Abstract
Background and purpose:
C-type natriuretic peptide (CNP) has been proposed to make a fundamental contribution in arterial endothelium-dependent hyperpolarization to acetylcholine. The present study was designed to address this hypothesis in the guinea-pig carotid artery.
Experimental approach:
The membrane potential of vascular smooth muscle cells was recorded in isolated arteries with intracellular microelectrodes.
Key results:
Acetylcholine induced endothelium-dependent hyperpolarizations in the presence or absence of N G-nitro-L-arginine, indomethacin and/or thiorphan, inhibitors of NO-synthases, cyclooxygenases or neutral endopeptidase, respectively. Acetycholine hyperpolarized smooth muscle cells in resting arteries and produced repolarizations in phenylephrine-stimulated arteries. CNP produced hyperpolarizations with variable amplitude. They were observed only in the presence of inhibitors of NO-synthases and cyclooxygenases and were endothelium-independent, maintained in phenylephrine-depolarized carotid arteries, and not affected by the additional presence of thiorphan. In arteries with endothelium, the hyperpolarizations produced by CNP were always significantly smaller than those induced by acetylcholine. Upon repeated administration, a significant tachyphylaxis of the hyperpolarizing effect of CNP was observed, while consecutive administration of acetycholine produced sustained responses. The hyperpolarizations evoked by acetylcholine were abolished by the combination of apamin plus charybdotoxin, but unaffected by glibenclamide or tertiapin. In contrast, CNP-induced hyperpolarizations were abolished by glibenclamide and unaffected by the combination of apamin plus charybdotoxin.
Conclusions and implications:
In the isolated carotid artery of the guinea-pig, CNP activates KATP and is a weak hyperpolarizing agent. In this artery, the contribution of CNP to EDHF-mediated responses is unlikely.
Keywords: EDHF-mediated responses, potassium channels, CNP, ANP
Introduction
The endothelium controls the tone of the underlying smooth muscle cells by releasing vasoactive substances such as prostacyclin (Moncada and Vane, 1979) and nitric oxide (Furchgott and Zawadzki, 1980). In some arteries, a pathway that requires the hyperpolarization of both the endothelial and smooth muscle cells (EDHF-mediated responses) contributes to endothelium-dependent relaxations. EDHF-mediated responses involve an increase in the endothelial intracellular calcium concentration and the opening of calcium-activated potassium channels of small and/or intermediate conductance (SKCa and IKCa). In many arteries, including the rat mesenteric artery, the endothelium-dependent hyperpolarization of the smooth muscle cells can be evoked by direct electrical coupling through myo-endothelial junctions and/or the accumulation of potassium ions in the intercellular space. Potassium ions hyperpolarize the smooth muscle cells by activating inward rectifying potassium channels (Kir) and/or Na+/K+-ATPase (Chaytor et al., 1998; Edwards et al., 1998, 1999; Sandow and Hill, 2000). Additionally, in some blood vessels, including large and small coronary arteries, the endothelium produces arachidonic acid metabolites derived from cytochrome P450 monooxygenases. The epoxyeicosatrienoic acids generated are not only intracellular messengers, but can also diffuse and hyperpolarize the smooth muscle cells by activating large conductance calcium-activated potassium channels (BKCa; Hecker et al., 1994; Campbell et al., 1996; Fleming, 2004).
However, recent experiments performed in isolated rat mesenteric arteries and in the isolated Langendorff-perfused heart preparation have been used to suggest that EDHF-mediated responses could involve the endothelial release of a hyperpolarizing factor, the C-type natriuretic peptide (CNP; Chauhan et al., 2003; Hobbs et al., 2004; Villar et al., 2007). Endothelial cells can theoretically synthesize numerous vasoactive peptides and among them CNP causes relaxation and hyperpolarization of arterial and venous smooth muscle cells (Koller and Goeddel, 1992; Stingo et al., 1992; Suga et al., 1993; Wei et al., 1994; Banks et al., 1996; Honing et al., 2001). The vasodilator effects of CNP are generally attributed to the activation of natriuretic peptide receptors of the B subtype (NPR-B) on the smooth muscle, followed by the stimulation of particulate guanylate cyclase, the accumulation of cyclic GMP and the subsequent opening of potassium channels, including BKCa and ATP-sensitive potassium channel, KATP (Wei et al., 1994; Banks et al., 1996; Garcha and Hughes, 2006). However, the mechanism underlying the relaxation of vascular smooth muscle caused by CNP during EDHF-mediated responses may involve a different pathway. CNP could activate the NPR-C receptors and produce a cyclic-GMP-independent hyperpolarization via the opening of a G-protein-regulated inward-rectifier potassium channel (GIRK; Chauhan et al., 2003; Ahluwalia and Hobbs, 2005).
The hypothesis that CNP is an endothelium-derived hyperpolarizing substance should be examined with caution, since it requires the validation of various concepts. First, if NPR-C, originally thought to be a clearance receptor, can mediate G-protein-dependent intracellular events such as the inhibition of adenylyl cyclase activity (Ahluwalia et al., 2004), it is unknown whether or not the CNP-dependent activation of NPR-C in vascular smooth muscle cells produces a cyclic GMP-independent, Pertussis toxin-sensitive signal. Second, whereas the functional expression of GIRK in neurons and cardiac myocytes is well documented, the protein expression and the complete characterization of this channel in vascular smooth muscle cells await full demonstration. And third, there is no evidence to date, in any cell type, that CNP can activate GIRK (Feletou and Vanhoutte, 2006; Sandow and Tare, 2007).
