified LDLs constitute new and suitable prognostic indicators of cardiovascular diseases, for example acute coronary syndromes, vulnerable plaques, pre-clinical or accelerated atherosclerosis and chronic renal failure in diabetes (Makita et al., 1996; Fraley and Tsimikas, 2006). Chronic renal failure complications including renal toxicity, ischaemia/reperfusion and myocardial injury result in a massive oxidative stress and the generation of MDA and 4-HNE, which deplete antioxidants defences, modify enzyme function and mitochondrial respiration by forming adducts on proteins (Siems et al., 2002). The presence of MDA and 4-HNE adducts in dialysed patients is considered as an additional risk factor for cardiovascular complications (Carluccio et al., 2002).
Coronary heart diseases and stroke due to accelerated atherosclerosis are the principal cause of mortality in diabetes. LDL modification by AGE and ALE precursors is detected in the plasma of diabetic patients (Makita et al., 1996) and is thought to play a role in the development and progression of accelerated atherosclerosis in diabetes. Moreover, diabetic cataract is associated with increased deposition of AGEs/ALEs-modified proteins in lens. The deposition of 4-HNE and MDA adducts in the lens of aged rats or submitted to high oxidative stress is directly correlated to the apoptosis of lens cells and cataract formation (Marsili et al., 2004). At last, 4-HNE could contribute to the mechanisms of obesity and insulin resistance, via the modification of adipose regulatory proteins (for example, adipocyte fatty acid-binding protein) (Grimsrud et al., 2007).
Neurodegenerative diseases
Increasing evidences suggest that oxidative stress is involved in the pathogenesis of neurodegenerative diseases, including Alzheimer (Lovell and Markesbery, 2006), Parkinson (Jenner, 2003), Creutzfeld–Jacob and prion diseases (Brown, 2005). It is likely that the mechanisms involved in neurodegeneration are multiple, and it is not clear so far if oxidative stress is the cause or consequence of the neuronal death, as it may occur during apoptosis and in the early steps or in advanced stages of the disease. Moreover, the detection of aldehyde adducts in the brain of neurodegenerative diseases strongly suggests the occurrence of an oxidative stress during brain degeneration (Zarkovic, 2003).
In Alzheimer's disease, amyloid β-peptide induces an oxidative stress, which results in the formation of 4-HNE and acrolein adducts, and the accumulation of modified proteins (Sayre et al., 1997; Smith et al., 1998). 4-HNE forms adducts on Lys residues of proteins of axonal neurofilaments (Wataya et al., 2002) and induces a dysfunction of membrane calcium-ATPases, and of glucose and glutamate transporters, which increases calcium influx, disrupts the synaptic calcium homoeostasis and finally leads to apoptosis (Mattson and Chan, 2003).
In Parkinson's disease, the oxidative stress contributes to mitochondrial dysfunction and degeneration of dopaminergic cells (Jenner, 2003). 4-HNE is formed during the lipid peroxidation process and reacts with proteins of the nigral neurons (Yoritaka et al., 1996). In addition, 4-HNE- and peroxynitrite-induced modification of proteins associated with the defect of the ubiquitin–proteasome system leads to the accumulation of modified proteins and contributes to neuronal cell death (Jenner, 2003).
The neurodegenerative process in prion diseases may result, at least in part, from a defect in antioxidant function of the mutant form of prion protein (Brown, 2005). 4-HNE is generated in brains of human patients affected with Creutzfeldt–Jakob disease, and in scrapie-infected mice. 4-HNE adducts are detected in astrocytes, not in neurons, and their role in the progression of the prion disease is not elucidated so far (Andreoletti et al., 2002).
Cancer
The role of lipid peroxidation in cancer is debated because, in the majority of tumours, the lipid composition of membrane is modified with decreased PUFA level and increased cholesterol content. Moreover, an increase in antioxidants and antioxidant defences is often observed, thus indicating that lipid peroxidation could be reduced in these diseases (Dianzani, 1989). In addition, in vivo studies on human colon adenocarcinoma show a reduced expression of TGFβ-1 correlated with a decrease in 4-HNE–protein adducts. This could mean that neoplastic progression is better under these conditions, since TGFβ-1 and 4-HNE cooperate for inducing the apoptosis of colon cell via activation of the JNK pathway (Biasi et al., 2006).
On the other hand, 4-HNE itself is mutagenic (it can form adducts on guanine) and carcinogenic for hepatocytes (Weber et al., 2003). Low 4-HNE concentrations trigger proliferative and inflammatory responses, which could play a role in tumour growth, this being supported by the detection of 4-HNE adducts in various tumour cells (Dianzani, 1989). Moreover, high heam-iron intake (reproduced by high meat intake) is associated with lipid peroxidation and 4-HNE generation, potentially involved in the promotion of colon cancer carcinogenesis (Pierre et al., 2007). The same authors propose the detection of an urinary metabolite of 4-HNE, the dihydroxynonane mercapturic acid, as a new biomarker suitable for the determination of pre-neoplasic lesions (Pierre et al., 2006).
Chronic inflammatory diseases
MDA and 4-HNE adducts are detected in osteoarthritic synovial cartilage (Shah et al., 2005), and contribute to cartilage degradation, by modifying type II collagen, which increases its degradation by metalloprotease MMP13 (Morquette et al., 2006). Moreover, 4-HNE alters osteoarthritic osteoblast activity by triggering diverse cellular responses such as increased osteocalcin and type I collagen synthesis, inhibition of alkaline phosphatase activity, induction of COX-2 expression and prostaglandin E2 release (Shi et al., 2006). Protein oxidation is involved in the pathogenesis of systemic lupus erythematosus (Morgan et al., 2005), and the detection of 4-HNE-modified proteins in the plasma of the affected patients has been proposed as a marker for the evolution of this disease (Grune et al., 1997).
Oxidative stress and lipid peroxidation contribute to the pathogenesis of asthma, acute lung inflammation and allergic airway inflammation (Boldogh et al., 2005; Castro et al., 2006). In these diseases, the exact role of 4-HNE is not yet established and its targets are not identified, but the overexpression of heme-oxygenase reduces oxidative stress, 4-HNE adducts formation and the progression of inflammation (Almolki et al., 2004). Increased MDA and 4-HNE protein adducts, correlated with decreased levels of GSH and antioxidants vitamins E and C, is a constant observation in chronic viral and alcoholic hepatitis (Loguercio and Federico, 2003). Conversely, the reduced expression of GST in bile duct leads to the accumulation of 4-HNE, and contributes to the pathogenic process of primary biliary cirrhosis (Tsuneyama et al., 2002).
Pharmacological inhibitors of ALE formation
The pharmacological inhibition of ALE formation involves several approaches including the use of transition metal chelators and antioxidants, which block oxidative stress, lipid peroxidation, and the generation of aldehydes, and the use of carbonyl scavengers, which interact more specifically with aldehydes and neutralize the formation of adducts. Most studies have been focussed on AGE inhibitors, as recently reviewed by Peyroux and Sternberg (2006), whereas ALE inhibitors have been less investigated so far. Although most inhibitors efficient on AGE formation may potentially inhibit ALE formation (Baynes and Thorpe, 2000), this review is focussed on the main pharmacological agents, which have been reported to neutralize ALE precursors generated from lipid peroxidation.
Hydrazine derivatives
Hydrazine
It is widely used in chemical synthesis as it condensates rapidly with the carbonyl group of ketone or aldehyde to form a methylene or methyl group via a hydrazone intermediate.
DNPH is a hydrazine derivative largely utilized for the quantitative detection of carbonyl groups (particularly 4-HNE) in tissues and biological fluids, using HPLC and spectrophotometric methods or for immunohistochemistry and western blotting using DNPH-labelled antibodies (Esterbauer et al., 1991; Waeg et al., 1996). DNPH reacts with aldehydes at acidic pH, which facilitates the rupture of the covalent bond aldehyde protein, and the binding of aldehyde on hydrazine, forming dinitrophenylhydrazones (Esterbauer et al., 1991). DNPH prevents in vitro the formation and the accumulation of acrolein and 4-HNE adducts on cellular proteins from cultured vascular cells, as well as the cytotoxicity of oxLDLs (Escargueil-Blanc et al., 2001). Anyway, the use of DNPH for in vivo trials in animals is limited because of its high mutagenic and toxic properties (Brooke et al., 1997).
Hydralazine (1-hydrazinophthalazine monohydrochloride)
It is a hydrazine derivative used as antihypertensive, and regarded for years as a drug of choice for the treatment of severe hypertension in pregnancy (Montan, 2004). Hydralazine and dihydralazine are efficient in trapping aldehyde-adducted proteins. It is particularly efficient in trapping acrolein, resulting in a strong protection against acrolein-induced hepatotoxicity (Burcham et al., 2000, 2002; Kaminskas et al., 2004). Hydralazine traps 4-HNE adducts formed in smooth muscle cells in the presence of oxLDL, particularly on PDGF receptor that reverses the inhibition of PDGF signalling (Vindis et al., 2006). Hydralazine reverses in vivo the formation of 4-HNE and acrolein adducts on tissue proteins, particularly the modification of PDGFR in atherosclerotic aortas of hypercholesterolemic rabbits and of apoE−/− mice. This protective effect could contribute to slow down the atherosclerotic process (Vindis et al., 2006). Hydralazine inhibits the modification of LDL induced by glyoxal and methylglyoxal, and prevents the formation and accumulation of foam cells (Brown et al., 2006), as well as renal damage in type II diabetes animal models (Nangaku et al., 2003). Hydralazine is a powerful antioxidant that inhibits the activation of NADH oxidase at the plasma membrane and the subsequent generation of ROS (Munzel et al., 1996).