In other arteries than the rat mesenteric artery, which also exhibit EDHF-mediated responses, the involvement of CNP has not been verified so far. For instance, in porcine coronary arteries, the hyperpolarizations elicited by CNP do not mimic endothelium-dependent hyperpolarizations (Barton et al., 1998), while in mesenteric arteries taken from mice deficient for the NPR-C gene, EDHF-mediated responses are not altered (McGuire et al., 2004). Finally, in a variety of arteries, CNP or activators of the NPR-C receptors do not evoke relaxation (McGuire et al., 2004; Boussery et al., 2005).
The purpose of the present work was to evaluate in the guinea-pig isolated carotid artery, one of the reference arteries used for studying EDHF-mediated responses (Busse et al., 2002; Félétou and Vanhoutte, 2005), the effect of natriuretic peptides on the membrane potential of smooth muscle cells and to determine in this artery whether or not CNP could contribute to the endothelium-dependent hyperpolarization evoked by acetylcholine.
Methods
All animal procedures were in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and the guidelines established by the Ethical Committee of the Institut de Recherches Servier.
Male Hartley guinea pigs (250–300 g; Charles River, l'Arbresle, France) were killed with an overdose of pentobarbitone (200 mg kg−1, intraperitoneal) and the internal carotid arteries with their branches were dissected free. Segments of artery (1 cm in length) were cleaned of adherent connective tissues and pinned down to the bottom of an organ chamber (0.5 ml in volume) superfused at a constant flow (2 ml min−1; 37 °C) with modified Krebs-Ringer bicarbonate solution of the following composition (in mM): NaCl 118.3, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, glucose 11.1 and EDTA 0.026 (buffered with 95% O2 and 5% CO2, pH 7.4). In some arteries, the endothelium was removed by infusing a saponin solution (1 mg ml−1, for 20 s) that was subsequently flushed with Krebs solution (Feletou and Teisseire, 1990). Transmembrane potentials were recorded from the adventitial side of the internal carotid arteries with glass capillary microelectrodes (tip resistance of 50–90 MΩ) filled with KCl (3 M) and connected to the headstage of a recording amplifier (World Precision Instrument intra 767, New Haven, CT, USA). An Ag/AgCl pellet, in contact with the bathing solution and directly connected to the amplifier, served as the reference electrode. The signal was continuously monitored on an oscilloscope (Gould DSO 405, Valley view, OH, USA) and was recorded by using a pClamp software (Axon instrument, Foster City, CA, USA). Successful impalements were signalled by a sudden negative drop in the potential from the baseline (zero potential reference), followed by a stable negative potential for at least 3 min. The acetylcholine-induced hyperpolarizations were analysed using pClampfit (Axon instrument, Foster City, CA, USA). Hyperpolarizations are expressed as the maximal amplitude between the resting membrane potential and that in the presence of the hyperpolarizing drugs (peak amplitude).
Drugs were added by continuous superfusion via the Krebs solution reservoir with the exception of the studies involving tertiapin. In this latter set of experiments, the prohibitive cost of this toxin prevented running experiments with continuous superfusion via the Krebs solution reservoir. Therefore, the membrane potential and the effect of acetylcholine were recorded first. Then, after washout of acetylcholine, the superfusion was stopped and the oxygenation (95% O2 and 5% CO2) was given directly in the chamber to maintain adequate pO2 and pH. The carotid arteries were incubated in the presence or absence of tertiapin (10 μM) for at least 20 min. An injection of a stock solution of acetylcholine diluted in Krebs solution (with or without tertiapin) was administered directly in the chamber to obtain a final concentration of 1 μM acetylcholine (Chataigneau et al., 1998a).
Statistics
Data are shown as mean±s.e.m.; n indicates the number of cells in which membrane potential was recorded. Comparisons vs control were performed statistically using a one-way analysis of variance, followed by Bonferroni's multiple comparison test for paired or unpaired experiments or by use of Student's t-test for paired or unpaired observations, as appropriate. Differences were considered to be statistically significant when the P-value was less than 0.05.
Drugs
Following drugs were used: acetylcholine, indomethacin, NG-nitro-L-arginine, phenylephrine, glibenclamide, levcromakalim, sodium nitroprusside and thiorphan (Sigma, La Verpillère, France); charybdotoxin and apamin (Latoxan, Valence, France); atrial natriuretic peptide (ANP), and CNP (Calbiochem, VWR, Fontenay/bois, France); tertiapin (Alomone labs, Jerusalem, Israel).
Results
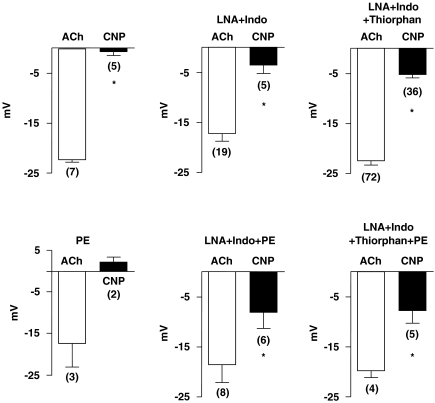
The membrane potential of the smooth muscle cells of the isolated guinea-pig carotid artery with endothelium was −55.4±2.8 mV (n=9). Acetylcholine (1 μM) produced a hyperpolarization, while CNP (1 μM) did not produce significant changes in membrane potential. Phenylephrine (0.5 μM) caused a depolarization (9.5 mV, n=2). In the presence of the α1-adrenergic agonist, CNP did not affect the membrane potential, while acetylcholine still hyperpolarized the smooth muscle cells (Figure 1).
Figure 1.
Acetylcholine (ACh: 1 μM)- and CNP (1 μM)-induced hyperpolarizations in guinea-pig carotid arteries with endothelium, under various experimental conditions. Phenylephrine (PE: 0.3–0.3 μM); NG-nitro-L-arginine (LNA: 100 μM); indomethacin (Indo: 5 μM); thiorphan (5 μM). Data are shown as means±s.e.m. The numbers in parenthesis indicate the number of cells recorded. * indicates a statistically significant difference between the amplitude of acetylcholine-induced hyperpolarization and that of CNP (t-test for unpaired experiments, P<0.05). CNP, C-type natriuretic peptide.
Effects of CNP in the presence of the combination of indomethacin (5 μM) plus NG-nitro-L-arginine (100 μM)
In the presence of the two inhibitors, the resting membrane potential was not significantly affected (−56.7±1.8 mV, n=18). Acetylcholine produced a similar hyperpolarization and CNP produced a small but measurable hyperpolarization in four out of five cells recorded from four different preparations. Phenylephrine (0.3 μM) caused a depolarization (membrane potential: −49.9±1.2 mV, n=15). The addition of acetylcholine hyperpolarized the cells and, in five out of six arteries, CNP produced hyperpolarizations of varying amplitudes and the mean value is shown in Figure 1.
Under these experimental conditions, ANP (1 μM) produced small but measurable hyperpolarizations, which never exceeded 7 mV (4.4±1.0 mV, n=5 and 4.2±0.2 mV, n=2, in resting and phenylephrine-depolarized carotid arteries, respectively).
Effects of CNP in the presence of the combination of indomethacin plus NG-nitro-L-arginine and thiorphan (5 μM)
Control conditions
In the presence of the three inhibitors, acetylcholine produced an endothelium-dependent hyperpolarization of the guinea-pig carotid arteries. CNP hyperpolarized 36 out of 42 preparations with endothelium (86%) and eight out of nine preparations without endothelium (89%). The changes in membrane potential induced by CNP varied widely from 0 to −17 mV in arteries with and from −1 to −12 mV in preparations without endothelium (Figure 1, Table 1). In arteries without endothelium, sodium nitroprusside (5 μM) hyperpolarized the smooth muscle cells (14.1±1.5 mV, n=7), as did levcromakalim (1 μM, Table 1). In the presence of phenylephrine (0.3 μM), acetylcholine produced hyperpolarizations and so did CNP in four out of five preparations (Figure 1, last panel).
Table 1.
Resting MP (mV) and hyperpolarizations (mV) induced by either acetylcholine (1 μM), CNP (1 μM), ANP (1 μM) and levcromakalim (1 μM) of smooth muscle cells of guinea-pig isolated carotid arteries with and without endothelium, in the presence of indomethacin (5 μM) plus Nω-nitro-L-arginine (100 μM) and thiorphan (5 μM).
| MP | Acetylcholine | CNP | ANP | Levcromakalim | |
|---|---|---|---|---|---|
| With endothelium | −51.1±0.7 | 22.4±0.6 | 5.2±0.7a | 1.1±0.7a | 19.4±1.5 |
| n=72 | n=72 | n=42 | n=3 | n=9 | |
| Without endothelium | −54.5±1.9 | 2.9±0.9b | 5.0±1.3 | 0.8±0.8 | 21.3±3.3a |
| n=12 | n=9 | n=9 | n=2 | n=6 |
Abbreviations: ANOVA, analysis of variance; ANP, atrial natriuretic factor; CNP, C-type natriuretic peptide; MP, membrane potential.
Data are shown as means±s.e.m. N indicates the number of cells recorded.
Statistically significant difference from the hyperpolarizations produced by acetylcholine.
A statistically significant difference between values obtained in arteries with and without endothelium (ANOVA followed by Bonferroni's post hoc test or unpaired t-test, P<0.05).
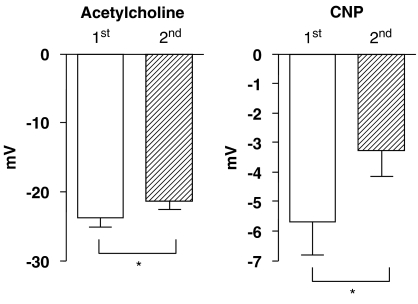
Upon repeated administration of CNP, during continuous recording from the same impaled cell, a significant tachyphylaxis of the hyperpolarization was observed. The CNP-induced hyperpolarization was significantly reduced but still measurable upon the second exposure (Figure 2). Under identical experimental conditions, the acetycholine-induced hyperpolarization, although significantly reduced by approximately 2.5 mV, remained robust (Figure 2).
Figure 2.
Amplitude of hyperpolarizations elicited by two consecutive administrations of acetylcholine (1 μM) and CNP (1 μM), as measured during a continuous recording from the same impaled cell, in guinea-pig carotid artery with endothelium, in the presence of NG-nitro-L-arginine (100 μM) plus indomethacin (5 μM) and thiorphan (5 μM). Data are shown as means±s.e.m. from measurements performed in 15 different cells from arteries taken from 15 different guinea-pigs. * indicates a statistically significant difference between the first and second administration (paired t-test, P<0.05). In both the cases, the amplitude of acetylcholine-induced hyperpolarization was significantly larger than that of CNP (unpaired t test, P<0.05). CNP, C-type natriuretic peptide.
Under the same conditions, ANP produced minimal changes in membrane potential in preparations with and without endothelium (Table 1).