Aminoguanidine (hydrazinecarboximidamide)
It is an early-stage inhibitor of AGE generation, and one of the most efficient drugs able to prevent proteins cross-linking induced by AGE precursors. Aminoguanidine reacts in vitro and in vivo with the α-oxoaldehydes methylglyoxal and glyoxal and ALE precursors (MDA) to form 3-amino-1,2,4-triazine derivatives (Thornalley et al., 2000; Thornalley, 2003). It is a highly nucleophilic agent that reacts rapidly with glucose and pyruvate, but also with pyridoxal phosphate, thereby decreasing the availability of vitamin B6 (Chen et al., 2004). Pyridoxal-aminoguanidine, a Schiff base formed between pyridoxal and aminoguanidine, prevents the decrease in pyridoxal phosphate, without altering and rather enhancing the carbonyl scavenger activities of aminoguanidine (Chen et al., 2004).
Most beneficial effects of aminoguanidine are related to its carbonyl scavenger properties, which protect in vitro and in vivo against the deleterious effects of AGE and ALE precursors (Peyroux and Sternberg, 2006). Aminoguanidine has been proved efficient in experimental animal models for diabetes, in inhibiting pathological complications, such as nephropathies (prevention of albuminuria and glomerulonephritis), accelerated atherosclerosis (inhibition of lipid peroxidation), cataract (inhibition of AGE deposition in lens) and neurovascular complications (Peyroux and Sternberg, 2006).
In addition, the protective effect of aminoguanidine is largely due to its antioxidant and chelating properties (Price et al., 2001). For instance, aminoguanidine reduces the expression of growth factors TGFβ-1 and PDGF-β and of proinflammatory cytokines (TNFα) (Peyroux and Sternberg, 2006), probably by preventing oxidative stress and NF-κB activation. Aminoguanidine inhibits the activity of the inducible nitric oxide synthase, which is upregulated in diabetes and generates high NO levels leading to peroxynitrite generation and endothelial dysfunction (Bardell and MacLeod, 2001). Conversely, aminoguanidine inhibits the semicarbazide-sensitive oxidase, which contributes to the generation of methylglyoxal and formaldehyde in diabetes (Yu and Zuo, 1997). Like hydralazine, aminoguanidine blocks the modification of LDL induced by methylglyoxal and MDA, thereby preventing their metabolic deviation towards macrophages (Brown et al., 2006). This protective effect (inhibition of apoB carbonylation) could result from its antioxidant properties, in addition to the carbonyl-scavenger activity (Jedidi et al., 2003).
Because aminoguanidine exhibits extensive beneficial effect in pre-clinical studies in experimental animal models for diabetes, several clinical trials in humans have been designed for evaluating its efficiency to slow down the progression of diabetes complications. These studies were not found conclusive, in part because of side-effects, and of weak carbonyl scavenger effects in human vascular tissues (Bolton et al., 2004).
OPB-9195 ((+/−)-2-isopropylidenehydrazono-4-oxo-thiazolidin-5-yla cetanilide)
It is a hydrazine derivative that inhibits the formation of AGEs (pentosidine and CML) in the plasma of uremic and diabetic patients (Miyata et al., 2000). OPB-9195 is more efficient than aminoguanidine for trapping 4-HNE and MDA from arachidonate oxidation (Miyata et al., 2000). As for aminoguanidine, clinical trials were not conclusive and were stopped because of the side-effects of this compound, particularly pyridoxal depletion which resulted in vitamin B6 deficiency (Peyroux and Sternberg, 2006).
Vitamin B6 derivatives
Vitamin B6
It exhibits antioxidant properties as it participates in the maintenance of reduced GSH, which is a major antioxidant and a natural ALE precursor scavenger. Vitamin B6 acts as a cofactor in the synthesis of Cys (the rate-limiting precursor for GSH biosynthesis) (Grimble, 1997). Deficiency or low levels in vitamin B6 result in an increased homocysteine level and oxidative stress potentially involved in accelerated atherosclerosis in vitamin B6-deficient rats (Endo et al., 2006).
Pyridoxamine
It is one of the three natural forms of vitamin B6 (with pyridoxal and pyridoxine). Pyridoxamine prevents the formation of AGEs such as CML and CEL, and is a potent inhibitor of Lys modification by 4-HNE and MDA generated during LDL oxidation by copper (Onorato et al., 2000). It is a potent MDA scavenger, which also blocks the accumulation of the fluorescent MDA-related pigment, lipofuscin (Kang et al., 2006). Pyridoxamine inhibits NO-mediated apoptosis of insulin secreting RINm5F cells, which occurs via the formation of ALE precursors (for example, MDA) (Cahuana et al., 2003). Moreover, pyridoxamine has a lipid-lowering effect and protects against the development of nephropathy, neuropathy and vaculopathy in streptozotocin-induced diabetic rats. Like aminoguanidine, pyridoxamine represents a potentially useful agent for the treatment of diseases involving hyperlipemia and oxidative stress (Voziyan and Hudson, 2005).
Pyridoxal isonicotinoyl hydrazones
These classes of agents exhibit potent chelating properties against iron and block iron-induced oxidative stress and lipid peroxidation, in particular MDA formation. Their use is reserved to iron overload diseases and neurodegenerative disorders (Whitnall and Richardson, 2006).
Amino-acid derivatives
Carnosine (β-alanyl-L-histidine)
It is a dipeptide of β-alanine and His, exhibiting antioxidant and carbonyl scavenger properties. Carnosine blocks the formation of MDA and methylglyoxal adducts in in vitro experimental models for lipid peroxidation and MDA-induced cytotoxicity in cultured brain endothelial cells (Hipkiss et al., 1997). At the cellular level, carnosine protected cultured human fibroblasts and rat brain endothelial cells against the toxic effects of MDA and AGEs. Interestingly, carnosine protects against amyloid peptide toxicity and DNA protein cross-linking in neurodegenerative disorders such as Alzheimer's disease, cardiovascular ischaemic damages and inflammatory diseases (Guiotto et al., 2005).
Histidyl hydrazide
It is a His analogue that selectively scavenges 4-HNE, and reduces the 4-HNE-induced apoptosis of cultured neurons, chemical hypoxia and glucose deprivation. This drug could be of interest in the treatment of related neurodegenerative diseases and 4-HNE-associated pathologies (Tang et al., 2007).
N-acetyl cysteine
It exhibits highly protective scavenging properties against 4-HNE and MDA. N-acetyl cysteine (NAC) pretreatment protects against MDA increase and GSH decrease in an animal model for Alzheimer (Fu et al., 2006), and prevents accelerated atherosclerosis in uremic apoE−/− mice (Ivanovski et al., 2005), as well as neuronal injury (Arakawa et al., 2006).
S-Adenosylmethionine
It is more potent than NAC for inhibiting 4-HNE adduct formation, and prevents efficiently hepatic toxicity induced by acetaminophen, which involves oxidative stress and lipid peroxidation (Valentovic et al., 2004).
Other therapeutic agents exhibiting protection against ALE formation
Angiotensin converting enzyme inhibitors
Angiotensin converting enzyme inhibitors exhibit antioxidant properties and block LDL oxidation, lipid peroxidation and the generation of MDA and 4-HNE (Hayek et al., 1999). ACE inhibitors (Captopril, Enalapril and Fosinopril) exhibit a potent antiatherogenic effect in apoE−/− mice, due to not only blood pressure reduction but also to their protective effect against LDL oxidation (Hayek et al., 1998, 1999). Moreover, a direct MDA-scavenging effect has been reported for Captopril (Altuntas et al., 1995).
AT1 angiotensin receptor inhibitors (Losartan, Candesartan)
These inhibit the formation of circulating MDA-modified LDLs in human diabetic patients and the modification by MDA of lungs in rats affected with bleomycin-induced pulmonary fibrosis (Hayek et al., 1999; Yao et al., 2006).
Antioxidants
These agents are efficient in preventing lipid peroxidation, thus the formation of ALEs on proteins. Among natural antioxidants involved in the detoxification of ALE precursors, GSH (Glu–Cys–Gly) exhibits a high reactivity for 4-HNE and is essential for maintaining antioxidants in an active state (α-tocopherol), and for protecting the cell against oxidative stress mediated by ROS (Petersen and Doorn, 2004). Though GSH can react spontaneously with 4-HNE via Michael addition, the reaction is much more rapid via the conjugation process catalysed by GST (EC 2.5.1.18), the highest activity being exhibited by GST A4-4 and GST 5.8 (Hubatsch et al., 1998; Petersen and Doorn, 2004). The conjugation catalysed by GSTs transforms 4-HNE into a GSH conjugate, thus decreases GSH intracellular concentration, while GSTs may also limit 4-HNE generation by reducing hydroperoxide formed during lipid peroxidation (Awasthi et al., 2004). GST4 activity is reduced in obese mice, which is correlated with the modification by 4-HNE of a fatty acid-binding protein on Cys, thus supporting the existence of links between oxidative stress and insulin resistance (Grimsrud et al., 2007). Interestingly, low doses of 4-HNE can induce the expression of the glutamate–Cys ligase (a rate-limiting enzyme in GSH biosynthesis), through a mechanism implying the EpRE–Nrf2 signalling pathway (Zhang et al., 2007).