Potassium channel blockers
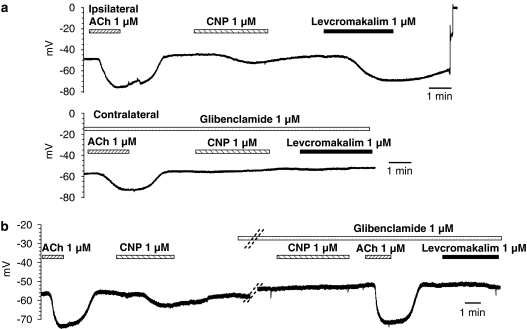
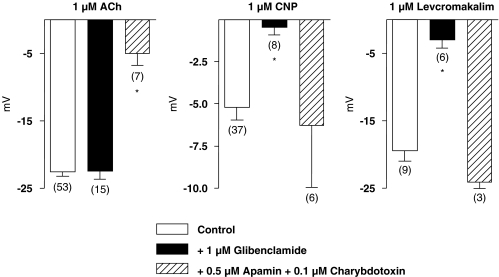
Glibenclamide (1 μM) did not affect the membrane potential of the vascular smooth muscle cells (−51.4±1.0. mV, n=14) or the hyperpolarization evoked by acetylcholine. However, it abolished both levcromakalim- (1 μM) and CNP-induced hyperpolarizations (Figures 3 and 4). The combination of charybdotoxin (0.1 μM) plus apamin (0.5 μM) produced a small depolarization (membrane potential: −48.0±3.6 mV, n=10), and inhibited the hyperpolarization to acetylcholine, but did not affect that to CNP or levcromakalim (Figure 4).
Figure 3.
Effect of glibenclamide (1 μM) on acetylcholine (ACh: 1 μM)-, CNP (1 μM)- and levcromakalim (1 μM)-induced hyperpolarizations in guinea-pig carotid artery with endothelium, in the presence of NG-nitro-L-arginine (100 μM) plus indomethacin (5 μM) and thiorphan (5 μM). Original recordings showing the effects of acetylcholine, CNP and levcromakalim under control conditions (top trace: ipsilateral carotid artery) and in the presence of glibenclamide (lower trace: contralateral carotid artery). Original recordings showing the effects of acetylcholine and CNP in the presence or absence of glibenclamide, as measured during a continuous recording from the same impaled cell. The parallel dotted lines indicate a 15-min incubation with glibenclamide. In both the cases, glibenclamide prevented the hyperpolarizations in response to CNP or levcromakalim. The bars shown under the name of each substance indicate the duration of exposure with the time points of administration and washout. CNP, C-type natriuretic peptide.
Figure 4.
Summary bar graphs showing the inhibitory effects of glibenclamide (1 μM) and that of the combination of charybdotoxin (0.1 μM) plus apamin (0.5 μM) on acetylcholine (ACh: 1 μM)-, CNP (1 μM)- and levcromakalim (1 μM)-induced hyperpolarizations, in guinea-pig carotid arteries with endothelium, in the presence of NG-nitro-L-arginine (100 μM) plus indomethacin (5 μM) and thiorphan (5 μM). Data are shown as means±s.e.m. The numbers in parenthesis indicate the number of cells recorded. Controls have been pooled for the sake of clarity. * indicates a statistically significant effect of an inhibitor (unpaired t-test, P<0.05). CNP, C-type natriuretic peptide.
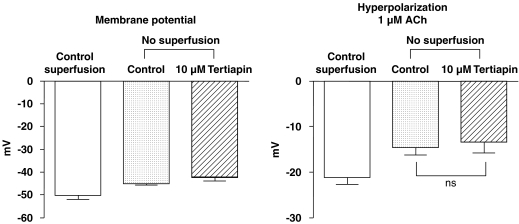
In the absence of superfusion while tertiapin is present or absent (10 μM), the membrane potential of the smooth muscle cells was not significantly different from that measured in control superfused arteries (Figure 5). Thus, the figure also shows that tertiapin did not significantly affect the acetylcholine-induced hyperpolarization.
Figure 5.
Summary bar graphs showing the effects of tertiapin (10 μM) on resting membrane potential (left panel) and acetylcholine (ACh: 1 μM; right panel)-induced hyperpolarization in guinea-pig carotid arteries with endothelium, in the presence of NG-nitro-L-arginine (100 μM) plus indomethacin (5 μM) and thiorphan (5 μM). Measurement of changes in resting membrane potential and hyperpolarization to acetylcholine in continuously superfused carotid arteries are also shown. Data are shown as means±s.e.m. of six different carotid arteries originating from six different guinea-pigs. NS indicates a not statistically significant difference (ANOVA, followed by Bonferroni post hoc test for paired experiments, P>0.05). ANOVA, analysis of variance.
Discussion
The present study confirms that, in the guinea-pig carotid artery with endothelium, acetylcholine produced hyperpolarizations of the smooth muscle in the presence or absence of NG-nitro-L-arginine, indomethacin and/or thiorphan, inhibitors of NO-synthases, cyclooxygenases or neutral endopeptidase, respectively. Furthermore, acetycholine not only produced hyperpolarizations in resting arteries, but also repolarized phenylephrine-depolarized smooth muscle cells. Finally, the hyperpolarizations evoked by acetylcholine were endothelium-dependent and abolished by the combination of the KCa channels blockers, apamin plus charybdotoxin, but unaffected by the KATP blocker, glibenclamide (Corriu et al., 1996; Chataigneau et al., 1998a; Quignard et al., 2000a; Gluais et al., 2005).
The observation that in the guinea-pig carotid artery, ANP inconsistently produced minor hyperpolarizations is in agreement with previous findings that, in the aorta of the same species, opening of potassium channels and changes in membrane potential are only minor contributors to relaxation following the activation of NPR-A receptors (Otsuka et al., 2002).
CNP causes endothelium-independent relaxations (Potter et al., 2006), although an endothelium-dependent component of the vasodilator response to the peptide has been described in some vascular beds, such as the rat coronary microcirculation (Brunner and Wolkart, 2001). In the present study, the effects of CNP were endothelium-independent and unmasked by the inhibition of NO synthases and/or cyclooxygenases. The potentiation of the response to natriuretic peptides (including CNP) by inhibitors of NO-synthases has been attributed to modulation of the particulate guanylate cyclase associated-receptors, NPR-A and NPR-B, by a NO- and cyclic-GMP-dependent mechanism (Madhani et al., 2003; Hobbs et al., 2004). This then suggests that, in the guinea-pig carotid artery, the hyperpolarizing effect of CNP involves particulate guanylate cyclase, possibly via the activation of NPR-B receptors. However, the amplitude of the hyperpolarization evoked by high concentrations of CNP, in the guinea-pig carotid artery, was highly variable and in average much smaller that observed in the rat mesenteric artery (Chauhan et al., 2003; Villar et al., 2007). However, the amplitude of CNP-induced hyperpolarization in the present study was similar to that reported previously in the porcine coronary artery (Barton et al., 1998).
The variability of the effects of CNP could have been explained by varying degree of enzymatic degradation. For instance in the guinea-pig carotid artery and under similar experimental conditions to those of the present study, hyperpolarization to substance P is observed only in the presence of thiorphan and perindoprilat, inhibitors of neutral endopeptidase 24.11 and angiotensin converting enzyme, respectively (Quignard et al., 2000a). CNP is the most susceptible of all natriuretic peptides to metabolism by neutral endopeptidase and is rapidly degraded by this enzyme (Kenny et al., 1993; Brandt et al., 1997). Thus, inhibitors of neutral endopeptidase can enhance the effects of CNP both in vivo and in vitro (Seymour et al., 1996; Honing et al., 2001; Marton et al., 2005; Garcha and Hughes, 2006), although this is not always the case (Kugiyama et al., 1996; Brandt et al., 1997; Kelsall et al., 2005). In agreement with this latter conclusion, in the present in vitro study, thiorphan did not enhance the CNP or the ANP-induced hyperpolarizations and did not reduce the large variability in amplitude of the response to CNP. Variations in the inactivation of CNP by the clearance receptor NPR-C and/or in the activity of phosphodiesterases controlling cGMP levels in smooth muscle cells may explain the phenomenon (Barber et al., 1998).
In the guinea-pig carotid artery, the acetylcholine-induced endothelium-dependent hyperpolarization is reproducible (Chataigneau et al., 1998b) as confirmed in the present study. By contrast, the hyperpolarization elicited by a second stimulation with CNP was attenuated. This is best explained by the observation that CNP produces rapid (in the minutes following a brief exposure) desensitization of the NPR-B receptor by a phosphorylation-dependent regulatory mechanism (Potter, 1998) and, after a prolonged exposure, induces the downregulation of the expression of NPR-B receptors (Rahmutula and Gardner, 2005).
CNP induces hyperpolarization of vascular smooth muscle by activating BKCa (Wei et al., 1994; Banks et al., 1996; Barber et al., 1998; Garcha and Hughes, 2006) and/or KATP (Wei et al., 1994; Barber et al., 1998) and possibly also GIRK (Chauhan et al., 2003; Villar et al., 2007). The present study demonstrates that glibenclamide, at a concentration that abolished the hyperpolarizing effect of levcromakalim, without affecting the endothelium-dependent hyperpolarization to acetylcholine, prevented the hyperpolarization elicited by CNP. This then indicates that CNP activated KATP and also demonstrates that, in the guinea-pig carotid artery, the natriuretic peptide was not involved in acetylcholine-induced endothelium-dependent hyperpolarization (Félétou and Vanhoutte, 2005). The inhibitory effect of glibenclamide probably is explained best if hyperpolarizations to CNP involve a cGMP-dependent component. Indeed, in the same preparation, various NO donors produce KATP-dependent and endothelium-independent hyperpolarizations (Corriu et al., 1996; Quignard et al., 2000b). The hyperpolarization in response to CNP was not influenced by the combination of charybdotoxin plus apamin, which abolished the endothelium-dependent hyperpolarization evoked by acetylcholine, confirming the specificity of the inhibition produced by glibenclamide. Additionally, since charybdotoxin is a nonspecific blocker of both IKCa and BKCa (Garcia et al., 1991), CNP did not activate BKCa in the smooth muscle of the guinea-pig carotid artery.
Tertiapin, a 21-aa peptide extracted from honeybee venom, is a potent and specific inhibitor of renal outer medullar potassium channel (ROMK1 or Kir1.1) and of GIRK1/4 (Kir3.1-Kir3.4; Jin and Lu, 1998). This toxin blocks muscarinic potassium channels in cardiac myocytes with an IC50 of approximately 8 nM (Kitamura et al., 2000). Chauhan et al. (2003), in the study that concluded that CNP can contribute to EDHF-mediated responses, used tertiapin at the concentration of 10 μM. In the present study, the same concentration of tertiapin did not affect the membrane potential of the guinea-pig carotid artery and did not affect the acetylcholine-induced hyperpolarization. These results contrast with those in the rat mesenteric artery (Chauhan et al., 2003). This discrepancy could possibly be explained by the fact that tertiapin, although selective for GIRK and ROMK, at concentrations up to 1 μM, can inhibit other populations of inwardly rectifying potassium channels at higher concentrations (Jin and Lu, 1998; Kitamura et al., 2000). EDHF-mediated responses are associated with the opening of endothelial IKCa and SKCa, which causes an efflux of potassium ions that accumulate in the intercellular space (Edwards et al., 1998; Burnham et al., 2002; Bychkov et al., 2002). In rat and murine mesenteric arteries, but not in the guinea-pig carotid artery, potassium ions activate smooth muscle Kir2.1 and this pathway contributes to the overall EDHF-mediated responses (Edwards et al., 1998, 1999; Quignard et al., 1999; McGuire et al., 2004). A nonspecific effect of an elevated concentration of tertiapin (10 μM) could explain, in the rat mesenteric artery, the partial inhibition of EDHF-mediated responses, which partially rely on Kir activation (Chauhan et al., 2003), while the same elevated concentration of tertiapin has no effect in the guinea-pig carotid artery. Although EDHF-mediated responses in the murine mesenteric artery partially rely on the activation of Kir, the concentration of tertiapin tested being much lower (100 nM) nonspecific effects of the inhibitor were not observed (McGuire et al., 2004).