Pretreatment of neurons with high NAC concentrations prevents 4-HNE-induced neuronal death, and suppresses the decrease in intracellular levels of GSH and sulfhydryl groups induced by 4-HNE. Lower NAC concentrations potentiated the protective effect of the seleno-organic GSH peroxidase mimetic Ebselen (Arakawa et al., 2006), though Ebselen itself prevents the generation of 4-HNE adducts formed during cerebral ischaemia (Imai et al., 2003), or alcohol-induced liver injury (Kono et al., 2001). These data are interesting as they propose a dual protective mechanism for these drugs via (i) their carbonyl scavenger effect against ALE precursors (4-HNE) and (ii) the maintenance of an efficient level in natural intracellular antioxidants (GSH), and the natural protection of the cells against oxidative stress.
However, antioxidants cannot neutralize the effects of ALE precursors once adducts are formed. For instance, trolox and phenolic acids cannot protect against the modification of TKRs induced by 4-HNE (Vacaresse et al., 2001). In vivo studies indicate that the protective effect of antioxidants on the formation of 4-HNE, MDA or acrolein in atherosclerotic plaques or in neurodegenerative diseases is variable, while agents efficient in vitro or in pre-clinical studies fail to protect significantly once tested in human patients (Peyroux and Sternberg, 2006). U-101033E (2,4-diaminopyrrolopyrimidine) is highly efficient in inhibiting 4-HNE or MDA generation (Rohn et al., 1998), but its inhibitory effect on ALE formation has not been studied in vivo. Vitamin E failed to protect humans from cardiovascular disease outcome and its antiatherogenic effect in apoE−/− is controversial (Suarna et al., 2006). Pyrrolidine dithiocarbamate blocks efficiently lipid peroxidation (MDA) in chronic inflammation (collagen-induced arthritis) (Cuzzocrea et al., 2002) and cerulein-induced pancreatitis (Virlos et al., 2003). Polyphenols reduce hyperlipemia and inhibit lipid peroxidation and atherosclerosis development in diabetic LDL receptor KO mice (Zang et al., 2006). Resveratrol, a red wine polyphenol, exhibits protective properties against lipid peroxidation and ALE formation in experimental models for numerous diseases including atherosclerosis, diabetes and Alzheimer diseases (Anekonda, 2006). However, most agents were not tested in human patients, and any correlation between their protective effect on ALE generation and the progression of the disease remains speculative.
Conclusion
ALEs are formed in a large variety of diseases and represent a suitable marker of lipid peroxidation. Their deleterious effect on proteins and their own signalling properties suggest that ALEs also contribute to initiate several diseases or aggravate their severity. Obviously, the early inhibition of lipid peroxidation and ALE formation by antioxidants blocks efficiently atherogenesis in animal models, whereas antioxidants fail to protect on more advanced states and in therapeutic human trials. Studies using AGE/ALE-precursor scavengers on the progression of diabetes complications were not conclusive in humans, and led to variable conclusions in animals. The effect of drugs like hydralazine on cardiovascular, inflammatory or neurodegenerative diseases has not been investigated in humans, thus it is not known if ALE-precursor scavengers could be beneficial or not on the late steps of ALEs-associated diseases. The synthesis and development of new drugs sharing both potent antioxidant and carbonyl scavenger properties should allow (i) to better understand the implication of ALEs in the advanced steps of diseases and (ii) to better evaluate the potential therapeutic interest of this class of carbonyl scavengers. The new therapeutical perspectives of these new drugs will also depend on their influence on natural cellular antioxidants and their ability to protect or regenerate their protective effect against oxidative stress.
Acknowledgments
This work was supported by grants from INSERM, Université Paul Sabatier-Toulouse III, Agence Nationale de la Recherche (ANR-05-PCOD-019-01-LiSA) and Groupement Lipides et Nutrition. C Coatrieux is the recipient of a fellowship from MENRT and Fondation pour la Recherche Médicale
Abbreviations
- AGE
advanced glycoxidation end products
- ALE
advanced lipid peroxidation end products
- MDA
malondialdehyde
- 4-HNE
4-hydroxynonenal
Conflict of interest
The authors state no conflict of interest.
References
- Aldini G, Dalle-Donne I, Colombo R, Maffei Facino R, Milzani A, Carini M. Lipoxidation-derived reactive carbonyl species as potential drug targets in preventing protein carbonylation and related cellular dysfunction. Chem Med Chem. 2006;1:1045–1058. doi: 10.1002/cmdc.200600075. [DOI] [PubMed] [Google Scholar]
- Almolki A, Taille C, Martin GF, Jose PJ, Zedda C, Conti M, et al. Heme oxygenase attenuates allergen-induced airway inflammation and hyperreactivity in guinea pigs. Am J Physiol Lung Cell Mol Physiol. 2004;287:L26–L34. doi: 10.1152/ajplung.00237.2003. [DOI] [PubMed] [Google Scholar]
- Altuntas Y, Guven M, Ince E, Acbay O, Caner M, Kanigur-Sultuybek G. The in vitro effects of captopril on the levels of lipid peroxidation and glutathione of erythrocytes in type II diabetes. J Basic Clin Physiol Pharmacol. 1995;6:281–288. doi: 10.1515/jbcpp.1995.6.3-4.281. [DOI] [PubMed] [Google Scholar]
- Andreoletti O, Levavasseur E, Uro-Coste E, Tabouret G, Sarradin P, Delisle MB, et al. Astrocytes accumulate 4-hydroxynonenal adducts in murine scrapie and human Creutzfeldt–Jakob disease. Neurobiol Dis. 2002;11:386–393. doi: 10.1006/nbdi.2002.0558. [DOI] [PubMed] [Google Scholar]
- Anekonda TS. Resveratrol—a boon for treating Alzheimer's disease. Brain Res Rev. 2006;52:316–326. doi: 10.1016/j.brainresrev.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Arakawa M, Ishimura A, Arai Y, Kawabe K, Suzuki S, Ishige K, et al. N-Acetylcysteine and ebselen but not nifedipine protected cerebellar granule neurons against 4-hydroxynonenal-induced neuronal death. Neurosci Res. 2006;57:220–229. doi: 10.1016/j.neures.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Awasthi YC, Yang Y, Tiwari NK, Patrick B, Sharma A, Li J, et al. Regulation of 4-hydroxynonenal-mediated signaling by glutathione S-transferases. Free Rad Biol Med. 2004;37:607–619. doi: 10.1016/j.freeradbiomed.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Bardell AL, MacLeod KM. Evidence for inducible nitric-oxide synthase expression and activity in vascular smooth muscle of streptozotocin-diabetic rats. J Pharmacol Exp Ther. 2001;296:252–259. [PubMed] [Google Scholar]
- Barrera G, Pizzimenti S, Dianzani MU. 4-hydroxynonenal and regulation of cell cycle: effects on the pRb/E2F pathway. Free Radic Biol Med. 2004;37:597–606. doi: 10.1016/j.freeradbiomed.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Barrera G, Pizzimenti S, Serra A, Feretti C, Fazio VM, Saglio G, et al. 4-hydroxynonenal specifically inhibits c-myb but does not affect c-fos expressions in HL-60 cells. Biochem Biophys Res Commun. 1996;227:589–593. doi: 10.1006/bbrc.1996.1550. [DOI] [PubMed] [Google Scholar]
- Baynes JW, Thorpe SR. Glycoxidation and lipoxidation in atherogenesis. Free Radic Biol Med. 2000;28:1708–1716. doi: 10.1016/s0891-5849(00)00228-8. [DOI] [PubMed] [Google Scholar]
- Biasi F, Vizio B, Mascia C, Gaia E, Zarkovic N, Chiarpotto E, et al. c-Jun N-terminal kinase upregulation as a key event in the proapoptotic interaction between transforming growth factor-beta1 and 4-hydroxynonenal in colon mucosa. Free Radic Biol Med. 2006;41:443–454. doi: 10.1016/j.freeradbiomed.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Boldogh I, Bacsi A, Choudhury BK, Dharajiya N, Alam R, Hazra TK, et al. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest. 2005;115:2169–2179. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton WK, Cattran DC, Williams ME, Adler SG, Appel GB, Cartwright K, et al. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol. 2004;24:32–40. doi: 10.1159/000075627. [DOI] [PubMed] [Google Scholar]
- Borovic S, Rabuzin F, Waeg G, Zarkovic N. Enzyme-linked immunosorbent assay for 4-HNE-histidine conjugates. Free Radic Res. 2006;40:809–820. doi: 10.1080/10715760600693422. [DOI] [PubMed] [Google Scholar]
- Brooke CJ, I, Cocker J, Groves J. Phenylhydrazine 1997. Sudbury, Suffolk, HSE Books, Risk Assessment Document EH 72/1
- Brown BE, Dean RT, Davies MJ. Glycation of low-density lipoproteins by methylglyoxal and glycolaldehyde gives rise to the in vitro formation of lipid-laden cells. Diabetologia. 2005;48:361–369. doi: 10.1007/s00125-004-1648-4. [DOI] [PubMed] [Google Scholar]
- Brown BE, Mahroof FM, Cook NL, van Reyk DM, Davies MJ. Hydrazine compounds inhibit glycation of low-density lipoproteins and prevent the in vitro formation of model foam cells from glycolaldehyde-modified low-density lipoproteins. Diabetologia. 2006;49:775–783. doi: 10.1007/s00125-006-0137-3. [DOI] [PubMed] [Google Scholar]
- Brown DR. Neurodegeneration and oxidative stress: prion disease results from loss of antioxidant defence. Folia Neuropathol. 2005;43:229–243. [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Burcham PC, Kaminskas LM, Fontaine FR, Petersen DR, Pyke SM. Aldehyde-sequestering drugs: tools for studying protein damage by lipid peroxidation products. Toxicology. 2002;181–182:229–236. doi: 10.1016/s0300-483x(02)00287-1. [DOI] [PubMed] [Google Scholar]
- Burcham PC, Kerr PG, Fontaine F. The antihypertensive hydralazine is an efficient scavenger of acrolein. Redox Rep. 2000;5:47–49. doi: 10.1179/rer.2000.5.1.47. [DOI] [PubMed] [Google Scholar]
- Buss H, Chan TP, Sluis KB, Domigan NM, Winterbourn CC. Protein carbonyl measurement by a sensitive ELISA method. Free Radic Biol Med. 1997;23:361–366. doi: 10.1016/s0891-5849(97)00104-4. [DOI] [PubMed] [Google Scholar]
- Cahuana GM, Tejedo JR, Jimenez J, Ramirez R, Sobrino F, Bedoya FJ. Involvement of advanced lipooxidation end products (ALEs) and protein oxidation in the apoptotic actions of nitric oxide in insulin secreting RINm5F cells. Biochem Pharmacol. 2003;66:1963–1971. doi: 10.1016/j.bcp.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Cantero AV, Portero-Otin M, Ayala V, Auge N, Sanson M, Elbaz M, et al. Methylglyoxal induces advanced glycation end product (AGEs) formation and dysfunction of PDGF receptor-{beta}: implications for diabetic atherosclerosis. FASEB J. 2007;21:1–11. doi: 10.1096/fj.06-7536com. [DOI] [PubMed] [Google Scholar]
- Carini M, Aldini G, Facino RM. Mass spectrometry for detection of 4-hydroxy-trans-2-nonenal (HNE) adducts with peptides and proteins. Mass Spectrom Rev. 2004;23:281–305. doi: 10.1002/mas.10076. [DOI] [PubMed] [Google Scholar]
- Carluccio F, Siems W, Stefanelli G, Sommerburg O, Grune T, Riedel E, et al. Homocysteine in chronic renal failure in relation to renal anemia and to oxidative stress parameters 4-hydroxynonenal and malondialdehyde. Clin Nephrol. 2002;58 Suppl 1:S26–S30. [PubMed] [Google Scholar]
- Castello L, Marengo B, Nitti M, Froio T, Domenicotti C, Biasi F, et al. 4-Hydroxynonenal signalling to apoptosis in isolated rat hepatocytes: the role of PKC-delta. Biochim Biophys Acta. 2005;1737:83–93. doi: 10.1016/j.bbalip.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Castro SM, Guerrero-Plata A, Suarez-Real G, Adegboyega PA, Colasurdo GN, Khan AM, et al. Antioxidant treatment ameliorates respiratory syncytial virus-induced disease and lung inflammation. Am J Respir Crit Care Med. 2006;174:1361–1369. doi: 10.1164/rccm.200603-319OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WH, Wu HJ, Shiao NH. Apoptotic signaling in methylglyoxal-treated human osteoblasts involves oxidative stress, c-Jun N-terminal kinase, caspase-3, and p21-activated kinase 2. J Cell Biochem. 2007;100:1056–1069. doi: 10.1002/jcb.21114. [DOI] [PubMed] [Google Scholar]
- Chen AS, Taguchi T, Sugiura M, Wakasugi Y, Kamei A, Wang MW, et al. Pyridoxal-aminoguanidine adduct is more effective than aminoguanidine in preventing neuropathy and cataract in diabetic rats. Horm Metab Res. 2004;36:183–187. doi: 10.1055/s-2004-814344. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Bertrand H, Yu BP. Inhibition of adenine nucleotide translocator by lipid peroxidation products. Free Radic Biol Med. 1995;19:583–590. doi: 10.1016/0891-5849(95)00066-7. [DOI] [PubMed] [Google Scholar]
- Chen ZH, Saito Y, Yoshida Y, Sekine A, Noguchi N, Niki E. 4-Hydroxynonenal induces adaptive response and enhances PC12 cell tolerance primarily through induction of thioredoxin reductase 1 via activation of Nrf2. J Biol Chem. 2005;280:41921–41927. doi: 10.1074/jbc.M508556200. [DOI] [PubMed] [Google Scholar]
- Cheng JZ, Singhal SS, Sharma A, Saini M, Yang Y, Awasthi S, et al. Transfection of mGSTA4 in HL-60 cells protects against 4-hydroxynonenal-induced apoptosis by inhibiting JNK-mediated signaling. Arch Biochem Biophys. 2001;392:197–207. doi: 10.1006/abbi.2001.2452. [DOI] [PubMed] [Google Scholar]
- Chisolm GM, Steinberg D. The oxidative modification hypothesis of atherogenesis: an overview. Free Radic Biol Med. 2000;28:1815–1826. doi: 10.1016/s0891-5849(00)00344-0. [DOI] [PubMed] [Google Scholar]
- Choudhary S, Zhang W, Zhou F, Campbell GA, Chan LL, Thompson EB, et al. Cellular lipid peroxidation end-products induce apoptosis in human lens epithelial cells. Free Radic Biol Med. 2002;32:360–369. doi: 10.1016/s0891-5849(01)00810-3. [DOI] [PubMed] [Google Scholar]
- Chowdhury PK, Halder M, Choudhury PK, Kraus GA, Desai MJ, Armstrong DW, et al. Generation of fluorescent adducts of malondialdehyde and amino acids: toward an understanding of lipofuscin. Photochem Photobiol. 2004;79:21–25. [PubMed] [Google Scholar]
- Cuzzocrea S, Chatterjee PK, Mazzon E, Dugo L, Serraino I, Britti D, et al. Pyrrolidine dithiocarbamate attenuates the development of acute and chronic inflammation. Br J Pharmacol. 2002;135:496–510. doi: 10.1038/sj.bjp.0704463. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A. Protein carbonylation in human diseases. Trends Mol Med. 2003;9:169–176. doi: 10.1016/s1471-4914(03)00031-5. [DOI] [PubMed] [Google Scholar]
- de Arriba SG, Krugel U, Regenthal R, Vissiennon Z, Verdaguer E, Lewerenz A, et al. Carbonyl stress and NMDA receptor activation contribute to methylglyoxal neurotoxicity. Free Radic Biol Med. 2006;40:779–790. doi: 10.1016/j.freeradbiomed.2005.09.038. [DOI] [PubMed] [Google Scholar]
- de Villiers WJ, Song Z, Nasser MS, Deaciuc IV, McClain CJ. 4-Hydroxynonenal-induced apoptosis in rat hepatic stellate cells: mechanistic approach. J Gastroenterol Hepatol. 2007;22:414–422. doi: 10.1111/j.1440-1746.2006.04625.x. [DOI] [PubMed] [Google Scholar]
- de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:904–914. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- Dianzani MU. Lipid peroxidation and cancer: a critical reconsideration. Tumori. 1989;75:351–357. doi: 10.1177/030089168907500410. [DOI] [PubMed] [Google Scholar]
- Donath B, Fischer C, Page S, Prebeck S, Jilg N, Weber M. Chlamydia pneumoniae activates IKK/I kappa B-mediated signaling, which is inhibited by 4-HNE and following primary exposure. Atherosclerosis. 2002;165:79–88. doi: 10.1016/s0021-9150(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Endo N, Nishiyama K, Otsuka A, Kanouchi H, Taga M, Oka T. Antioxidant activity of vitamin B6 delays homocysteine-induced atherosclerosis in rats. Br J Nutr. 2006;95:1088–1093. doi: 10.1079/bjn20061764. [DOI] [PubMed] [Google Scholar]
- Escargueil-Blanc I, Salvayre R, Vacaresse N, Jurgens G, Darblade B, Arnal JF, et al. Mildly oxidized LDL induces activation of platelet-derived growth factor beta-receptor pathway. Circulation. 2001;104:1814–1821. doi: 10.1161/hc4001.097179. [DOI] [PubMed] [Google Scholar]