In the guinea-pig carotid and in rat mesenteric arteries, acetylcholine produces a true hyperpolarization (that is, driving the membrane potential below the resting membrane potential toward the equilibrium value for potassium ions) in quiescent tissues and both repolarization and hyperpolarization in phenylephrine-depolarized tissue. EDHF-mediated responses can evoke both repolarization and hyperpolarization, since the activation of SKCa and IKCa is virtually voltage-independent (Burnham et al., 2002; Bychkov et al., 2002), although the contribution of each channel subtype in these different phenomena may depend on their subcellular localization (Crane et al., 2003; Sandow et al., 2006). Levcromakalim, the opener of KATP, can also produce both hyperpolarization and repolarization, since KATP shows only small inward rectification (Edwards and Weston, 1995). In the present study, CNP induced both true hyperpolarization and repolarization of phenylephrine-depolarized smooth muscle cells, an observation consistent with the activation of KATP. In rat mesenteric arteries, the studies performed by Chauhan et al. (2003) and Villar et al. (2007) involved the presence of a contractile agent that depolarized the smooth muscle cells. CNP repolarized the smooth muscle cells, bringing back the membrane potential close to the resting membrane potential. Whether or not CNP is able to truly hyperpolarize the smooth muscle of the rat mesenteric artery is unknown.
In conclusion, the present study demonstrates that natriuretic peptides are weak hyperpolarizing agents in the isolated carotid artery of the guinea-pig. CNP-induced hyperpolarizations involve probably a GMP-dependent mechanism via the activation of NPR-B receptors. In the guinea-pig carotid artery, as in porcine coronary and murine mesenteric arteries, the contribution of CNP to EDHF-mediated responses appears to be most unlikely.
Abbreviations
- ANP
atrial natriuretic peptide
- BKCa
large conductance calcium-activated potassium channel
- CNP
C-type natriuretic peptide
- IKCa
intermediate conductance calcium-activated potassium channel
- KATP
ATP-sensitive potassium channel
- GIRK
G-protein regulated inward-rectifier potassium channel
- SKCa
small conductance calcium-activated potassium channel
Conflict of interest
The authors state no conflict of interest.
References
- Ahluwalia A, Hobbs AJ. Endothelium-derived C-type natriuretic peptide: more than just a hyperpolarizing factor. Trends Pharmacol Sci. 2005;26:162–167. doi: 10.1016/j.tips.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Ahluwalia A, Macallister RJ, Hobbs AJ. Vascular actions of natriuretic peptides. Cyclic GMP-dependent and -independent mechanisms. Basic Res Cardiol. 2004;99:83–89. doi: 10.1007/s00395-004-0459-6. [DOI] [PubMed] [Google Scholar]
- Banks M, Wei CM, Kim CH, Burnett JC, Jr, Miller VM. Mechanism of relaxations to C-type natriuretic peptide in veins. Am J Physiol. 1996;271:H1907–H1911. doi: 10.1152/ajpheart.1996.271.5.H1907. [DOI] [PubMed] [Google Scholar]
- Barber DA, Burnett JC, Jr, Fitzpatrick LA, Sieck GC, Miller VM. Gender and relaxation to C-type natriuretic peptide in porcine coronary arteries. J Cardiovasc Pharmacol. 1998;32:5–11. doi: 10.1097/00005344-199807000-00002. [DOI] [PubMed] [Google Scholar]
- Barton M, Beny JL, D'uscio LV, Wyss T, Noll G, Luscher TF. Endothelium-independent relaxation and hyperpolarization to C-type natriuretic peptide in porcine coronary arteries. J Cardiovasc Pharmacol. 1998;31:377–383. doi: 10.1097/00005344-199803000-00008. [DOI] [PubMed] [Google Scholar]
- Boussery K, Delaey C, Van De Voorde J. The vasorelaxing effect of CGRP and natriuretic peptides in isolated bovine retinal arteries. Invest Ophthalmol Vis Sci. 2005;46:1420–1427. doi: 10.1167/iovs.04-1093. [DOI] [PubMed] [Google Scholar]
- Brandt RR, Mattingly MT, Clavell AL, Barclay PL, Burnett JC., Jr Neutral endopeptidase regulates C-type natriuretic peptide metabolism but does not potentiate its bioactivity in vivo. Hypertension. 1997;30:184–190. doi: 10.1161/01.hyp.30.2.184. [DOI] [PubMed] [Google Scholar]
- Brunner F, Wolkart G. Relaxant effect of C-type natriuretic peptide involves endothelium and nitric oxide-cGMP system in rat coronary microvasculature. Cardiovasc Res. 2001;51:577–584. doi: 10.1016/s0008-6363(01)00283-8. [DOI] [PubMed] [Google Scholar]
- Burnham MP, Bychkov R, Feletou M, Richards GR, Vanhoutte PM, Weston AH, et al. Characterization of an apamin-sensitive small conductance Ca2+-activated K+ channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol. 2002;135:1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. Endothelium-dependent hyperpolarization, bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- Bychkov R, Burnham MP, Richards GR, Edwards G, Weston AH, Félétou M, et al. Characterization of a charybdotoxin-sensitive intermediate conductance Ca2+-activated K+ channel in porcine coronary endothelium: relevance to EDHF. Br J Pharmacol. 2002;138:1346–1354. doi: 10.1038/sj.bjp.0705057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- Chataigneau T, Félétou M, Duhault J, Vanhoutte PM. Epoxyeicosatrienoic acids, potassium channel blockers and endothelium-dependent hyperpolarisation in the guinea-pig carotid artery. Br J Pharmacol. 