- Esterbauer H.Cytotoxicity and genotoxicity of lipid-oxidation products Am J Clin Nutr 199357779S–785S.discussion 785S–786S [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Dieber-Rotheneder M, Waeg G, Striegl G, Jurgens G. Biochemical, structural, and functional properties of oxidized low-density lipoprotein. Chem Res Toxicol. 1990;3:77–92. doi: 10.1021/tx00014a001. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Ferrington DA, Kapphahn RJ. Catalytic site-specific inhibition of the 20S proteasome by 4-hydroxynonenal. FEBS Lett. 2004;578:217–223. doi: 10.1016/j.febslet.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Fraley AE, Tsimikas S. Clinical applications of circulating oxidized low-density lipoprotein biomarkers in cardiovascular disease. Curr Opin Lipidol. 2006;17:502–509. doi: 10.1097/01.mol.0000245255.40634.b5. [DOI] [PubMed] [Google Scholar]
- Fredrikson GN, Berglund G, Alm R, Nilsson JA, Shah PK, Nilsson J. Identification of autoantibodies in human plasma recognizing an apoB-100 LDL receptor binding site peptide. J Lipid Res. 2006;47:2049–2054. doi: 10.1194/jlr.M600217-JLR200. [DOI] [PubMed] [Google Scholar]
- Friguet B. Oxidized protein degradation and repair in ageing and oxidative stress. FEBS Lett. 2006;580:2910–2916. doi: 10.1016/j.febslet.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Fu AL, Dong ZH, Sun MJ. Protective effect of N-acetyl-L-cysteine on amyloid beta-peptide-induced learning and memory deficits in mice. Brain Res. 2006;1109:201–206. doi: 10.1016/j.brainres.2006.06.042. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Miyata S, Higo S, Hamada Y, Ueyama S, Kasuga M. Methylglyoxal induces apoptosis through oxidative stress-mediated activation of p38 mitogen-activated protein kinase in rat Schwann cells. Ann NY Acad Sci. 2005;1043:151–157. doi: 10.1196/annals.1333.019. [DOI] [PubMed] [Google Scholar]
- Grimble RF. Effect of antioxidative vitamins on immune function with clinical applications. Int J Vitam Nutr Res. 1997;67:312–320. [PubMed] [Google Scholar]
- Grimsrud PA, Picklo MJ, Sr, Griffin TJ, Bernlohr DA. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Mol Cell Proteomics. 2007. [DOI] [PubMed]
- Grune T, Davies KJ. The proteasomal system and HNE-modified proteins. Mol Aspects Med. 2003;24:195–204. doi: 10.1016/s0098-2997(03)00014-1. [DOI] [PubMed] [Google Scholar]
- Grune T, Michel P, Sitte N, Eggert W, Albrecht-Nebe H, Esterbauer H, et al. Increased levels of 4-hydroxynonenal modified proteins in plasma of children with autoimmune diseases. Free Radic Biol Med. 1997;23:357–360. doi: 10.1016/s0891-5849(96)00586-2. [DOI] [PubMed] [Google Scholar]
- Guiotto A, Calderan A, Ruzza P, Borin G. Carnosine and carnosine-related antioxidants: a review. Curr Med Chem. 2005;12:2293–2315. doi: 10.2174/0929867054864796. [DOI] [PubMed] [Google Scholar]
- Hajjar DP, Haberland ME. Lipoprotein trafficking in vascular cells. Molecular Trojan horses and cellular saboteurs. J Biol Chem. 1997;272:22975–22978. doi: 10.1074/jbc.272.37.22975. [DOI] [PubMed] [Google Scholar]
- Hayek T, Attias J, Coleman R, Brodsky S, Smith J, Breslow JL, et al. The angiotensin-converting enzyme inhibitor, fosinopril, and the angiotensin II receptor antagonist, losartan, inhibit LDL oxidation and attenuate atherosclerosis independent of lowering blood pressure in apolipoprotein E deficient mice. Cardiovasc Res. 1999;44:579–587. doi: 10.1016/s0008-6363(99)00239-4. [DOI] [PubMed] [Google Scholar]
- Hayek T, Attias J, Smith J, Breslow JL, Keidar S. Antiatherosclerotic and antioxidative effects of captopril in apolipoprotein E-deficient mice. J Cardiovasc Pharmacol. 1998;31:540–544. doi: 10.1097/00005344-199804000-00011. [DOI] [PubMed] [Google Scholar]
- Hipkiss AR, Preston JE, Himswoth DT, Worthington VC, Abbot NJ. Protective effects of carnosine against malondialdehyde-induced toxicity towards cultured rat brain endothelial cells. Neurosci Lett. 1997;238:135–138. doi: 10.1016/s0304-3940(97)00873-2. [DOI] [PubMed] [Google Scholar]
- Hoppe G, O'Neil J, Hoff HF. Inactivation of lysosomal proteases by oxidized low density lipoprotein is partially responsible for its poor degradation by mouse peritoneal macrophages. J Clin Invest. 1994;94:1506–1512. doi: 10.1172/JCI117490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubatsch I, Ridderstrom M, Mannervik D. Human glutathione transferase A4-4: an alpha class enzyme with high catalytic efficiency in the conjugation of 4-hydroxynonenal and other genotoxic products of lipid peroxidation. Biochem J. 1998;330:175–179. doi: 10.1042/bj3300175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai H, Graham DI, Masayasu H, Macrae IM. Antioxidant ebselen reduces oxidative damage in fical cerebral ischemia. Free Rad Biol Med. 2003;34:56–63. doi: 10.1016/s0891-5849(02)01180-2. [DOI] [PubMed] [Google Scholar]
- Ivanovski O, Szumilak D, Nguyen-Khoa T, Ruellan N, Phan O, Lacour B, et al. The antioxidant N-acetylcysteine prevents accelerated atherosclerosis in uremic apolipoprotein E knockout mice. Kidney Int. 2005;67:2288–2294. doi: 10.1111/j.1523-1755.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- Jan CR, Chen CH, Wang SC, Kuo SY. Effect of methylglyoxal on intracellular calcium levels and viability in renal tubular cells. Cell Signal. 2005;17:847–855. doi: 10.1016/j.cellsig.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Je JH, Lee JY, Jung KJ, Sung B, Go EK, Yu BP, et al. NF-kappaB activation mechanism of 4-hydroxyhexenal via NIK/IKK and p38 MAPK pathway. FEBS Lett. 2004;566:183–189. doi: 10.1016/j.febslet.2004.04.037. [DOI] [PubMed] [Google Scholar]
- Jedidi I, Therond P, Zarev S, Cosson C, Couturier M, Massot C, et al. Paradoxical protective effect of aminoguanidine toward low-density lipoprotein oxidation: inhibition of apolipoprotein B fragmentation without preventing its carbonylation. Mechanism of action of aminoguanidine. Biochemistry. 2003;42:11356–11365. doi: 10.1021/bi034539w. [DOI] [PubMed] [Google Scholar]
- Jenner P.Oxidative stress in Parkinson's disease Ann Neurol 200353Suppl 3S26–S36.discussion S36–28 [DOI] [PubMed] [Google Scholar]
- Jurgens G, Ashy A, Esterbauer H. Detection of new epitopes formed upon oxidation of LDL, lipoprotein (a) and VLDL. Use of an antiserum against 4-HNE-modified LDL. Biochem J. 1990;265:605–608. doi: 10.1042/bj2650605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens G, Hoff HF, Chisolm GM, III, Esterbauer H. Modification of human serum low density lipoprotein by oxidation—characterization and pathophysiological implications. Chem Phys Lipids. 1987;45:315–336. doi: 10.1016/0009-3084(87)90070-3. [DOI] [PubMed] [Google Scholar]
- Jürgens G, Lang J, Esterbauer H. Modification of human LDL by the lipid peroxydation product 4-hydroxynonenal. Biochim Biophys Acta. 1986;875:103–114. doi: 10.1016/0005-2760(86)90016-0. [DOI] [PubMed] [Google Scholar]
- Kaminskas LM, Pyke SM, Burcham PC. Strong protein adduct trapping accompanies abolition of acrolein-mediated hepatotoxicity by hydralazine in mice. J Pharmacol Exp Ther. 2004;310:1003–1010. doi: 10.1124/jpet.104.067330. [DOI] [PubMed] [Google Scholar]
- Kang Z, Li H, Li G, Yin D. Reaction of pyridoxamine with malondialdehyde: mechanism of inhibition of formation of advanced lipoxidation end-products. Amino Acids. 2006;30:55–61. doi: 10.1007/s00726-005-0209-6. [DOI] [PubMed] [Google Scholar]
- Kapphahn RJ, Bigelow EJ, Ferrington DA. Age-dependent inhibition of proteasome chymotrypsin-like activity in the retina. Exp Eye Res. 2007;84:646–654. doi: 10.1016/j.exer.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Arteel GE, Rusyn I, Sies H, Thurman RG. Ebselen prevents early alcohol-induced liver injury in rats. Free Rad Biol Med. 2001;30:403–411. doi: 10.1016/s0891-5849(00)00490-1. [DOI] [PubMed] [Google Scholar]
- Kreuzer T, Grube R, Wutte A, Zarcovic N, Schaur RJ. 4-Hydroxynonenal modifies the effects of serum growth factors on the expression of the c-fos proto-oncogene and the proliferation of HeLa carcinoma cells. Free Radic Biol Med. 1998;25:42–49. doi: 10.1016/s0891-5849(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Kreuzer T, Zarcovic N, Grube R, Schaur RJ. Inhibition of HeLa cell proliferation by 4-hydroxynonenal is associated with enhanced expression of the c-fos oncogene. Cancer Biother Radiopharm. 1997;12:131–136. doi: 10.1089/cbr.1997.12.131. [DOI] [PubMed] [Google Scholar]
- Kristal BS, Park BK, Yu BP. 4-Hydroxyhexenal is a potent inducer of the mitochondrial permeability transition. J Biol Chem. 1996;271:6033–6038. doi: 10.1074/jbc.271.11.6033. [DOI] [PubMed] [Google Scholar]
- Kruman II, Mattson MP. Pivotal role of mitochondrial calcium uptake in neural cell apoptosis and necrosis. J Neurochem. 1999;72:529–540. doi: 10.1046/j.1471-4159.1999.0720529.x. [DOI] [PubMed] [Google Scholar]
- Lee JY, Je JH, Kim DH, Chung SW, Zou Y, Kim ND, et al. Induction of endothelial apoptosis by 4-hydroxyhexenal. Eur J Biochem. 2004;271:1339–1347. doi: 10.1111/j.1432-1033.2004.04042.x. [DOI] [PubMed] [Google Scholar]
- Lee JY, Jung GY, Heo HJ, Yun MR, Park JY, Bae SS, et al. 4-Hydroxynonenal induces vascular smooth muscle cell apoptosis through mitochondrial generation of reactive oxygen species. Toxicol Lett. 2006;166:212–221. doi: 10.1016/j.toxlet.2006.07.305. [DOI] [PubMed] [Google Scholar]
- Leonarduzzi G, Chiarpotto E, Biasi F, Poli G. 4-Hydroxynonenal and cholesterol oxidation products in atherosclerosis. Mol Nutr Food Res. 2005;49:1044–1049. doi: 10.1002/mnfr.200500090. [DOI] [PubMed] [Google Scholar]
- Leonarduzzi G, Robbesyn F, Poli G. Signaling kinases modulated by 4-hydroxynonenal. Free Radic Biol Med. 2004;37:1694–1702. doi: 10.1016/j.freeradbiomed.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Liu W, Akhand AA, Kato M, Yokoyama I, Miyata T, Kurokawa K, et al. 4-hydroxynonenal triggers an epidermal growth factor receptor-linked signal pathway for growth inhibition. J Cell Sci. 1999;112:2409–2417. doi: 10.1242/jcs.112.14.2409. [DOI] [PubMed] [Google Scholar]
- Loguercio C, Federico A. Oxidative stress in viral and alcoholic hepatitis. Free Radic Biol Med. 2003;34:1–10. doi: 10.1016/s0891-5849(02)01167-x. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Markesbery WR. Amyloid beta peptide, 4-hydroxynonenal and apoptosis. Curr Alzheimer Res. 2006;3:359–364. doi: 10.2174/156720506778249506. [DOI] [PubMed] [Google Scholar]
- Lyons TJ, Silvestri G, Dunn JA, Dyer DG, Baynes JW. Role of glycation in modification of lens crystallins in diabetic and nondiabetic senile cataracts. Diabetes. 1991;40:1010–1015. doi: 10.2337/diab.40.8.1010. [DOI] [PubMed] [Google Scholar]
- Makita Z, Yanagisawa K, Kuwajima S, Bucala R, Vlassara H, Koike T. The role of advanced glycosylation end-products in the pathogenesis of atherosclerosis. Nephrol Dial Transplant. 1996;11 Suppl 5:31–33. doi: 10.1093/ndt/11.supp5.31. [DOI] [PubMed] [Google Scholar]
- Marques C, Pereira P, Taylor A, Liang JN, Reddy VN, Szweda LI, et al. Ubiquitin-dependent lysosomal degradation of the HNE-modified proteins in lens epithelial cells. FASEB J. 2004;18:1424–1426. doi: 10.1096/fj.04-1743fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsili S, Salganik RI, Albright CD, Freel CD, Johnsen S, Peiffer RL, et al. Cataract formation in a strain of rats selected for high oxidative stress. Exp Eye Res. 2004;79:595–612. doi: 10.1016/j.exer.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Chan SL. Neuronal and glial calcium signaling in Alzheimer's disease. Cell Calcium. 2003;34:385–397. doi: 10.1016/s0143-4160(03)00128-3. [DOI] [PubMed] [Google Scholar]
- McEwen JE, Zimniak P, Mehta JL, Reis RJ. Molecular pathology of aging and its implications for senescent coronary atherosclerosis. Curr Opin Cardiol. 2005;20:399–406. doi: 10.1097/01.hco.0000175517.50181.89. [DOI] [PubMed] [Google Scholar]
- Miyata T, Ueda Y, Asahi K, Izuhara Y, Inagi R, Saito A, et al. Mechanism of the inhibitory effect of OPB-9195 [(+/−)-2-isopropylidenehydrazono-4-oxo-thiazolidin-5-yla cetanilide] on advanced glycation end product and advanced lipoxidation end product formation. J Am Soc Nephrol. 2000;11:1719–1725. doi: 10.1681/ASN.V1191719. [DOI] [PubMed] [Google Scholar]
- Montan S. Drugs used in hypertensive diseases in pregnancy. Curr Opin Obstet Gynecol. 2004;16:111–115. doi: 10.1097/00001703-200404000-00003. [DOI] [PubMed] [Google Scholar]
- Morgan PE, Sturgess AD, Davies MJ. Increased levels of serum protein oxidation and correlation with disease activity in systemic lupus erythematosus. Arthritis Rheum. 2005;52:2069–2079. doi: 10.1002/art.21130. [DOI] [PubMed] [Google Scholar]
- Morquette B, Shi Q, Lavigne P, Ranger P, Fernandes JC, Benderdour M. Production of lipid peroxidation products in osteoarthritic tissues: new evidence linking 4-hydroxynonenal to cartilage degradation. Arthritis Rheum. 2006;54:271–281. doi: 10.1002/art.21559. [DOI] [PubMed] [Google Scholar]
- Munzel T, Kurz S, Rajagopalan S, Thoenes M, Berrington WR, Thompson JA, et al. Hydralazine prevents nitroglycerin tolerance by inhibiting activation of a membrane-bound NADH oxidase. A new action for an old drug. J Clin Invest. 1996;98:1465–1470. doi: 10.1172/JCI118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni DV, Sayre LM. Structural definition of early lysine and histidine adduction chemistry of 4-hydroxynonenal. Chem Res Toxicol. 1995;8:284–291. doi: 10.1021/tx00044a014. [DOI] [PubMed] [Google Scholar]
- Nangaku M, Miyata T, Sada T, Mizuno M, Inagi R, Ueda Y, et al. Anti-hypertensive agents inhibit in vivo the formation of advanced glycation end products and improve renal damage in a type 2 diabetic nephropathy rat model. J Am Soc Nephrol. 2003;14:1212–1222. doi: 10.1097/01.asn.0000062961.76776.c1. [DOI] [PubMed] [Google Scholar]
- Nitti M, Domenicotti C, d'Abramo C, Assereto S, Cottalasso D, Melloni E, et al. Activation of PKC-beta isoforms mediates HNE-induced MCP-1 release by macrophages. Biochem Biophys Res Commun. 2002;294:547–552. doi: 10.1016/S0006-291X(02)00512-0. [DOI] [PubMed] [Google Scholar]
- Okada K, Wangpoengtrakul C, Osawa T, Toyokuni S, Tanaka K, Ushida K. 4-Hydroxy-2-nonenal-mediated impairment of intracellular proteolysis during oxidative stress. Identification of proteasomes as target molecules. J Biol Chem. 1999;274:23787–23793. doi: 10.1074/jbc.274.34.23787. [DOI] [PubMed] [Google Scholar]
- Onorato JM, Jenkins AJ, Thorpe SR, Baynes JW. Pyridoxamine, an inhibitor of advanced glycation reactions, also inhibits advanced lipoxidation reactions. Mechanism of action of pyridoxamine. J Biol Chem. 2000;275:21177–21184. doi: 10.1074/jbc.M003263200. [DOI] [PubMed] [Google Scholar]
- Page S, Fischer C, Baumgartner B, Haas M, Kreusel U, Loidl G, et al. 4-Hydroxynonenal prevents NF-kappaB activation and tumor necrosis factor expression by inhibiting IkappaB phosphorylation and subsequent proteolysis. J Biol Chem. 1999;274:11611–11618. doi: 10.1074/jbc.274.17.11611. [DOI] [PubMed] [Google Scholar]
- Palinski W, Rosenfeld ME, Yla-Herttuala S, Gurtner GC, Socher SS, Butler SW, et al. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci USA. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen DR, Doorn JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic Biol Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Peyroux J, Sternberg M. Advanced glycation endproducts (AGEs): pharmacological inhibition in diabetes. Pathol Biol (Paris) 2006;54:405–419. doi: 10.1016/j.patbio.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Pierre F, Peiro G, Tache S, Cross AJ, Bingham SA, Gasc N, et al. New marker of colon cancer risk associated with heme intake: 1,4-dihydroxynonane mercapturic acid. Cancer Epidemiol Biomarkers Prev. 2006;15:2274–2279. doi: 10.1158/1055-9965.EPI-06-0085. [DOI] [PubMed] [Google Scholar]