1998a;123:574–580. doi: 10.1038/sj.bjp.0701629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chataigneau T, Félétou M, Thollon C, Villeneuve N, Vilaine JP, Duhault J, et al. Cannabinoid CB1 receptor and endothelium-dependent hyperpolarisation in guinea-pig carotid, rat mesenteric and porcine coronary arteries. Br J Pharmacol. 1998b;123:968–974. doi: 10.1038/sj.bjp.0701690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci USA. 2003;100:1426–1431. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor AT, Evans WH, Griffith TM. Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries. J Physiol. 1998;508:561–573. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriu C, Félétou M, Canet E, Vanhoutte PM. Endothelium-derived factors and hyperpolarisations of the isolated carotid artery of the guinea-pig. Br J Pharmacol. 1996;119:959–964. doi: 10.1111/j.1476-5381.1996.tb15765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane GJ, Gallagher N, Dora KA, Garland CJ. Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J Physiol. 2003;553:183–189. doi: 10.1113/jphysiol.2003.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- Edwards G, Feletou M, Gardener MJ, Thollon C, Vanhoutte PM, Weston AH. Role of gap junctions in the responses to EDHF in rat and guinea-pig small arteries. Br J Pharmacol. 1999;128:1788–1794. doi: 10.1038/sj.bjp.0703009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G, Weston AH.Potassium channels in the regulation of vascular smooth muscle tone Pharmacologicol Control of Calcium and Potassium Homeostasis: Biological, Therapeutical and Clinical aspects 1995Kluwer Academic Press: Dordrecht; 85–93.In: Godfraind T, Mancia G, Abbracchio MP, Aguilar-Bryan L, Govoni S (eds) [Google Scholar]
- Feletou M, Teisseire B. Converting enzyme inhibition in isolated porcine resistance artery potentiates bradykinin relaxation. Eur J Pharmacol. 1990;190:159–166. doi: 10.1016/0014-2999(90)94122-e. [DOI] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. EDHF: the Complete Story. Taylor & Francis, CRC press: Boca Raton, Fl; 2005. [Google Scholar]
- Feletou M, Vanhoutte PM. EDHF: where are we now. Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- Fleming I. Cytochrome P450 epoxygenases as EDHF synthase(s) Pharmacol Res. 2004;49:525–533. doi: 10.1016/j.phrs.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of the endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garcha RS, Hughes AD. CNP, but not ANP or BNP, relax human isolated subcutaneous resistance arteries by an action involving cyclic GMP and BKCa channels. J Renin Angiotensin Aldosterone Syst. 2006;7:87–91. doi: 10.3317/jraas.2006.014. [DOI] [PubMed] [Google Scholar]
- Garcia ML, Galvez A, Garcia-Calvo M, King VF, Vazquez J, Kaczorowski GJ. Use of toxins to study potassium channels. J Bioenerg Biomembr. 1991;23:615–646. doi: 10.1007/BF00785814. [DOI] [PubMed] [Google Scholar]
- Gluais P, Edwards G, Weston AH, Falck JR, Vanhoutte PM, Félétou M. Role of Skca and ikca in the endothelium-dependent hyperpolarization of the guinea-pig isolated carotid artery. Br J Pharmacol. 2005;144:477–485. doi: 10.1038/sj.bjp.0706003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker M, Bara AT, Bauersachs J, Busse R. Characterization of endothelium-derived hyperpolarizing factor as a cytochrome P450-derived arachidonic acid metabolite in mammals. J Physiol. 1994;481:407–414. doi: 10.1113/jphysiol.1994.sp020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs A, Foster P, Prescott C, Scotland R, Ahluwalia A. Natriuretic peptide receptor-C regulates coronary blood flow and prevents myocardial ischemia/reperfusion injury: novel cardioprotective role for endothelium-derived C-type natriuretic peptide. Circulation. 2004;110:1231–1235. doi: 10.1161/01.CIR.0000141802.29945.34. [DOI] [PubMed] [Google Scholar]
- Honing ML, Smits P, Morrison PJ, Burnett JC, Jr, Rabelink TJ. C-type natriuretic peptide-induced vasodilation is dependent on hyperpolarization in human forearm resistance vessels. Hypertension. 2001;37:1179–1183. doi: 10.1161/01.hyp.37.4.1179. [DOI] [PubMed] [Google Scholar]
- Jin W, Lu Z. A novel high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry. 1998;37:13291–13299. doi: 10.1021/bi981178p. [DOI] [PubMed] [Google Scholar]
- Kelsall CJ, Chester AH, Amrani M, Singer DR. C-type natriuretic peptide relaxes human coronary artery bypass grafts preconstricted by endothelin-1. Ann Thorac Surg. 2005;80:1347–1351. doi: 10.1016/j.athoracsur.2005.01.069. [DOI] [PubMed] [Google Scholar]
- Kenny AJ, Bourne A, Ingram J. Hydrolysis of human and pig brain natriuretic peptides, urodilatin, C-type natriuretic peptide and some C-receptor ligands by endopeptidase-24.11. Biochem J. 1993;291:83–88. doi: 10.1042/bj2910083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura H, Yokoyama M, Akita H, Matsushita K, Kurachi Y, Yamada M. Tertiapin potently and selectively blocks muscarinic K(+) channels in rabbit cardiac myocytes. J Pharmacol Exp Ther. 2000;293:196–205. [PubMed] [Google Scholar]