- Pierre F, Tache S, Gueraud F, Rerole AL, Jourdan ML, Petit C. Apc mutation induces resistance of colonic cells to lipoperoxide-triggered apoptosis induced by faecal water from haem-fed rats. Carcinogenesis. 2007;28:321–327. doi: 10.1093/carcin/bgl127. [DOI] [PubMed] [Google Scholar]
- Poli G, Schaur RJ. 4-Hydroxynonenal in the pathomechanisms of oxidative stress. IUBMB Life. 2000;50:315–321. doi: 10.1080/713803726. [DOI] [PubMed] [Google Scholar]
- Portero-Otin M, Pamplona R, Bellmunt MJ, Ruiz MC, Prat J, Salvayre R, et al. Advanced glycation end product precursors impair epidermal growth factor receptor signaling. Diabetes. 2002;51:1535–1542. doi: 10.2337/diabetes.51.5.1535. [DOI] [PubMed] [Google Scholar]
- Price DL, Rhett PM, Thorpe SR, Baynes JW. Chelating activity of advanced glycation end-product inhibitors. J Biol Chem. 2001;276:48967–48972. doi: 10.1074/jbc.M108196200. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Phansalkar K, Audesirk G, West A, Cabell L. Differential regulation of c-jun and CREB by acrolein and 4-hydroxynonenal. Free Radic Biol Med. 2006;40:21–34. doi: 10.1016/j.freeradbiomed.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Yan SF, Schmidt AM. Methylglyoxal comes of AGE. Cell. 2006;124:258–260. doi: 10.1016/j.cell.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Raza H, John A. 4-hydroxynonenal induces mitochondrial oxidative stress, apoptosis and expression of glutathione S-transferase A4-4 and cytochrome P450 2E1 in PC12 cells. Toxicol Appl Pharmacol. 2006;216:309–318. doi: 10.1016/j.taap.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Riboulet-Chavey A, Pierron A, Durand I, Murdaca J, Giudicelli J, Van Obberghen E. Methylglyoxal impairs the insulin signaling pathways independently of the formation of intracellular reactive oxygen species. Diabetes. 2006;55:1289–1299. doi: 10.2337/db05-0857. [DOI] [PubMed] [Google Scholar]
- Robbesyn F, Garcia V, Auge N, Vieira O, Frisach MF, Salvayre R, et al. HDL counterbalance the proinflammatory effect of oxidized LDL by inhibiting intracellular reactive oxygen species rise, proteasome activation, and subsequent NF-kappaB activation in smooth muscle cells. FASEB J. 2003;17:743–745. doi: 10.1096/fj.02-0240fje. [DOI] [PubMed] [Google Scholar]
- Rockwell P, Yuan H, Magnusson R, Figueiredo-Pereira ME. Proteasome inhibition in neuronal cells induces a proinflammatory response manifested by upregulation of cyclooxygenase-2, its accumulation as ubiquitin conjugates, and production of the prostaglandin PGE(2) Arch Biochem Biophys. 2000;374:325–333. doi: 10.1006/abbi.1999.1646. [DOI] [PubMed] [Google Scholar]
- Roff M, Thompson J, Rodriguez MS, Jacque JM, Baleux F, Arenzana-Seisdedos F, et al. Role of IkappaBalpha ubiquitination in signal-induced activation of NFkappaB in vivo. J Biol Chem. 1996;271:7844–7850. doi: 10.1074/jbc.271.13.7844. [DOI] [PubMed] [Google Scholar]
- Rohn TT, Nelson LK, Waeg G, Quinn MT. U-101033E (2,4-diaminopyrrolopyrimidine), a potent inhibitor of membrane lipid peroxidation as assessed by the production of 4-hydroxynonenal, malondialdehyde, and 4-hydroxynonenal–protein adducts. Biochem Pharmacol. 1998;56:1371–1379. doi: 10.1016/s0006-2952(98)00266-4. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Satoh K, Yamada S, Koike Y, Igarashi Y, Toyokuni S, Kumano T, et al. A 1-hour enzyme-linked immunosorbent assay for quantitation of acrolein- and hydroxynonenal-modified proteins by epitope-bound casein matrix method. Anal Biochem. 1999;270:323–328. doi: 10.1006/abio.1999.4073. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Arora PK, Iyer RS, Salomon RG. Pyrrole formation from 4-hydroxynonenal and primary amines. Chem Res Toxicol. 1993;6:19–22. doi: 10.1021/tx00031a002. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Lin D, Yuan Q, Zhu X, Tang X. Protein adducts generated from products of lipid oxidation: focus on HNE and one. Drug Metab Rev. 2006;38:651–675. doi: 10.1080/03602530600959508. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer's disease. J Neurochem. 1997;68:2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- Schaur RJ. Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol Aspects Med. 2003;24:149–159. doi: 10.1016/s0098-2997(03)00009-8. [DOI] [PubMed] [Google Scholar]
- Shah R, Raska K, Jr, Tiku ML. The presence of molecular markers of in vivo lipid peroxidation in osteoarthritic cartilage: a pathogenic role in osteoarthritis. Arthritis Rheum. 2005;52:2799–2807. doi: 10.1002/art.21239. [DOI] [PubMed] [Google Scholar]
- Shi Q, Vaillancourt F, Cote V, Fahmi H, Lavigne P, Afif H, et al. Alterations of metabolic activity in human osteoarthritic osteoblasts by lipid peroxidation end product 4-hydroxynonenal. Arthritis Res Ther. 2006;8:R159. doi: 10.1186/ar2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibamoto T. Analytical methods for trace levels of reactive carbonyl compounds formed in lipid peroxidation systems. J Pharm Biomed Anal. 2006;41:12–25. doi: 10.1016/j.jpba.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Shringarpure R, Grune T, Mehlhase J, Davies KJ. Ubiquitin conjugation is not required for the degradation of oxidized proteins by proteasome. J Biol Chem. 2003;278:311–318. doi: 10.1074/jbc.M206279200. [DOI] [PubMed] [Google Scholar]
- Shringarpure R, Grune T, Sitte N, Davies KJ. 4-Hydroxynonenal-modified amyloid-beta peptide inhibits the proteasome: possible importance in Alzheimer's disease. Cell Mol Life Sci. 2000;57:1802–1809. doi: 10.1007/PL00000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siems W, Quast S, Carluccio F, Wiswedel I, Hirsch D, Augustin W, et al. Oxidative stress in chronic renal failure as a cardiovascular risk factor. Clin Nephrol. 2002;58 Suppl 1:S12–S19. [PubMed] [Google Scholar]
- Siow RC, Ishii T, Mann GE. Modulation of antioxidant gene expression by 4-hydroxynonenal: atheroprotective role of the Nrf2/ARE transcription pathway. Redox Rep. 2007;12:11–15. doi: 10.1179/135100007X162167. [DOI] [PubMed] [Google Scholar]
- Sitte N, Merker K, Von Zglinicki T, Grune T, Davies KJ. Protein oxidation and degradation during cellular senescence of human BJ fibroblasts: part I—effects of proliferative senescence. FASEB J. 2000;14:2495–2502. doi: 10.1096/fj.00-0209com. [DOI] [PubMed] [Google Scholar]
- Smit AJ, Lutgers HL. The clinical relevance of advanced glycation endproducts (AGE) and recent developments in pharmaceutics to reduce AGE accumulation. Curr Med Chem. 2004;11:2767–2784. doi: 10.2174/0929867043364342. [DOI] [PubMed] [Google Scholar]
- Smith MA, Sayre LM, Anderson VE, Harris PL, Beal MF, Kowall N, et al. Cytochemical demonstration of oxidative damage in Alzheimer disease by immunochemical enhancement of the carbonyl reaction with 2,4-dinitrophenylhydrazine. J Histochem Cytochem. 1998;46:731–735. doi: 10.1177/002215549804600605. [DOI] [PubMed] [Google Scholar]
- Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. 1997;272:20963–20966. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of LDL that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Steinbrecher UP. Receptors for oxidized low density lipoprotein. Biochim Biophys Acta. 1999;1436:279–298. doi: 10.1016/s0005-2760(98)00127-1. [DOI] [PubMed] [Google Scholar]
- Suarna C, Wu BJ, Choy K, Mori T, Croft K, Cynshi O, et al. Protective effect of vitamin E supplements on experimental atherosclerosis is modest and depends on preexisting vitamin E deficiency. Free Radic Biol Med. 2006;41:722–730. doi: 10.1016/j.freeradbiomed.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Suc I, Escargueil-Blanc I, Troly M, Salvayre R, Negre-Salvayre A. HDL and ApoA prevent cell death of endothelial cells induced by oxidized LDL. Arterioscler Thromb Vasc Biol. 1997;17:2158–2166. doi: 10.1161/01.atv.17.10.2158. [DOI] [PubMed] [Google Scholar]
- Suc I, Meilhac O, Lajoie-Mazenc I, Vandaele J, Jurgens G, Salvayre R. Activation of EGF receptor by oxidized LDL. FASEB J. 1998;12:665–671. doi: 10.1096/fasebj.12.9.665. [DOI] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Cutler RG, Jo DG, Magnus T, Chan SL, et al. Neuroprotective actions of a histidine analogue in models of ischemic stroke. J Neurochem. 2007;101:729–736. doi: 10.1111/j.1471-4159.2006.04412.x. [DOI] [PubMed] [Google Scholar]
- Thomas MC, Forbes JM, MacIsaac R, Jerums G, Cooper ME. Low-molecular weight advanced glycation end products: markers of tissue AGE accumulation and more. Ann NY Acad Sci. 2005;1043:644–654. doi: 10.1196/annals.1333.073. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch Biochem Biophys. 2003;419:31–40. doi: 10.1016/j.abb.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344 Part 1:109–116. [PMC free article] [PubMed] [Google Scholar]
- Thornalley PJ, Yurek-George A, Argirov OK. Kinetics and mechanism of the reaction of aminoguanidine with the alpha-oxoaldehydes glyoxal, methylglyoxal, and 3-deoxyglucosone under physiological conditions. Biochem Pharmacol. 2000;60:55–65. doi: 10.1016/s0006-2952(00)00287-2. [DOI] [PubMed] [Google Scholar]
- Torzewski M, Klouche M, Hock J, Messner M, Dorweiler B, Torzewski J, et al. Immunohistochemical demonstration of enzymatically modified human LDL and its colocalization with the terminal complement complex in the early atherosclerotic lesion. Arterioscler Thromb Vasc Biol. 1998;18:369–378. doi: 10.1161/01.atv.18.3.369. [DOI] [PubMed] [Google Scholar]
- Tsimikas S. Oxidized low-density lipoprotein biomarkers in atherosclerosis. Curr Atheroscler Rep. 2006;8:55–61. doi: 10.1007/s11883-006-0065-1. [DOI] [PubMed] [Google Scholar]
- Tsuneyama K, Harada K, Kono N, Sasaki M, Saito T, Gershwin ME, et al. Damaged interlobular bile ducts in primary biliary cirrhosis show reduced expression of glutathione-S-transferase-pi and aberrant expression of 4-hydroxynonenal. J Hepatol. 2002;37:176–183. doi: 10.1016/s0168-8278(02)00105-8. [DOI] [PubMed] [Google Scholar]
- Uchida K. Role of reactive aldehyde in cardiovascular diseases. Free Radic Biol Med. 2000;28:1685–1696. doi: 10.1016/s0891-5849(00)00226-4. [DOI] [PubMed] [Google Scholar]
- Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- Uchida K, Itakura K, Kawakishi S, Hiai H, Toyokuni S, Stadtman ER. Characterization of epitopes recognized by 4-hydroxy-2-nonenal specific antibodies. Arch Biochem Biophys. 1995;324:241–248. doi: 10.1006/abbi.1995.0036. [DOI] [PubMed] [Google Scholar]
- Uchida K, Kanematsu M, Morimitsu Y, Osawa T, Noguchi N, Niki E. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J Biol Chem. 1998;273:16058–16066. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- Vacaresse N, Vieira O, Robbesyn F, Jurgens G, Salvayre R, Negre-Salvayre A. Phenolic antioxidants trolex and caffeic acid modulate the oxidized LDL-induced EGF-receptor activation. Br J Pharmacol. 2001;132:1777–1788. doi: 10.1038/sj.bjp.0703981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valacchi G, Pagnin E, Phung A, Nardini M, Schock BC, Cross CE, et al. Inhibition of NFkappaB activation and IL-8 expression in human bronchial epithelial cells by acrolein. Antioxid Redox Signal. 2005;7:25–31. doi: 10.1089/ars.2005.7.25. [DOI] [PubMed] [Google Scholar]
- Valentovic M, Terneus M, Harmon RC, Carpenter AB. S-Adenosylmethionine (SAMe) attenuates acetaminophen hepatotoxicity in C57BL/6 mice. Toxicol Lett. 2004;154:165–174. doi: 10.1016/j.toxlet.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Van Kuijk FJ, Holte LL, Dratz EA. 4-Hydroxyhexenal: a lipid peroxidation product derived from oxidized docosahexaenoic acid. Biochim Biophys Acta. 1990;1043:116–118. doi: 10.1016/0005-2760(90)90118-h. [DOI] [PubMed] [Google Scholar]
- Vieira O, Escargueil-Blanc I, Jurgens G, Borner C, Almeida L, Salvayre R, et al. Oxidized LDLs alter the activity of the ubiquitin–proteasome pathway: potential role in oxidized LDL-induced apoptosis. FASEB J. 2000;14:532–542. doi: 10.1096/fasebj.14.3.532. [DOI] [PubMed] [Google Scholar]
- Vindis C, Escargueil-Blanc I, Elbaz M, Marcheix B, Grazide MH, Uchida K, et al. Desensitization of platelet-derived growth factor receptor-beta by oxidized lipids in vascular cells and atherosclerotic lesions: prevention by aldehyde scavengers. Circ Res. 2006;98:785–792. doi: 10.1161/01.RES.0000216288.93234.c3. [DOI] [PubMed] [Google Scholar]
- Virlos I, Mazzon E, Serraino I, Di Paola R, Genovese T, Britti D, et al. Pyrrolidine dithiocarbamate reduces the severity of cerulein-induced murine acute pancreatitis. Shock. 2003;20:544–550. doi: 10.1097/01.shk.0000093543.78705.aa. [DOI] [PubMed] [Google Scholar]
- Voss P, Siems W. Clinical oxidation parameters of aging. Free Radic Res. 2006;40:1339–1349. doi: 10.1080/10715760600953859. [DOI] [PubMed] [Google Scholar]
- Voziyan PA, Hudson BG. Pyridoxamine as a multifunctional pharmaceutical: targeting pathogenic glycation and oxidative damage. Cell Mol Life Sci. 2005;62:1671–1681. doi: 10.1007/s00018-005-5082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waeg G, Dimsity G, Esterbauer H. Monoclonal antibodies for detection of 4-hydroxynonenal modified proteins. Free Radic Res. 1996;25:149–159. doi: 10.3109/10715769609149920. [DOI] [PubMed] [Google Scholar]
- Wataya T, Nunomura A, Smith MA, Siedlak SL, Harris PL, Shimohama S, et al. High molecular weight neurofilament proteins are physiological substrates of adduction by the lipid peroxidation product hydroxynonenal. J Biol Chem. 2002;277:4644–4648. doi: 10.1074/jbc.M110913200. [DOI] [PubMed] [Google Scholar]
- Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- Whitnall M, Richardson DR. Iron: a new target for pharmacological intervention in neurodegenerative diseases. Semin Pediatr Neurol. 2006;13:186–197. doi: 10.1016/j.spen.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Xu G, Liu Y, Sayre LM. Polyclonal antibodies to a fluorescent 4-hydroxy-2-nonenal (HNE)-derived lysine-lysine cross-link: characterization and application to HNE-treated protein and in vitro oxidized low-density lipoprotein. Chem Res Toxicol. 2000;13:406–413. doi: 10.1021/tx990200s. [DOI] [PubMed] [Google Scholar]
- Yang Y, Sharma R, Sharma A, Awasthi S, Awasthi YC. Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochim Pol. 2003;50:319–336. [PubMed] [Google Scholar]
- Yao HW, Zhu JP, Zhao MH, Lu Y. Losartan attenuates bleomycin-induced pulmonary fibrosis in rats. Respiration. 2006;73:236–242. doi: 10.1159/000090140. [DOI] [PubMed] [Google Scholar]
- Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci USA. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PH, Zuo DM. Aminoguanidine inhibits semicarbazide-sensitive amine oxidase activity: implications for advanced glycation and diabetic complications. Diabetologia. 1997;40:1243–1250. doi: 10.1007/s001250050816. [DOI] [PubMed] [Google Scholar]
- Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, et al. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- Zarkovic N. 4-hydroxynonenal as a bioactive marker of pathophysiological processes. Mol Aspects Med. 2003;24:281–291. doi: 10.1016/s0098-2997(03)00023-2. [DOI] [PubMed] [Google Scholar]
- Zarkovic N, Ilic Z, Jurin M, Schaur RJ, Puhl H, Esterbauer H. Stimulation of HeLa cell growth by physiological concentrations of 4-hydroxynonenal. Cell Biochem Funct. 1993;11:279–286. doi: 10.1002/cbf.290110409. [DOI] [PubMed] [Google Scholar]
- Zhang H, Court N, Forman HJ. Submicromolar concentrations of 4-hydroxynonenal induce glutamate cysteine ligase expression in HBE1 cells. Redox Rep. 2007;12:101–106. doi: 10.1179/135100007X162266. [DOI] [PMC free article] [PubMed] [Google Scholar]