- Koller KJ, Goeddel DV. Molecular biology of the natriuretic peptides and their receptors. Circulation. 1992;86:1081–1088. doi: 10.1161/01.cir.86.4.1081. [DOI] [PubMed] [Google Scholar]
- Kugiyama K, Sugiyama S, Matsumura T, Ohta Y, Doi H, Yasue H. Suppression of atherosclerotic changes in cholesterol-fed rabbits treated with an oral inhibitor of neutral endopeptidase 24.11 (EC 3.4.24.11) Arterioscler Thromb Vasc Biol. 1996;16:1080–1087. doi: 10.1161/01.atv.16.8.1080. [DOI] [PubMed] [Google Scholar]
- Madhani M, Scotland RS, Macallister RJ, Hobbs AJ. Vascular natriuretic peptide receptor-linked particulate guanylate cyclases are modulated by nitric oxide-cyclic GMP signalling. Br J Pharmacol. 2003;139:1289–1296. doi: 10.1038/sj.bjp.0705365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton Z, Pataricza J, Krassoi I, Varro A, Papp JG. NEP inhibitors enhance C-type natriuretic peptide-induced relaxation in porcine isolated coronary artery. Vascul Pharmacol. 2005;43:207–212. doi: 10.1016/j.vph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- McGuire JJ, Hollenberg MD, Bennett BM, Triggle CR. Hyperpolarization of murine small caliber mesenteric arteries by activation of endothelial proteinase-activated receptor 2. Can J Physiol Pharmacol. 2004;82:1103–1112. doi: 10.1139/y04-121. [DOI] [PubMed] [Google Scholar]
- Moncada S, Vane JR. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2 and prostacyclin. Pharmacol Rev. 1979;30:293–331. [PubMed] [Google Scholar]
- Otsuka K, Tanaka H, Horinouchi T, Koike K, Shigenobu K, Tanaka Y. Functional contribution of voltage-dependent and Ca2+ activated K+ (BK(Ca)) channels to the relaxation of guinea-pig aorta in response to natriuretic peptides. J Smooth Muscle Res. 2002;38:117–129. doi: 10.1540/jsmr.38.117. [DOI] [PubMed] [Google Scholar]
- Potter LR. Phosphorylation-dependent regulation of the guanylyl cyclase-linked natriuretic peptide receptor B: dephosphorylation is a mechanism of desensitization. Biochemistry. 1998;37:2422–2429. doi: 10.1021/bi972303k. [DOI] [PubMed] [Google Scholar]
- Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- Quignard JF, Félétou M, Corriu C, Chataigneau T, Edwards G, Weston AH, et al. 3-Morpholinosydnonimine (SIN-1) and K+ channels in smooth muscle cells of the rabbit and guinea-pig carotid arteries. Eur J Pharmacol. 2000b;399:9–16. doi: 10.1016/s0014-2999(00)00372-1. [DOI] [PubMed] [Google Scholar]
- Quignard JF, Félétou M, Duhault J, Vanhoutte PM. Potassium ions as endothelium-derived hyperpolarizing factors in the isolated carotid artery of the guinea-pig. Br J Pharmacol. 1999;127:27–34. doi: 10.1038/sj.bjp.0702493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quignard JF, Feletou M, Edwards G, Duhault J, Weston AH, Vanhoutte PM. Role of endothelial cells hyperpolarization in EDHF-mediated responses in the guinea-pig carotid artery. Br J Pharmacol. 2000a;129:1103–1112. doi: 10.1038/sj.bjp.0703175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmutula D, Gardner DG. C-type natriuretic peptide down-regulates expression of its cognate receptor in rat aortic smooth muscle cells. Endocrinology. 2005;146:4968–4974. doi: 10.1210/en.2005-0262. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: possible relationship to vasodilator function. J Anat. 2006;209:689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow SL, Tare M. C-type natriuretic peptide: a new endothelium-derived hyperpolarizing factor. Trends Pharmacol Sci. 2007;28:61–67. doi: 10.1016/j.tips.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Seymour AA, Mathers PD, Abboa-Offei BE, Asaad MM, Weber H. Renal and depressor activity of C-natriuretic peptide in conscious monkeys: effects of enzyme inhibitors. J Cardiovasc Pharmacol. 1996;28:397–401. doi: 10.1097/00005344-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Stingo AJ, Clavell AL, Heublein DM, Wei CM, Pittlekow MR, Burnett JC., Jr Presence of C-type natriuretic peptide in cultured human endothelial cells and plasma. Am J Physiol. 1992;263:H1318–H1321. doi: 10.1152/ajpheart.1992.263.4.H1318. [DOI] [PubMed] [Google Scholar]
- Suga S, Itoh H, Komatsu Y, Ogawa Y, Hama N, Yoshimasa T, et al. Cytokine-induced C-type natriuretic peptide (CNP) secretion from vascular endothelial cells—evidence for CNP as a novel autocrine/paracrine regulator from endothelial cells. Endocrinology. 1993;133:3038–3041. doi: 10.1210/endo.133.6.8243333. [DOI] [PubMed] [Google Scholar]
- Villar IC, Panayiotou CM, Sheraz A, Madhani M, Scotland RS, Nobles M, et al. Definitive role for natriuretic peptide receptor-C in mediating the vasorelaxant activity of C-type natriuretic peptide and endothelium-derived hyperpolarising factor. Cardiovasc Res. 2007;74:515–525. doi: 10.1016/j.cardiores.2007.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CM, Hu S, Miller VM, Burnett JC., Jr Vascular actions of C-type natriuretic peptide in isolated porcine coronary arteries and coronary vascular smooth muscle cells. Biochem Biophys Res Comm. 1994;205:765–771. doi: 10.1006/bbrc.1994.2731. [DOI] [PubMed] [Google Scholar]